Abstract
Objective
To evaluate the effect of video laryngoscopy on the rate of endotracheal intubation on first laryngoscopy attempt among critically ill adults.
Design
A randomized, parallel-group, pragmatic trial of video compared with direct laryngoscopy for 150 adults undergoing endotracheal intubation by Pulmonary and Critical Care Medicine fellows.
Setting
Medical intensive care unit in a tertiary, academic medical center.
Patients
Critically ill patients ≥ 18 years old.
Interventions
Patients were randomized 1:1 to video or direct laryngoscopy for the first attempt at endotracheal intubation.
Measurements and Main Results
Patients assigned to video (n = 74) and direct (n = 76) laryngoscopy were similar at baseline. Despite better glottic visualization with video laryngoscopy, there was no difference in the primary outcome of intubation on the first laryngoscopy attempt (video 68.9% versus direct 65.8%, p = 0.68) in unadjusted analyses or after adjustment for the operator’s previous experience with the assigned device (odds ratio for video laryngoscopy on intubation on first attempt 2.02, 95% CI 0.82 – 5.02, p = 0.12). Secondary outcomes of time to intubation, lowest arterial oxygen saturation, complications, and in-hospital mortality were not different between video and direct laryngoscopy.
Conclusions
In critically ill adults undergoing endotracheal intubation, video laryngoscopy improves glottic visualization but does not appear to increase procedural success or decrease complications.
Trial Registration
clinicaltrials.gov Identifier: NCT02051816
Keywords: intubation, laryngoscopy, critical illness, respiratory failure
Introduction
As many as one third of critically ill patients undergoing endotracheal intubation experience a life-threatening complication (1-4). Patient illness and instability, limited preparation time, operator inexperience, and equipment limitations all contribute to procedure-related complications (2-4). Few of these factors are modifiable; hence, efforts to improve the safety of endotracheal intubation have focused on modifiable factors such as medications (5-8), preparation (1), positioning (9-11), preoxygenation (12-14), and equipment to improve glottic visualization (15-19).
Video laryngoscopes use a camera on the distal end of the blade oriented toward the glottis to improve visualization. While it is logical that a better glottic view might translate into easier or more rapid endotracheal intubation, data are conflicting as to whether video laryngoscopy results in increased intubations on the first attempt, decreased complications, or improved clinical outcomes (16, 18, 20-22). Along with the need to train operators on multiple devices, these conflicting results have limited the use of video laryngoscopy for the intubation of critically ill patients (23).
To address this uncertainty, we conducted a prospective randomized trial comparing the effect of video versus direct laryngoscopy on the rate of intubation on first attempt among critically ill adults. We hypothesized that video laryngoscopy would increase the rate of intubation on first attempt, adjusting for the operator's previous experience with the intubating device at the time of intubation.
Methods
The FELLOW (Facilitating EndotracheaL intubation by Laryngoscopy technique and apneic Oxygenation Within the intensive care unit) Study was a prospective, randomized trial of video laryngoscopy (VL) compared with direct laryngoscopy (DL) during endotracheal intubation of critically ill adults by Pulmonary and Critical Care Medicine (PCCM) fellows. The study was combined in a factorial design with a comparison of apneic oxygenation versus usual care, the results of which are reported separately (24). The protocol was approved by the Vanderbilt Institutional Review Board with a waiver of informed consent, the trial was registered on www.clinicaltrials.gov (NCT02051816), and the analysis plan was published online (https://starbrite.vanderbilt.edu/rocket/page/FELLOW) prior to completion of enrollment.
Study Patients
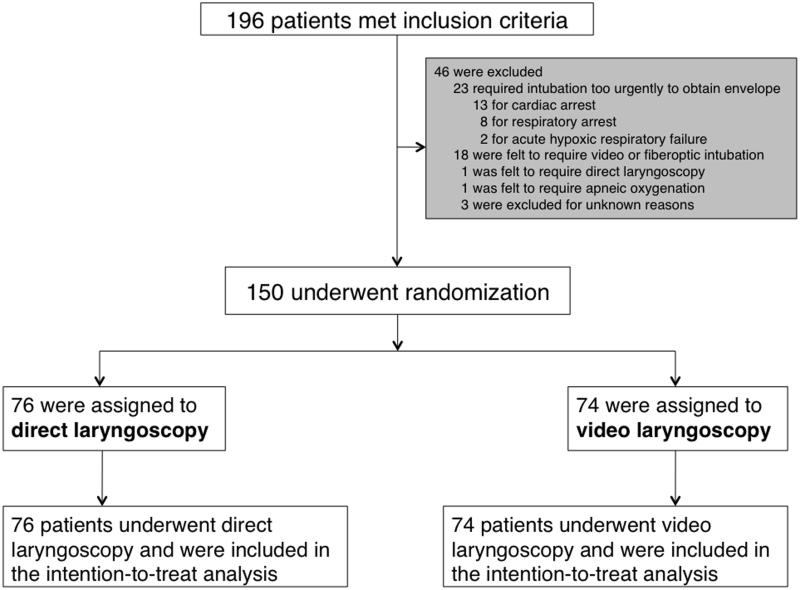
Between February 13, 2014 and February 11, 2015, all patients (≥ 18 years old) undergoing endotracheal intubation in the Vanderbilt University Medical Intensive Care Unit by a PCCM fellow were enrolled unless awake intubation was planned, intubation was required so emergently that a randomization envelope could not be obtained, or the treating clinicians felt a specific approach to intra-procedural oxygenation or a specific laryngoscopy device was mandated for the safe performance of the procedure (Figure 1).
