Abstract
We have shown previously that the cannabinoid receptor agonist CP55940 microinjected into the paraventricular nucleus of the hypothalamus (PVN) of urethane-anaesthetized rats induces depressor and pressor cardiovascular effects in the absence and presence of the CB1 antagonist AM251, respectively. The aim of our study was to examine whether the hypotension and/or hypertension induced by CP55940 given into the PVN results from its influence on glutamatergic and GABAergic neurotransmission. CP55940 was microinjected into the PVN of urethane-anaesthetized rats twice (S1 and S2, 20 min apart). Antagonists of the following receptors, NMDA (MK801), β2-adrenergic (ICI118551), thromboxane A2–TP (SQ29548), angiotensin II–AT1 (losartan) or GABAA (bicuculline), or the NO synthase inhibitor L-NAME were administered intravenously 5 min before S2 alone or together with AM251. The CP55940-induced hypotension was reversed into a pressor response by AM251, bicuculline and L-NAME, but not by the other antagonists. The CP55940-induced pressor effect examined in the presence of AM251 was completely reversed by losartan, reduced by about 50–60 % by MK801, ICI118551 and SQ29548, prevented by bilateral adrenalectomy but not modified by bicuculline and L-NAME. Parallel, but smaller, changes in heart rate accompanied the changes in blood pressure. The bi-directional CB1 receptor-mediated cardiovascular effects of cannabinoids microinjected into the PVN of anaesthetized rats depend on stimulatory glutamatergic and inhibitory GABAergic inputs to the sympathetic tone; the glutamatergic input is related to AT1, TP and β2-adrenergic receptors and catecholamine release from the adrenal medulla whereas the GABAergic input is reinforced by NO.
Keywords: Angiotensin AT1 receptor, β2-adrenoceptor, Cannabinoid CB1 receptor, GABAA receptor, NMDA receptor, Paraventricular nucleus of hypothalamus
Introduction
Cannabinoids act mainly via CB1 and CB2 receptors and influence multiple functions of the organism (for review, see Pertwee et al. 2010). Their complex cardiovascular effects are related to various peripheral and central mechanisms (for review, see Malinowska et al. 2012; Ibrahim and Abdel-Rahman 2014). Their most pronounced cardiovascular effect in anaesthetized rodents is a prolonged hypotension accompanied by a decrease in heart rate (HR) mediated mainly by peripheral presynaptic CB1 receptors on sympathetic nerve endings innervating resistance vessels and heart (Malinowska et al. 2010, 2012; Kwolek et al. 2005; Niederhoffer et al. 2003). The peripherally restricted CB1 antagonist AM6545 reversed the decreases in blood pressure (BP) and HR elicited by the intravenous injection (i.v.) of the cannabinoid agonist CP55940 into increases (Grzęda et al. 2015). Central CB1 receptors are also involved in the cannabinoid-induced decrease in BP in spontaneously hypertensive rats (SHR). Thus, the effect of AM3506 (which inhibits fatty acid amide hydrolase (FAAH), the main hydrolytic enzyme for the endocannabinoid anandamide (AEA)) was reduced by the brain-penetrant CB1 receptor antagonists rimonabant or AM251, but not by AM6545 i.v. (Godlewski et al. 2010).
The most distinct cardiovascular response to cannabinoids in conscious rodents is the pressor response (Gardiner et al. 2009), which can also be detected in anaesthetized rodents as the rapid phase that precedes the prolonged hypotension (e.g. Kwolek et al. 2005; Malinowska et al. 2010, 2012). This stimulatory effect, which has not been fully disclosed so far, is related to a central site of action. Thus, CB1 receptor-dependent increases in BP, plasma noradrenaline levels and/or renal sympathetic nerve activity were observed after intracisternal injection of WIN55212-2 and CP55940 to conscious rabbits (Niederhoffer and Szabo, 2000) and rats (Ibrahim and Abdel-Rahman 2011) and after injection of AEA, WIN55212-2 or HU-210 into the cisternal system (Pfitzer et al. 2004), the rostral ventrolateral medulla (RVLM) (Padley et al. 2003) or the dorsal periaqueductal gray (dPAG) (Dean 2011) of anaesthetized rats.
The paraventricular nucleus of the hypothalamus (PVN) represents one of the major integrative sites involved in the control of autonomic cardiovascular responses in the brain (Pyner 2009; Ferguson et al. 2008; Kc and Dick 2010). We found that AEA, its stable analogue methanandamide (MethAEA) or CP55940 injected intracerebroventricularly (i.c.v.) (Malinowska et al. 2010) or into the PVN (Grzęda et al. 2015) decreased BP and/or HR in urethane-anaesthetized rats. However, in the presence of the CB1 receptor antagonist AM251 i.v., the cardiodepressor effects of cannabinoids (given i.c.v. or into the PVN) were reversed into pure pressor and tachycardic responses, which were inhibited by the local microinjection of AM251. The CB2 receptor antagonist SR144528 i.v. did not modify the cardiovascular effects of CP55940 given into the PVN. Bilateral PVN lesion with kainic acid abolished pressor and depressor responses to CP55940 (Grzęda et al. 2015).
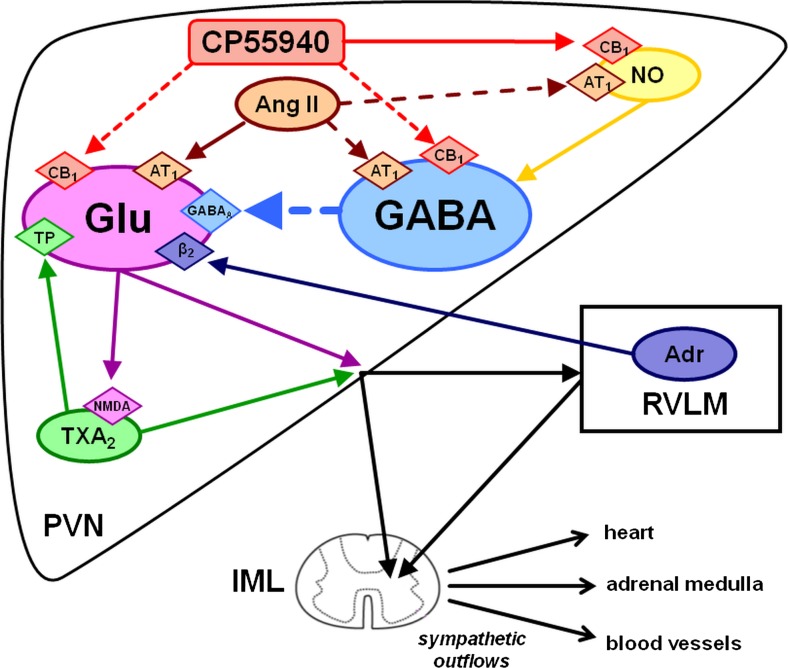
Cannabinoid CB1 receptors are located mainly presynaptically inhibiting the release of various neurotransmitters including glutamate and GABA (Schlicker and Kathmann 2001). Regulation of food intake and energy homeostasis (Busquets-Garcia et al. 2015) and stress response (Senst and Bains 2014) are influenced in opposite direction by CB1 receptors in the PVN according to their localization on different types of neurons (e.g. glutamatergic and GABAergic). Similarly, the sympathetic tone controlling the cardiovascular system results from the direct balance between stimulatory and inhibitory inputs, depending on glutamatergic and GABAergic neurotransmission in the PVN (Fig. 5), respectively. Thus, it is possible that the bi-directional CB1 receptor-mediated cardiovascular effects of CP55940 given into the PVN (Grzęda et al. 2015) result from the CB1 receptor-dependent modification of glutamatergic and GABAergic neurotransmission in the PVN. We have previously shown that the increase in BP induced by AEA (i.c.v.) was mediated by central β2-adrenergic, N-methyl-d-aspartate (NMDA) and thromboxane A2 (TXA2) TP receptors (Malinowska et al. 2010). The two main neurotransmission systems in the PVN are indirectly modified e.g. by β2, TP and angiotensin II (Ang II) AT1 receptors and by nitric oxide (NO) (Pyner 2009; Ferguson et al. 2008; Kc and Dick 2010). Thus, the aim of our study performed on urethane-anaesthetized rats was to examine whether the depressor and/or pressor cardiovascular effects induced by CB1 receptor activation by CP55940 in the PVN results from its influence on glutamatergic and GABAergic neurotransmission and/or additional factors (β2, TP and AT1 receptors as well as NO) indirectly modifying the latter two systems.
