Abstract
Background:
Multiple studies suggest a pivotal role of the endocannabinoid system in regulating the reinforcing effects of various substances of abuse. Rimonabant, a CB1 inverse agonist found to be effective for smoking cessation, was associated with an increased risk of anxiety and depression. Here we evaluated the effects of the CB1 neutral antagonist AM4113 on the abuse-related effects of nicotine and its effects on anxiety and depressive-like behavior in rats.
Methods:
Rats were trained to self-administer nicotine under a fixed-ratio 5 or progressive-ratio schedules of reinforcement. A control group was trained to self-administer food. The acute/chronic effects of AM4113 pretreatment were evaluated on nicotine taking, motivation for nicotine, and cue-, nicotine priming- and yohimbine-induced reinstatement of nicotine-seeking. The effects of AM4113 in the basal firing and bursting activity of midbrain dopamine neurons were evaluated in a separate group of animals treated with nicotine. Anxiety/depression-like effects of AM4113 and rimonabant were evaluated 24h after chronic (21 days) pretreatment (0, 1, 3, and 10mg/kg, 1/d).
Results:
AM4113 significantly attenuated nicotine taking, motivation for nicotine, as well as cue-, priming- and stress-induced reinstatement of nicotine-seeking behavior. These effects were accompanied by a decrease of the firing and burst rates in the ventral tegmental area dopamine neurons in response to nicotine. On the other hand, AM4113 pretreatment did not have effects on operant responding for food. Importantly, AM4113 did not have effects on anxiety and showed antidepressant-like effects.
Conclusion:
Our results indicate that AM4113 could be a promising therapeutic option for the prevention of relapse to nicotine-seeking while lacking anxiety/depression-like side effects.
Keywords: CB1 antagonism, nicotine reward, dopamine, anxiety, depression
Introduction
According to the 2013 World Health Organization report, 5 million deaths worldwide per year can be attributed directly to tobacco consumption, and by 2030 it is expected to reach 8 million (WHO, July, 2013). While there are several smoking cessation therapies available—nicotine replacement therapies, bupropion, varenicline (Cummings and Mahoney, 2006; Jorenby et al., 2006)—the success rate of these therapies after 1 year remains only about 20 to 25% (Gonzales et al., 2006). Current medications show limited efficacy and more novel effective therapeutic agents are required to help curb tobacco dependence (Lerman et al., 2007; Le Foll et al., 2014).
Behavioral studies in animal models have shown that the endogenous cannabinoid system plays a key role in brain mechanisms underlying the motivational effects of drug and drug-related stimuli (Cohen et al., 2002; Le Foll et al., 2014). Targeting the CB1 receptor for the treatment of nicotine addiction has garnered the greatest attention. This is mainly due to the early success and promise of rimonabant, an inverse agonist for the CB1 receptor that initially proved to be effective as an aid for smoking cessation. Rimonabant was able to reduce nicotine-taking behavior under fixed and progressive ratio (FR and PR) schedules of reinforcement and blocked nicotine-induce dopamine (DA) release in the nucleus accumbens (NAcc) shell and the bed nucleus of the stria terminalis (Cohen et al., 2002; Forget et al., 2009). Rimonabant blocked cue-induced reinstatement of nicotine-seeking behavior and prevents the establishment and expression of nicotine-induced conditioned place preference in rodents (Le Foll and Goldberg, 2004; Forget et al., 2005). Early clinical trials showed the efficacy of rimonabant in promoting smoking cessation (Cahill and Ussher, 2011). However, due to the occurrence of side effects, including depressive disorders, anxiety, insomnia, and thoughts of suicide (Janero and Makriyannis, 2009; Cahill and Ussher, 2011), the use of rimonabant was discontinued and all ongoing clinical studies involving rimonabant for other indications were halted (Sanofi Aventis, 2008; Janero and Makriyannis, 2009; Le Foll et al., 2014). In preclinical settings, under chronic administration paradigms, rimonabant also displayed anxiety-like (O’Brien et al., 2013) and depressive-like (Beyer et al., 2010) effects.
The idea of targeting the CB1 receptor via blockade still represents a promising strategy, since the use of CB1 inverse agonists has demonstrated the most consistent results in quitting efficacy and in preventing relapse. It has been suggested that the side effects of rimonabant might be due to its inverse agonist properties. Therefore, it would be logical to test if the blockade of the receptor with ligands lacking intrinsic activity per se at the cellular level, that is, not triggering changes in cellular signaling (no effect on cAMP accumulation [Chambers et al., 2007]) would indeed retain the same efficacy of rimonabant without its unwanted side effects (Kangas et al., 2013). AM4113 is a CB1 neutral antagonist and represents a novel class of drugs displaying high affinity for CB1 (0.8nM) and 100-fold selectivity for the CB1 receptor over CB2 (Chambers et al., 2007; Sink et al., 2008). AM4113 did not change the forskolin-stimulated cAMP accumulation in CB1-transfected HEK cells up to concentrations of 10 µM, which is indicative of its neutral antagonist properties (Sink et al., 2008).
The novel CB1 neutral antagonist AM4113 is able to block the CB1 receptor and shows no intrinsic activity (Sink et al., 2008), representing a great promise as a smoking cessation aid. We hence hypothesized here that AM4113 will modify the reinforcing effects of nicotine and preventrelapse to nicotine seeking, while retaining the efficacy of rimonabant, but will be devoid of its side effects (i.e., anxiety and depressive-like behaviors) (Le Foll et al., 2014).
In the present study, we evaluated the effects of AM4113 in the reinforcing effects of nicotine (i.e., nicotine-taking/seeking behavior) by using the i.v. self-administration paradigm. We also investigated the effects of AM4113 on the neuronal activity induced by nicotine in the ventral tegmental area (VTA). Finally, we tested the effects of AM4113 and rimonabant on behavior in the elevated plus maze (EPM) and forced swim test (FST) following its chronic administration in rats.
Materials and Methods
Animals
Male Long Evans or Wistar rats weighing 250 to 275g at the start of experiments were maintained on ~20g of rat chow daily (experiment 1) or free access to food (experiments 2 and 3) and ad libitum water. The animals were habituated for 7 days to the colony room and single-housed in a temperature-controlled room on a 12-h reverse light cycle with all behavioral testing occurring during the dark phase. All experimental procedures described were carried out in compliance with the guidelines of the Canadian Council on Animal Care and were reviewed and approved by the Animal Care Committee at CAMH.