Figure 1. Patient Screening, Randomization, and Follow-up.
Randomization and Blinding
Patients were randomly assigned in a 1:1 ratio to use of video or direct laryngoscopy on the first laryngoscopy attempt via random permuted blocks of 4, 8, and 12. Study assignment was concealed until after the decision had been made to intubate and the patient was enrolled in the trial. Because of the nature of the intervention, patients, clinicians, and study staff were aware of study group assignment after enrollment.
Study Interventions
Within the assigned laryngoscopy group, operators were free to select their preferred video laryngoscope (McGrath® Video Laryngoscope, GlideScope® Video Laryngoscope, or Olympus® Video Bronchoscope) or direct laryngoscope (curved MacIntosh or straight Miller blades). All other aspects of the procedure were at the discretion of the clinical team. All intubations were supervised by either a PCCM or Anesthesia attending physician who could offer feedback and guidance at any time during the procedure.
Data Collection
Study endpoints were collected by independent observers (ICU nurses or physicians trained in the definitions of each outcome) who were present in the patient’s room but not associated with the performance of the procedure. To confirm the accuracy of the data collected by the independent observers, the primary investigators concurrently assessed the same endpoints for a convenience sample of 10% of study intubations.
Measurement of Outcomes
The primary outcome was the rate of intubation on first attempt, adjusted for the operator’s previous experience with the intubating device at the time of the procedure. “Intubation on first attempt” was defined as successful placement of an endotracheal tube (Covidien™ Mallinckrodt™ Hi-Lo Oral/Nasal Tracheal Tube Cuffed) in the trachea during the first insertion of a laryngoscope into the oral cavity without removing the device from the mouth or using additional airway adjuncts. Adjustment for the operator’s previous device experience was performed by collecting the number of times the operator had previously used a video or direct laryngoscope at the time of each intubation event during the trial, such that the adjustment for prior experience with a specific device was updated constantly as the trial progressed. Suction devices and endotracheal tube stylets (Mallinckrodt™ Satin-Slip 14 french intubating stylet) were used routinely and not considered airway adjuncts. Secondary outcomes included time from induction to intubation, lowest arterial oxygen saturation (SpO2) measured between medication administration and 2 minutes after endotracheal tube placement, intubation on first attempt adjusted for patient age, severity of illness, body mass index, and operator device experience, the need for additional devices or operators, Cormack-Lehane grading of the glottic view (25), procedure-related complications, and in-hospital mortality.
Statistical Analysis
A prior study of endotracheal intubation by PCCM fellows in a similar population reported a rate of intubation on first attempt of 68% with DL and an improvement of 23% with use of VL (26). To have 90% statistical power to detect a difference in rate of intubation on the first attempt of 23% between VL and DL with a type I error rate of 0.05, we calculated a sample size of 142 patients. We planned to enroll a total of 150 patients to anticipating a small number of cases in which the primary endpoint would be unavailable.
Data are expressed as median and interquartile range for continuous variables and frequencies for categorical variables. Between-group comparisons were conducted using the Wilcoxon’s rank-sum test for continuous variables and Fisher’s exact test for categorical variables. Logistic regression models were created to analyze the effect of VL on intubation on first laryngosocpy attempt while adjusting for (1) previous experience with the device at the time of the procedure and (2) previous experience with the device plus pre-specified baseline confounders. IBM SPSS Statistics (version 22.0, Chicago, IL) was used for statistical analyses; a two-sided significance level of 0.05 was used for statistical inference.
Results
Of 196 critically ill adults intubated by PCCM fellows during the study period, 150 were enrolled and randomized to video or direct laryngoscopy (Figure 1). There was no crossover between study arms.
Baseline and Procedural Characteristics
Patients randomized to VL (n = 74) and DL (n = 76) were similar at baseline (Table 1). Sixteen PCCM fellows each performed a mean of 9.4 (SD ± 6.4) intubations as a part of the trial. Fellows’ prior total intubating experience and duration of training was similar between the VL and DL groups (Table 1). As anticipated, fellows had fewer prior intubations with VL (median 10, IQR 5 – 22) compared with DL (47, IQR 35 – 58) at the time of each procedure.
Table 1.
