Abstract
The bacterial pathogen Neisseria meningitidis expresses two major outer-membrane porins. PorA expression is subject to phase-variation (high frequency, random, on-off switching) and both PorA and PorB are antigenically variable between strains. PorA expression is variable and not correlated with meningococcal colonisation or invasive disease, whereas all naturally-occurring strains express PorB suggesting strong selection for expression. We have generated N. meningitidis strains lacking expression of both major porins, demonstrating that they are dispensable for bacterial growth in vitro. The porAB mutant strain has an exponential growth rate similar to the parental strain, as do the single porA or porB mutants, but the porAB mutant strain does not reach the same cell density in stationary phase. Proteomic analysis suggests that the double mutant strain exhibits compensatory expression changes in proteins associated with cellular redox state, energy/nutrient metabolism, and membrane stability. On solid media, there is obvious growth impairment that is rescued by addition of blood or serum from mammalian species, particularly heme. These porin mutants are not impaired in their capacity to inhibit both staurosporine-induced apoptosis and a phorbol 12-myristate 13-acetate -induced oxidative burst in human neutrophils suggesting that the porins are not the only bacterial factors that can modulate these processes in host cells.
Keywords: Neisseria meningitidis, Porins, PorA, PorB
Introduction
The pathogenic Neisseria, N. meningitidis and N. gonorrhoeae cause meningococcal disease and gonorrhoea, respectively. They are host-adapted organisms, residing only in humans. They must acquire all necessary nutrients from the host environment. Although closely related, there are significant differences between N. meningitidis and N. gonorrhoeae. One significant difference is that N. meningitidis expresses two major porins, PorA and PorB, whereas N. gonorrhoeae expresses a single major porin, PIB (most similar to PorB). Bacterial porins are integral major outer membrane proteins that allow small molecules to transit the outer membrane into the periplasm. PorA and PorB of N. meningitidis are trimeric voltage-gated pores that mediate ion exchange between the organism and its environment1. PorA is cation selective2, and different PorA proteins differ in their cation selectivity, perhaps as the result of changes in net charge of an extracellular loop3. PorB also possesses cation and anion translocation pathways4–5, and is reported to be more selective for anions (Cl−) over cations (Na+)5
A pseudogene ortholog of the porA gene of N. meningitidis is present in N. gonorrhoeae that is not expressed as a result of accumulated mutations, although rare strains of N. gonorrhoeae contain a meningococcal porA gene6. The PorA protein of N. meningitidis displays phase-variable expression (high frequency reversible on/off switching)7. Although most N. meningitidis strains express PorA, there are occasional reports of strains lacking PorA causing disease in humans8–9. There have been no reports of natural isolates of N. meningitidis or N. gonorrhoeae lacking PorB/PIB. Efforts to produce N. gonorrhoeae lacking PorB expression have been unsuccessful10. PorB expression has been experimentally abolished in N. meningitidis2, but this strain is reported to exhibit slightly reduced growth rates in vitro, whereas PorA mutants appear not to have growth defects2. PorA-dependent uptake of sugars is size dependent, with demonstrated transport of arabinose, glucose, and n-acetylglucosamine3. This is similar to the uptake of sugars by PorB using liposomes, with translocation of (in order of fastest translocation): glucose>galactose>arabinose11. PorB also has a minor role in antibiotic influx, where loss of this protein decreases susceptibility to cefsulodin, tetracycline2, doxycycline, ciprofloxacin, cefotaxime, ceftazidime, and cephalothin12. In contrast, PorA deletion has no effect on antimicrobial susceptibility2, 12. Taken together, both pathogens require PorB/PIB expression, but PorA expression by N. meningitidis is advantageous but not essential for human colonisation or disease.
In addition to their functions as voltage-gated pores, porins have also been shown to play additional roles in Neisseria biology. PorB purified from Neisseria gonorrhoeae was first proposed to induce apoptosis when HeLa and other cells were exposed to concentrations of 10 μg/mL purified PorB13. Purified gonococcal PorB, or PorB transferred into cells during bacterial infection by unknown mechanisms, was shown to target to the mitochondria and induce apoptosis14. Conversely, other studies have shown that purified meningococcal PorB interacts with Jurkat cell mitochondria to protect cells from apoptosis15. Furthermore, following infection of HeLa cells with N. meningitidis, PorB was shown to be physically associated with mitochondria, but RmpM, another major Neisseria protein, was not16. It was inferred from these observations that the anti-apoptotic effect is PorB-dependent. The PorB-dependent anti-apoptotic effect of N. meningitidis infection on macrophage cells was further found to be dependent on the presence of nitrosative stress17. More recently, Chen and Seifert made a series of N. gonorrhoeae PorB mutant strains, demonstrating that almost all PorB amino acids are mutable18. A subset of these mutant strains was tested for their ability to protect against apoptosis, and none of the strains were altered in their capacity to inhibit apoptosis compared to the parental strain18–19.
In this study, we generated meningococcal mutant strains lacking PorA, PorB or both PorA and PorB expression. We examined how depletion of both major porins affected growth rate, undertook proteomic and genomic analysis of these strains to assess compensatory regulatory or genetic changes, and examined their capacity to inhibit the proposed, porin-dependent PMN apoptosis and the oxidative burst in vitro.
Material and Methods
Bacterial strains and growth conditions: Escherichia coli was routinely grown on LB medium at 37 °C. Antibiotics were added as necessary at 100μg/mL (ampicillin), 10 μg/mL (tetracycline, chloramphenicol). N. meningitidis was routinely grown on BHI agar with 5% defibrinated horse blood, or with added Levinthal’s supplement (BHI and defibrinated horse blood equal volume, autoclaved at 121 °C, cooled and centrifuged, and added at 10% v/v to BHI). Antibiotics were added as necessary at 5 μg/mL (tetracycline), and 3–5 μg/mL (chloramphenicol). For experiments investigating growth of mutant strains on different media, N. meningitidis was cultured on gonococcal medium base (GCB; Difco) agar plus various supplements. These supplements included Kellogg’s supplements20, IsoVitalex (Becton Dickinson), defibrinated animal blood (Lampire Biologicals), animal serum (Lampire Biologicals), 10 μM bovin hemin (Sigma), and 5% human hemoglobin (Sigma). Animal serum was also treated with either 5% Chelex 100 (BioRad), 500 ug/ml proteinase K (Invitrogen), 10 ug/ml DNase (Worthington), 10 ug/ml RNase (Sigma), or 5 mM PMSF (Sigma) before being added to the GCB. Strains were cultured on agar medium for 16 to 18 hours at 37°C with 5% CO2.
Growth curves
N. meningitidis MC58 and derivatives from 16–18 hours growth on solid media were subcultured for 3–4 hours on solid media, resuspended to OD A600= 1, diluted 1:5 into 1ml Levinthal’s supplemented BHI in triplicate into individual sealed 2 mL cuvettes, and then incubated with shaking at 37 °C. Growth was monitored at hourly intervals. The experiment was repeated twice. Exponential growth phase was defined as between 120 min and 180 min. Samples at OD A600=1 were analysed by coomassie staining and Western blotting, PorA expression was monitored with anti P1.7 mAb MN14C11.6, and PorB with anti P3.15 mAb 2-P-15 (obtained from NIBSC, UK). Samples at OD A600=1 were serially diluted and colonies counted after 30 hours growth on Levinthal’s supplemented BHI agar.
