Abstract
Background:
Exposure to cocaine-associated stimuli triggers a robust rise in circulating glucocorticoid levels. Glucocorticoid receptors are richly expressed in the basolateral amygdala, a brain region that controls the reinstatement of cocaine-seeking behavior upon exposure to a previously cocaine-paired environmental context. In the present study, we investigated whether glucocorticoid receptor stimulation in the basolateral amygdala is integral to drug context-induced motivation to seek cocaine in a rat model of drug relapse.
Methods:
Rats were trained to lever press for cocaine reinforcement in a distinct environmental context and were then given daily extinction training sessions in a different context. At test, the rats received bilateral glucocorticoid receptor antagonist (mifepristone; 3 or 10ng/hemisphere) or vehicle microinfusions into either the basolateral amygdala or the overlying posterior caudate-putamen (anatomical control region). Immediately thereafter, drug-seeking behavior (i.e., nonreinforced lever presses) was assessed in the previously cocaine-paired context and locomotor activity was assessed in a novel context.
Results:
Intra-basolateral amygdala, but not intra-posterior caudate-putamen, mifepristone dose-dependently attenuated drug context-induced cocaine-seeking behavior relative to vehicle, such that responding was similar to that observed in the extinction context. In contrast, mifepristone treatment did not alter locomotor activity.
Conclusions:
These findings suggest that basolateral amygdala glucocorticoid receptor stimulation is necessary for drug context-induced motivation to seek cocaine.
Keywords: reinstatement, basolateral amygdala, glucocorticoid receptor, corticosterone
Introduction
Exposure to cocaine-associated stimuli, including drug paraphernalia and drug-taking environments, can trigger craving and increase the probability of drug relapse in cocaine users (Rohsenow et al., 1990; Childress et al., 1993) and cocaine-seeking behavior in laboratory animals (de Wit and Stewart, 1981). In cocaine users, exposure to drug-associated environmental stimuli precipitates reliable increases in the secretion of the adrenal glucocorticoid, cortisol (Berger et al., 1996; Sinha et al., 2000). Glucocorticoids have important influence on a number of cocaine-induced behaviors, and the glucocorticoid receptor (GR) has been proposed as a therapeutic target to reduce cocaine addiction (Deroche-Gamonet et al., 2003).
Significance Statement
Glucocorticoids, such as cortisol in humans and corticosterone in rats, promote cocaine-seeking behavior in rat models of drug relapse. However, the contribution of glucocorticoid receptor stimulation to these effects has not been evaluated fully, because previous research primarily utilized nonspecific manipulations of corticosterone levels, such as adrenalectomy, and nonselective manipulations, such as corticosterone synthesis inhibition. Furthermore, the role of glucocorticoids has not been investigated in cocaine-seeking behavior produced by exposure to a previously cocaine-paired environmental context in particular. Our findings indicate that glucocorticoid receptor stimulation within the basolateral amygdala is necessary for drug context-induced cocaine-seeking behavior. These findings may have important implications for the development of treatments for cocaine addiction.
GR stimulation regulates the acute motivational effects of cocaine. In strong support of this, adrenalectomy or GR gene inactivation disrupts cocaine-reinforced instrumental behavior under fixed ratio schedules of reinforcement (Deroche et al., 1997; Deroche-Gamonet et al., 2003). Furthermore, systemic GR antagonism dose-dependently attenuates the breaking point for cocaine reinforcement under progressive ratio schedules (Deroche-Gamonet et al., 2003). Corticosterone replacement is sufficient to restore or maintain cocaine self-administration rates following adrenalectomy (Deroche et al., 1997; Mantsch et al., 2008) but fails to eliminate impairments in the development of escalation in cocaine intake under long-access conditions (Mantsch et al., 2008). Overall, these findings indicate that GRs play a role in the acute reinforcing effects of cocaine, while other adrenal hormones or cocaine-related rapid increases in corticosterone underlie the neuroadaptations that give rise to the escalation phenomenon.