Fig. 5.
Possible mechanisms involved in the effect of the CB receptor agonist CP55940 topically administered to the paraventricular nucleus (PVN) on the sympathetic outflow and cardiovascular parameters. Activation of presynaptic inhibitory CB1 receptors on glutamatergic (Glu) neurones leads to a decrease in the sympathetic outflow (mainly to resistance vessels and heart) and a fall in blood pressure. Activation of presynaptic inhibitory CB1 receptors on GABAergic (γ-aminobutyric acid) neurons leads to an increase in the sympathetic outflow (mainly of the adrenal medulla) and in blood pressure. Activation of presynaptic facilitatory (i) angiotensin II (Ang II) AT1, (ii) thromboxane A2 (TXA2) TP and (iii) adrenaline (Adr) β2-adrenergic receptors increases whereas activation of GABAA receptors decreases the release of glutamate. Nitric oxide (NO) stimulates GABA release. For the sake of clarity, only interactions examined in the present study have been shown in this scheme. An inhibitory effect of Ang II and a facilitatory effect of CP55940 on NO production although opposite in direction to the effects usually obtained by AT1 and CB1 receptor activation, respectively, are also indicated.  stimulatory inputs;
stimulatory inputs;  inhibitory inputs. RVLM rostral ventrolateral medulla, IML intermediolateral column
inhibitory inputs. RVLM rostral ventrolateral medulla, IML intermediolateral column
Materials and methods
Male normotensive Wistar rats (weighing 280–350 g) with free access to food pellets and water were used. All surgical procedures and experimental protocols were in accordance with European and Polish legislation and were approved by the local Animal Ethics Committee in Białystok (Poland).
Placement of a cannula for drug administration into the PVN
Rats were anaesthetized intraperitoneally (i.p.) with pentobarbitone sodium (300 μmol/kg) and placed in a stereotaxic instrument (Stoelting WPI, Wood Dale, IL, USA). Stainless cannulae (outer and inner diameter of 0.5 and 0.3 mm, respectively) were stereotaxically implanted on the right side. The coordinates for the PVN were 1.5 mm caudal to the bregma, 0.5 mm lateral to the midline and 8 mm below the skull surface. Cannulae were fastened to the skull with acrylic cement. Rats were protected against infections by topical administration of the antibiotic doxycycline. The rats were then returned to their individual cages and allowed to recover.
Anaesthetized rats
At least 7 days later, rats were anaesthetized i.p. with urethane (14 mmol/kg). The trachea was cannulated. Systolic BP (SBP), mean BP (MBP) and diastolic BP (DBP) were measured from the right carotid artery via a transducer (ISOTEC; Hugo Sachs Elektronik–Harvard Apparatus GmbH, March, Germany). HR was recorded from the ECG by means of subcutaneous electrodes. Body temperature was maintained constant at approximately 37 °C using a heating pad (Bio-Sys-Tech, Białystok, Poland) and monitored by a rectal probe transducer (Physitemp BAT10; Physitemp Instruments, Inc., Clifton, NJ, USA). The left femoral vein was cannulated for i.v. injection of drugs administered in a volume of 0.5 mL/kg. The right femoral vein was prepared for infusion of prostaglandin F2α (PGF2α) by means of a Graseby 3100 syringe pump (Graseby Medical, Watford, Herts, UK). After surgical procedures, animals were gently placed on their abdomen and cardiovascular parameters were allowed to stabilize. Twenty minutes later, experiments were performed.
Experimental protocol
Agonists of NMDA receptors (NMDA; 1 mmol/rat, Kawabe et al. 2008) or cannabinoid receptors (CP55940; 0.1 nmol/rat, Grzęda et al. 2015) were administered into the PVN twice (S1 and S2, 20 min apart). PVN microinjections were administered slowly in a volume of 100 nL per rat and were completed within 1 min. We recorded agonist-induced maximal decreases or increases in the particular cardiovascular parameters that persisted for at least 5 s. Moreover, the non-selective nitric oxide synthase inhibitor L-NAME 37 μmol/kg (Gordish and Beierwaltes 2014) and the following antagonists were used: MK801 1 μmol/kg (NMDA receptor; Malinowska et al. 2010), ICI118551 1 μmol/kg (β2-adrenoceptor; Malinowska et al. 2010), SQ29548 1 μmol/kg (thromboxane A2 receptor (TP); Malinowska et al. 2010), losartan 10 μmol/kg (angiotensin II receptor (AT1); Kwolek et al. 2005) and bicuculline 5 μmol/kg (GABAA receptor; Elsersy et al. 2006). The latter antagonists/blockers or their solvents were administered i.v. alone or together with the CB1 receptor antagonist AM251 3 μmol/kg (Grzęda et al. 2015) 5 min before S2. In some experiments, bilateral acute adrenalectomy or a sham operation was performed 10 min before the second CP55940 administration. Adrenalectomy was done through two dorsolateral skin and muscular incisions. The adrenal glands were pulled out by holding the periadrenal fat and then excised. Sham operated animals were handled in the same way except that the adrenals were not removed. L-NAME increased DBP to about 80–90 mmHg. Since this effect did not recover during S2 and since the amplitude of vasopressor/vasodepressor effects is dependent on the basal BP (e.g. Kwolek et al. 2005), PGF2α (0.17–1.47 μmol/kg/h) was infused to control animals to ensure a basal DBP comparable to that of L-NAME-treated rats. At the end of the experiments, correct cannula placement was confirmed histologically and analysed by light microscopy. Only animals for which the correct placement of the guide cannula to the PVN was confirmed were included in this study.
Data analysis
Results are given as means ± SEM; n refers to the number of rats. In order to quantify the effects of antagonists on the cardiovascular effects of NMDA or CP55940, the agonist-induced maximal decreases or increases in BP and HR during S1 and S2 were calculated as percentage of the respective basal SBP, DBP, MBP and HR immediately before injection of the particular agonist. This procedure was chosen to minimize the influence of natural inter-subject variability on final data. For comparison of the mean values, the t test for paired and unpaired data was used, as appropriate. When two or more groups were compared with the same control, one-way analysis of variance (ANOVA) followed by Dunnett test was used. Differences were considered as significant when P < 0.05.
