Abstract
The functionality of stem cells is tightly regulated by cues from the niche, comprising both intrinsic and extrinsic cell signals. Besides chemical and growth factors, biophysical signals are important components of extrinsic signals that dictate the stem cell properties. The materials used in the fabrication of scaffolds provide the chemical cues whereas the shape of the scaffolds provides the biophysical cues. The effect of the chemical composition of the scaffolds on stem cell fate is well researched. Biophysical signals such as nanotopography, mechanical forces, stiffness of the matrix, and roughness of the biomaterial influence the fate of stem cells. However, not much is known about their role in signaling crosstalk, stem cell maintenance, and directed differentiation. Among the various techniques for scaffold design, nanotechnology has special significance. The role of nanoscale topography in scaffold design for the regulation of stem cell behavior has gained importance in regenerative medicine. Nanotechnology allows manipulation of highly advanced surfaces/scaffolds for optimal regulation of cellular behavior. Techniques such as electrospinning, soft lithography, microfluidics, carbon nanotubes, and nanostructured hydrogel are described in this review, along with their potential usage in regenerative medicine. We have also provided a brief insight into the potential signaling crosstalk that is triggered by nanomaterials that dictate a specific outcome of stem cells. This concise review compiles recent developments in nanoscale architecture and its importance in directing stem cell differentiation for prospective therapeutic applications.
Keywords: Biomaterial, Stem cell, Differentiation, Architecture, Scaffold
Background
The critical feature of stem cells is their ability to proliferate and differentiate using niche-dependent cues provided by signaling molecules, intercellular communication, and their neighboring extracellular matrix (ECM). Any of these components can be modulated to obtain specific lineage outcomes [1].
The insight in this review would provide reasonable approaches for researchers and clinicians to obtain a programmed cellular lineage by biomaterial structural modifications.
Stem cells and biomaterials
A key area of research that has gained significant attention over the past several years is tissue engineering—an allied field of regenerative medicine. The science of biomaterials has evolved from a cell carrier tool to one that can direct cellular differentiation. Biomaterials can now be molded into three-dimensional (3D) scaffolds to promote cell proliferation and/or differentiation for regeneration [2]. Mechanical factors such as matrix stiffness, matrix nanotopography, microgeometry, and extracellular forces significantly influence stem cell activities. Based on the source of derivation, biomaterials can be grouped under natural and synthetic polymers. Some of the natural scaffolds used in tissue engineering include collagen, silk fibroin, alginate, chitosan, keratin, and decellularized tissues such as de-epithelialized human amniotic membrane [3]. Biodegradability and a biologically active nature are the major advantages of natural scaffolds over synthetic scaffolds. Cells cultured on natural scaffolds reveal a good cellular response with enhanced tissue growth and host tissue integration on transplantation. One of the major drawbacks of natural scaffolds is their inherent ability to become cross contaminated from the source.
Synthetic scaffolds represent the largest group of biodegradable polymers with consistent properties apart from a high surface to volume ratio, versatility in chemical composition, and biological properties that show good malleability and processability [4, 5]. Polymers of diverse properties have been used for fabrication of scaffolds to be used for different applications. One of the major drawback of the synthetic scaffolds is the local inflammation initiated by the release of acids as their degradation byproduct [5].
Influence of the biophysical microenvironment on stem cell response
A cell responds to its environmental cues through the cellular mechanotransduction pathway. The soluble and insoluble cues regulate/modulate various genes and their downstream effectors. The physiological outcome of a cell growing on a scaffold is defined by three factors—biological, biochemical, and biomaterial. [6]. Different techniques with different architectures are used for synthesizing scaffolds for a specific biological or clinical application. (Figure 1). In the following section we have listed a few methods that impart architectural uniqueness to scaffold design and their limitations with respect to stem cell applications.
Fig. 1.
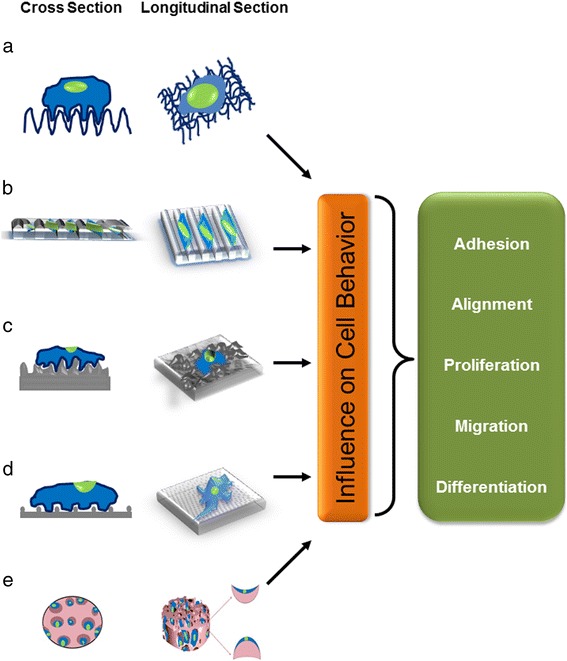
Cellular response to the biophysical microenvironment. Biomaterials with (a) fibrous architecture, (b) nano grooves/ridges, (c) surface roughness and varying nanotopographical features, (d) nanodotted surface, and (e) concave and convex curvatures inside a porous scaffold. These microenvironmental mechanical cues have the ability to influence cell adhesion, alignment, proliferation, differentiation, and migration
Nanoscale platforms
One of the major challenges in biomaterial science is to generate a substrate topography that mimics the in vivo microenvironment composed of pores, ridges, and channels that provide physical cues to cells at a nanoscale level. Scaffolds are generated by the techniques described below.
Electrospinning
Electrospinning is one of the most widely used fabrication techniques. Nanofibers of sub-microscale diameter are generated by ejecting electrically charged polymer solutions through a needle on to a grounded collector surface [7]. Usage-dependent customized nanofibers of different architectures and shapes can be fabricated using patterned collectors on electrospinning. Since the fiber diameter is lesser than the cell surface area, it is a perfect platform for the cells to organize and spread around the nanofibers with numerous focal adhesion points [6]. Pores of variable sizes can be introduced during the generation of electrospun scaffolds by incorporating porogens or sacrificial fibers that get dissolved after fabrication [8, 9]. A wide range of polymers of both natural and synthetic materials are employed for manufacturing scaffolds [10]. Though the biological materials promise better clinical applications it is difficult to maintain the integral chemical features of the material during the electrospinning process. For example, collagen loses its physicochemical properties when fluoroalcohol is used as a solvent to generate nanofibers [11]. It is much more challenging to precisely control the dimension and morphology of silk fibroin as 3D electrospun scaffolds for specific biomedical and dental applications [12]. Some drawbacks of this method are the possibility of cells squeezing into smaller pores thereby limiting their growth potential and the lack of precise control of fiber alignment. Modifications to the electric charges and the introduction of high-speed rotation mandrels have overcome this limitation [13]. Cells seeded on a nanofiber structure tend to maintain the phenotypic shape and a guided growth according to nanofiber orientation. Yin et al. differentiated human tendon stem/progenitor cells to tendons using electrospun poly-l-lactic acid (PLLA) nanofibers [14]. The cellular morphology was defined by the nano-architecture; cells were more stellate-patterned on random nanofibers and elongated and spindle-shaped fibroblastic phenotype with the aligned nanofibers [14]. Independent of the differentiation factors, the scaffold architecture is capable of directing lineage specification. For example, human bone marrow stromal cells (hBMSCs) cultured on nanofibrous poly-ε-caprolactone (PCL) scaffolds adopted an elongated, highly branched, osteogenic morphology [15]. Morelli et al. have reported that polylactic acid (PLA) and composite PLA-nanohydroxyapatite electrospun scaffolds were equally efficient in differentiating human mesenchymal stem cells (MSCs) to osteogenic and osteoclastogenic differentiation [16]. Kai et al. in a recent study demonstrated that electrospun composite PCL-gelatin scaffolds encapsulated with vascular endothelial growth factor promoted differentiation of MSCs for myocardial regeneration [17]. Furthermore, Mohtaram et al. found that electrospun PCL scaffolds with a smaller diameter loop mesh induced higher neuronal differentiation compared to thicker loops from neural progenitors [18]. Electrospun nanofibrous scaffolds of polylactic-co-glycolic acid (PLGA) and gelatin with embedded epidermal growth factors have been used for tissue engineered skin scaffolds [19]. Recently, Ortega et al. used a combination approach of electrospinning and microstereolithography to generate corneal membranes that mimicked the limbal region of the eye that harbor ocular stem cells [20]. Embedding guidance cues with gradient concentration on electrospun fibers can provide a polarized effect on the cultured cells and tissues. Similarly, techniques such as air brushing are now being used to eliminate the use of organic solvents for the preparation of polymer solutions. One of the salient feature of electrospinning is controlling the fiber alignment, which has been achieved for proper control of cellular functions through control of cellular morphology and alignment [21, 22]. The release of encapsulated drugs and biomolecules is being tailored through the use of core-shell fibers [23].
