Abstract
Objective:
The impact of lifetime dietary habits and their role in physical, mental, and social well-being has been the focus of considerable recent research. Omega-3 polyunsaturated fatty acids as a dietary constituent have been under the spotlight for decades. Omega-3 polyunsaturated fatty acids constitute key regulating factors of neurotransmission, neurogenesis, and neuroinflammation and are thereby fundamental for development, functioning, and aging of the CNS. Of note is the fact that these processes are altered in various psychiatric disorders, including attention deficit hyperactivity disorder, depression, and Alzheimer’s disease.
Design:
Relevant literature was identified through a search of MEDLINE via PubMed using the following words, “n-3 PUFAs,” “EPA,” and “DHA” in combination with “stress,” “cognition,” “ADHD,” “anxiety,” “depression,” “bipolar disorder,” “schizophrenia,” and “Alzheimer.” The principal focus was on the role of omega-3 polyunsaturated fatty acids throughout the lifespan and their implication for psychopathologies. Recommendations for future investigation on the potential clinical value of omega-3 polyunsaturated fatty acids were examined.
Results:
The inconsistent and inconclusive results from randomized clinical trials limits the usage of omega-3 polyunsaturated fatty acids in clinical practice. However, a body of literature demonstrates an inverse correlation between omega-3 polyunsaturated fatty acid levels and quality of life/ psychiatric diseases. Specifically, older healthy adults showing low habitual intake of omega-3 polyunsaturated fatty acids benefit most from consuming them, showing improved age-related cognitive decline.
Conclusions:
Although further studies are required, there is an exciting and growing body of research suggesting that omega-3 polyunsaturated fatty acids may have a potential clinical value in the prevention and treatment of psychopathologies.
Keywords: omega-3 polyunsaturated fatty acids (n-3 PUFAs), depression, schizophrenia, bipolar disorder, Alzheimer’s disease
Introduction
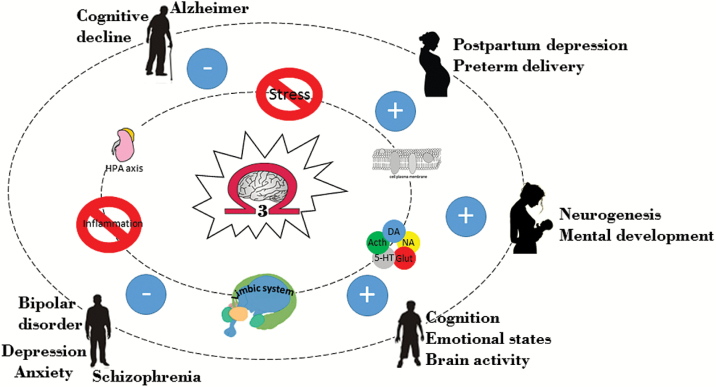
Despite the improved pharmacological approaches that moderately buffer the worldwide burden of poor mental health, a recent finding suggests that mental illnesses will continue to rise worldwide during the coming decades (Baxter et al., 2013). The general transition to more calorically dense, processed diets and reduced physical activity have had a significant impact on the overall health of individuals in developed nations and are associated with an increased incidence of psychopathologies such as anxiety and depression (Logan and Jacka, 2014). Accumulating translational evidence implicates the quality of diet as a crucial and common determinant for mental disorders (McNamara et al., 2015). Within the brain, omega-3 polyunsaturated fatty acids (n-3 PUFAs) have been under the spotlight for decades. However, only recently has research looked into the critical role of n-3 PUFAs in brain function and structure throughout the lifespan. n-3 PUFAs constitute key regulating factors of neurotransmission, neurogenesis, cell survival, and neuroinflammation and are thereby fundamental for development, functioning, and aging of the CNS (Mischoulon and Freeman, 2013). Importantly, these processes are altered in various psychiatric disorders, including attention deficit hyperactivity disorder (ADHD), schizophrenia, major depression, and Alzheimer’s disease (Sinn et al., 2010). Despite this evidence, the concept of n-3 PUFAs as a clear therapeutic compound for mental disorders still needs to be clarified. The purpose of this review is to further explore the role of n-3 PUFAs, which have been gaining increasing credence as potential targets for the development of novel strategies for the maintenance of mental health in the prevention and amelioration of psychopathology (Figure 1).
Figure 1.
Omega-3 polyunsaturated fatty acids [(n-3) PUFAs] throughout the lifespan. n-3 PUFAs represent essential components of the cellular membrane and constitute key regulating factors of neurotransmission, neurogenesis, stress response, inflammation, and emotional states. Thereby, they are fundamental for development, functioning, and aging of the CNS throughout the lifespan. n-3 PUFAs, mainly acting on the factors mentioned above, contribute to the maintenance of mental health, in the prevention and amelioration of psychopathology.
n-3 PUFAs in Early-Life: From Embryogenesis to Adolescence
Brain development is a sequential anatomical process characterized by specific well-defined stages of growth and maturation. It has become more evident that this process is influenced by n-3 PUFAs. Specifically, the levels of docosahexaenoic acid (DHA), the most abundant component of the n-3 PUFAs family, increase sharply along the perinatal period. In rats, the first important step of acquisition of DHA takes place in the embryonic phase and in the first 3 postnatal weeks of life, whereas in humans this period goes between the last trimester of gestation and the first 6 to 10 months after birth (Clandinin et al., 1980a, 1980a). At these stages of life, the foetal metabolic capability to convert the precursor of the n-3 PUFAs family, α-linolenic acid (ALA), to DHA is extremely limited (<0.2% in children). Indeed, it is the mother that guarantees an adequate delivery of DHA to the fetus through the placenta. (Innis, 2007).
The Perinatal Lipid Nutrition Project and The Early Nutrition Programming Project have recently developed consensus recommendations concerning dietary fat intake for pregnant and lactating women. They recommend a minimum DHA intake of 200mg/d (Koletzko et al., 2007). Interestingly, supplementation of n-3 PUFAs during pregnancy not only increases breast milk DHA content but also results in slightly longer gestation as well as reduced risk of preterm delivery (Singh, 2005). Accordingly, infants born prematurely show less accumulation of DHA in the brain (Barcelo-Coblijn and Murphy, 2009). However, the increased supply of DHA to the developing fetal nervous system leads to a progressive depletion of maternal plasma DHA (Smuts et al., 2003). Of relevance, in a cross-national ecological analysis, reduced levels of maternal milk DHA and lower seafood consumption correlated with higher rates of postpartum depression (Hibbeln, 2002). Therefore, diets enriched in n-3 PUFAs are recommended to both restore the depletion of DHA in the mother as well as to look after the needs of the fetus and suckling infant.
Animal studies reveal n-3 PUFAs as guarantors of proper brain development in early postnatal life (Lei et al., 2013). n-3 PUFA supplementation has been shown to protect from neuronal loss and decreased neurogenesis in the cerebral cortex and hippocampus of neonates (Lei et al., 2013). Furthermore, this effect can be long-lasting and show further benefits in neurocognitive function later in adulthood (Lei et al., 2013). In humans, Jensen et al. (2005) have shown a higher Psychomotor Development Index in breastfed infants of mothers who underwent DHA administration (200mg/d) for a period of 4 months. Psychomotor development, eye-hand coordination, and visual acuity were all improved after DHA algal-oil treatment. However, these improvements were limited to infants 30 months old and no further advantages on mental development were found. Although of relevance, this study has limitations in terms of treatment duration and assessment instruments. A different study measured IQ in children whose mothers underwent maternal supplementation with cod liver oil (1183mg/10mL DHA, 803mg/10mL eicosapentaenoic acid [EPA]) started from week 18 until 3 months after delivery (Helland et al., 2003). The scoring of the Mental Processing Composite of the Kaufman Assessment Battery for Children was increased by 4 points in 4-year-old children who were born to mothers who had taken cod liver oil enriched in DHA and EPA. However, only 84 children of the 590 pregnant women enrolled in the study completed the Kaufman Assessment Battery for Children at 4 years of age. Another similar study has shown higher mental processing scores and high degree of stereopsis and stereo acuity in children 3.5 years old whose mothers received a DHA-rich diet (Williams et al., 2001). Given the importance to maintain proper DHA levels during the neonatal period of life, the content and kind of fatty acids introduced in the body are of importance. In fact, infants fed formula with 0.4% or 2.4 % energy from ALA had 2.3±0.2 and 2.2±0.3g/100g fatty acids as DHA in plasma, respectively, despite the large difference in ALA intake, whereas infants fed formula with only 0.12 % energy from DHA had plasma DHA levels of 5.2±0.2g/100g (Innis, 2007).
n-3 PUFAs maintain their importance in brain development and functioning during adolescence (Table 1). At this stage of life, rats fed with an n-3 PUFA-deficient diet tend to have decreased n-3 PUFA mass (82% less) compared with control (Bondi et al., 2014). Such animals show less exploratory behavior, weaker memory performance, and increased tyrosine hydroxylase expression (dopamine [DA] precursor) in the dorsal striatum.
Table 1.
n-3 PUFA Impact in Healthy Adolescents
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| 6–11 years, low-income iron-deficient children, (n = 321, z n = 288). | (1) Iron + fish oil; (2) Iron + placebo; (3) Fish oil + placebo; (4) Placebo + placebo. Fish oil = 0.5g/d LC omega-3 (0.42g DHA + 0.08g EPA). | 8.5 months. | HVLT | LC omega-3 PUFA without iron had negative effects on working memory in children with iron deficiency anemia and long-term memory and retrieval in girls with iron deficiency, whereas boys with iron deficiency performed better. | (Baumgartner et al., 2012) |
| 7–9 years, low-income, marginally nourished indigenous children (n = 183, analysis on n = 155). | Fish flour bread spread provided at school (~0.89g/week DHA [0.13g/d]) vs control bread spread. | 6 months. | HVLT, spelling test, reading test | ↑ Verbal learning ability, memory, and spelling. Effects more pronounced in children with lower baseline performance scores. | (Dalton et al., 2009) |
| 10–12 years (n = 90, analysis on n = 86). | (1) Low-dose algal oil: 0.4g DHA; (2) High-dose algal oil: 1.0g/d DHA; (3) Placebo (vegetable oil). | 8 weeks. | Cognitive performance | Word recognition task: low dose: faster performance; high dose: slower performance. | (Kennedy et al., 2009) |
| 8–10 years (n = 450, analysis on n = 348). | Fish oil (0.4g DHA + 0.06g EPA)/d + micronutrients vs placebo (olive oil). | 16 weeks. | KBIT-2, WIAT-2, WMTB-C, creature counting, MFFT, comPET, SNAP-IV, SDQ. | No treatment effects. | (Kirby et al., 2010) |
| 8–10 year boys (n = 38, analysis on n = 33). | (1) Low-dose algal oil: 0.4g/d DHA; (2) High- dose algal oil: 1.2g/d DHA; (3) Placebo (corn oil). | 8 weeks. | Sustained attention test, fMRI | Both dosages ↑ activation of the dorsolateral prefrontal cortex during sustained attention task. No effect on attention or reaction time of attention. | (McNamara et al., 2010a) |
| 6–10 years, low-income, marginally nourished (n = 598, analysis on n = 550). | (1) High micronutrients + 0.93g ALA + 0.10g DHA/d; (2) High micronutrients + 0.14g/d ALA; (3) Low micronutrients + 0.93g ALA + 0.10g DHA/d (4) Low micronutrients + 0.14g/d ALA. | 12 months. | Cognitive test battery | No treatment effects. | (Muthayya et al., 2009) |
| 6–10 years Australia: well nourished, (n = 396, analyzed n = 276) Indonesia: marginally nourished (n = 384, analyzed n = 367). | (1) High micronutrients; (2) DHA + EPA (0.09g DHA + 0.02g EPA)/d; (3) Micronutrients + DHA + EPA (as above); (4) Placebo. | 12 months. | Cognitive test battery | No treatment effects. | (Osendarp et al., 2007) |
| 3–13 years, indigenous children with low literacy ability (n = 408). | Fish oil 0.75g LC omega-3 per school day (0.56g EPA + 0.17g DHA) plus 0.06g/d gamma linolenic acid vs placebo (palm oil). | 20 weeks. | WRAT4, DAP, MAP, CBRS | Nonverbal cognitive development (Draw-A Person): Improvements with strongest effects in 7- to 12-year olds. | (Parletta et al., 2013) |
| 8–12 years, mild- moderately malnourished (n = 59, analysis on n = 50). | Fish oil 0.45g/d LC omega-3 (0.18g EPA + 0.27g DHA) vs placebo (soybean oil). | 3 months. | Anthropometric measures, neuropsychological battery test | ↑ Processing speed, visual- perceptive capacity, attention, executive function. | (Portillo-Reyes et al., 2014) |
| 7–9 years, underperforming in reading (≤33rd centile) (n = 362). | Algal oil: 0.6g/d DHA vs placebo (corn/ soybean oil). | 16 weeks. | Age-standardized measures of reading, working memory, and parent- and teacher- rated behavior. | Treatment ↑ reading. | (Richardson et al., 2012) |
Abbreviations: CBRS, Comprehensive Behaviour Rating Scales; DAP, The Draw-A-Person; HVLT, Hopkins Verbal Learning Test; KBIT-2, Kaufman Brief Intelligence Test, Second Edition; MAP, Matrix Analogies Test; MFFT, Matching Familiar Figures Task; SDQ, Strengths and Difficulties Questionnaire; SNAP-IV, Swanson, Nolan, and Pelham rating scale for ADHD; WIAT-2, Wechsler Individual Achievement Test, Second Edition; WMTB-C, Working Memory Test Battery for Children; WRAT-4, Wide Range Achievement Test: Fourth Edition.