Several studies have examined the involvement of glucocorticoids in drug-seeking behavior. Only i.v. (0.37mg/kg), but not i.p. or s.c., corticosterone administration is sufficient to reinstate extinguished cocaine-seeking behavior (Deroche et al., 1997; Lee et al., 2003; Graf et al., 2013; Wong and Marinelli, 2016), unless s.c. corticosterone administration is preceded by adolescent cocaine exposure (Wong and Marinelli, 2016). This suggests that rapid increases in corticosterone levels or age-dependent cocaine-induced adaptations are required for glucocorticoid-induced reinstatement. Adrenalectomy or corticosterone synthesis inhibition prevents food deprivation-, foot shock-, and conditioned reinforcer-induced reinstatement of cocaine-seeking behavior (Piazza et al., 1994; Erb et al., 1998; Mantsch and Goeders, 1999b; Goeders and Clampitt, 2002; Shalev et al., 2003), with more modest or no effects observed on cocaine-primed reinstatement (Erb et al., 1998; Mantsch and Goeders, 1999a; Graf et al., 2013). Furthermore, adrenalectomy with corticosterone replacement is sufficient to restore stress-induced and cocaine-primed reinstatement, suggesting corticosterone plays a permissive role in these behavioral effects (Erb et al., 1998; Mantsch et al., 2008). Importantly, these studies do not provide conclusive evidence in support of the involvement of GRs in reinstatement, because adrenalectomy and corticosterone synthesis inhibitors also alter testosterone and corticotropin-releasing hormone levels (Smagin and Goeders, 2004), and corticosterone can act through GRs, mineralocorticoid receptors, and other cellular mechanisms (Graf et al., 2013). Furthermore, the involvement of GRs in reinstatement of cocaine-seeking behavior produced by exposure to a drug-paired environmental context has not been examined.
The basolateral amygdala (BLA) is a brain region that is abundant in GRs (Joels and Karst, 2012) and critical for cue-induced drug-seeking behaviors. Specifically, pharmacological inactivation of the BLA inhibits drug context-induced (Fuchs et al., 2005) and conditioned reinforcer-induced (Grimm and See, 2000) reinstatement of cocaine-seeking behavior. In the present study, we evaluated whether GR populations in the BLA contribute to drug context-induced cocaine-seeking behavior in an instrumental model of drug relapse. To this end, we administered mifepristone (RU38486), a potent competitive GR and progesterone receptor antagonist with no affinity for mineralocorticoid receptors (Cadepond et al., 1997), into the BLA just prior to reinstatement testing. We were able to selectively manipulate GRs using mifepristone due to the absence of progesterone receptor expression in the BLA of rats (Quadros et al., 2007; Forbes-Lorman et al., 2014).
Methods
Animals
Male Sprague-Dawley rats (Harlan/Envigo, Livermore, CA; 275–300g) were housed individually in a humidity-controlled vivarium on a reversed light-dark cycle (light on/off: 7:00 pm/7:00 am). Animals received 20 to 25g/d of rat chow, with water available ad libitum. Protocols for animal housing and experimentation followed the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, 2011) and were approved by the University of North Carolina and Washington State University Institutional Animal Care and Use Committees.
Food Training
To facilitate the acquisition of drug self-administration, rats were trained to lever press during a single overnight session. Each response on one (active) lever resulted in food reinforcement (45-mg grain-based food pellet; Bio-Serv., Flemington, NJ). Responses on a second (inactive) lever had no programmed consequences. Food training took place in sound-attenuated operant conditioning chambers (26x27x27cm, Coulbourn Instruments, Allentown, PA) equipped with 2 levers, stimulus lights above each lever, and a house light on the wall opposite to the levers. Importantly, the multi-modal sensory stimuli used subsequently for contextual conditioning were not presented to the animals during the food training session.
Surgery
Twenty-four hours after the food-training session, rats were anesthetized with a cocktail of ketamine and xylazine (80.0 and 5.0mg/kg, respectively, i.p.). Intravenous catheters were constructed in house as described previously (Fuchs et al., 2007). The catheters were implanted into the right jugular vein and exited in a port posterior to the rats’ scapulae. The catheter port was sealed with Tygon tubing and a cap (Plastics One, Roanoke, VA). Using standard stereotaxic procedures, 26-gauge stainless-steel guide cannulae (Plastics One,) were then aimed 2mm above the BLA (-2.7mm AP, 5.0mm ML, -6.7mm DV, relative to bregma) or the dorsally adjacent pCPu (-2.7mm AP, 5.0mm ML, -4.7mm DV). The guide cannulae were covered by stylets (Plastics One). To promote catheter patency, the catheters were flushed daily with 0.1mL of cefazolin (1.0mg/10mL, Henry Schein Animal Health, Tualatin, OR; dissolved in 70U/mL heparinized saline, Patterson Veterinary Supply, Sterling, MA) followed by 0.1mL of 10-U/mL heparinized saline. Animals received 5 days of postsurgical recovery before drug self-administration training. Catheter patency was verified periodically using propofol (10mg/0.1mL, Henry Schein), a short-active sedative-hypnotic that produces transient loss of muscle tone when administered i.v.