Though the technique is widely popular, the process of electrospinning depends on the polymer solution properties such as viscosity, surface tension, conductivity, and dielectric constant [24]. The viscosity of the solution maintains the ejecting fiber without breakages [25]. Voltage applied, flow rate of solution, type of collector, needle diameter, and the distance between the needle and the collector are factors that determine the pattern of the fibers [26]. Environmental factors such as temperature, humidity, and pressure can have some minor effects on the patterning of fibers by electrospinning [27]. The number of factors affecting the scaffold outcome generated by electrospinning is numerous, making it difficult to form a standard operating protocol for repeatability of scaffold architecture. The infiltration of cells within electrospun fibers is rather limited. The fibers are typically unable to serve as scaffolds for load-bearing tissues [28].
Soft lithography
Soft lithography fabrication uses elastomeric stamps, molds, and conformable photomasks ranging from micrometer to nanometer scale for scaffold generation. The synthesizer, based on the application, can customize the spatial distribution of polymer molecules placed on the substrate to aid specific outcomes such as nanodots, nanoridges, and grooves in the range from 30 nm to several microns [29, 30]. This spatial distribution of the polymer molecules also aids in spreading and shaping individual as well as groups of cells [31–33]. Embryonic stem cells are cultured in embryoid bodies and further differentiated using conditioned culture methods for lineage specificity [34, 35]. In an attempt to identify a stem cell delivery system, murine muscle satellite cells were cultured on 3D polyglycolic acid (PGA) scaffolds fabricated from a combination of soft lithography and thermal membrane lamination. Cells delivered by scaffold show higher integration to the damaged tibialis anterior muscles in comparison to cells injected intramuscularly [36]. Grooved patterns of micro- or nanoscale structures promote cell alignment and differentiation, especially with human embryonic stem cells, into neuronal lineage without the need for any supplements [37]. Hollow spheres are fabricated by injecting liquid drops into noncured polydimethylsiloxane (PDMS) mixtures. Furthermore, such drops provide a cell culture environment for growing embryoid bodies [38]. Micropatterned PDMS scaffolds generated by soft lithography have been used for mimicking musculoskeletal junctions connecting aligned myotubes with acetylcholine receptors [39]. However, the major limitation with this technique is that it provides a limited and narrow range of ECM signals for the cells to perceive, which can be highly inconsistent in comparison to the vast in vivo microenvironmental cues. This is further substantiated by the in vitro study where PLGA substrates of different groove depth promoted human tenocyte alignment with simultaneous upregulation in the expression of chondrogenic and osteogenic genes. On the contrary, in a rat patellar tendon model, neither of the grooved topographies induced ECM orientation parallel to the substrate. This indicates that cell phenotype maintenance is well established by two-dimensional (2D) imprinting technologies only in an in vitro condition. In an in vivo scenario, the neotissue formation and organization is established by multiple factors [40]. Another study deciphered the 2D imprinting technique exclusively to assess cell function in vitro for phenotype maintenance of human primary osteoblast phenotype on substrates of different grooves. Furthermore, in the in vivo sheep model, none of these topographies promoted osteogenesis [41].
Soft lithography is limited by distortion in the fabrication of single-layer structures [42]. The defects formed, which arise from dust particles, poor adhesion to the substrate and poor release from the stamp, must be controlled. Another drawback is the formation of a thin film of polymer under the nanometer-sized features. This layer is removed through ion etching but leads to damage of small nano-features generated on the fabricated scaffold. Integration of large and small features in phase-shift lithography is extremely difficult [43].
Microfluidics
Microfluidic devices make an excellent platform to study cells under various microenvironmental conditions such as stress capillary flow, chemical gradients, and the effects of single/low cell numbers on the temporal and spatial resolution. In microfluidics, the capillary flow maintains a constant soluble microenvironment and has a large surface area to volume ratio similar to biological systems [44]. This has been used extensively to study cell biological aspects such as cellular adhesion forces, the cytoskeleton, and for in vitro culture techniques. Microfluidics are used for high-throughput screening because of the capacity to culture a limited number of cells in a controlled manner. Such a system can thereby standardize culture conditions for differentiation without altering the cell number. Major limitations of microfluidics in long-term stem cell cultures are because of liquid evaporation, protein adsorption, leaching of non-reactive compounds, and hydrophobic recovery. Despite the aforementioned limitations, microfluidics provide a potential for simultaneous multi-parametric analysis with respect to the differentiation paradigm [45]. Mouse embryonic stem cell (mESC) differentiation studies using microfluidic systems have elucidated the decisive roles of fibroblast growth factor (FGF)4 and notch signaling during neuroectodermal lineage [46]. Three-dimensional microfluidics mimic the in vivo situation more closely, hence this microenvironment would be ideal for studying organogenesis and differentiation.
Much like soft lithography, the field of microfluidics employs strategies wherein the cells are grown on a substrate in 2D format and subjected to fluid flow. Designing microfluidic systems for 3D scaffolds remains a challenge and is just starting to be investigated. This fluidic strategy has to be utilized much more for clinical application [47].
Nanoparticles in nanotechnology
Nanoparticles have contributed immensely in altering the physicochemical properties of the scaffold because of their variable size and shapes. Most of the properties attributed to nanoparticles are driven by their high surface to volume ratio, improved solubility, electrical and heat conductivity, and improved catalytic activity on the surface [48]. These nanoparticles can be used for altering the scaffold architecture either by decorating the scaffold surface to impart surface features and varying surface chemistry as well as being incorporated in the matrix during scaffold synthesis to vary mechanical properties, electrical conductivity, and so forth. The most widely used nanoparticles in this field can be classified into five groups based on their nature: carbon based, inorganic base, metal based, nanostructured hydrogels and quantum dots based [49, 50].
Carbon nanotubes
Carbon nanotubes (CNTs) derived from graphene sheets are prepared with precise control of orientation, alignment, nanotube length, diameter, purity, and density. They are constructed as single-walled (SWNTs) or multi-walled carbon nanotubes (MWNTs). CNTs have tunable chemical and mechanical properties, like conductivity, biocompatibility, and nanoscale dimensions, that serve as topographical cues and to generate electrophysiological properties [51, 52]. Composites with polycarbonate membrane and collagen sponges promote the osteogenic potential of stem cells. Interactions with fibroblasts were noted to be enhanced in polyurethane composite scaffolds. Better adherence and enhanced proliferation could be observed in endothelial cells cultured on composite polyurethane scaffolds. Polyacrylic acid composites aided in neuronal differentiation from embryonic stem cells [52–56]. The major drawback of CNTs is the presence of impurities of carbonaceous particles such as nanocrystalline graphite, amorphous carbon, fullerenes, and different metals (typically Fe, Co, Mo or Ni) used as catalysts during the synthesis phase, and also concerns with toxicity as they are resistant to degradation in vivo [51, 57]. More recently, other forms of carbonaceous nanoparticles such as graphene and nanodiamonds are also being investigated [58, 59].