Drugs
AM251 [(N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide)] (Sigma-Aldrich, St. Louis, MO, USA); bicuculline, CP55940 [(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol], ICI118551 [(erythro-(±)-1-(7-methylindan-4-yloxy)-3-isopropylaminobutan-2-ol] (Tocris Cookson, Bristol, UK); L-NAME (Nω-nitro-l-arginine-methyl ester); losartan monopotassium salt (Cayman Chemicals, Ann Arbor, MI, USA); MK801 [((5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo(a,d)cyclohepten-5,10-imine hydrogen maleate], NMDA (N-methyl-d-aspartic acid) (Sigma-Aldrich); SQ29548 [([1S-[1α, 2α(Z), 3α, 4α]]-7-[3-[[2-[(phenylamino)carbonyl]hydrazino]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid] (Cayman Chemicals); pentobarbitone sodium (Biowet, Puławy, Poland); PGF2α; urethane (Sigma-Aldrich).
Drugs were dissolved in saline with the following exceptions: AM251 in a mixture of ethanol, Cremophor El, DMSO and saline (1:1:1:9.5); SQ29548 in a mixture of saline and DMSO (20:1); CP55940 was dissolved in 19 % solution of cyclodextrin. Solvents for agonists microinjected into the PVN or for particular antagonists/blockers given i.v. did not modify basal BP and HR.
Results
General
Basal SBP, DBP, MBP and HR measured immediately before the first (S1) and the second (S2) microinjection of NMDA or CP55940 into the PVN are given in Table 1. In animals not treated with any receptor antagonists, values of basal cardiovascular parameters were comparable before S1 and S2, confirming that the cardiovascular effects induced by agonists during S1 ceased before S2. Basal BP and HR were not altered by i.v. administration of the following antagonists: AM251 (CB1 receptors), MK801 (NMDA receptors), losartan (AT1 receptors), SQ29548 (TP receptors), ICI118551 (β2-adrenoceptors), and bicuculline (GABAA receptors) and by bilateral adrenalectomy. The NO synthesis inhibitor L-NAME given i.v. alone or together with AM251 increased SBP, DBP and MBP by about 20–30 mmHg but did not affect basal HR. In the related control group, PGF2α was infused to adjust basal BP values to those in rats treated with L-NAME (Table 1).
Table 1.
Basal systolic, diastolic and mean blood pressure (SBP, DBP and MBP in mmHg) and heart rate (HR in beats/min) immediately before S1 or S2 in urethane-anaesthetized rats
| Agonist | AM251 | Antagonist (i.v.) | Dosea | Number | Before S1 | Before S2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | MBP | HR | SBP | DBP | MBP | HR | |||||
| NMDA | − | − | − | 4 | 121 ± 8 | 49 ± 4 | 76 ± 9 | 376 ± 18 | 116 ± 14 | 48 ± 4 | 74 ± 8 | 380 ± 18 |
| + | − | − | 4 | 118 ± 5 | 67 ± 7 | 79 ± 7 | 378 ± 14 | 126 ± 4 | 60 ± 9 | 80 ± 7 | 396 ± 18 | |
| CP55940 | + | Solventb | − | 8 | 87 ± 8 | 54 ± 4 | 66 ± 5 | 334 ± 8 | 91 ± 7 | 57 ± 3 | 65 ± 4 | 334 ± 7 |
| − | MK801 | 1 | 4 | 103 ± 5 | 45 ± 3 | 60 ± 1 | 349 ± 18 | 102 ± 7 | 47 ± 3 | 64 ± 3 | 348 ± 13 | |
| + | MK801 | 1 | 4 | 117 ± 7 | 66 ± 7 | 85 ± 6 | 352 ± 9 | 118 ± 5 | 66 ± 5 | 85 ± 3 | 345 ± 9 | |
| − | ICI118551 | 1 | 4 | 96 ± 6 | 61 ± 8 | 75 ± 5 | 360 ± 6 | 96 ± 5 | 60 ± 6 | 76 ± 5 | 357 ± 3 | |
| + | ICI118551 | 1 | 5 | 87 ± 3 | 55 ± 2 | 66 ± 3 | 353 ± 6 | 86 ± 3 | 55 ± 2 | 66 ± 3 | 343 ± 9 | |
| − | Losartan | 10 | 4 | 104 ± 4 | 65 ± 6 | 73 ± 7 | 344 ± 6 | 101 ± 3 | 63 ± 5 | 73 ± 7 | 343 ± 5 | |
| + | Losartan | 10 | 4 | 101 ± 5 | 57 ± 4 | 72 ± 4 | 329 ± 12 | 98 ± 5 | 57 ± 4 | 72 ± 4 | 327 ± 12 | |
| − | Bicuculline | 5 | 4 | 84 ± 2 | 54 ± 4 | 66 ± 3 | 354 ± 8 | 83 ± 4 | 52 ± 4 | 66 ± 3 | 357 ± 8 | |
| + | Bicuculline | 5 | 4 | 73 ± 2 | 46 ± 1 | 59 ± 1 | 340 ± 26 | 77 ± 2 | 48 ± 1 | 62 ± 2 | 318 ± 16 | |
| + | Solventc | − | 4 | 89 ± 8 | 56 ± 6 | 70 ± 6 | 345 ± 16 | 93 ± 5 | 53 ± 4 | 67 ± 5 | 343 ± 13 | |
| − | SQ29548 | 1 | 4 | 98 ± 7 | 57 ± 5 | 71 ± 5 | 365 ± 11 | 96 ± 6 | 58 ± 4 | 73 ± 6 | 367 ± 11 | |
| + | SQ29548 | 1 | 4 | 87 ± 6 | 60 ± 8 | 72 ± 8 | 348 ± 17 | 87 ± 5 | 60 ± 7 | 71 ± 7 | 336 ± 9 | |
| − | L-NAMEd | 27 | 4 | 108 ± 6 | 71 ± 6 | 90 ± 6 | 394 ± 25 | 127 ± 8+,* | 94 ± 10+,* | 109 ± 11+,* | 398 ± 18 | |
| + | L-NAMEd | 27 | 4 | 87 ± 3 | 53 ± 4 | 61 ± 1 | 334 ± 14 | 110 ± 6+ | 79 ± 8+,* | 97 ± 8+,* | 332 ± 10 | |
| + | PGF2α d | − | 4 | 90 ± 4 | 58 ± 8 | 64 ± 13 | 336 ± 11 | 115 ± 9+ | 88 ± 9+,* | 95 ± 7+,* | 316 ± 4 | |