Cocaine Self-Administration Training
Rats were trained to lever press for cocaine infusions during 2-h sessions during the rats’ dark phase. Training continued until the rats reached the acquisition criterion (≥10 infusions/session on 10 days) (see schematic of experimental timeline in Figure 2). Training occurred in operant conditioning chambers arranged to form 1 of 2 distinct environmental contexts. Context 1 contained a red house light, intermittent pure tone (80 dB, 1kHz, 2 seconds on, 2 seconds off), pine-scented air freshener (Car Freshener Corp., Watertown, NY), and wire mesh flooring. Context 2 contained an intermittent white stimulus light (2 seconds on, 4 seconds off) located above the inactive lever, continuous pure tone (75 db, 2.5kHz), vanilla-scented air freshener (Scopus Products, Moorpark, CA), and a ceramic tile bisecting a bar floor. At the start of each self-administration training session, the rats’ jugular catheters were connected to an infusion pump (Coulbourn) via polyethylene 20 tubing and liquid swivels (Instech, Plymouth Meeting, PA). Tygon tubing connected the swivels to syringes that were mounted on programmable infusion pumps (Coulbourn). Active lever presses resulted in unsignaled cocaine infusions (0.15mg/0.05mL over 2 seconds, i.v.; NIDA Drug Supply Program, Research Triangle Park, NC) under a fixed ratio 1 schedule with a 20-second timeout period. Active lever presses during the timeout period and inactive lever presses during the session were recorded but had no programmed consequences. Reinforcer delivery and data collection were controlled using Graphic State Notation software 4.1.04 (Coulbourn).
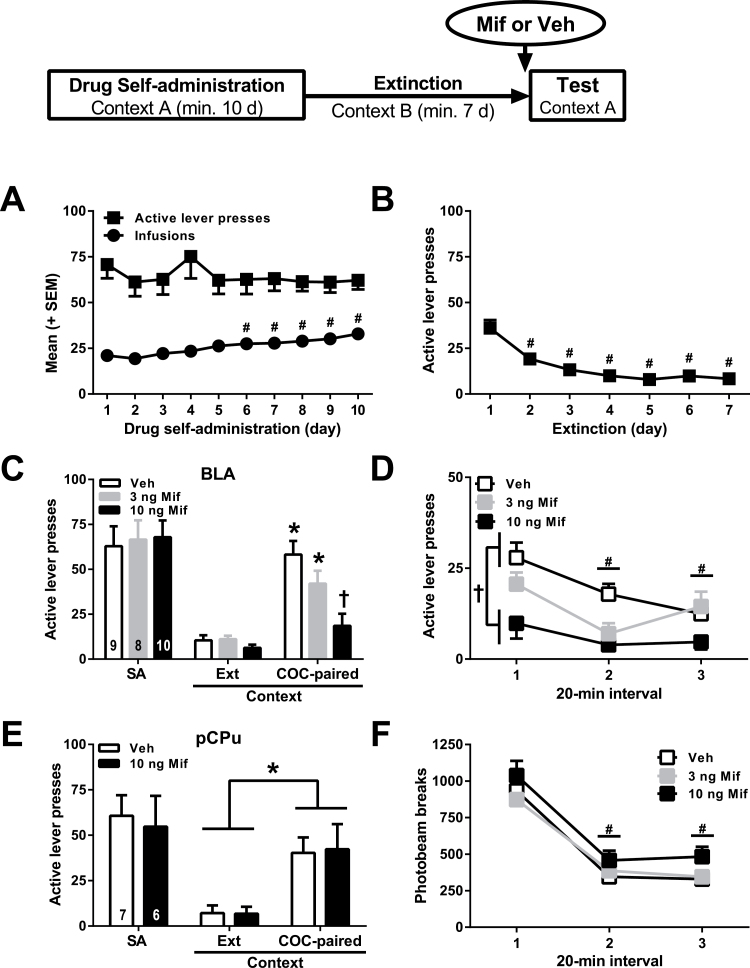
Figure 2.
Effects of intra-basolateral amygdala (BLA) or intra-posterior caudate-putamen (pCPu) mifepristone administration on drug context-induced reinstatement of cocaine-seeking behavior and locomotor activity. Top: Experimental timeline. In the schematic, context A represents the cocaine-paired context (i.e., context 1 or 2, counterbalanced across subjects, see Methods), context B represents the extinction context (i.e., context 2 and 1, respectively). (A) Time course of active lever presses (mean±SEM) and cocaine intake (mean number of infusions ± SEM) during the 10 criterion self-administration training days collapsed across the factor, group (n=40). (B) Time course of active lever presses (mean±SEM) during the first 7 extinction training days collapsed across the factor, group (n=40). (C) Active lever presses (mean±SEM) during cocaine self-administration (mean of last three 2-hour sessions), extinction training in the extinction context (1st hour of the last 2-hour session), and at test (1-h session) in the cocaine-paired context following intra-BLA administration of mifepristone or vehicle. The numbers in the bars indicate sample sizes. (D) Time course of active lever presses (mean/20 minutes±SEM) across time during the reinstatement test session (n=8–10/group as in A). (E) Active lever presses (mean±SEM) during each experimental phase in the pCPu anatomical control groups. (F) Locomotor activity in a novel context (mean photobeam breaks/20 minutes±SEM) (n=8–10/group as in A). Symbols represent difference from responding on day 1 (#, Tukey’s test, P<.05), from responding in the extinction context (*C, Tukey’s test, P<.05; E, ANOVA context main effect, P=.0001) or from the vehicle control group (†C, Tukey’s test, P<.05; D, ANOVA treatment main effect, P=.01, Tukey’s test, P<.05).