Metal and metal oxide nanoparticles
Metal oxide nanoparticles provide structural variabilities by exhibiting conductor or insulator characters. Oxide nanoparticles display unique chemical and physical properties with differential charge on the center and corner of the nanoparticle [60]. They have mostly been used in tracking stem cells post-transplantation [61–63]. MSCs incubated with magnetized iron oxide nanoparticles promoted calcium nodule formation in the presence of osteogenic culture medium [64]. Superparamagnetic iron oxide nanoparticles quench H2O2 and thereby promoted growth of MSCs [65]. Delcroix et al. showed that rat MSCs, when loaded with superparamagnetic iron oxide nanoparticles coated with 1-hydroxyethylidene-1.1-bisphosphonic acid and injected, showed migratory behavior only on creating a lesion [66]. Copper oxide nanoparticles did not show any effect on the differentiation potential of rat MSCs to osteogenic and chondrogenic lineage. Enhanced genotoxicity could be observed in the MSCs with increasing dosage of copper oxide nanoparticles [67].
Inorganic based
These are ceramic-based nanoparticles synthesized by a combination of a metal and a non-metal component. These are formed under higher temperature and pressure [68, 69]. These materials have high mechanical strength and low biodegradability. Hydroxyapatite and tricalcium phosphate nanoparticles have been shown to promote bone formation [70]. Silica nanoparticles enhance actin polymerization and promoted osteogenesis from MSCs [71]. Furthermore, these nanoparticles coated on scaffolds promote cellular growth of adipose-derived stem cells in culture through Erk kinase activation [72]. Fibrin-poly(lactide-caprolactone) nanoparticle-based scaffolds enhance the human adipose-derived stem cell seeding efficacy and promote cell growth and chondrogenic differentiation [73]. Embryonic stem cells cultured on polystyrene nanoparticles differ in their morphology based on their culture density. At lower density, the embryonic stem cells transform to embryoid bodies, whereas at higher density they became fibroblastic when cultured on polystyrene nanoparticles [74].
Quantum dots based
These are nano-sized semiconductors that can emit light in different colors. These comprise atoms for releasing electrons and cadmium as one of the chemicals. Most of their usage is limited to tracking stem cells undergoing differentiation and migration [75, 76]. These are photostable and have longer longevity. So far there have been no reports on the effect of quantum dots in altering stem cell proliferation or differentiation [77].
A major concern with the use of nanoparticles is their toxicity and environmental effects. The environmental effect during production of nanoparticles itself is a global concern [78]. Moreover, when used in scaffolds, the long-term effect in vivo is not well understood.
Nanostructured hydrogels
Hydrogels are 3D polymeric materials of a hydrophilic nature capable of holding large amounts of water. Co-polymerization/crosslinking free-radical polymerizations are commonly used to produce hydrogels by causing hydrophilic monomers to react with multifunctional crosslinkers to form a network. Hydrogels can be further classified into nanogels and micellar gels [79]. Nanogels are hydrophobic in nature and hence can be used to deliver products to cells. Nanostructured hydrogels are self-assembled injectable carriers of cells and proteins [80]. Chemically or physically crosslinked nanostructure scaffolds are fabricated by photo-irradiation of vinyl monomer conjugated to polyethlyne glycol, pluronic copolymers, and hyaluronic acid [80]. The degree of crosslinking determines the mechanical strength, durability, and swelling properties on the nanostructured hydrogels [81]. Most of the nanostructured hydrogels are primarily used for carrying genes and proteins to be delivered [82]. The environmental conditions are pivotal for crosslinking the monomers in a temperature- or pH-responsive crosslinking strategy [83]. This ability of the nanostructured hydrogels to transform from sol to gel form makes them the smart hydrogels. Mesenchymal stem cells cultured on nanostructured PEG-based hydrogels with nano-sized micelles showed an elevated gene expression of mesenchymal stem cell marker compared to those cultured without micelles [82]. Neural stem cells of human origin showed enhanced adhesion and proliferation when cultured on self-assembling peptide-based nanostructured hydrogels [84]. Nanostructured hydroxyapatite along with demineralized bone matrix were used for generating nanostructured hydrogels for growing mesenchymal stem cells. These cells showed increased osteocalcin production with alkaline phosphatase indicating higher osteogenic specific differentiation [85]. Nanostructured hydrogel with porous baghdadite shows sustained release of dexamethasone disodium phosphate, promoting osteogenic regeneration [86]. The in situ forming smart hydrogel can be functionalized by bioactive molecules to enhance growth and other functionality of stem cells. The sol-gel transition of the nanostructured hydrogels serve as carriers for drug and protein delivery in supporting regenerative medicine.
The spatial shape and alignment in stem cell function
All techniques used for generating scaffolds provide a geometrical control of the morphology of cells. The effects of geometrical forces on cells are explained using theoretical models of network and continuum mechanical models. While a continuum mechanical model describes the distribution of adherent cells, a network model is based on the contractile cytoskeleton deciphering the relationship between the force distribution and shape of adherent cells.
Studies have revealed modulation of cellular functions such as proliferation and differentiation based on cell-specific locations in tissues with certain geometry. Stem cells in a 3D matrix respond to 3D architectural features at different scales from nanometers to micrometers and further to millimeters with differing functions in apoptosis, proliferation, and differentiation. The dominating effect of matrix geometrical force on cell fate incitement is pivotal in tissue-specific regeneration (Fig. 2).
Fig. 2.
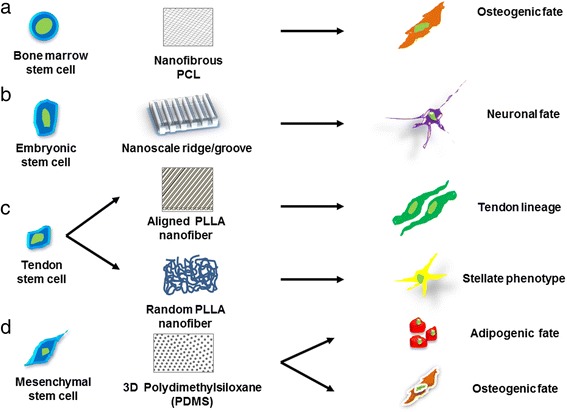
Schematic representation defining the importance of various scaffold architectures in determining the specific lineage of stem cells. Stem cells cultured on various nanostructured scaffolds yeild different differentiated cell types, such as a bone marrow stem cells grown on nanofibrous PCL scaffold promotes osteogenic fate, b embryonic stem cell cultured on annosclae ridge or groove promote neuronal fate, c tendon stem cells culutred on aligned and random PLLA directed tendon and stellate lineage, respectively, d mesenchymal stem cells on PDMS promote osteogenic as well as adipogenic fate.
Since cell shape and function are tightly linked together, scaffolds that modulate cell shape can dictate cell functions, for example the long body of neurons for effective delivery of signals, and the spherical shape of adipocytes for lipid storage. Human MSCs grown on microcontact-printed PDMS show osteogenic characteristics at the edges of the matrix and adipogenic nature towards the inner region of the scaffold [87].
Microenvironmental cues including mechanical forces are important for the formation of “stem cell niches”. Indeed, mechanical forces appear to either promote or block differentiation signals induced by growth factors and cytokines and they supersede the influence of soluble factors. To investigate the effects of mechanical forces on MSC differentiation, Kurpinski et al. used a micropatterned strip to align the cells along the direction of the uniaxial strain. They found an increased expression of calponin 1 (a smooth muscle marker) and a decrease in the expression of cartilage matrix marker. However, when the cells were aligned perpendicularly to the direction of the strain, the changes in gene expression were diminished [88]. This experiment suggests that mechanical strain has an important role in gene expression and fate of stem cells.