Baseline Characteristics
| Characteristic |
Video
Laryngoscopy n = 74 |
Direct Laryngoscopy
n = 76 |
|---|---|---|
| Age, median (IQR), years | 59 (49 - 68) | 60 (51 - 67) |
| Men, No. (%) | 47 (63.5%) | 44 (57.9%) |
| Caucasian, No. (%) | 63 (85.1%) | 62 (82.7%) |
| APACHE II score, median (IQR) | 22 (16.7 - 28) | 21 (15 - 25) |
| Body Mass Index, median (IQR), kg/m2 | 28.5 (23.4 - 32.7) | 28.8 (23.1 - 33.3) |
| ICU Diagnoses, No. (%) | ||
| Sepsis | 49 (66.2%) | 50 (65.8%) |
| Septic Shock | 21 (28.4%) | 13 (17.1%) |
| On Vasopressors | 11 (14.9%) | 9 (11.8%) |
| Cardiogenic Shock | 2 (2.7%) | 1 (1.3%) |
| Hemorrhagic Shock | 5 (6.8%) | 4 (5.3%) |
| Delirium | 34 (47.2%) | 34 (45.9%) |
| Hepatic Encephalopathy | 8 (10.8%) | 12 (15.8%) |
| COPD Exacerbation | 8 (10.8%) | 4 (5.3%) |
| Myocardial Infarction | 6 (8.1%) | 7 (9.2%) |
| Drug Overdose | 3 (4.2%) | 1 (1.3%) |
| Active Co-morbidities Complicating Intubation, No. (%) | ||
| BMI > 30 kg/m2 | 20 (27%) | 28 (36.8%) |
| Upper Gastrointestinal Bleeding | 6 (8.1%) | 7 (9.2%) |
| Limited Neck Mobility* | 3 (4.1%) | 2 (2.6%) |
| Limited Mouth Opening* | 3 (4.1%) | 3 (3.9%) |
| Head or Neck Radiation | 0 | 1 (1.3%) |
| Airway Mass or Infection | 1 (1.4%) | 0 |
| Witnessed Aspiration | 1 (1.4%) | 0 |
| Indication for Intubation, No. (%) | ||
| Hypoxic or Hypercarbic Respiratory Failure | 40 (54%) | 45 (59%) |
| Altered Mental Status or Encephalopathy | 20 (27%) | 19 (25%) |
| Other | 14 (19%) | 12 (16%) |
| Oxygen Saturation at Induction (%) median (IQR) | 99 (95 - 100) | 98 (93 - 100) |
| Operator Characteristics, median (IQR) | ||
| Number of times operator has previously used the assigned device at the time of intubation |
10 (5 - 22) | 47 (35 - 58) |
| Number of months of fellowship training completed at the time of the intubation |
23 (15 - 31) | 20 (14 - 30) |
| Number of Total Previous Intubations by Operators | 68 (52 - 69) | 56 (47 - 69) |
Data given as median (25th percentile - 75th percentile) or number (percentage) of patients.
APACHE = acute physiology and chronic health evaluation; ICU = intensive care unit; GI = gastrointestinal; COPD = chronic obstructive lung disease; MI = myocardial infarction; BMI = Body Mass Index; SpO2 = arterial oxygen saturation.
As reported by the fellow performing the intubation.
Post-randomization procedural characteristics including pre-oxygenation strategy, sedative medications, and laryngoscope blade size used were similar between groups (eTable 1). Only 2 patients in the VL arm and 3 patients in the DL arm were intubated without neuromuscular blockade. In the VL group, operators chose the McGrath® MAC (98.6%) and GlideScope® (1.4%) video laryngoscopes for the first intubation attempt. In the DL group, 97.4% of first intubation attempts were performed with a curved blade.
Primary Outcome
There was no difference in the rate of intubation on first attempt between video (68.9%) and direct (65.8%) laryngoscopy (unadjusted odds ratio (OR) of intubation on first attempt with VL 1.15, 95% CI 0.58 – 2.28, p = 0.68). Results were similar in analyses adjusting for operator experience with the assigned device at the time of intubation (adjusted OR 2.02, 95% CI 0.82 – 5.02, p = 0.12) and operator experience with the assigned device, APACHE II score, and BMI (OR 2.00, 95% CI 0.81 – 5.02, p = 0.12).
When intubation on the first attempt did not occur (23 of 74 patients in the VL group and 26 of 76 patients in the DL group), addition of only an endotracheal tube introducer (SunMed Introducer Adult Bougie with Coude tip, 15 french x 70 cm) allowed intubation for 7 (30%) of the failed VL patients compared with 4 (15%, p = 0.3) of the failed DL patients (eFigure 1). Of the 22 DL patients who required a second laryngoscopy attempt, half were intubated with VL and half with DL as opposed to the 16 VL patients requiring a second attempt, 14 of whom were intubated with VL and only 2 with DL.