In humans, Kuratko et al. (2013) provided a systematic review based on 15 publications regarding the influence of DHA in learning and behavior in healthy children. The studies differed in purpose and design, and some did not achieve a consistent conclusion regarding DHA’s effect on specific cognitive tests. However, the analysis found benefits of DHA supplementation in brain activity and school performance from over one-half of the considered studies. A landmark study showing improved brain activity after DHA supplementation dates back to 2010 (McNamara et al., 2010a). McNamara et al. showed for the first time regulation of cortical metabolic function and cognitive development exerted by DHA concentrations in the grey matter of healthy boys during sustained attention (McNamara et al., 2010a). The study was conducted in 33 subjects (9 years old) who were assigned to receive placebo or 1 of the 2 doses of DHA (400mg/d; 1200mg/d) for 8 weeks. A longitudinal study in arctic Quebec has revealed a relation within school-age children with higher DHA cord plasma concentration and memory function (Boucher et al., 2011). Despite this interesting finding, a previous study conducted in DHA-fed healthy children in the UK did not show any improvement on cognitive performance and learning (Kennedy et al., 2009). However, other studies have observed benefits of n-3 PUFAs, particularly in malnourished subjects. For instance, improvements in learning and cognitive performance have been shown in n-3 PUFA-supplemented, malnourished children 7 to 9 years old in South Africa (Dalton et al., 2009) and 8- to 12-year-old Mexican children (Portillo-Reyes et al., 2014), although, no benefits were found in 6- to 10-year-old malnourished children from India (Muthayya et al., 2009) and Indonesia (Osendarp et al., 2007). The discordance between these studies may be accounted for by differences in the extent of malnutrition. Moreover, further discrepancies can be due to differences in the experimental plan such as supplements used, different dosages, and duration of the trials. Overall, the current research supports the view that adequate intake of DHA since prenatal life may have a positive impact on brain activity, learning, and cognition in healthy children. Indeed, being components of cell membranes, n-3 PUFAs are involved in important phases of brain development such as neurogenesis, myelination, synaptogenesis, and dendritic arborisation, which are in continuous flux during development and learning (Wurtman, 2014). Thus, this raises the possibility that n-3 PUFAs may have a beneficial effect on neurodevelopmental psychiatric disorders (McNamara and Carlson, 2006).
n-3 PUFAs: Implication for Childhood Disorders
Deficits in n-3 PUFAs have been associated with higher risk of development of childhood disorders such as ADHD (Richardson and Ross, 2000). In a cross-sectional study, Burgess et al. (2000) observed that 40% of recruited ADHD subjects (53 ADHD 6- to 12-year-old boys) had significantly lower proportions of plasma DHA and EPA and greater frequency of n-3 PUFAsdeficiency syndrome such as thirst, frequent urination, and dry hair. Importantly, such physical signs linked with deficit in n-3 PUFAs are consistent in ADHD (Richardson et al., 2000). Moreover, behavioral and learning problems, such as anomalous visual, motor, attention, and language processing, have been linked to lower n-3 PUFAs plasma levels (Richardson, 2004). The reason for lower n-3 PUFAs levels in ADHD is unknown. Evidence suggests that testosterone can impair the biosynthesis of n-3 PUFAs while estrogens are positively related with the conversion of DHA from ALA both in rodents (Marra and de Alaniz, 1989) and humans (Childs et al., 2008). This may explain the higher prevalence of ADHD in males than in females.
In animal studies the spontaneously hypertensive rat is generally used as a model of ADHD (Meneses et al., 2011). Spontaneously hypertensive rat fed n-3 PUFA (EPA-DHA)-enriched diet (n-6:n-3 PUFAs ratio of 1:2.7), from gestational phase until postnatal day 50, partially ameliorated ADHD-like behavior by improving reinforcer-controlled activity, impulsiveness, and inattention (Dervola et al., 2012). Moreover, such a diet increased the DA and serotonin turnover ratio together with decreased levels of glutamate in the striatum of the same animals (Dervola et al., 2012). These neurotransmitters are thought to be altered in ADHD animal models (Gainetdinov et al., 2001) and ADHD young subjects (Paloyelis et al., 2012).
In humans, supplementation with PUFAs (480mg DHA, 80mg EPA, 40mg arachidonic acid [AA], 96mg γ-linolenic acids, and 24mg α-tocopheryl acetate) improved ADHD children’s oppositional defiant behavior from a clinical to a nonclinical range (Stevens et al., 2003). In this study, both parents and teachers rated improvement in conduct problems and attention difficulties after 4 months of treatment. However, more than one-half of the patients were prescribed medication for ADHD, making it difficult to attribute the observed improved symptoms to the n-3 PUFA treatment. A similar PUFA mixture (480mg DHA, 186mg EPA, 96mg γ-linolenic acids, 42mg AA, and 60 IU vitamin E per day), supplemented for 12 weeks, showed improvement in anxiety/shy tests, cognition, inattentiveness, hyperactivity/impulsiveness, total DSM-IV index, and Conners total global index in children with learning difficulties (Richardson and Puri, 2002). However, none of the subjects were formally diagnosed with ADHD and the sample size was small (41 children, 8–12 years old). Although the studies reported above seem to be encouraging, 3 recent systematic reviews have reported only minor n-3 PUFA effect in reducing ADHD symptoms. Grassman et al. (2013) reported mild behavioral and cognitive improvement in ADHD children after treatment with low doses of n-3 PUFAs. However, the studies included in their analysis were heterogeneous, had small sample sizes, and only a limited number were placebo controlled. Bloch and Qawasmi (2011) have reported a modest effect in the treatment of ADHD after treatment with high doses of n-3 PUFAs (especially EPA) in comparison with current pharmacotherapies such as psychostimulants, atomoxetine, or α(2) agonists. Gillies et al. (2012) reported improvement after combined n-3 PUFA and n-6 PUFA supplementation only in a minority of the studies that met the inclusion criteria of their review. Given its evidence of modest efficacy, it may be reasonable to investigate n-3 PUFAs as a supplement to traditional pharmacologic interventions. Future studies need to be adequately powered and placebo controlled and use adequate dosage, (Table 2).
Table 2.
n-3 PUFA Impact on Mental Illnesses in Early Life and Adolescence
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| 6–12 year old (78% boys); idiopathic ADHD diagnosis; were being treated successfully with medication (n = 54). | 345mg DHA (algae- derived) or undefined placebo. | 16 weeks. | CPRS; CBC; TOVA; CCT. | Treatment = placebo on all measures. | (Voigt et al., 2001) |
| 6–13 year old (78% boys); ADHD diagnosis; high FADS; some on medication (equally allocated to conditions) (n = 50). | 96mg GLA, 40mg AA, 80mg EPA, 480mg DHA, 24mg Vit E or olive oil placebo. | 16 weeks. | DBD; ASQ; CPT; WJPEB-R; FADS. | Treatment > placebo: DBD- Conduct (parents); DBD- Attention (teachers). Other 14 outcome measures nonsignificant. | (Stevens et al., 2003) |
| 6–12 year old (80% boys); ADHD diagnosis; 15% medicated; 82% comorbid conditions (n = 40). | 100mg EPA, 514mg DHA, or olive oil placebo (supplied in soymilk and bread). | 8 weeks. | DTVP; STM; CPT; Other. | Treatment = placebo on all measures (except that placebo > treatment on CPT and STM). | (Hirayama et al., 2004) |
| 8–12 year old (62% boys); normal IQ; low reading ability; above average ADHD scores on Conners’ Index; no participants in treatment for ADHD (n = 29). | 864mg LA, 42mg AA, 96mg ALA, 186mg EPA, 480mg DHA, 60 iµ Vit E or olive oil placebo. | 12 weeks. | CPRS. | Treatment > placebo: CPRS; Cognitive problems/ inattention; Anxious/shy; Conners’ global index; DSM inattention; DSM hyperactive/impulsive; Conners’ ADHD Index. | (Richardson and Puri, 2002) |
| 5–12 year old (77% boys); Developmental Coordination Disorder, one-third with ADHD symptoms in clinical range, not in treatment; IQ > 70 (n = 117). | 60mg AA, 10mg GLA, 558mg EPA, 174mg DHA, 9.6mg Vit E or olive oil placebo. | 12 weeks active vs placebo; 1-way crossover to active treatment for 12 weeks. | MABC; WORD; CTRS. | Treatment > placebo: WORD; CTRS Oppositional behavior; cognitive problems/inattention; hyperactivity; anxious/shy; perfectionism; social problems; Conners’ index; DSM-IV inattention, hyperactive/impulsive. | (Richardson and Montgomery, 2005) |
| 7–12 year old (74% boys); ADHD symptoms in clinical range; unmedicated (n= 132, questionnaire data available for 104). | 60mg AA, 10mg GLA, 558mg EPA, 174mg DHA, 9.6mg Vit E, or palm oil placebo. | 15 weeks active vs placebo; 1-way crossover to active treatment for 15 weeks. | CPRS, CTRS Vocabulary, subtests from WISC-III & TEA- ch, Stroop. | Treatment > placebo CPRS: cognitive problems/inattention; hyperactivity; ADHD Index; restless/impulsive; DSM-IV hyperactive/impulsive; oppositional. Treatment = placebo on other subscales and CTRS. Treatment > placebo on creature counting and vocabulary. Treatment = placebo on other cognitive tests. |
(Sinn and Bryan, 2007; Sinn et al., 2008) |
| 8–18 year old with diagnosed ADHD, unmedicated (85% males) (n = 75). | 60mg AA, 10mg GLA, 558mg EPA, 174mg DHA, 9.6mg Vit E or olive oil placebo. | 3 months active vs placebo; 1-way crossover to active treatment for 3 months. | Investigator-rated ADHD Rating Scale-IV; CGI. | Treatment = placebo overall Treatment > placebo in subgroups with inattentive subtype and comorbid neurodevelopmental disorders. | (Johnson et al., 2009) |
| 7–12 year old (79% male) with ADHD/ ADHD symptoms (50% diagnosed) (n = 54, 45 with bloods). | 1g EPA-rich oil, 1g DHA rich oil or sunflower oil placebo. | 3 x 3 crossover (4 months on each treatment). | CPRS, reading, writing, vocabulary, TEA-ch. | Treatment = placebo in 12-month crossover. Over 4 months erythrocyte DHA increases associated with improvements on CPRS - oppositional behavior, anxiety/ shyness – divided attention and reading. In subgroup with learning difficulties (n = 16 with blood) also on CPRS hyperactivity/ impulsivity and spelling. |
(Milte et al., 2011) |
| 5–17 years with Autistic Disorder (81.9% male) (n = 13). | 1.5g/d n-3 PUFA (0.84g EPA, 0.7g DHA), Vit E or coconut oil placebo. | 6 weeks parallel design. | Aberrant Behavior Checklist. | Treatment > placebo for stereotypy and hyperactivity (trends with large effect sizes). Treatment = placebo on 3 other subscales. |
(Amminger et al., 2007) |
| 6–12 year old; 25% girls children with MDD (n = 20). | 2g ethyl-EPA (96% from fish oil) or placebo, Vit E. | 4 weeks parallel design, adjunctive therapy. | HDRS. | Treatment > placebo at weeks 2, 3, and 4 on HDRS score and on core depressive symptom subscales. | (Nemets et al., 2006) |
| Mean-age = 16.4 with ultra-high risk (UHR) for psychosis, (n = 81 individuals, 27 males, 54 females). | ~1.2g ω-3 PUFAs (0.7g EPA, 0.48g DHA, 7.6mg vitamin E). | 12 weeks. | Gaussian Process Classification. | Treatment > placebo on GPC | (Sinn et al., 2010) |
| 8–14 year old, (n = 80 boys, 41 ADHD, 39 controls). | 10g of margarine daily, enriched with either 650mg of EPA/ DHA or placebo. | 16 weeks. | CBCL, SWAN, TRF, fMRI. | Treatment > parent- rated attention in both ADHD control. | (Bos et al., 2015) |
Abbreviations: ASQ, Conners’ Abbreviated Symptom Questionnaires; CBC, Child Behaviour Checklist; CCT, Children’s Colour Trails test; CGI-S, Clinical Global Impression-Severity; CPRS, Conners’ Parent Rating Scales; CPT, Conners’ Continuous Performance Test; CTRS, Conners’ Teacher Rating Scales; DBD, Disruptive Behaviour Disorders rating scale; FADS, fatty acid deficiency symptoms; GLA, γ-linolenic acids; HDRS, Hamilton Depression Rating Scale; MABC, Movement Assessment Battery for Children; MDD, major depressive disorder; Stroop, Stroop color-word test; STM, Short-term memory; TEA-ch, Test of Everyday Attention for children; TOVA, Test of Variables of Attention; WISC-III, Wechsler Intelligence Scale for Children, version 3; WORD, Wechsler Objective Reading Dimensions; WJPEB-R, Woodstock-Johnston Psycho-Educational Battery – Revised.
n-3 PUFAs in Adulthood: Stress Response
In adulthood, full brain development is already achieved. However, at this stage of life stressful life events can alter mood states and cognition, increasing susceptibility to psychopathologies (Cattaneo et al., 2015). n-3 PUFAs seem to play a role in the regulation of the stress-response influencing the activity of the hypothalamic–pituitary–adrenal (HPA) axis. In humans (7 volunteers), the stimulation by 30 minutes of mental stress (mental arithmetic’s and Stroop’s test) of plasma epinephrine, cortisol, and energy expenditure were all significantly blunted by 3 weeks of 7.2g/d fish oil administration (Delarue et al., 2003). However, the subjects were tested before and after 3 weeks of n-3 PUFA supplementation; thus, a possible effect of acclimatization due to the repetition of the testing procedure over time should be taken into account. Similarly, ACTH and cortisol plasma levels were blunted by 4 weeks of 7g/d fish oil supplementation prior lipopolysaccharide (2ng/kg)-induced neuroendocrine response (Michaeli et al., 2007). In contrast with the n-3 PUFA antiinflammatory effect (Grimm et al., 2002), high levels of cytokines were not reversed by n-3 PUFAs, perhaps due to a too low content of EPA (17%) and DHA (11%) in the fish oil supplement. Indeed, 2.5g/d of n-3 PUFAs (2g EPA, 0.35g DHA) supplementation showed reduction of stimulated interleukin 6 (IL-6) and tumour necrosis factor plasma levels in healthy medical students. Interestingly, this was associated with reduced anxiety symptoms in healthy students without an anxiety disorder diagnosis (Kiecolt-Glaser et al., 2011). This evidence acquires more interest, since stress can exert a pivotal role in memory and cognition (McEwen, 2007). Researchers at the University of Pittsburgh have determined that n-3 PUFAs can enhance cognition in young individuals. Healthy young adults (18–25 years of age) after 6 months of n-3 PUFA supplement, mostly DHA (750mg/d) and EPA (930mg/d), experienced improvement in working memory (Narendran et al., 2012). Working memory assessment was performed using a verbal n-back task that used 3 loads of working memory (1-, 2-, and 3-back) (Abi-Dargham et al., 2002). In addition to that, prior to supplementation of n-3 PUFAs, higher red blood cell DHA levels were significantly correlated with working memory in a group of young adults. Interestingly, a similar association between high DHA plasma levels and improved cognitive function was previously observed in a sample of 208 healthy subjects (30–54 years of age; Muldoon et al., 2010). DHA, but not other n-3 PUFAs, was associated with better scores on tests of nonverbal reasoning, mental flexibility, working memory, and vocabulary. Another study conducted in Australia showed 6 weeks of 6g/d fish oil intake (1.5g DHA, 360g EPA) were enough to ameliorate the Perceiving Stress Scale scoring in stressed university staff (Bradbury et al., 2004). Due to a lack of investigation, further research is warranted to elucidate the mechanisms by which n-3 PUFAs enhance cognitive performance in healthy individuals (Table 3).
