Abstract
The aim of this study is to measure GABA levels of perisylvian cortices in schizophrenia and bipolar disorder patients, using proton magnetic resonance spectroscopy (1H-MRS). Patients with schizophrenia (n=25), bipolar I disorder (BD-I; n=28) and bipolar II disorder (BD-II; n=20) were compared with healthy controls (n=30). 1H-MRS data was acquired using a Siemens 3 Tesla whole body scanner to quantify right and left perisylvian structures’ (including superior temporal lobes) GABA levels. Right perisylvian GABA values differed significantly between groups [χ2=9.62, df: 3, p = 0.022]. GABA levels were significantly higher in the schizophrenia group compared with the healthy control group (p=0.002). Furthermore, Chlorpromazine equivalent doses of antipsychotics correlated with right hemisphere GABA levels (r2=0.68, p=0.006, n=33). GABA levels are elevated in the right hemisphere in patients with schizophrenia in comparison to bipolar disorder and healthy controls. The balance between excitatory and inhibitory controls over the cortical circuits may have direct relationship with GABAergic functions in auditory cortices. In addition, GABA levels may be altered by brain regions of interest, psychotropic medications, and clinical stage in schizophrenia and bipolar disorder.
Keywords: Schizophrenia, Bipolar Disorder, GABA, Magnetic Resonance Spectroscopy, Auditory Cortex
1. Introduction
Several lines of evidence have converged that as an inhibitory neurotransmitter, Gamma-Amino Butyric Acid (GABA) neurotransmission serves for network integrity by facilitating neural synchronization in the brain [1]. Postmortem studies have shown abnormalities in GABAergic cells [2-4] and these findings suggested that disturbances in the early phases of brain development may lead to abnormalities of GABAergic neurotransmission possibly causing dysregulation in the inhibitory and excitatory neurotransmission in cortical circuitries [2]. Disturbed GABAergic neurotransmission may lead to abnormalities in integrative brain functions and cognitive dysfunction [5].
Irregularities in GABA neurotransmission have critical roles in the pathophysiology of schizophrenia and bipolar disorder [2]. Altered RNA, protein and neurochemical markers of interneurons [6], decreased number [7] and disturbed maturation of GABAergic cells [8] have indicated GABAergic dysfunction in schizophrenia and bipolar disorder. Measurements of GABA levels using proton magnetic resonance spectroscopy (1H-MRS) have reported altered GABA levels in schizophrenia [9,10] and bipolar disorder [11-13]. However, the findings are inconsistent possibly due to a number of reasons including different MRS methods, variability between brain regions of interest, medication effects and clinical course [10]. Most studies have focused on frontal, prefrontal, parietal or occipital cortices, medicated patients and clinically remitted patients, and all of these factors, including brain regions of interest, psychotropic medications, and clinical stage, may have significant effects on GABA levels.
The auditory cortices have a long and delicate developmental trajectory [14], which is vulnerable to the pathophysiology of schizophrenia and bipolar disorder [15]. Since auditory hallucinations are one of the most frequent symptoms of schizophrenia and abnormalities of the auditory cortices are associated with hallucinations [15], auditory cortices are among the most relevant brain regions in schizophrenia. In a recent 1H-MRS study, we have detected metabolic abnormalities within the left hemisphere superior temporal lobe in both schizophrenia and bipolar disorder [16]. Neural synchronization deficits with auditory tasks may indicate GABAergic abnormalities in auditory cortices in bipolar disorder and schizophrenia [17]. In addition, a recent 1H-MRS study report decreased GABA levels in the left perisylvian cortices in autism, this is consistent with the theory of excitatory-inhibitory balance dysregulation in autism spectrum disorders [18].
In this study, we aimed to investigate GABA levels within the auditory belt and parabelt regions located around the Sylvian (Lateral) Fissure, which host primary and association auditory cortices. To our knowledge, this is the first study that measure GABA levels at the perisylvian structures in schizophrenia and bipolar disorder. Since there are abnormalities in excitatory neurotransmission and GABAergic cells [2-13], we hypothesized that GABA levels might be altered in schizophrenia and bipolar disorder.