Extinction Training
Rats that self-administered cocaine in context 1 received daily 2-h extinction training sessions in context 2, and vice versa. During these sessions, active and inactive lever presses had no programmed consequences. Immediately before extinction session 4, rats were acclimated to the microinfusion procedure during a single sham infusion procedure. Stainless-steel injector cannulae (33-Ga, Plastics One) were inserted 2mm past the tip of the guide cannulae, but fluid was not infused in order to minimize tissue disruption. The injection cannulae remained in place for 4 minutes while the rats were gently held by an experimenter. Extinction training continued until the rats reached the extinction criterion (≤25 active lever presses/session on 2 consecutive days) after a minimum of 7 daily sessions.
Reinstatement Testing
On the test day, injector cannulae were inserted into the guide cannulae. The injector cannulae were connected via Tygon tubing to Hamilton syringes mounted on an infusion pump (Thermo-Fisher Scientific, Waltham, MA). The GR antagonist, mifepristone (3 or 10ng/0.5 μL/hemisphere; Sigma-Aldrich, St. Louis, MO) or vehicle (2% ethanol/phosphate buffered saline; 0.5 μL/hemisphere) was infused bilaterally over 2 minutes. Intra-BLA mifepristone doses were selected based on their effectiveness to site-selectively inhibit memory consolidation and reconsolidation (Roozendaal and McGaugh, 1997; Jin et al., 2007). The injection cannulae were left in place for 1 minute before and after infusion. Rats were then placed into the previously cocaine-paired context for a 1-hour test session. During the test session, rats were connected to the infusion apparatus to permit perception of, and similar interaction with, the cocaine-paired context as during self-administration training. However, fluid was not infused through the catheters. Active and inactive lever presses were recorded but had no programmed consequences.
Locomotor Activity Testing
To evaluate whether the effects of mifepristone on cocaine-seeking behavior were due to a change in general motor activity, BLA-cannulated rats from the reinstatement study were assigned to receive bilateral microinfusions of mifepristone or vehicle. Treatment assignment was randomized and testing commenced 2 to 7 days after the reinstatement test session. Immediately after the microinfusions, the rats were placed into novel Plexiglas chambers (42x20x20cm) equipped with 8 light sources and photodetectors. Photobeam breaks, produced by the horizontal movement of the animals, were measured for 1 hour by a computerized system (San Diego Instruments, San Diego, CA).
Histology
Rats were euthanized by ketamine/xylazine overdose (80/5 i.v. or 240/15mg/kg, i.p., depending on catheter patency). Rats were perfused transcardially with ice-cold sterile phosphate-buffered saline and 4% paraformaldehyde solution. The brains were dissected out and cryoprotected in 30% sucrose solution. Brain tissue was sectioned into 40-μm sections on a cryostat and stained with cresyl violet (Kodak, Rochester, NY) to visualize cannula placement.
Statistical Analyses
Separate ANOVAs or t tests were used to evaluate group differences in cocaine intake, lever responding, and locomotor activity with treatment (mifepristone doses, vehicle) as the between-subjects factor and time (days or 20-minute intervals) as the within-subject factor, where appropriate. Tukey’s posthoc tests were used to further investigate significant effects, when appropriate. Alpha was set at 0.05.
Results
Histology
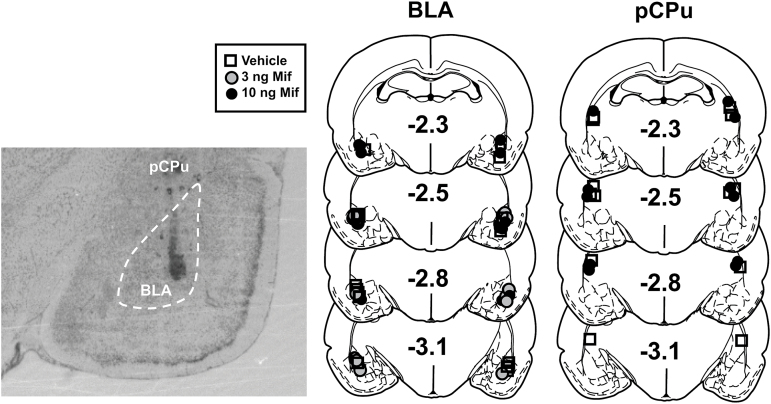
Cannula placements were located bilaterally in the lateral or basal nuclei of the amygdala or the overlying posterior caudate-putamen (pCPu) (Figure 1) without excessive gliosis or other abnormalities at the infusion sites. Data from 9 rats with incorrect cannula placements were excluded from statistical analyses. The resulting sample sizes are reported in Figure 2.
