Abstract
Background:
In vivo mapping by positron emission tomography of the serotonin 1A receptors has been hindered by the lack of suitable agonist positron emission tomography probes. 18F-labeled F13714 is a recently developed biased agonist positron emission tomography probe that preferentially targets subpopulations of serotonin 1A receptors in their “active state,” but its brain labeling pattern in nonhuman primate has not been described. In addition, a potential confound in the translatability of PET data between nonhuman animal and human arise from the use of anesthetics that may modify the binding profiles of target receptors.
Methods:
Positron emission tomography scans were conducted in a cohort of common marmosets (n=4) using the serotonin 1A receptor biased agonist radiotracer, 18F-F13714, compared with a well-characterized 18F-labeled antagonist radiotracer, 18F-MPPF. Experiments on each animal were performed under both consciousness and isoflurane-anesthesia conditions.
Results:
18F-F13714 binding distribution in marmosets by positron emission tomography differs markedly from that of the 18F-MPPF. Whereas 18F-MPPF showed highest binding in hippocampus and amygdala, 18F-F13714 showed highest labeling in other regions, including insular and cingulate cortex, thalamus, raphe, caudate nucleus, and putamen. The binding potential values of 18F-F13714 were about one-third of those observed with 18F-MPPF, with marked individual- and region-specific differences under isoflurane-anesthetized vs conscious conditions.
Conclusions:
These findings highlight the importance of investigating the brain imaging of serotonin 1A receptors using agonist probes such as 18F-F13714, which may preferentially target subpopulations of serotonin 1A receptors in specific brain regions of nonhuman primate as a biased agonist.
Keywords: 5-hydroxytryptamine, Callithrix jacchus, F13714, biased agonism
Significance Statement
Serotonin is a neurotransmitter substance in the brain that modulates numerous physiological functions, and the serotonin 1A receptor is a therapeutic target for neuropsychiatric and neurological disorders. Positron emission tomography has been used to characterize the serotonin 1A receptor in living brain, but there is still a lack of suitable agonist probe for studying the functional state of the serotonin 1A receptor. Here, we report the quantitative binding distribution in nonhuman primate brain of 18F-F13714, a recently developed agonist positron emission tomography probe for serotonin 1A receptor, and compare this with a well-characterized antagonist positron emission tomography probe, 18F-MPPF, under both consciousness and isoflurane-anesthesia. 18F-F13714 binding distribution in marmoset monkeys differs markedly from that of 18F-MPPF, with significant individual- and region-specific differences under anesthetized vs conscious conditions. Our findings highlight the importance of investigating the brain imaging of serotonin 1A receptors using agonist probes such as 18F-F13714, which may preferentially target subpopulations of serotonin 1A receptors as a biased agonist.
Introduction
Brain serotonin (5-hydroxytryptamine; 5-HT) is implicated in the control of cognitive and socioemotional abilities in various neuropsychiatric and neurological disorders (Kranz et al., 2010; Kiser et al., 2012; Olivier, 2015). Among the subtypes of 5-HT receptors, the 5-HT1A receptor is most widely distributed in the brain and is a therapeutic target for a variety of CNS disorders (Pazos and Palacios, 1985; Maurel et al., 2007; Celada et al., 2013; Billard et al., 2014
Recently, Lemoine et al. (2012) developed a 5-HT1A receptor agonist PET probe by 18F-labeling of F13714, a highly selective and efficacious 5-HT1A receptor agonist with very high binding affinity (Ki values <0.1nM) (Koek et al., 2001). F13714 exhibits high selectivity for 5-HT1A receptor in more than 40 other binding sites, including receptors, transporters, ion channels, and enzymes, which were tested by in vitro binding screening assays using both native rat and recombinant human binding sites (Assié et al., 2006) and shows high sensitivity to endogenous agonist, 5-HT, and G-protein uncoupling by Gpp(NH)p in the rat brain (Lemoine et al., 2012). Further, 18F-F13714 rapidly penetrates the blood–brain barrier with high lipophilicity and is only slightly metabolized in vivo (Lemoine et al., 2012). Intriguingly, a PET study using anesthetized cats indicated that 18F-F13714 preferentially binds to cortical rather than hippocampal 5-HT1A receptors, in contrast to findings with antagonist tracers showing a higher density in the hippocampus than in the cortex (Aznavour and Zimmer, 2007; Lemoine et al., 2012). These data reinforce the interpretation that the capacity to label “functional” 5-HT1A receptors may differ substantially between agonist and antagonist radiotracers. In addition, a variety of rat electrophysiological, neurochemical, and behavioral studies have indicated F13714 exhibits a biased agonist profile of activity at specific 5-HT1A receptor subpopulations, notably in the raphe region and the striatum (Newman-Tancredi et al., 2009; Iderberg et al., 2015; van Goethem et al., 2015; de Boer and Newman-Tancredi, 2016). A recent study in phMRI showed that F13714 and another biased agonist, F15599, induced specific pattern of changes in blood oxygen level dependent (BOLD) signal in a neuronal circuit in contrast to those induced by a classical agonist, 8-OH-DPAT, or a silent antagonist, MPPF, supporting the concept that biased agonists can preferentially target subpopulations of 5-HT1A receptors in specific brain regions (Becker et al., 2016). Investigation of brain imaging with 18F-F13714 is therefore desirable to better understand agonist interactions at subpopulations of 5-HT1A receptors.
Although previous experiments have demonstrated the utility of 18F-F13714 as a PET probe for the 5-HT1A receptor in cat (Assié et al., 2006; Maurel et al., 2007; Lemoine et al., 2012), the brain labelling pattern of 18F-F13714 in nonhuman primate has not been described. This would be highly informative, because the translatability of the novel biased agonist profile of F13714 to primate species (and particularly to human) remains to be established. Thus, use of nonhuman primates such as common marmosets, a species that shows human-like sociability involving serotoninergic neurotransmission (Yokoyama et al., 2013), is an attractive option for preclinical PET studies.
A supplementary issue that influences translatability of PET imaging data between animal and human studies is the use of anesthetics that may modify the binding profiles of target receptors (Elfving et al., 2003; Seeman and Kapur, 2003; Lancelot and Zimmer, 2010; Li et al., 2013). Carrying out marmoset PET without anesthesia may therefore be useful for identifying potential confounds in PET imaging due to anesthesia and for mapping the function of serotonin in vivo (Yokoyama et al., 2010; Yokoyama and Onoe, 2015).
The present study therefore had 2 aims. Firstly, to conduct PET scans in a cohort of marmosets using the agonist PET probe, 18F-F13714, and compare this with a well-characterized 18F-labeled antagonist PET probe, 18F-MPPF. Secondly, experiments were performed under both consciousness and isoflurane-anesthesia conditions. This study unveiled a differential binding distribution between agonist and antagonist PET probes, with differential impacts of isoflurane. Thus, the agonist PET probe, 18F-F13714, can provide functional binding profile of 5-HT1A receptors in the primate brain, which will help to further our understanding of the function of serotoninergic system in the human brain and its role in cognitive and socioemotional abilities.
MATERIALS AND METHODS
Subjects
Four adult male common marmosets (Callithrix jacchus) (including one pair of siblings), at 3.5 to 4.0 years of age at the start of experiments, were used in these studies. Animals were housed in pairs in home cages measuring 600×430×650mm under a 12-h-light/-dark cycle (light: 8:00 am to 8:00 pm). Each cage had 2 wooden perches, a food tray, and an automatic water dispenser. Animals were fed solid food (CMS-1, CLEA Japan, Inc., Tokyo, Japan) soaked in water and mixed with a suitable amount of powdered milk formula, honey, gluconic acid calcium, vitamin C, and lactobacillus probiotic twice a day, supplemented with chopped boiled eggs or bananas once a week. Each animal underwent PET scans 4 times with 18F-F13714 and 18F-MPPF within 1 year, both under consciousness and isoflurane anesthesia at a 3-week interval.
