Abstract
Inbred mouse strains have been used preferentially for behavioral testing over outbred counterparts, even though outbred mice reflect the genetic diversity in the human population better. Here, we compare the sociability of widely available outbred CD1 mice with the commonly used inbred C57BL/6J (C57) mice in the one-chamber social interaction test and the three-chamber sociability test. In the one-chamber task, intra-strain pairs of juvenile, non-littermate, male CD1 or C57 mice display a series of social and aggressive behaviors. While CD1 and C57 pairs spend equal amount of time socializing, CD1 pairs spend significantly more time engaged in aggressive behaviors than C57 mice. In the three-chamber task, sociability of C57 mice was less dependent on acclimation paradigms than CD1 mice. Following acclimation to all three chambers, both groups of age-matched male mice spent more time in the chamber containing a stranger mouse than in the empty chamber, suggesting that CD1 mice are sociable like C57 mice. However, the observed power suggests that it is easier to achieve statistical significance with C57 than CD1 mice. Because the stranger mouse could be considered as a novel object, we assessed for a novelty effect by adding an object. CD1 mice spend more time in the chamber with a stranger mouse than that a novel object, suggesting that their preference is social in nature. Thus, outbred CD1 mice are as appropriate as inbred C57 mice for studying social behavior using either the single or the three-chamber test using a specific acclimation paradigm.
Keywords: social behavior, autism, mouse strain, mouse model, wild type, autistic behavior, aggressivity
1. Introduction
Behavioral studies are commonly performed with strains of inbred versus outbred mice to reduce variability [1]. This is also thought to limit the number of animals to be used. However, examining the effects of genetic manipulations or drug treatments on behavior using inbred mice may be overestimated with respect to extrapolation of the results to humans because it does not account for inter-individual variability. Data obtained with inbred mice may thus need to be reproduced with outbred stocks. Alternatively, experiments may be carried out directly in outbred mice, such as commonly used CD1 mice. A major advantage of the outbred stocks is that they are excellent breeders and have large litters, which can accommodate several genotypes or treatments per litter (mean of 12 for CD1 [2]). In addition, some of the CD1 mouse behavior resemble that of wild mice [3], and they also have good visual acuity despite being albinos [4;5].
In this study, we set out to examine whether outbred CD1 mice are as suitable as C57BL/6J (C57) mice, a commonly used inbred strain, in social behavior testing. We focused our study on male mice to avoid variability related to the estrous cycle that would require additional accommodation with female mice[6–8]. Although the three-chamber sociability test has lately become the gold standard to examine social deficits in mice [9;10], a more classical single chamber task may be more sensitive in detecting social deficits (changes in social behavior) [11]. It is an overstatement to argue that these tests truly capture the range and nuance of the full spectrum of human social behavior, which includes a large spectrum of behavior and deficits that are common features in autism, schizophrenia, and depression. However, these tasks are the most accepted tests to examine impaired social interactions. In addition, the three-chamber sociability test allows for automatic analysis and quantification and thus removes personal biases compared to the one-chamber social interaction test. We found that CD1 mice display appropriate social behavior in the one-chamber and the three-chamber socialization tests using a specific habituation paradigm, suggesting that outbred CD1 mice can be used to examine the impact of drug treatments or genetic manipulations on social interactions.
2. Methods
Animals
Research protocols were approved by the Yale University Institutional Animal Care and Use Committee and experiments were carried out in accordance with the approved guidelines. Experiments were performed on 4–5 weeks old (three-chamber) and 5–6 weeks old (free social interaction) male CD1 (Charles River Laboratories) and C57BL/6Ncrl (Charles River Laboratories) mice. Mice were weaned and housed in groups of 3–5 in standard vented-rack cages in a 12:12 hour light:dark cycle with food and water available ad libitum. Behavioral experiments were conducted in a dedicated procedure room. All mice were habituated to the test room for at least 1 hour prior to the start of behavioral tasks.