An amalgamation of methods with a microengineered platform comprising of a soft hydrogel can be used for inducing differentiation of stem cells (Fig. 3). The outcome of stem cell lineage specification has been tabulated with specific biomaterials in Table 1.
Fig. 3.
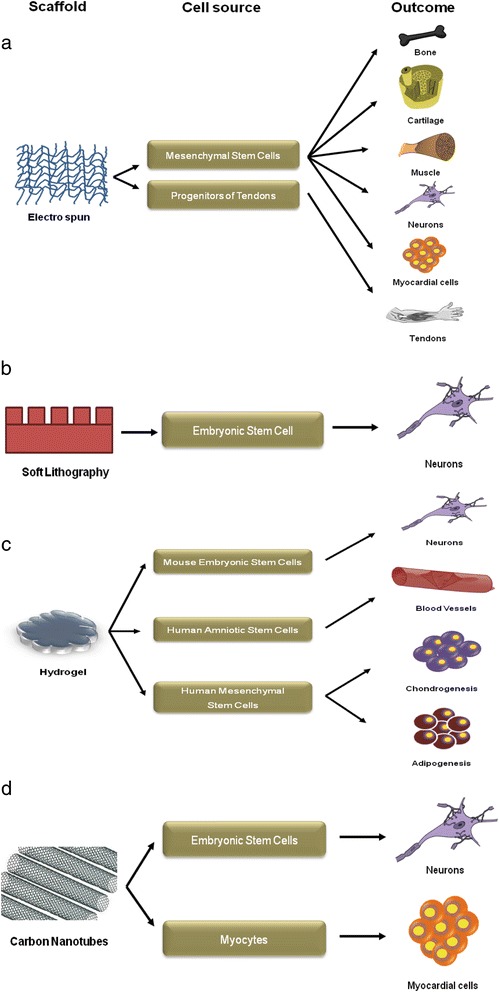
Various nanoscale platforms for directing stem cell fate. Scaffolds with (a) nanofibrous architecture, (b) soft lithography, (c) hydrogel, and (d) carbon nanotubes. These microenvironmental cues direct stem cell differentiation to a specific lineage
Table 1.
Strategic mode of directing the fate of stem cells through nanotopography of the synthetic scaffolds
| No. | Cells | Scaffolds | Differentiated cell type |
|---|---|---|---|
| 1 | Rat hair follicle stem cells (HFSCs) | Aligned poly-ε-caprolactone (PCL) nanofiber | Neuronal lineage |
| 2 | Mouse embryonic stem cells (ESCs) | Poly-l-lactic acid (PLLA) nanofiber | Osteogenic lineage |
| 3 | Retinal progenitor cells (RPCs) | Microfabricated PCL | Differentiated RPC |
| 4 | Mouse ESCs | Aligned PCL nanofibers | Neuronal lineage |
| 5 | Human mesenchymal stem cells (MSCs) | Type 1 collagen nanofiber | Osteogenic differentiation |
| 6 | Human MSCs | Poly(lactide-co-glycolide) nanofiber | Osteogenic lineage |
| 7 | Rat mesenchymal stem cells (MSCs) | PCL nanofiber | Osteogenesis |
| 8 | Human MSCs | Polydimethylsiloxane (PDMS) nanogroove | Neuronal lineage |
| 9 | Human MSCs | Polyacrylamide hydrogel | Neuronal lineage |
| 10 | Rat hippocampal progenitor cells | Micropatterned polystyrene with laminin | Neuronal lineage |
Biophysical regulators of stem cell fate
Stem cells respond to biophysical cues using cell signaling crosstalk, receptors and ligand interaction, protein modifications, protein-protein interactions, and transcriptional and translational regulations. The cell membrane enveloping the nanofeatures can result in increased intramembranous tension and rearrangement of cortical cytoskeleton thereby influencing cell morphology and behavior. Various mechanotransduction pathways have been proposed, such as the MAPK, the PI3K/Akt, RhoA/ROCK, Wnt/β-catenin, and the TGF-β pathways that rely mostly on the interaction of the cell with its biophysical environment. Significantly, all these mechanotransduction pathways are coupled with many other potent growth factor-mediated signaling pathways to regulate stem cell fate.
Mechanotransduction pathways to regulate stem cell fate
As an external signal, nanotopographical features of the ECM is capable of governing stem cell fate determination, but how this biophysical cue is translated into intracellular signaling remains elusive. Stem cells sense and respond to these insoluble biophysical signals through integrin-mediated adhesions and the interplay between integrin molecules is a controlling step in signal transduction. The force balance between the endogenous cytoskeleton contractility and external mechanical forces that are transmitted across cell ECM adhesions regulate such signaling. Through the arrangement of adhesion epitopes available to the cell, the topographical features on the substrates such as grooves/pillars of micrometer to nanometer size are sensed by cells [89, 90]. The size of the adhesive area is the most significant physical signal to determine cell fate as it is mediated by the integrins [91]. The various integrin-mediated signaling mechanisms are described below.
MAPK pathway
The Ras/MAPK pathway is activated when the biophysical signal is through integrin-mediated focal adhesion signaling. The key molecules that participate in this mechanotransduction system are focal adhesion kinase and Src family kinases (fyn) [92–94]. Furthermore, the Ras-Raf-MEK-ERK pathway gets triggered, but the exact molecular mechanism is not well known yet. Several possible pathways have been postulated, such as integrin-FAK-Grb2-SOS-Ras [95], integrin-fyn-Shc-Grb2-SOS-Ras [96], through the epidermal growth factor (EGF) receptor [97]. The MAPK pathway plays a critical role during the different stages of stem cell differentiation; for instance, temporal MAPK signaling dictates adipocyte differentiation [98]. Neural stem cells spontaneously differentiate into neurons when cultured on hydrogen terminated ultra-nanocrystalline diamond films with fibronectin integrin beat-1, focal adhesion kinase, and the MAPK pathway plays a decisive role [99]. Osteoblast differentiation on micro/nanotextured topography and titanium implant surface as well as magnesium alloy coated with porous b-tricalcium phosphate is modulated by the activation of the MAPK pathway [100, 101]. Gold nanoparticles interact with the cell membrane of MSCs inducing mechanical stress, thereby activating p38 MAPK signaling which promotes osteogenic specific gene expression in lieu of adipogenic signals [102]. Hence, MAPK signaling pathway plays a vital role in spatial and temporal differentiation of stem cells.
PI3K/Akt
This is a downstream pathway of Ras, and can also be activated through the integrin-mediated signaling in both embryonic stem cells and somatic stem cells [103–105]. Pharmacological blockage of the PI3K pathway reduces the expression of Nanog, a key transcription factor of pluripotency [106]. Mechanical strain induces integrin activation mediated by the PI3K pathway, enabling the binding of integrins to ECM proteins to activate the further downstream functionality [107]. In response to the extracellular signals, PI3K is crucial for inducing critical alteration to determine the cellular functions. An enumerate transcription factor, kinase, and regulatory molecule activity becomes regulated upon the phosphorylation of the key downstream effector of PI3K, i.e., serine-threonine kinase [108]. Upon binding to the cell surface receptors, growth factors such as insulin-like growth factor (IGF)-1 and neurotrophins (NGF) can trigger the activation of the PI3K pathway promoting survival and self-renewal of stem cells [109]. Woo et al. generated a composite scaffold of hydroxyapatite and polymer PLLA to study the properties of MSCs which survived better in the presence of an increased P13/Akt activity [110]. Zhang et al. also showed that a scaffold of PCL attached with Arg-Gly-Asp enhanced the proliferation of MSCs with the activation of the PI3K/Akt pathway via integrin [111]. Schwann cells cultured on carboxymethylated chitosan reveal that the proliferation is regulated by the intracellular signaling mechanism of Erk1/2 and PI3/Akt kinase pathways [112]. Mesenchymal stem cells under mechanical strain induced bone morphogenetic protein (BMP)2 that could be blocked in the presence of blockers of PI3 kinase [113]. Composite scaffolds of porous β-calcium silicate with poly-d,l-lactide-glycolide enhance the osteogenic and angiogenic potential of MSCs and endothelial cells by recruitment of AMP-activated protein kinase, Erk1/2 and PI3K/Akt pathways [114]. Polylactide-co-glycolide scaffolds impregnated with fibronectin and type I collagen induce osteogenic lineage of cultured MSCs via MAPK and PI3 kinase pathways [115].