To perform PET scans on conscious marmosets without causing discomfort to the animals, experiments were conducted according to a previously published method (Yokoyama et al., 2010). Briefly, animals were adequately trained for fixation on the chair with an acrylic head holder that was surgically attached to the skull surface before PET experiment. For PET scans under anesthesia, animals were maintained with isoflurane inhalation via a mask lying on a bed without the use of a head holder. MRI of the brain was acquired before surgery of the head holder attachment with a 3T MRI scanner (Magnetom Allegra, Siemens, Erlangen, Germany) under pentobarbital anesthetic for the anatomical alignment of PET data. Animals were maintained and handled in accordance with the recommendations of the United States National Institutes of Health, and all procedures were approved by the Animal Care and Use Committee of RIKEN Kobe Branch (MAH21-10).
PET Scans
PET scans in conscious marmosets were performed with a PET scanner (microPET Focus220; Siemens Medical Solutions, Knoxville, TN) as previously described (Yokoyama et al., 2010). After cannulation via a tail vein, animals were fixed in a sitting position (tilted to 45°) in the scanner. For PET scans in anesthetized animals, inhalation induction with sevoflurane and then isoflurane anesthesia was maintained at 1% concentration, with monitoring of heart rate (150–200 beats/min) and peripheral oxygen saturation (>98%) with pulse oximetry (BSM-3592; Nihon Kohden, Tokyo, Japan). Animals were laid in the face-up position on a bed with the heads softly fixed by round ended ear bars and placed horizontally in the PET scanner. For both conscious and anesthetized animals, a transmission scan with a 68Ge-68Ga pin source was performed for 30min before an emission scan.
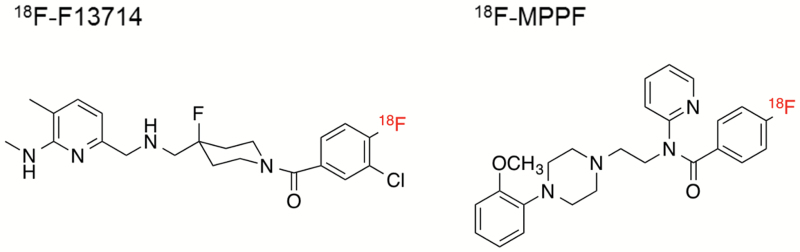
We used 2 kinds of PET probes: 18F-F13714 and 18F-MPPF (Figure 1). According to a previous method (Lemoine et al., 2012), 18F-F13714 was prepared by aromatic nucleophilic substitution reaction of the previously reported nitro precursor (3mg, 6.7 µmol) with 18F-KF/Kryptofix 2.2.2 in DMSO (200 μL). 18F-Fluoride anion was produced by a cyclotron (HM-12S; Sumitomo Heavy Industry Ltd., Tokyo, Japan). The radiochemical purity and chemical purity were >99% and >93%, respectively. The specific activity was 67.7±13.4 GBq/μmol at the end of synthesis. According to a previous method (Le Bars et al., 1998), 18F-MPPF was prepared by aromatic nucleophilic substitution reaction of the previously reported nitro precursor (5mg, 10.8 µmol) with 18F-KF/Kryptofix 2.2.2 in DMSO (500 μL). The radiochemical purity and chemical purity were both >99%. The specific activity was 182.5±65.6 GBq/μmol at the end of synthesis. In both tracers, PET scans were performed for 90 minutes immediately after bolus injection of radiolabeled tracers (18F-F13714: 162.2±21.4 MBq/kg; 18F-MPPF: 158.6±14.4 MBq/kg). Images were acquired with a 3-dimensional list-mode and reconstructed by a filtered back projection algorithm using a Hanning filter cut-off 0.5 cycles/pixels with the attenuation correction using blank and transmission images. A spatial resolution at the center of the field of view is approximately 1.35mm in full width at half-maximum. The dynamic histogram acquired during 90 minutes (6×10 seconds, 6×30 seconds, 11×60 seconds, 15×180 seconds, 3×600 seconds) was used for data analyses.
Figure 1.
Chemical structures of 18F-F13714 and 18F-MPPF.
Data Analysis
Reconstructed images were processed with image analysis software PMOD (version 3.5, PMOD Technologies Ltd., Zurich, Switzerland). First, individual PET images were aligned on each individual MR image by rigid matching using PMOD’s image registration and fusion tool (PFUS). Next, the template of the brain MR image that was previously prepared (Yokoyama et al., 2013) was used for normalization of individual PET images. PET images of the brain were deskulled according to the structural information from individual brain MR images. Individual brain MR images were then registered to the standard space of the template brain MR image by brain normalization. By using these registration matrices, PET images were transformed and normalized.
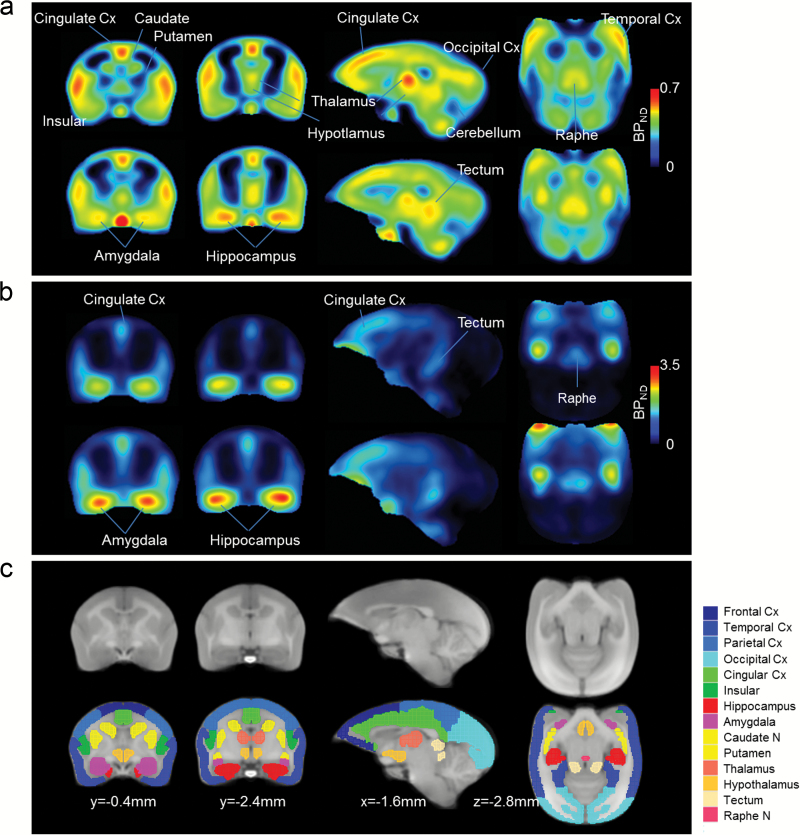
The normalized individual PET images were used to elucidate the quantitative 18F-F13714 and 18F-MPPF binding to 5-HT 1A receptors in the brain. We calculated binding potential (BPND) by quantifying the ratio at equilibrium of specifically bound radioligand to that of nondisplaceable radioligand in brain tissue using a nonlinear fitting method involving a simplified reference tissue model (SRTM), using cerebellum as a reference region and the cingulate cortex for 18F-F13714 or in the hippocampus for 18F-MPPF as a high expression region. Though in vitro autoradiography studies showed that 18F-F13714 and 18F-MPPF have almost no specific binding in cerebellum (Le Bars et al., 1998; Lemoine et al., 2012), there is controversy as to whether the cerebellum is devoid of the 5HT1A receptors in vivo (Parsey et al., 2010; Shrestha et al., 2012). However, we have confirmed in this study that the time activity curves of 18F-F13714 and 18F-MPPF in the cerebellum show the lowest uptake among brain regions, and also that when the ROI of cerebellum is divided into the gray and white matter, the shape of these TACs are very similar to each other. Therefore, using cerebellum as a reference region should be meaningful in our PET study at present. The parametric images of BPND were created by SRTM2 with fixing of k2’ estimated by SRTM. Regional BPND values were obtained by applying several anatomical regions of interest (ROIs) that were drawn manually in the template brain MR image with reference to the stereotaxic brain atlas of the common marmoset (Paxinos et al., 2011) (Figure 5) on the following regions: the frontal cortex, parietal cortex, temporal cortex, occipital cortex, cingulate cortex, insular cortex, caudate nucleus, putamen, amygdale, hippocampus, hypothalamus, thalamus, raphe, and tectum (Figure 5). Volumes of interest consisted of ROIs of 299.1mm3 on average, with 811.3mm3 at maximum in the temporal cortex and 1.3mm3 at minimum in the raphe.