Table 3.
n-3 PUFA Impact in Healthy Adults
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| University students, mean age ~22 years (n = 56, analyzed n = 54). | 2.3g/d fish oil (1.74g EPA + 0.25g DHA) vs placebo (olive oil). | 4 weeks. | Mini International Neuropsychiatric Interview, neutral and emotional information processing tests. | No effects on attention, memory or reaction time of attention. | (Antypa et al., 2009) |
| 22–51 years (n = 33). | 2.8g/d fish oil (1.6g EPA + 0.8g DHA). | 35 days. | Zimmermann and Fimm Attention Test procedure, EEG. | Improvements in sustained attention and reaction time of sustained attention. | (Fontani et al., 2005) |
| 18–35 years (n = 159, analyzed n = 140). | (1) DHA-rich fish oil (0.45g DHA + 0.09g EPA)/d; (2) EPA-rich fish oil (0.2g DHA + 0.3g EPA)/d; (3) Placebo (olive oil). |
12 weeks. | Cognitive performance and mood battery test. | No treatments effects. | (Jackson et al., 2012a) |
| 18–29 years (n = 65). | (1) Low-dose DHA fish oil (0.45g DHA + 0.09g EPA)/day; (2) High-dose DHA fish oil (0.9g DHA + 0.18g EPA)/d; (3) placebo (olive oil). | 12 weeks. | COMPASS, spatial working memory, numeric working memory, 3-back task, simple reaction time, Choice reaction time, Stroop task, RVIP. | Increased cerebral blood flow; cognitive tasks only assessed at end of study using comprehensive computerized cognitive test battery (episodic memory, working memory, attention, reaction time, executive function). Both dosages improved reaction times on 2 attention tasks, but effects were lost when correcting for multiple testing. | (Jackson et al., 2012b) |
| College students (mean age ~20±2 years) (n = 43, analyzed n = 41). | Fish oil (0.72g EPA + 0.48g DHA)/d vs placebo (coconut oil). | 4 weeks. | RAVLT, SCWT, TMT, PANAS. | No effects on verbal learning and memory, inhibition and executive control. | (Karr et al., 2012) |
| Mildly depressed adults, 18–70 years (average ± SD age 38±14 years) (n = 218, analyzed n = 190). | Fish oil 1.5g/d LC omega-3 (0.85g DHA + 0.63g EPA) vs placebo (olive oil). | 12 weeks. | DASS, BDI, GHQ, STAXI-2. | No treatment effects. | (Rogers et al., 2008) |
| 18–45 years (n = 228, analyzed n = 176). | Fish oil (1.2g DHA + 0.17g EPA)/d vs placebo (high oleic acid sunflower oil). | 6 months. | Computerized cognitive test battery (episodic and working memory, attention, reaction time (RT) of episodic and working memory, and attention and processing speed). | Improvement in reaction times and working memory. RBC DHA increased by 2.6% (to ~7.9%); RBC EPA increased by 0.2% (to ~0.81%). |
(Stonehouse et al., 2013) |
Abbreviations: BDI, Beck Depression Inventory; DAS, Differential Ability Scales; GHQ, General Health Questionnaire; PANAS, Positive and Negative Affect Schedule; RAVLT, Rey Auditory Verbal Learning Test; RBC, red blood cell; RVIP, Rapid Visual Information Processing; SCWT, Stroop Color Word Test; STAXI-2, State-Trait Anger Expression Inventory-2; TMT, treadmill test.
n-3 PUFAs and Psychopathologies
Major Depressive Disorder
Since the 19th century, the incidence of major depressive disorder (MDD) in Western countries seems to have increased. In contrast, the dietary intake of n-3 PUFAs has dramatically declined in favor of n-6 PUFA intake (Molendi-Coste et al., 2011). Joseph Hibbeln was one of the first investigators to draw attention to the importance of n-3 PUFAs in psychiatric disorders. In 1998, Hibbeln showed a cross-national significant negative correlation between worldwide fish consumption and prevalence of MDD (Hibbeln, 1998). Moreover, higher n-6/n-3 ratios, such as the AA/EPA ratio, has been detected in blood samples (Lin et al., 2010) and red blood cell phospholipids (Logan, 2003) of depressed patients. Accordingly, n-3 PUFA concentrations in the blood reflect an accurate, although not identical, representation of n-3 PUFAs levels in the brain (Horrobin, 2001). Lower DHA levels have been found in the postmortem orbitofrontal cortex of MDD patients (McNamara et al., 2007). Moreover, lower n-3PUFA levels have been found in chronic hepatitis C viral infection patients that developed interferon (IFN)-induced depression after IFN-α intervention. This finding identifies both n-3 PUFA-related genotypes and n-3 PUFA levels as risk factors for IFN-induced depression (Su et al., 2010). However, these findings do not show that fish consumption can cause differences in the prevalence of MDD or that eating fish or fish oils is useful in treatment. One double-blind, placebo-controlled study investigated the effect of EPA as adjunct to antidepressant therapy in a group of 20 MDD diagnosed patients. Although this was a small study (17 women, 3 men), the addition of 2g of EPA to standard antidepressant medication showed highly significant benefits by week 3 of treatment. Primarily, EPA showed effects on insomnia, depressed mood, and feelings of guilt and worthlessness (Nemets et al., 2002). In 2002, Peet and Horrobin observed that a specific EPA dosage (1g/d) was effective in ameliorating depressive symptoms in subjects with persistent depression despite ongoing treatment with antidepressant. In this 12-week, randomized, double-blind, placebo-controlled trial, 53% of the subjects who received EPA (17 subjects) achieved a 50% reduction on the Hamilton Depression Rating Scale score. In addition, the EPA had a broad-spectrum positive effect leading to improvements in anxiety, sleep, lassitude, libido, and suicidal ideation (Peet and Horrobin, 2002). Moreover, a 2-week, double-blind, placebo-controlled trial conducted in a group of 162 patients showed n-3 PUFA efficacy in the prevention of IFN-induced depression in hepatitis C virus patients (Su et al., 2014). Both DHA (1.75g/d) and EPA (3.5g/d) significantly delayed the onset of IFN-induced depression after 24 weeks of IFN-α treatment. Despite that, only EPA-treated patients showed lower incident rates of IFN-induced depression (10% vs 30% for placebo [oleic oil, 4g/d], P=.037). Although clinical outcomes on the effect of EPA on major depression seem to be promising, trials using DHA are inconclusive. Thirty-six subjects with major depression assigned to receive DHA (2g/d) for 6 weeks did not show differences in the score of the Montgomery-Asberg Depression Rating Scale compared with the placebo-treated group (Marangell et al., 2003). A recent meta-analysis focused on the hypothesis that EPA represents the key compound of the n-3 PUFA family having effects in the treatment of major depression. Fifteen trials (916 total participants) using n-3 PUFAs as either a mono or adjunctive therapy were analyzed. Studies were selected based on prospective, randomized, double-blinded, placebo-controlled study design, if depressive episode was the primary complaint with or without comorbid medical conditions and, if appropriate outcome measures were used to assess depressed mood. This meta-analysis concluded that n-3 PUFA supplements with >60% of EPA (in a dose range of 200 to 2200mg/d in excess of DHA) ameliorated the clinical condition. However, doses containing primarily DHA or <60% EPA were not effective against primary depression. Moreover, trial duration (4–16 weeks) was not a predictor of outcomes, suggesting that EPA improvements may not be limited to the initial treatment period (Sublette et al., 2011). Albeit, it is not possible to recommend n-3 PUFAs as either a mono or adjunctive therapy in major depression as yet, though the current research is strong enough to justify further studies.
Bipolar Disorder and Schizophrenia
To date, the link between n-3 PUFA levels and bipolar disorder/schizophrenia is poorly understood. n-3 PUFAs can have a slight beneficial effect on depressive symptoms when added to an existing psychopharmacological maintenance treatment for bipolar disorder. For instance, 30 patients with bipolar disorder were randomized to receive 9.6g/d of n-3 PUFAs (EPA 6.2g, DHA 3.2g) or placebo (olive oil) in addition to their ongoing usual treatment. The n-3 PUFAs patient group showed a significantly longer period of remission than the placebo group as assessed by the Kaplan-Meier survival analysis (Stoll et al., 1999). In a second study, patients with bipolar disorder were randomized to receive either 1to 2g/d ethyl-EPA (n = 24–25, respectively) or placebo (paraffin oil, n = 26) in addition to their ongoing usual treatment (Frangou et al., 2006). The EPA treatment, without apparent benefit of 2g over 1g EPA, significantly improved the Hamilton Rating Scale for Depression together with the Clinical Global Impression Scale. Nevertheless, current data on the efficacy of DHA and EPA in the treatment of bipolar disorder are insufficient for us to draw definite conclusions that can guide clinical practice.
Despite the large paucity of data, it seems that n-3 PUFAs may be of help in reducing psychotic-like symptoms (Schlogelhofer et al., 2014). Eighty-one participants with subthreshold psychosis were followed for 12 months to investigate the rate of progression to first-episode psychotic disorder. Previously, one-half of the group underwent a 12-week intervention period of 1.2g/d n-3 PUFAs or placebo. Only 5% of the n-3 PUFA group had transitioned to psychotic disorder in contrast to a 27.5 % in the control group. Moreover, n-3 PUFA intake ameliorated positive, negative, and general symptoms assessed by the Positive and Negative Syndrome Scale (Amminger et al., 2010). Overall, well-designed and executed randomized controlled trials in this field are clearly lacking, and the need for such high-quality primary research is acute. Study duration should be long enough to ensure that the n-3 PUFAs can be fully absorbed into brain cell membranes and therefore should ideally be 3 months at a minimum. Finally, the dose and composition of the n-3 PUFA supplement should be modelled on current evidence, which suggests that 1 to 2g/d of EPA or majority EPA supplement may be the most effective form of the treatment, although further research into the efficacy of varied compositions and doses of n-3 PUFA treatment is also necessary (Table 4).