| + | Sham | − | 4 | 78 ± 4 | 52 ± 2 | 61 ± 3 | 335 ± 7 | 78 ± 3 | 54 ± 1 | 64 ± 3 | 332 ± 6 | |
| + | Adrenalectomy | − | 4 | 81 ± 3 | 45 ± 2 | 56 ± 3 | 317 ± 8 | 80 ± 7 | 45 ± 3 | 57 ± 4 | 324 ± 9 | |
NMDA (1 mmol/rat) or CP55940 (0.1 nmol/rat) was injected into the paraventricular nucleus twice (S1-S2) 20 min apart. Antagonists or their solvents (alone or in combination with AM251 3 μmol/kg) were given 5 min before S2. Data are given as the means ± SEM of n experiments
aDoses of antagonists/blockers are given in μmol/kg
bSolvents for MK801, ICI118551, losartan and bicuculline
cSolvent for SQ29548. PGF2α was infused at a dose of 0.17–1.47 μmol/kg/h
dBasal SBP, MBP and DBP values before S2 (i) are higher (+ P < at least 0.05) than the respective values before S1 and (ii) are higher (*P < at least 0.05) or tend to be higher than the respective values before S2 in the presence of solventb (for the sake of simplicity, comparators are marked with italics in the table)
Influence of the CB1 receptor antagonist AM251 on the cardiovascular effects of NMDA and CP55940 given into the PVN
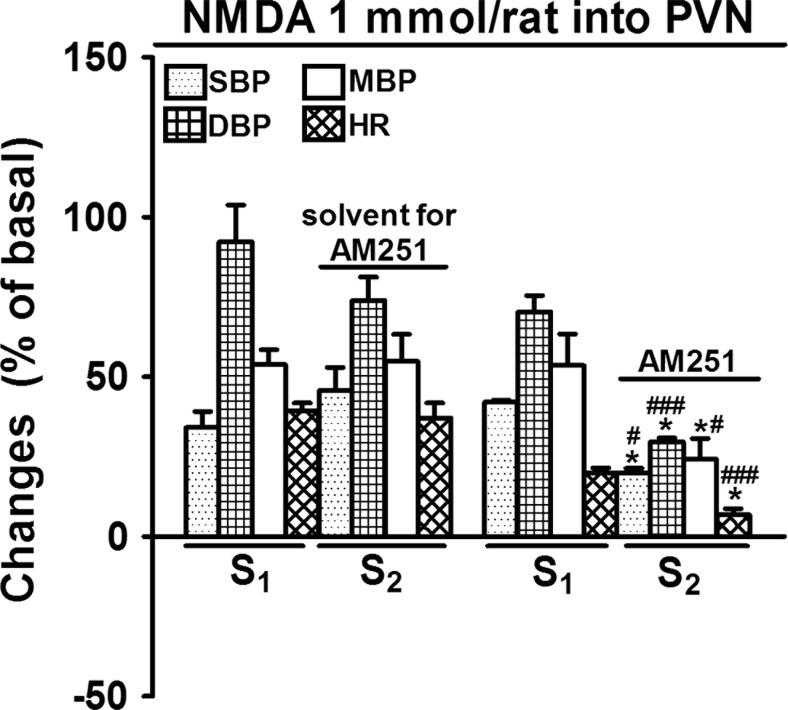
As shown in Fig. 1, the first (S1) microinjection of NMDA (1 mmol/rat) into the PVN increased SBP, DBP, MBP and HR by about 34, 92, 54 and 39 % of basal values (i.e. by 41 ± 6; 45 ± 5 and 41 ± 4 mmHg and 148 ± 10 beats/min; n = 4, respectively). The second administration of the agonist (S2) into the PVN of the same rat induced comparable increases in BP and HR. The pressor response of NMDA lasted for 550 ± 87 s, and the increase in HR for 724 ± 46 s (n = 4). The cannabinoid CB1 antagonist AM251 3 μmol/kg i.v. diminished the NMDA-induced increases in SBP, DBP, MBP and HR by about 50–60 % (Fig. 1).
Fig. 1.
Influence of AM251 on the increases in systolic, diastolic and mean blood pressure (SBP, DBP, MBP) and heart rate (HR) induced by NMDA microinjected into the paraventricular nucleus (PVN) in urethane-anaesthetized rats. NMDA was administered twice (S1 and S2, 20 min apart). AM251 3 μmol/kg or its solvent was administered i.v. 5 min before S2. Results are calculated as percentage of basal values determined immediately before S1 and S2 (see Table 1). Means ± SEM of 4 rats. *P < 0.001 compared to the corresponding S1; # P < 0.05; ### P < 0.001 compared to the corresponding S2 values without AM251
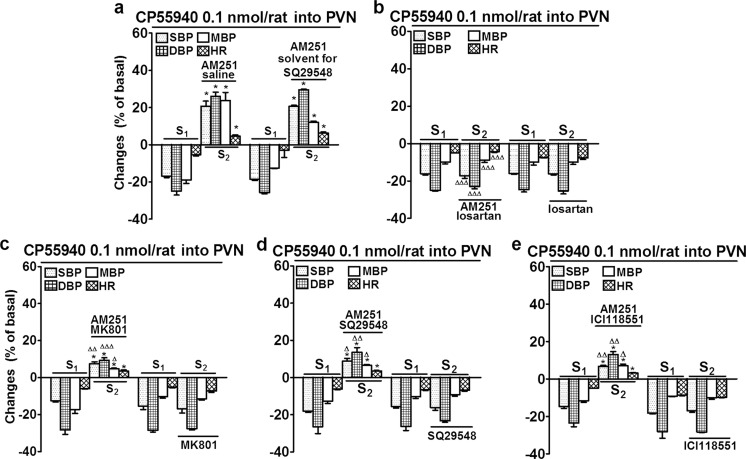
Similarly to our previous observations (Grzęda et al. 2015), the microinjection of CP55940 (0.1 nmol/rat) into the PVN decreased SBP, DBP, MBP and HR during S1 by about 15, 25, 20 and 5 % of basal values (i.e. by 16 ± 1; 13 ± 1 and 14 ± 1 mmHg and 17 ± 1 beats/min, respectively; n = 8; Fig. 2a). The cardiovascular effects of CP55940 lasted for about 2 min (for details, see Grzęda et al. 2015). Similar decreases in BP and HR were obtained during S1 in response to microinjection of CP55940 into the PVN before administration of particular antagonists (see S1 in Figs. 2, 3, and 4). However, in the presence of AM251 3 μmol/kg (independent of two various solvents for particular antagonists) i.v., CP55940 increased SBP, DBP, MBP and HR by about 20, 25, 25 and 5 %, (i.e. by 18 ± 2; 14 ± 1 and 15 ± 1 mmHg and 16 ± 1 beats/min, respectively; n = 8) (Figs. 2a, 3a, and 4).
Fig. 2.
Influence of AM251 alone (a) and of AM251 plus losartan (b), MK801 (c), SQ29548 (d) and ICI118551 (e) on the increases in systolic, diastolic and mean blood pressure (SBP, DBP, MBP) and heart rate (HR) induced by CP55940 microinjected into the paraventricular nucleus (PVN) in urethane-anaesthetized rats. CP55940 was administered twice (S1 and S2, 20 min apart). AM251 3 μmol/kg was administered i.v. 5 min before S2 either together with saline (solvent for losartan, MK801 and ICI118551) or the solvent for SQ29548 (a) or together with losartan 10 μmol/kg, MK801 1 μmol/kg, SQ29548 1 μmol/kg or ICI118551 1 μmol/kg (b–e). Results are calculated as percentage of basal values determined immediately before S1 and S2 (see Table 1). Means ± SEM of 4–8 rats. *P < 0.001 compared to the corresponding S1; Δ P < 0.05; ΔΔ P < 0.01; ΔΔΔ P < 0.001 compared to the S2 with AM251 and the respective solvent
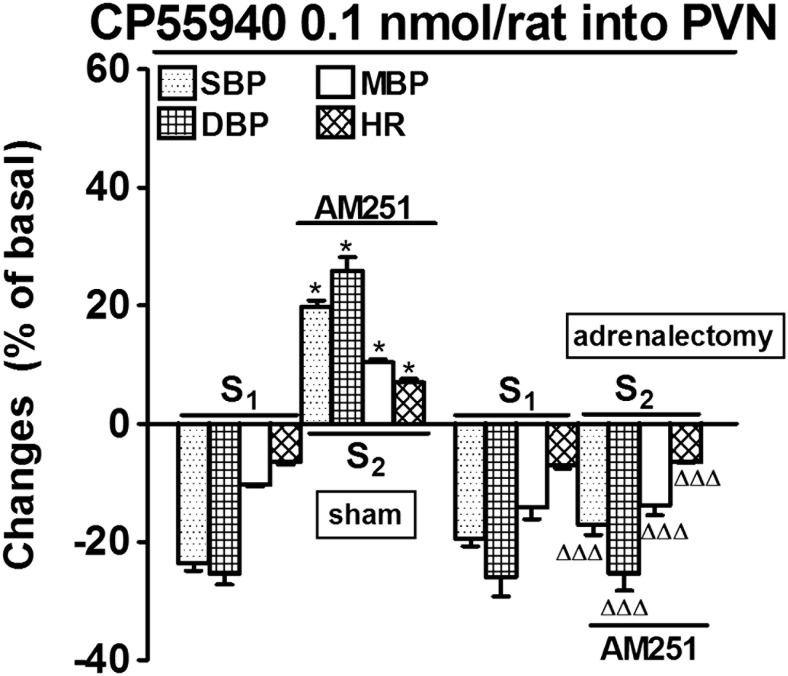
Fig. 4.