Secondary Outcomes
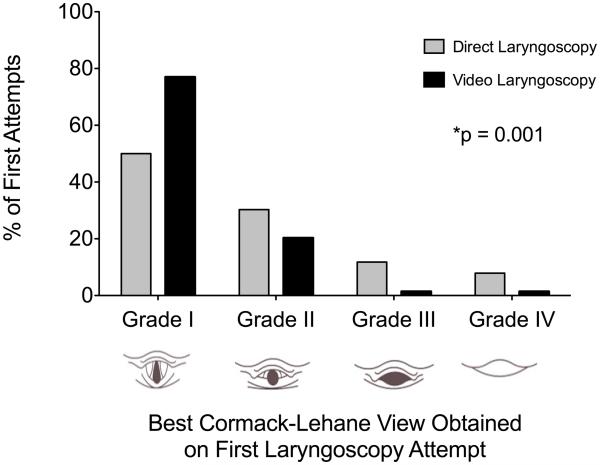
Despite significantly improving the Cormack-Lehane grade of glottic view (Figure 2), VL did not decrease time to intubation (126 seconds, IQR 89 – 197) compared with DL (153 seconds, IQR 93 – 253, p = 0.13) overall or in patients requiring only one attempt (105 seconds, IQR 75 – 150 versus 112 seconds, IQR 86 – 156, p = 0.45) (Table 2). There was no difference between VL and DL in lowest arterial oxygen saturation (91%, IQR 82 – 98% versus 90%, IQR 82 – 97%, p = 0.75) or decrease in SpO2 from baseline (4%, IQR 14 - 1% versus 4%, IQR 11 - 0%, p = 0.39). In-hospital mortality (VL 41.9% versus DL 42.1%, p = 1) and procedure-related complications (aspiration, esophageal intubation, new hypoxia, new hypotension, cardiac arrest, airway trauma) were similar between groups (Table 2). There were no differences in duration of mechanical ventilation (VL 3 days, IQR 1 - 11 versus DL 3 days, IQR 1 -8, p = 0.69) or intensive care unit length of stay (VL 6 days, IQR 2 - 11 versus DL 4 days, IQR 3 – 9, p = 0.41). In a 10% convenience sample, intubation on first attempt recorded concurrently by the independent observer and the primary investigators showed perfect interrater agreement (κ = 1.0, p = 0.001).
Figure 2. Cormack-Lehane Glottic Views Obtained on the First Laryngoscopy Attempt.
Video laryngoscopy results in better glottic views during the first laryngoscopy attempt compared with direct laryngoscopy (p = 0.001, Chi-square for a trend).
Table 2.
Secondary Clinical Outcomes for the Video vs Direct Laryngoscopy Groups
| Clinical Outcomes |
Video
Laryngoscopy (n = 74) |
Direct
Laryngoscopy (n = 76) |
p-value |
|---|---|---|---|
| Intubation on First Laryngoscopy Attempt, No. (%) | 51 (68.9%) | 50 (65.8%) | 0.68 |
| Number of Laryngoscopy Attempts, median (IQR) | 1 (1 - 1) | 1 (1 - 2) | 0.24 |
| Time from induction to intubation, median (IQR), seconds | 126 (89 - 197) | 153 (93 - 253) | 0.13 |
|
Time to intubation when only one attempt, median (IQR),
seconds (n=101) |
105 (75 - 150) | 112 (86 - 156) | 0.45 |
| Lowest arterial oxygen saturation, median (IQR), % | 91% (82 - 98) | 90% (82 - 97) | 0.75 |
|
Change in arterial oxygen saturation from baseline,
median (IQR), % |
− 4% (−14.5 to −1) | − 4% (−11 to 0) | 0.39 |
|
Best Cormack-Lehane view obtained on first attempt*,
No. (%) |
|||
| Grade I | 57 (77%) | 38 (50%) | 0.001 |
| Grade II | 15 (20.3%) | 23 (30.3%) | |
| Grade III | 1 (1.4%) | 9 (11.8%) | |
| Grade IV | 1 (1.4%) | 6 (7.9%) | |
| Difficulty of Intubation*, No. (%) | |||
| Easy | 64 (86.5%) | 54 (71.1%) | 0.051 |
| Moderate | 9 (12.2%) | 17 (22.4%) | |
| Difficult | 1 (1.4%) | 5 (6.6%) | |
| Procedural Complications*, Total No. (%) | 26 (35.1%) | 29 (38.1%) | 0.73 |
| Aspiration | 1 (1.4%) | 1 (1.3%) | 1 |
| Esophageal Intubation | 1 (1.4%) | 4 (5.3%) | 0.36 |
| SBP Less Than 80 mm Hg | 8 (10.8%) | 7 (9.2%) | 0.79 |
| SpO2 < 80% | 14 (19.4%) | 16 (21.1%) | 0.84 |
| Cardiac Arrest | 1 (1.4%) | 1 (1.3%) | 1 |
| Airway Trauma | 1 (1.4%) | 0 | 0.49 |
| Duration of Mechanical Ventilation, median (IQR), days | 3 (1 -11) | 3 (1 - 8) | 0.69 |
| ICU Length of Stay, median (IQR), days | 6 (2 -11) | 4 (3 - 9) | 0.41 |
| In-hospital Mortality, No. (%) | 31 (41%) | 32 (42%) | 1 |
Data given as median (25th percentile - 75th percentile) or number (percentage) of patients.
p-value = Mann-Whitney U Test, Pearson's Chi Square, or Chi-square for a trend SpO2 = arterial oxygen saturation; SBP = systolic blood pressure
Grade of view, airway difficulty description, and complications were reported by the operator.