Experiment 1
Training procedures and surgical techniques were similar to those previously reported (Forget et al., 2010; Khaled et al., 2010). Animals were initially trained on a schedule in which each lever press resulted in the delivery of a food pellet (no associated cues) (FR-1). Operant training sessions terminated at the 60-minute time point or earlier whenever 100 food pellets (45mg Dustless Precision Pellets, BioServ, Frenchtown, NJ) were obtained. Animals received a minimum of 5 sessions of training and by the 5th session, animals had to achieve 100 food pellets within 20 minutes in order to continue on to nicotine i.v. self-administration experiments. Once trained, each rat was implanted with an i.v. catheter in the jugular vein exiting in the mid-scapular region as previously described (Khaled et al., 2010). Penicillin G (30000 units, s.c.) was given within 24 hours before surgical procedures. Anesthesia was induced by a mixture of xylazine and ketamine hydrochloride (10 and 75mg/kg, respectively, i.p.). Postsurgical analgesia was provided using buprenorphine (0.01mg/kg, s.c.). Following surgery, animals were allowed to recover over a minimum of 7 days in their home cages before any other experimental procedure.
Acquisition of the Nicotine or Food Self-Administration
The self-administration sessions were carried out in experimental chambers equipped with 2 levers (Med Associates, St. Albans, VT). The start of the session was signaled by illumination of a house light; switching off this light indicated the time-out (TO) period during which lever responding was recorded but had no consequences. Nicotine (30-µg/kg/infusion) was delivered (1-second delivery time) using Med Associates pumps (Model PHM-104), and nicotine infusion speed was adjusted based on the weight of each rat. A cue light was present above the active lever and would illuminate for the duration of the 60-second TO period following each reinforcement. Rats underwent 5 sessions of self-administration under a FR-1 schedule of reinforcement, followed by 3 sessions under a FR-2 schedule and then 7 sessions under a FR-5 schedule (i.e., 5 active lever presses required for each reinforcement).
Testing Under FR
Two separate group of rats were considered to have acquired stable food or nicotine self-administration when they pressed the active lever more than twice the number of times they pressed the inactive lever, and received a minimum of 10 infusions/1-h session with <20% variation in the number of infusions/pellets earned per session during 2 consecutive sessions. Once stable responding was achieved, rats were tested using either acute i.p. injections of AM4113 (0, 1, 3, and 10mg/kg in a counter-balanced within subject design with at least 2 washout sessions) or chronic i.p. injections of AM4113 (10mg/kg, 10 days).
Testing under the PR
Two additional groups of rats were trained to acquire food or nicotine self-administration behavior under a FR as described above and then were switched to a PR schedule where the response requirement progressively increased according to the formula 5e(0.25*inf number)-5 (Roberts and Bennett, 1993) with the first response requirements being 5 and 10. The break point was defined as the highest ratio completed prior to the first 30-minute period without any response on the active lever. PR sessions lasted a maximum of 4 hours. The animals were allowed 10 days of nicotine self-administration under the PR schedule before testing with acute or chronic AM4113 (0.3, 1, 3, and 10mg/kg, 10 days), respectively.
Extinction
The effects of AM4113 on reinstatement of nicotine were tested in separate groups. The extinction phase (≥7sessions) consisted of withholding nicotine and its associated cues (house light stayed on and cue lights stayed off throughout the session). During extinction, following stable nicotine self-administration as described above, responses on the active and inactive levers were recorded but had no programmed consequences. The criterion for extinction was <20 active lever presses per 1-hour session over 2 consecutive sessions.
Reinstatement of Nicotine Seeking
For the cue-induced reinstatement tests (n=7), active lever presses resulted in presentation of the light cues as during nicotine self-administration under FR-5 60-second TO, with the exemption that no drug was delivered and a single presentation of the visual cue (light above the active lever on and house light off for 60 seconds) was delivered response independently immediately at the start of the session. The procedure for cue-induced reinstatement was chosen based on previous studies showing high levels of reinstatement (Liu et al., 2007; Forget et al., 2010; Gamaleddin et al., 2012).
Another group of rats (n=8) underwent nicotine-induced reinstatement tests as previously reported (Forget et al., 2010). Briefly, rats were injected with nicotine (0.15mg/kg, s.c.) 10 minutes before the test session.
An additional group of rats (n=15) underwent stress-induced reinstatement tests that used methodology previously described (Marinelli et al., 2007; Le et al., 2009). Briefly, rats were injected with yohimbine (an alpha-2 adrenoceptor antagonist that provokes stress- and anxiety-like responses in both humans and laboratory animals) (2.5mg/kg, s.c.) 30 minutes before the test session. Similar to the extinction phase, during nicotine- or stress-induced reinstatement testing, the cue light above the active lever was always off.
In the all reinstatement procedures described above (FR-5, 60-second TO), rats were pretreated with vehicle or AM4113 (0.3, 1, 3, and 10mg/kg), in a counterbalanced order, using a within-subject design, 60 minutes prior to the 1-h reinstatement session. Testing days were separated by at least three extinction sessions and required meeting criteria for stable extinction during 2 consecutive sessions.
Experiment 2
Single-Cell Extracellular DA Recording
In vivo single-cell extracellular recording of VTA dopaminergic neurons was performed in urethane-anesthetized animals. To determine if AM4113 (3mg/kg) was able to reduce the effects of nicotine (0.15mg/kg, s.c.) on VTA DA firing activity, we sampled a total of 38 VTA DA neurons (vehicle/saline, n=10; vehicle/nicotine, n=10; AM4113/nicotine, n=10; AM4113/saline, n=9) as previously described (Tan et al., 2009) (Supplemental Information).
Experiment 3
Chronic Treatment and Body Weight Gain
After habituating the animals to the housing room for one and one-half weeks, the rats were weight-matched between the 7 different groups: vehicle, AM4113 (1, 3, and 10mg/kg), or rimonabant (1, 3, and 10mg/kg). The animals were weighted every day before daily injections (days 1–21) were administered. At the end of this chronic treatment, rats were assessed using a battery of behavioral tests to evaluate the effects of the chronic treatment on anxiety/depressive like-behaviors.
EPM
The EPM was conducted 24 hours after the end of the chronic treatment (day 22). The maze consisted of a center platform (10x10cm) elevated 50cm off the floor, with 4 arms (50x10cm) at 90° to each other. Two parallel arms had walls (30cm tall) enclosing the platform. Dim lighting was provided to the platform, with a mean (±SEM) light intensity: 7.9 (±0.9), 7.5 (±0.6), and 1.6 (±0.2) lx to the central platform, open and closed arms, respectively. An experimenter, blind to the experimental conditions, measured the total time each subject spent in the central, open, and closed areas.
FST
The FST consisted of a Plexiglas cylinder (60-cm height, 20-cm diameter) filled with water (40cm, 24±1°C). On day 1, the animals were individually placed in the cylinder and left to swim. After 15 minutes, the animals were removed from the cylinder and dried with towels before being returned to their home cages. On day 2, the rats were placed in the same cylinder for 5 minutes. An experimenter, blind to the experimental conditions, scored immobility and climbing time. The animals were randomly assigned to 1 of 7 groups: vehicle, AM4113 (3 doses), or rimonabant (3 doses). To evaluate acute effects of AM4113 and rimonabant on FST, AM4113 and rimonabant (1, 3, and 10mg/kg) were administered 23 hours, 5 hours, and 1 hour before the FST sessions. Chronic effects of AM4113 and rimonabant were also evaluated by administering AM4113 and rimonabant (1, 3, and 10mg/kg) over 21 days before FST.