2. Materials and methods
2.1. Participants
The local Ethical Committee of Ankara Yıldırım Beyazıt University Medical School has approved the study. All participants provided written consent after the study procedures were fully explained. Remitted patients with schizophrenia (n=25), bipolar I disorder (BD-I) (n=28), bipolar II disorder (BD-II) (n=20) and a healthy control group (HC) (n=30) were enrolled. Socio-demographic features are presented in Table 1. Exclusion criteria were history of brain damage or surgery, MR incompatible metallic implants or prosthesis, systemic diseases, hearing disability, lifetime history of psychiatric comorbidity and/or substance abuse. All medications were allowed except benzodiazepines. The following clinical evaluations were administered by MIA: Structured Clinical Interview according for the DSM-IV (SCID-I) [19], Young Mania Rating Scale (YMRS) [20], Hamilton Depression Rating Scale (HDRS) [21], Scale for the Assessment of Positive Symptoms (SAPS) [22], Scale for the Assessment of Negative Symptoms (SANS) [23] and Brief Psychiatric Rating Scale (BPRS) [24]. All subjects completed an MR data acquisition session immediately following the clinical evaluations.
Table 1.
Clinical characteristics of the participants.
| SZ (n = 25) | BD-I (n = 28) | BD-II (n = 20) | HCs (n = 30) | F/χ2/t | P | ||
|---|---|---|---|---|---|---|---|
| Age (Years) | 38.4 ± 13.25 | 35.32 ± 9.13 | 38.85 ± 14.03 | 32.77 ± 10.65 | 1.58 | 0.207 | |
| Sex (M) | 13 | 13 | 9 | 13 | 0.44 | 0.931 | |
| Education | 9.16 ± 3.85 | 10.89 ± 4.85 | 12.40 ± 3.95 | 12.20 ± 3.36 | 2.67 | 0.052 | |
| Age at Onset of Disorder (years) | 22.58 ± 6.89 | 23.57 ± 8.66 | 24.85 ± 9.86 | 0.389 | 0.679 | ||
| Duration of Disorder (months) | 142.54 ± 130.37 | 95.21 ± 107.69 | 156.85 ± 127.86 | 1.95 | 0.151 | ||
| Number of Hospitalizations | 1.36 ± 1.77 | 1.36 ± 1.76 | 0.40 ± 0.68 | 2.20 | 1.18 | ||
| Number of Episodes | Total | 7.82 ± 5.64 | 8.68 ± 7.00 | −0.474 | 0.638 | ||
| Manic* | 2.77 ± 2.18 | 3.63 ± 3.56 | −1.006 | 0.320 | |||
| Depressive | 4.22 ± 3.42 | 4.95 ± 4.26 | −0.639 | 0.526 | |||
| BPRS | 8.00 ± 4.41 | 2.43 ± 2.86 | 2.25 ± 1.77 | 24.51 | < 0.001 | ||
| YMRS | 1.07 ± 1.61 | 1.55 ± 1.50 | −1.044 | 0.302 | |||
| HDRS | 3.46 ± 3.51 | 3.35 ± 3.28 | 0.114 | 0.910 | |||
| SAPS | Total | 11.52 ± 6.90 | |||||
| SANS | Total | 13.28 ± 9.04 | |||||
SZ: Schizophrenia, BD-I: Bipolar I Disorder, BD-II: Bipolar II Disorder, HCs: Healthy Controls, BPRS: Brief Psychiatric Rating Scale, HDRS: Hamilton Depression Rating Scale, SANS: Schedule for the Assessment of Negative Symptoms, SAPS: Schedule for the Assessment of Positive Symptoms, YMRS: Young Mania Rating Scale.
Hypomania for the Bipolar II Disorder.
2.2. Magnetic Resonance Imaging Data acquisition
Data were acquired on a 3.0 Tesla Siemens MAGNETOM TIM Trio whole-body MR system (Siemens, Erlangen, Germany) with a thirty two-channel phased-array head coil at the UMRAM National Magnetic Resonance Research Center, Ankara, Turkey.
