Abstract
The present study aims to clarify the possible involvement of parvovirus B19 (B19V) infection in rheumatoid arthritis (RA) pathogenesis by investigating the presence of B19V infection markers (genomic sequences and virus-specific antibodies) in association with the level of cytokines and RA clinical activity and aggressiveness. A total of 118 RA patients and 49 age- and sex-matched healthy volunteers were enrolled in the study. Nested PCR was used to detect B19V sequences in whole blood and cell-free plasma DNA, ELISA to detect virus-specific antibodies and cytokine levels in plasma and recomLine dot blot assay for antibodies to separate B19V antigens. The detection frequency of B19V DNA was higher in patients with RA (25.4 %) in comparison with healthy persons (18.4 %). B19V DNA in cell-free plasma (B19+p) was detected significantly often in RA patients in comparison with healthy controls (13.6 vs 2 %; P=0.0002). RA B19+p patients had higher disease activity and aggressiveness, decreased haemoglobin and increased erythrocyte sedimentation rates. IL-6 plasma levels were significantly higher in RA patients than in controls. Within the RA patients’ group the IL-6 level was significantly increased in B19+p patients with disease activity scores of DAS28>5.2, high C-reactive protein and low haemoglobin. Contrary to the healthy controls, the majority of RA B19+p patients did not have antibodies to VP-1S (VP1u) and VP-N (N-terminal half of structural proteins VP1 and VP2), which correspond to the epitopes of neutralizing antibodies. These results indicate that B19V infection at least in some patients is involved in RA pathogenesis.
Keywords: Parvovirus B19, rheumatoid arthritis, antibodies, IL-6
Introduction
Human parvovirus B19 (B19V) is a small DNA virus with a worldwide distribution. The genome of B19V consists of single-stranded 5.6 kb DNA that encodes three large proteins. The non-structural protein NS1 is composed of 671 aa. Structural capsid proteins VP1 and VP2 are encoded in the same ORF with production of proteins of 84 and 58 kDa, respectively. VP1 and VP2 are identical, with the exception of 227 aa at the amino-terminal end of the VP1 protein, the so-called VP1-unique region (VP1u) (Cotmore et al., 1986; Ozawa & Young, 1987; Ozawa et al., 1987). B19V is normally transmitted through the respiratory route, but can also be transmitted from the mother to the foetus (Berry et al., 1992; Brown et al., 1984; Clewley et al., 1987; Kinney et al., 1988; Musiani et al., 1999), through bone marrow (Cohen et al., 1997; Heegaard & Laub Petersen, 2000; Ozawa & Young, 1987) and organ transplantations (Bertoni et al., 1997; Lui et al., 2001), and via transfused blood products (Juhl et al., 2014). B19V replicates in red blood cell precursors in the bone marrow, however, it could persist in other cells (Bratslavska et al., 2015; Millá et al., 1993; Morey & Fleming, 1992; Söderlund-Venermo et al., 2002). B19V DNA was found in peripheral blood and synovial fluid cells (Kozireva et al., 2008), and VP1 protein was detected in synovial cells including lymphocytes, macrophages and neutrophils (Takahashi et al., 1998). Although antibodies to conformational and linear epitopes of VP1 and VP2 capsid proteins were detected (Azzi et al., 2004; Corcoran et al., 2000; Manaresi et al., 1999, 2004; Söderlund et al., 1995), the most important are neutralizing antibodies directed to the VP1u and VP1–VP2 junction regions (Saikawa et al., 1993; Zuffi et al., 2001).
B19V is associated with many clinical disorders. This virus causes erythema infectiosum (fifth disease) in children. B19V infection can be asymptomatic or can cause various complications such as arthralgia, arthritis, transient aplastic crisis and chronic anaemia in adults (Chorba et al., 1986; Dijkmans et al., 1988; Lefrère et al., 1986; Reid et al., 1985; White et al., 1985). There is some evidence that B19V infection is more frequent in patients with certain autoimmune diseases [juvenile rheumatoid arthritis (RA) (Oğuz et al., 2002), systemic lupus erythematosus (Hsu & Tsay, 2001), Sjögren's syndrome (Ramos-Casals et al., 2000), thyroiditis (Lehmann et al., 2008; Wang et al., 2010) and chronic fatigue syndrome (Chapenko et al., 2012)] and can even play a role in pathogenesis. However, some publications suggesting that B19V does not play any role in the pathogenesis, but may present a clinical and serological tableau making it difficult to distinguish between a viral infection and systemic lupus erythematosus (Moore et al., 1999; Sève et al., 2005). Some authors did not find a link between B19V infection and Sjögren's syndrome (De Re et al., 2002; De Stefano et al., 2003) or juvenile arthritis (Söderlund et al., 1997). B19V infection is often associated with chronic arthritis, although aetiologic associations with RA are conflicting (Kerr, 2000; Takahashi et al., 1998).
RA is one of the most common inflammatory autoimmune diseases characterized by persistent synovitis, systemic inflammation and production of autoantibodies (Scott et al., 2010). The molecular mechanisms of RA pathogenesis are not fully understood. It is believed that approximately half of the risk factors for RA are attributable to genetic factors such as the HLA alleles, while the other half of the risks are environmental factors including infection and smoking (Arleevskaya et al., 2014; McInnes & Schett, 2011; Okada et al., 2014). Clinical and animal model studies have suggested that infections by many micro-organisms, such as Porphyromonas gingivalis (Bartold et al., 2010; Hitchon et al., 2010), Proteus mirabilis (Arabski et al., 2012), Epstein–Barr virus (Mourgues et al., 2016) and mycoplasma contribute to the aetiopathogenesis of RA (Durigon et al., 1993). Higher rates of serological evidence of infections with Epstein–Barr virus and cytomegalovirus were detected in RA patients than in population controls (Magnusson et al., 2010; Meron et al., 2010).
The aim of the present study was to clarify the possible involvement of B19V infection in RA pathogenesis by investigating the presence of B19V infection markers in association with RA clinical activity and the level of cytokines.
Results
Frequency of B19V DNA in RA patients and healthy controls
In this study we investigated the frequency of B19V infection markers in the blood of RA patients. The B19V genomic DNA was determined in whole blood and cell-free plasma (Table 1). Persons in whose blood or plasma B19V DNA was detected were named B19+. If virus DNA was not detected, such persons were named B19−. The mean age of B19+ and B19− RA patients and control groups’ individuals was 58.3±13.6 and 58.3±13.0 years and 55.3±9.0 and 49.2±11.3 years, respectively. The frequency of B19V DNA was higher in patients with RA (30/118; 25.4 %) in comparison with control group individuals (9/49; 18.4 %). The same tendency was observed in both males [6/19 (31.6 %) of RA patients and 2/12 (16.7 %) of controls were B19V DNA positive] and females [24/99 (24.2 %) of RA patients and 7/37 (18.9 %) of controls were B19V DNA positive]. B19V DNA was detected in whole blood (B19+b), cell-free plasma (B19+p) or both whole blood and plasma (B19+b/p). Table 1 shows the localization of virus DNA in all investigated groups. In RA patients B19V DNA was mostly found in the cell-free plasma (53 % from all B19+), while in the control group the virus DNA was found mostly in the whole blood (89 % from all B19+). Our results showed that the frequency of B19V latent/persistent (B19V sequences presented only in DNA extracted from the whole blood) and active (B19V sequences presented in DNA extracted from the cell-free blood plasma) infection markers is different in RA patients and healthy people (Table S1, available in the online Supplementary Material). We investigated how the localization of virus DNA (blood and plasma, blood or plasma) correlates with B19V seroprevalence and antibody spectrum, the level of cytokines in plasma and disease clinical activity. Table 2 shows the clinical characteristics [white blood cells (WBC), haemoglobin (HgB), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), cyclic citrullinated peptide antibody (anti-CCP), disease activity score (DAS28), disease duration and rheumatoid factor (RF) result] of RA patients according to virus DNA localization. RA patients (68.8 %) from the B19+p group were strongly positive (>60 EU ml−1) for anti-CCPs, while only 25 % of B19+b/p, 33.3 % of B19+b and 48.9 % of B19− patients had anti-CCP levels >60 EU ml−1 (Table 2). Also, 68.8 % of RA patients from the B19+p group had high disease activity scores (DAS28>5.2). There were no more than 50 % of patients with DAS28>5.2 in the groups B19+b, B19+b/p and B19−. A higher percentage of patients from the RA B19+p group had decreased HgB (68.8 %) and increased ESR (87.5 %), in comparison with persons in the other groups. The presence of B19V DNA in RA patients did not influence the expression of RF, disease duration or CRP level (Table 2).