Influence of adrenalectomy on the increases in systolic, diastolic and mean blood pressure (SBP, DBP, MBP) and heart rate (HR) induced by CP55940 microinjected into the paraventricular nucleus (PVN) in urethane-anaesthetized rats. Adrenalectomy or sham operation was performed about 15 min before the second CP55940 microinjection. AM251 3 μmol/kg was given i.v. 5 min before S2. Results are calculated as percentage of basal values determined immediately before S1 and S2 (see Table 1). Means ± SEM of 4 rats. *P < 0.001 compared to the corresponding S1; ΔΔΔ P < 0.001 compared to the S2 values in sham operated rats
Fig. 3.
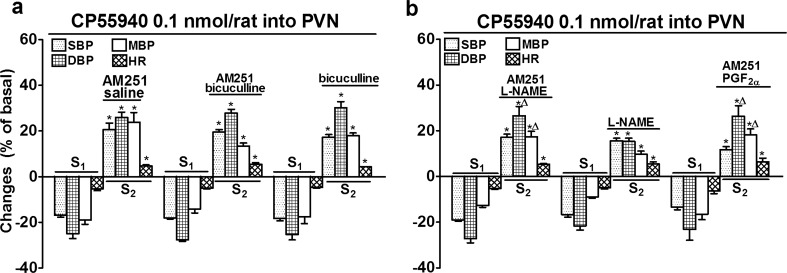
Influence of AM251, bicuculline (a) and L-NAME (b) on the increases in systolic, diastolic and mean blood pressure (SBP, DBP, MBP) and heart rate (HR) induced by CP55940 microinjected into the paraventricular nucleus (PVN) in urethane-anaesthetized rats. CP55940 was administered twice (S1 and S2, 20 min apart). AM251 3 μmol/kg [given with saline (solvent for bicuculline, L-NAME and PGF2α)], bicuculline 5 μmol/kg and/or L-NAME 37 μmol/kg were administered i.v. 5 min before S2. In one series (b) with AM251 (but without L-NAME), PGF2α 0.17–1.47 μmol/kg/h was infused to adjust basal parameters before S2 to those in rats treated with L-NAME. Results are calculated as percent of basal values determined immediately before S1 and S2 (see Table 1). Means ± SEM of 4–8 rats. *P < 0.001 compared to the corresponding S1 values; Δ P < 0.05 compared to S2 with L-NAME only
Influence of NMDA, angiotensin AT1, thromboxane TP and β2-adrenoceptor antagonists on the cardiovascular effects of CP55940
In contrast to AM251, the hypotension and the bradycardia induced by microinjection of CP55940 (0.1 nmol/rat) into the PVN (S1) were not modified (see S2) by the i.v. administration of antagonists of AT1, NMDA, TP receptors and β2-adrenoceptors, i.e., losartan (10 μmol/kg; Fig. 2b), MK801 (1 μmol/kg; Fig. 2c), SQ29584 (1 μmol/kg; Fig. 2d) and ICI118551 (1 μmol/kg; Fig. 2e), respectively.
Next, we examined how the pressor and tachycardic effects of CP55940 (0.1 nmol/rat) given into the PVN obtained in the presence of AM251 are affected by the four antagonists. Losartan completely reversed the CP55940-stimulated increases in BP and HR to hypotensive and bradycardic effects which were very similar to the responses induced by CP55940 in the absence of AM251 (Fig. 2b). MK801 (Fig. 2c), SQ29584 (Fig. 2d) and ICI118551 (Fig. 2e) diminished the CP55940-induced increases in SBP, DBP and MBP by about 60–70, 50–60 and 70–80 %, respectively but they did not affect the CP55940-stimulated increases in HR.
Influence of a GABAA receptor antagonist and an NO synthase inhibitor on the cardiovascular effects of CP55940
The GABAA receptor antagonist bicuculline (5 μmol/kg, i.v.; Fig. 3a) and the NO synthase inhibitor L-NAME (37 μmol/kg, i.v.; Fig. 3b) like AM251 reversed the depressant effects of CP55940 (0.1 nmol/rat given into the PVN) on SBP, DBP, MBP and HR into stimulatory ones. The CP55940-induced increases in SBP, DBP and MBP and HR in the presence of bicuculline were not affected when, in addition, AM251 (3 μmol/kg) was given i.v. (Fig. 3a) and they were comparable to those obtained in the presence of AM251 only.
By contrast, the CP55940-induced increases in DBP and MBP (but not those in SBP and HR) obtained in the presence of L-NAME were by about 45 and 65 % lower than those determined in the presence of AM251 (compare Fig. 3a, b). Moreover, the CB1 receptor antagonist enhanced the CP55940-induced increases in DBP and MBP (but not those in SBP and HR) obtained in the presence of L-NAME by about 75 and 80 %, respectively (Fig. 3b). Since L-NAME increased the level of the basal BP by about 20–30 mmHg (Table 1), additional experiments were carried out in which the baseline level of DBP was increased to about 90 mmHg by i.v. infusion of PGF2α. Similar effects of AM251 on the CP55940-induced increases in BP and HR occurred in the absence (Fig. 3a) and presence of PGF2α (Fig. 3b). PGF2α plus AM251 enhanced the CP55940-induced increases in DBP and MBP (but not those in SBP and HR) obtained in the presence of L-NAME by about 70 and 85 %, respectively (Fig. 3b).
Influence of bilateral adrenalectomy on the cardiovascular effects of CP55940
As shown in Fig. 4, bilateral adrenalectomy did not affect the hypotensive and bradycardic responses to CP55940 (0.1 nmol/rat) given into the PVN but completely prevented the pressor and tachycardic effects of CP55940 (given into the PVN) observed after previous i.v. administration of AM251. In sham operated animals, the CP55940-induced increases in SBP, DBP and MBP and HR in the presence of AM251 were similar to the changes observed under control conditions (compare Figs. 4, 2a, and 3a).
Discussion
General
The present study was carried out to clarify whether the hypotension and/or hypertension induced by CP55940 given into the PVN results from its influence on the two main neurotransmission systems (glutamatergic and GABAergic) and/or is related to additional receptors and NO indirectly modifying the two above systems. We performed experiments on anaesthetized rats to continue our previous experiments (Malinowska et al. 2010; Grzęda et al. 2015) and to reduce stress since the activation of CB1 receptors inhibits the stress-relevant neurons in the PVN (Crosby and Bains 2012). Like in our previous studies, urethane was used as anaesthetic and CP55940 served as cannabinoid receptor agonist.