Table 4.
n-3 PUFA Impact on Mental Illnesses in Adulthood
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| 18–70 years depressed (>15 on HDRS), medicated (n = 70). | Ethyl-EPA – 1, 2, or 4g/d or placebo. | 12 weeks parallel design, adjunctive therapy. | HDRS, MADRS, BDI. | Treatment > placebo on all 3 rating scales with 1g/d EPA – strong effects for core depressive symptoms. Treatment = placebo on 2g and 4g/d (nonsignificant trends). | (Peet and Horrobin, 2002) |
| 28–73 years diagnosed MDD (85% women) HDRS score > 18 (n = 20). | 2g ethyl-EPA (96% from fish oil) or placebo, Vit E. | 4 weeks parallel design, adjunctive therapy. | HDRS. | Treatment > placebo at weeks 2, 3, and 4 on HDRS score and on core depressive symptom subscales. | (Nemets et al., 2002) |
| 18–60 years outpatients with MDD; HDRS score > 18, medicated, (n = 22). | 3.3g/d n-3 PUFA (2.2g DHA, 1.1g EPA). | 8 weeks parallel design, adjunctive therapy. | HDRS. | Treatment > placebo on HDRS. | (Su et al., 2003) |
| 18–65 years MDD diagnosis; HDRS score > 16 (80% female) (n = 35). | 2g/d DHA or placebo. | 6 weeks parallel. | MADRS, HDRS, GAFS. | Treatment = placebo on outcome measures. | (Marangell et al., 2003) |
| 18–65 years recruited, (mean age 38), treated for current depressive episode (53% female) (n =77). | 3g/d n-3 PUFA (2.4g DHA; 0.6g EPA) + Vit E or olive oil placebo. | 12 weeks parallel, adjunctive therapy. | HDRS short form, BDI. | Treatment = placebo on outcome measures (improvements in both groups at week 2). | (Silvers et al., 2005) |
| 18–72 years outpatients with major depression diagnosis (n = 83, 45 males). | 3g/d n-3 PUFA (2.2g DHA, 0.6g EPA) + Vit E or olive oil placebo. | 4 month parallel design, adjunctive therapy. | HDRS, BDI, GAFS. | Treatment = placebo on outcome measures (improvements in both groups). | (Grenyer et al., 2007) |
| 18–40 years with MDD during pregnancy (n = 24). | 2.2g EPA + 1.2g DHA or placebo, both with tocopherols and orange flavor. | 8 weeks, parallel design. | HDRS, EPDS, GDI. | Treatment > placebo on outcome measures. | (Su et al., 2008) |
| 18–70 years recruited, (mean age = 38); people from GP surgeries or public with mild-moderate depression (77% female) (n = 190). | 630mg EPA, 850mg DHA, 870mg olive oil, or olive oil placebo (both with tocopherols and orange oil). | 12 weeks parallel design. | DASS, BDI, STAEI, mood using diary and visual probe task, cognitive function. | Treatment = placebo on outcome measures (improvements in both groups). | (Rogers et al., 2008) |
| 40–55 years recruited, (mean age 49) postmenopausal women with psychological distress and depressive symptoms (n = 120). | 1.5g ethyl-EPA, 0.5g ethyl-DHA. | 8 weeks parallel design. | PGWB, HSCL-D-20, HDRS. | Treatment = placebo on all measures (improvements in both groups). Treatment > placebo in women without MDE (major depressive episode diagnosis). | (Lucas et al., 2009) |
| Major depression + coronary heart disease (n = 122). | 930mg ethyl-EPA + 750mg ethyl DHA/d or corn oil placebo. | 10 weeks parallel design, adjunctive therapy. | BDI-II, HDRS. | Treatment = placebo on outcome measures (improvements in both groups). | (Carney et al., 2009) |
| 18–65 years inpatients with bipolar disorder (n = 30). | 9.6g/d n-3 PUFA (6.2g EPA, 3.4g DHA) or olive oil esther placebo. | 4 month parallel design; adjunctive therapy. | HDRS, YMRS, CGI-S, GAS. | Treatment > placebo on GAS, HDRS and CGI; treatment = placebo on YMRS. | (Stoll et al., 1999) |
| 57 Bipolar depressed and 59 rapid cycling (mean age 45) (n = 116, 51% male). | 6g/d ethyl-EPA or liquid paraffin placebo. | 4 month parallel design; adjunctive therapy. | IDS, YMRS, CGI- BP (bipolar disorder). | Treatment = placebo on outcome measures. | (Keck et al., 2006) |
| Outpatients with bipolar depression + scores > 17 on HDRS, (mean age 47) (n = 75, 76% female). | 1g/d ethyl EPA (n = 24); 2g/d ethyl EPA (n = 25) or paraffin placebo. | 12 week parallel design, adjunctive therapy. | HDRS, YMRS, CGI. | Treatment > placebo on HDRS & CGI on 1g and 2g/d. Treatment = placebo on YMRS. | (Frangou et al., 2007) |
| 16–64 years presenting after act of repeated self-harm (n = 49, 65% women). | 1.2g/d EPA + 0.9g DHA or corn oil placebo (with 1% EPA/DHA). | 12 weeks parallel design in addition to standard care. | BDI, HDRS, OAS-M, IMT/ DMT, PSS, DHUS. | Treatment > placebo on BDI, HDRS, PSS, DHUS. Treatment = placebo on OAS-M and IMT/DMT (hostility/aggression, memory). | (Hallahan et al., 2007) |
| Study 1: schizophrenic patients, PANSS score > 40, mean age 44 (n = 45). Study 2: diagnosed schizophrenia, untreated, mean age 35, (n = 30). |
2g/d EPA or corn oil placebo. | 3 months parallel, single therapy unless drugs needed. | PANSS; need for antipsychotic medication. | Treatment > placebo, particularly on positive subscale; 12/12 placebo and 8/14 EPA patients took medication. | (Peet et al., 2001) |
| (18–65 years, M = 40; 61% male) diagnosed schizophrenia or schizoaffective disorder (n = 30). | 3g/d ethyl EPA + Vit E or mineral oil + Vit E placebo. | 16 weeks parallel design, adjunctive therapy. | PANSS, CGI, MADRS, RBANS, AIMS, SARS. | Treatment = placebo on outcome measures (some showed improvements in both groups). | (Fenton et al., 2001) |
| 18–55 years schizophrenic, treatment resistant patients, PANSS score > 10 (n = 40, mean age 45). | 3g/d ethyl-EPA or liquid paraffin placebo. | 12 weeks parallel design, adjunctive therapy. | PANSS, ESRS. | Treatment > placebo on PANSS and dyskinesia subscale of ESRS. Treatment = placebo on other ESRS subscales. | (Emsley et al., 2002) |
| 20–62 years treatment- resistant schizophrenia; PANSS > 50, (n = 115, mean age 37, 66% male). | 1, 2, or 4g/d ethyl- EPA or liquid paraffin placebo. | 12 weeks parallel design, adjunctive therapy. | PANSS, LUNSERS, MADRS, AIMS, BAS, SARS. | Treatment = placebo on all rating scales; 2g treatment > placebo for patients on clozapine (associated with ↑AA). | (Peet et al., 2002) |
| First-episode psychosis patients (n = 69, mean age 21, 76% male). | 2g/d ethyl-EPA or mineral oil placebo not absorbed by intestinal tract (both with Vit E). | 12 weeks parallel design, adjunctive therapy. | BPRS, SANS, CDSS, CGI, GAF, SOFAS. | Treatment = placebo on all outcome measures. Treatment > placebo on CGI co-varying for duration of untreated psychosis; treatment > placebo at weeks 4–6. |
(Berger et al., 2007) |
| 13–25 years met defined risk factors for psychosis (n = 81, mean age 16, 40% male). | 1.2g/d n-3 PUFAs 0.7g EPA, 0.48g DHA, and 7.6mg of vitamin E). | 12 weeks. | PANSS, MADRS, GAF. | Treatment > placebo on PANSS and GAF at 12 weeks, 6 and 12 months. | (Amminger et al., 2010) |
| 19–30 years university students (study measured aggression and executive function) (n= 41, 70% female). | 1.5–1.8g/d DHA or 97% soybean oil + 3% fish oil placebo capsules. | 3 months parallel design. | P-F Study; Stroop; Dementia- detecting test. | Treatment > placebo on aggression (increased in placebo group during exam time); treatment = placebo on other measures. | (Hamazaki et al., 1996) |
| 18–40 years females with moderately severe borderline personality disorder (n= 30, mean age 26). | 1g/d ethyl-EPA or mineral oil placebo. | 8 weeks parallel design. | OAS-M; MADRS. | Treatment > placebo aggression and depressive symptoms | (Zanarini and Frankenburg, 2003) |
| MDD patients (n= 154). | (1) EPA 1g/d; (2) DHA 1g/d; (3) Placebo. |
8 weeks. | HDRS-17, QIDS- SR-16, CGI-S. | Treatments and placebo improved HDRS-17, QIDS- SR-16, CGI-S. | (Mischoulon et al., 2015) |
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; BDI, Beck Depression Inventory; BPRS, Brief Psychiatric Rating Scale; CBC, Child Behaviour Checklist; CDSS, Calgary Depression Scale for Schizophrenia; CGI-S, Clinical Global Impression-Severity; DASS, Depression & Anxiety Stress Scale; DHUS, Daily Hassles & Uplifts Scale; DMT, Delayed Memory Task; ESRS, Extrapyramidal Symptom Rating Scale; GAFS, Global Assessment of Functioning Scale (revised GAS); GAS, Global Assessment Scale; HDRS, Hamilton Depression Rating Scale; HSCL-D-20, 20-item Hopkins Symptom Checklist Depression Scale; IDS, Inventory for Depressive Symptomology; IMT, Immediate Memory Task; LUNSERS, Liverpool University Neuroleptic Side-Effects Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale; MDD, major depressive disorder; OAS-M, The Overt Aggression Scale, Modified; PANSS, Positive and Negative Syndrome Scale; PGWB, Psychological General Well-Being Schedule; PSS, Perceived Stress Scale; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SANS, Scale for the Assessment of Negative Symptoms; SARS, Simpson-Angus Rating Scale; SOFAS, Social and Occupational Functioning Assessment Scale; STAEI, State-Trait Anger Expression Inventory; YMRS, Young Mania Rating Scale.
n-3 PUFAs in Elderly
Clinical studies have been carried out to elucidate the role of n-3 PUFAs in healthy older subjects (Table 5). One of the largest randomized, controlled trials to date recruited 867 cognitively healthy subjects (70–79 years old) and did not reveal improved cognitive functioning in the California Verbal Learning Test after 24 months of treatment (200mg EPA plus 500mg DHA) (Dangour et al., 2010), even though at the end of the study, the n-3 PUFA serum levels were higher compared with the placebo group (olive oil) and the n-3:n-6 PUFA ratio was relatively high in both groups. Moreover, all the recruited subjects showed a high cognitive functioning at the beginning of the study, as assessed by the Mini-Mental State Examination. This, the authors suggest, is the reason for the negative findings. In another study, higher administration of n-3 PUFAs (900mg DHA) for 24 weeks showed improvements in verbal recognition memory and visuospatial learning in elderly subjects with low habitual intake of DHA (Yurko-Mauro et al., 2010). Accordingly, higher concentration (1.3g DHA and 0.45g EPA) and longer duration (12 months) of n-3 PUFAs showed improvement in different cognitive domains of a neuropsychological battery (Lee et al., 2013). In this study, the choice of subjects could have been critical. Thirty-five healthy elderly women with mild cognitive impairment (MCI) from a low socioeconomic background were recruited. Moreover, in this group the habitual intake of n-3 PUFAs was inadequate for financial reasons. These findings are in agreement with a double-blind, randomized control trial conducted by Sinn et al. (2012) in which 40 MCI subjects (over 65 years old) with low fish intake were divided into 3 experimental groups to receive a supplement rich in EPA (1.67g EPA plus 0.16g DHA), DHA (1.55g DHA + 0.40g EPA), or LA (2.2g). After 6 months of n-3 PUFA supplementation, depressive symptoms, assessed by the Geriatric Depression Scale, verbal fluency (Initial Letter Fluency), and self-reported physical health,were improved, especially in the DHA group. It is likely that MCI and low n-3 PUFA consumption offer the best prospect of cognitive improvement. Interestingly, DHA seems to be more of benefit in both memory and cognition than EPA, or their combination, as observed in adulthood. This discrepancy could be due to the phospholipid degradation occurring at this last stage of life. Since DHA constitutes the most abundant fatty acid in the brain and due to its importance in the formation and functionality of the CNS, it is plausible to think that DHA can ameliorate cognitive performance more than other PUFAs in older subjects.
Table 5.
n-3 PUFA Impact in Healthy Elderly
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| 70–75 years, cognitively healthy, MMSE ≥ 24 (median = 29) (n = 867, analysis on n =748). | Ethyl ester fish oil (0.2g EPA + 0.5g DHA)/d vs placebo (olive oil). | 24 months. | CVLT. | No effect on global cognitive function, memory, processing speed, executive function, global delay score. | (Dangour et al., 2010) |
| 60–80 years, stable MI patients, MMSE >21 (average ± SD 28±1.6 points). | (1) 0.4g/d EPA + DHA; (2) 2g/d ALA; (3) EPA + DHA + ALA; (4) Placebo. | 40 months. | MMSE. | No effect on MMSE. | (Geleijnse et al., 2012) |
| 60–80 years, healthy women (n = 57, analyzed n = 49). | (1) 0.8g/d DHA (algal oil); (2) 12mg/d lutein; (3) DHA + lutein; (4) Placebo. | 4 months. | Cognitive test battery measuring verbal fluency, memory, processing speed and accuracy. | Treatments (1), (2), (3) improved verbal fluency. DHA + lutein improved rate of learning and memory in 1 of 6 recall tests. | (Johnson et al., 2008) |
| ≥60 years, MCI, MMSE = 26.4 (25–28), middle to low- socioeconomic status (n = 36, analyzed n = 35). | Fish oil (1.3g DHA + 0.45g EPA)/d vs placebo (corn oil). | 12 months. | MMSE, RAVLT. | Improved memory (short-term memory, working memory, immediate visual memory, delayed recall). | (Lee et al., 2013) |
| 51–72 years, healthy (n = 44, analyzed n = 38. | Fish oil (1.05g DHA + 1.50g EPA)/d vs placebo. | 5 weeks. | Working memory. | Improved working memory. TNF-α inversely related to working memory performance. | (Nilsson et al., 2012) |
| >65 years, MCI, MMSE ≥ 22 (average ~27±2.5) (n = 50). | (1) EPA-rich fish oil (1.67g EPA + 0.16g DHA)/d); (2) DHA- rich fish oil: (1.55g DHA + 0.40g EPA)/d; (3) Placebo (safflower oil). | 6 months. | Cognitive battery. | DHA improved verbal fluency (test of fluid thinking/semantic memory). Only 1 of 11 cognitive assessments affected. | (Sinn et al., 2012) |
| 45–77 years (average ~56±8.7 years), healthy (n = 112, analyzed n = 75). | Tuna oil (0.25g DHA + 0.06g EPA)/d vs placebo (soybean oil). | 90 days. | CDR, visual acuity. | No treatment effects. | (Stough et al., 2012) |
| ≥65 years, cognitively healthy, median (25, 75 percentile) MMSE = 28, ranged from 23 to 30 (n = 302). | (1) Low-dose fish oil (0.26g EPA + 0.18g DHA)/d; (2) High dose fish oil (1.09g EPA + 0.85g DHA)/d; (3) Placebo (oleic acid). | 26 weeks. | Cognitive test battery. | Treatment improved attention in APOE4 allele carriers. Treatment–gender interactions: Attention improved in men. | (van de Rest et al., 2008a) |
| 50–90 years, nondemented participants with memory complaints, MMSE ≥ 27 (average ~28.5±1.11) (n = 157, analyzed n = 122). | PS containing LC omega-3: 300mg PS + 0.08g (DHA + EPA)/d. | 15 weeks. | Immediate and delayed verbal recall, learning abilities, and time to copy complex figure. | Improved verbal immediate recall. A subset of participants with higher baseline cognitive status performed better on immediate and delayed verbal recall, learning abilities and time to copy a complex figure. | (Vakhapova et al., 2010) |
| 50–75 years, MMSE < 26 (average ~29±1.0, ranged from 26 to 30) (n = 80, z n = 65). | Fish oil 2.2g/d LC omega-3 (1.32g EPA + 0.88g DHA) vs placebo (sunflower oil). | 26 weeks. | Stroop Color- Word test, TMT, AVLT. | Improved executive function. Subset who showed greatest increase in n-3 index showed improved memory. Improved white matter microstructural integrity, grey matter volume in frontal, temporal, parietal and limbic areas. Improvements in executive function associated with peripheral BDNF and inversely with fasting insulin. | (Witte et al., 2014) |
| ≥55 years (average ~70±9 years), subjective memory complaints with ARCD, MMSE >26 (n = 485). | 0.9g/d DHA from algal oil vs placebo (corn + soy oil). | 24 weeks. | CANTAB PAL, MMSE. | Improved visuospatial learning and episodic memory, immediate and delayed verbal recognition memory. | (Witte et al., 2014) |
Abbreviations: AVLT, Auditory Verbal Learning Test; CANTAB-PAL, Cambridge Neuropsychological Test Automated Battery - Paired Associates Learning; CDR, Clinical Dementia Rating; CVLT, California Verbal Learning Test; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; TNF- α, tumour necrosis factor-α.