Behavioral tests
Two cohorts of age-matched, sexually naïve juvenile mice were used for testing: one cohort for free social interaction in a one-chamber test and a second cohort for the three-chamber sociability test. Tests were conducted between the hours of 09:00 and 14:00 and, unless otherwise noted, the room was brightly lit with a modest and constant white background noise (60 dB). Investigators could not be blinded to the mouse strain due to the difference in coat colors, but the three-chamber sociability test was performed with ANY-maze video tracking software (Stoelting, Wood Dale, IL, USA) using an overhead video camera system to automate behavioral testing and provide unbiased data analyses. The one-chamber social interaction test requires manual scoring and was analyzed by an individual with no knowledge of the questions. All components of the behavioral apparatuses were wiped thoroughly with 1.5% NaClO followed by double distilled water between tests to disinfect and eliminate olfactory cues between mice.
One-chamber social interaction test
Each mouse (5–6 week-old) was individually acclimated in the same social arena (43 cm long x 22 cm wide x 21 cm tall) for 10 min while basal activity is being monitored. After each pair of non-littermate mice has been independently acclimated, they are placed together in the arena for 10 min. Mouse behavior was video-recorded with two high-definition IP cameras (MegaVideo AV2115DNAIv1, Arecont Vision, Glendale, CA, USA) at a 20 Hz acquisition rate. Cameras were mounted directly above as well as to the side the chamber, with a 30° angle, to obtain complete coverage of the mouse behavior. Videos were recorded using Blue Iris video security software full version and the behavior was viewed using VLC media player (freeware). Behavior was manually scored using the following criteria: nose-to-nose sniffing, following, body sniffing, and anogenital sniffing, and the following aggressive criteria: posturing, mounting, rough grooming, and fighting.
Three-chamber sociability test
4–5 week-old male mice were tested in a modified three-chambered social choice task as described previously [9;10]. The three-chambered apparatus consisted of an opaque rectangular plexiglass box (60 cm long x 44 cm wide x 40 cm tall) divided into three compartments (44 cm long x 20 cm wide) by clear plexiglass walls with small, retractable entryways. Each side chamber contained an inverted wire cup (10.5 cm tall x 10.5 cm diameter bottom x 7.6 cm diameter top, 1 cm bar spacing). We used two 10 min-long phases of the task: (1) habituation and (2) socialization. During habituation, the test mouse was allowed to either explore the middle chamber only (version 1) or freely explore the entire apparatus, with the side chambers containing only empty cups (version 2). During socialization, a stranger mouse was placed under a cup in one side while the other remained empty and a test mouse was allowed to then freely explore the apparatus. To segregate social behavior from novelty seeking bias in the chamber preference using habituation version 2, we added a novel object of similar size and color to the stanger mouse in one of the chambers. To mitigate innate chamber bias in test mice, strangers are randomly introduced in either chamber in an alternating pattern. Activity in each chamber and in close proximity to either the mouse or cup (front 25% of the mouse <1 inch margin around the cup) was recorded. Socialization index was calculated as the time in the mouse chamber divided by total time in the mouse chamber and cup zone (15 cm diameter including the cup). All stranger mice were non-littermate, age and strain-matched male mice that had been previously habituated to being under the cup.
Statistics
Analysis was performed on N indicating the number of animals. Data were presented in GraphPad Prism 6. Statistical significance was determined using paired Student t-test when comparing time spent in stranger versus empty chamber or cup, within a single strain. Two way ANOVA followed by Tukey post-test was used in two variable comparisons, such as time spent engaging in behavior or in chamber, between two strains of mice. Significance was set at P < 0.05 for significance. Data are presented as mean ± standard error of the mean (SEM).
Post-hoc power analysis
The observed effect size or Cohen’s d (see equation below), for the three-chamber sociability test was computed using the average time spent in the stranger versus the empty chamber and their respective standard deviation in the paired t-test. Observed power was estimated using the following equations, Cohen’s d effect size, number of subjects, and target confidence level (P-value).