Figure 5.
Serotonin (5-HT)1A receptor binding potential (BPND) images with 18F-F13714 (a) and 18F-MPPF (b) in conscious (top) and isoflurane (bottom) conditions. Note that the pseudo-color BPND scales differ between the 2 radiotracers: 0 to 0.7 for 18F-F13714: 0 to 3.5 for 18F-MPPF. MRI images corresponding to the anatomical regions shown in the positron emission tomography (PET) images are shown in (c). Names of separately colored areas on the lowermost MRI images are listed on the right, which represent regions of interest (ROIs) for evaluating BPND values in Tables 1 and 2 and in Figures 2 to 4 . Numerical representations as x, y, z are the coordinates for the anterior commissure on the ac-pc line in the standard marmoset MRI prepared in our laboratory.
The between-subject variability of BPND in each region was assessed for both conditions as the CV as follows:
The within-subject variability across conditions was assessed for each region as follows:
The reliability of the measurement per region was evaluated by comparing the within-subject variability (WS) to the between-subject variability (BS) in terms of intraclass correlation coefficient ICC (1,1) as follows, where the MSS represents mean sum of squares and is calculated for the WS and the BS situation:
Statistics
The effect of isoflurane on regional BPND was analyzed separately by 2-way ANOVA (region × condition) for 18F-F13714 and 18F-MPPF. Differences in BPND between conscious and anesthesia scans were tested using a paired t test in each ROI. The comparison regression between 18F-F13714 and 18F-MPPF across ROIs used the Deming regression model, taking measurement errors for both parameters separately on conscious and anesthetic scans. Statistical analyses were performed using R2.8.1, and a P value of .05 was defined as the threshold of statistical significance.
RESULTS
PET Scans with 18F-F13714
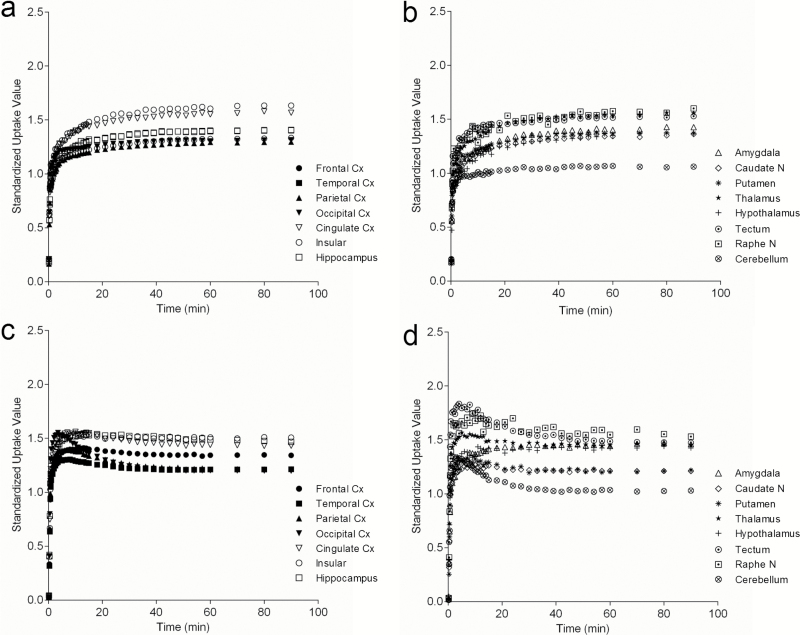
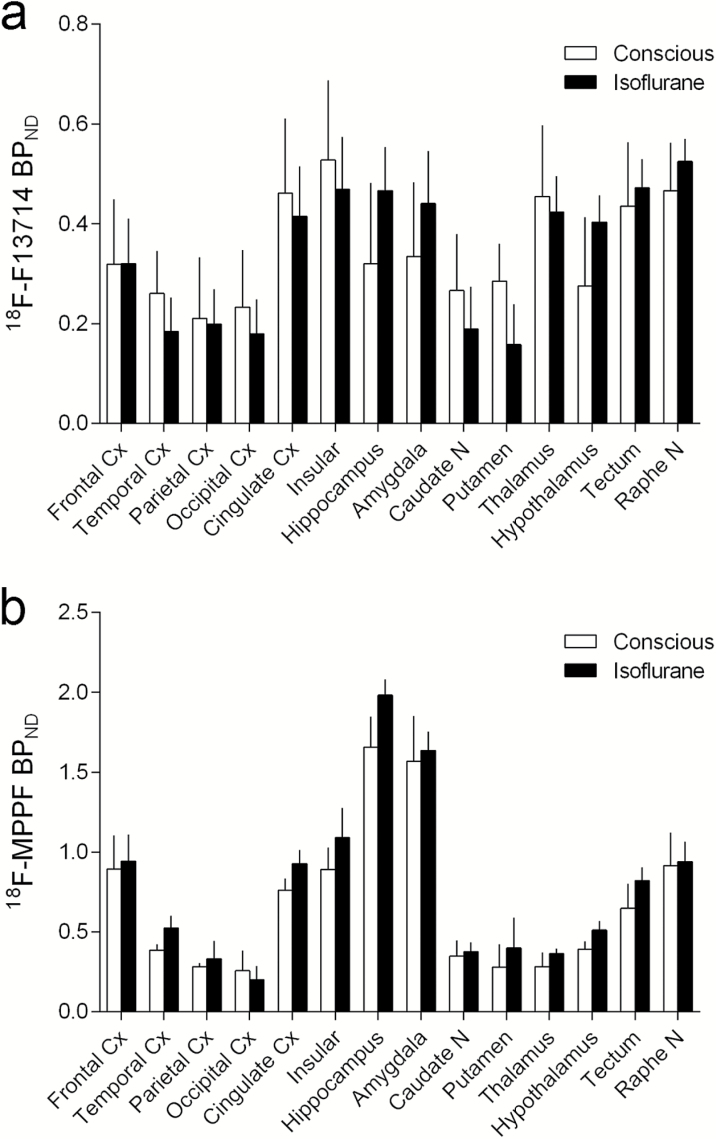
18F-F13714 uptake in the marmoset brain was rapid and showed apparently quasi-irreversible kinetics over the observation period (Figure 2), suggesting very high affinity binding of this tracer to the 5-HT1A receptor or, potentially, a rapid internalization of the receptors, although this remains speculative. In conscious animals, the distributed radioactivity indicated a distinctive pattern with a high level of uptake in the cingulate and insular cortices, raphe nucleus, thalamus, and tectum. Intermediate uptake was observed in the hippocampus, other cortical areas, amygdala, and hypothalamus, but also in the caudate and putamen. Low uptake was observed in the cerebellum (Figure 2a).
Figure 2.
Time activity curves of 18F-F13714 uptake in cortical (a and c) and subcortical (b and d) regions of interest in conscious (a-b) and isoflurane anesthetized (c-d) conditions. Anatomical locations of regions of interest refer to Figure 5. Data are mean values (n=4) to allow easy viewing, but relatively higher variability is found in each point of value in conscious (a-b) than in isoflurane anesthetized (c-d) conditions.
In animals anesthetized with isoflurane, there were subtle changes in the shape of the time activity curves, with an initial rapid rise followed by a slight decrease before showing uptake levels and quasi-irreversible kinetics similar to those observed in conscious animals (Figure 2b). There were differences in the uptake level in the various brain regions in anesthetized animals compared with conscious animals, with higher uptake in the hippocampus, amygdala, and hypothalamus and lower uptake in the caudate nucleus and putamen (see below for statistical information).