Drugs
(−)Nicotine-hydrogen-tartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in saline, the pH was adjusted to 7.0 (±0.2), and the solution was filtered through a 0.22-mm syringe filter (Fisher Scientific, Pittsburgh, PA). AM4113 (N-piperidin-1-yl-2,4-dichlorophenyl-1H-pyrazole-3-carboxamide) was synthesized at the Center for Drug Discovery, Northeastern University, Boston, MA. AM4113 was dissolved in dimethylsulfoxide, Tween 80, and saline as previously described (Hodge et al., 2008; Sink et al., 2010). Rimonabant [SR141716; N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide)] (NIDA Drug Supply Program, Bethesda, MD) was dissolved in methyl cellulose and Tween 80. The different doses of AM4113 (0, 0.3, 1, 3, and 10mg/kg) and rimonabant (0, 1, 3, and 10mg/kg) were i.p. administered using a counterbalanced within-subject design (acute treatment) 60 minutes prior to the start of the session (administration volume: 1mL/kg) (Sink et al., 2008; Cluny et al., 2011). For chronic studies, treatment consisted of the repeated administration of the same dose.
Yohimbine was obtained from Sigma-Aldrich and dissolved in distilled water. The doses of yohimbine and the pretreatment time were based on previous studies (Marinelli et al., 2007; Le et al., 2009).
Data Analysis
Difference in responses between the active and inactive levers during the acquisition of nicotine self-administration or during reinstatement was analyzed by 2-way ANOVA with repeated measures. The effects of AM4113 and rimonabant on the number of nicotine infusions and food pellets earned under the FR-5 and PR schedules were analyzed using 1-way ANOVA. Similarly, the effects of AM4113 and rimonabant in the EPM and the FST and the effects of AM4113 on VTA DA neuronal activity were analyzed using 1-way ANOVA followed by Bonferroni’s posthoc for multiple test comparisons. Statistical analyses were conducted using the statistical package Stat Soft. Inc. version 10. P<.05 was considered to be statistically significant.
Results
Effects of Acute AM4113 on Nicotine and Food SA under FR-5 and PR Schedules
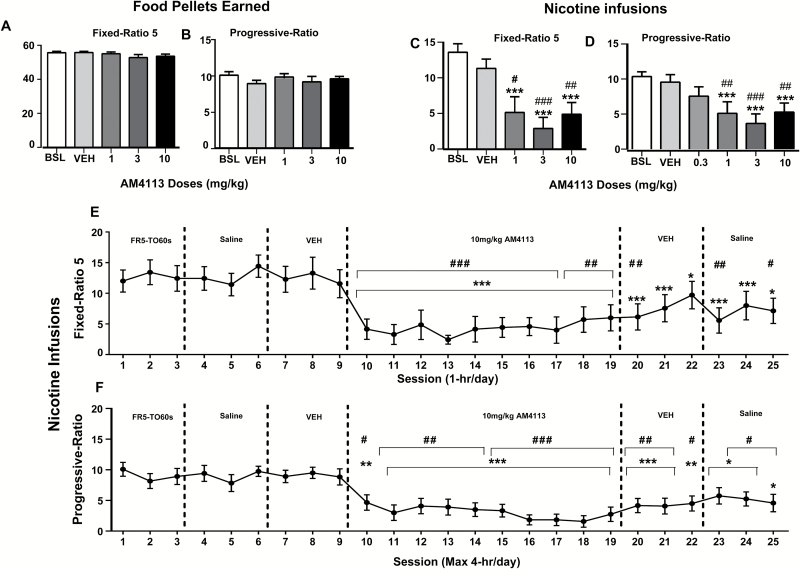
One-way ANOVA analysis with repeated measures revealed no significant effects of AM4113 pretreatment on food self-administration in either FR-5 or during PR schedules [(F(4,9) = 2.21, P>.05), (F4,9 = 1.18, P>.05)], respectively (Figure 1A-B). On the other hand, 1-way ANOVA with repeated measures showed the main effect of AM4113 on nicotine self-administration during FR-5 (F4,7=13.68, P<.001) and PR (F5,8=10.77, P<.001) schedules of reinforcement. Posthoc analyses showed that AM4113 significantly reduced nicotine intake (FR-5) relative to saline-baseline and vehicle controls (AM4113 1mg/kg (P<.001 and P<.05), 3mg/kg (P<.001 and P<.001), and 10mg/kg (P<.001 and P<.01), respectively) (Figure 1C). Similarly, posthoc tests showed that AM4113 significantly reduced nicotine intake relative to saline-baseline and vehicle controls under the PR schedule (AM4113 1mg/kg (P<.001 and P<.01), 3mg/kg (P<.001 and P<.001), and 10mg/kg (P<.001 and P<.01), respectively) (Figure 1D). No significant differences were observed between the saline-baseline and vehicle control groups (P>.05).
Figure 1.
Effects of acute AM4113 (60-minute pretreatment time) on food and nicotine-taking behavior under fixed ratio-5 (FR-5) and progressive ratio (PR) schedules of reinforcement. Bars represent average responding (±SEM) during FR-5 (1-h sessions, A and C) and PR (≤4-h sessions, B and D) schedules following treatment with saline (baseline, BSL), vehicle (VEH), or AM4113 (1, 3, and 10mg/kg). (A) Number of food pellets earned under FR-5 (n=10). (B) Food pellets earned under PR (n=10). (C) Nicotine infusions earned under FR-5 (n=8). (D) Nicotine infusions earned under PR (n=9). ***P<.001 vs saline BSL; #P<.05 vs VEH; ##P<.01 vs VEH; ###P<.001 vs VEH (1-way repeated-measures ANOVA followed by Bonferroni posthoc tests). Chronic administration of AM4113 on nicotine-taking behavior under FR-5 (n=7) (E) and PR schedules of reinforcement (n=12) (F). Each session represents average responding (±SEM). * and #, P<.05 vs saline-3 (session 6) and VEH-3 (session 9), respectively; ** and ##, P<.01 vs saline-3 (session 6) and VEH-3 (session 9); *** and ###, P<.001 vs saline-3 (session 6) and VEH-3 (session 9), respectively (1-way repeated-measures ANOVA followed by Bonferroni posthoc test).