Generation of plasmid constructs
The porA gene and flanking sequence was amplified from MC58 genomic DNA using primers PorAFor1 (5′-GTTTCGGTCGTTTCCGATAA-3′) and PorAR1 (5′-TTGAAACCCTGACCCTCTG-3′) and cloned into pGEMTeasy (Promega), to generate pPorA (fig 1A). Inverse PCR on pPorA using primers DelPorA-UP 5′-TCGCATATC GGCTTCCTTTTGTAAATTTGA-3′ and DelPorA-DOWN 5′-TCC GTC GGT TTG CGC CAC AAA TTC-3′ was performed to delete the porA ORF and to insert a SmaI site. The tetracycline resistance cassette from pGemTetMB21 was cloned into the SmaI site of pPorA to generate pPorA::Tet (fig 1C). After amplification and cloning of porB from MC58 genomic DNA with PorBF1 (5′-GCCCTCCAATACCCTCCCGAGTA-3′) and PorBR1 (5′-TGCCGTCTGAAGACTTCAGACGGCCGACAGGCTTTTTGTTGATACC-3′) (see fig. 1C), a BglII site was introduced by PCR. The chloramphenicol resistance determinant was amplified from pCmGFP22 with primers including BamHI sites (Cat5′Bam: 5′-GTGGATCCACACAATCTGCCCTTT-3′, Cat3′Bam: 5′-GGATCCGCACCAATAACTGCCTTA-3′ and the cat gene was cloned into BglII digested pPorB to generate pPorB:Cat (fig 1D), and plasmid sequenced to confirm site of cat insertion. RNA was extracted from bacteria grown in Levinthal’s supplemented BHI liquid media (grown to A600 OD1) using QIAGEN RNeasy kit, prior to conversion to cDNA, and quantitative real-time PCR, using primers Nm16S_For : 5′-CGTGGGTGCGAGCGTTAATC-3′, Nm16S_Rev: 5′-CTGCCTTCGCCATCGGTATTCCT-3′, PorA_RT_For: 5′-TAAGGGGAGTGAGGATTTGGGC-3′, PorA_RT_Rev: 5′-ATCAATGGCTTGGCTGGCATCG-3′, PorB_RT_For: 5′-TCAAACCGAAGTTGCCGCTACC-3′, and PorB_RT_Rev: 5′-TTGGAGAAGTCGTATTCCGCACC-3′. Abundance of transcripts for porA and porB was calculated for strains MC58, 58ΔA and 58ΔB relative to 16sRNA.
Figure 1. Plasmids generated for deletion of porin genes.
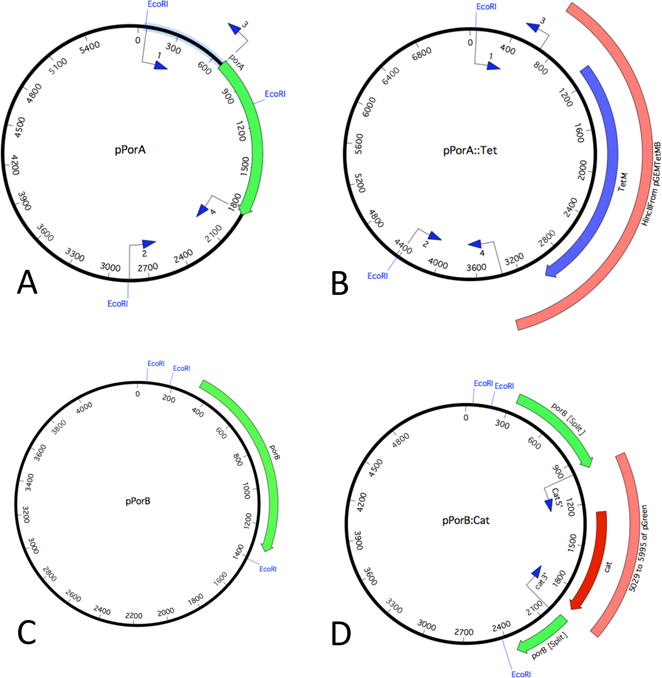
The porA and porB genes were amplified and cloned into pGEMTeasy (A,C). B: After deletion of porA by PCR, an HincII fragment (orange box) from pGem-TetMB containing tetracycline resistance (blue gene)21 was cloned into pPorA to generate pPorA::Tet. D: A fragment was amplified from pCmGFP (pGreen, orange box) containing the chloramphenicol acetyl transferase gene (red gene) and introduced into a PCR engineered site in porB, to generatepPorB:Cat.
Protein analysis
Bacteria were harvested from liquid media growth at OD A600= 1 and resuspended in 10 mM Tris pH 8.0. After separation on 4–12% Bis-Tris acrylamide gels, proteins were analysed using coomassie staining or western blotting. PorA and PorB expression was confirmed using goat polyclonal anti-PorA (sc-17396, Santa Cruz Biotechhnology), or murine mAb 8B5-5-G9 (anti-PorB, P3.15, obtained from NIBSC, UK). Alkaline-phosphatase-conjugated donkey anti-goat, and goat anti-mouse antibodies (Sigma-Aldrich) were used with colorimetric reagents to detect binding. For protein identification of bands excised from coomassie-stained gels, outer-membrane proteins were enriched using 1% sarkosyl detergent extraction, and LC-MS/MS was carried out as previously described23
Mass spectrometry (MS)
For SWATH-MS, 3–5 colonies were harvested into 300 μL 6M guanidinium chloride, 50 mM Tris-HCl pH 8, 10 mM DTT and incubated at 30 °C for 30 min. Cysteines were alkylated by addition of acrylamide to 25 mM and incubation at 30 °C for 1 hour, followed by further addition of DTT to 5 mM. Proteins were precipitated by addition of 1.2 mL 1:1 methanol:acetone, incubation at −20 °C for 16 hours, and centrifugation at 18,000 rcf for 10 min. The air-dried protein pellet was resuspended in 100 μL 50 mM Tris-HCl pH 8 with 1 μg trypsin (proteomics grade, Sigma-Aldrich) and incubated at 37 °C for 16 hours. Peptides were desalted with C18 ZipTips (Millipore). Proteins were identified by information dependent acquisition LC-ESI-MS/MS analysis performed as previously described24 using a Prominence nanoLC system (Shimadzu) and TripleTof 5600 mass spectrometer with a Nanospray III interface (AB SCIEX). Briefly, approximately 2 μg peptides were desalted on an Aglient C18 trap and then separated on a Vydac EVEREST reversed-phase C18 HPLC column at a flow rate of 1 μL/min. Separation used a gradient of 10–60% buffer B over 45 min, with buffer A (1 % acetonitrile and 0.1 % formic acid) and buffer B (80 % acetonitrile and 0.1 % formic acid). An MS TOF scan was performed from an m/z of 350–1800 for 0.5 s followed by information dependent acquisition of MS/MS of the top 20 peptides from m/z 40–1800 for 0.05 s per spectrum, with automated CE selection. Identical LC conditions were used for SWATH-MS (Sequential Window Acquisition of all THeoretical Mass Spectra)25. SWATH-MS of triplicate biological replicates was performed as previously described26 with an MS-TOF scan from an m/z of 350–1800 for 0.05 s, followed by high sensitivity information-independent acquisition with 26 m/z isolation windows with 1 m/z window overlap each for 0.1 s across an m/z range of 400–1250. Collision energy was automatically assigned by Analyst software (AB SCIEX) based on m/z window ranges. Proteins were identified essentially as described27 using ProteinPilot (AB SCIEX), searching a database with all predicted N. meningitidis MC58 proteins and common contaminants with standard settings: Sample type, identification; Cysteine alkylation, acrylamide; ID focus, biological modifications; Enzyme, Trypsin, Search effort, thorough ID. False discovery rate analysis was performed on all searches. ProteinPilot search results were used as ion libraries for SWATH analyses. The abundance of proteins was measured automatically using PeakView (AB SCIEX) with standard settings. Comparison of protein relative abundance was performed based on protein intensities26, or ion intensities using a linear mixed-effects model with the MSstats package in R28. Proteins with greater than 30 % changes in abundance and with adjusted P-values < 0.05 were considered significant.