Figure 1.
Photomicrograph and schematics illustrating cannula placement. Symbols represent the most ventral point of injection cannula tracts for rats that received mifepristone (Mif) or vehicle microinfusions into the basolateral amygdala (BLA) or posterior caudate-putamen (pCPu) immediately before reinstatement testing. Numbers represent distance from bregma in millimeters according to the rat brain atlas (Paxinos and Watson, 1997).
Behavioral History
There were no differences between the groups that were trained in context 1 vs context 2 in any dependent measure (data not shown). There were also no preexisting differences between the BLA-cannulated or pCPu-cannulated groups in cocaine intake, active lever responding, or inactive lever responding leading up to the test session. Inactive lever presses remained low at test. Descriptive statistics for these measures are reported in Table 1 and Figure 2. The ANOVA of active lever presses during the 10 criterion self-administration training days indicated no group or time main or interaction effects (F4-36,35-315=0.36–0.98, P=.46-0.95) (Figure 2A). The ANOVA of drug infusions during the last 10 days of self-administration indicated a time main effect (F9,315=11.62, P=.0001) but no group main effect or group x time interaction (F4-36,35-315=1.03–1.16, P=.34-0.43). Accordingly, collapsed across group, cocaine intake slightly increased across time (day 1 < day 6–10, Tukey’s tests, P<.05) (Figure 2A). Finally, the ANOVA of nonreinforced active lever presses during the first 7 extinction training days indicated a time main effect F(6,210=22.01, P=.0001), but no group main effect or group x time interaction (F4-24,35-210=0.25–1.16, P=.91-0.94). Thus, active lever responding gradually declined during extinction training (day 1 > 2–7, Tukey’s tests, P=.01) (Figure 2B).
Table 1.
Descriptive Statistics
| Exp./Group | Active Lever | Inactive Lever | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cocaine Intake | SA | EXT 1 | EXT 7 | SA | EXT 1 | EXT 7 | Test | ||
| BLA | Vehicle | 27.9±1.9 | 62.9±11.1 | 36.6±11.1 | 10.4±2.8 | 1.3±0.4 | 9.8±3.6 | 2.6±0.7 | 4.1±1.7 |
| 3ng Mif | 29.1±3.0 | 66.5±10.7 | 44.1±11.6 | 11.13±1.9 | 2.6±1.6 | 10.0±4.4 | 4.8±2.1 | 6.1±2.2 | |
| 10ng Mif | 32.5±3.2 | 67.9±9.4 | 36.5±11.8 | 6.3±1.8 | 1.53±0.6 | 8.8±4.7 | 2.1±0.5 | 3.1±1.5 | |
| pCPu | Vehicle | 32.0±2.7 | 60.7±11.3 | 32.3±11.2 | 7.1±1.4 | 0.1±0.1 | 9.3±4.0 | 0.5±0.2 | 1.1±1.0 |
| 10ng Mif | 28.7±2.0 | 54.8±7.0 | 28.5±11.8 | 6.8±1.6 | 3.0±1.4 | 9.1±4.2 | 1.0±0.4 | 2.0±1.1 | |
Cocaine intake (mean infusions/session for the last 3 training sessions ± SEM) and active and/or inactive lever responses during self-administration training (SA; mean ± SEM for the last 3 training sessions), during the first (EXT 1) and last (EXT 7) extinction training sessions, and during reinstatement testing in the cocaine-paired context (Test), where appropriate. Means are provided for rats that received mifepristone (Mif) or vehicle into the BLA or pCPu immediately before testing. Sample sizes and active lever data for the test session are shown in Figure 2.
Intra-BLA Mifepristone Inhibits Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior
Intra-BLA mifepristone pretreatment attenuated cocaine-seeking behavior in the cocaine-paired context (Figure 2C). The ANOVA of active lever presses during the last extinction session and the test session indicated significant treatment x time interaction (F1,24=7.57, P=.03), time main (F1,24=61.81, P <.0001), and treatment main (F2,24=7.56, P=.003) effects. The vehicle and 3-ng mifepristone groups exhibited more active lever responding upon reexposure to the cocaine-paired context at test relative to responding during the preceding extinction session (Tukey’s test, P <.05), whereas the 10-ng mifepristone group did not. Furthermore, the 10-ng mifepristone group exhibited less active lever responding than the other 2 groups in the cocaine-paired context at test (Tukey’s test, P <.05) but not during the preceding extinction session. The time-course analysis indicated that active lever responding gradually declined in all groups during the test session in the cocaine-paired context (Figure 2D; ANOVA time main effect: F2,48=14.90, P=.0001). Overall, the 10-ng mifepristone group exhibited less active lever responding than the other 2 groups throughout the test session (treatment main effect: F2,24=8.302, P=.002, Tukey’s tests, P <.05), and the 3-ng mifepristone group exhibited a trend for less active lever responding than the vehicle group (treatment x time interaction: F2,48=2.47, P=.06).