Under chronic treatment, the 1-way ANOVA analysis with repeated measures revealed a main effect of AM4113 on nicotine self-administration during FR-5 (F24,6=15.78, P<.001) and PR (F24,26=10.43, P<.001) schedules of reinforcement. Under FR-5, Bonferroni multiple comparison tests revealed significant differences between the third saline dose (session 6 of the experimental sequence) and AM4113 (sessions 10–19) treatments on nicotine intake (Figure 1E). Bonferonni multiple comparison tests also revealed significant differences between the third dose of vehicle (session 9) and AM4113 treatment (sessions 10–17, P<.001, sessions 18–19, P<.01). The decrease on nicotine intake persisted after the treatment with AM4113 when rats were treated with either vehicle or saline (sessions 20–25 vs saline on session 6, P<.001-0.05 and sessions 20, 23, and 25 vs vehicle on session 9, P<.01, P<.001, and P<.5, respectively) (Figure 1E).
Similarly, Bonferroni multiple comparison tests showed a significant decrease in nicotine intake during treatment with AM4113, under the PR schedule, compared with the third saline dose (session 6) vs sessions 10 to 19 (P<.001-0.01) or the third vehicle dose (session 9) vs sessions 10 to 19 (P<.001-0.01). This effect of AM4113 persisted under vehicle and saline treatments (P<.001-0.01) (Figure 1F). No differences were observed during the third dose of saline (session 6) or the third dose of vehicle (session 9), before AM4113 treatment, or under FR-5 or PR.
Effects of AM4113 on Reinstatement of Nicotine Seeking
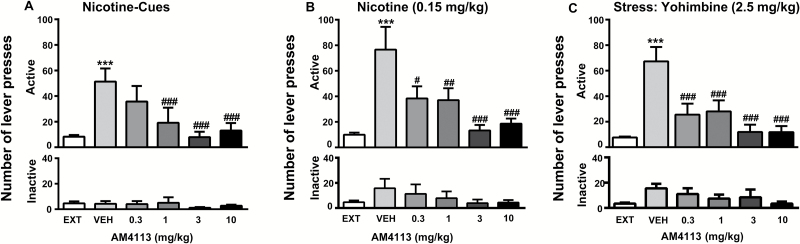
Two-way ANOVA analysis with repeated measures for active lever presses indicated a main effect of treatment on nicotine-seeking behavior compared with extinction conditions (P<.001) under light-associated cues, nicotine-priming, and stress (Figure 2A-C, top). ANOVA analysis revealed a main effect of treatment under all 3 conditions (light cues: F5,60 = 6.72; P<.001; nicotine priming: F5,70 = 4.33; P<.05; and yohimbine: F5,14 = 6.71; P<.001). Bonferroni posthoc tests showed that pretreatment with AM4113 (1, 3, and 10mg/kg) during light cue conditions (P<.001) attenuated the reinstatement induced by nicotine-associated cues. AM4113 at doses 0.3 (P<.05), 1, 3, and 10mg/kg (P<.01, P<.001, P<.001) reduced reinstatement induced by nicotine priming. Similarly, AM4113 attenuated reinstatement induced by stress (P<.001) at all doses (0.3; 1, 3, and 10mg/kg). Neither presentation of nicotine-associated cues nor nicotine-priming nor stress had an effect on responding on the inactive lever (Figure 2A-C, bottom).
Figure 2.
Effects of AM4113 (0.3, 1, 3, and 10mg/kg, i.p. 60-minute pretreatment time) on reinstatement of nicotine-seeking behavior induced by presentation of nicotine-associated cues (A), by a priming dose of nicotine (B), and pharmacological stressor yohimbine (C). Data are expressed as the number of active (A-C, top) and inactive (A-C, bottom) lever presses (mean±SEM). Effects of presentation of nicotine-associated cues alone, a priming dose of nicotine, or stress by yohimbine under vehicle treatment on reinstatement of nicotine-seeking behavior (***P<.001 compared with baseline behavior [EXT]). Pretreatment with AM4113 blocked reinstatement of nicotine-seeking behavior (#P<.05, ##P<.01, and ###P<.001). No differences were observed on inactive lever presses (cue condition, n=7, (nicotine-priming condition, n=8, and stress condition, n=15).
Effects of AM4113 on VTA Neuronal Activity
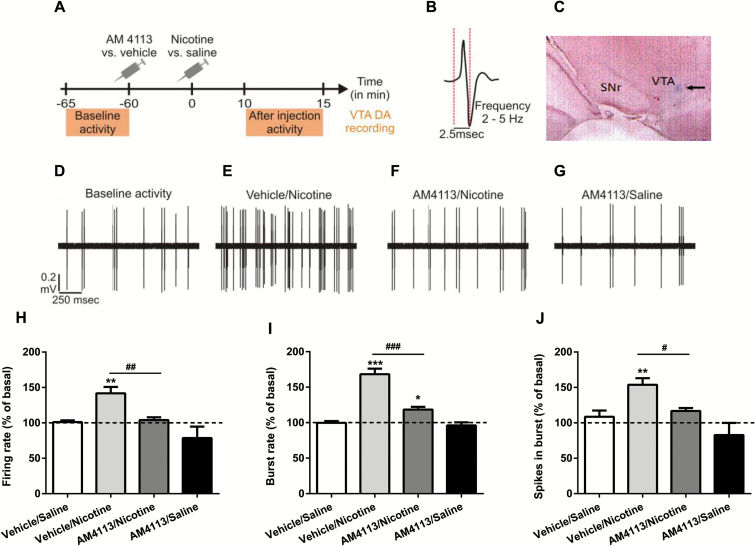
One-way ANOVA analysis indicated the main effect of AM4113 on VTA DA neuronal activity induced by nicotine. ANOVA revealed the main effect of treatment on FR (F 3,38 = 8.309, P<.001; Figure 3H), bursting rate (F 3,38 = 42.988 P<.001; Figure 3I), and spikes firing in burst (F 3,38 = 7.573, P<.001; Figure 3J). Posthoc analyses showed significant difference between vehicle/nicotine, AM4113/nicotine, AM4113/saline treatment (*P<.05, **P<.01, ***P<.001), and vehicle/saline. Moreover, the posthoc tests revealed significant differences in neuronal activity between the vehicle/nicotine and AM4113/nicotine treatments (#P<.05, ##P<.01, ###P<.001).
Figure 3.
AM4113 blocks nicotine-induced increases of ventral tegmental area (VTA) dopamine (DA) neuronal activity. (A) Timeline of the experiment. (B) Typical DA action potential waveform. (C) Microphotograph of coronal section through the VTA showing the deposit of pontamine sky blue done at the end of recordings. (D) Raw traces showing an example of baseline activity. (E) Increase in the activity observed 10 minutes after the injection of nicotine in the vehicle/nicotine group. (F) Blocking effect of AM4113 in the AM4113-/nicotine-treated group. (G) Effects of AM4113 (3mg/kg) on VTA DA neuronal firing rate in the vehicle/nicotine (n=10), AM4113/nicotine (n=10), and AM4113/vehicle (n=9) groups. In (H), burst rate (I) and spikes firing in burst (J) (vs vehicle/saline-treated group, n=10), *P<.05, **P<.01, ***P<.001 from vehicle/saline. Note the significant differences in neuronal activity between the vehicle/nicotine and AM4113/nicotine treatments (#P<.05, ##P<.01, ###P<.001). Bars represent mean ± SEM.
