Abstract
Structural imaging studies investigating the relationship between hippocampal volume (HCV) and peripheral measures of glucocorticoids (GCs) have produced conflicting results in both normal populations and in individuals with MDD, raising the possibility of other modulating factors. In preclinical studies, dehydroepiandrosterone (DHEA) and its sulfate ester (DHEAS; together abbreviated, DHEA(S)) have been shown to antagonize the actions of GCs on the central nervous system. Therefore, considering the relationship of HCV to both of these hormones simultaneously may be important, although it has rarely been done in human populations. Using high-resolution magnetic resonance imaging (MRI), the present pilot study examined the relationship between morning serum cortisol, DHEA(S), and HCV in nineteen normal controls and eighteen unmedicated subjects with Major Depressive Disorder (MDD). Serum cortisol and DHEA(S) were not significantly correlated with HCV across all subjects (cortisol: r = −0.165, p = 0.33; DHEA: r = 0.164, p = 0.35; DHEAS: r = 0.211, p = 0.22, respectively). However, the ratios of cortisol/DHEA(S) were significantly negatively correlated with HCV in combined group (Cortisol/DHEA: r = −0.461, p = 0.005; Cortisol/DHEAS: r = −0.363, p = 0.03). Significant or near-significant correlations were found between some hormonal measurements and HCV in the MDDs alone (DHEA: r = 0.482, p = 0.059; DHEAS: r = 0.507, p = 0.045; cort/DHEA: r = −0.589, p = 0.02; cort/DHEAS: r = −0.424 p = 0.10), but not in the controls alone (DHEA: r = 0.070, p = 0.79; DHEAS: r = 0.077, p = 0.77; cort/DHEA: r = −0.427, p = 0.09; cort/DHEAS: r = −0.331, p = 0.19). However, Group (MDDs vs controls) did not have a significant effect on the relationship between cortisol, DHEA(S), and their ratios with HCV (p > 0.475 in all analyses). Although the exact relationship between serum and central steroid concentrations as well as their effects on the human hippocampus remains not known, these preliminary results suggest that the ratio of cortisol to DHEA(S), compared to serum cortisol alone, may convey additional information about “net steroid activity” with relation to HCV.
Keywords: Cortisol, Dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), Hippocampus, Hippocampal volume, Glucocorticoid, Depression, Major depressive disorder (MDD), Magnetic resonance imaging (MRI)
1. Introduction
Glucocorticoids (GCs) have well-documented effects on the hippocampus (HC) in animals and in experimental models (Sapolsky, 2000). These effects can vary according to chronicity and level of exposure (Sapolsky, 2000). Although some conflicting data exist (Leverenz et al., 1999), long-term high dose GC exposure has been generally shown to exert both direct and indirect effects on the brain, especially in the hippocampus, an area of high GC receptor density (Armanini et al., 1990; Bodnoff et al., 1995; Sapolsky, 2000; Uno et al., 1994). Some of these effects include dendritic atrophy, decreased neurogenesis, and predisposition of neurons to other further insults (Armanini et al., 1990; Newman et al., 2010; Uno et al., 1994).
Unlike the findings in animal models of chronically elevated GC exposure, the majority of which support neuroendangerment or neurotoxicity, the human findings have been more nuanced. Clinical studies have often assessed this relationship using peripheral cortisol measures and magnetic resonance imaging (MRI)-estimates of HC volume (HCV) (Colla et al., 2007; Lupien et al., 1998; O’Brien et al., 2004; Pruessner et al., 2007; Sapolsky, 2000; Starkman et al., 1999; Vythilingam et al., 2004). In patients with Cushing’s syndrome, HC atrophy is directly correlated with plasma cortisol levels, with HC volume partially recovering following treatment-induced cortisol decrease (Starkman et al., 1999). Within relatively normal limits of cortisol, however, studies have found positive, negative, or no associations between peripheral cortisol levels and HCV in normal populations and in populations with neuropsychiatric diagnoses, such as Major Depressive Disorder (MDD) (Colla et al., 2007; Lupien et al., 1998; O’Brien et al., 2004; Pruessner et al., 2007; Sapolsky, 2000; Vythilingam et al., 2004). MDD is often, but not always, reported to be associated with hypercortisolemia and with HC atrophy separately (Sapolsky, 2000). Yet, of the few studies that have examined baseline cortisol and HCV conjointly in this population, most have failed to identify a direct association (Axelson et al., 1993; Colla et al., 2007; O’Brien et al., 2004; Sapolsky, 2000; Videbech and Ravnkilde, 2004; Vythilingam et al., 2004). Additionally, no studies have evaluated cortisol in the context of other steroid hormones that might bear a relationship with HCV. Therefore, more studies are necessary to characterize this relationship between steroids and HCV in MDDs with relations to controls.
The inconsistency in published human findings between peripheral cortisol and HCV suggests that peripheral cortisol measures alone may not provide an adequate picture of GC relationships with the HC. Although the cause of this inconsistency is most likely multifactorial, it may be due in part to the assessment of cortisol in isolation of other steroid hormones. GC effects in the HC and elsewhere may be altered by the presence of other steroids, some of which may lessen GC effects and toxicity and confer resiliency to stress (Bodnoff et al., 1995; Russo et al., 2012). In particular, dehydroepiandrosterone (DHEA) and its sulfate (DHEAS; together abbreviated here as “DHEA(S)”) have been shown to have certain functional “anti-glucocorticoid” effects that may offset the deleterious effects of increased cortisol exposure (Bodnoff et al., 1995; Goodyer et al., 2001; Maninger et al., 2009; Wolkowitz et al., 2001).