T1-weighted anatomical MRI (MPRAGE sequence, 256 X 256 voxels, TR: 2000 msec, TE: 3.02 msec, FOV read: 215, FOV phase: 100, slice thickness: 0.84, 192 slices) were collected for diagnostic and localization purposes. Proton Magnetic Resonance Spectroscopy (1H MRS) data was acquired using the single voxel Point REsolved Spectroscopy Sequence (PRESS) (TE = 30 msec, TR = 2000 msec) to quantify brain creatine (Cr) levels and MEscher-GArwood Point-REsolved Spectroscopy Sequence (MEGAPRESS) [25,26] (TE = 68 msec, TR = 2000 msec) to quantify brain GABA levels. Voxels (PRESS: 20 mm X 20 mm X 20 mm; MEGAPRESS: 30 mm X 30 mm X 20 mm) were placed in the structures around Sylvian Fissure including superior temporal lobe and inferior parietal lobe.
2.3. Magnetic Resonance Imaging Data analysis
The proton spectra were fit using LCModel (Version 6.3.0) to quantify the creatine levels [27,28] and GANNET software to quantify the GABA-to-creatine ratio (GABA/Cr) [29-34].
The structural T1-weighted images were segmented using SPM8 [Statistical Parameter Mapping– Welcome Department of Imaging Neuroscience, London, UK; (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/)] to determine the gray matter, white matter and CSF contributions to the voxel of interest. Absolute Cr values [16] were corrected for voxel tissue content and then multiplied with the GABA-to-Cr to determine the absolute GABA levels [35].
2.4. Statistical Analysis
Statistical analyses were performed using SPSS 22.0 software (Chicago, Illinois, USA). Outlier analysis was conducted and GABA values two standard deviations away from the mean of the corresponding groups were eliminated from further analysis. Chi-square test was used for the comparison of categorical variables. Shapiro-Wilk's tests for normality were performed for continuous variables. Two tailed independent samples t-test or Mann-Whitney U test were used for comparisons between independent groups. Group comparisons including more than two groups were performed by Univariate ANOVA or Kruskal-Wallis tests. Mann-Whitney U tests were performed for post-hoc comparisons after Kruskal-Wallis test. Since we had 4 groups and performed 6 Mann-Whitney U tests for posthoc comparisons between groups, we determined significance level as 0.0083 (0.05 / 6 = 0.0083) according to Bonferroni correction. In addition, Pearson's correlation analysis was performed to determine the relationship between GABA levels and clinical assessments.
3. Results
The demographic and clinical characteristics of the sample are listed in Table 1. There were no significant demographic differences in sociodemographic variables. All patients were clinically stable. However, schizophrenia patients scored significantly higher than the bipolar disorder groups on BPRS (F(2,63)=21.76, p<0.0001).
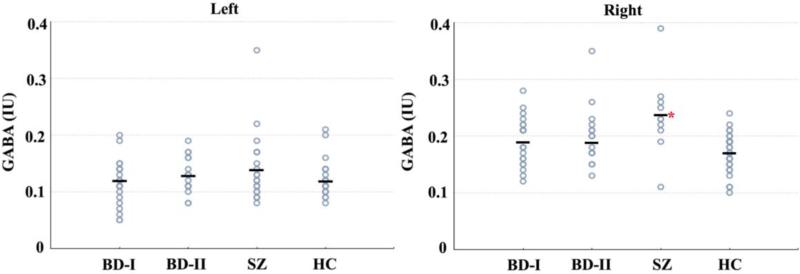
GABA levels at the right hemisphere significantly differed between the groups [χ2=9.62, df: 3, p=0.022] (Table 2). Posthoc comparisons revealed that GABA levels in the schizophrenia group were significantly higher than the BD-I (p=0.02), BD-II (p=0.02) and HC (p=0.002, Z=−3.08) groups (Figure 1). Difference between the groups was significant only between schizophrenia and HC after Bonferroni correction (p=0.002). There were no significant differences in the left hemisphere GABA levels between the groups [χ2=1.63, df: 3, p=0.652] (Table 2). GABA levels did not differ between the hemispheres within each group (p>0.05).
Table 2.