Genome sequencing and assembly
The genome assembly was carried out using the Spades Genome Assembler in pair end mode with – careful preset29. BayesHammer was used for read (150 bp) error correction prior to assembly with default parameters. In the Spades genome assembly module, k-mer lengths of 21, 33, 55 and 77 were used to build an iterative genome assembly. Post processing of assembled contigs/scaffolds for mismatch correction was done with Burrows Wheeler Aligner (BWA tool)30. For SNP analysis, paired-end reads were mapped against the N. meningitidis MC58 reference genome (NC_003112.2) using Bowtie2 with -sensitive preset. MarkDuplicates from Picard tool set was used to remove duplicate reads and SAMtools for transforming data into mpileup format. Single nucleotide polymorphisms were then called using VarScan 2.3.630–31 with only those reported that had evidence from reads in both orientations.
Eukaryotic cell assays
The ability of N. meningitidis porin mutants to inhibit apoptosis and ROS production was determined using HL-60 cells differentiated down the granulocytic pathway as previously described [17]. HL-60 cells were differentiated in 0.7% dimethylformamide (Sigma) for a period of 5 days, after which cells were infected with N. meningitidis strains. For the apoptosis inhibition assays, differentiated HL-60 cells were infected at a multiplicity of infection (MOI) of 50 for 3 hours, followed by treatment of cells with 1 μM staurosporine for 3 hours to induce apoptosis. Cells were washed with phosphate-buffered saline (PBS) before being treated with 50 μL cell lysis buffer (BD Pharmingen) to harvest cell lysates. To measure caspase-3 activity in the lysates, 5 μL reconstituted caspase-3 substrate (N-acetyl–Asp–Glu—Val–Asp–7-amino-4-methylcoumarin [Ac-DEVD-AMC]; BD Pharmingen) at a concentration of 1 mg/ml was incubated with assay buffer and 25 μL cell lysates for 60 min at 37°C. 7-Amino-4-methylcoumarin (AMC) fluorescence was measured using an excitation wavelength of 380 nm, an emission wavelength of 440 nm, and a SpectraMax M5 plate reader (Molecular Devices). Luminol-dependent chemiluminescence (LDCL) assays were performed to examine ROS production by infected HL-60 cells [17]. Assays were carried out in a total volume of 0.2 ml PBS supplemented with 0.9 mM CaCl2, 0.5 mM MgCl2, and 7.5 mM glucose (PBSG) in black-bottom 96-well plates (Nunc). HL-60 cells were resuspended at a concentration of 4 × 107 cells/ml, and 106 cells were seeded in the presence of 100 μM luminol. The cells were then stimulated with 100 ng/ml phorbol 12-myristate 13-acetate (PMA) and/or N. meningitidis strains that had been grown overnight on GCB + 5% horse blood. Following stimulation, LDCL was measured every 2 min over a total period of 60 min at 37°C using a SpectraMax M5 plate reader (Molecular Devices).
Results
Generation and characterisation of N. meningitidis porin mutants
Mutations in each porin gene of N. meningitidis were made by allelic replacement via homologous recombination during natural DNA transformation with an inactivated allele carrying an antibiotic resistance marker. Plasmids pPorA::Tet and pPorB:Cat (Fig 1) were linearized and transformed into MC58 to generate strains 58ΔA and 58ΔB respectively. The linearized pGEMporB::Cat was subsequently transformed into 58ΔA to generate 58ΔAB. Acapsulate strains lacking expression of either PorA or PorB had been previously generated12 designated ¢9DPorA and ¢9DPorB. Strain ¢9DPorAB was generated by transformation of ¢9DPorA with pPorB:Cat, demonstrating that capsular polysaccharide is not required to generate meningococci lacking both major porins, strain ¢9DPorAB.
Sequencing of PCR products from porB regions of the transformants confirmed replacement of the wild-type porB allele with the porB::cat allele, and full genome and sequence analysis confirmed no disruption or alteration in upstream or downstream genes, nor evidence of other chromosomal rearrangements or duplications: the double porin mutant strain 58ΔAB contained no unique SNPs or INDELs (data not shown). SDS-PAGE analysis confirmed that expression of PorA, PorB, or both had been abolished in the respective strains (Fig 2). Western immunoblot (Fig 2) and MS analysis of major bands confirmed the identity of the major bands (Supplementary Figure 1).
Figure 2. Confirmation of abolition of Porin expression.
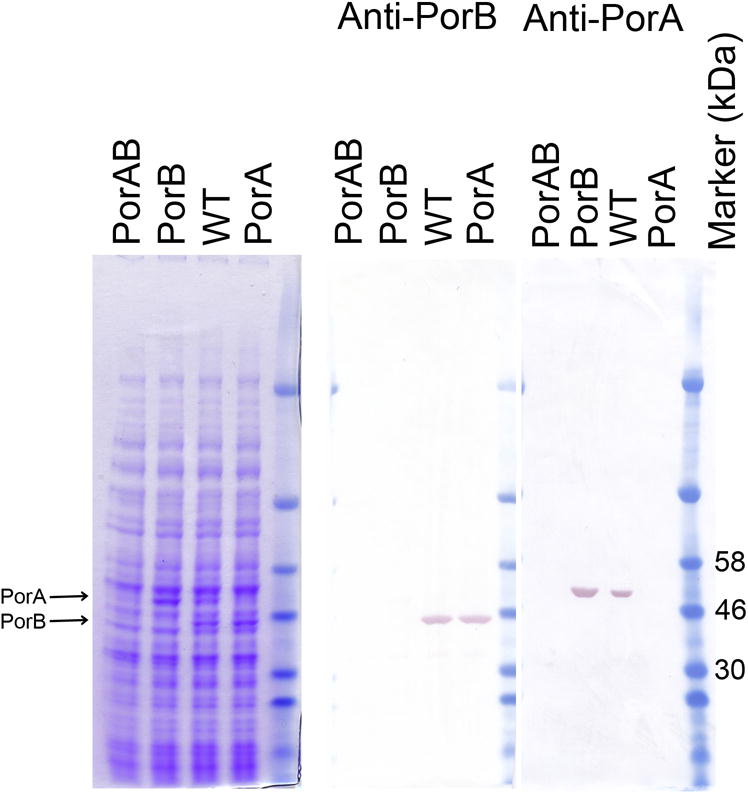
Bacteria were harvested from liquid cultures (OD A600=1) and separated electrophoretically. Gels containing total cellular protein from strains MC58, 58ΔPorA, 58ΔPorB and 58ΔPorAB (labelled WT, PorA, PorB, or PorAB respectively) were commassie stained, or western blotted and probed with antibodies specific for PorA or PorB.