Cohen's d effect size for a t-test:
Beta function:
Gamma function:
Lower incomplete beta function:
Noncentral t-distribution cumulative distribution function:
Noncentral t-distribution noncentrality parameter:
Regularized lower incomplete beta function:
3. Results
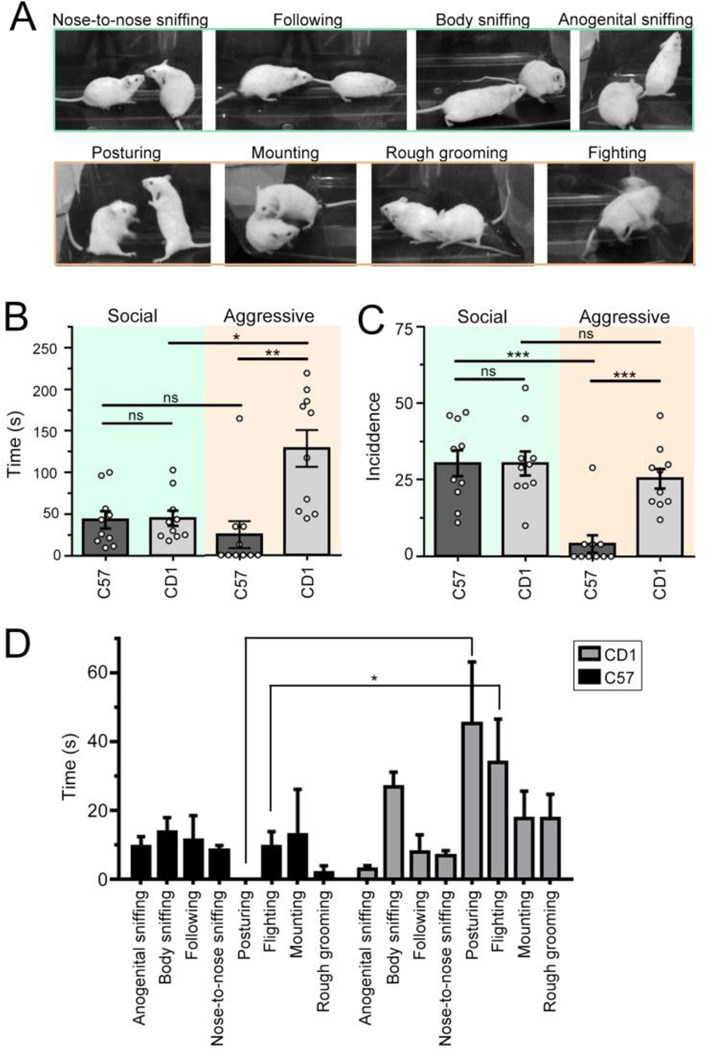
The one-chamber social interaction test has classically been the test of choice for its simplicity and perhaps its sensitivity over other social tests [11]. We thus first compared the sociability of outbred CD1 mice to that of inbred C57 mice in the one-chamber test, where individuals are free to initiate and reciprocate interactions with the other animal in the arena for 10 mins. 4 behaviors were classified as social interactions - nose to nose, anogenital, and body sniffing, and following - and 4 as aggressive interactions - posturing, mounting, rough grooming, and fighting (Fig. 1A). When considering social interactions, CD1 mice spent on average the same amount of time socializing in the same number of bouts as C57 mice did (CD1: 45.0 ± 9.1 sec in 30.3 ± 3.9 bouts of interactions, N=10 and C57: 43.2 ± 10.4 sec in 30.4 ± 4.2 bouts, N=10, Fig. 1B and C). Consistent with previous reports ( [12] for review and references), CD1 mice spent on average more time engaged in aggressive behavior than C57 mice (CD1: 128.2 ± 22.0 sec in 25.4 ± 3.2 bouts, N=10; C57: 24.6 ± 16.1 sec in 4.1 ± 2.8 bouts, N=10, one-way ANOVA, P < 0.001, F(3,36)=9.12 (time) and P < 0.001, F(3,36)=12.17 (incidence), Fig. 1B and C). CD1 mice also spent more time engaged in aggressive than social behaviors (P < 0.001, Fig. 1B) but share similar incident rate between social and aggressive interactions (Fig. 1C). Among the different types of scored aggressive behaviors, posturing and fighting in CD1s stand out most significantly over C57 mice (posturing: P < 0.0001, fighting: P<0.05 by two-way ANOVA; Fig. 1D). Together, these data suggest that CD1s are as social as inbred strain of mice, but that aggressive behaviors need to be taken into account when designing the one-chamber social task or alternatively use another task, such as the three-chamber test of sociability.