GABA levels.
| SZ | BD-I | BD-II | HCs | χ2 (df) | P | |
|---|---|---|---|---|---|---|
| Left GABA | 0.12 (0.11 - 0.17) | 0.11 (0.09 - 0.14) | 0.13 (0.11 - 0.16) | 0.12 (0.10 - 0.14) | 1.63 (3) | 0.652 |
| Right GABA | 0.23 (0.20 - 0.27) | 0.18 (0.16 - 0.22) | 0.18 (0.15 - 0.21) | 0.18 (0.15 - 0.20) | 9.62 (3) | 0.022 |
Kruskal-Wallis Test. Median (25-75 percentiles) values are reported. SZ: Schizophrenia, BD-I: Bipolar I Disorder, BD-II: Bipolar II Disorder, HCs: Healthy Controls.
Figure 1.
GABA levels in groups. There was significant difference between groups at right hemisphere. Schizophrenia group had significantly higher GABA levels in comparison to healthy controls. There was no significant difference between groups at left hemisphere. BD-I: Bipolar I Disorder, BD-II: Bipolar II Disorder, SZ: Schizophrenia, HC: Healthy Controls. GABA levels are in international units (IU).
Patients with schizophrenia were on significantly more atypical anti-psychotics than the BD-I or BD-II groups (χ2(2,73)=8.874, p<0.012) (Table 3). Chlorpromazine equivalents of antipsychotic doses were correlated positively with right hemisphere GABA levels (r2=0.68, p=0.006, n=33: schizophrenia, BD-I and BD-II groups). Serum valproate levels correlated positively with left hemisphere GABA levels (r2 =0.8, p=0.016, n=14: BD-I and BD-II groups).
Table 3.
Medication statuses of the patient groups.
| SZ (n = 25) | BD-I (n = 28) | BD-II (n = 20) | χ2/Z | P | |
|---|---|---|---|---|---|
| Atypical Antipsychotics (n) | 22 | 14 | 12 | 8.87 | 0.012 |
| Chlorpromazine Equivalent (mg) | 225 (145.75 - 400) | 267 (133 - 400) | 150 (50 - 267) | 6.39 (2) | 0.041 |
| Lithium (n) | 0 | 11 | 10 | 0.54 | 0.461 |
| Serum Lithium Levels (mEq/L) | 0.70 (0.45 - 0.85) | 0.70 (0.45 - 0.85) | −0.47 | 0.658 | |
| Valproate (n) | 0 | 11 | 7 | −0.86 | 0.650 |
| Serum Valproate Levels (▪g/ml) | 72.2 (56 - 99.35) | 57.3 (44.8 - 83.6) | 0.91 | 0.386 |
Kruskal-Wallis test, Chi-square test and Mann-Whitney U test. Median (25-75 percentiles) values are reported. In the post-hoc comparisons of the chlorpromazine equivalent doses of antipsychotics, there were no significant differences between the groups.
There was a correlation between left hemisphere GABA levels and the alogia subscale of the SANS (r2=0.8, p<0.05, n=10). There was no significant correlation between GABA levels and YMRS (r 2=0.48, p=0.158, n=39) however, there was a trend for a negative correlation with HDRS (r2=0.53, p=0.08, n=39) in the bipolar disorder groups.
4. Discussion
GABA levels in the right perisylvian structures were higher in schizophrenia patients in comparison to bipolar disorder and healthy control groups. There was positive correlation between antipsychotic medications and GABA levels at right hemisphere. Previous 1H-MRS studies have reported inconsistent results regarding GABA levels in schizophrenia. In first episode psychosis patients, GABA levels were lower within left basal ganglia [38] and bilateral calcarine sulci [37] and approximately the same within frontal and parieto-occipital lobes [35] in comparison to healthy controls. Moreover, a study comparing young schizophrenia patients and healthy controls reported that GABA levels were lower within anterior cingulate region for the schizophrenia patients and same within centrum semiovale [38]. Whereas in chronic schizophrenia patients, GABA levels were higher within anterior cingulate and parieto-occipital cortices [9], and normal within anterior cingulate cortex and left basal ganglia regions [39]. Variations of the GABA levels might be due to differences in brain regions, psychotropic medications, and clinical states in the previous 1H-MRS studies [10,12,40].