Antagonists were administered i.v. since only a small volume could be microinjected into the PVN and only in this way, we were able to examine the CP55940-induced decreases (S1) and increases (S2) in cardiovascular parameters in one rat. On the other hand, this route of administration does not allow us to exclude that the effects of the antagonists may occur on central sites apart from the PVN. The effectiveness of the antagonists on centrally mediated effects after their i.v. application was confirmed in our previous publications (MK801, ICI118551 and SQ29548; Malinowska et al. 2010; Grzęda et al. 2015) or by other investigators (L-NAME, bicuculline and losartan; Turnbull et al. 1998; Elsersy et al. 2006; Busnardo et al. 2014). Moreover, (1) we have shown previously that central but not peripheral NMDA, β2 and TP receptors are involved in the pressor effect of cannabinoids since the effects of AEA or MetAEA given i.v. were reduced by NMDA, β2 and TP receptor antagonists in “intact” but not in pithed rats (i.e. in a model in which the effects of drugs involve peripheral sites only; Kwolek et al. 2005; Malinowska et al. 2010). (2) The pressor responses to AEA i.c.v. (obtained in the presence of a CB1 antagonist) were diminished by i.v. administration of NMDA, β2 and TP antagonists (Malinowska et al. 2010). (3) The AT1 receptor antagonist losartan i.v. did not modify the cardiovascular effects of AEA i.v. (including its pressor effect; Kwolek et al. 2005). Moreover, subcutaneous injection of losartan inhibits AT1 receptors (labelled by 125I-Ang II binding) in the rat PVN (Wang et al. 2003). To the best of our knowledge, peripheral GABAA receptors do not participate in the regulation of the rat cardiovascular system.
Modification of the CP55940-induced hypotension and bradycardia
Two main observations are in line with our working hypothesis that the decrease in BP and HR in response to the microinjection of CP55940 into the PVN is related to the activation of presynaptic inhibitory CB1 receptors on glutamatergic neurons. Firstly, we confirmed that AM251 i.v. reversed the depressant effects of CP55940 on BP and HR into stimulatory ones (Grzęda et al. 2015). We cannot exclude the possibility that the effect of AM251 i.v. is partially related to the inhibition of peripheral presynaptic inhibitory CB1 on sympathetic nerve endings. However, the pressor effect of CP55940 given into the PVN in the presence of AM251 i.v. was completely reversed by AM251 microinjected into the PVN (Grzęda et al. 2015). The final integration of the sympathetic outflow by the PVN results from the balance between stimulatory and inhibitory inputs depending on glutamate and GABA, respectively (Fig. 5; Pyner 2009; Ferguson et al. 2008; Kc and Dick 2010). In the PVN, presynaptic inhibitory CB1 receptors are localized both on glutamatergic and GABAergic synapses (Senst and Bains 2014); activation of CB1 receptors on GABAergic neurons should result in hypertension. The question arises why in our hands CP55940 primarily activates CB1 receptors on glutamatergic neurons? Different feeding (Busquets-Garcia et al. 2015) or emotional states (Senst and Bains 2014) determine the direction of final effects of endocannabinoids on CB1 receptors on glutamatergic and GABAergic transmission in the PVN on food intake or stress. In our previous paper (Grzęda et al. 2015), we suggested that the higher resting sympathetic tone in urethane-anaesthetized rats (Carruba et al. 1987) may lead to a stronger inhibitory effect of cannabinoids on glutamate than on GABA release.
Secondly, similarly to AM251 (present paper and Grzęda et al. 2015), blockade of GABAA receptors and NO synthesis, i.e. of mechanisms that have an inhibitory influence on the glutamatergic neurotransmission (Pyner 2009; Kc and Dick 2010), reversed the CP55940-induced hypotension and bradycardia into pressor and tachycardic responses. As shown in Fig. 5, GABA inhibits the stimulatory glutamatergic influence via GABAA receptors (Li et al. 2006) and NO potentiates the inhibitory effect of GABAergic transmission (Pyner 2009; Kc and Dick 2010). Thus, the respective blockers bicuculline and L-NAME dis-inhibited the effects induced by GABA and NO and reversed the depressor influence of CP55940 on cardiovascular parameters into a stimulatory one. The type of NO synthase is unclear since L-NAME is a non-selective inhibitor and both neuronal and endothelial NO synthase in the PVN are involved in the modulation of the sympathetic tone (Lu et al. 2015).
Bilateral adrenalectomy did not affect the CP55940-induced hypotension and bradycardia. These results are in line with our previous study and allow us to exclude the possibility that catecholamines released from the adrenal medulla contributed to the neurogenic cardiovascular responses (Malinowska et al. 2001).
Modification of the CP55940-induced pressor and tachycardic responses
The pressor responses to CP55940 given into the PVN were routinely examined in the presence of AM251, which, according to our hypothesis, primarily diminishes the inhibitory effect of CB1 receptors on glutamatergic neurons. The activation of presynaptic inhibitory CB1 receptors on GABAergic neurons that tonically inhibit glutamatergic neurotransmission should decrease the GABAergic inhibitory input leading to increases in BP and HR. Indeed, AM251 did not modify the CP55940-induced increase in BP obtained in the presence of bicuculline and enhanced that determined in the presence of L-NAME, suggesting that the CP55940 increased BP acting predominantly at inhibitory presynaptic CB1 receptors on GABAergic neurones or by increasing NO production. Similarly, the microinjection of the CB1 receptor agonist WIN55212-2 into the RVLM (Ibrahim and Abdel-Rahman 2011) or AEA into the dPAG (Dean 2011) increased BP via inhibition of brainstem GABAergic transmission and by increasing the NO level in the RVLM (Ibrahim and Abdel-Rahman 2012). Colocalization of CB1 receptors and NO synthase has been shown in the rat PVN (Zou et al. 2015). The glutamate-based stimulatory output of the PVN is subject to a tonic inhibition arising from GABA and NO (Pyner 2009; Kc and Dick 2010). Both bicuculline (Li et al. 2006) and AM251 (Gyombolai et al. 2012) given into the PVN caused increases in BP and/or HR, suggesting that the sympathetic tone is tonically inhibited not only by the GABAergic but also by the endocannabinergic system. The fact that the above antagonists had no influence on basal cardiovascular parameters in our study is probably related to the fact that they were administered i.v. Since L-NAME increased BP by itself, we performed additional experiments in which basal BP was increased by PGF2α infusion to the level obtained after L-NAME application. Our data show that compared to the L-NAME group, AM251 further increased the CP55940-induced effect on DBP and MBP regardless of whether the basal BP was increased by L-NAME or PGF2α.
The AT1 antagonist losartan completely reversed the CP55940-induced pressor and tachycardic effects into hypotension and bradycardia. The NMDA, TP and β2 receptor antagonists MK801, SQ29584, and ICI118551, respectively, diminished the CP55940-induced pressor effects by about 50–60 % without affecting the CP55940-stimulated increases in HR. As shown in Fig. 5, AT1, TP and β2 receptors enhance glutamatergic transmission, which is additionally increased by the activation of NMDA receptors.