Subgroup Analyses
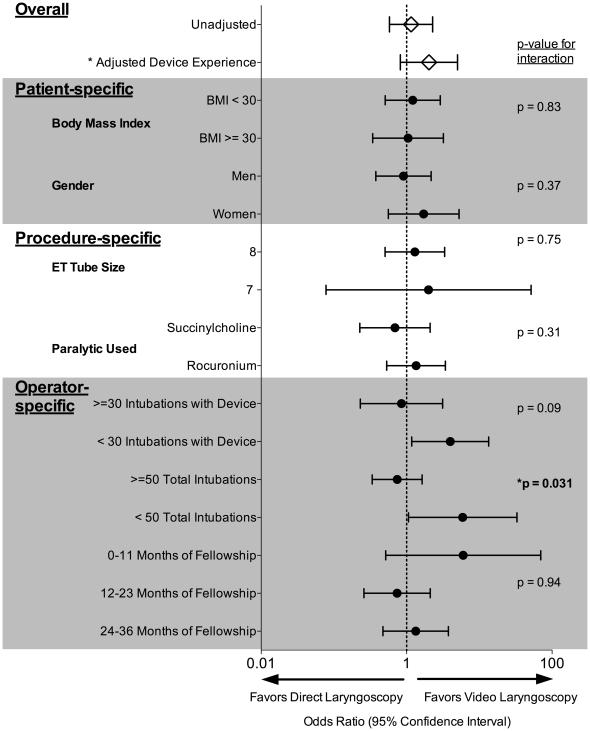
There was no significant increase in the odds of intubation on first attempt with VL in any of the pre-specified subgroups (Figure 3). The presence or absence of apneic oxygenation (24) did not modify the effect of video laryngoscopy on intubation on first attempt (p-value for interaction = 0.77). An additional post hoc subgroup analysis suggested that operators’ total prior intubating experience might modify the effect of VL on intubation on first attempt (eFigure2). Operators with less than 50 total prior intubations had higher odds of intubation on first attempt with VL compared with more experienced operators (≥ 50 total prior intubations) who had no increased odds of intubation on first attempt with VL (p-value for interaction = 0.031). Given potential confounding between total intubating experience and experience with a given laryngoscopy device, we fit a multivariable logistic regression model adjusting for prior experience with the assigned device and found there was no longer a statistically significant interaction between previous total intubation experience and VL for the outcome of intubation on first attempt (p-value for interaction = 0.17) (eFigure 2).
Figure 3. Subgroup Analyses and Evaluation for Effect Modification.
Subgroup analyses were conducted by patient, procedure, and operator-specific variables. Odds ratios and 95% confidence intervals for the outcome of intubation on first attempt using video laryngoscopy are displayed for the overall study at the top of the figure followed by all subgroups. * Adjusted Device Experience represents the primary outcome for reference. The right justified columns are P-values for interaction terms entered into the logistic regression model to test for effect modification of any of the subgroup variables. There were no patient- or procedure-specific subgroups that benefitted from intubation with video laryngoscopy nor were there any statistically significant interactions detected. Regarding the operator specific variables of previous experience with the assigned intubating device, previous total intubating experience, and previous fellowship training experience, operators less experienced with the assigned device (< 30 previous uses of the device, median) and the intubation procedure (< 50 total intubations, lowest quartile of experience) had a higher odds of intubation on first attempt with video laryngoscopy. However, only previous total intubating experience (< 50 total intubations) modified the effect of video laryngoscopy on intubation on first attempt (p = 0.031).
Discussion
This randomized trial comparing video and direct laryngoscopy for endotracheal intubation of critically ill adults by PCCM fellows did not demonstrate an increase in intubation on first attempt with video laryngoscopy. The lack of effect persisted after adjustment for the operator’s previous experience with the intubating device and across all pre-specified subgroups.
The results of the current trial are in contrast with results of prior studies demonstrating improved procedural success with VL (15, 18, 26). There are several potential explanations for this difference. Prior studies limited to non-critically ill populations (17) may not apply to the patient, operator, and procedural conditions surrounding intubation in the ICU. Un-blinded observational study designs (3, 18, 27, 28), non-random patient assignments (15), and “before-after” quality improvement studies (26) may suffer from confounding by selection bias, changes in practice over time, and the observer bias associated with self-reported data. Observer bias is of particular concern in studies reporting unexpectedly high rates of intubation failures with direct laryngoscopy compared with video laryngoscopy (15, 18, 21, 26). A lack of accounting of the experience of the operator at the time of the procedure (15, 16, 18, 26, 28) may also confound the results of prior work. Neuromuscular blockade, which is associated with improved glottic view and reduced intubation attempts with DL (8, 29, 30), was used in 96% of intubations in the current trial but less frequently (18, 26, 28) or not at all (15) in past studies.