Intra-pCPu Mifepristone Does Not Alter Cocaine-Seeking Behavior
Intra-pCPu administration of 10ng of mifepristone (behaviorally effective BLA dose) failed to alter cocaine-seeking behavior in the cocaine-paired context (Figure 2E). The ANOVA of active lever presses during the last extinction session and the test session indicated a time main effect only (F1,10=38.72, P=.0001) and no differences between the 10-ng mifepristone and vehicle control groups in either context (treatment main and interaction effects: F1,10=0.23–0.35, P=.57-0.64).
Intra-BLA Mifepristone Does Not Alter General Motor Activity
Locomotor activity declined gradually during the 1-hour session (Figure 2F, ANOVA time main effect: F2,44=156.23, P <.0001). Furthermore, the groups did not significantly differ in locomotor activity, although the 10-ng mifepristone group exhibited a trend for more locomotion than the other groups (treatment x time interaction: F4,44=0.53, P=.71; treatment main effect: F2,22=3.22, P=.06).
Discussion
Exposure to cocaine-associated stimuli elicits a reliable increase in self-reports of craving and induces robust hypothalamic pituitary adrenal (HPA) axis activation in cocaine users (Berger et al., 1996). Extending these observations, findings in the present study establish that there is a causal relationship between increased GR stimulation and drug context-induced motivated behavior in rats. Furthermore, a critical site of the corresponding GR-dependent glucocorticoid effects is the BLA. Supporting these conclusions, we report that intra-BLA administration of mifepristone inhibited drug context-induced reinstatement of extinguished cocaine-seeking behavior in a dose-dependent (Figure 2C-D) and anatomically selective manner (Figure 2E).
Previous studies indicate that an inverted U-shaped relationship exists between HPA axis activation and reinstatement performance (Deroche et al., 1997). Systemic corticosterone administration alone elicits maximal reinstatement of cocaine-seeking behavior at a dose (0.37mg/kg, i.v.) that produces a rise to approximately 600ng/mL in plasma corticosterone concentrations, similar to foot-shock stress (as cited in Deroche et al., 1997), with negligible behavioral response elicited above and below this dose (Deroche et al., 1997). In comparison, we reported in a recent study of cocaine memory reconsolidation (S. J. Stringfield and R. A. Fuchs, unpublished observations) that brief (15-minute) nonreinforced exposure to a cocaine-paired context identical to that utilized in the present study elicits a rise in blood serum corticosterone concentrations from 130ng/mL (baseline) to 250ng/mL. Further, it can be assumed that 2-hour exposure to the cocaine-paired context resulted in a similar corticosterone response, approaching levels produced by restraint stress in drug-naïve rats (Mantsch et al., 2007a; Mantsch et al., 2007b). Sensitized HPA axis function following withdrawal from cocaine, reported earlier (Mantsch et al., 2007a; Zhou et al., 2011), may contribute to similar corticosterone responses to reward-predictive stimuli and physically aversive stressors. Nevertheless, drug context-induced corticosterone serum concentrations alone would have been insufficient to produce significant reinstatement of extinguished cocaine-seeking behavior in the present study.
The mifepristone-induced dose-dependent decrease in cocaine-seeking behavior in the present study (Figure 2C) indicates that corticosterone-induced GR stimulation in the BLA upon exposure to the cocaine-paired context is necessary for cocaine-seeking behavior. The mifepristone-induced decrease in cocaine-seeking behavior was not due to a nonspecific deficit in instrumental performance, as the behavioral effective dose of mifepristone did not alter inactive lever responding in the cocaine-paired context (Table 1), relative to vehicle. Notably, inactive lever pressing is not a sensitive measure of hypoactivity due to floor effects. However, mitigating the possibility that mifepristone acted by suppressing motor activity, the behaviorally effective dose of mifepristone produced a trend for an increase, as opposed to a decrease, in locomotion in a novel context (Figure 2F). Mifepristone effects were also anatomically selective to the BLA, since mifepristone administration into the dorsally adjacent pCPu did not inhibit reinstatement (Figure 2E).
