Abstract
Background:
Current data on antidepressant action of the N-methyl-D-aspartate receptor antagonist, (+)-MK-801, is inconsistent. This study was conducted to examine the effects of (+)-MK-801 and its less potent stereoisomer, (-)-MK-801, in the social defeat stress model of depression.
Methods:
The antidepressant effects of (+)-MK-801 (0.1mg/kg) and (-)-MK-801 (0.1mg/kg) in the social defeat stress model were examined.
Results:
In the tail suspension and forced swimming tests, both stereoisomers significantly attenuated increased immobility time in susceptible mice. In the sucrose preference test, (+)-MK-801, but not (-)-MK-801, significantly enhanced reduced sucrose consumption 2 or 4 days after a single dose. However, no antianhedonia effects were detected 7 days after a single dose of either stereoisomer.
Conclusions:
Both stereoisomers of MK-801 induced rapid antidepressant effects in the social defeat stress model, although neither produced a long-lasting effect (7 days).
Key words: antidepressant, ketamine, MK-801, NMDA receptor, stereoisomer
Introduction
Multiple lines of evidence suggest that glutamatergic neurotransmission via the N-methyl-D-aspartate (NMDA) receptor is pivotal in the pathogenesis of depression and that the NMDA receptor antagonist, ketamine, induces rapid and sustained antidepressant effects in both treatment-resistant major depression and bipolar depression (Hashimoto, 2009; Skolnick et al., 2009; Tokita et al., 2012; Hashimoto et al., 2013; Niciu et al., 2014; Ohgi et al., 2015; Gerhard et al., 2016). A number of clinical findings for ketamine have been reverse-translated into preclinical models in an effort to understand its antidepressant action. (+)-MK-801 is a high affinity, noncompetitive antagonist at the NMDA receptor (Wong et al., 1986). Although there are consistent data for the antidepressant action of ketamine in preclinical models of depression, the results on antidepressant activity for (+)-MK-801 in rodents are inconsistent. Autry et al. (2011) reported that (+)-MK-801 at 0.1mg/kg significantly decreased immobility time during the forced swimming test (FST) in control mice. Unlike ketamine, the antidepressant action of (+)-MK-801 was short lived, persisting for 3 hours after a single dose (Autry et al., 2011). Very recently, Zanos et al. (2016) reported that (+)-MK-801 (0.03 and 0.1mg/kg) failed to elicit antidepressant effects lasting 24 hours in the FST, although (+)-MK-801 showed antidepressant activity lasting for 1 hour in control mice. Thus, it seems that (+)-MK-801 produces a fast-acting but nonsustainable antidepressant response in control mice (Autry et al., 2011; Zanos et al., 2016). Unlike ketamine (10mg/kg), (+)-MK-801 (0.1mg/kg) did not reverse social avoidance induced by repeated social defeat stress, indicating a lack of antidepressant activity for (+)-MK-801 in the social defeat stress model (Zanos et al., 2016). The authors conclude that there are NMDA receptor inhibition-independent mechanisms underlying ketamine’s antidepressant effects (Zanos et al., 2016).
It has been shown that (-)-MK-801 is one-seventh as potent as its stereoisomer, (+)-MK-801 (Ki = 211.7nM vs Ki = 30.5nM, respectively), at NMDA receptors (Wong et al., 1986). The purpose of this study was to compare the rapid and sustained antidepressant effects of (+)-MK-801 and (-)-MK-801 in the social defeat stress model of depression.
Methods
Animals
Male adult C57BL/6 mice, aged 8 weeks (body weight 20–25g, Japan SLC, Inc., Hamamatsu, Japan) and male adult CD1 mice, aged 13 to 15 weeks (body weight >40g, Japan SLC, Inc) were used in the experiments. Animals were housed under controlled temperatures and 12-hour-light/-dark cycles (lights on between 7:00 am and 7:00 pm) with ad libitum food and water. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee. All efforts were made to minimize suffering.
Drugs and Drug Administration
On the day of injection, vehicle (10mL/kg, 0.9% saline), (+)-MK-801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate, 0.1mg/kg, Sigma-Aldrich, St. Louis, MO] or (-)-MK-801 [(5S,10R)-(-)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate, 0.1mg/kg, Sigma-Aldrich] was administered i.p.. The dose of (+)-MK-801 (0.1mg/kg) and (-)-MK-801 (0.1mg/kg) was used as reported previously (Autry et al., 2011; Zanos et al., 2016).