The balance of the excitatory and inhibitory impulses may determine the GABAergic cell activity [18,41]. Correlation between GABA and glutamate layers in prefrontal cortices [41] might be an indicator of the relationship between excitatory and inhibitory neurotransmission. Since the excitatory neurotransmission is degraded in neurodevelopmental disorders, activity of the GABAergic cells and GABA levels might be altered in order to protect the balance between excitatory and inhibitory neurotransmission [41-43].
In addition, GABA receptors are highly susceptible to rapid neuroplastic changes and various mechanisms such as phosphorylation of synaptic proteins [44]. These findings are suggestive of dynamic modulation of GABAergic neurotransmission according to the dynamics of the synapse or the network. Taken together, these findings may also explain the variability of GABA levels reported in previous studies using 1H-MRS, as GABA neurotransmission is modulated by several clinical factors prone to rapid changes. On the other hand, GABA receptors are often not saturated [45] and therefore the determinant of GABAergic signaling is synthesis of GABA from glutamate [46]. Therefore, activity level of the enzyme glutamic-acid decarboxylase (GAD) 65 and 67 isoenzymes, which catalyze the rate limiting step of GABA synthesis, determine the level of GABAergic activity. Although postmortem studies have reported decreased expression of GAD67 [47], several long term modulations may also alter GABAergic signaling as well as short term changes [48] and deficiency of GAD67 might be compensated upon long term modulations [46].
Abnormalities in the left hemispheric auditory cortices are associated with linguistic functions and specific symptoms of psychotic spectrum disorders [15,16] and developmental disorders [18]. However, GABA levels were higher in right hemisphere in schizophrenia and were positively correlated with antipsychotic doses in this study. This finding might be suggesting that antipsychotics could have enhanced GABA levels only at right hemisphere and could not enhanced left hemisphere GABA levels due to a stronger neuropathology in left hemisphere. In addition, valproate serum levels were correlated with GABA levels at right hemisphere in bipolar disorder. This is in line with a previous study [8], which has reported that mood stabilizer anticonvulsants adjunctive to antipsychotics have increased GABA levels at parieto-occipital lobe in schizophrenia. On the other hand, studies investigating the relationship between antipsychotics and GABA levels have been indicated both decrease [39,49] and no effect [9,36] in schizophrenia, as a result of the medication. Further controlled studies with specific designs to investigate the effects of medications on GABA levels are needed to obtain more consistent and reliable results using 1H-MRS. A current concept suggests that antipsychotics may restore the disturbances (disrupted myelination, reverse the loss of dendritic spines, enhance synaptic connections) of the excitatory neurotransmission that projects to GABAergic cells and stimulate oligodendrocyte maturation and increase the efficiency of GABAergic cells [43,49]. To this end, antipsychotics may ameliorate the pathology of the GABAergic cells in schizophrenia and bipolar disorder. However, it is not possible to predict the ultimate effects of antipsychotics on GABAergic functions currently, as there are several other determinants for GABAergic functions (such as excitatory/inhibitory balance, receptor phosphorylation, ion channel physiology), but yet GABA level is currently the only measurable in vivo response of the cell.
Although cortical GABA content as quantified by 1H-MRS has been found to predict the functional status of GABA-mediated processes in previous neurophysiology and pharmacological studies [40], normal GABA levels do not imply regulated GABAergic function. Determinants of GABA levels and mechanisms of compensatory changes in GABAergic activity are future directions for further clarification of GABAergic abnormalities in schizophrenia and bipolar disorder. While 1H-MRS utilizing edited pulse sequences such as MEGAPRESS is considered a reliable and reproducible method for measuring brain GABA [50], 1H-MRS cannot discriminate between intra and extracellular GABA levels. Therefore, these results should be viewed cautiously.
To conclude, higher GABA levels observed in the right auditory cortex of schizophrenia patients could be a compensatory mechanism to obtain the balance between excitatory and inhibitory impulses in the cerebral cortex. Due to pharmacological and physiopathological influences on this balance, in this investigation, we may be capturing a certain phase of GABA metabolism that could be modulated in a dynamic process. Dynamic modulation of the GABAergic activity might be the underlying reason of the variable results of the 1H-MRS studies measuring GABA levels.
Highlights.
GABAergic neurotransmission is disturbed in histopathological examinations and neuroimaging studies schizophrenia and bipolar disorder.