Variability was observed in 18F-F13714 binding between individual animals in both conscious and anesthetic conditions (Table 1). Regional BPND values had a moderate variation across subjects in conscious animals (CV: 26.1–58.0%, average 38.4%), but a higher variability in anesthetized animals (CV: 17.4–103.6%, average 53.7%). Two-way ANOVA revealed that isoflurane did not significantly change overall 18F-F13714 BPND (F(1, 42)=0.0013, P=.971), as the effects of anesthesia were region specific and not unidirectional. A significant interaction effect of region and condition was observed (F(13, 42)=4.392, P=.0001). Tukey’s posthoc comparison after Bonferroni correction showed that isoflurane significantly increased 18F-F13714 BPND in the hippocampus (t=3.65, P<.01) and hypothalamus (t=3.19, P<.05) and decreased in the putamen (t=3.19, P<.05). The high ICC (0.37–0.95, average 0.73) indicated a good reliability of individual variations in regional 18F-F13714 binding, even across conscious and anesthetic conditions.
Table 1.
Regional 18F-F13714 BPND Across Individuals under Conscious and Isoflurane Anesthesia Conditions
| Regions | Conscious | Isoflurane | VAR (%, mean±SD) | ICC | Paired t | ||
|---|---|---|---|---|---|---|---|
| BPND (mean±SD) | CV (%) | BPND (mean±SD) | CV (%) | ||||
| Frontal Cx | 0.32±0.13 | 40.8 | 0.32±0.18 | 56.6 | 17.4±10.0 | 0.92 | 0.021 |
| Temporal Cx | 0.26±0.08 | 32.5 | 0.18±0.14 | 73.4 | 48.3±41.1 | 0.63 | 1.900 |
| Parietal Cx | 0.21±0.12 | 58.0 | 0.20±0.14 | 70.8 | 24.7±22.7 | 0.93 | 0.286 |
| Occipital Cx | 0.23±0.11 | 49.0 | 0.18±0.14 | 77.2 | 54.5±60.8 | 0.81 | 1.339 |
| Cingulate Cx | 0.46±0.15 | 32.5 | 0.42±0.20 | 48.3 | 17.2±18.4 | 0.89 | 1.141 |
| Insular | 0.53±0.16 | 30.3 | 0.47±0.21 | 45.0 | 16.0±22.1 | 0.84 | 1.480 |
| Hippocampus | 0.32±0.16 | 50.5 | 0.47±0.17 | 37.2 | 40.6±23.1 | 0.62 | 3.653* |
| Amygdala | 0.33±0.15 | 44.4 | 0.44±0.21 | 47.6 | 27.2±16.6 | 0.76 | 2.660 |
| Caudate N | 0.27±0.11 | 42.3 | 0.19±0.17 | 89.5 | 55.2±46.8 | 0.78 | 1.935 |
| Putamen | 0.29±0.07 | 26.1 | 0.16±0.16 | 103.6 | 93.4±82.6 | 0.37 | 3.186* |
| Thalamus | 0.45±0.14 | 31.3 | 0.42±0.14 | 33.9 | 15.2±12.6 | 0.88 | 0.778 |
| Hypothalamus | 0.28±0.14 | 49.9 | 0.40±0.11 | 26.4 | 42.8±32.1 | 0.41 | 3.193* |
| Tectum | 0.44±0.13 | 29.3 | 0.47±0.12 | 24.6 | 14.0±11.8 | 0.83 | 0.907 |
| Raphe N | 0.47±0.10 | 20.8 | 0.52±0.09 | 17.4 | 17.7±11.1 | 0.51 | 1.477 |
| Mean | 38.4 | 53.7 | 0.73 | ||||
PET Scans with 18F-MPPF
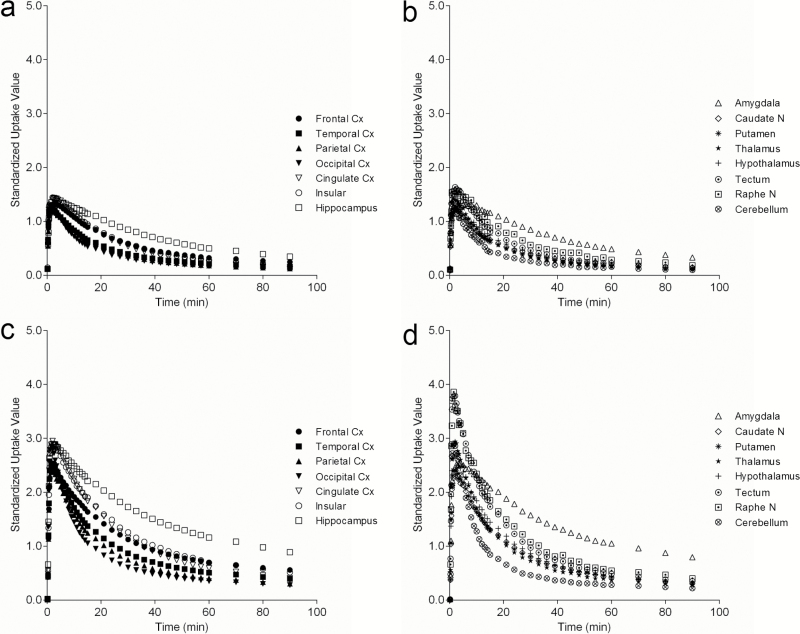
18F-MPPF uptake was similar in shape of the time activity curves between the conscious and anesthetic conditions, with a rapid rise and gradual decline, although the uptake level and variance was generally increased in anesthetized animals (Figure 3). A high level of uptake was observed in the hippocampus and amygdala; moderate uptake in the cingulate, insular, and frontal cortices, raphe nucleus, and tectum; lower uptake in the thalamus, striatum, hypothalamus, and other cortical areas; and low uptake in the cerebellum. There was some variation between individual animals in 18F-MPPF regional BPND values (although this was lower than that observed with 18F-F13714): under conscious conditions, CV values were 9.4% to 48.4%, average 22.5%; under anesthetized conditions: CV values were 10.1% to 94.5%, average 36.5% (Table 2).
Figure 3.
Time activity curves of 18F-MPPF uptake in cortical (a and c) and subcortical (b and d) regions of interest in conscious (a-b) and isoflurane-anesthetized (c-d) conditions. Anatomical locations of regions of interest (ROIs) refer to Figure 5. Data are mean values (n=4) to allow easy viewing, but there is similar variability in each point of value in conscious (a-b) than in isoflurane-anesthetized (c-d) conditions.
Table 2.
Regional 18F-MPPF BPND across Individuals under Conscious and Isoflurane Anesthesia Conditions
| Regions | Conscious | Isoflurane | VAR (%, mean±SD) | ICC | Paired t | ||
|---|---|---|---|---|---|---|---|
| BPND (mean±SD) | CV (%) | BPND (mean±SD) | CV (%) | ||||
| Frontal Cx | 0.89±0.21 | 23.6 | 0.94±0.33 | 35.3 | 30.6±23.7 | -0.54 | 0.385 |
| Temporal Cx | 0.39±0.04 | 9.4 | 0.53±0.15 | 28.9 | 30.8±18.0 | -0.12 | 1.107 |
| Parietal Cx | 0.28±0.02 | 8.0 | 0.33±0.23 | 68.0 | 58.7±45.0 | 0.07 | 0.387 |
| Occipital Cx | 0.26±0.12 | 48.4 | 0.20±0.17 | 87.2 | 92.0±65.9 | -0.72 | 0.462 |
| Cingulate Cx | 0.76±0.07 | 9.8 | 0.93±0.17 | 18.8 | 25.3±14.0 | -0.54 | 1.335 |
| Insular | 0.89±0.14 | 15.3 | 1.09±0.37 | 34.2 | 23.8±20.2 | 0.10 | 1.590 |
| Hippocampus | 1.66±0.19 | 11.6 | 1.98±0.20 | 10.1 | 18.0±7.3 | 0.03 | 2.593 |
| Amygdala | 1.57±0.28 | 18.0 | 1.64±0.24 | 14.6 | 5.4±3.7 | 0.93 | 0.539 |
| Caudate N | 0.35±0.10 | 28.0 | 0.38±0.12 | 30.7 | 25.0±19.0 | 0.16 | 0.227 |
| Putamen | 0.28±0.15 | 52.0 | 0.40±0.38 | 94.5 | 45.6±39.5 | 0.21 | 0.966 |
| Thalamus | 0.28±0.09 | 31.1 | 0.36±0.06 | 17.4 | 27.8±16.6 | 0.39 | 0.643 |
| Hypothalamus | 0.39±0.05 | 13.0 | 0.51±0.12 | 22.8 | 25.5±21.9 | -0.30 | 0.962 |
| Tectum | 0.65±0.15 | 23.8 | 0.82±0.17 | 20.2 | 24.1±13.1 | 0.35 | 1.382 |
| Raphe N | 0.92±0.21 | 22.4 | 0.94±0.25 | 26.3 | 21.2±8.7 | 0.56 | 0.193 |
| Mean | 22.5 | 36.3 | 0.04 | ||||
Two-way ANOVA revealed that isoflurane significantly increased 18F-MPPF BPND (F(1, 42)=10.03, P=.0029), while there was no statistically significant interaction effect for individual brain regions (F(13, 42)=0.56 P=.87). Tukey’s posthoc comparison after Bonferroni correction indicated no significant effect of isoflurane on 18F-MPPF BPND in any ROIs. The very low ICC (average 0.04), except for the amygdala (0.93), suggests that the individual variation of regional 18F-MPPF binding was inconsistent between conscious and anesthetic conditions.
