Abstract
Background:
Oral lichen planus (OLP) is associated with various other systemic conditions such as hypertension, diabetes mellitus (DM). This study evaluated the prevalence of OLP in DM patients compared with non-diabetic control subjects in a meta-analysis study.
Methods:
In this study from January 1973 to August 2016, we searched the studies in Web of Science, Medline/PubMed, Scopus, Science direct, SID (Scientific Information Database), Cochrane and Embase databases. Strategy search was the Medical Subject Heading (MeSH) term oral lichen planus or oral mucosa combined with diabetes in PubMed and this search in other databases. Heterogeneity between estimates was evaluated by the Q and I2 statistic. Also, publication bias was assessed through funnel plot analysis with the Kendall’s and Egger’s tests.
Results:
From 831 studies were identified with different search strategies, 11 studies met the criteria to be included in meta-analysis (11 case-control studies). The overall prevalence of OLP in 11 studies with 4937 DM patients and 3698 control subjectswas 1.5% and 0.75%, respectively. In this meta-analysis, the OR in prevalence of OLP in DM patients compared with control subjects was 1.584 (95%CI1.013-2.477; P=0.044) with a low level of heterogeneity (I2 = 0%) that the result showed the prevalence of OLP in DM patients is significantly more than control subjects.
Conclusions:
This meta-analysis study showed an association between OLP with DM, whereas this association was no significant in previous studies, it was probably because different selecting of age, sex, type of DM, medications and criteria. Totally, the meta-analysis showed the risk of OLP in DM was higher compared with control subjects.
Keywords: Oral lichen planus, Diabetes mellitus, Meta-analysis study
1. INTRODUCTION
Oral lichen planus (OLP) is a chronic inflammatory disease that its incidence is more in women than men with different age range in around the world (1). The prevalence of OLP in the general population varies from 1-2% (2). Clinically OLP is divided into six forms: reticular, papular, plaque like, atrophic, erosive and bullous types (3). Smokers and/or patients with alcohol abuse show a higher prevalence of OLP lesions (4). Numerous different topical and general treatments have been suggested for OLP such as corticosteroids, immunosuppressants such as cyclosporin, tacrolimusand retinoids (5). Diabetes mellitus (DM) is a chronic disease with serious long-term, debilitating complications and no known cure (6) that is characterized by disturbances in carbohydrate, fat and protein metabolism (7). There are two types of diabetes: type I (insulin-dependent) and type II (non-insulin-dependent) (8). Nowadays, there are different treatments; oral and injectable, available for the treatment of type II diabetes (9), but insulin is the only antihyperglycemic therapy for type I diabetes. Because of varied clinical forms of OLP, it is associated with various other systemic conditions such as diabetes mellitus (10). This association can be due to the endocrine dysfunction in DM that may be related to an immunological defect and contribution to the development of OLP (11). Antidiabetic drugs and certain antidiabetic drugs in DM patients can be caused an allergic manifestation to produce lichenoid reaction (12). Consideration to the incidence and characteristics of oral mucosal lesions among DM patients can be useful for the planning, prevention and reducing the incidence of these lesions. The aim of this study was to evaluate the prevalence of OLP in DMpatients compared with control subjects in a meta-analysis study.
2. PATIENTS AND METHODS
Eligibility criteria
The studies were searched for the finding of the prevalence of OLP in DM compared with non-DM group (control subjects). We selected full text articles based on following inclusion criteria: a) only original articles of case-control studies in English’s abstract; b) it must evaluate the prevalence of OLP in DM patients; c) for meta-analysis, the results must be compared with control subjects; d) OLP must be in DM and non-DM. After that, the criteria for eligible studies were:
Diagnosis of OLP was based on clinical, histological methods or both;
The classification of DM was made according to WHO (World Health Organization);
Diagnosis of DM patients and control subjects was based on FBS, HbA1c or both;
The control subjects did not have DM or OLP and any cutaneous dermatological or systematic disease;
The DM patients had no any systematic disease.
Search Strategy
We searched the articles in Web of Science, Medline/PubMed, Scopus, Science direct, SID (Scientific Information Database), Cochrane and Embase databases from January 1973 to August 2016, using the Medical Subject Heading (MeSH) term oral lichen planus or oral mucosa combined with diabetes in PubMed and this strategy in other databases.