DHEA(S) is the most abundant steroid hormone in humans, but its effects on the central nervous system are not as widely studied as glucocorticoids (Maninger et al., 2009). DHEA(S) has been shown, in animal models, to have multiple effects on the central nervous system, including anti-inflammation, neurogenesis, protection against NMDA excitotoxicity, oxidative stress and GC-induced cytotoxicity (Maninger et al., 2009). Outside of these pre-clinical studies, to our knowledge, only one study has directly examined the relationship between serum DHEA levels and HCV in humans. Magri et al. (2000) found serum DHEA levels to be positively correlated with HCV in the elderly and in Alzheimer patients. Several human studies have also provided some evidence of serum DHEA(S) relationships with hippocampal functionality. Using single photon emission computed tomography (SPECT), Murialdo et al. (2000) found serum DHEAS levels to be positively correlated with bilateral hippocampal perfusion in Alzheimer’s Disease patients. Similarly, several studies found positive correlations between DHEA(S) levels and memory functions, while identifying negative correlations between cortisol levels and memory function tasks (Carlson et al., 1999; Kalmijn et al., 1998). Supporting these findings of opposing effects of cortisol and DHEA(S) at an electrophysiological level, do Vale et al. (2015) found that higher salivary cortisol levels were associated with increased distractibility, whereas higher DHEA levels were associated with less distractibility and with improved working memory in women. The relationship between DHEA(S) levels in serum and DHEA(S) levels or actions in the HC is not fully known, but these studies suggest that peripheral levels of DHEA(S) in humans may be relevant markers of HC volume, perfusion and/or function, at least in certain disease states. Additional studies that concurrently assessed cortisol and DHEA(S) provide some preliminary evidence of interactions between cortisol and DHEA at the level of hippocampus (Carlson et al., 1999; do Vale et al., 2015; Kalmijn et al., 1998).
To our knowledge, no studies in humans have yet examined the interactive effect of serum cortisol and DHEA(S) levels on HCV. In the present pilot study, we examined the relationship between serum cortisol, DHEA(S), the cortisol/DHEA(S) ratios and HCV using high-resolution MRI. We hypothesized that the cortisol/DHEA(S) ratios would be inversely correlated with HCV across all subjects, and that this ratio would be more predictive of HCV than would levels of either steroid hormone alone. As the MDD population has often, but not always, been found to be associated with smaller HCVs and with greater hormonal dysfunction, such as higher serum levels of cortisol and higher cortisol/DHEA ratios (Goodyer et al., 2001; Maninger et al., 2009; Mocking et al., 2015; Videbech and Ravnkilde, 2004; Wolkowitz et al., 2009), we also explored whether these relationships would differ in unmedicated subjects with MDD compared to healthy controls.
2. Materials and methods
2.1. Subjects
Eighteen subjects with MDD and nineteen healthy controls were recruited for this pilot study. The study was approved by the UCSF Committee on Human Research, and subjects were paid for their participation. Depressed subjects were all outpatients. All subjects, MDD and controls, were recruited by fliers, bulletin board notices, Craigslist postings, newspaper ads and, in the case of MDD subjects, clinical referrals. Structured Clinical Interviews for DSM Disorders (SCID) were conducted and were clinically verified by a separate psychiatric interview with a Board-certified psychiatrist, to confirm the diagnosis of MDD for the MDD group and the absence of any Axis I disorder for the HCs. Depressed subjects with psychosis or bipolar histories were excluded, although co-morbid anxiety disorders, except PTSD, were allowed when the depressive diagnosis was considered to be the primary diagnosis. Subjects with PTSD were excluded, since PTSD may have important differences, compared to MDD, in stress hormone regulation (Yehuda, 2006). Potential subjects were also excluded if they met SCID criteria for alcohol or substance abuse within 6 months of entering the study. Subjects in both groups were medically healthy as assessed by physical examination, review of systems and screening laboratory tests, had no acute illnesses or infections, and had not had any vaccinations within 6 weeks of entering the study. All subjects were free of any psychotropic medication, including antidepressants, antipsychotics and mood stabilizers, as well as any hormone supplements, steroid-containing birth control or other interfering medications (e.g. statins) or vitamin supplements above the U.S. Recommended Daily Allowances, for a minimum of 6 weeks before entry into the study, with the exception of short-acting sedative-hypnotics, as needed, up to a maximum of 3 times per week, but none within one week prior to testing. An additional inclusion criterion for the MDD subjects was a minimum Hamilton Depression Rating Scale (17-item version, HDRS) score of 17, which has been found to maximize sensitivity and specificity for differentiating moderate to severe range depression from mild to no depression (Zimmerman et al., 2013). While HDRS was used as an inclusion criterion for the MDD group, the Quick Inventory of Depressive Symptoms was used to compare depressive symptom severity between the MDDs and the HCs, as it has greater sensitivity in the expected lower range of the controls (Rush et al., 1996). The total score for QIDS ranges from 0 to 27, with scores >11 correlating to moderate to severe depression. To characterize co-morbid anxiety symptoms in the MDD subject group, the Hamilton Anxiety Rating Scale (HAM-A) was administered (Thompson, 2015). The total score ranges from 0 to 56, with 17–25 correlating to mild and >25 correlating to moderate to severe anxiety.
2.2. Blood sampling and assays
Subjects were admitted as outpatients to the UCSF Clinical Translational Science Institute at 0800 h, having fasted (except for water) since 0000 h the night before. Urine toxicology screening and urine pregnancy test (when applicable) were performed on all subjects and confirmed negative on the morning of testing. Serum was collected into serum separator tubes for assay of cortisol, DHEA, and DHEAS by radioimmunoassay (Diagnostic Products Cooperation, DPC, Los Angeles, CA). All serum samples were assayed in the same batches. Intra-and inter-assay coefficients of variation (CV)’s for cortisol, DHEA and DHEAS were 3.5%, 3.7% and 6.2% and 4.5%, 4.3%, and 6.6%, respectively.