Comparison of 18F-F13714 and 18F-MPPF Binding Distribution
There were notable differences in BPND between 18F-F13714 and 18F-MPPF across the ROIs (Figure 4). 18F-MPPF showed a markedly higher contrast than 18F-F13714, especially in the binding-rich regions, with an approximately 3 times higher BPND. Thus maximal BPND for 18F-MPPF amounted to 1.5 to 2.0 in the hippocampus and amygdala, whereas maximal BPND for 18F-F13714 amounted to about 0.5 in the insular cortex and raphe nucleus. Other brain regions did not show marked differences in BPND between PET probes: both 18F-F13714 and 18F-MPPF yielded BPND values of about 0.2 to 0.3 in the caudate nucleus, putamen, and thalamus (Figure 4).
Figure 4.
Serotonin (5-HT)1A receptor binding potential values with 18F-F13714 (a) and 18F-MPPF (b) in conscious (open column) and isoflurane anesthetized (filled column) conditions. Anatomical locations of regions of interest (ROIs) refer to Figure 4. Data are mean ± SD (n=4).
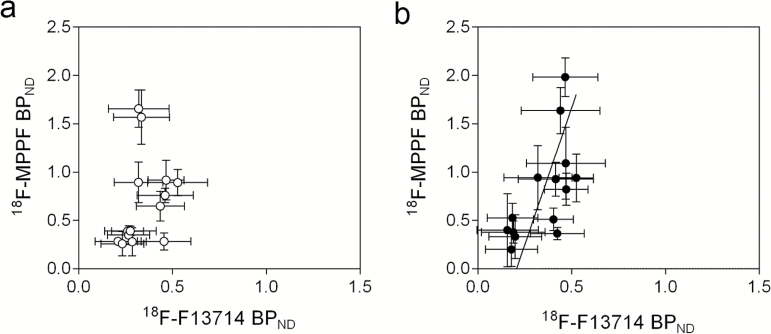
Averaged BPND images from all subjects for 18F-F13714 and 18F-MPPF are shown in Figure 5. Notably, there was no correlation of BPND between the 2 tracers in conscious animals (F(1,12)=1.19, P=.30), while a significant correlation was observed under isoflurane anesthesia (F(1,12)=8.92, P=.01) (Figure 6). These results suggest that the population of 5-HT1A receptors identified by 18F-F13714 was proportional to that identified by 18F-MPPF under anesthesia conditions, but not when PET experiments were carried out in conscious marmosets.
Figure 6.
Correlation of Serotonin (5-HT)1A receptor binding potential values with an agonist tracer 18F-F13714 and an antagonist tracer 18F-MPPF in conscious (open circle) (a) and isoflurane-anesthetized (filled circle) conditions (b). Data are mean ± SD (n=4).
Discussion
This is the first report on PET imaging of 18F-F13714, a novel, selective 5-HT1A receptor agonist PET probe, in nonhuman primate brain. The results indicate that it displays a highly distinctive brain distribution pattern in conscious and anesthetized common marmosets that differs markedly from that of a well-characterized antagonist PET probe, 18F-MPPF.
The overall brain distribution pattern of 18F-F13714 binding in marmosets exhibited some consistency with previous reports of 5-HT1A receptor densities in rats, baboons, rhesus monkeys, and humans determined with other PET probes, including 18F-MPPF (Pike et al., 1996; Maeda et al., 2001; Costes et al., 2007; Milak et al., 2010, 2011; Wooten et al., 2011). Thus, higher binding of 18F-F13714 was observed in regions classically associated with 5-HT1A receptor expression, including the cortical regions, hippocampus, amygdala, hypothalamus, and raphe nucleus (Figures 4 and 5). However, the intensity of the binding distribution in these brain regions was strikingly different from that seen with 18F-MPPF. Indeed, 18F-F13714 showed lower binding in the hippocampus and amygdala than in cingulate and insular cortices, particularly in conscious marmosets. This is reminiscent of the preferential binding in cortical areas previously reported in cats using 18F-F13714 and another agonist PET probe, 18F-F15599 (Lemoine et al., 2010, 2012). In contrast, the antagonist tracer, 18F-MPPF showed almost 2-fold higher binding to the hippocampus and amygdala compared with the cingulate and insular cortices, consistent with previous reports in rats, cats, and humans (Plenevaux et al., 2000; Passchier et al., 2001; Aznavour et al., 2006; Costes et al., 2007; Sibon et al., 2008). For 18F-F13714, BPND value in the raphe nucleus was similar to that in the hippocampus; in contrast, the BPND value in the raphe nucleus for 18F-MPPF was only one-half of that in the hippocampus (Figure 4). Further, 18F-F13714 exhibited pronounced binding to the thalamus and hypothalamus, whereas these brain regions were only modestly labeled by 18F-MPPF. Finally, 18F-F13714 exhibited a noticeable labelling of the caudate nucleus and putamen, approaching that observed in the frontal cortex. In contrast, binding of 18F-MPPF in the caudate and putamen amounted to less than one-half of that seen in the frontal cortex.
As discussed previously (Lemoine et al., 2012), F13714 is highly selective for 5-HT1A receptors, and its neurochemical and behavioral effects are blocked by a 5-HT1A receptor antagonist (WAY100635) (Assié et al., 2006; Iderberg et al., 2015). Accordingly, the in vitro autoradiographic labeling seen with 18F-F13714 on rat brain sections was completely blocked by co-incubation with WAY-100635, and in vivo cat brain PET labelling was prevented by preinjection of WAY-100635 (Lemoine et al., 2012). These considerations suggest that differences in labeling patterns between the 2 tracers may relate to the agonist properties of 18F-F13714 that enable it to distinguish a 5-HT1A receptor subpopulation of high-affinity sites (Assié et al., 2006; Maurel et al., 2007; Lemoine et al., 2012), a concept described previously for the 5-HT1A receptor in baboons with 11C-CUMI-101 (agonist) and 11C-WAY100635 (antagonist) (Kumar et al., 2012). In the case of F13714, extensive evidence supports the notion that it acts as a biased agonist that preferentially targets 5-HT1A receptors in specific brain regions, including the raphe, striatum, and thalamus (Assié et al., 2006; Iderberg et al., 2015; Becker et al., 2016).