n-3 PUFAs and Alzheimer’s Disease
Postmortem analysis of AD subjects shows lower n-3 PUFAs levels in the hippocampus and frontal lobe together with decreased hippocampal size (Soderberg et al., 1991; Yehuda et al., 2002). Moreover, reduced risk of senile dementia and AD has been reported to be related to higher fish consumption (Morris et al., 2003). However, no cognitive improvements were observed in subjects affected by moderate AD after 24 weeks of n-3 PUFAs (EPA 1080mg plus DHA 720mg) (Chiu et al., 2008). Neither has a longer trial, up to 1 year of n-3 PUFA supplementation, shown neuropsychiatric improvements in AD subjects (Freund-Levi et al., 2008). On the other hand, Yehuda et al. (1996) have shown improvements in mood, cooperation, appetite, sleep, ability to navigate in the home, and short-term memory after only 4 weeks of an ALA:LA mixture (1:4). Furthermore, within 174 AD subjects that underwent n-3 PUFA administration for a total period of 1 year, only 15% of them showed reduction in cognitive decline (Freund-Levi et al., 2006). The impact of n-3 PUFAs on mental illnesses in elderly are reported in Table 6.
Table 6.
n-3 PUFA Impact on Mental Illnesses in Elderly
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| >65 years nondepressed community dwelling adults (n = 302, mean age 70, 55% male). | 1.8g/d EPA + DHA, 400mg/d EPA + DHA. | 26 weeks. | CES-D, MADRS, GDS-15, HADQ. |
Treatment = placebo on outcome measures. | (van de Rest et al., 2008b) |
| People with AD living in own homes, on stable treatment with acetylcholine esterase inhibitors, (n = 204, mean age 73). | 1.72g DHA + 600mg EPA/ day. | 6 months parallel + 1-way crossover to fish oil for 6 months. | NPI, MADRS, CGB, DAD. | Treatment > placebo on MADRS in non- apoE-4 carriers and agitation in apoE-4 carriers. | (Freund-Levi et al., 2008) |
| 50–73 years AD patients, (n = 100, 21% females). | 0.5g ALA:LA, 1:4 ratio. | 4 weeks, adjunctive therapy. | 12-item quality of life questionnaire (caregiver), clinician interview. | Treatment > placebo on 12-item QOL questionnaire. | (Yehuda et al., 1996) |
| Nursing home residents with mild-moderate vascular dementia (n = 20, mean age 83). | 4.32g/d DHA. | 12 months. | MMSE; HDS-R; clinical evaluation. | Treatment > placebo on outcome measures after 3 and 6 months, associated with DHA increases. | (Terano et al., 1999) |
| n = 21, mean age 68; 57% male. | 240mg/d AA+DHA or olive oil placebo. | 3 months. | RBANS (Japanese version). | Treatment > placebo on immediate memory and attention. | (Kotani et al., 2006) |
| n = 178, mean age 74. | 1.72g DHA + 600mg EPA/d. | 6 months, adjunctive therapy. | MMSE, ADAS-cog; global function on bCDRS. | Treatment > placebo on MMSE in mild MCI group (n = 27). | (Freund-Levi et al., 2006) |
| n = 35, mean age 74, 57% female. | 1.08g EPA + .72g DHA or olive oil placebo. | 6 months. | CIBIC-plus; ADAS- cog; MMSE; HDRS. | Treatment > placebo on CIBIC-plus. Treatment > placebo on ADAS- cog in MCI sub-group. | (Chiu et al., 2008) |
| n = 302 (mean age 70, 55% male. | 1.8g/d EPA+DHA; 400mg/d EPA + DHA. | 26 weeks. | Word Learning Task; Digit Span; Trail Making; Stroop; Verbal Fluency. | Treatment = placebo on outcome measures; treatment > placebo on attention for apoE- 4 carriers and males. | (van de Rest et al., 2008a) |
Abbreviations: ADAS-cog, cognitive portion of the Alzheimer’s Disease Assessment Scale; CES-D, Centre for Epidemiologic Studies Depression Scale; CGB, Caregivers Burden Scale; CIBIC-plus, Clinician’s Interview-Based Impression of Change Scale; DAD, Disability Assessment for Dementia scale; GDS, Geriatric Depression Scale; HADQ, Hospital Anxiety and Depression Questionnaire; HDRS, Hamilton Depression Rating Scale; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
n-3 PUFAs: Mechanisms of Action
Inflammation
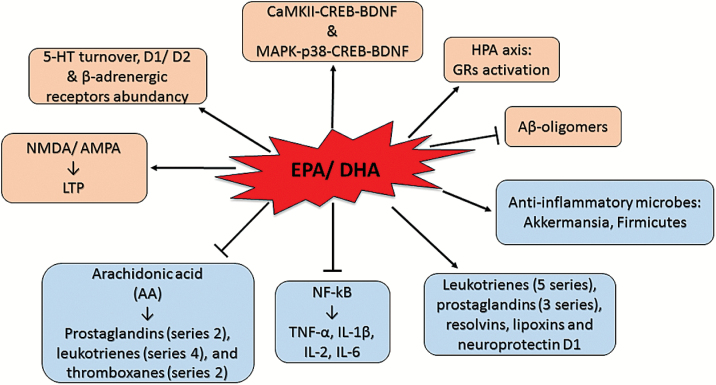
As outlined in the previous sections, n-3 PUFAs have multiple implications in several conditions. Therefore, they are likely to act via multiple mechanisms (Figure 2). The antiinflammatory effect exerted by n-3 PUFAs represents one of the most investigated mechanisms. Moreover, chronic inflammation is now considered to be central to the pathogenesis of stress-related disorders such as depression (Barnes et al., 2016). Primarily EPA and in part DHA metabolize into antiinflammatory compounds such as leukotrienes (5 series), prostaglandins (3 series), resolvins, lipoxins, and neuroprotectin D1. Competing with n-6 PUFAs for metabolism, both EPA and DHA may interfere with the production of the n-6 PUFA arachidonic acid-derived proinflammatory eicosanoids such as prostaglandins (series 2), leukotrienes (series 4), and thromboxanes (series 2) (Das, 2006). DHA and EPA have been also shown to inhibit the release of proinflammatory cytokines, such as interferon-γ, tumour necrosis factor-α, IL-1β, IL-2, and IL-6, directly acting on the transcriptional factor NF-kB (Kang and Weylandt, 2008).
Figure 2.
Omega-3 polyunsaturated fatty acid [(n-3) PUFA] mechanisms of action. Schematic representing the possible mechanisms of action of n-3 PUFAs.
HPA Axis
n-3 PUFAs seem to play a role in the regulation of the stress-response influencing the activity of the HPA axis. It has been proposed that n-3 PUFA deficiency may induce a chronic stress state by disruption of glucocorticoid receptors (GR)-mediated negative feedback (Larrieu et al., 2014, 2016). Controversially, the same authors observed that supplementation of n-3 PUFAs (9 weeks) prevented detrimental chronic social defeat stress-induced emotional and neuronal impairments by attenuating HPA dysfunction (Larrieu et al., 2014). Similarly, n-3 PUFA (EPA 12%, DHA 18%; 16 weeks) supplementation decreased stress-induced high plasma corticosterone levels and decreased anxiety- and depressive-like behaviors, as assessed by the elevated plus maze and the forced swim test, while increasing cognition as assessed by the Morris water maze in rats that underwent restrain stress (Ferraz et al., 2011). Accordingly, a study from our laboratory recently revealed n-3 PUFA (EPA 80%, DHA 20%; 12 weeks)-induced hippocampal GR activation correlated with cognitive and mood state improvements in adult female rats assessed by the novel object recognition, elevated plus maze, and forced swim test (Pusceddu et al., 2015b) (Table 7). Another study from our laboratory showed the protective effects of DHA against corticosterone-induced neuronal death as well as astrocyte overgrowth in cortical primary cultures (Pusceddu et al., 2015c). Furthermore, in the same study, we observed that the DHA was able to reverse the corticosterone-induced downregulation of GR expression levels in neurons. The regulation of GRs may represent a novel mechanism exerted by n-3 PUFAs which is worth to investigate further.
Table 7.
Effect of n-3 PUFAs on Adult Rodent Behavior
| Animals | Treatment | Length of Treatment | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| C57Bl6/J mice 2nd generation. | (1) control; (2) n-3 def diet; (3) n-3 suppl diet. |
3 months old. | Chronic social defeat test, open field, forced swim test. | (3) ameliorated chronic social defeat stress-induced emotional and neuronal impairments by impeding HPA axis hyperactivity. | (Larrieu et al., 2014) |
| C57BL6/J female mice. | (1) n-3 def diet; (2) n-3 suppl diet. |
After weaning, both groups were fed with a control diet. | Social investigation, forced swim test, open field. | Anxiety-like behaviour induced by (1) was abolished by the cannabinoid agonist WIN55,212-2. | (Larrieu et al., 2012) |
| C57Bl/6 mice. | (1) control; (2) n-3 def diet + (1) after weaning. |
Until 14 weeks old. | Open-field, object recognition, light-dark transition, elevated plus maze, social interaction tests. | (2) reduced anxiety-like behavior compared to (1). | (Palsdottir et al., 2012) |
| Wistar rats 2nd generation. | (1) n-3 adeq diet; (2) n-3 def diet. |
Until 60 days old. | Inhibitor avoidance task, flinch-jump task, open-field, elevated plus maze. | (1) Improved inhibitor avoidance task and elevated plus maze performances compared to (2). | (Moreira et al., 2010) |
| Long Evans rats. | 3 generations (F): (1) n-3 adeq diet; (2) n-3 def diet; (3) n-3 def till birth of 3F; (4) n-3 def till weaning of 3F; (5) n-3 def till 7 weeks of 3F. |
Until 9 or 13 weeks of age. | MWM, motor activity. | (3), (4) similar MWM outcomes and DHA brain levels to (1). | (Moriguchi and Salem, 2003) |
| Wistar rats, 2nd generation. | (1) Control; (2) n-3 def diet; (3) same as (2) + DHA/AA at weaning. |
After lactation all groups received (1). | Passive-avoidance test. | (3) reversed learning impairments observed in (2). | (Garcia- Calatayud et al., 2005) |
| Wistar Imamichi rats, 2nd generation. | (1) Control; (2) n-3 def diet; (3) same as (2) + DHA (300mg/kg/d). |
DHA was administrated 1 week prior behavioral test. | Elevated plus maze, fear conditioning. | (3) reversed behavioral impairments observed in (2). | (Takeuchi et al., 2003) |
| Wistar rats, 2nd generation. | (1) Control; (2) fish oil. |
2 months. | Elevated plus maze, ambulatory activity test. | (2) improved animal behaviors. | (Chalon et al., 1998) |
| Long Evans rats. | (1) def diet; (2) adeq diet. |
15 weeks. | Forced swim test, resident intruder test, open field. | (2) Improved forced swim and resident intruder test. | (DeMar et al., 2006) |
| Long Evans rats. | (1) Control; (2) artificial rearing: n-3 def diet; (3) artificial rearing: n-3 adeq diet. |
Until 9 weeks. | Motor activity, elevated plus- maze, Morris water maze. | (3) Improved spatial learning compared to (2). | (Lim et al., 2005b) |
| Long Evans rats. | (1) Control; (2) n-3 def diet; (3) same as (2) + DHA enriched diet after weaning. |
56 days. | Locomotor activity, thermal stimulus. | (3) reversed behavioral impairment compared to (2). | (Levant et al., 2004) |
| Long Evans rats, 2nd and 3rd generation. | (1) n-3 def diet; (2) n-3 adeq diet. |
8 weeks. | Motor activity, elevated plus maze, Morris water maze. | (2) reversed behavioral impairment compared to (1). | (Moriguchi et al., 2000) |
| Donryu rats, 2nd generation. | (1) Control; (2) n-3 def diet; (3) same as (2) + DHA after weaning. |
7 wks. | Brightness- discrimination learning test. | (3) reversed behavioural impairment compared to (2). | (Ikemoto et al., 2001) |
BDNF
DHA regulation of BDNF protein levels has gotten the attention of several researchers. This is of importance, since stress-related pathologies are strongly associated with decreased levels of BDNF (Lee and Kim, 2010). Accordingly, decreased BDNF levels are found in rats fed a diet containing inadequate n-3 PUFAs (Rao et al., 2007). Indeed, DHA supplementation to cortical astrocytes cells increased cAMP response element-binding protein and BDNF protein levels via a p38 mitogen-activated protein kinase-dependent mechanism (Rao et al., 2007). Likewise, DHA-induced hippocampal calcium–calmodulin protein kinase II-cAMP response element-binding protein-BDNF pathway activation enhanced synaptic plasticity, memory, and learning in rats (Wu et al., 2008).