2.3. Magnetic resonance imaging and processing
The following sequences were acquired on a Bruker MedSpec 4T system equipped with a USA instruments eight-channel array coil: (1) For measurement of total hippocampal volume a volumetric T1-weighted gradient echo MRI (MPRAGE) TR/TE/TI = 2300/3/950 ms, 1.0 mm × 1.0 mm × 1.0 mm resolution. (2) For determination of the intracranial volume (ICV), a T2-weighted turbospin echo sequence, TR/TE: 8390/70 ms, 0.9 mm × 0.9 mm × 3 mm resolution, 54 slices. The volume of the total hippocampus was determined from the T1 image using the hippocampal masks provided by the FreeSurfer subcortical parcellation routine (Fischl et al., 2002). All maps were visually checked for accuracy by the expert rater who was blinded to the diagnosis and manually corrected by overlaying the label generated in FreeSurfer onto the T1 image in review (http://www.colin-studholme.net/software/software.html). This procedure generated a map of comparable accuracy as obtained by a manual marking scheme (ICC for manual correction of the Freesurfer labels: 0.9). The intracranial volume (ICV) was determined using the BET program (FMRIB Image Analysis Group, Oxford University, www.fmrib.ox.ac.uk/fsl). The resulting skull stripped imaged was checked by overlaying it onto the image with skull to ensure that all extracranial and skull structures were removed and all intracranial structures fully preserved. To correct for volume differences due to different head sizes, all volumes were normalized to the ICV using the following formula: normalized volume = raw volume × 1000 ccm/ICV ccm,
2.4. Statistical analyses
We first assessed the impact of age, sex, body-mass index (BMI), household income, and current tobacco use as potential confounds; we found significant effects of age and sex on DHEA and DHEAS. Consequently, all analyses including DHEA and DHEAS were controlled for age and sex. Before analyzing the data, distributions were examined for normality. Cortisol, DHEA, DHEAS, and their ratios were found to be non-normally distributed and subsequently natural log transformed or square-root transformed. Between-group comparison of the demographic variables was by independent sample t-tests, Chi square tests and independent sample Kruskal-Wallis tests. Other between-group data, when covariates were applied, were analyzed by analysis of covariance (ANCOVA). Correlations between variables were assessed by partial correlation, covarying for age and sex. Further moderator analyses were conducted to assess for group (control versus MDD) effects using linear regression, with HCV as the dependent variable and cortisol, DHEA(S), and their ratios individually being the independent variable. Age and sex were included as covariates in these analyses. All tests were 2-tailed with an alpha = 0.05. Probability values between 0.05 and 0.10 are reported as trends. 95% confidence intervals (CIs) were calculated for each correlation using VassarStats calculator (Lowry, 2015). Due to the exploratory nature of this pilot study and the small sample size, the alpha was not adjusted for multiple comparisons (three hormones and two hormone ratios), and the results are interpreted accordingly as hypothesis-generating.
3. Results
3.1. Demographics and covariates
Detailed characteristics of the depressed and healthy control subjects are presented in Table 1. The mean age of the MDD and HC subjects did not significantly differ (37.3 + 11.2 vs. 36.6 + 12.1 years [range 25–69 years], respectively), nor did the sex distribution (67% vs 63% female, respectively), ethnicity distribution or body-mass index (24.8 + 4 vs. 26.7 + 4 [kg/m2, respectively). The subject groups also did not significantly differ in current and past alcohol and nicotine consumption, marital status, highest educational level attained or self-rated socioeconomic status [100], although mean household income was significantly lower in the depressed subjects than in the controls (t = 2.59, p = 0.014, df = 32).
Table 1.
Descriptive Statistics of Study Subjects.
| All | MDDs | Controls | ||
|---|---|---|---|---|
| N | 37 | 18 | 19 | |
| Age | 36.9 ± 11.4 | 37.3 ± 11.2 | 36.5 ± 12.1 | t = −0.195; p = 0.846 |
| Sex (m/f) | 13/25 | 6/12 | 7/12 | χ2 = 0.117; p = 0.9897 |
| Education | 31% Advanced degrees 51% BA or AA 17% Some college | 29% Advanced degrees 53% BA or AA 18% Some college | 33% Advanced degrees 50% BA or AA 17% Some college | χ2 = 0.174; p = 0.917 |
| Current Tobacco Use | 18.9% | 22.2% | 15.7% | χ2 = 0.249; bp = 0.618 |
| BMI (kg/m2)a | 24.6 ± 4.1 | 24.7 ± 4.5 | 24.6 ± 3.8 | F = 0.021;p = 0.885 |
| Hippocampal Volume | 4.51 ± 0.68 | 4.41 ± 0.74 | 4.60 ± 0.61 | t = 0.842;p = 0.405 |
| Hamilton Depression | 19.5 ± 3.1 | N/A | ||
| Quick IDS | 8.2 ± 6.8 | 14.3 ± 4.2 | 2.4 ± 1.5 | t = −11.73; p = 0.00 |
| Serum cortisol (ng/mL) | 130 ± 81.2 | 127 ± 69.0 | 132 ± 93.1 | t = 0.185; p = 0.85 |
| DHEA (ng/mL)a | 125 ± 87.5 | 128 ± 81.8 | 123 ± 946 | F = 0.148, p = 0.70 |
| DHEAS (ng/mL)a | 178 ± 115 | 190 ± 126 | 167 ± 105 | F = 0.764, p = 0.39 |
| Cort/DHEAa | 1.32 ± 0.874 | 1.35 ± 0.856 | 1.30 ± 0.913 | F = 0.415, p = 0.524 |
| Cort/DHEASa | 0.927 ± 0.629 | 0.856 ± 0.426 | 0.995 ± 0.781 | F = 0.065 p = 0.801 |
Statistics were performed on normalized values, covarying for age and sex.
The mean and standard deviation for BMI, DHEA(S), and Cort/DHEA(S) shown in table are calculated from raw values.
As expected, depressed participants had higher QIDS scores than controls (t = −11.73, p < 0.0001), and the mean HAMD score in the depressed participants was 19.5 ± 3.1 (range: 17–26), reflecting a relatively moderate degree of depressive symptom severity. The MDD subjects had a lifetime mean of 2 ± 1 (range: 1–5) depressive episodes, a total of 5217 ± 4451 (range: 282–12775) days. Twelve of the 18 MDD subjects were previously treated for depression for an average of 362 ± 378 (range: 0–1025) days, although all subjects have been free of any psychotropic drugs and on a stable psychotherapy regimen for a minimum of 6 weeks prior to study entry. In evaluation of symptoms of co-morbid anxiety, the MDD subjects had mean HAM-A score of 16.5 ± 6.2 (range: 4–29), indicating a normal to mild range of anxiety on average. In our MDD population, co-morbid anxiety disorders included unspecified anxiety disorder in four subjects, obsessive-compulsive disorder in two subjects, binge eating disorder in two subjects, and social phobia disorder in one subject.