Our data suggest that AT1 receptors seem to play a major role in the CP55940-induced stimulatory cardiovascular responses. The particularly strong effect of losartan might result from the fact that Ang II in the PVN does not only increase the glutamatergic tone (Pyner 2009; Kc and Dick 2010; Ferguson et al. 2008; Nunn et al. 2011) but also inhibits the GABAergic tone (via an inhibitory influence on NO or GABA; Chen and Pan 2007; Nunn et al. 2011). Our results are in line with findings by Gyombolai et al. (2012) who showed that CB1 receptors play a role in the hypertensive effects of angiotensin II in the rat paraventricular nucleus since co-administration of AM251 together with Ang II abolished the well-known pressor effect of Ang II given into the PVN. Gyombolai et al. (2012) suggested that the Ang II-induced hypertension is connected with the activation of CB1 receptors, e.g. due to (1) the heterodimerization of CB1 and AT1 receptors (Rozenfeld et al. 2011) or (2) Ang II–induced endocannabinoid release that inhibits the GABAergic tone. In another study on the rat PVN (Chen and Pan 2007), a direct inhibitory effect of Ang II on GABAergic transmission, involving Gi/o proteins and superoxide formation, has been suggested.
Electrical stimulation of the C1 area of the rostral ventrolateral medulla increased BP in rats in a manner sensitive to intra-hypothalamic microinjection of the β2 antagonist ICI118551 but not to the β1 antagonist atenolol (Ward-Routledge et al. 1988). In addition, the PVN is richly innervated by noradrenergic nerve terminals originating from the brainstem, especially A1, A2 and A6 cell groups (Cunningham and Sawchenko 1988). It contains more β2- than β1-adrenoceptors (Rainbow et al. 1984). However, the microinjection of the β2-adrenoceptor agonist fenoterol did not modify BP and HR in anaesthetized rats, but authors have used one dose of the agonist (5 nmol/rat) only (Tsushima et al. 1994). On the other hand, noradrenaline microinjected into the PVN increased BP (Bachelard et al. 1992). Moreover, ICI118551 but not atenolol diminished the pressor effect of endothelin i.c.v., which may indirectly act via β2-adrenoceptors (Ono and Kaneko 1995).
Bilateral adrenalectomy completely reversed the pressor and tachycardic effects of CP55940 to hypotension and bradycardia of a comparable magnitude as they were before AM251 administration. Thus, we can conclude that the pressor effect of CP55940 given into the PVN is completely related to catecholamine release from the adrenal medulla but not from sympathetic nerve endings. Similarly, microinjection of a TXA2 mimetic into the PVN elevated plasma levels of adrenaline but had little effect on plasma levels of noradrenaline, suggesting that TP receptors are involved in the central adrenomedullary outflow in rats (Murakami et al. 2002).
Perfusion of the PVN with NMDA increased, in a manner sensitive to MK801, the local level of thromboxane B2 (the inactive metabolite of TXA2), glutamate and GABA, the plasma level of catecholamines (Okada et al. 2000; Kondo et al. 2015) and BP (Li and Pan 2007). We confirmed that microinjection of NMDA into the PVN increased BP and HR (e.g. Kawabe et al. 2008) and showed for the first time that both cardiovascular effects were diminished by AM251 given i.v. We can exclude the possibility that AM251 inhibits presynaptic inhibitory CB1 receptors located mainly on peripheral sympathetic nerve endings innervating resistance vessels and heart (for review, see Malinowska et al. 2010) or on central glutamatergic neurons (for review, see Schlicker and Kathmann 2001) since in both cases an increase in BP would be expected. The most plausible explanation of our data is that AM251 inhibits presynaptic inhibitory CB1 receptors located on central GABAergic neurons, thereby dis-inhibiting the GABAergic tone and inhibiting the NMDA-induced stimulatory effects. The fact that AM251 increases basal BP in the study by Gyombolai et al. (2012) but reduces the NMDA-induced increase in BP in the present one might be related to a normal and enhanced sympathetic tone, respectively (discussed in Grzęda et al. 2015).
Conclusions
Stimulation of presynaptic inhibitory CB1 receptors on glutamatergic neurons in the PVN decreases BP and HR. Even if due to the i.v. administration of the antagonists/blockers, other locations cannot be excluded with absolute certainty that our data provide evidence that glutamatergic neurotransmission is probably increased by presynaptic facilitatory AT1, TP and β2-adrenergic heteroreceptors but inhibited by postsynaptic GABAA receptors (Fig. 5). On the other hand, stimulation of presynaptic CB1 receptors on GABAergic neurones in the PVN might inhibit their inhibitory influence on glutamatergic neurotransmission, thereby increasing the sympathetic tone and ultimately leading to increases in BP and HR. The tone of the GABAergic neurones might in turn be increased by NO. The final effect, i.e. depression or stimulation of cardiovascular parameters, probably depends on the level of the sympathetic tone. The increase and decrease in BP (and HR) induced by the activation of CB1 receptors in the PVN are dependent and independent of catecholamine release from the adrenal medulla, respectively.
Acknowledgments
The work has been supported by the Medical University of Białystok (Poland; grant Nos. 133-13561F and 143-13794F).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Bachelard H, Harland D, Gardiner SM, Kemp PA, Bennett T. Regional haemodynamic effects of noradrenaline injected into the hypothalamic paraventricular nuclei of conscious, unrestrained rats: possible mechanisms of action. Neuroscience. 1992;47:941–957. doi: 10.1016/0306-4522(92)90042-Z. [DOI] [PubMed] [Google Scholar]
- Busnardo C, Tavares RF, Correa FM. Angiotensinergic neurotransmission in the paraventricular nucleus of the hypothalamus modulates the pressor response to acute restraint stress in rats. Neuroscience. 2014;270:12–19. doi: 10.1016/j.neuroscience.2014.03.064. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Desprez T, Metna-Laurent M, Bellocchio L, Marsicano G, Soria-Gomez E. Dissecting the cannabinergic control of behavior: the where matters. BioEssays. 2015;37:1215–1225. doi: 10.1002/bies.201500046. [DOI] [PubMed] [Google Scholar]
- Carruba MO, Bondiolotti G, Picotti GB, Catteruccia N, Da Prada M. Effects of diethyl ether, halothane, ketamine and urethane on sympathetic activity in the rat. Eur J Pharmacol. 1987;134:15–24. doi: 10.1016/0014-2999(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Chen Q, Pan HL. Signaling mechanisms of angiotensin II–induced attenuation of GABAergic input to hypothalamic presympathetic neurons. J Neurophysiol. 2007;97:3279–3287. doi: 10.1152/jn.01329.2006. [DOI] [PubMed] [Google Scholar]
- Crosby KM, Bains JS (2012) The intricate link between glucocorticoids and endocannabinoids at stressrelevant synapses in the hypothalamus. Neuroscience 204:31–7 [DOI] [PubMed]
- Cunningham ET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Dean C. Cannabinoid and GABA modulation of sympathetic nerve activity and blood pressure in the dorsal periaqueductal gray of the rat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1765–R1772. doi: 10.1152/ajpregu.00398.2011. [DOI] [PubMed] [Google Scholar]
