Abstract
Reversed-phase chromatography is a method that is often used for glycan separation. For this, glycans are often derivatized with a hydrophobic tag to achieve retention on hydrophobic stationary phases. The separation and elution order of glycans in reversed-phase chromatography is highly dependent on the hydrophobicity of the tag and the contribution of the glycan itself to the retention. The contribution of the different monosaccharides to the retention strongly depends on the position and linkage, and isomer separation may be achieved. The influence of sialic acids and fucoses on the retention of glycans is still incompletely understood and deserves further study. Analysis of complex samples may come with incomplete separation of glycan species, thereby complicating reversed-phase chromatography with fluorescence or UV detection, whereas coupling with mass spectrometry detection allows the resolution of complex mixtures. Depending on the column properties, eluents, and run time, separation of isomeric and isobaric structures can be accomplished with reversed-phase chromatography. Alternatively, porous graphitized carbon chromatography and hydrophilic interaction liquid chromatography are also able to separate isomeric and isobaric structures, generally without the necessity of glycan labeling. Hydrophilic interaction liquid chromatography, porous graphitized carbon chromatography, and reversed-phase chromatography all serve different research purposes and thus can be used for different research questions. A great advantage of reversed-phase chromatography is its broad distribution as it is used in virtually every bioanalytical research laboratory, making it an attracting platform for glycan analysis.
Graphical Abstract.
Glycan isomer separation by reversed phase liquid chromatography
Keywords: Glycan, Reversed phase, Liquid chromatography, Separation
Introduction
Glycosylation is a frequently observed posttranslational modification in proteins. Many membrane and secretory proteins are glycosylated while passing through the endoplasmic reticulum and Golgi system [1]. Glycans are composed of monosaccharides that contain many chiral centers and are connected by glycosidic linkages. They may have very complex three-dimensional structures [2], and stereoisomerism can have a substantial influence on the function of these molecules [3]. Various glycans are found in human cells; for example, N-glycans or O-glycans that are linked to proteins, next to lipid-linked glycans and free molecules [4]. Structural and conformational differences in proteins can be caused by glycans, which may result in modulated protein activity and protein interactions [3, 5, 6]. These molecules participate in many different biological processes, such as cell signaling and recognition, immune defense, and parasitic infections [3].
The analysis of protein glycosylation can be performed on different levels—intact glycoproteins [7, 8], glycopeptides [9–12], and released glycans [13–15]—each resulting in slightly different information on the glycoprotein. A disadvantage of the analysis of intact glycoproteins is that good separation of the different glycoforms of a glycoprotein is hard to achieve, especially for proteins with many glycosylation sites and in complex samples [3]. The analysis of glycopeptides has the advantage that the glycosylation can be assigned to specific locations on the protein. This site-specific information can be used to assign specific glycan structures to distinct glycosylation sites. Furthermore, it can contribute to the understanding of the molecular structure of the protein [9, 16].
In this review the focus is on released glycans. Glycans can be released from proteins and peptides in an enzymatic and chemical way [17, 18]. For N-glycans, various different enzymatic release methods are available, but for O-glycans, generally chemical release methods need to be used. The use of a chemical release method for glycans has several limitations: For example, the reducing-end aldehyde of the glycan can be reduced to an alditol by reductive β-elimination [19], thereby prohibiting subsequent labeling of the reducing end. After release, a derivatization step is often performed to improve the properties of the glycans for analysis. In addition, when one is working with complex biological samples, enrichment of the glycans needs to be performed. Hydrophilic interaction liquid chromatography (HILIC), graphitized carbon chromatography, and reversed-phase solid-phase extraction are the most used methods for enrichment of glycans [20–26]. Besides these methods, methods based on graphene have been developed [27, 28].
Information on the released glycans can be gathered with several different techniques. Separation techniques such as capillary electrophoresis (CE) and liquid chromatography (LC) are often used in combination with mass spectrometry (MS), fluorescence, or UV detection. In addition, matrix-assisted laser desorption/ionization MS is used for glycan analysis without separation or in combination with LC fractionation. Also, gel electrophoresis is a commonly used technique for glycan analysis. Most of these techniques can be used for analysis of glycans in both their native form and their derivatized form.