3.2. Between-Group comparisons of hormones and hippocampal volume
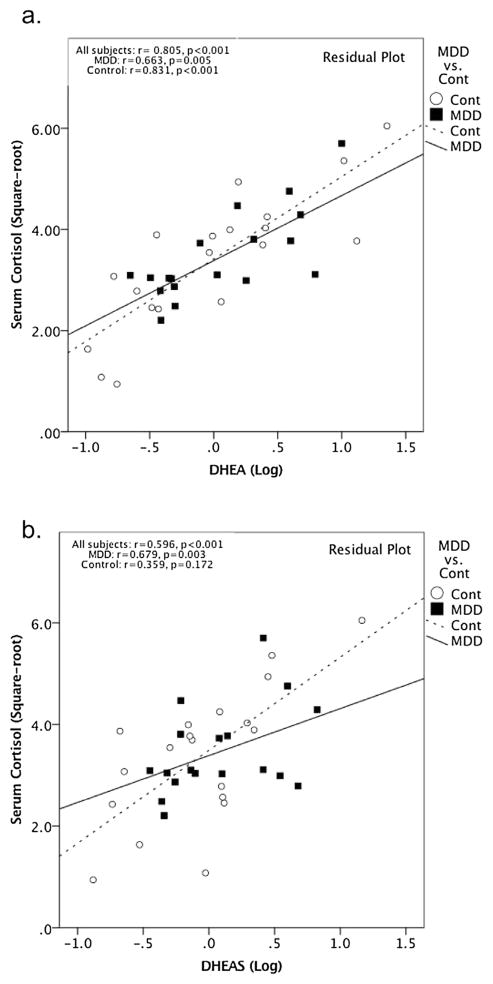
Serum cortisol, DHEA, DHEA-S and their ratios did not significantly differ between MDD subjects and HC’s (Table 1). Hippocampal volume similarly did not differ between MDD subjects and HC’s (Table 1). Interestingly, serum cortisol was positively correlated with DHEA across all subjects (r = 0.805, p < 0.001, 95% CI [0.651, 0.895], df = 33) as well as within the MDD (r = 0.664, p = 0.005, 95% CI [0.286, 0.863], df = 14) and the control group (r = 0.831, p < 0.001, 95% CI [0.606, 0.933], df = 15) separately (Fig. 1). Similarly, serum cortisol levels were positively correlated with serum DHEAS, across all subjects (r = 0.596, p < 0.001, 95% CI [0.338, 0.771], df = 33) as well as within the MDDs (r = 0.679, p = 0.003, 95% CI [0.311, 0.87], df = 14) but not within the controls (r = 0.359, p = 0.172, 95% CI [−0.113, 0.699], df = 15) (Fig. 1).
Fig. 1.
a) Serum cortisol was positively correlated with DHEA across all subjects (r = 0.805, p < 0.001, 95% CI [0.651, 0.895], df = 33) as well as within the MDD (r = 0.664, p = 0.005, 95% CI [0.286, 0.863], df = 14) and the control group (r = 0.831, p < 0.001, 95% CI [0.606, 0.933], df = 15) separately. b) Serum cortisol levels were positively correlated with serum DHEAS, after covarying for age and sex across all subjects (r = 0.596, p < 0.001, 95% CI [0.338, 0.771], df = 33) as well as within the MDDs (r = 0.679, p = 0.003, 95% CI [0.311, 0.87], df = 14) but not within the controls (r = 0.359, p = 0.172, 95% CI [−0.113, 0.699], df = 15).
3.3. Relationship between serum cortisol, DHEA(S) levels, and the Cortisol/DHEA(S) ratios, and hippocampal volume
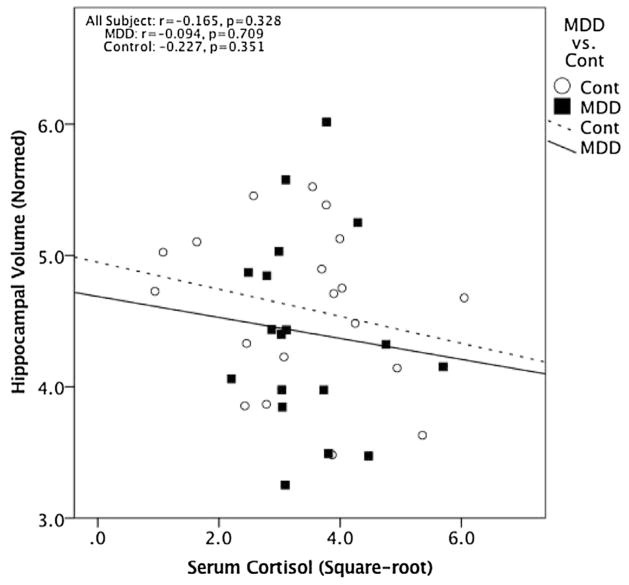
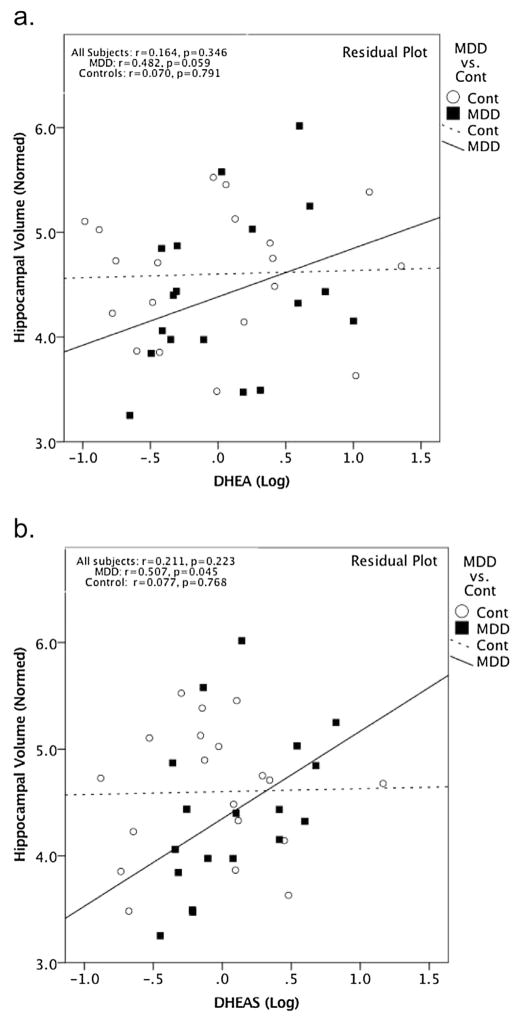
Serum cortisol levels were not significantly correlated with HCV across all subjects (r = −0.165, p = 0.328, 95% CI [−0.464, 0.268], df = 36) or within the MDD subjects (r = −0.094, p = 0.709, 95% CI [−0.537, 0.389], df = 17) or control subjects (r = −0.227, p = 0.351, 95% CI [−0.617, 0.253], df = 18) separately (Fig. 2). Serum DHEA and DHEAS levels were also not significantly correlated with HCV across all subjects (r = 0.164, p = 0.346, 95% CI [−0.168, 0.463], df = 33; r = 0.211, p = 0.223, 95% CI [−0.121, 0.500], df = 33, respectively) (Fig. 3). However, in MDD subjects alone, serum DHEAS levels significantly positively correlated with HCV (r = 0.507, p = 0.045, 95% CI [0.053, 0.787], df = 14) and serum DHEA showed a near-significant trend-level correlation with HCV (r = 0.482, p = 0.059, 95% CI [0.020, 0.774], df = 14) (Fig. 3). These relationships between HCV and DHEA or DHEAS were not seen in the HC group alone (r = 0.070, p = 0.791, 95% CI [−0.396, 0.508], df = 15; r = 0.077, p = 0.768, 95% CI [−0.39, 0.513], df = 15, respectively) (Fig. 3). Although the relationship between HCV and DHEA(S) was significant only in the MDD group, moderator analysis did not show a significant effect of group on the relationship between DHEA(S) and HCV (DHEA: t = 0.346, p = 0.732; DHEAS: t = 0.060, p = 0.953).