The acute effects of mifepristone on drug context-induced reinstatement were likely mediated by membrane-bound GRs on glutamatergic and/or GABAergic neurons within the BLA (Johnson et al., 2005). Importantly, the effects of glucocorticoids on neural activity vary as a function of circulating corticosteroid history and brain region (Karst et al., 2010; Tasker and Herman, 2011). In the presence of baseline corticosterone levels, GR stimulation in the BLA increases the excitability of BLA principal neurons and decreases the GABA-mediated synaptic inhibition of these neurons (Duvarci and Pare, 2007). Conversely, after recent corticosterone application or restraint stress, glucocorticoids decreased the excitability of BLA pyramidal neurons (Karst et al., 2010). Low circulating corticosteroid concentrations prior to and at the onset of the test session, as we have reported (S. J. Stringfield and R. A. Fuchs, unpublished observations), likely favored the corticosterone-induced excitation of glutamatergic principal neurons in the BLA and mediated the mifepristone-induced inhibitory effects on cocaine-seeking behavior in the present study. Specifically, via monosynaptic as well as polysynaptic connections between BLA principal neurons and elements of the relapse circuitry, corticosterone at BLA GRs could control glutamate and/or dopamine neurotransmission in the prefrontal cortex (Lasseter et al., 2014), nucleus accumbens (Xie et al., 2012), and dorsal hippocampus (Xie et al., 2010, 2013) that is required for drug context-induced cocaine-seeking behavior. Recent optogenetic studies support this conclusion, indicating that photoinhibition of BLA projections to the prelimbic cortex or nucleus accumbens core disrupt cue-induced cocaine-seeking behavior (Stefanik and Kalivas, 2013), although similar studies have not been conducted using the contextual reinstatement model.
The cellular mechanisms by which exposure to a cocaine-paired context elicits GR-dependent reinstatement have not been characterized. Lidocaine or D1 antagonist administration into the rostral, but not the caudal, BLA impairs cue-induced cocaine-seeking behavior (Kantak et al., 2002; Mashhoon et al., 2009). GRs are selectively expressed in the rostral, but not the caudal, BLA (Johnson et al., 2005; Sivukhina et al., 2013), and mifepristone microinfusions were aimed at the anterior region of the BLA in the present study. Therefore, differential GR expression may be a source of functional heterogeneity in cue-induced reinstatement within the BLA, and cue-induced reinstatement may involve interactions between corticosterone and dopamine. In further support of the latter hypothesis, exposure to cocaine-predictive environmental stimuli elicits conditioned dopamine release in the amygdala of rats (Weiss et al., 2000). Similar to cocaine itself, cocaine context exposure may enhances HPA activity and circulating corticosterone levels via D1- and D2-like dopamine receptor-dependent (Borowsky and Kuhn, 1991; Ikemoto and Goeders, 1998), as well as N-methyl-D-aspartate receptor-dependent (Damianopoulos and Carey, 1995), mechanisms. In addition, corticosterone may be able to amplify dopamine neurotransmission in the BLA via GR-independent mechanisms that involve the inhibition of corticosterone-sensitive monoamine transporters and thus the impairment of dopamine clearance (Graf et al., 2013; Hill and Gasser, 2013). Such GR-independent processes in the BLA or elsewhere may potentiate drug context-induced reinstatement of cocaine-seeking behavior, but GR-dependent mechanisms play a requisite role in the BLA in this phenomenon.
Findings from the present study concur with earlier research suggesting that GRs may be suitable therapeutic targets for the treatment of cocaine addiction (Deroche-Gamonet et al., 2003). This conclusion is offered with the caveat that rats received limited access to cocaine in the present study (2h/d), although they obtained 256 infusions of cocaine in average (Figure 2A) and exhibited escalation in drug intake (approximately 33% increase in the number of infusions/d over the last 10 days of training). We have shown previously that conditioned stimulus-induced reinstatement is transiently enhanced by a long-access cocaine self-administration regimen (6h/d) or by a short access regimen coupled with yoked cocaine exposure (1 + 5h/d, respectively), relative to a short access cocaine regimen (1h/d; Kippin et al., 2006). Hence, future studies will need to evaluate the impact of more extensive cocaine regimens on drug context-induced reinstatement and on the recruitment of glucocorticoids and GRs in this phenomenon. Furthermore, given that these receptors are distributed throughout the human body, continuing efforts to understand the site-specific cellular mechanisms by which GRs mediate drug context-induced cocaine-seeking behavior will be useful for the development of nuanced, anatomically targeted therapeutic interventions in the future. In the short term, it is encouraging that systemic mifepristone administration attenuates self-reports of craving in response to alcohol-associated cues as well as subsequent alcohol intake in alcoholics (Vendruscolo et al., 2015).
Statement of Interest
None.
Acknowledgments
The authors thank Drs Ryan McLaughlin and Ilia Karatsoreos for insightful comments on a draft of this manuscript and Dr Kelley Harmon and Nicole Jones for technical assistance with data collection.
This work was supported by the National Institute on Drug Abuse (R01 DA025646 to R.A.F.) and the National Institute of Neurological Disorders and Stroke (T32 NS007431 to S.J.S.).