Table 1. Presence of B19V genomic sequence in the blood DNA of RA patients and healthy persons.
The values marked by stars are significant according to Fisher’s exact tests: *P<0.05, **P<0.0005. B19V, Parvovirus B19; B19+, parvovirus B19 DNA positive; B19−, parvovirus B19 DNA negative; RA, rheumatoid arthritis; Contr., healthy persons.
| Investigated persons (total) | Age±sd (years) | B19+ samples | B19− samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole blood | Cell-free blood plasma |
Whole blood and cell-free blood plasma | All positive | N | % Total | |||||||
| N | % Total | N | % Total | N | % Total | N | % Total | |||||
| Females | ||||||||||||
| Contr. | 37 | 50.3±10.4 | 6 | 16.2 | 1 | 2.7 | 0 | 0 | 7 | 18.92 | 30 | 81.08 |
| RA | 99 | 57.3±13.0 | 6 | 6.1 | 12 | 12.1 | 6 | 6.1 | 24 | 24.24 | 75 | 75.76 |
| Males | ||||||||||||
| Contr. | 12 | 49.8±13.8 | 2 | 16.66 | 0 | 0 | 0 | 0 | 2 | 16.66 | 10 | 83.33 |
| RA | 19 | 63.5±12.7 | 0 | 0 | 4 | 21.1 | 2 | 10.5 | 6 | 31.58 | 13 | 68.42 |
| All investigated (males+females) | ||||||||||||
| Contr. | 49 | 50.2±11.3 | 8 | 16.3** | 1 | 2.0* | 0 | 0 | 9 | 18.37 | 40 | 81.63 |
| RA | 118 | 58.3±13.0 | 6 | 5.1** | 16 | 13.6* | 8 | 6.8 | 30 | 25.42 | 88 | 75.58 |
Table 2. Clinical characteristics of rheumatoid arthritis patients.
Table 2 shows the number of patients with normal values (norm.), higher than normal (>norm.), lower than normal (<norm.) or with appropriate indicated values. The percentage of patients with indicated parameters are shown. WBC, White blood cells; HgB, haemoglobin; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; anti-CCP, cyclic citrullinated peptide antibody; DAS28, disease activity score; RF, rheumatoid factor.
| Clinical data | Parvovirus B19 DNA positive in | Parvovirus B19 DNA negative | |||
|---|---|---|---|---|---|
| Blood and plasma | Blood | Plasma | |||
| WBC (×109 l−1) | Norm. | 5 | 3 | 11 | 46 |
| >Norm. | 3 | 3 | 5 | 42 | |
| % >Norm. | 37.5 | 50.0 | 31.3 | 47.7 | |
| HgB (g l−1) | Norm. | 3 | 4 | 5 | 48 |
| <Norm. | 5 | 2 | 11 | 40 | |
| % <Norm. | 62.5 | 33.3 | 68.8 | 45.5 | |
| ESR (mm h−1) | Norm. | 3 | 3 | 2 | 19 |
| >Norm. | 5 | 3 | 14 | 68 | |
| % >Norm. | 62.5 | 50.0 | 87.5 | 77.3 | |
| CRP (mg l−1) | Norm. | 3 | 2 | 5 | 22 |
| >Norm. | 5 | 4 | 11 | 66 | |
| % >Norm. | 62.5 | 66.7 | 68.8 | 75.0 | |
| Anti-CCP (EU ml−1) | 1–60 | 6 | 4 | 5 | 45 |
| >60 | 2 | 2 | 11 | 43 | |
| % >60 | 25.0 | 33.3 | 68.8 | 48.9 | |
| DAS28 | <5.2 | 4 | 5 | 5 | 43 |
| >5.2 | 4 | 1 | 11 | 43 | |
| % >5.2 | 50.0 | 16.7 | 68.8 | 50.0 | |
| Disease duration (years) | 0–10 | 5 | 2 | 8 | 44 |
| >10 | 3 | 4 | 8 | 44 | |
| % >10 | 37.5 | 66.7 | 50.0 | 50.0 | |
| RF (U ml−1) | 1–100 | 3 | 3 | 9 | 50 |
| >100 | 5 | 3 | 7 | 38 | |
| % >100 | 62.5 | 50.0 | 43.8 | 44.0 | |
Antibodies to B19V antigens in the plasma of RA patients and healthy controls
Antibodies to the main capsid protein VP2 were determined in the plasma of all investigated (118 RA and 49 controls) persons using the Biotrin ELISA kit (Tables 3 and S2). The percentages of RA and control persons carrying antibodies to VP-2P, VPN, VP-1S (VP1u), VP-2r, VP-C and NS-1 are shown in Table 3. The individual raw data are shown in Table S2. Table 3 shows that the majority (78–90 %) of subjects (healthy controls and RA patients, B19V DNA positive or negative) had IgG class antibodies to VP2 protein; however they did not have IgM class antibodies. Part of the plasma samples (55 from RA patients and 28 from healthy persons) were analysed using the Mikrogen Diagnostik's recomLine dot blot kit. According to manufacturer’s (Mikrogen Diagnostik) manual, recomLine dot blot enables determination of antibodies to the various virus antigens: VP-2P (main capsid antigen, conformation epitope), VP-N (the N-terminal half of the structural proteins VP1 and VP2), VP-1S [corresponds to the unique region of VP1 (VP1u)], VP-2r (main capsid antigen, linear epitope), VP-C (the C-terminal half of the structural proteins VP1 and VP2) and NS-1 (non-structural protein) (Pfrepper et al., 2005). VP-2P protein in recomLine dot blot corresponds to the VP2 protein in Biotrin ELISA. Although IgG ELISA seems to be more sensitive [77 samples out of 83 were positive in IgG VP2 ELISA, whereas only 59 samples out of 83 were positive in IgG VP-2P dot blot (Table S2)], the results obtained using Biotrin VP2 IgG ELISA and recomLine with IgG VP-2P dot blot are comparable according to the Spearman's correlation test (r=0.909, P<0.0001). The IgG class antibodies to VP2/VP-2P proteins were detected in the plasma of majority of RA patients’ and controls’ plasma samples (to VP2 in 91 % of RA B19+, 94 % of RA B19−, 79 % of B19+ controls and 100 % of B19− controls; to VP-2P in 77 % of RA B19+, 67 % of RA B19−, 78 % of B19+ controls and 68 % of B19− controls). The seroprevalence of IgG antibodies to VP2/VP-2P did not differ significantly between the groups of RA patients and healthy controls. However, the seroprevalence of IgG antibodies to VP-N and VP-1S was different between RA B19+b and RA B19+p patients. In the RA B19+b group, 80 % of patients had IgG antibodies to VP-N and VP-1S, while in the RA B19+p group, only 46 and 38 % of patients had antibodies to VP-N and VP-1S, respectively. Only a limited number of persons had IgG antibodies to VP-2r (five RA B19+, five RA B19− and three B19− controls), VP-C (one RA B19− and one B19− control) and NS-1 (two B19− controls) (see Tables 3 and S2). Although we did not detect IgM antibodies to VP2 protein by ELISA in the plasma of RA patients and healthy controls, recomLine dot blot assay showed the presence of IgM class antibodies in some RA patients. IgM antibodies to VP-2P were detected in the plasma of 11, to VP-N in 7 and to VP-1S and VP-2r in 2 RA patients (Table S2). IgM antibodies to the proteins mentioned were not detected in the plasma of healthy controls (Tables 3 and S1). The presence of IgM class antibodies in RA did not show an association with the presence of B19 DNA in plasma or whole blood.