For released glycan analysis, various LC stationary phases are used, including high-pH anion-exchange [29, 30], HILIC [31–33], porous graphitized carbon (PGC) [34–36], and reversed-phase stationary phases (see Table 1). In high-pH anion exchange, deprotonation of hydroxyl groups is achieved, which contributes to the separation of the glycans. Both native and derivatized glycans can be separated with this technique [29]. PGC separation is based on hydrophobic and polar interactions [34, 37]. Native glycans are retained on the stationary phase and are eluted with water and acetonitrile [34]. Strong acidic or basic eluents can be used, because the columns are more hydrophobic and chemically stabler than reversed-phase columns [37].
Table 1.
Reversed-phase (RP) liquid chromatography (LC) methods for glycan analysis
| Column and flow | Solvents | pH | Samples | Derivatization | Separation | Detection | Ionization mode | Remarks | References | Year |
|---|---|---|---|---|---|---|---|---|---|---|
| RP nano column: C18 StableBond Zorbax 5 μm; 75 μm × 150 mm (0.250 μL/min) | Gradient of 97 % water–3 % acetonitrile–0.1 % formic acid with 0.5 mM sodium acetate and 97 % acetonitrile–3 % water–0.1 % formic acid with 0.5 mM sodium acetate | Acidic | N-Glycans from bovine fetuin and human blood serum samples | Reduction and permethylation | Separation was performed to minimize negative effects from competitive ionization | ESI-MS | + | [48] | 2010 | |
| RP precolumn: Acclaim PepMap100 C18 nano trap column. Analytical RP column: Acclaim PepMap100 C18; 75 μm × 150 mm (0.300 μL/min) |
Gradient of 2 % acetonitrile–0.1 % formic acid in water and 0.1 % formic acid in acetonitrile | Acidic | N-Glycans from model glycoproteins and from human blood serum | Reduction and permethylation | RP separation was used as the sample purification method | ESI-MS | + | Analysis of small amounts (low picomole to femtomolerange) is challenging | [49] | 2011 |
| RP nano column: PepMap; 75 μm × 150 mm (0.350 μL/min) | Gradient of 2 % acetonitrile in water with 0.1 % formic acid and acetonitrile with 0.1 % formic acid | Acidic | Released permethylated N-glycans from model glycoproteins (RNase B and porcine thyroglobulin) and human blood serum | Reduction and permethylation | Different glycan compositions were baseline-separated, but this was not the case for all samples | ESI-MS MALDI-TOF-MS |
+ | No detection of low-abundance structures | [43] | 2012 |
| RP precolumn: NanoEase Atlantis C18 5 μm; 100 Å, 180 μm × 23.5 mm (10 μL/min). Analytical RP column: PepMap 100 3 μm; 75 μm × 150 mm (0.300 μL/min) |
Gradient of 10 % acetonitrile in 0.1 % formic acid and sodium hydroxide and 90 % acetonitrile and 10 % 2-propanol in 0.1 % formic acid | Acidic | Purified glycan standards (sialyl Lewis X and sialyl Lewis A) and N-glycans originating from α-1-acid glycoprotein and IgG | Reduction and permethylation | Isomers of glycans were separated | ESI-MS | + | Sodium hydroxide was added to the eluent to induce sodium adduct formation. Glycans were analyzed in the low femtomole range |
[44] | 2013 |
| Acclaim C18 nano column and HSS T3 C18 nano UPLC column (350 nL/min) | Gradient of 0.1 % formic acid in 2 % acetonitrile and 0.1 % formic acid in 100 % acetonitrile | Acidic | Released N-glycans from RNase B, fetuin, and human blood serum | Reduction and permethylation | Isomer separation was achieved at high temperatures | ESI-MS | + | Separation was performed at different temperatures: ambient to 75 °C | [50] | 2016 |
| Nano-LC RP trap column: PepMap 3 μm; 75 μm × 20 mm. RP nano column: Acclaim PepMap C18 75 μm × 150 mm (300 nL/min) |
Gradient of 0.1 % formic acid in 2 % acetonitrile and 0.1 % formic acid in 100 % acetonitrile | Acidic | Released N-glycans from RNase B, fetuin, and human blood serum | Reduction and permethylation | Glycans were not fully separated but were spread over a retention time range of 20–50 min. From MS-detection the different co-eluting glycans could be identified | ESI-MS | + | [52] | 2016 | |