Social Defeat Procedure
The social defeat procedure was performed as previously reported (Yang et al, 2015; Zhang et al, 2015; Ren et al, 2016). Every day, the C57BL/6 mice were exposed to a different CD1 aggressor mouse for 10 minutes for a total of 10 days. When the social defeat session ended, the resident CD1 mouse and the intruder mouse were housed in one half of the cage separated by a perforated Plexiglas divider to allow visual, olfactory, and auditory contact for the remainder of the 24-hour period. At 24 hours after the last session, all mice were housed individually. On day 11, a social avoidance test was performed to identify subgroups of mice that were susceptible and unsusceptible to social defeat stress. This was accomplished by placing mice in an interaction test box (42×42cm) with an empty wire-mesh cage (10×4.5cm) located at one end. The movement of the mice was tracked for 2.5 minutes followed by 2.5 minutes in the presence of an unfamiliar aggressor confined in the wire-mesh cage. The duration of the subject’s presence in the “interaction zone” (defined as the 8-cm-wide area surrounding the wiremesh cage) was recorded by a stopwatch. The interaction ratio was calculated as time spent in an interaction zone with an aggressor/time spent in an interaction zone without an aggressor. An interaction ratio of 1 was set as the cutoff: mice with scores <1 were defined as susceptible to social defeat stress and those with scores ≥1 were defined as unsusceptible. Only susceptible mice were used in the experiments.
Behavioral Tests
Behavioral tests were performed as reported previously (Yang et al, 2015; Zhang et al, 2015; Ren et al, 2016).
Locomotion
The locomotor activity was measured by an animal movement analysis system SCANETMV-40 (MELQUEST Co., Ltd., Toyama, Japan), and the mice were placed in experimental cages (length × width × height: 560×560×330mm). The cumulative exercise was recorded for 60 minutes. Cages were cleaned between testing session.
Tail Suspension Test (TST)
A small piece of adhesive tape was placed approximately 2cm from the tip of the tail for mouse. A single hole was punched in the tape and mice were hung individually on a hook. The immobility time was recorded for 10 minutes. Mice were considered immobile only when they hung passively and completely motionless.
FST
The FST was tested by an automated forced-swim apparatus SCANETMV-40 (MELQUEST). The mice were placed individually in a cylinder (23cm diameter, 31cm high) containing 15cm of water maintained at 23°C ± 1°C. Immobility time from activity time as (total) – (active) time was calculated by the apparatus analysis software. The immobility time for mouse was recorded for 6 minutes.
Sucrose Preference Test (SPT)
Mice were exposed to water and 1% sucrose solution for 48 hours followed by 4 hours of water and food deprivation and a 1-hour exposure to 2 identical bottles, one water and the other a 1% sucrose solution. The bottles containing water and sucrose were weighed before and at the end of this period, and the sucrose preference was determined.
Statistical Analysis
The data are shown as the mean ± SEM. Analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS). Comparisons between groups were performed using the 1-way ANOVA followed by posthoc Tukey test. P< .05 was considered statistically significant.