Effects of Acute and Chronic Treatment of AM4113 on Body Weight Gain, Anxiety, and Depressive-Like Behavior
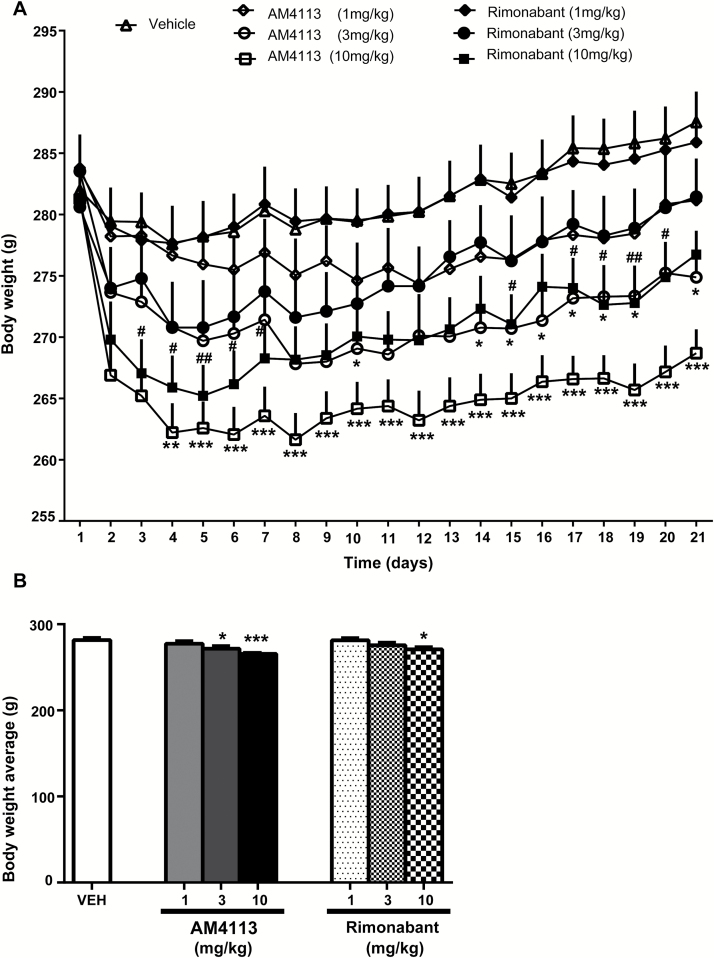
ANOVA analysis with repeated measures revealed an overall effect of treatment (F6,142 = 2.88, P=.05) and of days (F20,29 = 86.002, P<.001) and an interaction between days and treatment (F126,29 = 4.91, P<.001). Posthoc tests showed decreased body weight during chronic treatment with AM4113 at doses of 3mg/kg (P<.05) and 10mg/kg (P<.01 and P<.001) and rimonabant at the highest dose tested (10mg/kg) (P<.05 and P<.01) (Figure 4A). One-way ANOVA analysis revealed the main effect of treatment on mean body weight (F6,141=4.98, P<.001). Bonferroni comparisons showed significant differences between the vehicle, AM4113 (P<.05 and P<.001 at 3 and 10mg/kg, respectively), and rimonabant (P<.05 at 10mg/kg) (Figure 4B).
Figure 4.
Effects of chronic AM4113 or rimonabant treatment (21 days) on body weight. (A) Chronic treatment with AM4113 (3 and 10mg/kg) or rimonabant (10mg/kg) decreased body weight. *P<.05, **P<.01, *** P<.001, significant differences between vehicle and AM4113 groups, respectively; #P<.05 and ##P<.01, significant differences between vehicle and rimonabant groups, respectively. (B) Chronic treatment with either AM4113 (1, 3, and 10mg/kg, n=18, n=17, and n=19, respectively) or rimonabant (1, 3, and 10mg/kg, n=18, n=18, and n=19, respectively) decreased mean body weight during the 21-day treatment (bar graph). *P<.05 and ***P<.001, significant differences compared with vehicle (n=40, Dunnett’s Multiple Comparison Test).
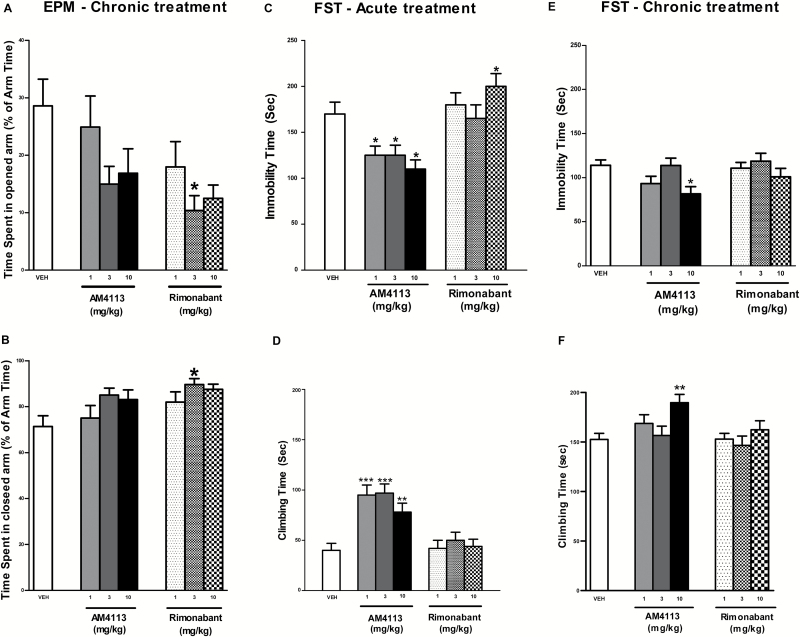
One-way ANOVA analysis revealed an overall effect of treatment (F6,142 = 2.61 P<.05) on the EPM. For the main effect of group, posthoc comparisons showed that significantly less time was spent in the opened arm when rats were treated with rimonabant (3mg/kg) compared with the vehicle group. This is consistent with the relatively greater time spent in the closed arm. Following chronic treatment with AM4113, rats did not display any significant difference in the open and closed arm time compared with the vehicle group (P>.05) (Figure 5A-B).
Figure 5.
Effects of chronic AM4113 and rimonabant treatments on anxiety-like behavior using the elevated plus maze (EPM) test. Bars represent means ± SEM. Rimonabant (1, 3, and 10mg/kg, n=18, n=18, and n=19, respectively) dose dependently decreased time spent in the open arm (A) and increased time spent in the closed arm (B). No effects were observed following AM4113 (1, 3, and 10mg/kg, n=18, n=17, and n=19, respectively). *Significantly different vs vehicle, P<.05. Effect of acute (C-D) and chronic (E-F) AM4113 treatment on immobility and climbing time. *P<.05, **P<.01, ***P<.001 significant difference compared to vehicle (n=40), Dunnett’s Multiple Comparison Test).