Figure 1. CD1 and C57 spent equal amount of time in social behavior in the one-chamber social test, but spent more time in aggressive behaviors.
(A) Snap-shots of the different aggressive and social behaviors. (B and C) Bar graphs of the time spent (B) and the frequency of interactions (incidence, C) in social (green) or aggressive (salmon) interactions for C57 (dark grey) and CD1 (light grey) mice. (D) Bar graphs of the time spent in each type of social and aggressive behavior for C57 and CD1 mice. Bar: mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 with one way ANOVA.
To assess sociability without the confound from aggressive behaviors in CD1 mice, we used the three-chamber sociability task, which is now broadly used for testing mouse sociability [9;10]. The task consists of habituation and socialization. These phases are illustrated in an experimental schematic in Fig. 2A. There are two published variations of the test with different habituation regimens that work equally well in C57 animals [13–16]. The first version allows the subject animal to habituate only in the middle chamber without access to side chambers, while the second version allows the animal to freely explore all three chambers, including the empty cups, one of which would later hold a stranger animal.
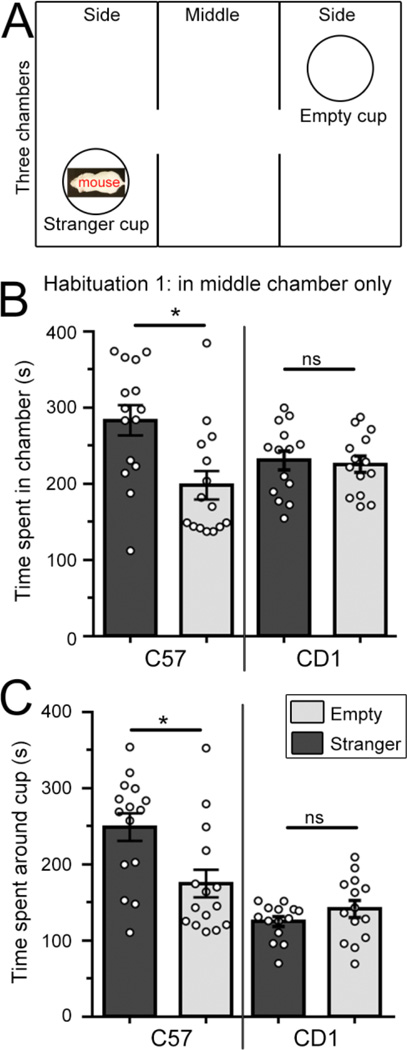
Figure 2. CD1 and C57 mice differ in their sociability in the three-chamber social task following habituation only in the middle chamber.
(A). Diagram of the three-chamber apparatus. (B and C) Using version 1 of the habituation protocol (only in middle chamber), bar graphs of the time spent in each side chamber (B) or in close proximity to the cup (C) containing a stranger mouse (dark) or being empty (light grey) for C57 and CD1 mice. *, P < 0.05, two way ANOVA, ns: not significant. Bar: mean ± SEM.