Results
Antidepressant Effects of (+)-MK-801 and (-)-MK-801 in the Social Defeat Stress Model of Depression
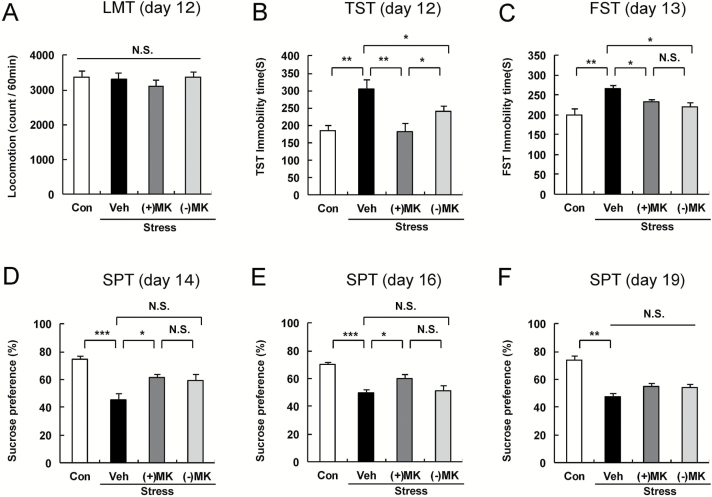
In this study, we compared the antidepressant effects of (+)-MK-801 (0.1mg/kg) and (-)-MK-801 (0.1mg/kg) in the social defeat stress model (Figure 1). Locomotion showed no difference (F3,32 = 0.047, P=.986) among the 4 groups (Figure 2A). In the TST and FST, (+)-MK-801 (0.1mg/kg) and (-)-MK-801 (0.1mg/kg) significantly attenuated the increased immobility times in susceptible mice after social defeat stress (Figure 2B-C). One-way ANOVA showed statistical significance in both the TST and FST (TST: F3,32 = 8.398, P<.001; FST: F3,32 = 6.734, P=.003) among the 4 groups (Figure 2B-C). Posthoc tests showed that antidepressant effect of (+)-MK-801 was significantly more potent than that of (-)-MK-801 in the TST, but not FST (Figure 2B-C). In the SPT, the sucrose preference of mice treated with (+)-MK-801, but not (-)-MK-801, was significantly higher (2 days after a single dose: F3,32 = 12.11, P<.001, 4 days after a single dose: F3,32 = 9.559, P<.001) than that of the vehicle-treated group (Figure 2D-E). Seven days after a single dose, there was statistically significant difference (F3,32 = 3.132, P=.035) among the 4 groups (Fig. 2F). Posthoc test showed that sucrose preference of mice treated with (+)-MK-801 or (-)-MK-801 was not different from vehicle-treated mice, indicating no antianhedonia effect 7 days after a single dose (Figure 2F). These behavioral data suggest that both (+)-MK-801 and (-)-MK-801 promote a rapid antidepressant effect in the social defeat stress model, although both stereoisomers do not show a sustained antidepressant effect.
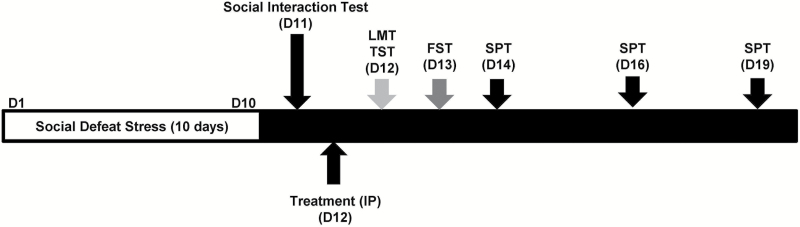
Figure 1.
The schedule of social defeat stress, drug administration, and behavioral tests. Repeated social defeat stress was performed for 10 days (day 1 to day 10). Social interaction test was performed day 11, and susceptible mice were used in the subsequent experiments. Vehicle, (+)-MK-801 (0.1mg/kg), or (-)-MK-801 (0.1mg/kg) was administered i.p. (day 12). Locomotion test (LMT) and tail suspension test (TST) were performed 4 and 6 hours after a single injection, respectively (day 12). Forced swimming test (FST) was performed 1 day after injection (day 13). A 1% sucrose preference test (SPT) was performed 2 days (day 14), 4 days (day 15), and 7 days (day 19) after a single injection.
Figure 2.
Antidepressant effects of (+)-MK-801 and (-)-MK-801 in social defeat stress model. Behavioral tests were performed as described in the Figure 1. A: LMT (day 12), B: TST (day 12), C: FST (day 13), D: SPT (day 14) E: SPT (day 16) and F: SPT (day 19). The values represent the mean ± SEM (n = 9 or 10). *P < .05, ** P < .01, *** P < .001 compared with the vehicle-treated stress group. Con: control; Veh: vehicle; (+)MK: (+)-MK-801; (-)MK: (-)-MK-801; LMT: locomotion test; TST: tail suspension test; FST: forced swimming test; SPT: 1% sucrose preference test.