Table 3. B19V seroprevalence among RA patients and healthy persons.
Percentage of persons with antibodies to parvovirus B19 VP2 protein (detected using Biotrin ELISA kit) and to various parvovirus B19 antigens (detected using Mikrogen recomLine kit). B19+ RA patients were divided into the groups according to the viral DNA findings: in blood and cell-free blood plasma (b/p), blood (b) and cell-free blood plasma (p). N, Number of persons; B19+, parvovirus B19 DNA positive; B19−, parvovirus B19 DNA negative; Contr., healthy controls.
| All investigated samples | Samples tested by recomLine dot blot | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Positive in VP2 ELISA | N | Positive in VP2 ELISA | Positive in recomLine dot blot | ||||||||||||||
| VP-2P | VP-N | VP-1S | VP-2r | VP-C | NS-1 | |||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |||
| IgG antibodies to B19V proteins | ||||||||||||||||||
| RA B19+b/p | 8 | 7 | 87.5 | 4 | 3 | 75.0 | 3 | 75.0 | 2 | 50.0 | 2 | 50.0 | 2 | 50.0 | 0 | 0 | 0 | 0 |
| RA B19+b | 6 | 5 | 83.3 | 5 | 5 | 100 | 5 | 100 | 4 | 80.0 | 4 | 80.0 | 1 | 20.0 | 0 | 0 | 0 | 0 |
| RA B19+p | 16 | 15 | 93.8 | 13 | 12 | 92.3 | 9 | 69.2 | 6 | 46.2 | 5 | 38.5 | 2 | 15.4 | 0 | 0 | 0 | 0 |
| RA B19+ | 30 | 27 | 90.0 | 22 | 20 | 90.9 | 17 | 77.3 | 12 | 54.5 | 11 | 50.0 | 5 | 22.7 | 0 | 0 | 0 | 0 |
| RA B19− | 88 | 76 | 86.4 | 33 | 31 | 93.9 | 22 | 66.7 | 18 | 54.5 | 14 | 42.4 | 5 | 15.2 | 1 | 3.0 | 1 | 3.0 |
| Contr. B19+ | 9 | 7 | 77.8 | 9 | 7 | 77.8 | 7 | 77.8 | 6 | 66.7 | 6 | 66.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Contr. B19− | 44 | 36 | 81.8 | 19 | 19 | 100 | 13 | 68.4 | 11 | 57.9 | 11 | 57.9 | 3 | 15.8 | 1 | 5.3 | 2 | 10.5 |
| IgM antibodies to B19V proteins | ||||||||||||||||||
| RA B19+b/p | 8 | 0 | 0 | 4 | 0 | 0 | 2 | 50.0 | 2 | 50.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| RA B19+b | 6 | 0 | 0 | 5 | 0 | 0 | 2 | 40.0 | 0 | 0 | 0 | 0 | 1 | 20.0 | 0 | 0 | 0 | 0 |
| RA B19+p | 16 | 0 | 0 | 9 | 0 | 0 | 2 | 22.2 | 3 | 33.3 | 2 | 22.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| RA B19+ | 30 | 0 | 0 | 18 | 0 | 0 | 6 | 33.3 | 5 | 27.8 | 2 | 11.1 | 1 | 5.6 | 0 | 0 | 0 | 0 |
| RA B19− | 88 | 0 | 0 | 12 | 0 | 0 | 5 | 41.7 | 2 | 16.7 | 0 | 0 | 1 | 8.3 | 0 | 0 | 0 | 0 |
| Contr. B19+ | 9 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Contr. B19− | 44 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Levels of IL-6 in the plasma of RA patients and healthy controls
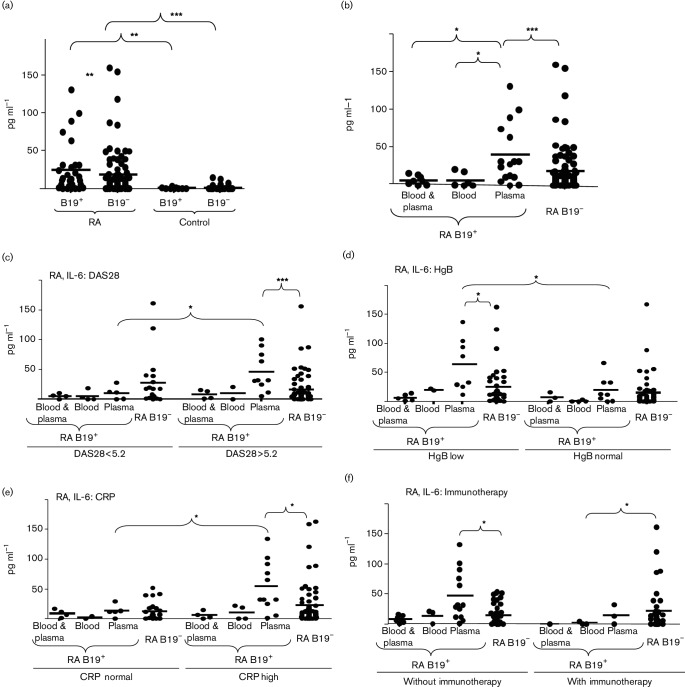
The levels of IL-6 were determined in the blood plasma of all investigated RA patients and controls. The levels of IL-6 were significantly higher in the plasma of RA patients (25.0±33.6 pg ml−1 in B19+ group and 19.0±30.5 pg ml−1 in B19− group) in comparison with the persons in the control group (0.8±1.0 pg ml−1 in B19+ group and 1.6±3.4 pg ml−1 in B19− group) (Fig. 1a). There were no significant differences between the plasma level of IL-6 in B19+ and B19− controls. However, RA B19+ patients had significantly higher average levels of IL-6 than RA B19− patients. Importantly, we found that the IL-6 levels were particularly increased in RA B19+p patients’ group (40.6±39.7 pg ml−1) (Fig. 1b). The levels of IL-6 in patients from the RA B19+b and RA B19+b/p groups were 7.2±9.8 and 7.0±5.9 pg ml−1, respectively. Our data showed that some clinical data (anti-CCP, DAS28, HgB and ESR) differ when B19V DNA was detected in the plasma (Table 2). Therefore, the cytokine levels were analysed according to the findings in respect of clinical parameters. The levels of IL-6 in the plasma of RA B19+ patients were investigated according to RA disease activity. The patients were divided according to DAS28 in two groups: DAS28<5.2 (low disease activity or remission) and DAS28>5.2 (high disease activity) (Fig. 1c). Fig. 1(c) shows that the level of IL-6 is increased in RA B19+p patients with DAS28>5.2 (IL-6 54.4±40.7 pg ml−1), but not in patients with DAS28<5.2 (IL-6 10.6±11.4 pg ml−1). Also, the RA patients were divided into groups according to HgB levels. The levels of IL-6 were similarly low in all B19+ and B19− RA patients' groups with normal values of HgB. However, the levels of IL-6 were increased in RA B19+p patients with lower values of HgB. IL-6 concentration in the plasma of RA B19+p patients was 61.6±43.6 pg ml−1 when the level of HgB was low and 19.7±21.8 pg ml−1 when the level of HgB was normal (Fig. 1d). We investigated IL-6 levels in RA patients according to CRP level (Fig. 1e). The IL-6 concentration in the plasma was lower in B19+p RA patients with normal levels of CRP (IL-6 12.7±10.0 pg ml−1). RA B19+p patients with increased CRP levels had increased IL-6 concentrations (IL-6 53.4±41.9 pg ml−1) in comparison with RA B19+b/p, B19+b and B19− patients' groups (the average IL-6 concentration varied from 1.8 to 12 pg ml−1). The concentrations of IL-6 were significantly higher also in RA B19+ and RA B19−patients' groups that fulfilled the following criteria: low HgB, high ESR, DAS28>5.2 in comparison to RA B19+ and RA B19−patients' groups that have normal HgB, normal ESR and DAS28<5.2 (Fig. S1). The IL-6 concentration in the plasma was also analysed taking into account the method of treatment. The concentration of IL-6 was higher (46.6±41.5 pg ml−1) in RA B19+p patients without immunotherapy. However, RA B19+p patients after immunotherapy had lower (14.8±16.0 pg ml−1) levels of IL-6. IL-6 plasma levels in RA B19+b and RA B19+b/p patients were low, whether or not they had had immunotherapy (up to 14 pg ml−1) (Fig. 1f). The patients were divided into different groups with low, normal or