Using the first version of the test, we found that C57 mice spent significantly more time in the chamber where the stranger mouse was kept compared to the chamber with an empty cup (N=15 mice, P<0.05, two way ANOVA, Fig. 2B), as previously published [13–16]. In addition, C57 mice spent more time in close proximity to the cup containing the stranger mouse (N=15 mice, P<0.05, two way ANOVA, Fig. 2C). However, CD1 mice spent equal amounts of time in the chamber with stranger 1 compared to the chamber with an empty cup (N=14 mice, Fig. 2B), suggesting that CD1 mice display a deficient social preference towards stranger mice compared to C57 mice using version one of the three-chamber social task. The lack of social preference prompted us to assess CD1 mice with the second habituation version of the task.
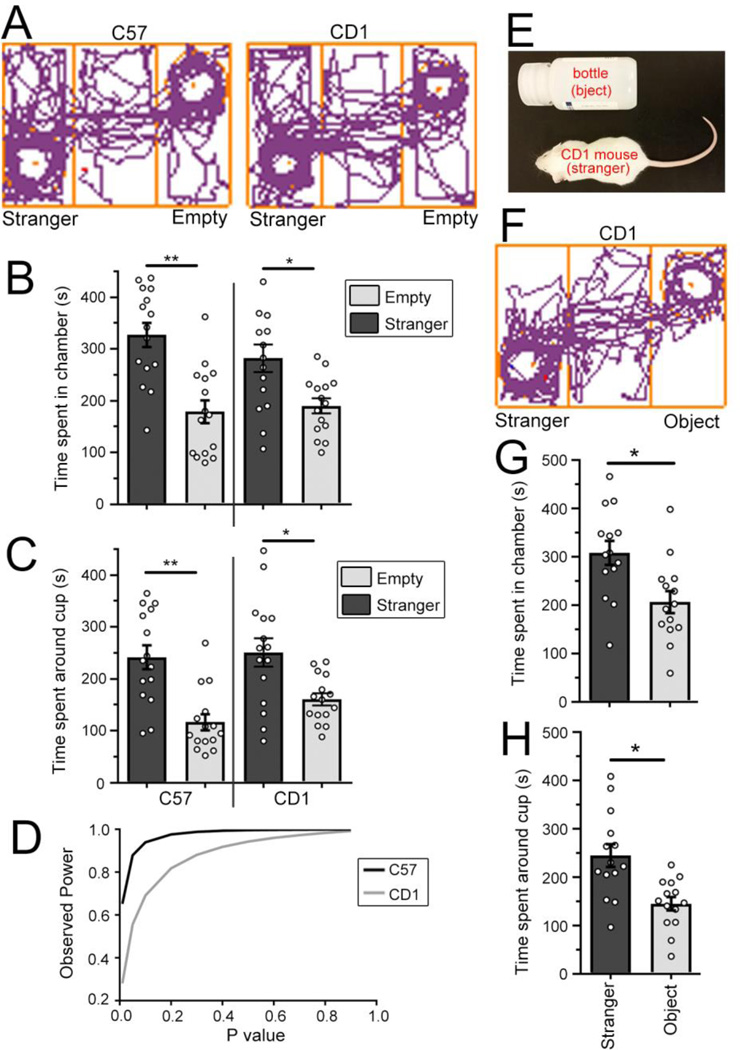
When given the chance to explore all three chambers and cups in the arena during the habituation phase, both CD1 and C57 mice showed similar track paths (Fig. 3A) and preference toward the stranger mouse (Fig. 3B and C). Both CD1 and C57 mice spent significantly more time in the chamber where the stranger mouse was kept than the chamber with an empty cup (N=15 mice for each group, P<0.01 for C57 and P<0.05 for CD1, two way ANOVA, Fig. 3B). Furthermore, both strains spent more time investigating the cup containing the stranger mouse than the empty cup (N=15 mice, P<0.01 for C57 and P<0.05 for CD1, two way ANOVA, Fig. 3C). Thus, by using a different habituation protocol, CD1 mice showed a similar preference towards the stranger mouse compared to the object (empty cup) as C57 mice. Together, these data suggest that CD1s are as appropriate as C57s when using in the three-chamber sociability test with habituation to all three chambers.