Auditory cortices are one of the most relevant brain regions in schizophrenia and bipolar disorder.
Right hemisphere GABA concentrations were higher in schizophrenia in comparison to the healthy control group.
GABA concentrations might be altered by several clinical and pharmacological mechanisms in psychiatric disorders.
GABAergic neurotransmission is prone to rapid changes stimulated by certain dynamics of the receptor, synapse or network.
Acknowledgement
We would like to thank Prof. Dr. Ergin Atalar from Bilkent University (Turkey) and Ali Avcı from Siemens, Turkey. We also appreciate technical help regarding voxel segmentation provided by Dinesh Deelchand, Dr. Uzay Emir and Dr. Gülin Öz from the Center for Magnetic Resonance Research, Minneapolis, MN, USA.
Funding: This study was funded by Scientific Research Projects Committee of the Ankara Yıldırım Beyazıt University (Project No: 803), and NIMH grant to CMM (MH073998) and K24 MH104449 from the NIH to DÖ. Dr. Phillips acknowledged the support of the Pittsburgh Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 2.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa M, Mizukami K, Iwakiri M, Asada T. Immunohistochemical and immunoblot analysis of gamma-aminobutyric acid B receptor in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci. Lett. 2005;383:272–277. doi: 10.1016/j.neulet.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Mizukami K, Sasaki M, Ishikawa M, Iwakiri M, Hidaka S, Shiraishi H, Iritani S. Immunohistochemical localization of gamma-aminobutyric acid (B) receptor in the hippocampus of subjects with schizophrenia. Neurosci. Lett. 2000;283:101–104. doi: 10.1016/s0304-3940(00)00939-3. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural. Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Wang AY, Lohmann KM, Yang CK, Zimmerman EI, Pantazopoulos H, Herring N, et al. Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta. Neuropathol. 2011;122:615–626. doi: 10.1007/s00401-011-0881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandal MJ, Nesbitt AM, Mccurdy RM, Alter MD. Measuring the maturity of the fast-spiking interneuron transcriptional program in autism, schizophrenia, and bipolar disorder. PLoS ONE. 2012;7:e41215. doi: 10.1371/journal.pone.0041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Öngür D, Prescot AP, Mccarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol. Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: Application to schizophrenia. Neurosci. Biobehav. Rev. 2015;51:276–295. doi: 10.1016/j.neubiorev.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, et al. Reduction in occipital cortex γ-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol. Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Brady RO, Mccarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, et al. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar. Disord. 2013;15:434–439. doi: 10.1111/bdi.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE, et al. Brain GABA levels in patients with bipolar disorder. Prog Neuropsychopharmacol Biol. Psychiatry. 2009;33:427–434. doi: 10.1016/j.pnpbp.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Moore DR. Auditory development and the role of experience. Br. Med. Bull. 2002;63:171–181. doi: 10.1093/bmb/63.1.171. [DOI] [PubMed] [Google Scholar]
- 15.Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- 16.Atagün MI, Şıkoğlu EM, Can SS, Karakaş-Uğurlu G, Ulusoy-Kaymak S, Çayköylü A, et al. Investigation of Heschl's gyrus and planum temporale in patients with schizophrenia and bipolar disorder: A proton magnetic resonance spectroscopy study. Schizophr. Res. 2015;161:202–209. doi: 10.1016/j.schres.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atagün MI. Brain oscillations in bipolar disorder and lithium-induced changes. Neuropsychiatr. Dis. Treat. 2016;12:589–601. doi: 10.2147/NDT.S100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014;86:28–34. doi: 10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First MD, Gibbon M, Spitzer RL, Robert L, Gibbon M, Williams JBW. Biometrics. Research. New York: 1996. User's guide for the structured interview for DSM-IV axis I disorders research version (SCID-I, version 2.0) [Google Scholar]
- 20.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreasen NC. The scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City IA: 1984. [Google Scholar]
- 23.Andreasen NC. The scale for assessment of negative symptoms (SANS) University of Iowa; Iowa City IA: 1983. [Google Scholar]
- 24.Overall JE. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962;10:799. [Google Scholar]