SWATH-MS proteomic analysis
We were able to generate N. meningitidis cells with abolition of expression of both major porins, and with no detectable genomic changes associated with deletion of PorA and PorB. In order to determine if any compensatory protein expression changes were apparent in the double porin mutant, and to gain insights into the normal role of PorA and PorB, we performed relative quantification of the global cellular proteomes of MC58, 58ΔA, 58ΔB and 58ΔAB using SWATH-MS. Whole cell proteomics of these strains identified a total of 413 unique proteins (Supplementary Table S1), 400 of which were used in SWATH-MS analysis. SWATH-MS data was analysed considering proteins which showed significant (P<0.05) and large (greater than 30%) changes in expression. Relative quantification of cellular proteomes using SWATH-MS identified 9 proteins (2.3%) with higher abundance, and 2 proteins (0.5%) with lower abundance in 58ΔA than MC58 (Supplementary Table S2, Fig 3A). In contrast, the 58ΔB strain showed substantially more changes in global proteome relative to MC58, with 12 proteins (3%) with higher abundance, and 14 proteins (3.5%) with lower abundance (Supplementary Table S3, Fig 3B). Deletion of both porA and porB (strain 58ΔAB) showed many large changes in expression relative to the parental MC58, with 33 proteins (8.3%) with higher abundance and 34 (8.5%) with lower abundance (Supplementary Table S4, Fig 3C). Consistent with this, 2 proteins (0.5%) had higher abundance and 9 proteins (2.2%) had lower abundance in 58ΔB compared with 58ΔA (Supplementary Table S5, Fig 3D). Untargeted SWATH-MS relative quantification of global cellular proteomes confirmed the absence of PorA and PorB expression where expected, as these proteins showed the largest fold change reductions in expression in the respective mutant strains (Supplementary Tables S2, S3 and S4, Fig 3F,G). Residual apparent detection of PorA or PorB protein in respective deletion strains was due to measurement of background inherent in SWATH-MS measurements. Expression of PorA and PorB were upregulated in the reciprocal mutants, suggesting partial functional complementation (Fig 3F, G). This was not an artefact due to the abundance of PorA and PorB, as these proteins contribute only ~5% of total MS ion intensity to the global peptide intensity count. Examination of RNA levels indicated that the porA transcript was 5.5 fold more abundant in strain 58ΔB than in the parental MC58 strain (relative to 16S RNA), whereas porB transcript was similar in MC58 and 58ΔA.
Figure 3.
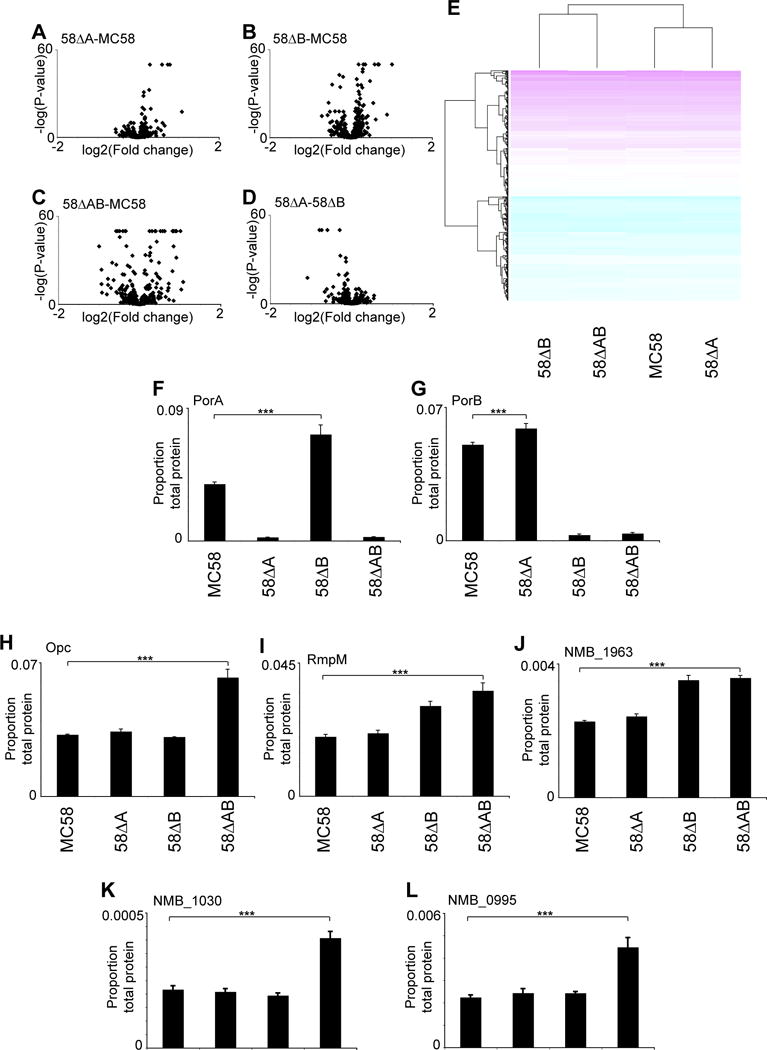
SWATH-MS relative quantification of PorA and PorB-dependent changes in global meningococcal proteome. Volcano plots for (A) 58ΔA versus MC58, (B) 58ΔB versus MC58, (C) 58ΔAB versus MC58, (D) 58ΔA versus 58ΔB. (E) Clustered heat map of protein expression in MC58, 58ΔA, 58ΔB and 58ΔAB. Protein abundance in MC58, 58ΔA, 58ΔB and 58ΔAB for (F) PorA, (G) PorB, (H) Opc, (I) RmpM, (J) NMB1963, (K) NMB1030, and (L) NMB0995. For F-L, ***, P< 0.001
Amongst other proteins that were significantly more abundant (P<10−10) in strains lacking PorB (58ΔB and 58ΔAB) relative to MC58 and 58ΔA strains, NMB1963 encodes a putative ABC transporter with similarity to a toluene tolerance family, and NMB1963 and its associated operon has been implicated in glutamate uptake in low sodium ion environments32. RmpM, a protein that forms complexes with porins, and stabilises Neisseria outer membrane proteins33–34, also was over-represented in 58ΔB and 58ΔAB relative to MC58 and 58ΔA strains. Opc, a trimeric beta-barrel protein35, was identified as being more abundant in the double porin knockout compared with all other strains. NMB0995, (macrophage infectivity potentiator-related protein) was also increased in strain 58ΔAB compared with all other strains, as was NMB1030 (Fig 3K), recently characterised as a ubiquinone binding protein36.
Determination of growth requirement of porin mutants
Growth in Levinthal’s-supplemented BHI broth was assessed. Abolition of both porins did not reduce growth rate to a large extent in this rich medium (which contains heated, clarified horse serum), with the growth rate in the exponential phase not significantly different between any of the strains when calculated by comparing slope of the curve (Fig 4). Calculation of generation times revealed small but significantly increased generation times for 58ΔB and 58ΔAB compared to MC58 and 58ΔA (supplementary table S6). At each time point the 58ΔAB strain exhibited lower OD than MC58 (P<0.05, independent t-Test), and reached lower OD at stationary phase (Fig 4). Bacterial numbers were similar in samples taken from these cultures at A600= 1.
Figure 4.