| Acclaim PepMap C18 75 μm × 150 mm (350 nL/min) | Gradient of 0.1 % formic acid in 2 % acetonitrile and 0.1 % formic acid in 100 % acetonitrile | Acidic | Human, bovine and goat milk free oligosaccharides and N-glycans | Reduction and permethylation | Glycans were not fully separated but spread over a retention time range of 15 to 55 minutes. From MS detection the different coeluted glycans could be identified. Isomers of glycans were also separated | ESI-MS | + | [51] | 2016 | |
| Alltech Adsorbosphere RP C18 column | Isocratic methanol–water (80:20) containing 1 % acetic acid | Acidic | Oligosaccharides | Permethylation | RP chromatography was only used to separate glycans from salt contaminants | ESI-MS | + | [121] | 1997 | |
| Hypersil C18; 100 mm × 2.1 mm (0.2–0.4 mL/min) | Gradient and isocratic measurements with water and methanol and/or acetonitrile buffered with 1 mM sodium acetate | Acidic | Permethylated oligosaccharide mixtures | 2-Aminobenzamide and permethylation | α and β anomers were differentiated in some cases, but in other measurements the separation of diantennary, triantennary and tetraantennary glycans was poor | ESI-MS | + | [122] | 2001 | |
| Hypersil ODS C18 3 μm; 150 mm × 4.6 mm (0.5–1.5 mL/min) | Gradient of 50 mM formic acid in water adjusted to pH 5 with triethylamine and 50:50 first solvent–acetonitrile | 5.0 | O-Glycans from bovine serum fetuin, human serum IgA1, human secretory IgA, human neutrophil gelatinase B, and human glycophorin A | 2-Aminobenzamide | Low peak capacity, glycan species were not separated individually | FL (excitation 330 nm, emission 420 nm) | An ion-pairing reagent (triethylamine) was added to separate glycans containing sialic acids. Glycans were analyzed in the low femtomole range |
[60] | 2002 | |
| Hypersil ODS C18 3 μm; 4.6 mm × 150 mm (0.5–1.5 mL/min) | Gradient of 50 mM formic acid in water adjusted to pH 5 with triethylamine and 50:50 first solvent–acetonitrile | 5.0 | N-Glycans and O-glycans from apolipoprotein (a) | 2-Aminobenzamide | Glycans were separated but the run time was 180 min | FL (excitation 330 nm, emission 420 nm) | [84] | 2001 | ||
| Acquity UPLC BEH C18 1.7 μm; 100 mm × 2.1 mm (0.350 mL/min) | Gradient of water and 25:75 methanol–water both containing 20 mM diethylamine (ion-pairing agent) and 50 mM formic acid | Acidic | Released N-glycans from monoclonal antibodies, fetuin, and RNase B | 2-Aminobenzamide | Selectivity for glycans is low and low peak capacity | FL (excitation 250 nm, emission 428 nm) | An ion-pairing reagent (diethylamine) was added to separate glycans containing sialic acids | [61] | 2011 | |
| Nano-LC RP trap column: PepMap 100 3 μm; 300 μm × 5 mm. RP nano column: PepMap C18 100 3 μm; 75 μm × 150 mm (gradient pump 150 nL/min and microflow pump 10 μL/min) |
Gradient pump: gradient of 0.4 % acetonitrile in water with 0.1 % formic acid and water–acetonitrile (5:95 v/v) containing 0.1 % formic acid. Microflow pump: 0.4 % acetonitrile in water with 0.1 % formic acid | 4.4 | Glycan pools | 2-Aminobenzamide | ND | UV absorbance (254 nm) FL (excitation 360 nm, emission 425 nm) ESI-MS |
+ and − | Protocol for RP separation only or as a second dimension after HILIC separation. Glycans were analyzed in the femtomole range |
[38] | 2009 |
| Nano-LC RP guard column: PepMap; 300 μm × 10 mm. RP nano column: PepMap C18 3 μm; 75 μm × 150 mm (150 nL/min) |
Gradient of water–acetonitrile (95:5 v/v) containing 0.1 % formic acid and water–acetonitrile (5:95 v/v) containing 0.1 % formic acid | Acidic | Released glycans from glycoproteins from Schistosoma mansoni worms | 2-Aminobenzamide | With RP separation in the second dimension after HILIC separation, the different glycans coeluted in HILIC were not fully separated | ESI-MS | + | Method for RP separation only and for separation in the second dimension after HILIC separation | [45] | 2006 |