References
- Berger SP, Hall S, Mickalian JD, Reid MS, Crawford CA, Delucchi K, Carr K, Hall S. (1996) Haloperidol antagonism of cue-elicited cocaine craving. Lancet 347:504–508. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Kuhn CM. (1991) Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther 256:204–210. [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE. (1997) RU486 (mifepristone): mechanisms of action and clinical uses. Ann Rev Med 48:129–156. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. (1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137:73–95. [PubMed] [Google Scholar]
- Damianopoulos EN, Carey RJ. (1995) Evidence for N-methyl-D-aspartate receptor mediation of cocaine induced corticosterone release and cocaine conditioned stimulant effects. Beh Brain Res 68:219–228. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV. (1997) Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther 281:1401–1407. [PubMed] [Google Scholar]
- Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, Kellendonk C, Le Moal M, Spanagel R, Schutz G, Tronche F, Piazza PV. (2003) The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci 23:4785–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. (2007) Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci 27:4482–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. (1998) The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 18:5529–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes-Lorman R, Auger AP, Auger CJ. (2014) Neonatal RU-486 (mifepristone) exposure increases androgen receptor immunoreactivity and sexual behavior in male rats. Brain Res 1543:143–150. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. (2007) Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26:487–498. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. (2002) Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology 161:222–232. [DOI] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robble MA, Vranjkovic O, Wheeler DS, Mantsch JR, Gasser PJ. (2013) Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci 33:11800–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE. (2000) Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22:473–479. [DOI] [PubMed] [Google Scholar]
- Hill JE, Gasser PJ. (2013) Organic cation transporter 3 is densely expressed in the intercalated cell groups of the amygdala: anatomical evidence for a stress hormone-sensitive dopamine clearance system. J Chem Neuroanat 52:36–43. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Goeders NE. (1998) Microinjections of dopamine agonists and cocaine elevate plasma corticosterone: dissociation effects among the ventral and dorsal striatum and medial prefrontal cortex. Brain Res 814:171–178. [DOI] [PubMed] [Google Scholar]
- Jin XC, Lu YF, Yang XF, Ma L, Li BM. (2007) Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. Eur J Neurosci 25:3702–3712. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H. (2012) Corticosteroid effects on calcium signaling in limbic neurons. Cell Calc 51:277–283. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. (2005) Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience 136:289–299. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. (2002) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22:1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schutz G, Joels M. (2010) Metaplasticity of amygdalar responses to the stress hormone corticosterone. Pro Nat Acad Sci 107:14449–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. (2006). Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology 187:60–7. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Arguello AA, Wells AM, Hodges MA, Fuchs RA. (2014) Contribution of a mesocorticolimbic subcircuit to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 39:660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. (2003) Role of the hypothalamic-pituitary-adrenal axis in reinstatement of cocaine-seeking behavior in squirrel monkeys. Psychopharmacology 168:177–183. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Serge JP, Hoks MA, Francis DM, Katz ES. (2008) Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacology 33:814–826. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. (2007. a) Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res 1167:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. (1999. a) Ketoconazole does not block cocaine discrimination or the cocaine-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav 64:65–73. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. (1999. b) Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology 142:399–407. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, Cullinan WE, Ziegler DR. (2007. b) Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett 415:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Tsikitas LA, Kantak KM. (2009) Dissociable effects of cocaine-seeking behavior following D1 receptor activation and blockade within the caudal and rostral basolateral amygdala in rats. Eur J Neurosci 29:1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1997) The rat brain in stereotaxic coordinates. New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Marinelli M, Jodogne C, Deroche V, Rouge-Pont F, Maccari S, Le Moal M, Simon H. (1994) Inhibition of corticosterone synthesis by Metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Res 658:259–264. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Wagner CK. (2007) Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol 504:42–56. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. (1990) Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict 25:957–993. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. (1997) Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobio Learn Mem 67:176–179. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. (2003) The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology 168:170–176. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. (2000) Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 152:140–148. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Goeders NE. (2004) Effects of acute and chronic ketoconazole administration on hypothalamo--pituitary--adrenal axis activity and brain corticotropin-releasing hormone. Psychoneuroendocrinology 29:1223–1228. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. (2013) Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci 7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Herman JP. (2011) Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 14:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. (2015) Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Investig 125:3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. (2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Nat Acad Sci 97:4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WC, Marinelli M. (2016) Adolescent-onset of cocaine use is associated with heightened stress-induced reinstatement of cocaine seeking. Addict Biol 21:634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Arguello AA, Wells AM, Reittinger AM, Fuchs RA. (2013) Role of a hippocampal SRC-family kinase-mediated glutamatergic mechanism in drug context-induced cocaine seeking. Neuropsychopharmacology 38:2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. (2012) Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol 17:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. (2010) Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology 208:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Litvin Y, Piras AP, Pfaff DW, Kreek MJ. (2011) Persistent increase in hypothalamic arginine vasopressin gene expression during protracted withdrawal from chronic escalating-dose cocaine in rodents. Neuropsychopharmacology 36:2062–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]