More specific to the patient population of interest, the findings in the current trial are also in conflict with those of the only prior controlled trial of VL versus DL in the ICU (15); however both results may be true. Operator view is improved when neuromuscular blockade is used for intubation (8, 29) and use of neuromuscular blockade in 96% of patients in the current trial compared with 0% in the prior trial may explain the higher rate of success with DL in the current trial (66% versus 40%, respectively). Alternatively, the near-exclusive use of the McGrath® MAC video laryngoscope in the current trial compared to the GlideScope® video laryngoscope used in the prior trial might contribute to the difference in findings; however, these two video laryngoscopes performed similarly in both trials regarding rates of grade I or II glottic view (greater than 90%) and intubation on first attempt (around 70%) in the VL arm of each trial.
Because uncertainty regarding the role of routine VL use in ICU intubations persisted despite previous studies, we incorporated three design features into our trial to more definitively test this key question. Study group assignments were concealed until after enrollment and randomization had occurred. Randomization using variable-sized permuted blocks prevented operator or study personnel from knowing which device would be assigned next. Observer bias was minimized by employing trained, independent data collectors uninvolved in the procedure, and validating the quality of their data collection by concurrent collection of the same data by study personnel. We also deliberately used a relatively homogenous operator population (PCCM fellows) but nonetheless anticipated that operator experience would not remain balanced over the course of the study and adjusted for continuously updated imbalances in operator device experience at the time when the procedure was performed.
There are a number of possible reasons why improving glottic view with VL does not translate into procedural success. Our study suggests that despite obtaining a better view of the glottis, there may be more difficulty inserting an endotracheal tube with VL as evidenced by the higher use of endotracheal tube introducers in the VL group. Additionally, as a result of accounting for the operator’s previous experience, our data contain the intriguing suggestion that improving glottic view with VL may only matter to less experienced operators (Figure 3, eFigure 2). However, this was a post hoc analysis which was not significant after rigorously accounting for experience with each device and should only be considered hypothesis-generating. Finally, the effect of video laryngoscopy on intubation on first attempt was not modified by the apneic oxygenation half of the factorial design as the p-value for interaction was 0.77 and apneic oxygenation did not increase the lowest arterial oxygen saturation compared to usual care (24).
The current trial has some limitations. First, exclusions of intubations where the operator believed the patient needed a specific laryngoscopy device for the safe performance of the procedure was necessary for safety reasons but may limit the extrapolation of these results to all critically ill patients and data were not collected as to how operators came to this decision. The rate of intubation on first attempt with VL and DL in these excluded patients was not collected. Although the proportion of eligible patients excluded by physician preference criteria (9%) was similar to prior interventional critical care trials (31-33), it means our study findings apply only to the 90% of ICU intubations for which there is not an a priori indication for use of a specific laryngoscopy device. Specifically, our results cannot inform how VL and DL would compare among patients anticipated to have difficult upper airway anatomy or other potential indications for VL. Second, although a clear definition of the primary outcome and trained, independent observers collecting the data mitigates observer bias, intubation on first attempt is not directly linked to patient-centered clinical outcomes. However, patient-centered clinical outcomes were collected and we found no difference between study groups. Third, although operators were allowed to choose the specific VL and DL devices, this was largely a trial of the McGrath® MAC video laryngoscope and curved intubating blades. Therefore, these data may not be generalizable to operators using video laryngoscopes other than the McGrath® MAC and direct laryngoscopes with straight blades. Additionally, once patients were randomized to VL or DL, patients in the VL group were more likely to receive rocuronium rather than succinylcholine for neuromuscular blockade (eTable 1), which may influence measures of procedural success. However, in a subgroup analysis (Figure 3) there was no significant effect modification of the paralytic medication chosen on intubation on first attempt. Finally, although designed with 90% statistical power to detect a difference between arms of 23% based on a past study of PCCM fellows performing endotracheal intubation (26), our trial was not powered to detect small differences between arms.
Where do these results leave the question of routine VL use for trainees performing endotracheal intubation outside of the operating room? Despite a wealth of observational data promoting VL for the intubation of critically ill adults (16-18, 26, 28), the two largest randomized trials including 623 critically ill adults with trauma (20) and the current trial of 150 critically ill adults in the MICU have not shown benefit. Considering the results of large randomized trials and importance of exposure to various techniques during training (23), exclusive use of VL in out-of-OR airway management appears premature. Future studies should employ rigorous trial designs with true randomization, objectively collected data, and careful accounting of patient, operator, and device characteristics to definitively determine the circumstances under which VL should be the first line for airway management in the critically ill patient.
Conclusions
Among critically ill adults undergoing endotracheal intubation by PCCM fellows, video laryngoscopy does not improve the rate of intubation on first attempt. These results do not support the routine use of video laryngoscopy for all ICU intubations. Future study should focus on whether video laryngoscopy improves the rate of intubation on first attempt in operators with limited intubating experience.