Monoaminergic System
Associations between monoaminergic dysfunction in stress-related pathologies and lower dietary levels of n-3 PUFAs have been observed. Male rats with a 61% decrease in brain DHA, induced by feeding a diet deficient in n-3 PUFAs from birth, exhibited lower expression of the serotonin-synthesizing enzyme tryptophan hydroxylase in the midbrain and higher serotonin turnover in the prefrontal cortex compared with controls (McNamara et al., 2010b). Controversially, feeding a diet containing ALA reversed the effect on serotonin turnover (McNamara et al., 2010b). Consistent with this, adult rats supplemented with DHA and EPA exhibited increased concentrations of serotonin in the frontal cortex and hippocampus (Vines et al., 2012). Similarly, in mice, the decrease in brain serotonin levels induced by unpredictable chronic mild stress was reversed by an n-3 PUFA diet supplementation (Vancassel et al., 2008). Likewise, the role of n-3 PUFAs in modulation of noradrenergic neurotransmission has recently received attention. Studies in cultured SH-SY5Y neuroblastoma cells suggest that either brief exposure to, or incorporation of, DHA increased basal, but not KCl-evoked release of [3H]- norepinephrine by a mechanism involving enhanced exocytosis (Mathieu et al., 2010). DHA treatment also increased the density of β-receptors on rat astrocytes in primary culture (Joardar et al., 2006). Also, the DA system is affected by variation in dietary n-3 PUFA content. Virgin females with lower tissue DHA levels had altered abundancy of D1 and D2 DA receptors in the caudate nucleus relative to virgin females with normal DHA (Davis et al., 2010). These receptor alterations have been found in rodent models of depression and are consistent with the proposed hypodopaminergic basis for anhedonia and motivational deficits in depression (Kram et al., 2002). The effects of n-3 PUFAs on adult rodents’ behavior are reported in Table 7.
Glutamatergic System
It has been observed that n-3 PUFAs can regulate the functionality of the glutamatergic system, which can undergo dysregulation during aging. Indeed, n-3 PUFAs are involved in the regulation of the post-synaptic 2-amino-3-propionic acid and N-methyl-d-aspartate receptors (Nishikawa et al., 1994). This suggests that n-3 PUFAs may play an important role in the genesis of long-term potentiation, which is involved in memory formation and restoration of synaptic plasticity (Dyall et al., 2007; Lynch et al., 2007; Kelly et al., 2011). Excess glutamate can lead to excessive release of AA, which initiates a proinflammatory cascade of events involving the production of eicosanoids via the activation of inducible cyclooxigenase and lipoxigenases and the production of proinflammatory cytokines (Bazan, 2007; Farooqui et al., 2007).
Aβ Oligomers and Antiapoptosis
In vitro studies have shown DHA preventive effects against Aβ oligomer-induced neurotoxicity both in cortical and hippocampal cultures (Florent et al., 2006; Wang et al., 2010). Moreover, DHA has been found to promote cellular survival and prevent cortical neuronal apoptosis in primary cultures in a physiological condition (Cao et al., 2005) and after chronic corticosterone treatment (Pusceddu et al., 2015c). Moreover, similar results have been found in animal models of AD fed with n-3 PUFA-enriched diet (Lim et al., 2005a; Green et al., 2007). Interestingly, learning memory performance improved in Aβ-infused adult rats supplemented upon DHA intervention (Hashimoto et al., 2002; Hashimoto et al., 2005, 2009) (Table 8). These findings indicate that n-3 PUFAs decrease Aβ levels and have antioxidative stress and antiapoptosis effects, leading to neuron protection and maintenance of learning memory ability.
Table 8.
Effect of n-3 PUFAs in Aged Rodents
| Animals | Treatment | Length of Treatment | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| C57BL/6 mice. | (1) n-3: n-6 = 1: 29 ratio; (2) n-3: n-6 = 1: 3.6 ratio; (3) n-3: n-6 = 2: 1 ratio. |
From age 3 to 7 months. | Open field, Barnes maze; Serum and hippocampal cytokines; Hippocampal Ki67, DCX, GFAP, IBA-1, oxo8dG/oxo8G staining. |
(3) ↑ Anxiety, hippocampal dependent spatial memory vs (1); (3) ↑ hippocampal PUFA, ↓ hippocampal and serum TNF-α, ↑ KI67 and DCX vs (1); |
(Grundy et al., 2014) |
| C57B6/J mice. | Gavage: (1) n-3 PUFA mixture (440mg/kg); (2) olive oil; (3) Control. |
From 19 to 27 months. | MWM, NOR, SYM, CFC, EPM; BrdU, DCX, GFAP, Ki67, Iba-1; DG, CA1, CA3 volume, cell numbers, neurons, dendrites. |
(1) ↑ NOR, MWM, SYM, CFC; (1) ↑ neurogenesis, dendritic arborization of DG newborn neurons, hippocampal volumes and cell density, microglia. (1) ↓ apoptosis, astrocytosis. |
(Cutuli et al., 2014) |
| Sprague-Dawley rats. | Gavage: (1) Control (2) Control + fish oil; (3) Deficient; (4) Deficient + fish oil. |
Max 140 days. | MWM. | (3) ↓ MWM performance; (4) partially ↑ MWM; (2) ↑ MWM. |
(Chung et al., 2008) |
| SAMP8 mice 2nd generation. | (1) n-3 def diet; (2) n-3 enriched diet. |
Until 28 weeks old. | Sidman active avoidance task, light and dark discrimination learning test. | (2) ↑ Performance in discrimination learning test. | (Umezawa et al., 1995) |
| Wistar rats. | (1) control; (2) DPA or EPA enriched diet. |
56 days. | Morris water maze; LTP, caspase-3, microglial activity. |
(2) ↑ Spatial learning, ↓ microglial activation. | (Kelly et al., 2011) |
| C57B6/J mice. | (1) control; (2) DHA enriched diet. (3) n-3 def diet + DHA enriched at age 8 months. |
Entire lifespan. | Open field, light/dark test, Morris water maze | (2) ↑ Anxiety-like behavior and memory compared to (3). | (Carrie et al., 2002) |
| CD1 mice 2nd generation. | (1) n-3 def diet; (2) n-3 enriched diet. |
Until 23 months. | Forced swim test, Y-maze test, cytokines. | (2) ↓ Depressive-like behavior. | (Moranis et al., 2012) |
| Wistar rats 3rd generation. | (1) n-3 def diet; (2) n-3 def diet + oral DHA for 10 weeks prior behavior. |
10 weeks. | 8-arm radial maze | (2) ↑ Memory compared to (1). | (Gamoh et al., 2001) |
Abbreviations: LTP, long-term potentiation; MWM, Morris water maze; NOR, novel object recognition; EPM, elevated plus maze; CFC, contextual fear conditioning; SYM, spatial Y-maze; DCX, doublecortin; DG, dentate gyrus; CA1, cornu ammonis 1; CA3, cornu ammonis 3.
New Horizons: The Gut Microbiome
Parallel with these findings, we recently shed light on the role of n-3 PUFAs in regulating the gut microbiome, which is involved in a bidirectional communication with and influence over the brain both in rodents and humans (Cryan and Dinan, 2012). Indeed, there is a corpus of evidence to support the view that the gut microbiome plays an important role in stress-related psychopathologies such as depression (Dinan and Cryan, 2016). A first study conducted in our laboratory showed that EPA/DHA combination (1g/kg/d) was able to normalize early-life stress-induced disruption of female rat gut microbiome (Pusceddu et al., 2015a). This microbial disruption seems to be mainly due to a shift in Bacteroidetes:Firmicutes, the alteration of which in human stool specimen has been revealed in depressed individuals (Jiang et al., 2015). The early-life stress-induced microbial disruption also altered abundance of members of the gut microbiome known to have inflammatory effects such as Akkermansia (Stecher et al., 2007) and Flexibacter (Frank et al., 2007), and this was correlated with alteration of the corticosterone response to acute stress. Notably, EPA/DHA administration was beneficial for restoring members of the gut microbiome with immunoregulatory functions, suggesting a possible prevention to an overly robust stress-induced inflammatory response that may contribute to the onset of mental illnesses. Subsequent to this first evidence, a second study revealed a correlation between neurobehavioral outcomes and gut microbiome composition in mice fed with diets containing altered n-3 PUFA status (Robertson et al., 2016). Although the impact of n-3 PUFAs on the gut microbiome is still at its infancy, understanding the mechanisms behind their potential interplay may open up novel attractive strategies to prevent the onset of psychopathologies.
Conclusions
Despite strong evidence pointing out an inverse correlation between n-3 PUFAs levels and quality of life/psychiatric diseases, the introduction of n-3 PUFAs in clinical practice is still in its infancy. The primary reason for this is largely because of inconsistent and inconclusive randomized clinical trials. Inadequate dosing, inadequate duration, and lack of placebo control are major weaknesses in many studies. Use of insensitive and inappropriate clinical rating measures is another cause for concern. Nonetheless, there are some clear recommendations emerging. Low habitual intake of n-3 PUFAs is associated with poorer mental health and, in children, low literacy ability. A major finding is the positive evidence within older healthy adults with age-related cognitive decline. They benefit most from consuming n-3 PUFAs, particularly DHA. However, with the development of senile dementia or Alzheimer’s disease, n-3 PUFAs lose efficacy. While MDD still remains one of the most discussed and investigated forms of psychopathology, a clear identification of n-3 PUFAs as a treatment or adjunct therapy has not yet emerged. The evidence base is still weak and RCTs have been inconsistent with many study design limitations. Hence, a major challenge ahead is to design and conduct rigorous RCTs to provide proper evidence of n-3 PUFA application in clinical conditions, such as MDD. Further studies are also required to definitively demonstrate that n-3 PUFAs reduce the risk of transit from a prepsychotic state to overt schizophrenia. On the other hand, n-3 PUFAs are safe and well-tolerated supplements with only mild transient side effects. Moreover, being considered as a “natural remedy” and due to their relatively low cost, n-3 PUFAs represent an appealing option for individuals. While the evidence is not entirely conclusive to make specific recommendations for dietary intake of n-3 PUFAs, the limited ability of synthesis of n-3 PUFAs de novo and their critical role in brain development and functioning make it reasonable to include n-3 PUFAs in a balanced daily diet.
Statement of Interest
J.F.C. declares research funding: Mead Johnson Nutrition, Cremo, Suntory Wellness Danone-Nutritia, 4D Pharma. Speaker’s Bureau: Yakult, Mead Johnson, Janssen, Boehringer Ingelheim, Research Consultant: Alkermes and Mead Johnson.