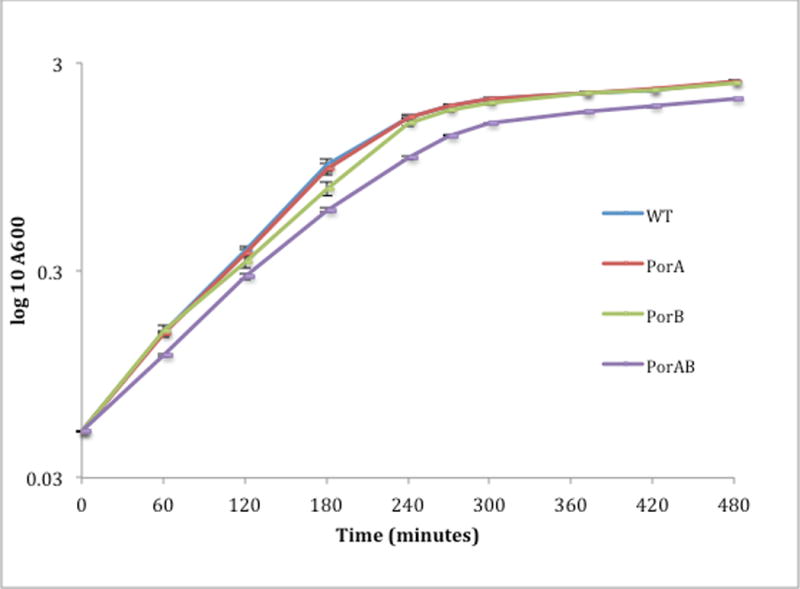
Growth curve of parental and porin deficient strains in rich media. Each data point represents the mean of three replicate cultures, ± SD.
To further characterise growth, bacterial colony growth was observed on solid media. Growth was poor on GCB plates containing Kellogg’s supplements or IsoVitalex (Fig 5), which are both supplements that are routinely used for growth of Neisseria. As NMB1030, NMB0995, and Opc are all upregulated during growth in human blood37, and these proteins were each upregulated in the 58ΔAB strain (Fig 4), we assessed growth with blood and blood derivatives. Qualitative observations indicated that strain 58ΔAB grew reasonably well on medium containing 5% equine blood or serum (Fig 5). Treatment of serum with chelex or nucleases to remove divalent cations and DNA/RNA, respectively did not reduce growth. Growth was similar on GCB when supplemented with porcine or bovine blood, and on media supplemented with human hemoglobin, porcine or fetal bovine serum (not shown), whereas colony size was not enhanced with bovine hemin (Fig 5). These observations suggest a nutrition acquisition defect for the 58ΔAB strain that is complemented by growth with blood or blood derivative.
Figure 5.
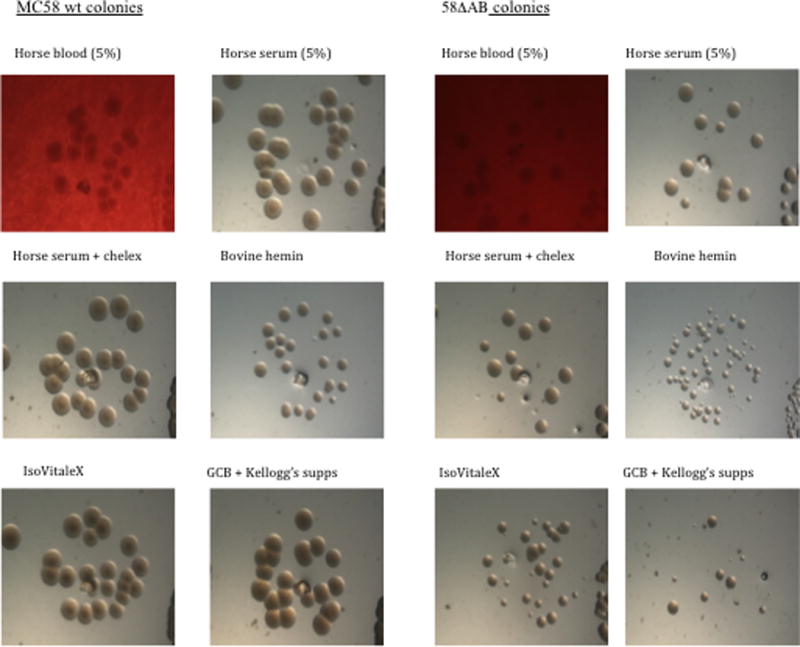
Growth of N. meningitidis 58ΔAB on solid media. Bacteria were grown overnight on solid GCB that contained supplementation as indicated, and imaged using a stereoscope.
Effect on host cells: modification of oxidative burst
Porin purified from N. gonorrhoeae has previously been demonstrated to directly affect the oxidative burst of human neutrophils38, and gonococcal infection of HL-60 cells inhibits PMA-induced oxidative burst39. We tested the ability of meningococci lacking both PorA and PorB to inhibit luminol-dependent chemiluminescence induced by PMA in differentiated HL-60 cells, using N. gonorrhoeae as a positive control (Fig 6A). The porin mutant strain lacking both PorA and PorB did not differ in its ability to inhibit PMA-induced ROS production relative to wild-type N. gonorrhoeae or N. meningitidis. Meningococcal mutants lacking either PorA or PorB singly also inhibited PMA-induced ROS production to the same extent as the wild-type strain, and none of the strains tested induced ROS production from HL-60 cells in the absence of PMA (data not shown).
Figure 6.
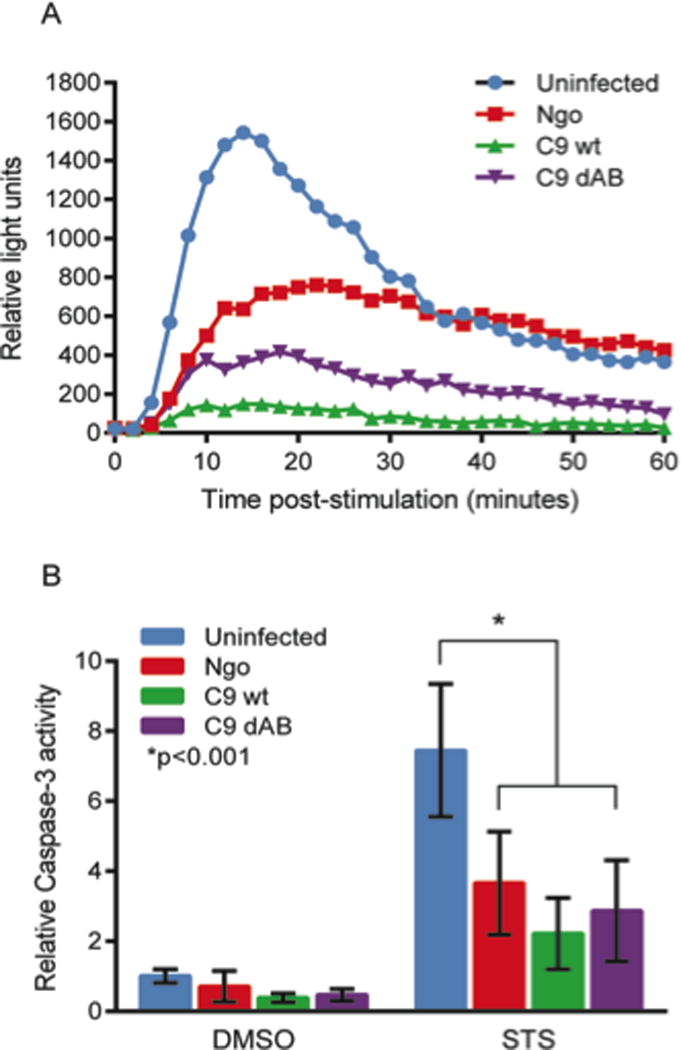
Effect of porin mutations on (A) the PMA-induced oxidative burst, or (B) staurosporine (STS)-induced caspase-3 activity in differentiated HL-60 cells. Meningococcal strains ¢9 (labelled C9 wt) and ¢9ΔPorAB (labelled C9 dAB) were compared with N. gonorrhoeae (labelled Ngo). (A) Differentiated HL-60 cells were stimulated with PMA to induce an oxidative burst and infected with N. gonorrhoeae or N. meningitidis strains. Luminol-dependent chemiluminescence was measured over a period of 60 minutes. (B) Differentiated HL-60 cells were infected with N. gonorrhoeae or N. meningitidis strains for 3 hours, then treated with either STS to induce apoptosis or DMSO as a control for a further 3 hours. Caspase-3 activity was measured using the fluorogenic substrate Ac-DEVD-AMC, and data are presented as the caspase-3 activity relative to that of uninfected, DMSO-treated controls.