For the FST, 1-way ANOVA analysis revealed a main effect of drug during acute [immobility: F6,61=5.2, P<.001; climbing: F6,61=8.32, P<.001) and chronic treatments [immobility: F6,142=2.61 P<.05; climbing: time F6,142 = 2.95 P<.001]. Posthoc analyses showed that immobility time was further increased (10mg/kg, P<.05) following acute rimonabant administration, while immobility decreased (P<.05) and climbing increased (P<.01) following acute AM4113 (1, 3, and 10mg/kg) administration (Figure 5C-D) compared with vehicle. Posthoc tests showed that immobility was decreased (P<.05) and climbing increased (P<.05) following chronic AM4113 (10mg/kg) administration compared with vehicle (Figure 5E-F). No significant effects were found under chronic rimonabant treatment (P>.05).
Discussion
In the present study, acute administration of AM4113 dose dependently decreased nicotine intake and motivation to obtain nicotine without affecting food-maintained responding. Moreover, the reduction in both nicotine intake and the motivation to obtain nicotine was maintained during the chronic administration of AM4113. Interestingly, the effects of the chronic administration of AM4113 on nicotine intake and motivation for nicotine remained even after discontinuing AM4113 treatment.
AM4113 was also able to dose dependently block reinstatement of nicotine seeking induced by the reintroduction of the cues previously paired with nicotine, a priming injection of nicotine, or a pharmacological stressor. On the other hand, AM4113 significantly reduced both the firing frequency and bursting activity induced by nicotine on VTA DA cells. Similar to rimonabant, chronic treatment with AM4113 decreased body weight but did not induce anxiety or depressive-like behaviors as measured in the EPM and FST, respectively.
Previous studies have shown that AM4113 affects food-taking behavior (Hodge et al., 2008; Sink et al., 2009b). In the present study, we did not observe modified food-taking behaviors following AM4113 administration. These divergent results on food-taking might be explained firstly by the methodological differences with respect to previous studies (e.g., 60-minute vs 30-minute sessions; Sink et al., 2009b) or the different strains (e.g., Long Evans vs Sprague Dawley rats; Hodge et al., 2008; Sink et al., 2009b). Secondly, and a more likely explanation for the discrepancy, is the influence of the light/dark cycle on behavioral results. Our experiments were carried out during the dark period (active phase) in contrast to the Hodge study, where experiments were conducted during the light period (the resting phase for the animals). Our findings on the acute effects of AM4113 on nicotine self-administration are in agreement with previous studies using CB1 antagonists. Therefore, both rimonabant and SLV330 were shown to be effective at reducing nicotine intake (Cohen et al., 2002; de Bruin et al., 2011). However, Cohen and colleagues (2002) showed nicotine taking at low dose (0.3mg/kg) during the second consecutive day of treatment. In our study, rats received a single injection of each dose; this difference of magnitude is certainly due to carryover effects in Cohen studies. Rimonabant is also able to block the development of nicotine-induced conditioned place preference (Le Foll and Goldberg, 2004; Hashemizadeh et al., 2014). On the other hand, drugs increasing brain anandamide levels seem to enhance nicotine’s rewarding/reinforcing effects (Merritt et al., 2008).
The effects of chronic AM4113 on both nicotine intake and motivation for nicotine resemble previous findings (Forget et al., 2009) using rimonabant. Interestingly, in our study the chronic administration of AM4113 significantly attenuated nicotine-taking during the course of the 10-day treatment period. Similarly, studies using a shorter chronic treatment (3 days) with rimonabant or CB1 inverse agonist AM251 found comparable results (Shoaib, 2008; Forget et al., 2009). Shoaib (2008) found that AM251 significantly reduced responding, but no significant effects on responding post-AM251 treatment were observed. In the study by Forget et al. (2009), rimonabant was found effective to decrease motivation; however, they did not evaluate the possible existence of posttreatment effects. In our study, we found that both nicotine intake and motivation for nicotine remained significantly below baseline levels after the discontinuation of the chronic treatment with AM4113. Several hypotheses might be proposed in an attempt to explain the postchronic effects of AM4113. A first hypothesis might be that AM4113 is accumulated within the animals’ system, having carry-over effects on the post-AM4113 treatment sessions. This possibility seems not very likely, as preliminary (unpublished) studies have shown that AM4113 has a 2-hour half-life and can be eliminated within approximately 10 hours from the time of injection and hence would not be present in the animals’ system during the next experimental session. An alternative hypothesis might be that a chronic treatment with AM4113 results in adaptations on the mesocorticolimbic reward neurocircuitry. CB1 receptors do not appear to be expressed on DA neurons (Lupica and Riegel, 2005), but are expressed on GABAergic (VTA) and glutamatergic (VTA/NAcc) afferents in the mesocorticolimbic system (Maldonado et al., 2006). Therefore, endocannabinoids are able to modulate DA transmission indirectly through interaction with GABA and glutamate neurons (Cohen et al., 2005a; Maldonado et al., 2006). In this study we found that AM4113 significantly reduced the nicotine-induced bursting/firing rate and firing frequency on VTA DA cells. Previous studies have reported that rimonabant was able to block nicotine-induced DA release in the shell of the NAcc and the bed nucleus of the stria terminalis, showing that CB1 blockade is able to reduce nicotine-induced elevation of DA levels in key mesocorticolimbic structures (e.g., NAcc). However, to further investigate AM4113’s mechanism of action on nicotine-reinforcing effects, it will be interesting to study the effects of the intra-VTA administration of AM4113. In fact, similar studies were already conducted to evaluate the effects of the intracerebroventricular administration of AM4113 on operant responding for food (Sink et al., 2009a).
AM4113 dose dependently reduced cue-, nicotine-, and stress-induced reinstatement of nicotine seeking. In our study, AM4113 did not affect food-maintained responding. Our results clearly revealed that AM4113 did not modify the number of inactive lever presses that act as a control for nonspecific motor effects of the drug and whether the drug is controlling the behavior. Our results on nicotine seeking are in agreement with previous studies using rimonabant (Cohen et al., 2005b; Forget et al., 2009). Indeed, a large body of evidence has shown that blockade of CB1 receptors blocks reinstatement of drug-seeking behavior and that this effect is seen with a large variety of drugs of abuse (De Vries and Schoffelmeer, 2005; Le Foll and Goldberg, 2005). Similar to what was described above for nicotine taking, the effects of AM4113 on cue-induced reinstatement might be due to its actions on the mesocorticolimbic dopaminergic system. Previous studies have suggested that the endocannabinoid transmission is critically involved in triggering conditioned nicotine-seeking behavior in key cortico-limbic regions (Kodas et al., 2007). Therefore, rimonabant dose dependently reduced cue-induced nicotine-seeking behavior when injected into the NAcc (Kodas et al., 2007).