Data Extraction
The relevant data extracted from every study wasthe name of author, year of publication, type of study, number of DM patients, number of control subjects, number of OLP patients, percentage of sex, the mean age, type of DM and duration of DM. One reviewer (M.S) searched the articles and then the second reviewer (H.R.M) blinded to the first reviewer. If there was any disagreement between two reviewers, third reviewer (R.S) resolved the problem.
Analysis
The odds ratios (ORs) of the studies were calculated in comparison of the risk estimate of OLP in DM patients compared with control subjects by using meta-analysis. Heterogeneity between estimates was evaluated by the Q and I2 statistic and for the Q statistic, heterogeneity was considered for P<0.1. In this study, confidence interval (CI) was 95% and 2-sided P<0.05 was considered to be statistically significant. Publication bias was assessed through funnel plot analysis with the Kendall’s and Egger’s tests.
3. RESULTS
From 831studies identified with different search strategies, 20 eligible studies were found. Out of 20studies, one study was case report, 3 studies of animal, 3 studies of review and 3 studies of cross-sectional that were excluded (Figure 1). Therefore, 11studies met the criteria to be included in meta-analysis (11 case-control studies) (Table 1).
Figure 1.
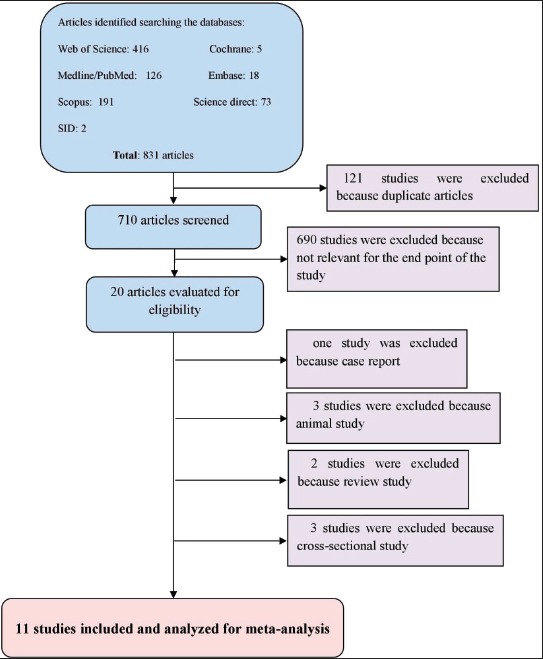
Flow diagram of study
Table 1.
Characteristics of the analyzed studies in the review investigating the prevalence of lichen planus patients in diabetes mellitus patient Abbreviation: OLP, oral lichen planus; DM, diabetes mellitus.
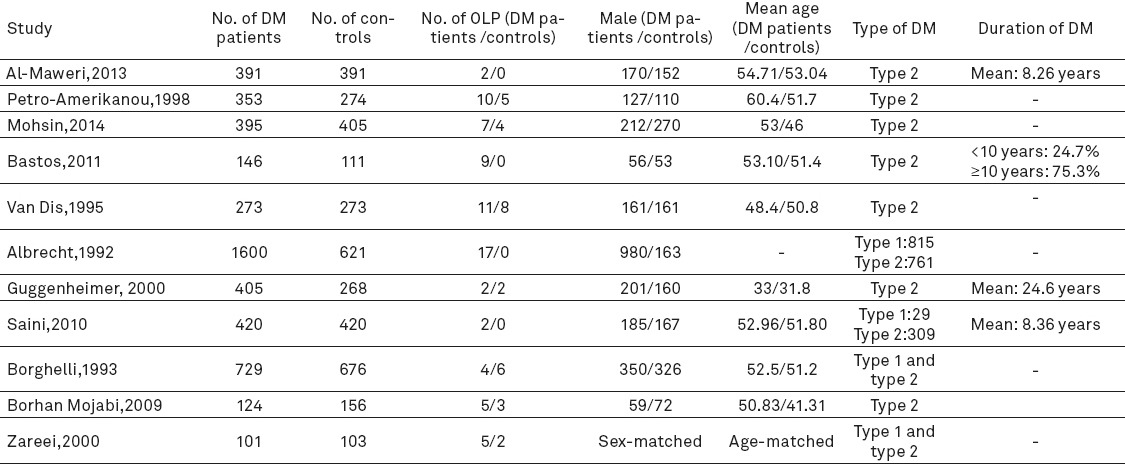
The age, sex and prevalence of OLP of the included studies in meta-analysis
In previous studies in this review, the prevalence rate of OLP was reported 0.5 to 9.3% in DM patients and 0 to 1.8% in control subjects. The overall prevalence of OLP in 11 studies (13, 11, 14-18, 19, 20-22) with 4937 DM patients and 3698 control subjectswas 74 (1.5%) and 28 (0.75%), respectively (Table 1). The percentage of males in these studies was between 36 to 61.2% in DM patients and 26.2 to 59.7% in control subjects. Overall, the minimum age was 8 years. Seven studies (13, 14, 16, 17, 19, 20, 22) were age-matched. The mean age of DM patients and control subjects was 51 and 47.7 years, respectively. Out of 11 studies, type II diabetes alone was reported in 7 studies and other four studies were included both type I and type II. The duration of DM has been shown in Table 1.