| PepMap C18 3 μm; 75 μm × 150 mm (150 nL/min) | Gradient of 0.8 mM sodium hydroxide in water–acetonitrile (95:5 v/v) containing 0.1 % formic acid and water–acetonitrile (5:95 v/v) containing 0.1 % formic acid | Acidic | Egg-derived oligosaccharides from urine from individuals infected with S. mansoni | 2-Aminobenzamide | RP separation was used to obtain fragmentation spectra of major oligosaccharides | ESI-MS | + | Sodium hydroxide was added to the eluent to induce sodium adduct formation | [46] | 2007 |
| Nano-LC RP guard column: PepMap; 300 μm × 10 mm. RP nano column: PepMap C18 3 μm; 75 μm × 150 mm (150 nL/min) |
Gradient of 0.4 % acetonitrile in water with 0.1 % formic acid and water–acetonitrile (5:95 v/v) containing 0.1 % formic acid | Acidic | Released glycans from glycoproteins from S. mansoni worms and released N-glycans in tobacco plants | 2-Aminobenzamide | Low peak capacity, elution of glycans was spread over time, but there was no clear separation. With RP separation in the second dimension after HILIC separation, the different glycans coeluted in HILIC were separated |
ESI-MS MALDI-TOF |
+ | Method for RP separation only and for separation in the second dimension after HILIC separation | [47] | 2006 |
| Thermo Scientific C18 3 μm; 250 mm × 4 mm (0.2 mL/min) | Gradient of water and 10:90 acetonitrile–water with both containing 0.1 % acetic acid | Acidic | Released N-glycans from recombinant IgG antibodies | 2-Aminobenzamide | Glycans were separated but the run time was ≥140 min | FL (excitation 330 nm, emission 420 nm) ESI-MS |
+ | Glycans were analyzed in the femtomole range | [80] | 2007 |
| Thermo Scientific C18 3 μm; 250 mm × 4 mm (0.2 mL/min) | Gradient of water and 10:90 acetonitrile–water with both containing 0.1 % acetic acid | Acidic | Released N-glycans from RNase B from bovine pancreas, ovalbumin (grade VII) from chicken egg, and fetuin from fetal calf serum | 2-Aminobenzamide | Glycans were separated but the run time was ≥160 min | FL (excitation 330 nm, emission 420 nm) ESI-MS |
+ and − | Glycans were analyzed in the femtomole range | [81] | 2009 |
| Waters T3 C18 1.7 μm; 150 mm × 2.1 mm | Gradient of water and acetonitrile both containing 0.1 % formic acid | Acidic | N-Glycans from human, bovine, equine, and canine IgGs | 2-Aminobenzamide | Different types of glycans were separated, but assignment of individual glycans was difficult. Sialylated glycans could not be separated | FL (excitation 330 nm, emission 420 nm) | [82] | 2014 | ||
| Zorbax rapid resolution SB-C18 1.8 μm; 50 mm × 2.1 mm (0.333 mL/min) | Gradient of water and 5 % acetonitrile in water both containing 0.1 % acetic acid | Acidic | Released N-glycans from recombinant IgG | 2-Aminobenzamide | Separation of isomers was observed in a runtime of 50 min | FL ESI-MS |
+ | A rapid resolution column was used. The limit of detection was less than 10 fmol | [83] | 2009 |
| Xterra column C18 3.5 μm; 2.1 mm × 150 mm (0.15 mL/min) | Gradient of 2 % acetonitrile in 0.1 % trifluoroacetic acid and 20 % acetonitrile in 0.1 % trifluoroacetic acid | Acidic | Purified oligosaccharides | 2-Aminobenzamide | Isomers of glycans were separated but the run time was ≥180 min | UV absorbance (230 nm) ESI-MS |
+ | [125] | 2005 | |
| Acquity UPLC BEH C18 1.7 μm; 2.1 mm × 150 mm (0.3 mL/min) | Anthranilic acid: gradient of 1.0 % formic acid in water and 1.0 % formic acid in 50 % acetonitrile. 2-Aminobenzamide: gradient of 0.5 % formic acid in water and 0.5 % formic acid in 5 % acetonitrile |
Acidic | N-Glycans from monoclonal antibodies | Anthranilic acid and 2-aminobenzamide | Isomers of glycans were separated but the run time was 80 minutes. Coelution of glycans was observed | FL (excitation 250 nm, emission 425 nm) ESI-MS |
+ | [126] | 2013 | |
| Hypersil ODS column C18; 250 mm × 4 mm (1.2 mL/min) | Gradient of 50 mM ammonium formate and acetonitrile | 4.4 | Released N-glycans from bovine fibrin and IgG | 2-Aminopyridine and other fluorescent labels for oligosaccharides | Desialylated IgG N-glycans can be separated, but this is strongly dependent on the label | MALDI-TOF-MS ESI-MS |
+ and − | A less hydrophobic label increases the contribution of the glycan itself to the retention | [70] | 2009 |