Effect on host cells: inhibition of apoptosis
PorB has been cited as having a role in either inducing13–14 or protecting against apoptosis15–18, 39. We tested the effect of PorA and/or PorB mutation on the modulation of cellular apoptosis during infection. HL-60 cells were differentiated towards granulocyte phenotype for 5 days using 0.7% dimethylformamide as previously described39 and the capacity of meningococci to inhibit staurosporine-induced apoptosis was assessed in granulocyte-differentiated HL-60 cells (Fig 6B). As expected39, N. gonorrhoeae inhibited apoptosis induced by STS, indicated by decreased caspase-3 activity relative to STS-treated, uninfected cells, as did the porin-replete meningococcal strain. The acapsulate meningococcal ¢9DAB strain lacking both PorA and PorB was equally effective at inhibiting staurosporine-induced caspase-3 activity relative to both of the wild-type strains.
Discussion
This study is the first report of the generation of a N. meningitidis strain that lacks both major porins. This was unexpected as it is not possible to generate a mutant of the closely related N. gonorrhoeae that lacks its single, major porin, PIB. A major difference between the two species is the presence of a capsular polysaccharide in N. meningitidis. However, capsule is not required for survival of N. meningitidis in the absence of major porins, as we were able to generate an acapsulate strain lacking both PorA and PorB expression (Supplementary Fig 1). Western immunoblot of capsulate strains indicated the absence of the porins (Fig 2), and MS analysis of total cellular protein confirmed that the major porins were absent (Fig 3). In the single porin mutant strains, the relative proportion of the reciprocal porin was higher, suggesting compensatory upregulation (Fig 3F, G). The increased transcript level of porA in the 58ΔB strain indicates transcriptional compensation. Conversely, in the 58ΔA strain, the slight increase in PorB protein level detected by SWATH-MS was not matched by an increased porB transcript level, suggesting that PorB protein content is influenced on a post-transcriptional mechanism.
We analysed the proteome of the strains to determine whether compensatory changes occur in the absence of porins. Lack of PorA resulted in few changes whereas deletion of PorB resulted in an increased set of differentially expressed proteins. Loss of both major porins resulted in even further changes. These results are consistent with the observation that PorA is more dispensable than PorB: naturally occurring PorB-lacking strains have not been reported, and PorB has an important in vivo function, whereas clinical isolates lacking PorA are described, and PorA is naturally phase-variable.
The Neisseria protein RmpM protein was over-represented in total cellular protein, as assessed by quantitative- SWATH-MS analysis, in strains lacking either PorB alone, or both PorB and PorA. This suggests that in the absence of the major porin PorB, RmpM is upregulated and may be required to stabilise other protein complexes, or may be required for membrane stabilisation. However, it is of note that RmpM is not apparent as a major band in sarkosyl-extracted outer-membrane proteins of a strain lacking both major porins (Supplementary figure 1). RmpM forms complexes with porins and other outer membrane proteins3, 33, 40, and is associated with Omp85/β-barrel assembly machinery34, and the absence of PorA and PorB may thus reduce the association of this protein with membrane proteins in this detergent. PorA, PorB, and RmpM are reported to be upregulated after prolonged growth with human epithelial cells41, indicating an important role for these proteins in interactions with host cells. In strain 58ΔAB, Opc is more abundant. The Opc protein is phase-variably expressed through alterations in length of a poly(C) tract in its promoter42. Genome sequence analysis revealed no alterations in the promoter region of the opc gene in the 58ΔAB strain, suggesting that the increased abundance of this protein (Fig 4H) is the result of regulatory changes rather than the result of phase-variation. Indeed, opc is reported as induced during growth in serum37.
NMB1963 is part of a glutamate uptake operon32. The protein encoded by this gene was more abundant in strains 58ΔB and 58ΔAB, both of which lack PorB. N. meningitidis does not contain a glutamine synthase gene, and must therefore rely on glutamate uptake. The upregulation of NMB1963 may be an indication of compensatory upregulation to rescue general nutrient stress when major porins are absent, or may suggest that PorB has an unrecognised role in amino acid uptake. Other proteins with altered abundance indicative of changes in nutrient and/or energy metabolism included SucA (NMB0995) and SucC (NMB0959), downregulated in the PorAB mutant strain, as well as reductions of PpsA (phosphoenolpyruvate synthase, NMB0618), AdhP (alcohol dehydrogenase, propanol-preferring, NMB0546), and AcnB (aconitate hydratase, NMB1572.) Cellular processes associated with proline also appear to be affected, with downregulation of ProS (NMB0133) and PutA (NMB0401) in the double mutant, strain 58ΔAB.
As an alternate role for the upregulation of NMB1963, glutamate has several cellular fates after uptake, including synthesis of glutathione, an important molecule in maintenance of intracellular redox state. A mutant of the glutamate GltT ABC type transporter (NMB1965, in the same operon as NMB1963) has recently been reported to have significantly reduced glutathione content. This strain was attenuated in vivo, and GltT was shown to have a significant role in bacterial resistance to neutrophil oxidative burst43. Mutants of GltT were significantly more susceptible to H2O243. The protein encoded by NMB0995 was also over-represented in the total protein sample of strain 58ΔAB compared with all other strains. This protein belongs to the family of macrophage infectivity potentiator-related proteins. The exact function of these proteins are unclear, but MIP-related proteins contain conserved domains of the TIGR01926 family, some of which are known to act as peroxidases, or correlate with resistance to oxidative stress. Other proteins were upregulated in the double porins mutant strain that are indicative of an alteration of intracellular redox state: NMB1044 (fpr1), NMB0946 (encoding peroxiredoxin 2 family protein). The increased abundance of these proteins indicate that cells may be under additional oxidative stress as a result of PorAB abrogation, but this link remains to be experimentally assessed. DnaK (molecular chaperone, NMB0554) and DsbA2 were also upregulated, suggesting protein synthesis and stabilisation is affected.
In light of these obvious changes in protein expression (which were not attributable to selection of compensatory mutations in the double porin mutant), it was noted that growth on solid media was improved when blood products were added, specifically blood, serum, or human hemoglobin. Growth in a rich liquid medium containing Levinthal’s supplement (which includes horse blood) is slightly reduced in the absence of PorB, and stationary phase was reached at a lower OD for 58ΔAB. PorB mutants have previously been described as having “slightly reduced” growth rates2.
We next investigated the effect of porin deletion on interactions between N. meningitidis and granulocytes. Considerable evidence exists to suggest that porins of Neisseria, in particular PorB, can function to modulate apoptotic signaling in various eukaryotic cell types13–16, 44–46. Porin has also been shown to affect the oxidative burst in phagocytic cells38–39, 47–48, and for these reasons we investigated the ability of the N. meningitidis double mutants to inhibit both apoptosis and the oxidative burst in granulocytic HL-60 cells. Our results suggest that meningococci lacking both PorA and PorB are still able to inhibit both STS-induced apoptosis and a PMA-induced oxidative burst to the same extent as the parental strain, contrary to our expectations. Potential explanations for our lack of observed phenotype are either (i) an alternate neisserial outer membrane protein is complementing the function of PorB (and/or PorA) with respect its effects on eukaryotic cell survival and function, or (ii) PorA or PorB do not play a significant role for either apoptosis inhibition or regulation of ROS production. Future analysis will be required to test between these hypotheses.