Treatment and duration of DM in the review
In study of Al-Maweri (13), 261 DM patients were not treated medications, 91 cardiovascular agents, 10 antibiotics, 14 non-steroidal anti-inflammatory drugs (NSAIDs), 6 antiasthmatic and 9 others. Also, 327 control subjects were not treated medications, 42 cardiovascular agents, 4 antibiotics, 6 NSAIDs, 7 antiasthmatics and 5 others. In study of Bastos, 67.1% patients took hypoglacemic, 19.8% insulin and 19% both (14). In study of Van Dis, 65% DM patients and 75% control subjects were not taking medications, 21% and 8% had angiotensin-converting enzyme inhibitors, 7% and 8% NSAIDs, 5% and 4% furosemide, 9% and 8% thiazide derivatives, 0.3% and 0.3% sulfonamide, 1% and 1% propranolol, 0.3% and 0% levamisole and also 0% and 0.3% tetracycline (17). In the study of Saini (19), 68.1% DM patients and 84.3% control subjects were not taking medications, 22.4% and 10% cardiovascular agents, 2.4% and 1% antibiotics, 3.3% and 4.1% NSAIDs, 1.4% and 1.7% antiasthmatics, and also 2.4% and 1.7% others. Other studies didn’t report any drugs for the groups.
Meta-analysis
Eleven case-control studies were included in meta-analysis (Figure 2). The OR in prevalence of OLP in DM patients compared with control subjects was 1.584 (95% CI 1.013-2.477; P=0.044) with a low level of heterogeneity (I2=0%). Therefore, the prevalence of OLP in DM patients is significantly more than control subjects.
Figure 2.
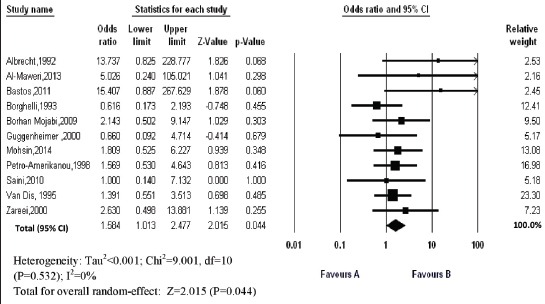
Forest plot of odds ratio of prevalence of oral lichen planus (95% confidence intervals) in diabetes mellitus patients compared with control subjects (Favours A: Patients; Favours B: Controls)
Bias indicators
For the risk of bias, we used below two tests (Figure 3): Kendall’s test; tau =0.418, z-value of 1.79 and p-value of 0.073 and Egger’s test as 1.42 (95% CI=-0.18 to 3.03), standard error 0.7114, t=2.00, p=0.076. Therefore, there was no evidence for the risk of bias.
Figure 3.