high values of WBC, ESR, anti-CCP and RF. The level of IL-6 was increased in the RA B19+p patients group, but we did not observe significant differences in the concentration of IL-6 by comparing B19+p RA patients with normal or increased/decreased values of WBC, ESR, anti-CCP and RF (Fig. S2). Also, we did not find significant changes in IL-2, IL-4, IL-10, IL-12, IL-17, TNF-α and IFN-γ levels in the plasma of RA B19+p patients (Table S3). Next, we analysed the correlation between cytokine concentrations in the plasma and clinical parameters according to the B19V DNA localization (Table S4). We observed some significant correlations that were characteristic only of patients from RA B19+p group. There were positive correlations between IL-2 and IL-12 levels (r=0.91, P<0.0005), IL-10 and IL-12 levels (r=0.71, P<0.005), IL-12 level and disease duration (r=0.54, P<0.05), IL-12 level and DAS28 (r=0.53, P<0.05), IL-10 level and CRP concentration (r=0.6, P<0.05), IL-6 level and CRP concentration (r=0.7, P<0.005) and IFN-γ level and CRP concentration (r=0.58, P<0.05). Negative correlations were observed between IL-4 and HgB levels (r=−0.5, P<0.05) and IL-6 and HgB levels (r=−0.69, P<0.005). Such correlations were not observed when B19V DNA was detected in blood and plasma, or blood or not detected at all.
Fig. 1.
Amount of IL-6 in the plasma of parvovirus B19 DNA positive (B19+) and parvovirus B19 DNA negative (B19−) persons. IL-6 in the plasma of rheumatoid arthritis (RA) patients and healthy age-matched persons (control) (a). IL-6 in the plasma of RA patients, divided according to the localization of virus DNA (b). RA patients are divided into the groups according to the findings of virus DNA (in blood, in cell-free blood plasma and in both blood and cell-free plasma) and DAS28<5.2 and DAS28>5.2 (c); HgB lower than normal and HgB normal (d); normal values of CRP and CRP high (e) and patients with and without immunotherapy (f). One symbol shows the amount of IL-6 (pg ml−1) of one person. Results are significant according to Mann–Whitney U test: *P<0.05, **P<0.005 or ***P<0.0005.
We calculated correlations between amount of antibodies and levels of cytokines for various indices of clinical data. We found significant negative correlations between the amount of antibodies to VP-C and levels of IL-6 in plasma (r=−0.6, P<0.05), between antibodies to VP-N, VP-1S, VP-2r and age (r=−0.6, P<0.05) and between antibodies to VP-2r and RF (r=−0.59, P<0.5). A significant positive correlation was noticed between levels of antibodies to VP2 and levels of IFN-γ (r=0.59, P<0.5). Such correlations were observed only in RA B19+p patients and were not noticeable in the groups of RA B19+b, RA B19+b/p, RA B19−, control B19+ and control B19− persons.
Discussion
B19V infection in RA patients was investigated in this study. Our results show that the presence of B19V DNA is more frequent in RA patients than in age- and sex-matched people that do not have RA and do not suffer from the pain in the joints. In addition, in contrast to persons in the control group where B19V DNA is found mostly in whole blood (persistent infection in latent phase), the majority of B19V DNA-positive RA patients have virus DNA in cell-free blood plasma, i.e. they have persistent infection in the active phase. Our data coincide with previously published data, in showing that the prevalence of B19V DNA in serum, plasma or peripheral blood cells was significantly higher in patients with RA than in controls (Caliskan et al., 2005; Chen et al., 2006; Kozireva et al., 2008; Tzang et al., 2009b) and that RA activity was higher in patients with latent/persistent B19V infection (Kakurina et al., 2015). However, some authors concluded that B19V is not involved in the aetiopathogenesis of RA (Kerr et al., 1995; Nikkari et al., 1995). Paradoxically, we find that B19V DNA is present only in the cell-free blood plasma of some RA patients but not in their whole blood which also includes plasma. This could be due to low copies of virus DNA in the plasma. When DNA is isolated from the cell-free plasma, viral DNA is concentrated and could be detectable. However, when DNA is purified from whole blood, the majority of the DNA comes from blood cells, and the viral DNA (present in plasma) is diluted and is not detectable due to low copies and insufficient PCR sensitivity. Therefore, it is most likely that the B19V DNA detected in whole blood represents viral DNA from B19V containing blood cells. Moreover, the presence of B19V DNA in peripheral blood cells has been shown previously by Kakurina et al. (2015), while the presence of VP1 protein in synovial cells including lymphocytes, macrophages and neutrophils (e.g. cells present in the blood) was shown by Takahashi et al. (1998).
Since B19V DNA in RA patients dominates in the plasma, we analysed how it affects the clinical data, the antibody response to various virus proteins and the levels of cytokine expression in the blood. Our data show that RA patients with B19V DNA in cell-free plasma have also higher levels of anti-CCP and higher scores of DAS28, indicating higher disease aggressiveness and activity, respectively. Many of the RA patients with B19V DNA sequences in plasma DNA also have decreased HgB (68.8 %) and increased ESR (87.5 %), in comparison with the RA patients who have virus sequences in whole blood DNA or do not have it at all. This is consistent with previously known data that persistent B19V infection in humans may cause chronic anaemia (Kurtzman et al., 1989) and, as our data suggest, that B19V may play a certain role in the pathogenesis of RA.