Fig. 2.
No significant correlation was found between cortisol (square-root transformed) and normed HCV (r = −0.165, p = 0.328, 95% CI [−0.464, 0.268], df = 36) across the overall group or within the MDD subjects (r = −0.094, p = 0.709, 95% CI [−0.537, 0.389], df = 17) or control subjects alone (r = −0.227, p = 0.351, 95% CI [−0.617, 0.253], df = 18).
Fig. 3.
All plots are residual plots, covarying for age and sex. a. DHEA showed a positive trend with normed HCV within the MDD group (r = 0.482, p = 0.059, 95% CI [0.020, 0.774], df = 14) but not within the control group (r = 0.070, p = 0.791, 95% CI [−0.396, 0.508], df = 15) or across the overall group (r = 0.164, p = 0.346, 95% CI [−0.168, 0.463], df = 33). b. DHEAS was found to be positively correlated with HCV within the MDD group (r = 0.507, p = 0.045, 95% CI [0.053, 0.787], df = 14), but not within the control group (r = 0.077, p = 0.768, 95% CI [−0.39, 0.513], df = 15, respectively) or across the overall group (r = 0.211, p = 0.223, 95% CI [−0.121, 0.500], df = 33).
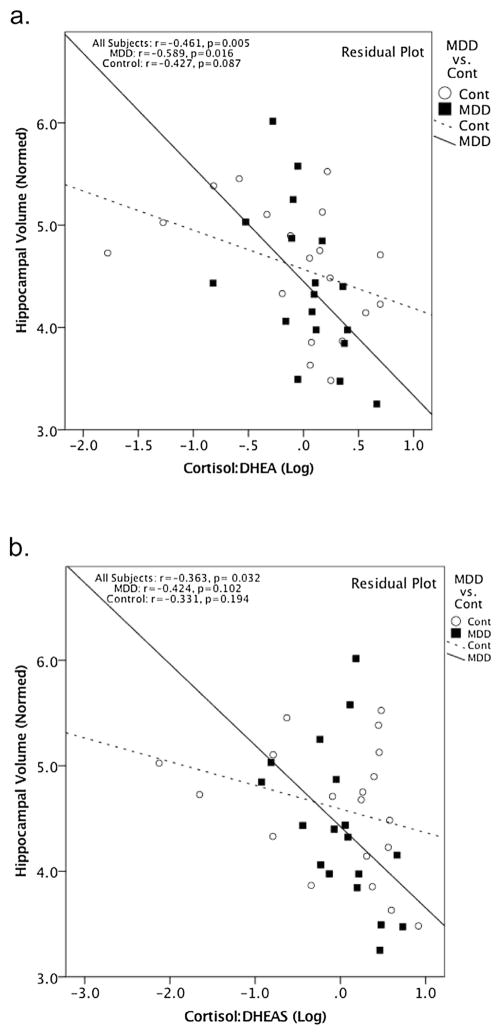
Considering these steroid hormone levels as ratios revealed stronger correlations with HCV compared to considering their levels in isolation. Specifically, the cortisol/DHEA and cortisol/DHEAS ratios were significantly negatively correlated with HCV across all subjects (Cortisol/DHEA: r = −0.461, p = 0.005, 95% CI [−0.682, −0.162], df = 33; Cortisol/DHEAS: r = −0.363, p = 0.032, 95% CI [−0.614, −0.045], df = 33, respectively) (Fig. 4). When examining the groups separately, a significant negative correlation was found between the cortisol/DHEA ratio and HCV in MDDs (r = −0.589, p = 0.016, 95% CI [−0.828, −0.169], df = 14) (Fig. 4), but this was significant only at the trend level in the controls (r = −0.427, p = 0.087, 95% CI [−0.738, 0.033], df = 15) (Fig. 4). The cortisol/DHEAS ratio did not show significant correlations with HCV in either group alone (MDD group: r = −0.424, p = 0.102, 95% CI [−0.743, 0.053], df = 14; controls: r = −0.331, p = 0.194, 95% CI [−0.682, 0.145], df = 15) (Fig. 4). Group was not found to be a significant moderator of the relationship between the ratios and HCV (Cort/DHEAS: t = −0.723, p = 0.475; Cort/DHEA: t = −0.576, p = 0.569).
Fig. 4.