Acknowledgments
The APC Microbiome Institute has conducted studies in collaboration with several companies including GSK, Pfizer, Cremo, Suntory Wellness, Wyeth, Nutritica, and Mead Johnson. T.G.D. and J.F.C. have spoken at meetings sponsored by food and pharmaceutical companies.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22:3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amminger GP, Berger GE, Schafer MR, Klier C, Friedrich MH, Feucht M. (2007) Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Biol Psychiatry 61:551–553. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. (2010) Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 67:146–154. [DOI] [PubMed] [Google Scholar]
- Antypa N, Van der Does AJ, Smelt AH, Rogers RD. (2009) Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J Psychopharmacol 23:831–840. [DOI] [PubMed] [Google Scholar]
- Barcelo-Coblijn G, Murphy EJ. (2009) Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res 48:355–374. [DOI] [PubMed] [Google Scholar]
- Barnes J, Mondelli V, Pariante CM. (2016) NPPR: genetic contributions of inflammation to depression. Neuropsychopharmacology. Advance online publication. doi: 10.1038/npp.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J, Smuts CM, Malan L, Kvalsvig J, van Stuijvenberg ME, Hurrell RF, Zimmermann MB. (2012) Effects of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: a randomized, double-blind, placebo-controlled intervention in South Africa. Am J Clin Nutr 96:1327–1338. [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Patton G, Scott KM, Degenhardt L, Whiteford HA. (2013) Global epidemiology of mental disorders: what are we missing? PLoS One 8:e65514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. (2007) Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care 10:136–141. [DOI] [PubMed] [Google Scholar]
- Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. (2007) Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry 68:1867–1875. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Qawasmi A. (2011) Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 50:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Taha AY, Tock JL, Totah NK, Cheon Y, Torres GE, Rapoport SI, Moghaddam B. (2014) Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol Psychiatry 75:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos DJ, Oranje B, Veerhoek ES, Van Diepen RM, Weusten JM, Demmelmair H, Koletzko B, de Sain-van der Velden MG, Eilander A, Hoeksma M, Durston S. (2015) Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology 40:2298–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. (2011) Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am J Clin Nutr 93:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J, Myers SP, Oliver C. (2004) An adaptogenic role for omega-3 fatty acids in stress; a randomised placebo controlled double blind intervention study (pilot) [ISRCTN22569553]. Nutr J 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess JR, Stevens L, Zhang W, Peck L. (2000) Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr 71:327–330. [DOI] [PubMed] [Google Scholar]
- Cao D, Xue R, Xu J, Liu Z. (2005) Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutr Biochem 16:538–546. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. (2009) Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA 302:1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie I, Smirnova M, Clement M, De JD, Frances H, Bourre JM. (2002) Docosahexaenoic acid-rich phospholipid supplementation: effect on behavior, learning ability, and retinal function in control and n-3 polyunsaturated fatty acid deficient old mice. Nutr Neurosci 5:43–52. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Macchi F, Plazzotta G, Veronica B, Bocchio-Chiavetto L, Riva MA, Pariante CM. (2015) Inflammation and neuronal plasticity: a link between childhood trauma and depression pathogenesis. Front Cell Neurosci 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalon S, Delion-Vancassel S, Belzung C, Guilloteau D, Leguisquet AM, Besnard JC, Durand G. (1998) Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J Nutr 128:2512–2519. [DOI] [PubMed] [Google Scholar]
- Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. (2008) Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc 67:19–27. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. (2008) The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 32:1538–1544. [DOI] [PubMed] [Google Scholar]
- Chung WL, Chen JJ, Su HM. (2008) Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J Nutr 138:1165–1171. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. (1980. a) Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum Dev 4:131–138. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. (1980. b) Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev 4:121–129. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712. [DOI] [PubMed] [Google Scholar]
- Cutuli D, De Bartolo P, Caporali P, Laricchiuta D, Foti F, Ronci M, Rossi C, Neri C, Spalletta G, Caltagirone C, Farioli-Vecchioli S, Petrosini L. (2014) n-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front Aging Neurosci 6:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A, Wolmarans P, Witthuhn RC, van Stuijvenberg ME, Swanevelder SA, Smuts CM. (2009) A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostaglandins Leukot Essent Fatty Acids 80:143–149. [DOI] [PubMed] [Google Scholar]
- Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, Holder GE, Knight R, Letley L, Richards M, Uauy R. (2010) Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr 91:1725–1732. [DOI] [PubMed] [Google Scholar]
- Das UN. (2006) Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J 1:420–439. [DOI] [PubMed] [Google Scholar]
- Davis PF, Ozias MK, Carlson SE, Reed GA, Winter MK, McCarson KE, Levant B. (2010) Dopamine receptor alterations in female rats with diet-induced decreased brain docosahexaenoic acid (DHA): interactions with reproductive status. Nutr Neurosci 13:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue J, Matzinger O, Binnert C, Schneiter P, Chiolero R, Tappy L. (2003) Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab 29:289–295. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. (2006) One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res 47:172–180. [DOI] [PubMed] [Google Scholar]
- Dervola KS, Roberg BA, Woien G, Bogen IL, Sandvik TH, Sagvolden T, Drevon CA, Johansen EB, Walaas SI. (2012) Marine Omicron-3 polyunsaturated fatty acids induce sex-specific changes in reinforcer-controlled behaviour and neurotransmitter metabolism in a spontaneously hypertensive rat model of ADHD. Behav Brain Funct 8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. (2016) Mood by microbe: towards clinical translation. Genome Med 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT. (2007) Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol Aging 28:424–439. [DOI] [PubMed] [Google Scholar]
- Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. (2002) Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry 159:1596–1598. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. (2007) Modulation of inflammation in brain: a matter of fat. J Neurochem 101:577–599. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. (2001) A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry 158:2071–2074. [DOI] [PubMed] [Google Scholar]
- Ferraz AC, Delattre AM, Almendra RG, Sonagli M, Borges C, Araujo P, Andersen ML, Tufik S, Lima MM. (2011) Chronic omega-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav Brain Res 219:116–122. [DOI] [PubMed] [Google Scholar]
- Florent S, Malaplate-Armand C, Youssef I, Kriem B, Koziel V, Escanye MC, Fifre A, Sponne I, Leininger-Muller B, Olivier JL, Pillot T, Oster T. (2006) Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J Neurochem 96:385–395. [DOI] [PubMed] [Google Scholar]
- Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S, Lodi L. (2005) Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur J Clin Invest 35:691–699. [DOI] [PubMed] [Google Scholar]
- Frangou S, Lewis M, McCrone P. (2006) Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry 188:46–50. [DOI] [PubMed] [Google Scholar]
- Frangou S, Lewis M, Wollard J, Simmons A. (2007) Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol 21:435–439. [DOI] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104:13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Levi Y, Basun H, Cederholm T, Faxen-Irving G, Garlind A, Grut M, Vedin I, Palmblad J, Wahlund LO, Eriksdotter-Jonhagen M. (2008) Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry 23:161–169. [DOI] [PubMed] [Google Scholar]
- Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. (2006) Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 63:1402–1408. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. (2001) Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci U S A 98:11047–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamoh S, Hashimoto M, Hossain S, Masumura S. (2001) Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol 28:266–270. [DOI] [PubMed] [Google Scholar]
- Garcia-Calatayud S, Redondo C, Martin E, Ruiz JI, Garcia-Fuentes M, Sanjurjo P. (2005) Brain docosahexaenoic acid status and learning in young rats submitted to dietary long-chain polyunsaturated fatty acid deficiency and supplementation limited to lactation. Pediatr Res 57:719–723. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Giltay EJ, Kromhout D. (2012) Effects of n-3 fatty acids on cognitive decline: a randomized, double-blind, placebo-controlled trial in stable myocardial infarction patients. Alzheimers Dement 8:278–287. [DOI] [PubMed] [Google Scholar]
- Gillies D, Sinn J, Lad SS, Leach MJ, Ross MJ. (2012) Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 7:CD007986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann V, Santos-Galduroz RF, Galduroz JC. (2013) Effects of low doses of polyunsaturated fatty acids on the attention deficit/hyperactivity disorder of children: a systematic review. Curr Neuropharmacol 11:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM. (2007) Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci 27:4385–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenyer BF, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P, Howe PR. (2007) Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 31:1393–1396. [DOI] [PubMed] [Google Scholar]
- Grimm H, Mayer K, Mayser P, Eigenbrodt E. (2002) Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br J Nutr 87:59–67. [DOI] [PubMed] [Google Scholar]
- Grundy T, Toben C, Jaehne EJ, Corrigan F, Baune BT. (2014) Long-term omega-3 supplementation modulates behavior, hippocampal fatty acid concentration, neuronal progenitor proliferation and central TNF-alpha expression in 7 month old unchallenged mice. Front Cell Neurosci 8:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan B, Hibbeln JR, Davis JM, Garland MR. (2007) Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry 190:118–122. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Sawazaki S, Itomura M, Asaoka E, Nagao Y, Nishimura N, Yazawa K, Kuwamori T, Kobayashi M. (1996) The effect of docosahexaenoic acid on aggression in young adults. A placebo-controlled double-blind study. J Clin Invest 97:1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y, Oka J, Shido O. (2002) Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem 81:1084–1091. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hossain S, Tanabe Y, Kawashima A, Harada T, Yano T, Mizuguchi K, Shido O. (2009) The protective effect of dietary eicosapentaenoic acid against impairment of spatial cognition learning ability in rats infused with amyloid beta(1–40). J Nutr Biochem 20:965–973. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. (2005) Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr 135:549–555. [DOI] [PubMed] [Google Scholar]
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. (2003) Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 111:e39–44. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. (1998) Fish consumption and major depression. Lancet 351:1213. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. (2002) Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord 69:15–29. [DOI] [PubMed] [Google Scholar]
- Hirayama S, Hamazaki T, Terasawa K. (2004) Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder - a placebo-controlled double-blind study. Eur J Clin Nutr 58:467–473. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. (2001) Phospholipid metabolism and depression: the possible roles of phospholipase A2 and coenzyme A-independent transacylase. Hum Psychopharmacol 16:45–52. [DOI] [PubMed] [Google Scholar]
- Ikemoto A, Ohishi M, Sato Y, Hata N, Misawa Y, Fujii Y, Okuyama H. (2001) Reversibility of n-3 fatty acid deficiency-induced alterations of learning behavior in the rat: level of n-6 fatty acids as another critical factor. J Lipid Res 42:1655–1663. [PubMed] [Google Scholar]
- Innis SM. (2007) Dietary (n-3) fatty acids and brain development. J Nutr 137:855–859. [DOI] [PubMed] [Google Scholar]
- Jackson PA, Deary ME, Reay JL, Scholey AB, Kennedy DO. (2012. a) No effect of 12 weeks’ supplementation with 1g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–35 years. Br J Nutr 107:1232–1243. [DOI] [PubMed] [Google Scholar]
- Jackson PA, Reay JL, Scholey AB, Kennedy DO. (2012. b) Docosahexaenoic acid-rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biol Psychol 89:183–190. [DOI] [PubMed] [Google Scholar]
- Jensen CL, Voigt RG, Prager TC, Zou YL, Fraley JK, Rozelle JC, Turcich MR, Llorente AM, Anderson RE, Heird WC. (2005) Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am J Clin Nutr 82:125–132. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194. [DOI] [PubMed] [Google Scholar]
- Joardar A, Sen AK, Das S. (2006) Docosahexaenoic acid facilitates cell maturation and beta-adrenergic transmission in astrocytes. J Lipid Res 47:571–581. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, McDonald K, Caldarella SM, Chung HY, Troen AM, Snodderly DM. (2008) Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci 11:75–83. [DOI] [PubMed] [Google Scholar]
- Johnson M, Ostlund S, Fransson G, Kadesjo B, Gillberg C. (2009) Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: a randomized placebo-controlled trial in children and adolescents. J Atten Disord 12:394–401. [DOI] [PubMed] [Google Scholar]
- Kang JX, Weylandt KH. (2008) Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem 49:133–143. [DOI] [PubMed] [Google Scholar]
- Karr JE, Grindstaff TR, Alexander JE. (2012) Omega-3 polyunsaturated fatty acids and cognition in a college-aged population. Exp Clin Psychopharmacol 20:236–242. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, Altshuler LL, Kupka R, Nolen WA, Leverich GS, Denicoff KD, Grunze H, Duan N, Post RM. (2006) Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry 60:1020–1022. [DOI] [PubMed] [Google Scholar]
- Kelly L, Grehan B, Chiesa AD, O’Mara SM, Downer E, Sahyoun G, Massey KA, Nicolaou A, Lynch MA. (2011) The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol Aging 32:2318 e2311–2315. [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Jackson PA, Elliott JM, Scholey AB, Robertson BC, Greer J, Tiplady B, Buchanan T, Haskell CF. (2009) Cognitive and mood effects of 8 weeks’ supplementation with 400mg or 1000mg of the omega-3 essential fatty acid docosahexaenoic acid (DHA) in healthy children aged 10–12 years. Nutr Neurosci 12:48–56. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. (2011) Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun 25:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A, Woodward A, Jackson S, Wang Y, Crawford MA. (2010) A double-blind, placebo-controlled study investigating the effects of omega-3 supplementation in children aged 8–10 years from a mainstream school population. Res Dev Disabil 31:718–730. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Cetin I, Brenna JT, Perinatal Lipid Intake Working Group; Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Association of Perinatal Medicine; European Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenterology, Hepatology and Nutrition, Committee on Nutrition; International Federation of Placenta Associations; International Society for the Study of Fatty Acids and Lipids (2007) Dietary fat intakes for pregnant and lactating women. Br J Nutr 98:873–877. [DOI] [PubMed] [Google Scholar]
- Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, Sakakibara M, Yoshimoto T, Guo J, Yamashima T. (2006) Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res 56:159–164. [DOI] [PubMed] [Google Scholar]
- Kram ML, Kramer GL, Ronan PJ, Steciuk M, Petty F. (2002) Dopamine receptors and learned helplessness in the rat: an autoradiographic study. Prog Neuropsychopharmacol Biol Psychiatry 26:639–645. [DOI] [PubMed] [Google Scholar]
- Kuratko CN, Barrett EC, Nelson EB, Salem N., Jr (2013) The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: a review. Nutrients 5:2777–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu T, Hilal LM, Fourrier C, De Smedt-Peyrusse V, Sans N, Capuron L, Laye S. (2014) Nutritional omega-3 modulates neuronal morphology in the prefrontal cortex along with depression-related behaviour through corticosterone secretion. Transl Psychiatry 4:e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu T, Hilal ML, De Smedt-Peyrusse V, Sans N, Laye S. (2016) Nutritional omega-3 deficiency alters glucocorticoid receptor-signaling pathway and neuronal morphology in regionally distinct brain structures associated with emotional deficits. Neural Plast 2016:8574830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu T, Madore C, Joffre C, Laye S. (2012) Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J Physiol Biochem 68:671–681. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. (2010) The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig 7:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LK, Shahar S, Chin AV, Yusoff NA. (2013) Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology (Berl) 225:605–612. [DOI] [PubMed] [Google Scholar]