Figure 3. CD1 and C57 mice display similar sociability in the three-chamber social task following habituation in all three chambers.
(A) Track paths for CD1 and C57 mice using habituation version 2. (B and C) Bar graphs of the time spent in the chamber (B) or in close proximity of the cup (C) for C57 and CD1 mice using version 2 of the habituation protocol. **, P<0.01; *, P<0.05, two way ANOVA. (D) Graph of the observed power against the P value C57 and CD1 mice from data shown in B. (E) Photograph of a stanger mouse and the novel object used for the novelty test. (F) Track paths for CD1 mice using the three-chamber test with a novel object and a stanger CD1 mouse in the side chamber. (G and H) Bar graphs of the time spent in the chamber (G) or in close proximity of the cup (H) for CD1 mice. *, P<0.05, paired t-test. Bar: mean ± SEM.
Variability has always been a major concern in using outbred animals for behavior testing. While CD1s appear to share a similar variability to C57s in their preference in time spent in chamber with the stranger mouse, the significance levels of the two differ. To properly assess and compare the statistical powers of these two mouse strains, we performed a post-hoc effect size and power analysis on the three-chamber data acquired with habituation 2 (Fig. 3D). The effect size (Cohen’s d) computed using average time an animal spent in each chamber and their respective standard deviations (C57s: stranger , SD=90.6, empty , SD=85.1; CD1s: stranger , SD=103.2, empty , SD=56.4) showed that C57 mice confer a greater Cohen’s d than CD1 mice (1.689 and 1.103, repectively). The observed power in our three-chamber study, estimated using effect and sample size (N=15), suggests that it is easier to achieve statistical significance at P<0.05 with C57 than CD1 mice (83% and 47% power, respectively, Fig. 3D).
Using the habituation paradigm 2, CD1 may perceive the stranger mouse as a novel object considering that CD1 mice are innately curious. To test this hypothesis, we asked CD1 animals to freely choose between a novel object similar in size, mass, and color as the stranger mouse (Fig. 3E) in a modified three-chamber sociability test. With habituation 2, CD1s spend more time in the chamber with the stranger mouse than in the chamber with the novel object (N=14, P<0.05, paired t-test, Fig. 3F-H), suggesting that the underlying nature of CD1’s preference of stranger over empty chamber is based on social preference, notnovelty.
4. Discussion
Our novel finding is that juvenile male outbred CD1 mice display similarly suitable behaviors as inbred C57 mice in the two social tests as long as a specific habituation pragrigm is used for one of the tests. Neverthtless, although both inbred and outbred strains achieved statistical significance with 15 animals, the inbred strain requires fewer animals than their outbred counterparts in the three-chamber sociability task based on the observed power.
Here we only used male C57 and CD1 mice. However, female mice display differences in their behavior, including social behavior, compared to male mice [17;18]. Considering that autism does not only affect male, it will be important to examine the social behavior of CD1 females. This would, however, require careful accommodation of the estrous cycle on social behaviors since the it can affect mouse behavior in general[6–8]. We chose to use juvenile mice for several reasons. One reason is that several research groups perform genetic manipulations in embryonic mice using in utero electroporation and knockdown strategies that may only last 5–6 weeks after birth. A second reason is to reduce some of the financial burden of housing mice. A third reason is that with respect of autism and many psychiatric disorders, there is no need to study adult mice as autism or oher psychiatric disorders associated with social deficits manifess itself in infants or adolescents.
Using a one-chamber social test, CD1 and C57 mice spent the same amount of time socializing. However, CD1 mice displayed more aggressive behavior than C57 mice. This provides a strong limitation in using this task for testing sociability. Nevertheless, it has been reported that castration can eliminate the aggressive behavior of CD1 mice and could be an option for using that social test [19].