All plots are residual plots, covarying for age and sex. The Cort/DHEA ratio negatively correlated with hippocampal volume across the combined group (r = −0.461, p = 0.005, 95% CI [−0.682, −0.162], df = 33). When looking at the two groups separately, a negative correlation was seen in the MDD group (r = −0.589, p = 0.016, 95% CI [−0.828, −0.169], df = 14) and a trend is seen in the control group (r = −0.427, p = 0.087, 95% CI [−0.738, 0.033], df = 15). b. Cort/DHEAS ratio negatively correlated with hippocampal volume in the combined group (r = −0.363, p = 0.032, 95% CI [−0.614, −0.045], df = 33). When looking at the two groups separately, no significant correlations were seen (MDD: r = −0.424, p = 0.102, 95% CI [−0.743, 0.053], df = 14; controls: r = −0.331, p = 0.194, 95% CI [−0.682, 0.145], df = 15).
4. Discussion
In this pilot study, we examined the relationship between HCV and serum hormone levels of morning resting cortisol and DHEA(S) in un-medicated MDD subjects and in healthy controls. Across all subjects, as well as within each subject group separately, serum cortisol level was not significantly correlated with HCV. Serum DHEA(S) levels were also not significantly correlated with HCV across all subjects or within the healthy controls, but they were positively correlated (statistically significant for DHEAS and positive trend for DHEA) with HCV in subjects with MDD. Importantly, the ratios of cortisol/DHEA(S) showed stronger relationships with HCV compared to the individual hormone levels, showing significant negative correlations with HCV across all subjects. Group did not have a significant effect on the relationship between the baseline hormone levels, their ratios and HCV. Of note, in our data, the MDD and HC groups did not significant differ in baseline hormonal levels or in HCV, which is consistent with some (Goodyer et al., 1998; Videbech and Ravnkilde, 2004; Vythilingam et al., 2004), but not other studies in MDDs (Markopoulou et al., 2009; O’Brien et al., 2004; Sapolsky, 2000). The significance of this is not known but our findings may not extend to MDDs with more pronounced baseline differences.
The lack of a significant relationship between serum cortisol levels and HCV is consistent with several prior studies but not with several others (Sapolsky, 2000; Vythilingam et al., 2004). Reasons for these between-study discrepancies are not fully known but may relate to subject-specific differences including differences in medication status, sample collection procedural and timing differences, and differences in methods of assessing HCV between studies (Sindi et al., 2014). In the present study, all subjects had single blood samples drawn in the morning under resting and fasted conditions, and all subjects were free of interfering medical conditions or medications. In particular, all subjects were free of antidepressants and any steroid-containing medication for a minimum of 6 weeks prior to participation. To control for extraneous sources of variability, age and sex were co-varied in all analyses.
The major finding in the present study is that the ratios of cortisol/DHEA(S) had qualitatively stronger and more consistent negative associations with HCV than either hormone alone. This was seen across all subjects, in MDDs alone, and, to a lesser extent, in the controls alone. This finding is consistent with the hypothesis that DHEA(S) may have protective roles in the hippocampus when interacting with cortisol activity (Karishma and Herbert, 2002; Kimonides et al., 1998; Wada et al., 2014), although, as discussed below, the relationship between serum steroid concentrations and those in the HC are largely unknown. A growing literature suggests that DHEA(S) can buffer the potentially neurotoxic effects of cortisol (Goodyer et al., 2001; Maninger et al., 2009). The ratios between cortisol and DHEA(S) may reflect a “catabolic/anabolic balance” within the body (Mocking et al., 2015). Accordingly, considering cortisol as a ratio with DHEA(S) may yield a more complete picture of “net” steroid activity, compared to either peripheral steroid hormone level alone. It is important to consider there are other systems that also interact with the HPA axis, which can further complicate steroid interactions in the hippocampus, such as the neurosteroid allopregnanolone and the enzyme 11β-hydroxysteroid dehydro-genase (11β-HSD) type I (Apostolova et al., 2005; Patchev and Almeida, 1996)
The finding of a significant or near-significant positive correlation between DHEA(S) and HCV in the MDD subjects, but not in the controls or in the combined sample, is a novel observation. However, this qualitative difference between the groups must be interpreted cautiously as no statistically significant group effect was found on the relationship between DHEA(S) and HCV in our small study. With our available data and limited power, we cannot determine whether there is in fact a meaningful difference between the groups. To the extent MDDs might have a stronger relationship between cortisol, DHEA(S), and HCV, this could relate to a heightened “neuroprotective” effect of DHEA in stress-related conditions or in conditions characterized by glutamatergic, GABAergic, serotonergic or hormonal dysregulations (Maninger et al., 2009), as might be seen in MDD (Bielau et al., 2007; Mathews et al., 2012; Naughton et al., 2000). This hypothesis may be investigated further in future studies with larger sample sizes.
Several animal studies have looked at the effects of DHEA(S) on the central nervous system (Karishma and Herbert, 2002; Kimonides et al., 1998; Wada et al., 2014). Several in vitro studies have shown that DHEA(S) may exert direct effects on dendritic growth, neurogenesis, neuronal survival via NMDA and TrkA (nerve growth factor) receptors (Compagnone and Mellon, 2000; Lazaridis et al., 2011). In vivo studies, using rats and song birds, found peripheral administration of low-concentrations of DHEA(S) to have protective properties against CNS damage from ischemia, oxidative stress, glutamate, and GCs (Karishma and Herbert, 2002; Li et al., 2001; Li et al., 2009; Newman et al., 2010). More specifically related to GC, DHEA(S) have demonstrated, in both in vitro and in vivo studies, to directly oppose the suppression of long-term potentiation and neurogenesis by GCs (Kaminska et al., 2000; Karishma and Herbert, 2002; Newman et al., 2010), although some studies report no opposing effect (Gubba et al., 2004).