| Shim-pack VP-ODS C18, 2 mm ID, and Shim-pack CLC-ODS C18, 6 mm ID | Gradient of water with 10 mM ammonium formate and water with 10 mM ammonium formate containing 0.5 % 1-butanol | 4.0 | Released N-glycans from human IgG from serum | 2-Aminopyridine | Isomers of glycans were separated in a run time of 60 min | MALDI-TOF-MS | [88] | 2009 | ||
| Shim-pack HRC-ODS-silica C18; 150 mm × 6 mm (1.0 mL/min) | Gradient of 10 mM sodium phosphate buffer and 10 mM sodium phosphate buffer containing 0.5 % 1-butanol | 3.8 | Released N-glycans from human serum glycoproteins | 2-Aminopyridine | Broad peaks and run time of ≥70 min, but separation of glycans | FL (excitation 320 nm, emission 400 nm) MALDI-TOF-MS |
+ | [89] | 2007 | |
| Stainless steel column 4 mm × 250 mm packed with TSKgel (5 μm, C18). Cosmosil 5C18-P column (0.46 cm × 15 cm or 25 cm). μBondasphere 5 μm C18 300 Å column (0.39 cm × 15 cm) (1.5 mL/min) |
Gradient of 0.1 M ammonium acetate buffer and 0.1 M ammonium acetate buffer containing 0.5 % 1-butanol | 4.0 | N-Glycans | 2-Aminopyridine | Different glycans were separated, but separation by gel filtration is needed before RP analysis (1986) [90, 91]. The method was used to purify glycans from glycoproteins (1990) [95]. Coelution of glycans was observed |
FL (excitation 320 nm, emission 400 nm) | Prediction of retention times of glycans in RP chromatography | [90, 91, 95] | 1986, 1990 | |
| Stainless steel column 4 mm × 250 mm packed with TSKgel (5 μm, C18) (1.6 mL/min) | 0.1 M phosphate buffer | 3.8 | Glucose, lactose, laminaribiose, maltose, gentiobiose, cellobiose, and isomaltooligosaccharides | 2-Aminopyridine | Isomers of glycans were separated, but not all glycans were baseline-separated | FL (excitation 320 nm, emission 400 nm) | Glycans were analyzed in the picomole range | [92] | 1981 | |
| Cosmosil 5C18-P column (250 mm × 1.5 mm) (150 μL/min) | 20 mM ammonium acetate buffer containing 0.075 % 1-butanol, with increasing concentration of 1-butanol during the run | 4.0 | N-Glycans | 2-Aminopyridine | Coelution of glycans was observed | FL (excitation 320 nm, emission 400 nm) | Prediction of retention times of glycans in RP chromatography | [93] | 1998 | |
| Shim-pack CLC-ODS-silica C18; 6 mm × 150 mm (1.0 mL/min) | ND | ND | N-Glycans from human IgG | 2-Aminopyridine | Glycans were not all separated individually and the run time was ≥60 min | FL | [94] | 2006 | ||
| AquaSep C8 5 μm; 250 mm × 4.6 mm (1.0 mL/min) | Gradient of 0.05 % trifluoroacetic acid in water and acetonitrile | Acidic | Lactose and maltopentaose | 2-Amino-5-bromopyridine | Low peak capacity | UV absorbance (200-320 nm) ESI-MS |
+ | The method was not optimized for RP separation. The limit of detection was approximately 14 pmol |
[39] | 2003 |
| Various C18 phases with 3-μm particle size; 200 mm × 75 μm (0.3 mL/min) | Gradients of 5 mM ammonium acetate in water and acetonitrile | 6.5 | Dextrin 20, dextran from Leuconostoc ssp., glucose, and maltose | 4-Aminobenzoic acid methyl ester, 4-aminobenzoic acid butyl ester, aminobenzoic acid ethyl ester, and 4-n-heptyloxyaniline | Low peak capacity, species were not separated individually | ESI-MS UV absorbance MALDI-TOF-MS |
+ | [59] | 2002 | |
| Symmetry C18; 4.6 mm × 250 mm (1.0 L/min) | Gradient of 100 mM ammonium acetate (pH 6.69) and acetonitrile | Acidic | N-linked glycans released from α1-acid glycoprotein and IgG | 2-Aminoacridone | Low peak capacity, species were not separated individually. | FL (excitation 442 nm, emission 520 nm) MALDI-TOF-MS |
+ | [100] | 1997 | |
| Zorbax Eclipse XDB C18 5 μm; 150 mm × 4.6 mm (1 mL/min) | Gradient of 0.1 M ammonium acetate in water and methanol | Acidic | Disaccharides from rat liver proteoglycans | 2-Aminoacridone | Low peak capacity compared with SAX-HPLC, but labeled saccharides were separated | FL (excitation 425 nm, emission 520 nm) | Glycans were analyzed in the femtomole range | [102] | 2008 | |