RhoA/ROCK
RhoA acts through Rho-kinase (ROCK) and is a key molecular regulator of actin cytoskeleton tension and focal adhesion formation. By the activation of focal adhesion kinase through integrin-mediated signaling, this pathway acts as a downstream target [116]. RhoA can be activated by different growth factors and cytokines as well as biophysical signals from the cellular microenvironment [117]. High RhoA activity associated with high actomyosin contractility induces osteogenesis, while low RhoA activity leads to adipogenesis [118]. Parekh et al. have demonstrated that the osteogenic differentiation of hBMSCs in 2D polyethylene glycol hydrogel in the absence of supplements is triggered through elevated expression levels of actin and myosin filaments [119]. The RhoA/ROCK pathway influences the stem cell differentiation through the regulation of Sox-9 as the transcription factor [120].
Wnt/β-catenin
Wnt/β-catenin signaling regulates the fate decisions of almost all stem cell types in a spatio-temporal regulated manner. For example, a dosage-dependent Wnt signaling results in either maintenance of the pluripotency or promotion of neural differentiation [121]. Biophysical signals have been shown to directly regulate Wnt signaling as demonstrated in osteoblasts in a time-dependent manner [122]. The signaling crosstalk between Wnt and integrin signaling has been postulated through two independent frameworks, where integrin-linked kinases and focal adhesion kinases are ascertained to play a significant role [123]. Both the pathways promote the accumulation and translocation of β-catenin in the nucleus independently [124].
Conclusions
Tissue engineering is a promising field that has been developing intensely due to its potential for clinical application in cellular maintenance/differentiation. Scaffold functionalization tuned for specific application and cell response is the targeted approach. Despite advances in the development of biomimetic nanofibrous scaffolds for tissue engineering applications, several challenges still remain. Various factors from the extracellular environment known to control cell adhesion, proliferation, and differentiation have been incorporated into the design of biomaterials to achieve the objective of creating increased communication between biomaterials and their surrounding biological environment. The effects of these material modifications on cell activity are dose dependent as well as spatio-temporal dependent. One of the major challenges is to develop complex clinically relevant 3D porous scaffolds using a composite combination of materials. Additionally, it is crucial to develop a strategy to produce fibers with a diameter identical to that of native ECM fibers while maintaining high porosity for cell infiltration and migration. Research is now focused on developing an efficient biocompatible scaffold using combinatorial approaches, which could pave the way for developing scaffolds mimicking tissue junctions such as neuromuscular junctions and bone-cartilage junctions. Developing combinatorial signaling crosstalk with a dosage gradient would be a big asset in the branch of developmental biology where the axis, orientation, as well as polarization of the cells and matrix are important. These scaffolds could be used for studies related to gradient signaling, which is essential in tissue junctions. These areas of new and expanding research demonstrate the vastness as well as the challenges encountered in this multidisciplinary field. Newer technologies can offer better clinical outcomes and expand commercial arenas.
Acknowledgements
The authors would like to thank the Narayana Nethralaya Foundation, Department of Science and Technology (DST), Govt. of India (SR/SO/HS-228/2012) for providing financial support to conduct this review. The authors would like to thank Dr. P. Narendra and Dr. Arkasubhra Ghosh for their administrative support. The authors convey their gratitude to Dr. K Bhujang Shetty for providing all the logistics needed for this review.
Funding
The study was supported by the Narayana Nethralaya Foundation, Department of Science and Technology (DST), Govt. of India (SR/SO/HS-228/2012). KC thanks DST for the Ramanujan fellowship.
Availability of data and materials
Not applicable.
Authors’ contributions
LK, KD, CJ, KC, RS, and DD wrote and revised the manuscript. DD conceptualized the review. CJ, DD, and SSK provided valuable suggestions and literature for the review. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interest.
Consent for publication
All the authors have provided their consent for publication of this review manuscript.
Ethical approval and consent to participate
Not Applicable.
Abbreviations
- 2D
Two-dimensional
- 3D
Three-dimensional
- BMP
Bone morphogenetic protein
- CNT
Carbon nanotube
- ECM
Extracellular matrix
- FGF
Fibroblast growth factor
- hBMSC
Human bone marrow stromal cell
- IGF
Insulin-like growth factor
- MAPK
Mitogen-activated protein kinase
- mESC
Mouse embryonic stem cell
- MSC
Mesenchymal stem cell
- MWNT
Multi-walled carbon nanotube
- NGF
Neurotrophins
- P13K/Akt
Phosphoinositide 3-kinase/protein kinase B
- PCL
Poly-ε-caprolactone
- PDMS
Polydimethylsiloxane
- PGA
Polyglycolic acid
- PLA
Polylactic acid
- PLGA
Polylactic-co-glycolic acid
- PLLA
Poly-l-lactic acid
- RhoA/ROCK
Rho-associated protein kinase
- SWNT
Single-walled carbon nanotube
- TGF
Transforming growth factor
Contributor Information
Lekshmi Krishna, Email: lekshmikrshn@yahoo.co.in.
Kamesh Dhamodaran, Email: kameshd@narayananethralaya.com.
Chaitra Jayadev, Email: drchaitra@hotmail.com.
Kaushik Chatterjee, Email: kchatterjee@materials.iisc.ernet.in.
Rohit Shetty, Email: drrohitshetty@yahoo.com.
S. S. Khora, Email: sskhora@vit.ac.in
Debashish Das, Phone: 91 80 6666 0722, Email: dasdebashish@yahoo.co.uk, Email: drdebashish@narayananethralaya.com.