In summary, we report that N. meningitidis lacking the major porins are viable. This differs from the related N. gonorrhoeae which is not viable without PorB. Proteomic analysis indicates that deletion of both major porins results in compensatory alterations in metabolism, specifically likely affecting intracellular redox state. Future studies will focus on further characterising the functional importance of porins in the biology of the pathogenic Neisseria, and their interaction with host cells.
Supplementary Material
Supporting Information Available:
Supplementary Fig 1: commassie gel and peptide assignments of major proteins
Supp Table S1: Proteins identified by LC-ESI-MS/MS of whole cell extracts of N. meningitidis.
Supp Table S2: Proteins with differential abundance in 58ΔA compared with MC58 as determined by SWATH-MS
Supp Table S3: Proteins with differential abundance in 58ΔB compared with MC58 as determined by SWATH-MS.
Supp Table S4: Proteins with differential abundance in 58ΔAB compared with MC58 as determined by SWATH-MS.
Supp Table 5: Proteins with differential abundance in 58ΔB compared with 58ΔA as determined by SWATH-MS.
Supp Table 6: Generation times of MC58, 58ΔA, 58ΔB, and 58ΔAB
Acknowledgments
MPJ received funding from NHMRC program grant 565526, NHMRC program grant 1071659, and ARC Discovery Project grant 13010314. HSS received funding from NIH grants R01 AI044239 and R37 AI033493 to HSS
Footnotes
This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Massari P, Ram S, Macleod H, Wetzler LM. The role of porins in neisserial pathogenesis and immunity. Trends in microbiology. 2003;11(2):87–93. doi: 10.1016/s0966-842x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 2.Tommassen J, Vermeij P, Struyve M, Benz R, Poolman JT. Isolation of Neisseria meningitidis mutants deficient in class 1 (porA) and class 3 (porB) outer membrane proteins. Infection and immunity. 1990;58(5):1355–9. doi: 10.1128/iai.58.5.1355-1359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen C, Wiese A, Reubsaet L, Dekker N, de Cock H, Seydel U, Tommassen J. Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochimica et biophysica acta. 2000;1464(2):284–98. doi: 10.1016/s0005-2736(00)00155-3. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe M, Nimigean CM, Iverson TM. Structural basis for solute transport, nucleotide regulation and immunological recognition of Neisseria meningitidis PorB. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(15):6811–6. doi: 10.1073/pnas.0912115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song J, Minetti CA, Blake MS, Colombini M. Successful recovery of the normal electrophysiological properties of PorB (class 3) porin from Neisseria meningitidis after expression in Escherichia coli and renaturation. Biochimica et biophysica acta. 1998;1370(2):289–98. doi: 10.1016/s0005-2736(97)00279-4. [DOI] [PubMed] [Google Scholar]
- 6.Whiley DM, Limnios A, Moon NJ, Gehrig N, Goire N, Hogan T, Lam A, Jacob K, Lambert SB, Nissen MD, Sloots TP. False-negative results using Neisseria gonorrhoeae porA pseudogene PCR – a clinical gonococcal isolate with an N. meningitidis porA sequence, Australia, March 2011. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2011;16(21) [PubMed] [Google Scholar]
- 7.van der Ende A, Hopman CT, Zaat S, Essink BB, Berkhout B, Dankert J. Variable expression of class 1 outer membrane protein in Neisseria meningitidis is caused by variation in the spacing between the −10 and −35 regions of the promoter. Journal of bacteriology. 1995;177(9):2475–80. doi: 10.1128/jb.177.9.2475-2480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Ende A, Hopman CT, Keijzers WC, Spanjaard L, Lodder EB, van Keulen PH, Dankert J. Outbreak of meningococcal disease caused by PorA-deficient meningococci. The Journal of infectious diseases. 2003;187(5):869–71. doi: 10.1086/367899. [DOI] [PubMed] [Google Scholar]
- 9.Crowe BA, Wall RA, Kusecek B, Neumann B, Olyhoek T, Abdillahi H, Hassan-King M, Greenwood BM, Poolman JT, Achtman M. Clonal and variable properties of Neisseria meningitidis isolated from cases and carriers during and after an epidemic in The Gambia, West Africa. The Journal of infectious diseases. 1989;159(4):686–700. doi: 10.1093/infdis/159.4.686. [DOI] [PubMed] [Google Scholar]
- 10.Bauer FJ, Rudel T, Stein M, Meyer TF. Mutagenesis of the Neisseria gonorrhoeae porin reduces invasion in epithelial cells and enhances phagocyte responsiveness. Molecular microbiology. 1999;31(3):903–13. doi: 10.1046/j.1365-2958.1999.01230.x. [DOI] [PubMed] [Google Scholar]
- 11.Rudel T, Schmid A, Benz R, Kolb HA, Lang F, Meyer TF. Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell. 1996;85(3):391–402. doi: 10.1016/s0092-8674(00)81117-4. [DOI] [PubMed] [Google Scholar]
- 12.Peak IR, Jennings CD, Jen FE, Jennings MP. Role of Neisseria meningitidis PorA and PorB expression in antimicrobial susceptibility. Antimicrobial agents and chemotherapy. 2014;58(1):614–6. doi: 10.1128/AAC.02506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller A, Gunther D, Dux F, Naumann M, Meyer TF, Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. The EMBO journal. 1999;18(2):339–52. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller A, Gunther D, Brinkmann V, Hurwitz R, Meyer TF, Rudel T. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. The EMBO journal. 2000;19(20):5332–43. doi: 10.1093/emboj/19.20.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massari P, Ho Y, Wetzler LM. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(16):9070–5. doi: 10.1073/pnas.97.16.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massari P, King CA, Ho AY, Wetzler LM. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cellular microbiology. 2003;5(2):99–109. doi: 10.1046/j.1462-5822.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- 17.Tunbridge AJ, Stevanin TM, Lee M, Marriott HM, Moir JW, Read RC, Dockrell DH. Inhibition of macrophage apoptosis by Neisseria meningitidis requires nitric oxide detoxification mechanisms. Infection and immunity. 2006;74(1):729–33. doi: 10.1128/IAI.74.1.729-733.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A, Seifert HS. Saturating mutagenesis of an essential gene: a majority of the Neisseria gonorrhoeae major outer membrane porin (PorB) is mutable. Journal of bacteriology. 2014;196(3):540–7. doi: 10.1128/JB.01073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen A, Seifert HS. Structure-function studies of the Neisseria gonorrhoeae major outer membrane porin. Infection and immunity. 2013;81(12):4383–91. doi: 10.1128/IAI.00367-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. Neisseria gonorrhoeae. I. Virulence Genetically Linked to Clonal Variation. Journal of bacteriology. 1963;85:1274–9. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren MJ, Roddam LF, Power PM, Terry TD, Jennings MP. Analysis of the role of pglI in pilin glycosylation of Neisseria meningitidis. FEMS immunology and medical microbiology. 2004;41(1):43–50. doi: 10.1016/j.femsim.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Srikhanta YN, Dowideit SJ, Edwards JL, Falsetta ML, Wu HJ, Harrison OB, Fox KL, Seib KL, Maguire TL, Wang AH, Maiden MC, Grimmond SM, Apicella MA, Jennings MP. Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS pathogens. 2009;5(4):e1000400. doi: 10.1371/journal.ppat.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blakeway LV, Power PM, Jen FE, Worboys SR, Boitano M, Clark TA, Korlach J, Bakaletz LO, Jennings MP, Peak IR, Seib KL. ModM DNA methyltransferase methylome analysis reveals a potential role for Moraxella catarrhalis phasevarions in otitis media. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-256578. [DOI] [PubMed] [Google Scholar]