Although the majority of RA patients in our study have IgG class B19V-specific antibodies, the spectrum of antibodies varies. A smaller percentage of RA B19+p patients, compared with healthy controls or RA B19+b patients, have VP-1S- and VP-2P-specific antibodies. VP-1S and VP-N antigens overlap with the unique region of VP1. It is known that the epitopes of neutralizing antibodies are located within the VP1u and VP1–VP2 junction regions (Saikawa et al., 1993), and also within the amino-terminal portion of VP2 (Sato et al., 1991). Neutralizing antibodies are produced shortly after infection (von Poblotzki et al., 1995) and elicit a long-lasting immune response (Zuffi et al., 2001). The production of neutralizing antibodies to VP1u is very important for immune protection against viral infection, while VP1u is essential for B19V binding and internalization (Leisi et al., 2013). In our study, the lack of neutralizing antibodies in RA B19+p patients could explain the higher frequency of B19V persistent infection in the active phase or viraemia (cell-free virus in the blood stream), because the production of neutralizing antibody to capsid protein in persistently infected subjects plays a major role in limiting B19V infection in human (Kurtzman et al., 1989). B19+p RA patients differ from other (B19+b/p, B19+b and B19−) RA patients and from healthy controls, in that not only do the majority of them not have antibodies to VP-1S and VP-N, but also in that the amount of antibodies to VP-N, VP-1S and VP-2r negatively correlates with age, the amount of antibodies to VP-2r negatively correlates with RF, the amount of antibodies to VP-C negatively correlates with levels of IL-6 and HgB values negatively correlate with levels of IL-4 and IL-6. Antibodies to VP-2P and VP2 positively correlate with WBC and IFN-γ, respectively. Positive correlations were observed between the levels of IL-2 and IL-12, IL-10 and IL-12, IL-12 and disease duration, IL-12 and DAS28, IL-10 and CRP, IL-6 and CRP and IFN-γ and CRP. Such correlations were not observed in other groups of RA patients and healthy persons. The presence of these correlations, in the RA B19+p patient group only, also indicates that B19V plays a role in RA pathogenesis.
Our data and previous (Manicourt et al., 1993; Stabler et al., 2004) data show that levels of IL-6 are increased in the serum of patients with RA compared with controls. Our data indicate significantly increased IL-6 concentrations in the plasma of B19V-infected RA patients, but only in those who have a virus genomic sequence in cell-free plasma DNA. The levels of IL-6 in RA B19+b and RA B19+b/p patients are even lower than in RA B19− patients, and are similar to levels in the plasma of healthy controls. Also, IL-6 levels are increased in RA patients with DAS28>5.2, low HgB and high CRP and in those not receiving immunotherapy.
Serum IL-6 level as a potential biomarker reflecting RA disease activity is described previously (Shimamoto et al., 2013). B19V infection also could cause the increased IL-6 concentration in blood. Increased levels of IL-6 mRNA or increased serum IL-6 levels are detected during acute B19 infection, but not by follow-up (Kerr et al., 2001,2004). The levels of IL-6 are raised in B19-infected patients with chronic fatigue syndrome (Chapenko et al., 2012).
It is shown that B19V proteins NS1 (Hsu et al., 2006; Kerr et al., 2001) and VP1u (Tzang et al., 2009a) induce IL-6 mRNA expression. B19V proteins during viraemia could induce IL-6 production in RA patients. Takahashi et al. (1998) have shown in vitro that B19V induced IL-6 production could be suppressed by the addition of neutralizing anti-VP1 antibody. However, the majority of RA patients do not have neutralizing antibodies to the VP1 N-terminal part, and this could be a reason for B19V infection activity and increased levels of IL-6 in blood. The active phase of persistent B19V infection in RA patients is associated with increased disease activity, an increased amount of anti-CCP, decreased HgB and increased ESR. In summary, our study suggests that B19V infection, at least in some patients, plays a role in pathogenesis of RA.
Methods
Blood samples of patients.
A total of 118 patients with RA (99 females and 19 males, mean age 58.3±13.0 years) and 49 age- and sex-matched healthy volunteers (37 females and 14 males, mean age 50.2±11.3 years) as the control group were enrolled in this study. Participants in the study were selected from patients seen at the Vilnius University Hospital Santariskiu Clinics. RA was diagnosed according to 2010 ACR/EULAR (American College of Rheumatology/ European League Against Rheumatism) RA Classification Criteria for the classification of RA by expert rheumatologists (Cotmore et al., 1986). We did not include chronic (over 6 months) B19V-induced polyarthritis with transient RF in this study as RA. Also, reactive arthritis induced by parvovirus infection does not fulfil the RA Classification Criteria for the classification of RA. RA group patients were treated by immunosupressants. Some of the RA patients have comorbidities (e.g. cardiovascular diseases). All patients underwent an extensive medical examination, whole blood analysis and extensive serologic evaluation which included tests for WBC, HgB, ESR, CRP, anti-CCP, DAS28 and RF. Blood samples were collected in vacutainers with K2EDTA and processed within 4 h of collection. The whole blood samples at aliquots of 0.5 ml and the plasma samples at aliquots of 0.2 ml were frozen and stored at −80 °C until used for analysis.
This study was approved by the Vilnius Regional Biomedical Research Ethics Committee.
DNA extraction and PCRs.
DNA was isolated from 0.5 ml of the whole blood and from 0.2 ml of cell-free plasma. The sample was digested by proteinase K and extracted by a standard phenol–chloroform technique. B19V genomic DNA was detected by a nested PCR. The sequences of B19V primers were as described by Durigon et al. (1993). The sequences of the primers were: F-out AATACACTGTGGTTTTATGGGCCG, R-out CCATTGCTGGTTATAACCACAGGT; F-in GAAAACTTTCCATTTAATGATGTAG, R-in CTAAAATGGCTTTTGCAGCTTCTAC. The PCR was performed using Maxima Hot Start Polymerase (Thermo Scientific) according to the manufacturer’s recommendations. Positive and negative (DNA without B19V genomic sequences) controls were included in every PCR as well as water controls after every third sample. The cycling conditions of the first reaction were: 95 °C 10 min, 40 cycles: 95 °C 45 s, 55 °C 45 s, 75 °C 1 min and elongation 75 °C 2 min. Two microlitres of the product from first PCR was subjected to the second reaction of PCR. The cycling conditions of the second reaction were the following: 95 °C 10 min, 40 cycles: 95 °C 45 s, 56 °C 45 s, 75 °C 45 s and elongation 75 °C 2 min. The PCR products (284 bp) were analysed in 3 % agarose gel.
Detection of antibodies to B19V antigens.
IgM and IgG antibodies to B19V antigens were detected in blood plasma. Antibodies to VP2 protein were detected using Parvovirus B19 IgM and IgG Enzyme Immunoassay kits (Biotrin). The assays were performed and the results were calculated according to the manufacturer's instructions. Data comparison between different assay runs was facilitated by using an index value. The index was calculated as the ratio of the sample’s optical density (or OD450 nm) measurements to the cutoff’s OD450 nm. An index value <0.9 or >1.1 indicated sample negativity or positivity, respectively. Equivocality was indicated if the index value was in the range 0.9–1.1.
The antibodies to various virus proteins were determined using recomLine Parvovirus B19 IgG and IgM kits (Mikrogen). IgM and IgG class antibodies to VP-2P (main capsid antigen, conformation epitope), VP-N (N-terminal half of the structural proteins VP1 and VP2), VP-1S (VP1u), VP-2r (main capsid antigen, linear epitope), VP-C (C-terminal half of the structural proteins VP1 and VP2) and NS-1 (non-structural protein) were determined. The assays were performed according to the manufacturer’s instructions. The bands of the blots were scanned and the band density was quantified using ImageJ 1.49 software.
Determination of the cytokine concentration in the plasma.