Thus, F13714 potently inhibits 5-HT release in cortex and striatum (Assié et al., 2006; Iderberg et al., 2015), reverses L-DOPA-induced dyskinesia in Parkinsonian rats, and stimulates a BOLD signal in the raphe, striatum, and motor cortex (Becker et al., 2016), results that are consistent with the present study showing accentuated binding to these brain regions in marmoset brain. Although the molecular basis for this remains to be clarified, it is likely due to a preferential interaction of 18F-F13714 with 5-HT1A receptors that are coupled to specific G-protein subtypes and/or intracellular signaling mechanisms (Newman-Tancredi, 2011). The locus of these effects remains under investigation, but a potent activation of somatodendritic 5-HT1A autoreceptors appears important. The present PET data clearly indicate that 18F-F13714 binds to sites in the raphe (Figures 2, 4, and 5). Microdialysis studies show that F13714 potently inhibits 5-HT release in both hippocampus and striatum (Assié et al., 2006; Iderberg et al., 2015), responses controlled by somatodendritic 5 HT1A receptors. An ex vivo immediate early gene expression study showed that F13714 potently elicits increases in c-fos expression in dorsal and median raphe (Cussac et al., 2007). Taken together, these observations suggest that 18F-F13714 has an accentuated activity at presynaptic 5-HT1A receptors. However, it should be noted that, although binding profile of F13714 has been extensively characterized in the rat, in the marmoset brain it is still under investigation. Some caution is therefore warranted until further studies of this issue have been carried out.
Some additional points should be noted. Firstly, although the agonistic profile of 18F-F13714 has not been directly demonstrated in marmoset brain, it may be inferred by the distinctive binding kinetics we observed in anesthetized animals. Indeed, whereas isoflurane caused a global increase in 18F-MPPF uptake throughout the brain (Figure 3), binding kinetics of 18F-F13714 showed region-specific changes. The binding in tectum and occipital cortex increased rapidly, peaked, and then decreased slightly before stabilizing. In contrast, binding in amygdala and frontal cortex did not show this temporal profile and resembled that observed in conscious marmosets (Figure 2). It is tempting to speculate that these different profiles may be caused by changes in endogenous serotonin levels, which, by competing for binding to 5-HT1A receptors, could change the binding potential of 18F-F13714. Indeed, isoflurane was reported to decrease extracellular 5-HT in the hippocampus and the frontal cortex (Whittington and Virág, 2006; Mukaida et al., 2007) and inhibit 5-HT neuron firing in the medullary raphe (Johansen et al., 2015). It may therefore be surmised that, because its agonist activity, 18F-F13714 may have a higher sensitivity than for 18F-MPPF for measuring receptor occupancy by endogenous synaptic 5-HT release.
The assertion that agonist PET probes can identify a subset of 5-HT1A receptors whereas antagonist tracers label the total receptor population (Aznavour et al., 2006; Lemoine et al., 2010; Kumar et al., 2012; Billard et al., 2014) is supported by the observation that 18F-F13714 showed lower BPND values than 18F-MPPF in all brain regions. Linear regression of the binding potential values of 2 tracers in anesthetized marmosets achieved statistical significance (see Figure 6 and Results) and suggested that 5-HT1A receptors labelled by 18F-F13714 constitute about one-third of the total receptor population (Figure 6). This implies a ratio of approximately 1:2 for G-protein-coupled receptors in a high-affinity state to those in a low-affinity state. This ratio is similar to that reported using 11C-CUMI-101 and 11C-WAY100635 in anesthetized baboons, although the agonist activity of 11C-CUMI-101 may be questionable (Hendry et al., 2011; Kumar et al., 2012; Shrestha et al., 2014). By contrast, there was no correlation in binding potential values between the 2 PET probes in conscious animals, possibly reflecting changes in extracellular 5-HT levels due to vigilance state. Indeed, consciousness affects serotonergic neurotransmission in forebrain structures including the hippocampus and hypothalamus (Portas et al., 1998; Park et al., 1999; Shouse et al., 2000; Python et al., 2001). Given that human PET studies are normally performed without an anesthetic, these results strengthen the rationale for testing both agonist and antagonist PET probes when quantifying 5-HT1A receptor function.
It is surmised that agonist PET probes such as 18F-F13714 may be more suitable than 18F-MPPF for detecting changes in 5-HT1A receptor function elicited by therapeutic agents such as antidepressants or antipsychotics, many of which act as agonists at this target (see Introduction). Agonist PET probes would also be desirable when assessing the association between 5-HT1A receptors and behavioral phenotypes. The involvement of the 5-HT1A receptor in various aspects of cognition, depression, and anxiety has been amply demonstrated (Harder and Ridley, 2000; Gingrich and Hen, 2001; Albert et al., 2014; Stiedl et al., 2015), but a human database of 5-HT1A receptor binding by PET with an antagonist probe 11C-WAY100635 showed high between-subject variability and failed to find any correlations with personality variables (Rabiner et al., 2002), while several studies reported an association with anxious or aggressive traits in smaller sample sizes (Tauscher et al., 2001; Parsey et al., 2002).
Some limitations of the present study should be mentioned. Firstly, the data herein are based on a small cohort of marmosets (4 animals). Nevertheless, using the same animals in all conditions allowed us to show clear differences between the 5-HT agonist and antagonist and between conscious and isoflurane conditions. Secondly, there are no data directly showing the selectivity and agonist behavior of 18F-F13714 binding for 5-HT1A receptors in the marmoset brain, although the selectivity of F13714 has been documented in vitro (Assié et al., 2006). Thirdly, the existence of 2 distinct affinity states of 5-HT1A receptors (high affinity, “functional” state and lower affinity, nonfunctional state) have not yet been directly demonstrated in vivo and therefore warrants further evaluation (Cumming et al., 2002; Skinbjerg et al., 2012).
Conclusions
We successfully evaluated 5-HT1A receptor binding distribution in the marmoset brain using a novel biased agonist PET probe, 18F-F13714, in comparison with a well-characterized antagonist PET probe, 18F-MPPF. There were striking differences in the pattern of binding between the 2 PET probes. Whereas 18F-MPPF showed highest binding in hippocampus and amygdala, 18F-F13714 showed highest labeling in other regions, including insular and cingulate cortex, thalamus, and raphe nucleus. Marked binding was noted in caudate nucleus and putamen. This distinctive pattern of labeling correlates with results from phMRI brain imaging (Becker et al., 2016) and underpin the remarkably potent antidyskinetic activity of F13714 in rat models of Parkinson’s disease (Iderberg et al., 2015).
The present study also found marked individual- and region-specific differences under isoflurane-anesthetized vs conscious conditions of PET imaging. Notably, 18F-F13714 showed a marked difference to 18F-MPPF with respect to susceptibility to isoflurane, suggesting that the functional state of 5-HT1A receptors is strongly influenced by anesthesia.
Taken together, these findings highlight the importance of investigating the brain imaging of 5-HT1A receptors using agonist PET probes, such as 18F-F13714, and suggest that caution is necessary when interpreting results on serotonin receptor PET imaging when using anesthetized animals. Further studies are required to extend the present observations to human: if biased agonist activity at 5-HT1A receptors can also be observed under clinical conditions, new opportunities may emerge for treatment of neuropsychiatric and neurological disorders involving serotonergic systems.
Statement of Interest
A.N.-T. is an employee and stock-holder of Neurolixis Inc. In the last 3 years he has received consulting honoraria from Lundbeck, Adamed, and Theranexus.
Acknowledgments
We thank Dr T. Hayashi and Dr Y. Wada for their assistance in performing animal MRI and PET and Mr T. Ose and Ms E. Hayashinaka for technical assistance.
This work was supported in part by a grant of the Bilateral Joint Research Project from Japan Society for the Promotion of Science (JSPS), Japanese Government (to H.O.), JSPS KAKENHI 25293254 and 26118517 (to C.Y.), and the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and Development.