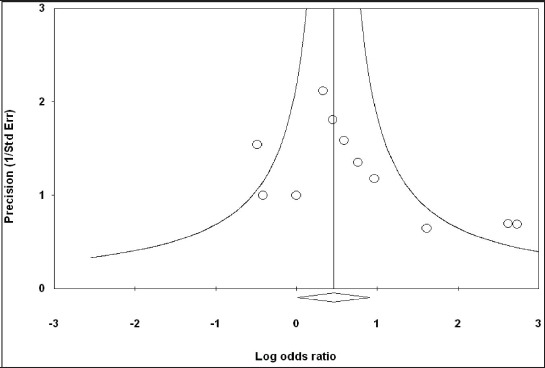
Funnel plot of the included studies in the meta-analysis investigating the prevalence of lichen planus in diabetes mellitus patients
4. DISCUSSION
A number of studies reported the prevalence of OLP in DM patients with different percentages. The studies showed that the prevalence rate of OLP was 0.5 to 9.3% in DM patients and 0 to 1.8% in control subjects. Van Dis et al., (17) Potts et al. (23) and Robertson & Wray (24), concluded that the type of medication was associated with the presence of OLP lesions. Age is a risk factor for OLP. Ara et al. (25) and Bastos et al. (14) reported the correlation between age and OLP that majority of OLP patients had age 40-50 years. Also, the report of Bastos et al. (14) showed that more OLP patients had DM for a period more than 5 years. The OLP was estimated to affect 0.5% to 2.0% of the general population. This disease has most often been reported in middle-aged patients with 30-60 years of age and is more common in women than in men (26). The relative risk of OLP is 3.7% in people with mixed oral habits, lowest (0.3%) in non-users of tobacco and highest (13.7%) among those who smoked and chewed tobacco (27). Al-Maweri et al. (13) reported that in DM patients and control subjects with no oral habits such as smoking, alcohol consumption or tobacco or betel nut chewing, the prevalence rate of OLP was 0.5 and 0%, respectively, and Ahmed et al. (28) showed this rate in DM patients without a history of addiction was 9.3%. Two studies (14, 15), checked the prevalence rate of OLP in DM patients/control subjects with smoking that concluded the rate was 6.2%/0% and 1.06%/0%, respectively, and this rate in study of Guggenheimer et al. (16) with smoking and alcohol consumption was 0.5%/0.74%. Therefore, the results showed that alcohol consumption, smoking or tobacco or chewing betel nutcan increase risk of OLP in DM patients compared with control subjects. One study (13), reported that this higher prevalence rate of oral mucosa lesions in DM patients may be due to slower healing rates in these patients that leads to a longer duration of a given lesion and not be due to an increase in the incidence. Therefore, if a lesion takes two months to cure in a DM patient and one month in control subject, the prevalence will be more in DM patients at a given point of time. Poor metabolic control can correlate with various diabetic complications (29) that this control may lead to many pathological changes and increase the susceptibility of oral tissues to infection and local irritants (30). A number of researchers indicated that OLP in DM patients could be linked with compromised immune system in these patients (11) or may be connected to a number of oral hypoglycemic medications taken particularly by older people (31). This meta-analysis study evaluated the prevalence of OLP in a total of 14 studies and comparison of OLP rate in DM patients compared with control subjects that the results showed the prevalence of OLP was 1.37% in DM patients and 0.75% in control subjects. However, previous studies concluded that there was no significant difference between the prevalence of OLP and DM patients compared with control subjects, but the meta-analysis showed the difference was statistically significant(P<0.05) and the prevalence of OLP was significantly higher in DM patients than control subjects that a very important factor can be type of medications and reducing of immune system in DM patients. To better and more understanding about the reason of increasing the OLP in DM patients should be done cross-sectional and clinical trials studies in DM patients in future, especially considering to the type and duration of treatment, changes in the immune system and also type of diabetes.
Limitations
There were several limitationsin this study. First, we could not evaluate some studies that published in other languages. Second, some studies in meta-analysis had added the patients with type I diabetes to study and therefore, we couldn’t report the prevalence of OLP in type II diabetes alone. Third, treatment duration can be effective in incidence of OLP in DM patients, but a number of studies had not reported it. Forth, diagnosis of diabetes in some studies was just based on fasting blood sugar level, whereas HbA1c test is complementary test for more accurate diagnosis of diabetes and it can be because the price of the more that can’t be check in all patients. At last, a number of effective factors on the incidence of OLP such as age, sex, oral habits, smoking and alcohol consumption had not been controlled in some studies and type of treatments were different.