- 25.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR. Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Waddell KW, Avison MJ, Joers JM, Gore JC. A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn. Reson. Imaging. 2007;25:1032–1038. doi: 10.1016/j.mri.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 28.Provencher SW. Automatic quantitation of localized in vivo1H spectra with LCModel. NMR. Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 29.Dydak U, Jiang Y-M, Long L-L, Zhu H, Chen J, Li W-M, et al. In Vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ. Health. Perspect. 2010;119:219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edden RA, Barker PB. Spatial effects in the detection of γ-aminobutyric acid: Improved sensitivity at high fields using inner volume saturation. Magn. Reson. Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 31.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging. 2013;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu H, Edden RAE, Ouwerkerk R, Barker PB. High resolution spectroscopic imaging of GABA at 3 Tesla. Magn. Reson. Med. 2011;65:603–609. doi: 10.1002/mrm.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikoglu EM, Navarro AAL, Starr D, Dvir Y, Nwosu BU, Czerniak SM, et al. Vitamin D 3 supplemental treatment for mania in youth with bipolar spectrum disorders. J. Child. Adolesc. Psychopharmacol. 2015;25:415–424. doi: 10.1089/cap.2014.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochran DM, Sikoglu EM, Hodge SM, Edden RA, Foley A, Kennedy DN, et al. Relationship among glutamine, γ-aminobutyric acid, and social cognition in autism spectrum disorders. J. Child. Adolesc. Psychopharmacol. 2015;25:314–322. doi: 10.1089/cap.2014.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhury FA, O'gorman RL, Nashef L, Elwes RD, Edden RA, Murdoch JB, et al. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J. Magn. Reson. Imaging. 2014;41:694–699. doi: 10.1002/jmri.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto N, Yoshimura R, Kakeda S, Moriya J, Hori H, Hayashi K, et al. No alterations of brain GABA after 6 months of treatment with atypical antipsychotic drugs in early-stage first-episode schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:1480–1483. doi: 10.1016/j.pnpbp.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Kelemen O, Kiss I, Benedek G, Kéri S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: The potential impact of GABA concentration in the visual cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;47:13–19. doi: 10.1016/j.pnpbp.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Rowland LM, Edden RA, Kontson K, Zhu H, Barker PB, Hong LE. GABA predicts inhibition of frequency-specific oscillations in schizophrenia. J Neuropsychiatry. Clin. Neurosci. 2013;25:83–87. doi: 10.1176/appi.neuropsych.11120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr. Res. 2010;117:83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Maddock RJ, Buonocore MH. MR Spectroscopic studies of the brain in psychiatric disorders. Curr. Top. Behav. Neurosci. 2011;11:199–251. doi: 10.1007/7854_2011_197. [DOI] [PubMed] [Google Scholar]
- 41.Brix MK, Ersland L, Hugdahl K, Grüner R, Posserud M-B, Hammar Å, et al. Brain MR spectroscopy in autism spectrum disorder—the GABA excitatory/inhibitory imbalance theory revisited. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramamoorthi K, Lin Y. The contribution of gabaergic dysfunction to neurodevelopmental disorders. Trends. Mol. Med. 2011;17:452–462. doi: 10.1016/j.molmed.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int. J. Neuropsychopharm. 2012;16:691–700. doi: 10.1017/S1461145712001095. [DOI] [PubMed] [Google Scholar]
- 44.Stelzer A, Kay A, Wong R. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988;241:339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- 45.Cohen AS, Lin DD, Coulter DA. Protracted postnatal development of inhibitory synaptic transmission in rat hippocampal area CA1 neurons. J. Neurophysiol. 2000;84:2465–2476. doi: 10.1152/jn.2000.84.5.2465. [DOI] [PubMed] [Google Scholar]
- 46.Lazarus MS, Krishnan K, Huang ZJ. GAD67 deficiency in parvalbumin interneurons produces deficits in inhibitory transmission and network disinhibition in Mouse prefrontal cortex. Cereb. Cortex. 2015;25:1290–1296. doi: 10.1093/cercor/bht322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 48.Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 2012;287:40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao X. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 2012;69:449. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 50.Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, et al. In vivo quantification of intracerebral GABA by single-voxel 1H-MRS—how reproducible are the results? Eur. J. Radiol. 2010;73:526–531. doi: 10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]