Using the well-accepted three-chamber sociability task, we found that CD1 and C57 mice display differences in their social behavior. Following habituation to only the middle chamber, CD1 mice did not show social preference while C57 did. The lack of social preference using this habituation paradigm is unclear. Following habituation to all three chambers, both CD1 and C57 mice display similar social preference, however, reaching significance is easier with C57 than CD1 mice as expected for an inbred versus outbred strains. Because CD1 mice are curious by nature, we examined whether the stanger mouse would act as a novel object. We thus added a novel object in one side chamber and a stanger mouse in the other side chamber. Using this paradigm, CD1 mice still displayed preference to the stranger mouse as opposed to the novel object suggesting that their preference was social in nature.
In conclusion, our study reports that outbred CD1 mice are suitable for social testing using the one-chamber social test acknowledging the aggression limitation and the three-chamber sociability task using the habituation paradigm that invovles all three chambers. Considering that CD1 mice display a more representative variability as seen in the human population, we suggest that this stain of outbred mice be preferentially used for social testing when possible.
Highlights.
Outbred CD1 mice are more aggressive than inbred C57BL/6J (C57) mice.
CD1 mice are as appropriate as C57 mice for studying social behavior
It is easier to achieve statistical significance with C57 than CD1 mice.
Acknowledgments
This work was supported by grant R01NS093704 from NIH/NINDS. We thank the lab members for helpful discussion and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat. Genet. 2005;37:1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka T. Effects of litter size on behavioral development in mice. Reprod. Toxicol. 1998;12:613–617. doi: 10.1016/s0890-6238(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 3.Parmigiani S, Ferrari PF, Palanza P. An evolutionary approach to behavioral pharmacology: using drugs to understand proximate and ultimate mechanisms of different forms of aggression in mice. Neurosci. Biobehav. Rev. 1998;23:143–153. doi: 10.1016/s0149-7634(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 4.Fellini L, Morellini F. Mice create what-where-when hippocampus-dependent memories of unique experiences. J. Neurosci. 2013;33:1038–1043. doi: 10.1523/JNEUROSCI.2280-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fellini L, Morellini F. Geometric information is required for allothetic navigation in mice. Behav. Brain Res. 2011;222:380–384. doi: 10.1016/j.bbr.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 6.Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol. Biochem. Behav. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 8.Schneider T, Popik P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology. 2007;32:651–659. doi: 10.1016/j.psyneuen.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D'Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato A, Kasai S, Kobayashi T, Takamatsu Y, Hino O, Ikeda K, Mizuguchi M. Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat. Commun. 2012;3:1292. doi: 10.1038/ncomms2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Loo PL, Van Zutphen LF, Baumans V. Male management: Coping with aggression problems in male laboratory mice. Lab Anim. 2003;37:300–313. doi: 10.1258/002367703322389870. [DOI] [PubMed] [Google Scholar]
- 13.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 14.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, Toyama K, Takao K, Miyakawa T. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol. Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh Y, Endo S, Nakata T, Kobayashi Y, Yamada K, Ikeda T, Takeuchi A, Hiramoto T, Watanabe Y, Kazama T. ERK2 contributes to the control of social behaviors in mice. Neurosci. J. 2011;31:11953–11967. doi: 10.1523/JNEUROSCI.2349-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex-specific ways. Horm. Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Holmes MM, Niel L, Anyan JJ, Griffith AT, Monks DA, Forger NG. Effects of Bax gene deletion on social behaviors and neural response to olfactory cues in mice. Eur. J. Neurosci. 2011;34:1492–1499. doi: 10.1111/j.1460-9568.2011.07881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesel ML. Castration eliminates conspecific aggression in group-housed CD1 male surveillance mice (Mus musculus) J. Am. Assoc. Lab Anim Sci. 2013;52:8. [PMC free article] [PubMed] [Google Scholar]