| Symmetry C18; 4.6 mm × 250 mm (1.0 L/min) | Gradient of 100 mM ammonium acetate and acetonitrile | 6.6 | Released glycans from bovine RNase B and α-acid glycoprotein and a dextran ladder | 2-Aminoacridone and 3-(acetylamino)-6-aminoacridine | Low peak capacity, species were not separated individually | FL (excitation 442 nm, emission 525 nm) MALDI-TOF-MS |
+ and − | Glycans were analyzed in the femtomole range | [103] | 2000 |
| Symmetry C18; 150 mm × 1.0 mm (0.050 mL/min) | Gradient of 10 mM triethylammonium acetate in water and 10 mM triethylammonium acetate in methanol | 7.0 | Released glycans from bovine fetuin, bovine RNase B, and chicken ovalbumin | 8-Aminonaphthalene-1,3,6-trisulfonic acid | Low peak capacity, but separation of several isomers was achieved | UV absorbance (220, 262, and 354 nm) ESI-MS |
− | Glycans were analyzed in the femtomole range | [104] | 2003 |
| Vydac 218-TP54 C18 5 μm; 250 mm × 4.6 mm (1.0 mL/min) | Gradient of acetonitrile and water with a constant concentration of 0.04 % trifluoroacetic acid | Acidic | Released N-glycans from ovalbumin | 1-Phenyl-3-methyl-5-pyrazolone | No efficient separation, sugars were eluted within the same range in 6 min | ESI-MS | + and − | RP-HPLC was used as a desalting method because of inefficient separation. The detection limit was 2 nmol |
[110] | 2001 |
| Vydac 218-TP54 C18 5 μm; 250 mm × 4.6 mm (1.0 mL/min) | Gradient of 2:1 tert-butyl alcohol–acetonitrile and water with a constant concentration of 0.05 % trifluoroacetic acid | Acidic | N-Glycan standards | 1-Phenyl-3-methyl-5-pyrazolone | Low peak capacity, but glycan standards were separated | ESI-MS | + | [108] | 1999 | |
| Vydac 218-TP54 C18 5 μm; 250 mm × 4.6 mm (1.0 mL/min) | Gradient of acetonitrile and water with a constant concentration of 0.04 % trifluoroacetic acid | Acidic | Tetraglucose and N-linked oligosaccharides | 1-Phenyl-3-methyl-5-pyrazolone | ND | ESI-MS | + and − | RP-HPLC was used as a desalting method. Glycans were analyzed in the picomole range |
[134] | 1999 |
| Alltima C18-LL 5 μm; 150 mm × 2.1 mm. Guard column: Exsil C18 5 μm; 2 mm (0.2 mL/min) |
Gradient of acetonitrile–water in a ratio of 1:99 v/v and acetonitrile–water in a ratio of 80:20 v/v both containing 16 mM ammonium acetate, 24 mM acetic acid, and 0.5 mM triethylamine | 4.5 | Isolated oligosaccharides from MPS type IIIA urine | 1-Phenyl-3-methyl-5-pyrazolone | Monosaccharides to octasaccharides were separated | UV absorbance (254 nm) ESI-MS |
− | [107] | 2006 | |
| Vydac 218-TP54 C18 5 μm; 250 mm × 4.6 mm (0.3–1.0 mL/min) | First series: gradient of 0.05 M acetic acid in water and 0.05 M acetic acid in acetonitrile. Second series: gradient of water and 0.1 M acetic acid in 80 % acetonitrile in water | Acidic | Released N-glycans from hen ovalbumin (grades V and VII) | Phenylhydrazine | Low peak capacity, species were not separated individually | ESI-MS | + | [112] | 2003 | |
| Vydac 218-TP54 C18 5 μm; 250 mm × 4.6 mm (0.5 mL/min) | Gradient of 5 % acetonitrile in water and 90 % acetonitrile in water with 0.1 % trifluoroacetic acid | Acidic | Released N-glycans from mouse serum | Phenylhydrazine | Only used to separate types of glycans for better MALDI-MS analysis | UV absorbance (254 nm) MALDI-MS/MS |
+ | [113] | 2008 | |
| Zorbax 300-SB C8 5 μm; 150 mm × 4.6 mm (0.5 mL/min) | Gradient of 0.1 M acetic acid in 10:90 acetonitrile–water and 0.1 M acetic acid in 25:75 acetonitrile–water | 7.0 | Small saccharides: arabinose, galactose, glucose, GalNAc, GlcNAc, and lactose | Phenylhydrazine | Low peak capacity, but glycan standards were separated | UV absorbance (254 nm) ESI-MS |
+ | Glycans were analyzed in the picomole range | [40] | 2003 |
| μBondapak C18; 4 mm × 30 mm (2 mL/min) | Isocratic 22 % acetonitrile and 78 % water | Monosaccharides | Dansylhydrazine | Low peak capacity, species were not separated individually | UV absorbance (254 nm) FL (excitation 240 nm, emission 550 nm) |
Glycans were analyzed in the nanomole range | [114] | 1981 |
BEH bridged ethyl hybrid, ESI electrospray ionization, FL fluorescence, GalNAc N-acetylgalactosamine, GlcNAc N-acetylglucosamine, HILIC hydrophilic interaction liquid chromatography, HPLC high-performance liquid chromatography, ID internal diameter, MALDI matrix-assisted laser desorption/ionization, MS mass spectrometry, MPS mucopolysaccharidosis, ND no data, ODS octadecylsilyl, SAX strong anion exchange, TOF time of flight