5. CONCLUSIONS
This meta-analysis study showed an association between OLP with DM, whereas this association was no significant in previous studies, it was probably because different selecting of age, sex, type of DM, medications and criteria. Totally, the meta-analysis showed the risk of OLP in DM was more compared with control subjects (high prevalence 9.3% in DM patients compared with 1.8% in control subjects).
Footnotes
• Conflict of interest: none declared
REFERENCES
- 1.Mozaffari HR, Rahmani M, Rezaei F, Sadeghi M, Sharifi R, Ejtehadi A. Evaluation of oral lichen planus frequency in patients referred to pathology centers of Kermanshah city, during 2008 to 2011. Sch J App Med Sci. 2016;4(6E):2200–2. [Google Scholar]
- 2.Carazzo M, Thorpe R. Oral lichen planus: a review. Minerva Stomatol. 2009;58(10):519–37. [PubMed] [Google Scholar]
- 3.Shen ZY, Liu W, Zhu LK, Feng JQ, Tang GY, Zhou ZT. A retrospective clinicopathological study on oral lichen planus and malignant transformation: Analysis of 518 cases. Med Oral Patol Oral Cir Bucal. 2012;17(6):e943–7. doi: 10.4317/medoral.17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torrente-Castells E, Figueiredo R, Berini-Aytés L, Gay-Escoda C. Clinical features of oral lichen planus. A retrospective study of 65 cases. Med Oral Patol Oral Cir Bucal. 2010;15(5):e685–90. doi: 10.4317/medoral.15.e685. [DOI] [PubMed] [Google Scholar]
- 5.Canter PH, Wider B, Ernst E. The antioxidant Vitamins A, C, E and selenium in the treatment of arthritis: A systematic review of randomized clinical trials. Rheumatology (Oxford) 2007;46(8):1223–33. doi: 10.1093/rheumatology/kem116. [DOI] [PubMed] [Google Scholar]
- 6.Arrieta-Blanco JJ, Bartolomé-Villar B, Jiménez-Martinez E, Saavedra-Vallejo P, Arrieta-Blanco FJ. Bucco-dental problems in patients with Diabetes Mellitus (I) :Index of plaque and dental caries. Med Oral. 2003;8(2):97–109. [PubMed] [Google Scholar]
- 7.Black JM, Hawks JH. Medical-Surgical Nursing-Clinical Management for Positive Outcomes. 8th ed. London: Saunders; 2009. p. 1062. [Google Scholar]
- 8.Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016;92(1084):63–9. doi: 10.1136/postgradmedj-2015-133281. [DOI] [PubMed] [Google Scholar]
- 9.Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354–95. doi: 10.4239/wjd.v7.i17.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krupaa RJ, Sankari SL, Masthan KM, Rajesh E. Oral lichen planus: An overview. J Pharm Bioallied Sci. 2015;7(Suppl 1):S158–61. doi: 10.4103/0975-7406.155873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrou-Amerikanou C, Markopoulos AK, Belazi M, Karamitsos D, Papanayotou P. Prevalence of oral lichen planus in diabetes mellitus according to the type of diabetes. Oral Dis. 1998;4(1):37–40. doi: 10.1111/j.1601-0825.1998.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaomongkolgit R. Oral lichenoid drug reaction associated with antihypertensive and hypoglycemic drugs. J Drugs Dermatol. 2010;9(1):73–5. [PubMed] [Google Scholar]
- 13.Al-Maweri SA, Ismail NM, Ismail AR, Al-Ghashm A. Prevalence of oral mucosal lesions in patients with type 2 diabetes attending hospital universiti sains malaysia. Malays J Med Sci. 2013;20(4):39–46. [PMC free article] [PubMed] [Google Scholar]