The IL-2, IL-4, IL-6, IL-10, IL-12, IL-17 and TNF-α levels in the plasma were detected using human IL-2, IL-4, Il-6, IL-10, IL-12 (p70), IL-17 and TNF-α ELISA MAX Standard Sets (BioLegend) according to the manufacturer’s recommendations. The IFN-γ level was detected by two-site ELISA using home-made murine mAbs to human IFN-γ and recombinant human IFN-γ (Life Technologies), as the standard as described before (Voll et al., 1997). The plasma for ELISA was diluted 1 : 3. The results were calculated using ‘Gen5’ software.
Statistical analysis.
Fisher's exact test was used to calculate rates and proportions between the groups. Mann–Whitney U test was used to compare two groups of patients with non-normal distribution of data. The Spearman’s rank correlation coefficient was used to discover the strength of correlation between two sets of data. P<0.05 was considered significant.
Acknowledgements
This work was supported in part by the Research Council of Lithuania (TAP LLT 02/201), the Taiwan–Latvia–Lithuania Cooperation project 2012–2014 and 7FP project ‘Unlocking infectious diseases research potential at Riga Stradiņš University’, Baltinfect, agreement number 316275. We thank Professor Derek Pheby, Visiting Professor of Epidemiology at Buckinghamshire New University, UK, for advising on the use of English in this paper.
Footnotes
Four supplementary tables and two supplementary figures are available with the online Supplementary Material.
Supplementary Data
References
- Arabski M., Fudala R., Koza A., Wasik S., Futoma-Koloch B., Bugla-Ploskonska G., Kaca W.(2012). The presence of anti-LPS antibodies and human serum activity against Proteus mirabilis S/R forms in correlation with TLR4 (Thr399Ile) gene polymorphism in rheumatoid arthritis. Clin Biochem 451374–1382. 10.1016/j.clinbiochem.2012.06.021 [DOI] [PubMed] [Google Scholar]
- Arleevskaya M. I., Gabdoulkhakova A. G., Filina Y. V., Miftakhova R. R., Bredberg A., Tsybulkin A. P.(2014). A transient peak of infections during onset of rheumatoid arthritis: a 10-year prospective cohort study. BMJ Open 4:e005254. 10.1136/bmjopen-2014-005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A., Manaresi E., Zakrzewska K., DeSantis R., Musiani M., Zerbini M.(2004). Antibody response to B19 parvovirus VP1 and VP2 linear epitopes in patients with haemophilic arthritis. J Med Virol 72679–682. 10.1002/jmv.20031 [DOI] [PubMed] [Google Scholar]
- Bartold P. M., Marino V., Cantley M., Haynes D. R.(2010). Effect of Porphyromonas gingivalis-induced inflammation on the development of rheumatoid arthritis. J Clin Periodontol 37405–411. 10.1111/j.1600-051X.2010.01552.x [DOI] [PubMed] [Google Scholar]
- Berry P. J., Gray E. S., Porter H. J., Burton P. A.(1992). Parvovirus infection of the human fetus and newborn. Semin Diagn Pathol 94–12. [PubMed] [Google Scholar]
- Bertoni E., Rosati A., Zanazzi M., Azzi A., Zakrzewska K., Guidi S., Fanci R., Salvadori M.(1997). Aplastic anemia due to B19 parvovirus infection in cadaveric renal transplant recipients: an underestimated infectious disease in the immunocompromised host. J Nephrol 10152–156. [PubMed] [Google Scholar]
- Bratslavska O., Kozireva S., Baryshev M., Russev R., Alexandrov M., Uzameckis D., Todorova K., Dimitrov P., Murovska M.(2015). Parvovirus B19 infection increases proliferative activity of non-permissive cells. Virologie 6849–58. [Google Scholar]
- Brown T., Anand A., Ritchie L. D., Clewley J. P., Reid T. M.(1984). Intrauterine parvovirus infection associated with hydrops fetalis. Lancet 21033–1034. [DOI] [PubMed] [Google Scholar]
- Caliskan R., Masatlioglu S., Aslan M., Altun S., Saribas S., Ergin S., Uckan E., Koksal V., Oz V., et al. (2005). The relationship between arthritis and human parvovirus B19 infection. Rheumatol Int 267–11. 10.1007/s00296-004-0494-5 [DOI] [PubMed] [Google Scholar]
- Chapenko S., Krumina A., Logina I., Rasa S., Chistjakovs M., Sultanova A., Viksna L., Murovska M.(2012). Association of active human herpesvirus-6, -7 and parvovirus b19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Adv Virol 2012205085. 10.1155/2012/205085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. S., Chou P. H., Li S. N., Tsai W. C., Lin K. H., Tsai K. B., Yen J. H., Liu H. W.(2006). Parvovirus B19 infection in patients with rheumatoid arthritis in Taiwan. J Rheumatol 33887–891. [PubMed] [Google Scholar]
- Chorba T., Coccia P., Holman R. C., Tattersall P., Anderson L. J., Sudman J., Young N. S., Kurczynski E., Saarinen U. M., et al. (1986). The role of parvovirus B19 in aplastic crisis and erythema infectiosum (fifth disease). J Infect Dis 154383–393. 10.1093/infdis/154.3.383 [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Cohen B. J., Field A. M.(1987). Detection of parvovirus B19 DNA, antigen, and particles in the human fetus. J Med Virol 23367–376. 10.1002/jmv.1890230409 [DOI] [PubMed] [Google Scholar]
- Cohen B. J., Beard S., Knowles W. A., Ellis J. S., Joske D., Goldman J. M., Hewitt P., Ward K. N.(1997). Chronic anemia due to parvovirus B19 infection in a bone marrow transplant patient after platelet transfusion. Transfusion 37947–952. 10.1046/j.1537-2995.1997.37997454023.x [DOI] [PubMed] [Google Scholar]
- Corcoran A., Doyle S., Waldron D., Nicholson A., Mahon B. P.(2000). Impaired gamma interferon responses against parvovirus B19 by recently infected children. J Virol 749903–9910. 10.1128/JVI.74.21.9903-9910.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., McKie V. C., Anderson L. J., Astell C. R., Tattersall P.(1986). Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J Virol 60548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Re V., De Vita S., Battistella V., Marzotto A., Libra M., Ferraccioli G., Boiocchi M.(2002). Absence of human parvovirus B19 DNA in myoepithelial sialadenitis of primary Sjögren's syndrome. Ann Rheum Dis 61855–856. 10.1136/ard.61.9.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano R., Manganelli S., Frati E., Selvi E., Azzi A., Zakrzewska K., Marcolongo R.(2003). No association between human parvovirus B19 infection and Sjögren's syndrome. Ann Rheum Dis 6286–87. 10.1136/ard.62.1.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkmans B. A., van Elsacker-Niele A. M., Salimans M. M., van Albada-Kuipers G. A., de Vries E., Weiland H. T.(1988). Human parvovirus B19 DNA in synovial fluid. Arthritis Rheum 31279–281. 10.1002/art.1780310218 [DOI] [PubMed] [Google Scholar]
- Durigon E. L., Erdman D. D., Gary G. W., Pallansch M. A., Torok T. J., Anderson L. J.(1993). Multiple primer pairs for polymerase chain reaction (PCR) amplification of human parvovirus B19 DNA. J Virol Methods 44155–165. 10.1016/0166-0934(93)90051-R [DOI] [PubMed] [Google Scholar]