Supplementary Material
Acknowledgements
The authors would like to thank the patients, nurses, respiratory therapists, residents, and attending physicians of the Vanderbilt Medical Intensive Care Unit. Critical review of the manuscript was provided by Drs. James R. Sheller and Arthur P. Wheeler.
Sources of Funding: Investigators conducting this study were supported by a National Heart, Lung, and Blood Institute (NHLBI) T32 award (HL087738). Data collection utilized the Research Electronic Data Capture (REDCap) tool developed and maintained with Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH).
Abbreviations
- ICU
Intensive Care Unit
- VL
video laryngoscopy
- DL
direct laryngoscopy
- PCCM
Pulmonary and Critical Care Medicine
- BMI
body mass index
- SpO2
arterial oxygen saturation
- APACHE
Acute Physiology and Chronic Health Evaluation
Footnotes
Conflict of Interest Statement
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declare no potential conflicts of interest with the current study; however T.W.R. reported serving on an advisory board for Avisa Pharma, LLC and as a DSMB member for GlaxoSmithKline PLC.
Authors contributions: M.W.S., D.R.J., and T.W.R, study concept and design. D.R.J, M.W.S., R.J.L, D.T.M, B.C.N, T.R.A., R.D.K., B.A.F., M.J.N., C.M.S, B.W.R., J.Z.B., acquisition of the data. D.R.J, M.W.S., R.J.L, D.T.M, B.C.N, T.R.A., R.D.K., B.A.F., M.J.N., C.M.S, B.W.R., J.Z.B, T.W.R., data analysis and interpretation. D.J.R., M.W.S., T.W.R, manuscript preparation and drafting. D.R.J., M.W.S., and T.W.R, statistical methods, statistical data analysis. D.R.J, M.W.S., R.J.L, D.T.M, B.C.N, T.R.A., R.D.K., B.A.F., M.J.N., C.M.S, B.W.R., J.Z.B, T.W.R., manuscript critique and review. All authors approved the manuscript submitted. D.R.J., M.W.S., and T.W.R. take responsibility for the integrity of the work as a whole.
Copyright form disclosures: Dr. Janz received support for article research from the National Institutes of Health (NIH). Drs. Semler, Lentz, Assad, and Shaver received support for article research from the NIH. Their institutions received funding (Investigators conducting this study were supported by a National Heart, Lung, and Blood Institute [NHLBI] T32 award [HL087738]). Dr. Norman received support for article research from the NIH. Dr. Keriwala’s institution received funding (Investigators conducting this study were supported by a National Heart, Lung, and Blood Institute [NHLBI] T32 award [HL087738]). Dr. Richmond received support for article research from the NIH. Dr. Zinggeler-Berg received support for article research from the NIH and disclosed other support (Investigators conducting this study were supported by a National Heart, Lung, and Blood Institute [NHLBI} T32 award [HL087738]). Dr. Rice received support for article research from the NIH and received funding from GlaxoSmithKline, LLC and AVISA Pharma, LLC. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Jaber S, Jung B, Corne P, et al. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med. 2010;36:248–255. doi: 10.1007/s00134-009-1717-8. [DOI] [PubMed] [Google Scholar]
- 2.Cook TM, Woodall N, Harper J, et al. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth. 2011;106:632–642. doi: 10.1093/bja/aer059. [DOI] [PubMed] [Google Scholar]
- 3.Simpson GD, Ross MJ, McKeown DW, et al. Tracheal intubation in the critically ill: a multi-centre national study of practice and complications. Br J Anaesth. 2012;108:792–799. doi: 10.1093/bja/aer504. [DOI] [PubMed] [Google Scholar]
- 4.Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth. 2004;16:508–516. doi: 10.1016/j.jclinane.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Jabre P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300. doi: 10.1016/S0140-6736(09)60949-1. [DOI] [PubMed] [Google Scholar]
- 6.Lyon RM, Perkins ZB, Chatterjee D, et al. Significant modification of traditional rapid sequence induction improves safety and effectiveness of pre-hospital trauma anaesthesia. Crit Care. 2015;19:134. doi: 10.1186/s13054-015-0872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu W-J, Wang F, Tang L, et al. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Chest. 2015;147:335–346. doi: 10.1378/chest.14-1012. [DOI] [PubMed] [Google Scholar]
- 8.Mosier JM, Sakles JC, Stolz U, et al. Neuromuscular Blockade Improves First Attempt Success for Intubation in the Intensive Care Unit: A Propensity Matched Analysis. Ann Am Thorac Soc. 2015;12:150226125024007–741. doi: 10.1513/AnnalsATS.201411-517OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adnet F, Baillard C, Borron SW, et al. Randomized study comparing the “sniffing position” with simple head extension for laryngoscopic view in elective surgery patients. Anesthesiology. 2001;95:836–841. doi: 10.1097/00000542-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Prakash S, Rapsang AG, Mahajan S, et al. Comparative evaluation of the sniffing position with simple head extension for laryngoscopic view and intubation difficulty in adults undergoing elective surgery. Anesthesiol Res Pract. 2011;2011:297913–6. doi: 10.1155/2011/297913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins JS, Lemmens HJM, Brodsky JB, et al. Laryngoscopy and morbid obesity: a comparison of the "sniff" and “ramped” positions. Obes Surg. 2004;14:1171–1175. doi: 10.1381/0960892042386869. [DOI] [PubMed] [Google Scholar]