In this study we also evaluated the effects of AM4113 on nicotine-priming induced reinstatement of nicotine seeking. As demonstrated previously, acute exposure to nicotine effectively reinstated nicotine-seeking behavior in saline-extinguished rats (Chiamulera et al., 1996; Shoaib, 2006). AM4113 was able to decrease the effect of priming doses of nicotine, which most likely involves a DA-dependent mechanism (Cohen et al., 2005a). In our study, AM 41113 was also able to reduce nicotine seeking induced by the pharmacological stressor yohimbine. Interestingly, a recent study has reported that the chronic stimulation of the endogenous anandamide tone was also able to reduce stress- (yohimbine) induced relapse (of cocaine seeking) in rats (Chauvet et al., 2014). On the other hand, rimonabant was not able to reduce alcohol- (Economidou et al., 2006) or cocaine-seeking behavior (De Vries et al., 2001) when using the foot-shock as stressor to induce drug seeking. The different results obtained in studies using AM4113 or rimonabant might be due the different drugs studied (alcohol or cocaine vs nicotine) or/and the type of stressor (pharmacological vs foot-shock). In fact, previous studies have shown the existence of separate brain circuits responsible for the effects of yohimbine and foot-shock induced stress. Therefore, yohimbine effects on stress-induced relapse might be mediated by its effects on D2 (Scatton et al., 1980), α-1 (Doxey et al., 1984), and 5-HT1a (Winter and Rabin, 1992) receptors, while foot-shock effects seem to be mediated by the corticotropin-releasing hormone projections from the central amygdala to the bed nucleus of the stria terminalis (BNST) (Erb et al., 2001).
In the present study, AM4113 and rimonabant (0, 1, 3, or 10mg/kg) were administered to rats once daily for 21 consecutive days. This treatment was sufficient to produce a significant reduction in rat body weight at the dose of 10mg/kg for rimonabant and at 3 and 10mg/kg for AM4113, an effect that is consistent with the antiobesity activity reported in clinical studies using this CB1 receptor antagonist/inverse agonist (Padwal and Majumdar, 2007). Indeed both CB1 neutral antagonists and CB1 inverse agonists have been successfully adopted in weight control in obesity animal models (Mastinu et al., 2012; Meye et al., 2013) and clinical trials (Pi-Sunyer et al., 2006; Van Gaal et al., 2008). The effects on body weight of both CB1 inverse agonists and neutral antagonists might be linked to their capacity of blocking the synthesis of new adipose tissue (Mastinu et al., 2013) and to decrease food intake (Cluny et al. 2011).
The side effects associated with rimonabant prevented its use in clinic (i.e., anxiety and depressive side effects). Therefore, in this study we tested whether a chronic administration of AM4113 will have effects on behavioral tests for anxiety and depressions. In contrast to rimonabant, chronic AM4113 did not produce anxiogenic-like effects on the EPM, and acute treatment with either drug did not affect anxiety-related indices in the open-field test (supplementary Figure 2). Previous studies have shown similar results in rats using the EPM (Sink et al., 2010). Similarly, studies using other tests for anxiety-like behavior (e.g., the light-dark immersion), found that rimonabant, but not the cannabis-derived CB1 neutral antagonist tetrahydrocannabivarin, produced an anxiogenic-like reaction following its acute or chronic administration (O’Brien et al., 2013).
In the present study, we observed antidepressant-like behaviors in the FST following AM4113 administration (either when 3 times within 24 hours or chronic). Rimonabant administration resulted in depressive-like behaviors as measured in the FST, while it did not affect locomotor activity. Similar to rimonabant, AM4113 did not affect locomotor activity as measured in the open-field test (supplementary Figure 2). In line with our findings, Jutkiewicz and colleagues (2010) reported depressive-like effects following rimonabant but not after AM4113 treatments. Likewise, previous studies have shown that rimonabant, but not the neutral antagonist tetrahydrocannabivarin, reduced saccharin hedonic reactions in the taste reactivity test of palatability processing (O’Brien et al., 2013). The absence of depressive and anxiety-like behavior side effects following chronic AM4113 can be attributed to the lack of inverse agonism on the CB1 receptor. In fact, the anxiogenic properties of rimonabant (O’Brien et al., 2013) seem to be also present in other CB1 inverse agonists as AM251 (Sink et al., 2010). Previous studies have shown depressive-like effects after chronic rimonabant (Beyer et al., 2010). However, in our study we did not observe increased depressive-like behaviors following the chronic treatment with rimonabant. In the Beyer and colleagues study (Beyer et al., 2010), a 21-day rimonabant treatment induced depressive-like behavior. The discrepancy between the present study and the Beyer study can be attributed to the methodology. Indeed, in the Beyer study, immobility time was evaluated during a 15-minute period on the first day, and in our study, immobility time was evaluated during a 5-minute period on the second day of the FST.
In summary, the current study shows a great potential for AM4113 as a pharmacological therapeutic tool for nicotine addiction. AM4113 effects seem to be specific for nicotine-reinforcing effects (vs food) rather than a general decrease in motivation. Moreover, AM4113 decreased nicotine intake and motivation for nicotine for prolonged periods of time when given chronically, and the effects persisted after discontinuation of treatment. The behavioral effects of AM4113 seem to correlate with its effects on the dopaminergic activity in the VTA in response to nicotine. AM4113 also attenuated nicotine seeking induced by cue, priming, or a pharmacological stressor. Interestingly, AM4113 seems to lack anxiogenic or depression-like effects associated with the inverse agonism at CB1 receptors. In conclusion, CB1 neutral antagonists represent a great promise as smoking-cessation and weight-loss aids and should be considered for clinical studies.
Statement of Interest
None.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research and NIDA, NIH.