- Lei X, Zhang W, Liu T, Xiao H, Liang W, Xia W, Zhang J. (2013) Perinatal supplementation with omega-3 polyunsaturated fatty acids improves sevoflurane-induced neurodegeneration and memory impairment in neonatal rats. PLoS One 8:e70645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levant B, Radel JD, Carlson SE. (2004) Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res 152:49–57. [DOI] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. (2005. a) A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci 25:3032–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Hoshiba J, Moriguchi T, Salem N., Jr (2005. b) N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr Res 58:741–748. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. (2010) A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 68:140–147. [DOI] [PubMed] [Google Scholar]
- Logan AC. (2003) Neurobehavioral aspects of omega-3 fatty acids: possible mechanisms and therapeutic value in major depression. Altern Med Rev 8:410–425. [PubMed] [Google Scholar]
- Logan AC, Jacka FN. (2014) Nutritional psychiatry research: an emerging discipline and its intersection with global urbanization, environmental challenges and the evolutionary mismatch. J Physiol Anthropol 33:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. (2009) Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am J Clin Nutr 89:641–651. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, Roche OJ, O’Connell F, Lynch MA. (2007) Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging 28:845–855. [DOI] [PubMed] [Google Scholar]
- Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. (2003) A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 160:996–998. [DOI] [PubMed] [Google Scholar]
- Marra CA, de Alaniz MJ. (1989) Influence of testosterone administration on the biosynthesis of unsaturated fatty acids in male and female rats. Lipids 24:1014–1019. [DOI] [PubMed] [Google Scholar]
- Mathieu G, Denis S, Langelier B, Denis I, Lavialle M, Vancassel S. (2010) DHA enhances the noradrenaline release by SH-SY5Y cells. Neurochem Int 56:94–100. [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, Alfieri D, Weber W, Jarvis K, DelBello MP, Strakowski SM, Adler CM. (2010. a) Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr 91:1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Carlson SE. (2006) Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 75:329–349. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. (2007) Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry 62:17–24. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. (2010. b) Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: relationship with central serotonin turnover. Prostaglandins Leukot Essent Fatty Acids 83:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Vannest JJ, Valentine CJ. (2015) Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: mechanisms and implications for psychopathology. World J Psychiatry 5:15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A, Perez-Garcia G, Ponce-Lopez T, Tellez R, Gallegos-Cari A, Castillo C. (2011) Spontaneously hypertensive rat (SHR) as an animal model for ADHD: a short overview. Rev Neurosci 22:365–371. [DOI] [PubMed] [Google Scholar]
- Michaeli B, Berger MM, Revelly JP, Tappy L, Chiolero R. (2007) Effects of fish oil on the neuro-endocrine responses to an endotoxin challenge in healthy volunteers. Clin Nutr 26:70–77. [DOI] [PubMed] [Google Scholar]
- Milte CM, Sinn N, Buckley JD, Coates AM, Young RM, Howe PR. (2011) Polyunsaturated fatty acids, cognition and literacy in children with ADHD with and without learning difficulties. J Child Health Care 15:299–311. [DOI] [PubMed] [Google Scholar]
- Mischoulon D, Freeman MP. (2013) Omega-3 fatty acids in psychiatry. Psychiatr Clin North Am 36:15–23. [DOI] [PubMed] [Google Scholar]
- Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead BL, Fehling K, Martinson MA, Hyman Rapaport M. (2015) A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry 76:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendi-Coste O, Legry V, Leclercq IA. (2011) Why and how meet n-3 PUFA dietary recommendations? Gastroenterol Res Pract 2011:364040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranis A, Delpech JC, De Smedt-Peyrusse V, Aubert A, Guesnet P, Lavialle M, Joffre C, Laye S. (2012) Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav Immun 26:721–731. [DOI] [PubMed] [Google Scholar]
- Moreira JD, Knorr L, Ganzella M, Thomazi AP, de Souza CG, de Souza DG, Pitta CF, Mello e Souza T, Wofchuk S, Elisabetsky E, Vinade L, Perry ML, Souza DO. (2010) Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: relevance for behavior alterations. Neurochem Int 56:753–759. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Greiner RS, Salem N., Jr (2000) Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem 75:2563–2573. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Salem N., Jr (2003) Recovery of brain docosahexaenoate leads to recovery of spatial task performance. J Neurochem 87:297–309. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. (2003) Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 60:940–946. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Ryan CM, Sheu L, Yao JK, Conklin SM, Manuck SB. (2010) Serum phospholipid docosahexaenonic acid is associated with cognitive functioning during middle adulthood. J Nutr 140:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthayya S, Eilander A, Transler C, Thomas T, van der Knaap HC, Srinivasan K, van Klinken BJ, Osendarp SJ, Kurpad AV. (2009) Effect of fortification with multiple micronutrients and n-3 fatty acids on growth and cognitive performance in Indian schoolchildren: the CHAMPION (Children’s Health and Mental Performance Influenced by Optimal Nutrition) Study. Am J Clin Nutr 89:1766–1775. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Muldoon MF, Moghaddam B. (2012) Improved working memory but no effect on striatal vesicular monoamine transporter type 2 after omega-3 polyunsaturated fatty acid supplementation. PLoS One 7:e46832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemets B, Stahl Z, Belmaker RH. (2002) Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 159:477–479. [DOI] [PubMed] [Google Scholar]
- Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. (2006) Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 163:1098–1100. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Radeborg K, Salo I, Bjorck I. (2012) Effects of supplementation with n-3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: a randomized controlled cross-over study. Nutr J 11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M, Kimura S, Akaike N. (1994) Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol 475:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, Karyadi SJ, van Klinken BJ, van der Knaap HC, Lukito W, Mikarsa W, Transler C, Wilson C, Group NS. (2007) Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am J Clin Nutr 86:1082–1093. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J. (2012) Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51:722–732 e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsdottir V, Mansson JE, Blomqvist M, Egecioglu E, Olsson B. (2012) Long-term effects of perinatal essential fatty acid deficiency on anxiety-related behavior in mice. Behav Neurosci 126:361–369. [DOI] [PubMed] [Google Scholar]
- Parletta N, Cooper P, Gent DN, Petkov J, O’Dea K. (2013) Effects of fish oil supplementation on learning and behaviour of children from Australian Indigenous remote community schools: a randomised controlled trial. Prostaglandins Leukot Essent Fatty Acids 89:71–79. [DOI] [PubMed] [Google Scholar]
- Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. (2001) Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res 49:243–251. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF. (2002) A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 59:913–919. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF, Group EEMS. (2002) A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res 36:7–18. [DOI] [PubMed] [Google Scholar]
- Portillo-Reyes V, Perez-Garcia M, Loya-Mendez Y, Puente AE. (2014) Clinical significance of neuropsychological improvement after supplementation with omega-3 in 8–12 years old malnourished Mexican children: a randomized, double-blind, placebo and treatment clinical trial. Res Dev Disabil 35:861–870. [DOI] [PubMed] [Google Scholar]
- Pusceddu MM, El Aidy S, Crispie F, O’Sullivan O, Cotter P, Stanton C, Kelly P, Cryan JF, Dinan TG. (2015. a) N-3 polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS One 10:e0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu MM, Kelly P, Ariffin N, Cryan JF, Clarke G, Dinan TG. (2015. b) n-3 PUFAs have beneficial effects on anxiety and cognition in female rats: Effects of early life stress. Psychoneuroendocrinology 58:79–90. [DOI] [PubMed] [Google Scholar]
- Pusceddu MM, Nolan YM, Green HF, Robertson RC, Stanton C, Kelly P, Cryan JF, Dinan TG. (2015. c) The omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) reverses corticosterone-induced changes in cortical neurons. Int J Neuropsychopharmacol. Advance online publication. doi: 10.1093/ijnp/pyv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. (2007) n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry 12:36–46. [DOI] [PubMed] [Google Scholar]
- Richardson AJ. (2004) Long-chain polyunsaturated fatty acids in childhood developmental and psychiatric disorders. Lipids 39:1215–1222. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P. (2012) Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: a randomized, controlled trial (the DOLAB Study). PLoS One 7:e43909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AJ, Calvin CM, Clisby C, Schoenheimer DR, Montgomery P, Hall JA, Hebb G, Westwood E, Talcott JB, Stein JF. (2000) Fatty acid deficiency signs predict the severity of reading and related difficulties in dyslexic children. Prostaglandins Leukot Essent Fatty Acids 63:69–74. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Montgomery P. (2005) The Oxford-Durham study: a randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics 115:1360–1366. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Puri BK. (2002) A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuropsychopharmacol Biol Psychiatry 26:233–239. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Ross MA. (2000) Fatty acid metabolism in neurodevelopmental disorder: a new perspective on associations between attention-deficit/hyperactivity disorder, dyslexia, dyspraxia and the autistic spectrum. Prostaglandins Leukot Essent Fatty Acids 63:1–9. [DOI] [PubMed] [Google Scholar]
- Robertson RC, Seira Oriach C, Murphy K, Moloney GM, Cryan JF, Dinan TG, Paul Ross R, Stanton C. (2016) Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, Heatherley SV, Christian LM, McNaughton SA, Ness AR. (2008) No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr 99:421–431. [DOI] [PubMed] [Google Scholar]
- Schlogelhofer M, Amminger GP, Schaefer MR, Fusar-Poli P, Smesny S, McGorry P, Berger G, Mossaheb N. (2014) Polyunsaturated fatty acids in emerging psychosis: a safer alternative? Early Interv Psychiatry 8:199–208. [DOI] [PubMed] [Google Scholar]
- Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. (2005) Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids 72:211–218. [DOI] [PubMed] [Google Scholar]
- Singh M. (2005) Essential fatty acids, DHA and human brain. Indian J Pediatr 72:239–242. [PubMed] [Google Scholar]
- Sinn N, Bryan J. (2007) Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr 28:82–91. [DOI] [PubMed] [Google Scholar]
- Sinn N, Bryan J, Wilson C. (2008) Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: a randomised controlled trial. Prostaglandins Leukot Essent Fatty Acids 78:311–326. [DOI] [PubMed] [Google Scholar]
- Sinn N, Milte C, Howe PR. (2010) Oiling the brain: a review of randomized controlled trials of omega-3 fatty acids in psychopathology across the lifespan. Nutrients 2:128–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, Howe PR. (2012) Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr 107:1682–1693. [DOI] [PubMed] [Google Scholar]
- Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. (2003) A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol 101:469–479. [DOI] [PubMed] [Google Scholar]
- Soderberg M, Edlund C, Kristensson K, Dallner G. (1991) Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 26:421–425. [DOI] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD. (2007) Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5:2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L, Zhang W, Peck L, Kuczek T, Grevstad N, Mahon A, Zentall SS, Arnold LE, Burgess JR. (2003) EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids 38:1007–1021. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. (1999) Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 56:407–412. [DOI] [PubMed] [Google Scholar]
- Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, Kennedy D. (2013) DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr 97:1134–1143. [DOI] [PubMed] [Google Scholar]
- Stough C, Downey L, Silber B, Lloyd J, Kure C, Wesnes K, Camfield D. (2012) The effects of 90-day supplementation with the omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiol Aging 33:824 e821–823. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu CC, Shen WW. (2003) Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 13:267–271. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, Pariante CM. (2008) Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 69:644–651. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Peng CY, Lai HC, Huang CL, Chen YC, Aitchison KJ, Pariante CM. (2010) Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry 67:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, Chang HC, Pariante CM. (2014) Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry 76:559–566. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Ellis SP, Geant AL, Mann JJ. (2011) Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry 72:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Iwanaga M, Harada E. (2003) Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res 964:136–143. [DOI] [PubMed] [Google Scholar]
- Terano T, Fujishiro S, Ban T, Yamamoto K, Tanaka T, Noguchi Y, Tamura Y, Yazawa K, Hirayama T. (1999) Docosahexaenoic acid supplementation improves the moderately severe dementia from thrombotic cerebrovascular diseases. Lipids 34 Suppl:S345–346. [DOI] [PubMed] [Google Scholar]
- Umezawa M, Ohta A, Tojo H, Yagi H, Hosokawa M, Takeda T. (1995) Dietary alpha-linolenate/linoleate balance influences learning and memory in the senescence-accelerated mouse (SAM). Brain Res 669:225–233. [DOI] [PubMed] [Google Scholar]
- Vakhapova V, Cohen T, Richter Y, Herzog Y, Korczyn AD. (2010) Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dement Geriatr Cogn Disord 29:467–474. [DOI] [PubMed] [Google Scholar]
- van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, Beekman AT, de Groot CP. (2008. a) Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 71:430–438. [DOI] [PubMed] [Google Scholar]
- van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Hoefnagels WH, Beekman AT, de Groot LC. (2008. b) Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 88:706–713. [DOI] [PubMed] [Google Scholar]
- Vancassel S, Leman S, Hanonick L, Denis S, Roger J, Nollet M, Bodard S, Kousignian I, Belzung C, Chalon S. (2008) n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J Lipid Res 49:340–348. [DOI] [PubMed] [Google Scholar]
- Vines A, Delattre AM, Lima MM, Rodrigues LS, Suchecki D, Machado RB, Tufik S, Pereira SI, Zanata SM, Ferraz AC. (2012) The role of 5-HT(1)A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: a possible antidepressant mechanism. Neuropharmacology 62:184–191. [DOI] [PubMed] [Google Scholar]
- Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. (2001) A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr 139:189–196. [DOI] [PubMed] [Google Scholar]
- Wang PY, Chen JJ, Su HM. (2010) Docosahexaenoic acid supplementation of primary rat hippocampal neurons attenuates the neurotoxicity induced by aggregated amyloid beta protein(42) and up-regulates cytoskeletal protein expression. J Nutr Biochem 21:345–350. [DOI] [PubMed] [Google Scholar]
- Williams C, Birch EE, Emmett PM, Northstone K. (2001) Stereoacuity at age 3.5 y in children born full-term is associated with prenatal and postnatal dietary factors: a report from a population-based cohort study. Am J Clin Nutr 73:316–322. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Hermannstadter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, Hahn A, Floel A. (2014) Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex 24:3059–3068. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. (2008) Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 155:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ. (2014) A nutrient combination that can affect synapse formation. Nutrients 6:1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. (2002) The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging 23:843–853. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Rabinovtz S, Carasso RL, Mostofsky DI. (1996) Essential fatty acids preparation (SR-3) improves Alzheimer’s patients quality of life. Int J Neurosci 87:141–149. [DOI] [PubMed] [Google Scholar]
- Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N, Jr, Stedman M, Investigators M. (2010) Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement 6:456–464. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR. (2003) omega-3 Fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry 160:167–169. [DOI] [PubMed] [Google Scholar]