- Heegaard E. D., Laub Petersen B.(2000). Parvovirus B19 transmitted by bone marrow. Br J Haematol 111659–661. 10.1046/j.1365-2141.2000.02407.x [DOI] [PubMed] [Google Scholar]
- Hitchon C. A., Chandad F., Ferucci E. D., Willemze A., Ioan-Facsinay A., van der Woude D., Markland J., Robinson D., Elias B., et al. (2010). Antibodies to Porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol 371105–1112. 10.3899/jrheum.091323 [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Tsay G. J.(2001). Human parvovirus B19 infection in patients with systemic lupus erythematosus. Rheumatology 40152–157. 10.1093/rheumatology/40.2.152 [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Tzang B. S., Huang C. N., Lee Y. J., Liu G. Y., Chen M. C., Tsay G. J.(2006). Increased expression and secretion of interleukin-6 in human parvovirus B19 non-structural protein (NS1) transfected COS-7 epithelial cells. Clin Exp Immunol 144152–157. 10.1111/j.1365-2249.2006.03023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl D., Steppat D., Görg S., Hennig H.(2014). Parvovirus b19 infections and blood counts in blood donors. Transfus Med Hemother 4152–59. 10.1159/000357650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakurina N., Kadisa A., Lejnieks A., Mikazane H., Kozireva S., Murovska M.(2015). Use of exploratory factor analysis to ascertain the correlation between the activities of rheumatoid arthritis and infection by human parvovirus B19. Medicina 5118–24. 10.1016/j.medici.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Kerr J. R.(2000). Pathogenesis of human parvovirus B19 in rheumatic disease. Ann Rheum Dis 59672–683. 10.1136/ard.59.9.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. R., Cartron J. P., Curran M. D., Moore J. E., Elliott J. R., Mollan R. A.(1995). A study of the role of parvovirus B19 in rheumatoid arthritis. Br J Rheumatol 34809–813. [DOI] [PubMed] [Google Scholar]
- Kerr J. R., Barah F., Mattey D. L., Laing I., Hopkins S. J., Hutchinson I. V., Tyrrell D. A.(2001). Circulating tumour necrosis factor-alpha and interferon-gamma are detectable during acute and convalescent parvovirus B19 infection and are associated with prolonged and chronic fatigue. J Gen Virol 823011–3019. 10.1099/0022-1317-82-12-3011 [DOI] [PubMed] [Google Scholar]
- Kerr J. R., Cunniffe V. S., Kelleher P., Coats A. J., Mattey D. L.(2004). Circulating cytokines and chemokines in acute symptomatic parvovirus B19 infection: negative association between levels of pro-inflammatory cytokines and development of B19-associated arthritis. J Med Virol 74147–155. 10.1002/jmv.20158 [DOI] [PubMed] [Google Scholar]
- Kinney J. S., Anderson L. J., Farrar J., Strikas R. A., Kumar M. L., Kliegman R. M., Sever J. L., Hurwitz E. S., Sikes R. K.(1988). Risk of adverse outcomes of pregnancy after human parvovirus B19 infection. J Infect Dis 157663–667. 10.1093/infdis/157.4.663 [DOI] [PubMed] [Google Scholar]
- Kozireva S. V., Zestkova J. V., Mikazane H. J., Kadisa A. L., Kakurina N. A., Lejnieks A. A., Danilane I. N., Murovska M. F.(2008). Incidence and clinical significance of parvovirus B19 infection in patients with rheumatoid arthritis. J Rheumatol 351265–1270. [PubMed] [Google Scholar]
- Kurtzman G. J., Cohen B. J., Field A. M., Oseas R., Blaese R. M., Young N. S.(1989). Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J Clin Invest 841114–1123. 10.1172/JCI114274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrère J. J., Couroucé A. M., Bertrand Y., Girot R., Soulier J. P.(1986). Human parvovirus and aplastic crisis in chronic hemolytic anemias: a study of 24 observations. Am J Hematol 23271–275. 10.1002/ajh.2830230311 [DOI] [PubMed] [Google Scholar]
- Lehmann H. W., Lutterbüse N., Plentz A., Akkurt I., Albers N., Hauffa B. P., Hiort O., Schoenau E., Modrow S.(2008). Association of parvovirus B19 infection and Hashimoto's thyroiditis in children. Viral Immunol 21379–383. 10.1089/vim.2008.0001 [DOI] [PubMed] [Google Scholar]
- Leisi R., Ruprecht N., Kempf C., Ros C.(2013). Parvovirus B19 uptake is a highly selective process controlled by VP1u, a novel determinant of viral tropism. J Virol 8713161–13167. 10.1128/JVI.02548-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S. L., Luk W. K., Cheung C. Y., Chan T. M., Lai K. N., Peiris J. S.(2001). Nosocomial outbreak of parvovirus B19 infection in a renal transplant unit. Transplantation 7159–64. [DOI] [PubMed] [Google Scholar]
- Magnusson M., Brisslert M., Zendjanchi K., Lindh M., Bokarewa M. I.(2010). Epstein-Barr virus in bone marrow of rheumatoid arthritis patients predicts response to rituximab treatment. Rheumatology 491911–1919. 10.1093/rheumatology/keq159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaresi E., Gallinella G., Zerbini M., Venturoli S., Gentilomi G., Musiani M.(1999). IgG immune response to B19 parvovirus VP1 and VP2 linear epitopes by immunoblot assay. J Med Virol 57174–178. [DOI] [PubMed] [Google Scholar]
- Manaresi E., Gallinella G., Venturoli S., Zerbini M., Musiani M.(2004). Detection of parvovirus B19 IgG: choice of antigens and serological tests. J Clin Virol 2951–53. 10.1016/S1386-6532(03)00088-X [DOI] [PubMed] [Google Scholar]
- Manicourt D. H., Triki R., Fukuda K., Devogelaer J. P., Nagant de Deuxchaisnes C., Thonar E. J.(1993). Levels of circulating tumor necrosis factor alpha and interleukin-6 in patients with rheumatoid arthritis. Relationship to serum levels of hyaluronan and antigenic keratan sulfate. Arthritis Rheum 36490–499. 10.1002/art.1780360409 [DOI] [PubMed] [Google Scholar]
- McInnes I. B., Schett G.(2011). The pathogenesis of rheumatoid arthritis. N Engl J Med 3652205–2219. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- Meron M. K., Amital H., Shepshelovich D., Barzilai O., Ram M., Anaya J. M., Gerli R., Bizzaro N., Nicola B., Shoenfeld Y.(2010). Infectious aspects and the etiopathogenesis of rheumatoid arthritis. Clin Rev Allergy Immunol 38287–291. 10.1007/s12016-009-8158-6 [DOI] [PubMed] [Google Scholar]
- Millá F., Feliu E., Ribera J. M., Juncà J., Flores A., Vidal J., Zarco M. A., Masat T.(1993). Electron microscopic identification of parvovirus virions in erythroid and granulocytic-line cells in a patient with human parvovirus B19 induced pancytopenia. Leuk Lymphoma 10483–487. 10.3109/10428199309148206 [DOI] [PubMed] [Google Scholar]
- Moore T. L., Bandlamudi R., Alam S. M., Nesher G.(1999). Parvovirus infection mimicking systemic lupus erythematosus in a pediatric population. Semin Arthritis Rheum 28314–318. 10.1016/S0049-0172(99)80015-8 [DOI] [PubMed] [Google Scholar]
- Morey A. L., Fleming K. A.(1992). Immunophenotyping of fetal haemopoietic cells permissive for human parvovirus B19 replication in vitro. Br J Haematol 82302–309. 10.1111/j.1365-2141.1992.tb06422.x [DOI] [PubMed] [Google Scholar]