The molecular mechanisms by which DHEA(S) may attenuate certain effects of cortisol are not fully known, but several suggestive leads exist (Maninger et al., 2009). In a study by Morfin and Starka (2001), DHEA was found to directly prevent the nuclear uptake of GC receptors in neurons. Kimonides et al. (1998) found that DHEA administration can directly lower corticosterone-induced nuclear translocation of stress-activated protein kinase-3, a protein implicated in neuronal death. DHEA can also alter glu-cocorticoid metabolism via its effects on 11β-HSD, type I and type II (Apostolova et al., 2005). In addition to directly interacting with GCs, DHEA(S) can indirectly buffer the excitotoxicity of the glutaminergic pathway via N-methyl-D-aspartic acid (NMDA), a pathway that is activated by GCs (Armanini et al., 1990; Kimonides et al., 1998) Taken together, these results suggest the potential for a multifaceted neuroprotective role for DHEA(S) against the physiological stress response or GCs (Maninger et al., 2009)
We note that, while DHEA and DHEAS are readily interconverted, these closely related steroids may have somewhat different biological actions (Maninger et al., 2009). In this pilot study, the relationships of DHEA and DHEAS with HCV were generally similar, but were somewhat stronger with DHEA. While there may be differences in HCV relationships with DHEA versus DHEAS, our study was not sufficiently powered to detect such differences.
Because our study is a cross-sectional study, we cannot determine the direction of causality, if any, between steroid hormones and HCV (Sapolsky et al., 1986). However, it is possible that the relationship is bi-directional. The “glucocorticoid cascade” theory succinctly incorporates both theories by stating that hippocampal atrophy results in lessened GC feedback inhibition, which, in turn, could lead to cumulative increase in GC exposure and HC damage (Sapolsky et al., 1986). To add to this theory, our data raise the possibility that the cascade involves not only GCs, but may be modulated by other steroids such as DHEA(S).
The strengths of this study include the use of well-characterized medication-free subjects and the simultaneous blood sampling for cortisol and DHEA(S). Limitations of this study include the small sample size, the use of single time point blood samples, and the aforementioned cross-sectional design of this study. Because of these limitations, the results of this study are considered exploratory, hypotheses-generating, and require replication. Additionally, the relationship between serum, brain and CSF steroid levels is unclear (Guazzo et al., 1996; Maninger et al., 2009). Therefore, any interpretations of our data that posit local actions of these steroids in the HC are largely speculative. Finally, post-hoc corrections for multiple hormone correlations were not applied due to the exploratory nature of the study.
In conclusion, HCV in our preliminary study was significantly negatively correlated with the cort/DHEA(S) ratios across all subjects, whereas it was not significantly correlated with serum individual levels of cortisol or DHEA(S) across all subjects. It is possible that serum DHEA(S) levels may be more relevant to HCV in MDDs, compared to healthy individuals. However, our study did not identify a significant difference between the groups and was not sufficiently powered to do so. Important next steps include large-scale replication studies on cortisol, DHEA(S) and HCV in normal and pathologic states. Finally, published data on correlations between cortisol and HCV might be re-considered in light of possible interactive effects of other steroids that may not have been assessed.
Acknowledgments
Role of the funding
Source: This study was funded by an NIMH R01 grant (R01 MH083784) and donations from the Tinberg Family Foundation and from the O’shaughnessy Foundation. This study was also supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. None of the granting or funding agencies had a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript.
This study was funded by an NIMH R01 grant (R01 MH083784) and donations from the Tinberg Family Foundation and from the O’shaughnessy Foundation. This study was also supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors thank the participants of the study and Kevin Delucchi, PhD, of UCSF School of Medicine for statistical analyses support.
Footnotes
Contributors
Each author materially participated in the research and/or article preparation. All authors have approved the final article.
The authors’ individual contributions are as follows:
Rowen O. Jin: Lead author, wrote first and subsequent drafts of manuscripts, performed all scientific analyses, formulated initial hypothesis to be tested, researched background literature. Sara Mason: Co-lead on project. Refined initial hypotheses, did literature searches, performed statistical analyses, contributed to writing manuscript.
Synthia H. Mellon: Co-PI on R01 grant supporting this study. Contributed expertise in DHEA to refine hypotheses and to interpret data, helped write manuscript. Elissa S. Epel: Co-PI on R01 grant supporting this study, assisted in designing study, refining hypotheses, interpreting data and writing manuscript.
Victor I. Reus: Contributed PNE expertise to refine hypotheses and interpret data, contributed to writing manuscript, provide clinical support for depressed subjects Laura Mahan: Study Coordinator, responsibility for all aspects of subject recruitment and running; assisted in data analysis and interpretation and manuscript preparation Rebecca L. Rosser: Study Coordinator, responsibility for all aspects of subject recruitment and running; assisted in data analysis and interpretation and manuscript preparation.
Christina Hough: Study Coordinator, responsibility for all aspects of subject recruitment and running; assisted in data analysis and interpretation and manuscript preparation Heather M. Burke: Study Coordinator, responsibility for all aspects of subject recruitment and running; assisted in data analysis and interpretation and manuscript preparation.
Susanne G. Mueller: Overall responsibility for neuroimaging aspect of the study, performed hippocampal voluming on all subjects, analyzed and interpreted data and contribute to manuscript preparation Owen M. Wolkowitz: Co-PI on R01 grant supporting this study. Developed overall study, developed hypotheses in tandem with Rowen Jin, clinical responsibility for subjects, performed statistical analyses and data interpretation, reviewed and helped write multiple drafts of manuscript.
Owen M. Wolkowitz: Co-PI on R01 grant supporting this study. Developed overall study, developed hypotheses in tandem with Rowen Jin, clinical responsibility for subjects, performed statistical analyses and data interpretation, reviewed and helped write multiple drafts of manuscript.