Discussion
In this study, we found that both stereoisomers of MK-801 were capable of promoting rapid antidepressant effects, although neither compound produced a long-lasting response of 7 days in the social defeat stress model. By contrast, Zanos et al. (2016) reported that (+)-MK-801 did not reverse social interaction deficits induced by chronic social defeat stress, indicating a lack of antidepressant action in this model for this compound. Here, we also found that with the exception of the TST, the antidepressant effects of (-)-MK-801 were similar to those of (+)-MK-801, despite (-)-MK-801 having an affinity one-seventh that of (+)-MK-801 at the NMDA receptor. The reasons underlying the lack of stereoselectivity of MK-801 on depression-like behaviors are currently unknown. Previously, we reported that (R)-ketamine potency at the NMDA receptor (Ki = 1400nM) was greater than that of (S)-ketamine (Ki = 300nM) in rodent models of depression (Zhang et al., 2014; Yang et al., 2015; Hashimoto, 2016). Thus, it is possible that NMDA receptor inhibition may not play a major role in the long-lasting (7 days) antidepressant actions of ketamine (Hashimoto, 2014, 2016). To our knowledge, this is the first report comparing the antidepressant effects of (+)-MK-801 and (-)-MK-801 in the social defeat stress model.
It is reported that the NMDA receptor antagonists, including ketamine (1mg/kg) and (+)-MK-801 (0.05mg/kg), are able to attenuate the depression-like phenotype seen in the early-life social isolation stress model (Haj-Mirzaian et al., 2015), suggesting involvement of the NMDA receptor in the depression-like behaviors induced in this model. Furthermore, the NMDA receptor antagonists, such as (+)-MK-801 (0.3mg/kg) and CGP 37849 (5mg/kg), improved deficits in sucrose intake under the chronic mild stress model (Papp and Moryl, 1994), indicating possible antianhedonia effects for the NMDA receptor antagonists. Taken together, it is likely that (+)-MK-801 promotes antidepressant effects in rodent models of depression (Papp and Moryl, 1994; Haj-Mirzaian et al., 2015; this study), although these data are inconsistent with a recent report from Zanos et al. (2016).
It is reported that other NMDA receptor antagonists, such as Ro 25–6981, ifenprodil (GluN2B antagonist), CPP, and 7-chlorokynurenic acid (a prodrug of glycine site antagonist 7-chlorokynurenic acid) showed sustained (24 hours) antidepressant effects in rodents (Li et al., 2010; Autry et al., 2011; Miller et al., 2014; Zanos et al., 2015). These findings show that the NMDA receptor antagonists have sustained antidepressant actions in rodents. In addition, it is also reported that antidepressant effects of Ro 25–6981 were intact in mutants with global GluA1 deletion or GluN1 deletion in forebrain interneurons, but absent in mutants with global deletion of GluN2A. These findings suggest that GluN2B subtype could be a therapeutic target for depression (Kiselycznyk et al., 2015).
It has also been reported that ketamine and R-ketamine can promote sustained antidepressant effects in the chronic mild stress (Ma et al., 2013) and social defeat stress models (Yang et al., 2015; Zhang et al., 2015). Although both isomers of ketamine produced a sustained antidepressant effect 7 days after a single dose in the social defeat stress model, neither stereoisomer of MK-801 showed antidepressant effects at 7 days after a single dose. While the reasons underlying this discrepancy are unknown, it is likely that the differential actions of ketamine and MK-801 on synaptogenesis may contribute to differences in the duration of antidepressant action between these compounds. Further detailed studies investigating ketamine’s sustained antidepressant effects are needed. Given the varied affinities of MK-801 and ketamine stereoisomers for the NMDA receptor, it is unlikely that this receptor is critical for the long-lasting antidepressant effects of ketamine, although antagonism at this receptor may promote its rapid antidepressant action. To address this, further detailed studies examining the precise mechanisms driving the antidepressant effect of ketamine stereoisomers are currently underway.