- Elsersy H, Mixco J, Sheng H, Pearlstein RD, Warner DS. Selective gamma-aminobutyric acid type A receptor antagonism reverses isoflurane ischemic neuroprotection. Anesthesiology. 2006;105:81–90. doi: 10.1097/00000542-200607000-00016. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus–a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets. 2008;12:717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Factors influencing the regional haemodynamic responses to methanandamide and anandamide in conscious rats. Br J Pharmacol. 2009;158:1143–1152. doi: 10.1111/j.1476-5381.2009.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Alapafuja SO, Bátkai S, Nikas SP, Cinar R, Offertáler L, Osei-Hyiaman D, Liu J, Mukhopadhyay B, Harvey-White J, Tam J, Pacak K, Blankman JL, Cravatt BF, Makriyannis A, Kunos G. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol. 2010;17:1256–1266. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordish KL, Beierwaltes WH. Resveratrol induces acute endothelium-dependent renal vasodilation mediated through nitric oxide and reactive oxygen species scavenging. Am J Physiol Renal Physiol. 2014;306:F542–F550. doi: 10.1152/ajprenal.00437.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzęda E, Schlicker E, Luczaj W, Harasim E, Baranowska-Kuczko M, Malinowska B. Bi-directional CB1 receptor-mediated cardiovascular effects of cannabinoids in anaesthetized rats: role of the paraventricular nucleus. J Physiol Pharmacol. 2015;66:343–353. [PubMed] [Google Scholar]
- Gyombolai P, Pap D, Turu G, Catt KJ, Bagdy G, Hunyady L. Regulation of endocannabinoid release by G proteins: a paracrine mechanism of G protein-coupled receptor action. Mol Cell Endocrinol. 2012;353:29–36. doi: 10.1016/j.mce.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor responses in conscious rats. Brain Res. 2011;1414:1–9. doi: 10.1016/j.brainres.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressor response in conscious rats. J Pharmacol Exp Ther. 2012;341:579–586. doi: 10.1124/jpet.112.192369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. Cannabinoid receptor 1 signaling in cardiovascular regulating nuclei in the brainstem: a review. J Adv Res. 2014;5:137–145. doi: 10.1016/j.jare.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular function of a glutamatergic projection from the hypothalamic paraventricular nucleus to the nucleus tractus solitarius in the rat. Neuroscience. 2008;153:605–617. doi: 10.1016/j.neuroscience.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kc P, Dick TE. Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Res Physiol Neurobiol. 2010;174:55–64. doi: 10.1016/j.resp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo F, Tachi M, Gosho M, Fukayama M, Yoshikawa K, Okada S. Changes in hypothalamic neurotransmitter and prostanoid levels in response to NMDA, CRF, and GLP-1 stimulation. Anal Bioanal Chem. 2015;407:5261–5272. doi: 10.1007/s00216-015-8496-6. [DOI] [PubMed] [Google Scholar]
- Kwolek G, Zakrzeska A, Schlicker E, Göthert M, Godlewski G, Malinowska B. Central and peripheral components of the pressor effect of anandamide in urethane-anaesthetized rats. Br J Pharmacol. 2005;145:567–575. doi: 10.1038/sj.bjp.0706195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- Lu QB, Feng XM, Tong N, Sun HJ, Ding L, Wang YJ, Wang X, Zhou YB. Neuronal and endothelial nitric oxide synthases in the paraventricular nucleus modulate sympathetic overdrive in insulin-resistant rats. PLoS One. 2015;10:e0140762. doi: 10.1371/journal.pone.0140762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B, Baranowska-Kuczko M, Schlicker E. Triphasic blood pressure responses to cannabinoids: do we understand the mechanism? Br J Pharmacol. 2012;165:2073–2088. doi: 10.1111/j.1476-5381.2011.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B, Piszcz J, Koneczny B, Hryniewicz A, Schlicker E. Modulation of the cardiac autonomic transmission of pithed rats by presynaptic opioid OP4 and cannabinoid CB1 receptors. Naunyn Schmiedeberg's Arch Pharmacol. 2001;364:233–241. doi: 10.1007/s002100100450. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Zakrzeska A, Kurz CM, Göthert M, Kwolek G, Wielgat P, Braszko JJ, Schlicker E. Involvement of central β2-adrenergic, NMDA and thromboxane A2 receptors in the pressor effect of anandamide in rats. Naunyn Schmiedeberg's Arch Pharmacol. 2010;381:349–360. doi: 10.1007/s00210-010-0497-6. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Okada S, Nishihara M, Yokotani K. Roles of brain prostaglandin E2 and thromboxane A2 in the activation of the central symphato-adrenomedullary outflow in rats. Eur J Pharmacol. 2002;452:289–294. doi: 10.1016/S0014-2999(02)02308-7. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Cannabinoid cause central sympathoexcitation and bradycardia in rabbits. J Pharmacol Exp Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedeberg's Arch Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- Nunn N, Womack M, Dart C, Barrett-Jolley R. Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Curr Neuropharmacol. 2011;9:262–277. doi: 10.2174/157015911795596531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Murakami Y, Nishihara M, Yokotani K, Osumi Y. Perfusion of the hypothalamic paraventricular nucleus with N-methyl-D-aspartate produces thromboxane A2 and centrally activates adrenomedullary outflow in rats. Neuroscience. 2000;96:585–590. doi: 10.1016/S0306-4522(99)00598-9. [DOI] [PubMed] [Google Scholar]
- Ono N, Kaneko M. Influences of β-blocking agents on cardiovascular actions induced by endothelin-3 administered intracerebroventricularly in anesthetized rats. Life Sci. 1995;57:345–353. doi: 10.1016/0024-3205(95)00293-F. [DOI] [PubMed] [Google Scholar]
- Padley JR, Li Q, Pilowsky PM, Goodchild AK. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetized rats. Br J Pharmacol. 2003;140:384–394. doi: 10.1038/sj.bjp.0705422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631 International Union of Basic and Clinical Pharmacology. [DOI] [PMC free article] [PubMed]
- Pfitzer T, Niederhoffer N, Szabo B. Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetized rats. Br J Pharmacol. 2004;142:943–952. doi: 10.1038/sj.bjp.0705874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat. 2009;38:197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parson B, Wolf BB. Quantitative autoradiography of β1- and β2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Gupta A, Gagnidze K, Lim MP, Gomes I, Lee-Ramos D, Nieto N, Devi LA. AT1R–CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 2011;30:2350–2363. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/S0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Senst L, Bains J. Neuromodulators, stress and plasticity: a role for endocannabinoid signalling. J Exp Biol. 2014;217(Pt 1):102–108. doi: 10.1242/jeb.089730. [DOI] [PubMed] [Google Scholar]
- Tsushima H, Fujimoto S, Matsuda T. Effects of β1- and β2-adrenoceptor agonists applied into the hypothalamic paraventricular nuclei of spontaneously hypertensive rats on urine production. Jpn J Pharmacol. 1994;64:201–207. doi: 10.1254/jjp.64.201. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Kim CK, Lee S, Rivier CL. Influence of carbon monoxide, and its interaction with nitric oxide, on the adrenocorticotropin hormone response of the normal rat to a physico-emotional stress. J Neuroendocrinol. 1998;10:793–802. doi: 10.1046/j.1365-2826.1998.00266.x. [DOI] [PubMed] [Google Scholar]
- Wang JM, Tan J, Leenen FH. Central nervous system blockade by peripheral administration of AT1 receptor blockers. J Cardiovasc Pharmacol. 2003;41:593–599. doi: 10.1097/00005344-200304000-00012. [DOI] [PubMed] [Google Scholar]
- Ward-Routledge C, Marshall P, Marsden CA. Involvement of central α- and β-adrenoceptors in the pressor response to electrical stimulation of the rostral ventrolateral medulla in rats. Br J Pharmacol. 1988;94:609–619. doi: 10.1111/j.1476-5381.1988.tb11567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Somvanshi RK, Paik S, Kumar U. Colocalization of cannabinoid receptor 1 with somatostatin and neuronal nitric oxide synthase in rat brain hypothalamus. J Mol Neurosci. 2015;55:480–491. doi: 10.1007/s12031-014-0369-5. [DOI] [PubMed] [Google Scholar]