The objective of this review is to compare reversed-phase separation methods for glycan analysis. An overview of the literature on this subject is presented, with the emphasis on separation of the glycans investigated. Various labeling compounds are compared for their advantages in separation and detection. In addition, the elution orders of the glycans are discussed.
Column specifications and configurations
Reversed-phase chromatography is a widely used separation technique. An advantage of this technique is that it can be used in many laboratories, because only standard laboratory equipment is required [38]. In addition, various detection techniques can be used in combination with reversed-phase chromatography, depending on the labeling reagent used.
Reversed-phase separation is based on a noncovalent association between the nonpolar stationary phase and the nonpolar moieties of an analyte. The strength of this association depends on the polarity of the mobile phase [10]. The relative solubility of the analyte in the stationary phase and the mobile phase determines the degree of association of the analyte with the stationary phase and therefore the retention of the analyte. The retention is thus dependent on the competitive solubilization of the analyte between the stationary phase and the mobile phase.
An overview of the literature on reversed-phase separation of carbohydrates is presented in Table 1. As can be seen, in almost all methods a C18 reversed-phase column is used for separation. Only two of the methods use a C8 column to separate analytes [39, 40]. Although most methods are based on C18 separation, many different kinds of C18 columns are used. Reversed-phase chromatography is a commonly used analysis technique in chemistry and in other fields. Therefore an outstanding variety of C18 columns are commercially available. Columns with various different specifications are used. There are differences for example, in column length, internal diameter, and particle size, which may have a substantial influence on the separation efficiency. Differences between columns in terms of particle shape and bonded phase packing are illustrated by Snyder and Kirkland [41]. The hydrophobicity of the stationary phase also differs among columns [42]. In addition, the density and nature of the nonpolar groups immobilized on the silica surface will influence the selectivity [10].
Besides traditional and narrow-bore analytical reversed-phase columns, the use of analytical nanoscale reversed-phase columns is also described in several articles [38, 43–52]. Nano HPLC systems became commercially available in the 1990s. These nano HPLC columns typically have a dimension of 75 μm × 150 mm and a flow rate of around 300 nL/min. In addition, chip-based nano HPLC systems exist [53, 54]. Unfortunately, reduction of the internal diameter of the column will also limit the amount of sample that can be injected. To facilitate larger injection volumes, trapping columns are used. The analytes are trapped on a small column with relatively high flow rates and often large injection volumes followed by elution onto the longer analytical column for separation [55, 56]. By reduction of the internal diameter, the sensitivity of the measurements is increased with MS detection: sensitivities in the low femtomole range can be achieved in MS and MS/MS mode [53, 57, 58]. In addition, the use of nanoscale columns increases the separation efficiency and resolution, as was shown by Schmid et al. [59]. The coupling of nanoscale columns with MS detection is of importance for glycan analysis as the samples are often complex and full chromatographic separation is not obtained. Of note, separation of isobaric and isomeric glycan species is of particular value in combination with MS detection to achieve detailed characterization of complex glycan samples.