References
- 1.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–19. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elisseeff J, Ferran A, Hwang S, Varghese S, Zhang Z. The role of biomaterials in stem cell differentiation: applications in the musculoskeletal system. Stem Cells Dev. 2006;15:295–303. doi: 10.1089/scd.2006.15.295. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Borrelli M, Reichl S, Schrader S, Geerling G. Review of alternative carrier materials for ocular surface reconstruction. Curr Eye Res. 2014;39:541–52. doi: 10.3109/02713683.2013.853803. [DOI] [PubMed] [Google Scholar]
- 4.Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;2011:19. doi: 10.1155/2011/290602. [DOI] [Google Scholar]
- 5.Gunatillake P, Mayadunne R, Adhikari R. Recent developments in biodegradable synthetic polymers. Biotechnol Annu Rev. 2006;12:301–47. doi: 10.1016/S1387-2656(06)12009-8. [DOI] [PubMed] [Google Scholar]
- 6.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 7.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 8.Baker BM, Shah RP, Silverstein AM, Esterhai JL, Burdick JA, Mauck RL. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc Natl Acad Sci U S A. 2012;109:14176–81. doi: 10.1073/pnas.1206962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehraban M, Zadhoush A, Abdolkarim Hosseini Ravandi S, Bagheri R, Heidarkhan Tehrani A. Preparation of porous nanofibers from electrospun polyacrylonitrile/calcium carbonate composite nanofibers using porogen leaching technique. J Appl Polym Sci. 2013;128:926–33. doi: 10.1002/app.38091. [DOI] [Google Scholar]
- 10.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1:15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeugolis DI, Khew ST, Yew ES, Ekaputra AK, Tong YW, Yung LY, Hutmacher DW, Sheppard C, Raghunath M. Electro-spinning of pure collagen nano-fibres—just an expensive way to make gelatin? Biomaterials. 2008;29:2293–305. doi: 10.1016/j.biomaterials.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Zafar M, Najeeb S, Khurshid Z, Vazirzadeh M, Zohaib S, Najeeb B, Sefat F. Potential of electrospun nanofibers for biomedical and dental applications. Materials. 2016;9:73. doi: 10.3390/ma9020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nurfaizey A, Stanger J, Tucker N, Buunk N, Wallace A, Staiger M. Manipulation of electrospun fibres in flight: the principle of superposition of electric fields as a control method. J Mater Sci. 2012;47:1156–63. doi: 10.1007/s10853-011-5847-3. [DOI] [Google Scholar]
- 14.Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, Ouyang HW. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163–75. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 15.Kumar G, Tison CK, Chatterjee K, Pine PS, McDaniel JH, Salit ML, Young MF, Simon CG., Jr The determination of stem cell fate by 3D scaffold structures through the control of cell shape. Biomaterials. 2011;32:9188–96. doi: 10.1016/j.biomaterials.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morelli S, Salerno S, Holopainen J, Ritala M, De Bartolo L. Osteogenic and osteoclastogenic differentiation of co-cultured cells in Polylactic acid-nanohydroxyapatite fiber scaffolds. J Biotechnol. 2015;204:53–62. doi: 10.1016/j.jbiotec.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Kai D, Prabhakaran MP, Jin G, Tian L, Ramakrishna S. Potential of VEGF-encapsulated electrospun nanofibers for in vitro cardiomyogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med. 2015. doi:10.1002/term.1999. [DOI] [PubMed]
- 18.Mohtaram NK, Ko J, King C, Sun L, Muller N, Jun MB, Willerth SM. Electrospun biomaterial scaffolds with varied topographies for neuronal differentiation of human-induced pluripotent stem cells. J Biomed Mater Res A. 2015;103:2591–2601. doi: 10.1002/jbm.a.35392. [DOI] [PubMed] [Google Scholar]
- 19.Norouzi M, Shabani I, Ahvaz HH, Soleimani M. PLGA/gelatin hybrid nanofibrous scaffolds encapsulating EGF for skin regeneration. J Biomed Mater Res A. 2015;103:2225–35. doi: 10.1002/jbm.a.35355. [DOI] [PubMed] [Google Scholar]
- 20.Ortega I, Sefat F, Deshpande P, Paterson T, Ramachandran C, Ryan AJ, MacNeil S, Claeyssens F. Combination of microstereolithography and electrospinning to produce membranes equipped with niches for corneal regeneration. J Vis Exp. 2014;91:e5182. doi:10.3791/51826. [DOI] [PMC free article] [PubMed]
- 21.Kiselev P, Rosell-Llompart J. Highly aligned electrospun nanofibers by elimination of the whipping motion. J Appl Polym Sci. 2012;125:2433–41. doi: 10.1002/app.36519. [DOI] [Google Scholar]
- 22.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng Part B Rev. 2011;17:349–64. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperling LE, Reis KP, Pranke P, Wendorff JH. Advantages and challenges offered by biofunctional core-shell fiber systems for tissue engineering and drug delivery. Drug Discov Today. 2016;21:1243–56. doi: 10.1016/j.drudis.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Reneker DH, Yarin AL. Electrospinning jets and polymer nanofibers. Polymer. 2008;49:2387–425. doi: 10.1016/j.polymer.2008.02.002. [DOI] [Google Scholar]
- 25.Nezarati RM, Eifert MB, Cosgriff-Hernandez E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng Part C Methods. 2013;19:810–9. doi: 10.1089/ten.tec.2012.0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zargham S, Bazgir S, Tavakoli A, Rashidi AS, Damerchely R. The effect of flow rate on morphology and deposition area of electrospun nylon 6 nanofiber. J Eng Fabr Fibers. 2012;7:42–9. [Google Scholar]
- 27.Kai D, Liow SS, Loh XJ. Biodegradable polymers for electrospinning: towards biomedical applications. Mater Sci Eng C Mater Biol Appl. 2014;45:659–70. doi: 10.1016/j.msec.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 28.Ma B, Xie J, Jiang J, Shuler FD, Bartlett DE. Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration. Nanomedicine. 2013;8:1459–81. doi: 10.2217/nnm.13.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–76. doi: 10.1016/S0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 30.M. XYaWG. Soft Lithography. Ann Rev Mater Sci. 1998;28:153–84.
- 31.Suh KY, Seong J, Khademhosseini A, Laibinis PE, Langer R. A simple soft lithographic route to fabrication of poly(ethylene glycol) microstructures for protein and cell patterning. Biomaterials. 2004;25:557–63. doi: 10.1016/S0142-9612(03)00543-X. [DOI] [PubMed] [Google Scholar]
- 32.Khademhosseini AJ, S. Suh KY, Tran TNT, Eng G, Yeh J, Seong J, Langer R. Direct patterning of protein- and cell-resistant polymeric monolayers and microstructures. Adv Mater. 2003;15:1995–2000.
- 33.Harris GM, Shazly T, Jabbarzadeh E. Deciphering the combinatorial roles of geometric, mechanical, and adhesion cues in regulation of cell spreading. PLoS One. 2013;8:e81113. doi: 10.1371/journal.pone.0081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laperle A, Masters KS, Palecek SP. Influence of substrate composition on human embryonic stem cell differentiation and extracellular matrix production in embryoid bodies. Biotechnol Prog. 2015;31:212–9. doi: 10.1002/btpr.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon SH, Ju J, Park SJ, Bae D, Chung HM, Lee SH. Optimizing human embryonic stem cells differentiation efficiency by screening size-tunable homogenous embryoid bodies. Biomaterials. 2014;35:5987–97. doi: 10.1016/j.biomaterials.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Boldrin L, Elvassore N, Malerba A, Flaibani M, Cimetta E, Piccoli M, Baroni MD, Gazzola MV, Messina C, Gamba P, et al. Satellite cells delivered by micro-patterned scaffolds: a new strategy for cell transplantation in muscle diseases. Tissue Eng. 2007;13:253–62. doi: 10.1089/ten.2006.0093. [DOI] [PubMed] [Google Scholar]