- 24.Bailey UM, Jamaluddin MF, Schulz BL. Analysis of congenital disorder of glycosylation-Id in a yeast model system shows diverse site-specific under-glycosylation of glycoproteins. Journal of proteome research. 2012;11(11):5376–83. doi: 10.1021/pr300599f. [DOI] [PubMed] [Google Scholar]
- 25.Gillet LC, Navarro P, Tate S, Rost H, Selevsek N, Reiter L, Bonner R, Aebersold R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Molecular & cellular proteomics: MCP. 2012;11(6):O111 016717. doi: 10.1074/mcp.O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Bailey UM, Schulz BL. Automated measurement of site-specific N-glycosylation occupancy with SWATH-MS. Proteomics. 2015 doi: 10.1002/pmic.201400465. [DOI] [PubMed] [Google Scholar]
- 27.Bailey UM, Punyadeera C, Cooper-White JJ, Schulz BL. Analysis of the extreme diversity of salivary alpha-amylase isoforms generated by physiological proteolysis using liquid chromatography-tandem mass spectrometry. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2012;911:21–6. doi: 10.1016/j.jchromb.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Choi M, Chang CY, Clough T, Broudy D, Killeen T, MacLean B, Vitek O. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics. 2014;30(17):2524–6. doi: 10.1093/bioinformatics/btu305. [DOI] [PubMed] [Google Scholar]
- 29.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of computational biology: a journal of computational molecular cell biology. 2012;19(5):455–77. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolenko SI, Korobeynikov AI, Alekseyev MA. BayesHammer: Bayesian clustering for error correction in single-cell sequencing. BMC genomics. 2013;14(Suppl 1):S7. doi: 10.1186/1471-2164-14-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome research. 2012;22(3):568–76. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monaco C, Tala A, Spinosa MR, Progida C, De Nitto E, Gaballo A, Bruni CB, Bucci C, Alifano P. Identification of a meningococcal L-glutamate ABC transporter operon essential for growth in low-sodium environments. Infection and immunity. 2006;74(3):1725–40. doi: 10.1128/IAI.74.3.1725-1740.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzoa J, Abel A, Sanchez S, Chan H, Feavers I, Criado MT, Ferreiros CM. Analysis of outer membrane porin complexes of Neisseria meningitidis in wild-type and specific knock-out mutant strains. Proteomics. 2009;9(3):648–56. doi: 10.1002/pmic.200800486. [DOI] [PubMed] [Google Scholar]
- 34.Volokhina EB, Beckers F, Tommassen J, Bos MP. The beta-barrel outer membrane protein assembly complex of Neisseria meningitidis. Journal of bacteriology. 2009;191(22):7074–85. doi: 10.1128/JB.00737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins R, Achtman M, Ford R, Bullough P, Derrick J. Projection structure of reconstituted Opc outer membrane protein from Neisseria meningitidis. Molecular microbiology. 1999;32(1):217–9. doi: 10.1046/j.1365-2958.1999.01335.x. [DOI] [PubMed] [Google Scholar]
- 36.Donnarumma D, Golfieri G, Brier S, Castagnini M, Veggi D, Bottomley MJ, Delany I, Norais N. Neisseria meningitis GNA1030 is a ubiquinone-8 binding protein. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015 doi: 10.1096/fj.14-263954. [DOI] [PubMed] [Google Scholar]
- 37.Echenique-Rivera H, Muzzi A, Del Tordello E, Seib KL, Francois P, Rappuoli R, Pizza M, Serruto D. Transcriptome analysis of Neisseria meningitidis in human whole blood and mutagenesis studies identify virulence factors involved in blood survival. PLoS pathogens. 2011;7(5):e1002027. doi: 10.1371/journal.ppat.1002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenzen DR, Gunther D, Pandit J, Rudel T, Brandt E, Meyer TF. Neisseria gonorrhoeae porin modifies the oxidative burst of human professional phagocytes. Infection and immunity. 2000;68(11):6215–22. doi: 10.1128/iai.68.11.6215-6222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen A, Seifert HS. Neisseria gonorrhoeae-mediated inhibition of apoptotic signalling in polymorphonuclear leukocytes. Infection and immunity. 2011;79(11):4447–58. doi: 10.1128/IAI.01267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prinz T, Tommassen J. Association of iron-regulated outer membrane proteins of Neisseria meningitidis with the RmpM (class 4) protein. FEMS microbiology letters. 2000;183(1):49–53. doi: 10.1111/j.1574-6968.2000.tb08932.x. [DOI] [PubMed] [Google Scholar]
- 41.Hey A, Li MS, Hudson MJ, Langford PR, Kroll JS. Transcriptional profiling of Neisseria meningitidis interacting with human epithelial cells in a long-term in vitro colonization model. Infection and immunity. 2013;81(11):4149–59. doi: 10.1128/IAI.00397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkari J, Pandit N, Moxon ER, Achtman M. Variable expression of the Opc outer membrane protein in Neisseria meningitidis is caused by size variation of a promoter containing poly-cytidine. Molecular microbiology. 1994;13(2):207–17. doi: 10.1111/j.1365-2958.1994.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 43.Tala A, Monaco C, Nagorska K, Exley RM, Corbett A, Zychlinsky A, Alifano P, Tang CM. Glutamate utilization promotes meningococcal survival in vivo through avoidance of the neutrophil oxidative burst. Molecular microbiology. 2011;81(5):1330–42. doi: 10.1111/j.1365-2958.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller A, Rassow J, Grimm J, Machuy N, Meyer TF, Rudel T. VDAC and the bacterial porin PorB of Neisseria gonorrhoeae share mitochondrial import pathways. The EMBO journal. 2002;21(8):1916–29. doi: 10.1093/emboj/21.8.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binnicker MJ, Williams RD, Apicella MA. Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infection and immunity. 2004;72(11):6408–17. doi: 10.1128/IAI.72.11.6408-6417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozjak-Pavlovic V, Dian-Lothrop EA, Meinecke M, Kepp O, Ross K, Rajalingam K, Harsman A, Hauf E, Brinkmann V, Gunther D, Herrmann I, Hurwitz R, Rassow J, Wagner R, Rudel T. Bacterial porin disrupts mitochondrial membrane potential and sensitizes host cells to apoptosis. PLoS pathogens. 2009;5(10):e1000629. doi: 10.1371/journal.ppat.1000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjerknes R, Guttormsen HK, Solberg CO, Wetzler LM. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infection and immunity. 1995;63(1):160–7. doi: 10.1128/iai.63.1.160-167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Criss AK, Seifert HS. Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukocytes. Cellular microbiology. 2008;10(11):2257–70. doi: 10.1111/j.1462-5822.2008.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available:
Supplementary Fig 1: commassie gel and peptide assignments of major proteins
Supp Table S1: Proteins identified by LC-ESI-MS/MS of whole cell extracts of N. meningitidis.
Supp Table S2: Proteins with differential abundance in 58ΔA compared with MC58 as determined by SWATH-MS
Supp Table S3: Proteins with differential abundance in 58ΔB compared with MC58 as determined by SWATH-MS.
Supp Table S4: Proteins with differential abundance in 58ΔAB compared with MC58 as determined by SWATH-MS.
Supp Table 5: Proteins with differential abundance in 58ΔB compared with 58ΔA as determined by SWATH-MS.
Supp Table 6: Generation times of MC58, 58ΔA, 58ΔB, and 58ΔAB