- 14.Bastos AS, Leite AR, Spin-Neto R, Nassar PO, Massucato EM, Orrico SR. Diabetes mellitus and oral mucosa alterations: prevalence and risk factors. Diabetes Res Clin Pract. 2011;92(1):100–5. doi: 10.1016/j.diabres.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Albrecht M, Bánóczy J, Dinya E, Tamás G., Jr Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J Oral Pathol Med. 1992;21(8):364–6. doi: 10.1111/j.1600-0714.1992.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 16.Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, Block HM, et al. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: II. Prevalence and characteristics of Candida and Candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(5):570–6. doi: 10.1067/moe.2000.104477. [DOI] [PubMed] [Google Scholar]
- 17.Van Dis ML, Parks ET. Prevalence of oral lichen planus in patients with diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(6):696–700. doi: 10.1016/s1079-2104(05)80302-6. [DOI] [PubMed] [Google Scholar]
- 18.Mohsin SF, Ahmed SA, Fawwad A, Basit A. Prevalence of oral mucosal alterations in type 2. diabetes mellitus patients attending a diabetic center. Pak J Med Sci. 2014;30(4):716–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Saini R, Al-Maweri SA, Saini D, Ismail NM, Ismail AR. Oral mucosal lesions in non oral habit diabetic patients and association of diabetes mellitus with oral precancerous lesions. Diabetes Res Clin Pract. 2010;89(3):320–6. doi: 10.1016/j.diabres.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Borghelli RF, Pettinari IL, Chuchurru JA, Stirparo MA. Oral lichen planus in patients with diabetes. An epidemiologic study. Oral Surg Oral Med Oral Pathol. 1993;75(4):498–500. doi: 10.1016/0030-4220(93)90178-7. [DOI] [PubMed] [Google Scholar]
- 21.Borhan Mojabi K, Esfahani M, Bokharaee MM. Evaluation of median rhomboid glossitis and oral lichen planus in patients with diabetes mellitus. J Qazvin Univ Med Sci. 2009;13(1):56–60. [Google Scholar]
- 22.Zareei MR, Shirei R. Assessment of the prevalence of oral lichen planus in diabetic patients. Journal of Dental School. 2000;18(2):137–43. [Google Scholar]
- 23.Potts AJ, Hamburger J, Scully C. The medication of patients with oral lichen planus and the association of nonsteroidal anti-inflammatory drugs with erosive lesions. Oral Surg Oral Med Oral Pathol. 1987;64(5):541–3. doi: 10.1016/0030-4220(87)90029-6. [DOI] [PubMed] [Google Scholar]
- 24.Robertson WD, Wray D. Ingestion of medication among patients with oral keratoses including lichen planus. Oral Surg Oral Med Oral Pathol. 1992;74(2):183–5. doi: 10.1016/0030-4220(92)90380-9. [DOI] [PubMed] [Google Scholar]
- 25.Ara SA, Mamatha GP, Rao B. Incidence of diabetes mellitus in patients with diabetes mellitus. J Dental Clin. 2011;3(1):29–33. [Google Scholar]
- 26.Gupta S, Jawanda MK. Oral Lichen Planus: An Update on Etiology, Pathogenesis, Clinical Presentation, Diagnosis and Management. Indian J Dermatol. 201;60(3):222–9. doi: 10.4103/0019-5154.156315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafer WG, Hine MK, Levy BM. Shafer’s textbook of oral pathology. 6th ed. Noida, India: Elsevier publications; 2009. p. 800. [Google Scholar]
- 28.Ahmed I, Nasreen S, Jehangir U, Wahid Z. Frequency of oral lichen planus in patients withnoninsulin dependent diabetes mellitus. Journal of Pakistan Association of Dermatologists. 2012;22(1):30–34. [Google Scholar]
- 29.Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97(3):604–11. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 30.Sykes LM, Sukha A. Potential risk of serious oral infections in the diabetic patient: a clinical report. J Prosthet Dent. 2001;86(6):569–73. doi: 10.1067/mpr.2001.120200. [DOI] [PubMed] [Google Scholar]
- 31.Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, Migliorati CA, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(Suppl):S25e1–12. doi: 10.1016/j.tripleo.2006.11.001. [DOI] [PubMed] [Google Scholar]