References
- Albert PR, Vahid-Ansari F, Luckhart C. (2014) Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié M-B, Lomenech H, Ravailhe V, Faucillon V, Newman-Tancredi A. (2006) Rapid desensitization of somatodendritic 5-HT1A receptors by chronic administration of the high-efficacy 5-HT1A agonist, F13714: a microdialysis study in the rat. Br J Pharmacol 149:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznavour N, Rbah L, Léger L, Buda C, Sastre J- P, Imhof A, Charnay Y, Zimmer L. (2006) A comparison of in vivo and in vitro neuroimaging of 5-HT 1A receptor binding sites in the cat brain. J Chem Neuroanat 31:226–232. [DOI] [PubMed] [Google Scholar]
- Aznavour N, Zimmer L. (2007) [18F]MPPF as a tool for the in vivo imaging of 5-HT1A receptors in animal and human brain. Neuropharmacology 52:695–707. [DOI] [PubMed] [Google Scholar]
- Bantick RA, Montgomery AJ, Bench CJ, Choudhry T, Malek N, McKenna PJ, Quested DJ, Deakin JFW, Grasby PM. (2004. a) A positron emission tomography study of the 5-HT1A receptor in schizophrenia and during clozapine treatment. J Psychopharmacol (Oxf) 18:346–354. [DOI] [PubMed] [Google Scholar]
- Bantick RA, Rabiner EA, Hirani E, de Vries MH, Hume SP, Grasby PM. (2004. b) Occupancy of agonist drugs at the 5-HT1A receptor. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 29:847–859. [DOI] [PubMed] [Google Scholar]
- Becker G, Bolbos R, Costes N, Redoute J, Newman-Tancredi A, Zimmer L. (2016) Selective serotonin 5-HT1A receptor biased agonists elicit distinct brain activation patterns: a pharmacoMRI study. Sci Rep 6:26633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard T, Le Bars D, Zimmer L. (2014) PET radiotracers for molecular imaging of serotonin 5-HT1A receptors. Curr Med Chem 21:70–81. [DOI] [PubMed] [Google Scholar]
- Celada P, Bortolozzi A, Artigas F. (2013) Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 27:703–716. [DOI] [PubMed] [Google Scholar]
- Costes N, Zimmer L, Reilhac A, Lavenne F, Ryvlin P, Le Bars D. (2007) Test-retest reproducibility of 18F-MPPF PET in healthy humans: a reliability study. J Nucl Med 48:1279–1288. [DOI] [PubMed] [Google Scholar]
- Cumming P, Wong DF, Gillings N, Hilton J, Scheffel U, Gjedde A. (2002) Specific binding of [(11)C]raclopride and N-[(3)H]propyl-norapomorphine to dopamine receptors in living mouse striatum: occupancy by endogenous dopamine and guanosine triphosphate-free G protein. J Cereb Blood Flow Metab 22:596–604. [DOI] [PubMed] [Google Scholar]
- Cussac D, Lauressergues E, Berrichon G, Sammut M, Newman-Tancredi A, Buritova Y. (2007) F15599, a 5-HT1A agonist that preferentially targets post-synaptic receptors: 1) activity on ERK1/2 phosphorylation and c-fos induction. In: Abstract of Society for Neurosciences 170.7/EE10, San Diego. [Google Scholar]
- de Boer SF, Newman-Tancredi A. (2016) Anti-aggressive effects of the selective high-efficacy “biased” 5-HT1A receptor agonists F15599 and F13714 in male WTG rats. Psychopharmacology (Berl) 233:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfving B, Bjørnholm B, Knudsen GM. (2003) Interference of anaesthetics with radioligand binding in neuroreceptor studies. Eur J Nucl Med Mol Imaging 30:912–915. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. (2006) Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci 9:1362–1363. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. (2001) Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 155:1–10. [DOI] [PubMed] [Google Scholar]
- Harder JA, Ridley RM. (2000) The 5-HT1A antagonist, WAY 100 635, alleviates cognitive impairments induced by dizocilpine (MK-801) in monkeys. Neuropharmacology 39:547–552. [DOI] [PubMed] [Google Scholar]
- Hendry N, Christie I, Rabiner EA, Laruelle M, Watson J. (2011) In vitro assessment of the agonist properties of the novel 5-HT1A receptor ligand, CUMI-101 (MMP), in rat brain tissue. Nucl Med Biol 38:273–277. [DOI] [PubMed] [Google Scholar]
- Iderberg H, McCreary AC, Varney MA, Cenci MA, Newman-Tancredi A. (2015) Activity of serotonin 5-HT(1A) receptor “biased agonists” in rat models of Parkinson’s disease and L-DOPA-induced dyskinesia. Neuropharmacology 93:52–67. [DOI] [PubMed] [Google Scholar]
- Johansen SL, Iceman KE, Iceman CR, Taylor BE, Harris MB. (2015) Isoflurane causes concentration-dependent inhibition of medullary raphé 5-HT neurons in situ. Auton Neurosci Basic Clin 193:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser D, Steemers B, Branchi I, Homberg JR. (2012) The reciprocal interaction between serotonin and social behaviour. Neurosci Biobehav Rev 36:786–798. [DOI] [PubMed] [Google Scholar]
- Koek W, Vacher B, Cosi C, Assié MB, Patoiseau JF, Pauwels PJ, Colpaert FC. (2001) 5-HT1A receptor activation and antidepressant-like effects: F 13714 has high efficacy and marked antidepressant potential. Eur J Pharmacol 420:103–112. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. (2010) Reward and the serotonergic system. Neuroscience 166:1023–1035. [DOI] [PubMed] [Google Scholar]
- Kumar JSD, Majo VJ, Hsiung S-C, Millak MS, Liu K-P, Tamir H, Prabhakaran J, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV. (2006) Synthesis and in vivo validation of [O-methyl-11C]2-{4-[4-(7-methoxynaphthalen-1-yl)piperazin- 1-yl]butyl}-4-methyl-2H-[1,2,4]triazine-3,5-dione: a novel 5-HT1A receptor agonist positron emission tomography ligand. J Med Chem 49:125–134. [DOI] [PubMed] [Google Scholar]
- Kumar JSD, Milak MS, Majo VJ, Prabhakaran J, Mali P, Savenkova L, Mann JJ, Parsey RV. (2012) Comparison of high and low affinity serotonin 1A receptors by PET in vivo in nonhuman primates. J Pharmacol Sci 120:254–257. [DOI] [PubMed] [Google Scholar]
- Lancelot S, Zimmer L. (2010) Small-animal positron emission tomography as a tool for neuropharmacology. Trends Pharmacol Sci 31:411–417. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien L-K, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. (2007) Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry 61:1081–1089. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Lemaire C, Ginovart N, Plenevaux A, Aerts J, Brihaye C, Hassoun W, Leviel V, Mekhsian P, Weissmann D, Pujol JF, Luxen A, Comar D. (1998) High-yield radiosynthesis and preliminary in vivo evaluation of p-[18F]MPPF, a fluoro analog of WAY-100635. Nucl Med Biol 25:343–350. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Verdurand M, Vacher B, Blanc E, Le Bars D, Newman-Tancredi A, Zimmer L. (2010) [18F]F15599, a novel 5-HT1A receptor agonist, as a radioligand for PET neuroimaging. Eur J Nucl Med Mol Imaging 37:594–605. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Becker G, Vacher B, Billard T, Lancelot S, Newman-Tancredi A, Zimmer L. (2012) Radiosynthesis and preclinical evaluation of 18F-F13714 as a fluorinated 5-HT1A receptor agonist radioligand for PET neuroimaging. J Nucl Med 53:969–976. [DOI] [PubMed] [Google Scholar]
- Li C- X, Patel S, Auerbach EJ, Zhang X. (2013) Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett 541:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Ogawa M, Okauchi T, Kawabe K, Zhang MR, Semba J, Suzuki K. (2001) In vivo binding properties of [carbonyl-11C]WAY-100635: effect of endogenous serotonin. Synapse 40:122–129. [DOI] [PubMed] [Google Scholar]
- Maurel JL, Autin J-M, Funes P, Newman-Tancredi A, Colpaert F, Vacher B. (2007) High-efficacy 5-HT1A agonists for antidepressant treatment: a renewed opportunity. J Med Chem 50:5024–5033. [DOI] [PubMed] [Google Scholar]
- Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JSD, Majo VJ, Mann JJ, Parsey RV. (2010) In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. J Nucl Med 51:1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Severance AJ, Prabhakaran J, Kumar JSD, Majo VJ, Ogden RT, Mann JJ, Parsey RV. (2011) In vivo serotonin-sensitive binding of [11C]CUMI-101: a serotonin 1A receptor agonist positron emission tomography radiotracer. J Cereb Blood Flow Metab 31:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida K, Shichino T, Koyanagi S, Himukashi S, Fukuda K. (2007) Activity of the serotonergic system during isoflurane anesthesia. Anesth Analg 104:836–839. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Bajwa AK, Wooten DW, Hillmer AT, Pan M-L, Pandey SK, Saigal N, Christian BT. (2016) Comparative assessment of (18) F-Mefway as a serotonin 5-HT1A receptor PET imaging agent across species: Rodents, nonhuman primates, and humans. J Comp Neurol 524:1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A. (2011) Biased agonism at serotonin 5-HT1A receptors: preferential postsynaptic activity for improved therapy of CNS disorders. Neuropsychiatry 1:149–164. [Google Scholar]
- Newman-Tancredi A, Martel J-C, Assié M-B, Buritova J, Lauressergues E, Cosi C, Heusler P, Bruins Slot L, Colpaert FC, Vacher B, Cussac D. (2009) Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol 156:338–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B. (2015) Serotonin: a never-ending story. Eur J Pharmacol 753:2–18. [DOI] [PubMed] [Google Scholar]
- Park SP, Lopez-Rodriguez F, Wilson CL, Maidment N, Matsumoto Y, Engel J., Jr (1999) In vivo microdialysis measures of extracellular serotonin in the rat hippocampus during sleep–wakefulness. Brain Res 833:291–296. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. (2002) Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res 954:173–182. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mikhno A, Milak M, Zanderigo F, Sullivan GM, Oquendo MA, Mann JJ. (2010) Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry 68:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passchier J, van Waarde A. (2001) Visualisation of serotonin-1A (5-HT1A) receptors in the central nervous system. Eur J Nucl Med 28:113–129. [DOI] [PubMed] [Google Scholar]
- Passchier J, van Waarde A, Vaalburg W, Willemsen AT. (2001) On the quantification of [18F]MPPF binding to 5-HT1A receptors in the human brain. J Nucl Med Off Publ Soc Nucl Med 42:1025–1031. [PubMed] [Google Scholar]
- Paxinos G, Watson C, Petrides M, Rosa M, Tokuno H. (2011) The marmoset brain in stereotaxic coordinates, 1st edition. London; Waltham, MA: Academic Press. [Google Scholar]
- Pazos A, Palacios JM. (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res 346:205–230. [DOI] [PubMed] [Google Scholar]
- Pike VW, McCarron JA, Lammertsma AA, Osman S, Hume SP, Sargent PA, Bench CJ, Cliffe IA, Fletcher A, Grasby PM. (1996) Exquisite delineation of 5-HT1A receptors in human brain with PET and [carbonyl-11C]WAY-100635. Eur J Pharmacol 301:R5–7. [DOI] [PubMed] [Google Scholar]
- Plenevaux A, Weissmann D, Aerts J, Lemaire C, Brihaye C, Degueldre C, Le Bars D, Comar D, Pujol J, Luxen A. (2000) Tissue distribution, autoradiography, and metabolism of 4-(2’-methoxyphenyl)-1-[2’ -[N-2”-pyridinyl)-p-[(18)F]fluorobenzamido]ethyl]piperazine (p-[(18)F]MPPF), a new serotonin 5-HT(1A) antagonist for positron emission tomography: An In vivo study in rats. J Neurochem 75:803–811. [DOI] [PubMed] [Google Scholar]
- Portas CM, Bjorvatn B, Fagerland S, Grønli J, Mundal V, Sørensen E, Ursin R. (1998) On-line detection of extracellular levels of serotonin in dorsal raphe nucleus and frontal cortex over the sleep/wake cycle in the freely moving rat. Neuroscience 83:807–814. [DOI] [PubMed] [Google Scholar]
- Python A, Steimer T, de Saint Hilaire Z, Mikolajewski R, Nicolaidis S. (2001) Extracellular serotonin variations during vigilance states in the preoptic area of rats: a microdialysis study. Brain Res 910:49–54. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, Bench CJ, Gunn RN, Cowen P, Grasby PM. (2002) A database of [(11)C]WAY-100635 binding to 5-HT(1A) receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. NeuroImage 15:620–632. [DOI] [PubMed] [Google Scholar]
- Seeman P, Kapur S. (2003) Anesthetics inhibit high-affinity states of dopamine D2 and other G-linked receptors. Synapse 50:35–40. [DOI] [PubMed] [Google Scholar]
- Shouse MN, Staba RJ, Saquib SF, Farber PR. (2000) Monoamines and sleep: microdialysis findings in pons and amygdala. Brain Res 860:181–189. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Hirvonen J, Hines CS, Henter ID, Svenningsson P, Pike VW, Innis RB. (2012) Serotonin-1A receptors in major depression quantified using PET: controversies, confounds, and recommendations. NeuroImage 59:3243–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha SS, Liow J- S, Lu S, Jenko K, Gladding RL, Svenningsson P, Morse CL, Zoghbi SS, Pike VW, Innis RB. (2014) (11)C-CUMI-101, a PET radioligand, behaves as a serotonin 1A receptor antagonist and also binds to α(1) adrenoceptors in brain. J Nucl Med 55:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibon I, Benkelfat C, Gravel P, Aznavour N, Costes N, Mzengeza S, Booij L, Baker G, Soucy J- P, Zimmer L, Descarries L. (2008) Decreased [18F]MPPF binding potential in the dorsal raphe nucleus after a single oral dose of fluoxetine: a positron-emission tomography study in healthy volunteers. Biol Psychiatry 63:1135–1140. [DOI] [PubMed] [Google Scholar]
- Skinbjerg M, Sibley DR, Javitch JA, Abi-Dargham A. (2012) Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochem Pharmacol 83:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O, Pappa E, Konradsson-Geuken Å, Ögren SO. (2015) The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front Pharmacol 6:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. (2001) Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry 158:1326–1328. [DOI] [PubMed] [Google Scholar]
- van Goethem NP, Schreiber R, Newman-Tancredi A, Varney M, Prickaerts J. (2015) Divergent effects of the “biased” 5-HT1 A receptor agonists F15599 and F13714 in a novel object pattern separation task. Br J Pharmacol 172:2532–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington RA, Virág L. (2006) Isoflurane decreases extracellular serotonin in the mouse hippocampus. Anesth Analg 103:92–98, table of contents. [DOI] [PubMed] [Google Scholar]
- Wooten DW, Moraino JD, Hillmer AT, Engle JW, Dejesus OJ, Murali D, Barnhart TE, Nickles RJ, Davidson RJ, Schneider ML, Mukherjee J, Christian BT. (2011) In vivo kinetics of [F-18]MEFWAY: a comparison with [C-11]WAY100635 and [F-18]MPPF in the nonhuman primate. Synapse 65:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Kawasaki A, Hayashi T, Onoe H. (2013) Linkage between the midline cortical serotonergic system and social behavior traits: positron emission tomography studies of common marmosets. Cereb Cortex 1991 23:2136–2145. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Onoe H. (2015) Positron emission tomography imaging of the social brain of common marmosets. Neurosci Res 93:82–90. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Yamanaka H, Onoe K, Kawasaki A, Nagata H, Shirakami K, Doi H, Onoe H. (2010) Mapping of serotonin transporters by positron emission tomography with [11C]DASB in conscious common marmosets: comparison with rhesus monkeys. Synapse 64:594–601. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Fournet G, Benoît J, Guillaumet G, Le Bars D. (2003) Carbon-11 labelling of 8[[3-[4-(2-[(11)C]methoxyphenyl)piperazin-1-yl]-2-hydroxypropyl]oxy]thiochroman, a presynaptic 5-HT(1A) receptor agonist, and its in vivo evaluation in anaesthetised rat and in awake cat. Nucl Med Biol 30:541–546. [DOI] [PubMed] [Google Scholar]