- 37.Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K, Kim KS. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360–6. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Cosson S, Lutolf MP. Hydrogel microfluidics for the patterning of pluripotent stem cells. Sci Rep. 2014;4:4462. doi: 10.1038/srep04462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gingras J, Rioux RM, Cuvelier D, Geisse NA, Lichtman JW, Whitesides GM, Mahadevan L, Sanes JR. Controlling the orientation and synaptic differentiation of myotubes with micropatterned substrates. Biophys J. 2009;97:2771–9. doi: 10.1016/j.bpj.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.English A, Azeem A, Spanoudes K, Jones E, Tripathi B, Basu N, McNamara K, Tofail SAM, Rooney N, Riley G, et al. Substrate topography: a valuable in vitro tool, but a clinical red herring for in vivo tenogenesis. Acta Biomater. 2015;27:3–12. doi: 10.1016/j.actbio.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 41.Azeem A, English A, Kumar P, Satyam A, Biggs M, Jones E, Tripathi B, Basu N, Henkel J, Vaquette C, et al. The influence of anisotropic nano- to micro-topography on in vitro and in vivo osteogenesis. Nanomedicine. 2015;10:693–711. doi: 10.2217/nnm.14.218. [DOI] [PubMed] [Google Scholar]
- 42.Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 43.Xia Y, Whitesides GM. Soft Lithography. Annu Rev Mater Res. 1998;28:153–84. [Google Scholar]
- 44.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q, Austin RH. Applications of microfluidics in stem cell biology. Biogeoscience. 2012;2:277–86. doi: 10.1007/s12668-012-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–6. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 47.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–73. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 48.Sanvicens N, Marco MP. Multifunctional nanoparticles—properties and prospects for their use in human medicine. Trends Biotechnol. 2008;26:425–33. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2:3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116:2826–85. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- 51.Prasek J, Chomoucka J, Hubalek J, Jasek O, Adamc V, Kizek R. Methods for carbon nanotubes synthesis—review. J Mater Chem. 2011;21:15872–84. doi: 10.1039/c1jm12254a. [DOI] [Google Scholar]
- 52.Shao W, Arghya P, Yiyong M, Rodes L, Prakash S. Carbon nanotubes for use in medicine: potentials and limitations. InTech: Croatia; 2013. [Google Scholar]
- 53.Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A, Abasi M, Hanifehpour Y, Joo SW. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. 2014;9:393. doi: 10.1186/1556-276X-9-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stout DA, TJW Carbon nanotubes for stem cell control. Materialstoday. 2012;15:312–8. [Google Scholar]
- 55.Kawaguchi M, Fukushima T, Hayakawa T, Nakashima N, Inoue Y, Takeda S, Okamura K, Taniguchi K. Preparation of carbon nanotube-alginate nanocomposite gel for tissue engineering. Dent Mater J. 2006;25:719–25. doi: 10.4012/dmj.25.719. [DOI] [PubMed] [Google Scholar]
- 56.Chao TI, Xiang S, Chen CS, Chin WC, Nelson AJ, Wang C, Lu J. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2009;384:426–30. doi: 10.1016/j.bbrc.2009.04.157. [DOI] [PubMed] [Google Scholar]
- 57.Liao H, Paratala B, Sitharaman B, Wang Y. Applications of carbon nanotubes in biomedical studies. Methods Mol Biol. 2011;726:223–41. doi: 10.1007/978-1-61779-052-2_15. [DOI] [PubMed] [Google Scholar]
- 58.Brady MA, Renzing A, Douglas TE, Liu Q, Wille S, Parizek M, Bacakova L, Kromka A, Jarosova M, Godier G, Warnkel PH. Development of composite poly(lactide-co-glycolide)- nanodiamond scaffolds for bone cell growth. J Nanosci Nanotechnol. 2015;15:1060–9. doi: 10.1166/jnn.2015.9745. [DOI] [PubMed] [Google Scholar]
- 59.Kumar S, Raj S, Sarkar K, Chatterjee K. Engineering a multi-biofunctional composite using poly(ethylenimine) decorated graphene oxide for bone tissue regeneration. Nanoscale. 2016;8:6820–36. doi: 10.1039/C5NR06906H. [DOI] [PubMed] [Google Scholar]
- 60.Fernández-García M, Rodriguez JA. Metal oxide nanoparticles In: Encyclopedia of inorganic chemistry. John Wiley & Sons Ltd; 2011. doi:10.1002/9781119951438.eibc0331.
- 61.Nucci LP, Silva HR, Giampaoli V, Mamani JB, Nucci MP, Gamarra LF. Stem cells labeled with superparamagnetic iron oxide nanoparticles in a preclinical model of cerebral ischemia: a systematic review with meta-analysis. Stem Cell Res Ther. 2015;6:27. doi: 10.1186/s13287-015-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro EM. Biodegradable, polymer encapsulated, metal oxide particles for MRI-based cell tracking. Magn Reson Med. 2015;73:376–89. doi: 10.1002/mrm.25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hachani R, Lowdell M, Birchall M, Thanh NT. Tracking stem cells in tissue-engineered organs using magnetic nanoparticles. Nanoscale. 2013;5:11362–73. doi: 10.1039/c3nr03861k. [DOI] [PubMed] [Google Scholar]
- 64.Ito A, Hibino E, Honda H, Hata K-I, Kagami H, Ueda M, Kobayashi T. A new methodology of mesenchymal stem cell expansion using magnetic nanoparticles. Biochem Eng J. 2004;20:119–25. doi: 10.1016/j.bej.2003.09.018. [DOI] [Google Scholar]
- 65.Huang DM, Hsiao JK, Chen YC, Chien LY, Yao M, Chen YK, Ko BS, Hsu SC, Tai LA, Cheng HY, et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials. 2009;30:3645–51. doi: 10.1016/j.biomaterials.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 66.Delcroix GJ, Jacquart M, Lemaire L, Sindji L, Franconi F, Le Jeune JJ, Montero-Menei CN. Mesenchymal and neural stem cells labeled with HEDP-coated SPIO nanoparticles: in vitro characterization and migration potential in rat brain. Brain Res. 2009;1255:18–31. doi: 10.1016/j.brainres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 67.Zhang W, Jiang P, Chen W, Zheng B, Mao Z, Antipov A, Correia M, Larsen EH, Gao C. Genotoxicity of copper oxide nanoparticles with different surface chemistry on rat bone marrow mesenchymal stem cells. J Nanosci Nanotechnol. 2016;16:5489–97. doi: 10.1166/jnn.2016.11753. [DOI] [PubMed] [Google Scholar]
- 68.Chau DYS, Agashi K, Shakesheff KM. Microparticles as tissue engineering scaffolds: manufacture, modification and manipulation. Mater Sci Technol. 2008;24:1031–44. doi: 10.1179/174328408X341726. [DOI] [Google Scholar]
- 69.Camargo PHC, Satyanarayana KG, Wypych F. Nanocomposites: synthesis, structure, properties and new application opportunities. Mater Res. 2009;12:1–39. doi: 10.1590/S1516-14392009000100002. [DOI] [Google Scholar]
- 70.Manuel CM, Ferraz MP, Monteiro FJ. Synthesis of hydroxyapatite and tricalcium phosphate nanoparticles—preliminary studies. Key Eng Mater. 2003;240-242:555-58.
- 71.Huang DM, Chung TH, Hung Y, Lu F, Wu SH, Mou CY, Yao M, Chen YC. Internalization of mesoporous silica nanoparticles induces transient but not sufficient osteogenic signals in human mesenchymal stem cells. Toxicol Appl Pharmacol. 2008;231:208–15. doi: 10.1016/j.taap.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 72.Kim KJ, Joe YA, Kim MK, Lee SJ, Ryu YH, Cho DW, Rhie JW. Silica nanoparticles increase human adipose tissue-derived stem cell proliferation through ERK1/2 activation. Int J Nanomedicine. 2015;10:2261–72. doi: 10.2147/IJN.S71925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung Y, Chung YI, Kim SH, Tae G, Kim YH, Rhie JW, Kim SH, Kim SH. In situ chondrogenic differentiation of human adipose tissue-derived stem cells in a TGF-beta1 loaded fibrin-poly(lactide-caprolactone) nanoparticulate complex. Biomaterials. 2009;30:4657–64. doi: 10.1016/j.biomaterials.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 74.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 75.Collins MC, Gunst PR, Cascio WE, Kypson AP, Muller-Borer BJ. Labeling and imaging mesenchymal stem cells with quantum dots. Methods Mol Biol. 2012;906:199–210. doi: 10.1007/978-1-61779-953-2_15. [DOI] [PubMed] [Google Scholar]
- 76.Lin S, Xie X, Patel MR, Yang YH, Li Z, Cao F, Gheysens O, Zhang Y, Gambhir SS, Rao JH, Wu JC. Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 2007;7:67. doi: 10.1186/1472-6750-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Danner S, Benzin H, Vollbrandt T, Oder J, Richter A, Kruse C. Quantum dots do not alter the differentiation potential of pancreatic stem cells and are distributed randomly among daughter cells. Int J Cell Biol. 2013;2013:12. doi: 10.1155/2013/918242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J. 2016;20:1–11. doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moeinzadeh S, Jabbari E. Nanostructure formation in hydrogels. In: Handbook of nanomaterials properties. doi.org/10.1007/978-3-642-31107-9_62.