References
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, Chan K, Manners MT, Samad TA, Kennedy JD, Bingham B, Whiteside GT. (2010) Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis 39:148–155. [DOI] [PubMed] [Google Scholar]
- Cahill K, Ussher MH. (2011) Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database Syst Rev CD005353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. (2007) A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol 293:R2185–2193. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Nicolas C, Thiriet N, Lardeux MV, Duranti A, Solinas M. (2014) Chronic stimulation of the tone of endogenous anandamide reduces cue- and stress-induced relapse in rats. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M. (1996) Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology (Berl) 127:102–107. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Chambers AP, Vemuri VK, Wood JT, Eller LK, Freni C, Reimer RA, Makriyannis A, Sharkey KA. (2011) The neutral cannabinoid CB(1) receptor antagonist AM4113 regulates body weight through changes in energy intake in the rat. Pharmacol Biochem Behav 97:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Kodas E, Griebel G. (2005. a) CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav 81:387–395. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. (2005. b) Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 30:145–155. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. (2002) SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol 13:451–463. [DOI] [PubMed] [Google Scholar]
- Cummings KM, Mahoney M. (2006) Current and emerging treatment approaches for tobacco dependence. Current oncology reports 8:475–483. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, Lange JH, Kruse CG, Herremans AH, Schoffelmeer AN, van Drimmelen M, De Vries TJ. (2011) SLV330, a cannabinoid CB(1) receptor antagonist, attenuates ethanol and nicotine seeking and improves inhibitory response control in rats. Behav Brain Res 217:408–415. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN. (2005) Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci 26:420–426. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. (2001) A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7:1151–1154. [DOI] [PubMed] [Google Scholar]
- Doxey JC, Lane AC, Roach AG, Virdee NK. (1984) Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine. Naunyn Schmiedebergs Arch Pharmacol 325:136–144. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. (2006) Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology (Berl) 183:394–403. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. (2001) A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 158:360–365. [DOI] [PubMed] [Google Scholar]
- Forget B, Coen KM, Le Foll B. (2009) Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration--comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 205:613–624. [DOI] [PubMed] [Google Scholar]
- Forget B, Hamon M, Thiebot MH. (2005) Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology (Berl) 181:722–734. [DOI] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. (2010) Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biological Psychiatry 68:265–271. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, Goldberg SR, Le Foll B. (2012) Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol 17:47–61. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama 296:47–55. [DOI] [PubMed] [Google Scholar]
- Hashemizadeh S, Sardari M, Rezayof A. (2014) Basolateral amygdala CB1 cannabinoid receptors mediate nicotine-induced place preference. Prog Neuropsychopharmacol Biol Psychiatry 51:65–71. [DOI] [PubMed] [Google Scholar]
- Hodge J, Bow JP, Plyler KS, Vemuri VK, Wisniecki A, Salamone JD, Makriyannis A, McLaughlin PJ. (2008) The cannabinoid CB1 receptor inverse agonist AM 251 and antagonist AM 4113 produce similar effects on the behavioral satiety sequence in rats. Behav Brain Res 193:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero DR, Makriyannis A. (2009) Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs 14:43–65. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EMA, Vemuri VK, Bergman J. (2010) Pro-depressant-like effects of CB1 receptor inverse agonists/antagonists in male Sprague-Dawley rats. FASEB J 24:581.7. [Google Scholar]
- Kangas BD, Delatte MS, Vemuri VK, Thakur GA, Nikas SP, Subramanian KV, Shukla VG, Makriyannis A, Bergman J. (2013) Cannabinoid discrimination and antagonism by CB(1) neutral and inverse agonist antagonists. J Pharmacol Exp Ther 344:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, Gaal J, Le Foll B. (2010) The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacology 13:181–190. [DOI] [PubMed] [Google Scholar]
- Kodas E, Cohen C, Louis C, Griebel G. (2007) Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology (Berl) 194:161–171. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. (2009) The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 204:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. (2004) Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport 15:2139–2143. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. (2005) Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther 312:875–883. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Pushparaj A, Pryslawsky Y, Forget B, Vemuri K, Makriyannis A, Trigo JM. (2014) Translational strategies for therapeutic development in nicotine addiction: rethinking the conventional bench to bedside approach. Prog Neuropsychopharmacol Biol Psychiatry 52:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Lesage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. (2007) Translational research in medication development for nicotine dependence. Nat Rev Drug Discov 6:746–762. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE. (2007) Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology 32:710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC. (2005) Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48:1105–1116. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. (2006) Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. (2007) The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 195:345–355. [DOI] [PubMed] [Google Scholar]
- Mastinu A, Pira M, Pani L, Pinna GA, Lazzari P. (2012) NESS038C6, a novel selective CB1 antagonist agent with anti-obesity activity and improved molecular profile. Behav Brain Res 234:192–204. [DOI] [PubMed] [Google Scholar]
- Mastinu A, Pira M, Pinna GA, Pisu C, Casu MA, Reali R, Marcello S, Murineddu G, Lazzari P. (2013) NESS06SM reduces body weight with an improved profile relative to SR141716A. Pharmacol Res 74:94–108. [DOI] [PubMed] [Google Scholar]
- Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. (2008) The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther 326:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Trezza V, Vanderschuren LJ, Ramakers GM, Adan RA. (2013) Neutral antagonism at the cannabinoid 1 receptor: a safer treatment for obesity. Mol Psychiatry 18:1294–1301. [DOI] [PubMed] [Google Scholar]
- O’Brien LD, Wills KL, Segsworth B, Dashney B, Rock EM, Limebeer CL, Parker LA. (2013) Effect of chronic exposure to rimonabant and phytocannabinoids on anxiety-like behavior and saccharin palatability. Pharmacol Biochem Behav 103:597–602. [DOI] [PubMed] [Google Scholar]
- Padwal RS, Majumdar SR. (2007) Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 369:71–77. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, Group RI-NAS (2006) Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295:761–775. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA. (1993) Heroin self-administration in rats under a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 111:215–218. [DOI] [PubMed] [Google Scholar]
- Sanofi Aventis (2008) Sanofi-Aventis to discountinue all clinical trials with Rimonabant. http://en.sanofi.com/Images/14245_20081105_rimonabant_en.pdf. Paris, Retrieved November 5, 2008. [Google Scholar]
- Scatton B, Zivkovic B, Dedek J. (1980) Antidopaminergic properties of yohimbine. J Pharmacol Exp Ther 215:494–499. [PubMed] [Google Scholar]
- Shoaib M. (2006) Effects of isoarecolone, a nicotinic receptor agonist in rodent models of nicotine dependence. Psychopharmacology (Berl) 188:252–257. [DOI] [PubMed] [Google Scholar]
- Shoaib M. (2008) The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology 54:438–444. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. (2008) The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology 33:946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Nunes EJ, Collins LE, Vemuri VK, Thakur G, Makriyannis A, Salamone JD. (2009. a) Intracerebroventricular administration of cannabinoid CB1 receptor antagonists AM251 and AM4113 fails to alter food-reinforced behavior in rats. Psychopharmacology (Berl) 206:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Sink J, Randall PA, Collins LE, Correa M, Markus EJ, Vemuri VK, Makriyannis A, Salamone JD. (2010) Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. Eur Neuropsychopharmacol 20:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Wood J, Makriyannis A, Salamone JD. (2009. b) Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: effects on food-reinforced behavior and comparisons with AM4113. Pharmacol Biochem Behav 91:303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Bishop SF, Lauzon NM, Sun N, Laviolette SR. (2009) Chronic nicotine exposure switches the functional role of mesolimbic dopamine transmission in the processing of nicotine’s rewarding and aversive effects. Neuropharmacology 56:741–751. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O, Group RI-ES (2008) Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J 29:1761–1771. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. (1992) Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J Pharmacol Exp Ther 263:682–689. [PubMed] [Google Scholar]
- World Health Organization (WHO, July, 2013) http://www.who.int/mediacentre/factsheets/fs339/en/(www.who.int)(www.who.int(www.who.int)). Tobacco Fact sheet, Retrieved March 12, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.