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- Apostolova G, Schweizer RA, Balazs Z, Kostadinova RM, Odermatt A. Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E957–964. doi: 10.1152/ajpendo.00442.2004. [DOI] [PubMed] [Google Scholar]
- Armanini MP, Hutchins C, Stein BA, Sapolsky RM. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990;532:7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH, Jr, Krishnan KR. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res. 1993;47:163–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- Bielau H, Steiner J, Mawrin C, Trubner K, Brisch R, Meyer-Lotz G, Brodhun M, Dobrowolny H, Baumann B, Gos T, Bernstein HG, Bogerts B. Dysregulation of GABAergic neurotransmission in mood disorders: a postmortem study. Ann N Y Acad Sci. 2007;1096:157–169. doi: 10.1196/annals.1397.081. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB, Chertkow HM. Relationships between dehydroepiandrosterone sulfate (DHEAS) and cortisol (CRT) plasma levels and everyday memory in Alzheimer’s disease patients compared to healthy controls. Horm Behav. 1999;35:254–263. doi: 10.1006/hbeh.1999.1518. [DOI] [PubMed] [Google Scholar]
- Colla M, Kronenberg G, Deuschle M, Meichel K, Hagen T, Bohrer M, Heuser I. Hippocampal volume reduction and HPA-system activity in major depression. J Psychiatr Res. 2007;41:553–560. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PM. Adrenal steroid secretion and major depression in 8- to 16-year-olds: III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychol Med. 1998;28:265–273. doi: 10.1017/s0033291797006314. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. Br J Psychiatry. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. J Clin Endocrinol Metab. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- Gubba EM, Fawcett JW, Herbert J. The effects of corticosterone and dehydroepiandrosterone on neurotrophic factor mRNA expression in primary hippocampal and astrocyte cultures. Brain Res Mol Brain Res. 2004;127:48–59. doi: 10.1016/j.molbrainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Launer LJ, Stolk RP, de Jong FH, Pols HA, Hofman A, Breteler MM, Lamberts SW. A prospective study on cortisol, dehydroepiandrosterone sulfate, and cognitive function in the elderly. J Clin Endocrinol Metab. 1998;83:3487–3492. doi: 10.1210/jcem.83.10.5164. [DOI] [PubMed] [Google Scholar]
- Kaminska M, Harris J, Gijsbers K, Dubrovsky B. Dehydroepiandrosterone sulfate (DHEAS) counteracts decremental effects of corticosterone on dentate gyrus LTP. Implications for depression. Brain Res Bull. 2000;52:229–234. doi: 10.1016/s0361-9230(00)00251-3. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis I, Charalampopoulos I, Alexaki VI, Avlonitis N, Pediaditakis I, Efstathopoulos P, Calogeropoulou T, Castanas E, Gravanis A. Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biol. 2011;9:e1001051. doi: 10.1371/journal.pbio.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Klein G, Sun P, Buchan AM. Dehydroepiandrosterone (DHEA) reduces neuronal injury in a rat model of global cerebral ischemia. Brain Res. 2001;888:263–266. doi: 10.1016/s0006-8993(00)03077-8. [DOI] [PubMed] [Google Scholar]
- Li Z, Cui S, Zhang Z, Zhou R, Ge Y, Sokabe M, Chen L. DHEA-neuroprotection and -neurotoxicity after transient cerebral ischemia in rats. J Cereb Blood Flow Metab. 2009;29:287–296. doi: 10.1038/jcbfm.2008.118. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Magri F, Terenzi F, Ricciardi T, Fioravanti M, Solerte SB, Stabile M, Balza G, Gandini C, Villa M, Ferrari E. Association between changes in adrenal secretion and cerebral morphometric correlates in normal aging and senile dementia. Dement Geriatr Cogn Disord. 2000;11:90–99. doi: 10.1159/000017220. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markopoulou K, Papadopoulos A, Juruena MF, Poon L, Pariante CM, Cleare AJ. The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology. 2009;34:19–26. doi: 10.1016/j.psyneuen.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72:1313–1333. doi: 10.2165/11633130-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocking RJ, Pellikaan CM, Lok A, Assies J, Ruhe HG, Koeter MW, Visser I, Bockting CL, Olff M, Schene AH. DHEAS and cortisol/DHEAS-ratio in recurrent depression: state, or trait predicting 10-year recurrence? Psychoneuroendocrinology. 2015;59:91–101. doi: 10.1016/j.psyneuen.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Morfin R, Starka L. Neurosteroid 7-hydroxylation products in the brain. Int Rev Neurobiol. 2001;46:79–95. doi: 10.1016/s0074-7742(01)46059-4. [DOI] [PubMed] [Google Scholar]
- Murialdo G, Nobili F, Rollero A, Gianelli MV, Copello F, Rodriguez G, Polleri A. Hippocampal perfusion and pituitary-adrenal axis in Alzheimer’s disease. Neuropsychobiology. 2000;42:51–57. doi: 10.1159/000026672. [DOI] [PubMed] [Google Scholar]
- Naughton M, Mulrooney JB, Leonard BE. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol. 2000;15:397–415. doi: 10.1002/1099-1077(200008)15:6<397::AID-HUP212>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Newman AE, MacDougall-Shackleton SA, An YS, Kriengwatana B, Soma KK. Corticosterone and dehydroepiandrosterone have opposing effects on adult neuroplasticity in the avian song control system. J Comp Neurol. 2010;518:3662–3678. doi: 10.1002/cne.22395. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Almeida OF. Gonadal steroids exert facilitating and buffering effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci. 1996;16:7077–7084. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner M, Pruessner JC, Hellhammer DH, Bruce Pike G, Lupien SJ. The associations among hippocampal volume, cortisol reactivity, and memory performance in healthy young men. Psychiatry Res. 2007;155:1–10. doi: 10.1016/j.pscychresns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sindi S, Fiocco AJ, Juster RP, Lord C, Pruessner J, Lupien SJ. Now you see it, now you don’t: testing environments modulate the association between hippocampal volume and cortisol levels in young and older adults. Hippocampus. 2014;24:1623–1632. doi: 10.1002/hipo.22341. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Thompson E. Hamilton rating scale for anxiety (HAM-A) Occup Med (Lond) 2015;65:601. doi: 10.1093/occmed/kqv054. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wada H, Newman AE, Hall ZJ, Soma KK, MacDougall-Shackleton SA. Effects of corticosterone and DHEA on doublecortin immunoreactivity in the song control system and hippocampus of adult song sparrows. Dev Neurobiol. 2014;74:52–62. doi: 10.1002/dneu.22132. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2:115–143. doi: 10.3109/15622970109026799. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids. Mood memory, and mechanisms. Ann N Y Acad Sci. 2009;1179:19–40. doi: 10.1111/j.1749-6632.2009.04980.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N Y Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the hamilton depression rating scale. J Affect Disord. 2013;150:384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- do Vale S, Selinger L, Martins JM, Bicho M, do Carmo I, Escera C. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone-sulfate (DHEAS) and emotional processing—a behavioral and electrophysiological approach. Horm Behav. 2015;73:94–103. doi: 10.1016/j.yhbeh.2015.06.005. [DOI] [PubMed] [Google Scholar]