- 80.Chung HJ, Park TG. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today. 2009;4:429–37. doi: 10.1016/j.nantod.2009.08.008. [DOI] [Google Scholar]
- 81.Zheng Shu X, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–48. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Yang C, Khan M, Liu S, Hedrick JL, Yang YY, Ee PL. Nanostructured PEG-based hydrogels with tunable physical properties for gene delivery to human mesenchymal stem cells. Biomaterials. 2012;33:6533–41. doi: 10.1016/j.biomaterials.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 83.Montoro SR, Medeiros SF, Alves GM. Nanostructured polymer blends. Oxford: William Andrew Publishing; 2014. Nanostructured hydrogels (chapter 10) pp. 325–55. [Google Scholar]
- 84.Gelain F, Cigognini D, Caprini A, Silva D, Colleoni B, Donega M, Antonini S, Cohen BE, Vescovi A. New bioactive motifs and their use in functionalized self-assembling peptides for NSC differentiation and neural tissue engineering. Nanoscale. 2012;4:2946–57. doi: 10.1039/c2nr30220a. [DOI] [PubMed] [Google Scholar]
- 85.Lee JH, Lee KM, Baek HR, Jang SJ, Lee JH, Ryu HS. Combined effects of porous hydroxyapatite and demineralized bone matrix on bone induction: in vitro and in vivo study using a nude rat model. Biomed Mater. 2011;6:015008. doi: 10.1088/1748-6041/6/1/015008. [DOI] [PubMed] [Google Scholar]
- 86.Sehgal RR, Roohani-Esfahani SI, Zreiqat H, Banerjee R. Nanostructured gellan and xanthan hydrogel depot integrated within a baghdadite scaffold augments bone regeneration. J Tissue Eng Regen Med. 2015. doi:10.1002/term.2023. [DOI] [PubMed]
- 87.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010;107:4872–7. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2006;103:16095–100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13:558–69. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 91.Harris GM, Piroli ME, Jabbarzadeh E. Deconstructing the effects of matrix elasticity and geometry in mesenchymal stem cell lineage commitment. Adv Funct Mater. 2014;24:2396–403. doi: 10.1002/adfm.201303400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guan J-L. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–96. doi: 10.1016/S1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 93.Lehoux S, Esposito B, Merval R, Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation. 2005;111:643–9. doi: 10.1161/01.CIR.0000154548.16191.2F. [DOI] [PubMed] [Google Scholar]
- 94.Sastry SK, Burridge K. Focal Adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 95.Schlaepfer DD, Hanks SK, Hunter T, Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–91. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 96.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–34. doi: 10.1016/S0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 97.Cabodi S, Moro L, Bergatto E, Boeri Erba E, Di Stefano P, Turco E, Tarone G, Defilippi P. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem Soc Trans. 2004;32:438–42. doi: 10.1042/bst0320438. [DOI] [PubMed] [Google Scholar]
- 98.Bost F, Aouadi M, Caron L, Binetruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–6. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 99.Chen YC, Lee DC, Tsai TY, Hsiao CY, Liu JW, Kao CY, Lin HK, Chen HC, Palathinkal TJ, Pong WF, et al. Induction and regulation of differentiation in neural stem cells on ultra-nanocrystalline diamond films. Biomaterials. 2010;31:5575–87. doi: 10.1016/j.biomaterials.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 100.Wang W, Liu Q, Zhang Y, Zhao L. Involvement of ILK/ERK1/2 and ILK/p38 pathways in mediating the enhanced osteoblast differentiation by micro/nanotopography. Acta Biomater. 2014;10:3705–15. doi: 10.1016/j.actbio.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 101.Jiang T, Guo L, Ni S, Zhao Y. Upregulation of cell proliferation via Shc and ERK1/2 MAPK signaling in SaOS-2 osteoblasts grown on magnesium alloy surface coating with tricalcium phosphate. J Mater Sci Mater Med. 2015;26:158. doi: 10.1007/s10856-015-5479-2. [DOI] [PubMed] [Google Scholar]
- 102.Yi C, Liu D, Fong CC, Zhang J, Yang M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 2010;4:6439–48. doi: 10.1021/nn101373r. [DOI] [PubMed] [Google Scholar]
- 103.Chen HC, Guan JL. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1994;91:10148–52. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paling NRD, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–70. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 106.Storm MP, Bone HK, Beck CG, Bourillot P-Y, Schreiber V, Damiano T, Nelson A, Savatier P, Welham MJ. Regulation of nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem. 2007;282:6265–73. doi: 10.1074/jbc.M610906200. [DOI] [PubMed] [Google Scholar]
- 107.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280:16546–9. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 108.Paez J, Sellers WR. PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat Res. 2003;115:145–67. doi: 10.1007/0-306-48158-8_6. [DOI] [PubMed] [Google Scholar]
- 109.Sudha B, Jasty S, Krishnan S, Krishnakumar S. Signal transduction pathway involved in the ex vivo expansion of limbal epithelial cells cultured on various substrates. Indian J Med Res. 2009;129:382–9. [PubMed] [Google Scholar]
- 110.Woo KM, Seo J, Zhang R, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007;28:2622–30. doi: 10.1016/j.biomaterials.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H, Lin CY, Hollister SJ. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials. 2009;30:4063–9. doi: 10.1016/j.biomaterials.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He B, Liu SQ, Chen Q, Li HH, Ding WJ, Deng M. Carboxymethylated chitosan stimulates proliferation of Schwann cells in vitro via the activation of the ERK and Akt signaling pathways. Eur J Pharmacol. 2011;667:195–201. doi: 10.1016/j.ejphar.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 113.Chen K-D, Li Y-S, Kim M, Li S, Yuan S, Chien S, Shyy JY-J. Mechanotransduction in response to shear stress: roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 114.Wang C, Lin K, Chang J, Sun J. Osteogenesis and angiogenesis induced by porous beta-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34:64–77. doi: 10.1016/j.biomaterials.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 115.Kundu AK, Khatiwala CB, Putnam AJ. Extracellular matrix remodeling, integrin expression, and downstream signaling pathways influence the osteogenic differentiation of mesenchymal stem cells on poly(lactide-co-glycolide) substrates. Tissue Eng Part A. 2009;15:273–83. doi: 10.1089/ten.tea.2008.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–19. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys. 2012;41:519–42. doi: 10.1146/annurev-biophys-042910-155306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 119.Parekh SH, Chatterjee K, Lin-Gibson S, Moore NM, Cicerone MT, Young MF, Simon CG., Jr Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials. 2011;32:2256–64. doi: 10.1016/j.biomaterials.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626–34. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- 121.Muroyama Y, Kondoh H, Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun. 2004;313:915–21. doi: 10.1016/j.bbrc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 122.Jansen JH, Eijken M, Jahr H, Chiba H, Verhaar JA, van Leeuwen JP, Weinans H. Stretch-induced inhibition of Wnt/beta-catenin signaling in mineralizing osteoblasts. J Orthop Res. 2010;28:390–6. doi: 10.1002/jor.20991. [DOI] [PubMed] [Google Scholar]
- 123.Crampton SP, Wu B, Park EJ, Kim J-H, Solomon C, Waterman ML, Hughes CCW. Integration of the β-catenin-dependent Wnt pathway with integrin signaling through the adaptor molecule Grb2. PLoS ONE. 2009;4:e7841. doi: 10.1371/journal.pone.0007841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oloumi A, Syam S, Dedhar S. Modulation of Wnt3a-mediated nuclear [beta]-catenin accumulation and activation by integrin-linked kinase in mammalian cells. Oncogene. 2006;25:7747–57. doi: 10.1038/sj.onc.1209752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