- 12.Vourc'h M, Asfar P, Volteau C, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41:1538–1548. doi: 10.1007/s00134-015-3796-z. [DOI] [PubMed] [Google Scholar]
- 13.Baillard C, Fosse J-P, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174:171–177. doi: 10.1164/rccm.200509-1507OC. [DOI] [PubMed] [Google Scholar]
- 14.Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59:165–75. doi: 10.1016/j.annemergmed.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg MJ, Li N, Acquah SO, et al. Comparison of video laryngoscopy versus direct laryngoscopy during urgent endotracheal intubation: a randomized controlled trial. Crit Care Med. 2015;43:636–641. doi: 10.1097/CCM.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 16.van Zundert A, Pieters B, Doerges V, et al. Videolaryngoscopy allows a better view of the pharynx and larynx than classic laryngoscopy. Br J Anaesth. 2012;109:1014–1015. doi: 10.1093/bja/aes418. [DOI] [PubMed] [Google Scholar]
- 17.Su Y-C, Chen C-C, Lee Y-K, et al. Comparison of video laryngoscopes with direct laryngoscopy for tracheal intubation: a meta-analysis of randomised trials. Eur J Anaesthesiol. 2011;28:788–795. doi: 10.1097/EJA.0b013e32834a34f3. [DOI] [PubMed] [Google Scholar]
- 18.Mosier JM, Whitmore SP, Bloom JW, et al. Video laryngoscopy improves intubation success and reduces esophageal intubations compared to direct laryngoscopy in the medical intensive care unit. Crit Care. 2013;17:R237. doi: 10.1186/cc13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones BM, Agrawal A, Schulte TE. Assessing the efficacy of video versus direct laryngoscopy through retrospective comparison of 436 emergency intubation cases. J Anesth. 2013;27:927–930. doi: 10.1007/s00540-013-1651-3. [DOI] [PubMed] [Google Scholar]
- 20.Yeatts DJ, Dutton RP, Hu PF, et al. Effect of video laryngoscopy on trauma patient survival: a randomized controlled trial. J Trauma Acute Care Surg. 2013;75:212–219. doi: 10.1097/TA.0b013e318293103d. [DOI] [PubMed] [Google Scholar]
- 21.Hurford D, Cook T, Nolan J, et al. Control group bias: a potential cause of over-estimating the benefit of videolaryngoscopy on laryngeal view. Br J Anaesth. 2013;111:124–125. doi: 10.1093/bja/aet184. [DOI] [PubMed] [Google Scholar]
- 22.Wang P-K, Huang C-C, Lee Y, et al. Comparison of 3 video laryngoscopes with the Macintosh in a manikin with easy and difficult simulated airways. Am J Emerg Med. 2013;31:330–338. doi: 10.1016/j.ajem.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg MJ, Kory P. Survey of video laryngoscopy use by U.S. critical care fellowship training programs. Ann Am Thorac Soc. 2014;11:1225–1229. doi: 10.1513/AnnalsATS.201405-189OC. [DOI] [PubMed] [Google Scholar]
- 24.Semler MW, Janz DR, Lentz RJ, et al. Randomized Trial of Apneic Oxygenation during Endotracheal Intubation of the Critically Ill. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201507-1294OC. rccm.201507–1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–1111. [PubMed] [Google Scholar]
- 26.Kory P, Guevarra K, Mathew JP, et al. The impact of video laryngoscopy use during urgent endotracheal intubation in the critically ill. Anesth Analg. 2013;117:144–149. doi: 10.1213/ANE.0b013e3182917f2a. [DOI] [PubMed] [Google Scholar]
- 27.Jones BM, Agrawal A, Schulte TE. Assessing the efficacy of video versus direct laryngoscopy through retrospective comparison of 436 emergency intubation cases. J Anesth. doi: 10.1007/s00540-013-1651-3. [DOI] [PubMed] [Google Scholar]
- 28.Michailidou M, O'Keeffe T, Mosier JM, et al. A comparison of video laryngoscopy to direct laryngoscopy for the emergency intubation of trauma patients. World J Surg. 2015;39:782–788. doi: 10.1007/s00268-014-2845-z. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox SR, Bittner EA, Elmer J, et al. Neuromuscular blocking agent administration for emergent tracheal intubation is associated with decreased prevalence of procedure-related complications. Crit Care Med. 2012;40:1808–1813. doi: 10.1097/CCM.0b013e31824e0e67. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds SF, Heffner J. Airway management of the critically ill patient: rapid-sequence intubation. Chest. 2005;127:1397–1412. doi: 10.1378/chest.127.4.1397. [DOI] [PubMed] [Google Scholar]
- 31.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 32.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wheeler AP, Bernard GR, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 33.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.