- Mourgues C., Henquell C., Tatar Z., Pereira B., Nourisson C., Tournadre A., Soubrier M., Couderc M.(2016). Monitoring of Epstein-Barr virus (EBV)/cytomegalovirus (CMV)/varicella-zoster virus (VZV) load in patients receiving tocilizumab for rheumatoid arthritis. Joint Bone Spine 83412–415. 10.1016/j.jbspin.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Musiani M., Pasini P., Zerbini M., Gentilomi G., Roda A., Gallinella G., Manaresi E., Venturoli S.(1999). Prenatal diagnosis of parvovirus B19-induced hydrops fetalis by chemiluminescence in situ hybridization. J Clin Microbiol 372326–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkari S., Roivainen A., Hannonen P., Möttönen T., Luukkainen R., Yli-Jama T., Toivanen P.(1995). Persistence of parvovirus B19 in synovial fluid and bone marrow. Ann Rheum Dis 54597–600. 10.1136/ard.54.7.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oğuz F., Akdeniz C., Unüvar E., Küçükbasmaci O., Sidal M.(2002). Parvovirus B19 in the acute arthropathies and juvenile rheumatoid arthritis. J Paediatr Child Health 38358–362. [DOI] [PubMed] [Google Scholar]
- Okada Y., Kim K., Han B., Pillai N. E., Ong R. T., Saw W. Y., Luo M., Jiang L., Yin J., et al. (2014). Risk for ACPA-positive rheumatoid arthritis is driven by shared HLA amino acid polymorphisms in Asian and European populations. Hum Mol Genet 236916–6926. 10.1093/hmg/ddu387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Young N.(1987). Characterization of capsid and noncapsid proteins of B19 parvovirus propagated in human erythroid bone marrow cell cultures. J Virol 612627–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Hao Y. S., Kurtzman G., Shimada T., Young N.(1987). Novel transcription map for the B19 (human) pathogenic parvovirus. J Virol 612395–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrepper K. I., Enders M., Motz M.(2005). Human parvovirus B19 serology and avidity using a combination of recombinant antigens enables a differentiated picture of the current state of infection. J Vet Med B Infect Dis Vet Public Health 52362–365. 10.1111/j.1439-0450.2005.00874.x [DOI] [PubMed] [Google Scholar]
- Ramos-Casals M., Cervera R., García-Carrasco M., Vidal J., Trejo O., Jiménez S., Costa J., Font J., Ingelmo M.(2000). Cytopenia and past human parvovirus B19 infection in patients with primary Sjögren's syndrome. Semin Arthritis Rheum 29373–378. 10.1053/sarh.2000.7024 [DOI] [PubMed] [Google Scholar]
- Reid D. M., Reid T. M., Brown T., Rennie J. A., Eastmond C. J.(1985). Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet 1422–425. [DOI] [PubMed] [Google Scholar]
- Saikawa T., Anderson S., Momoeda M., Kajigaya S., Young N. S.(1993). Neutralizing linear epitopes of B19 parvovirus cluster in the VP1 unique and VP1-VP2 junction regions. J Virol 673004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Hirata J., Kuroda N., Shiraki H., Maeda Y., Okochi K.(1991). Identification and mapping of neutralizing epitopes of human parvovirus B19 by using human antibodies. J Virol 655485–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. L., Wolfe F., Huizinga T. W.(2010). Rheumatoid arthritis. Lancet 3761094–1108. 10.1016/S0140-6736(10)60826-4 [DOI] [PubMed] [Google Scholar]
- Sève P., Ferry T., Koenig M., Cathebras P., Rousset H., Broussolle C.(2005). Lupus-like presentation of parvovirus B19 infection. Semin Arthritis Rheum 34642–648. 10.1016/j.semarthrit.2004.07.008 [DOI] [PubMed] [Google Scholar]
- Shimamoto K., Ito T., Ozaki Y., Amuro H., Tanaka A., Nishizawa T., Son Y., Inaba M., Nomura S.(2013). Serum interleukin 6 before and after therapy with tocilizumab is a principal biomarker in patients with rheumatoid arthritis. J Rheumatol 401074–1081. 10.3899/jrheum.121389 [DOI] [PubMed] [Google Scholar]
- Söderlund M., Brown C. S., Spaan W. J., Hedman L., Hedman K.(1995). Epitope type-specific IgG responses to capsid proteins VP1 and VP2 of human parvovirus B19. J Infect Dis 1721431–1436. 10.1093/infdis/172.6.1431 [DOI] [PubMed] [Google Scholar]
- Söderlund M., von Essen R., Haapasaari J., Kiistala U., Kiviluoto O., Hedman K.(1997). Persistence of parvovirus B19 DNA in synovial membranes of young patients with and without chronic arthropathy. Lancet 3491063–1065. 10.1016/S0140-6736(96)09110-6 [DOI] [PubMed] [Google Scholar]
- Söderlund-Venermo M., Hokynar K., Nieminen J., Rautakorpi H., Hedman K.(2002). Persistence of human parvovirus B19 in human tissues. Pathol Biol 50307–316. 10.1016/S0369-8114(02)00307-3 [DOI] [PubMed] [Google Scholar]
- Stabler T., Piette J. C., Chevalier X., Marini-Portugal A., Kraus V. B.(2004). Serum cytokine profiles in relapsing polychondritis suggest monocyte/macrophage activation. Arthritis Rheum 503663–3667. 10.1002/art.20613 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Murai C., Shibata S., Munakata Y., Ishii T., Ishii K., Saitoh T., Sawai T., Sugamura K., Sasaki T.(1998). Human parvovirus B19 as a causative agent for rheumatoid arthritis. Proc Natl Acad Sci U S A 958227–8232. 10.1073/pnas.95.14.8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzang B. S., Chiu C. C., Tsai C. C., Lee Y. J., Lu I. J., Shi J. Y., Hsu T. C.(2009a). Effects of human parvovirus B19 VP1 unique region protein on macrophage responses. J Biomed Sci 1613. 10.1186/1423-0127-16-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzang B. S., Tsai C. C., Tsay G. J., Wang M., Sun Y. S., Hsu T. C.(2009b). Anti-human parvovirus B19 nonstructural protein antibodies in patients with rheumatoid arthritis. Clin Chim Acta 40576–82. 10.1016/j.cca.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Voll R. E., Roth E. A., Girkontaite I., Fehr H., Herrmann M., Lorenz H. M., Kalden J. R.(1997). Histone-specific Th0 and Th1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum 402162–2171. 10.1002/art.1780401210 [DOI] [PubMed] [Google Scholar]
- von Poblotzki A., Gigler A., Lang B., Wolf H., Modrow S.(1995). Antibodies to parvovirus B19 NS-1 protein in infected individuals. J Gen Virol 76519–527. 10.1099/0022-1317-76-3-519 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang W., Liu H., Wang D., Wang W., Li Y., Wang Z., Wang L., Zhang W., Huang G.(2010). Parvovirus B19 infection associated with Hashimoto's thyroiditis in adults. J Infect 60360–370. 10.1016/j.jinf.2010.02.006 [DOI] [PubMed] [Google Scholar]
- White D. G., Woolf A. D., Mortimer P. P., Cohen B. J., Blake D. R., Bacon P. A.(1985). Human parvovirus arthropathy. Lancet 1419–421. [DOI] [PubMed] [Google Scholar]
- Zuffi E., Manaresi E., Gallinella G., Gentilomi G. A., Venturoli S., Zerbini M., Musiani M.(2001). Identification of an immunodominant peptide in the parvovirus B19 VP1 unique region able to elicit a long-lasting immune response in humans. Viral Immunol 14151–158. 10.1089/088282401750234529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.