The pharmacokinetic profile of (+)-MK-801 in male SD rats has been reported. The elimination half-life in rat plasma after dosing with (+)-MK-801, administered at 2mg/kg i.p. is 1.9 hours (Vezzani et al., 1989), indicating a possible rapid clearance of (+)-MK-801. The pharmacokinetic profile of (-)-MK-801 in rodents is currently unknown. In this study, we did not detect an antidepressant response 7 days after a single dose of (+)-MK-801 or (-)-MK-801. The half-life of ketamine in mouse plasma is approximately 30 minutes, suggestive of rapid clearance from the body, similar to (+)-MK-801. Additionally, the 2 stereoisomers of ketamine share similar pharmacokinetic profiles (Domino, 2010). Therefore, it is unlikely that the differential duration of antidepressant effects seen between MK-801 and ketamine are due to differences in their pharmacokinetic profiles.
In conclusion, this study shows that a single dose of either (+)-MK-801 or (-)-MK-801 induces a rapid antidepressant effect in the social defeat stress model of depression, although this response is short lived.
Statement of Interest
Dr. Hashimoto is an inventor on a filed patent application on “The use of (R)-ketamine in the treatment of psychiatric diseases” by Chiba University. Dr. Hashimoto has received research support from Dainippon Sumitomo, Mochida, Otsuka, and Taisho. The other authors declare no conflict of interest.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.H.) and the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and development, AMED (to K.H.). Dr. Bangkun Yang was supported by China Scholarship Council. Dr. Qian Ren was supported by Research Fellowship of the Japan Society for the Promotion of Science (Tokyo, Japan). Min Ma was supported by the Nurture of Creative Research Leaders in Immune System Regulation and Innovative Therapeutics Program of Chiba University.
References
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF. (2010) Taming the ketamine tiger. 1965. Anesthesiology 113:678–684. [DOI] [PubMed] [Google Scholar]
- Gerhard DM, Wohleb ES, Duman RS. (2016) Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov. Today 21:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Mirzaian A, Kordjazy N, Haj-Mirzaian A, Ostadhadi S, Ghasemi M, Amiri S, Faizi M, Dehpour A. (2015) Evidence for the involvement of NMDA receptors in the antidepressant-like effect of nicotine in mouse forced swimming and tail suspension tests. Psychopharmacology (Berl) 232:3551–3561. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2009) Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 61:105–123. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2013) Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol 100:15–29. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2014) The R-stereoisomer of ketamine as an alternative for ketamine for treatment-resistant major depression. Clin Psychopharmacol Neurosci 12:72–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. (2016) Letter to the Editor: R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol Med 46:2449–2451. [DOI] [PubMed] [Google Scholar]
- Kiselycznyk C, Jury NJ, Halladay LR, Nakazawa K, Mishina M, Sprengel R, Grant SG, Svenningsson PA. (2015) NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism. Behav Brain Res 287:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J, Gao CG, Hashimoto K. (2013) Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One 8:e56053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, Hall BJ. (2014) GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife 3:e03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA, Jr, Charney DS. (2014) Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol 54:119–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgi Y, Futamura T, Hashimoto K. (2015) Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr Mol Med 15:206–221. [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E. (1994) Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol 263:1–7. [DOI] [PubMed] [Google Scholar]
- Ren Q, Ma M, Ishima T, Morisseau C, Yang J, Wagner KM, Zhang JC, Yang C, Yao W, Dong C, Han M, Hammock BD, Hashimoto K. (2016) Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci USA 113:E1944–E1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. (2009) Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci 30:563–569. [DOI] [PubMed] [Google Scholar]
- Tokita K, Yamaji T, Hashimoto K. (2012) Roles of glutamate signaling in preclinical and/or mechanistic models of depression. Pharmacol Biochem Behav 100:688–704. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Serafini R, Stasi MA, Caccia S, Conti I, Tridico RV, Samanin R. (1989) Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J Pharmacol Exp Ther 249:278–283. [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. (1986) The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist Proc Natl Acad Sci USA 83:7104–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K. (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Piantadosi SC, Wu HQ, Pribut HJ, Dell MJ, Can A, Snodgrass HR, Zarate CA, Jr, Schwarcz R, Gould TD. (2015) The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/glycineB-site inhibition. J Pharmacol Exp Ther 355:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX., Hashimoto K. (2014) R(−)-ketamine shows greater potency and longer lasting antidepressant effects than S(+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Hashimoto K. (2015) Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 232:4325–4335. [DOI] [PubMed] [Google Scholar]