Full chromatographic separation, or at least the separation of isomers, can be achieved by use of two analytical columns. In work reported in a few articles, a reversed-phase column was used in the second dimension of two-dimensional LC [38, 45–47]. In the first dimension, separation was performed in HILIC mode at analytical scale, resulting in an incomplete separation of the different glycans in the samples. By use of nanoscale reversed phase in the second dimension, separation of complex samples and isomer mixtures was achieved. With this two-dimensional separation with nanoscale MS detection, detailed characterization of complex glycan mixtures can be achieved with high sensitivity.
Solvents
As can be seen from Table 1, both gradient and isocratic runs are performed. In all cases the pH of the solvents is acidic or neutral. Most methods use a binary gradient, with solvent A consisting of mainly water and solvent B consisting of acetonitrile, methanol, or a mixture of one of these solvents with water. Solvent A may also contain up to 10 % acetonitrile. In addition, both solvents may contain low concentrations of a volatile acid (formic acid, acetic acid, or trifluoroacetic acid). Dependent on whether electrospray ionization (ESI) MS is used or not, volatile buffers (sodium acetate, triethylammonium acetate, ammonium acetate, or ammonium formate) or nonvolatile buffers (sodium phosphate) are used. In two cases, low amounts of sodium hydroxide were added to solvent A to induce sodium adduct formation in MS [44, 46]. In addition, in a few methods an ion-pairing reagent (diethylamine and triethylamine) is used to support retention of glycans with charged groups such as sialic acids [60, 61]. Dependent on which label was used, some adjustments in the solvents were made.
Derivatization and detection
Although techniques that do not require derivatization exist [62], generally glycans are derivatized before reversed-phase separation and analysis. Carbohydrates absorb light only at low wavelengths, which results in low sensitivity in UV and fluorescence detection. In addition, amperometric and refractive index detection also have a problem with sensitivity when carbohydrates are analyzed. Coupling UV-absorbing and fluorescent molecules to these analytes greatly enhances the detection sensitivity in, for example, HPLC and CE [29]. Derivatization can also be used to increase detectability in MS by use of labels with a substituent that can be charged; for example, an amine or carboxylic acid group [29]. Also, derivatization with a hydrophobic label can increase sensitivity in ESI-MS, which was examined by Williams et al. [63]. However, a hydrophobic bias, which depends on the chemical and physical properties of the analytes, is present in ESI-MS [64]. In 1993, Fenn [65] described that ion desorption from droplets is dependent on the surface activity of the analyte. He compared this with the hydrophobicity of the analyte and showed in an example that the MS response increases with the number of carbon atoms per alkyl chain in tetraalkylammonium halides. This hydrophobic bias has consequences for quantitation by ESI-MS [66]. In addition, the signal intensity in ESI-MS depends on the amount of organic solvent present. For instance, the use of 80 % acetonitrile as the solvent showed a sixfold increase in intensity in the study of Bleicher and Bayer [67]. Moreover, the total signal intensity per analyte is also decreased by their being multiple charge states [68]. Quantitation by MS detection may for these reasons be compromised, unless an internal standard with properties similar to those of the analyte (e.g., an isotopically labeled version of the analyte) is used for absolute quantitation [69].
To be able to analyze carbohydrates with reversed-phase chromatography, it is important to make the analytes more hydrophobic so they can interact with the alkyl chains in the stationary phase. As shown in Table 1, various labels are used in reversed-phase chromatography of glycans: 2-aminobenzamide (AB), anthranilic acid (AA), 2-aminopyridine (PA), 2-amino-5-bromopyridine (ABP), 4-aminobenzoic acid methyl ester (ABME), 4-aminobenzoic acid ethyl ester (ABEE), 4-aminobenzoic acid butyl ester (ABBE), 4-n-heptyloxyaniline (HOA), 2-aminoacridone (AMAC), 3-(acetylamino)-6-aminoacridine (AA-Ac), 2-aminonapthalene trisulfone (ANTS), 1-phenyl-3-methyl-5-pyrazolone (PMP), phenylhydrazine, dansylhydrazine, and the individuality normalization when labeling with isotopic glycan hydrazide tags (INLIGHT). The structures of these labels are shown in Fig. 1. Besides labeling of the reducing end, reduction of the reducing end and permethylation of the glycan were also performed (Table 1). Another reducing-end label gaining popularity for HILIC analysis with fluorescence detection is procainamide [70, 71], yet its use in reverse-phase chromatography has still to be established. Likewise, labels that target glycosylamines generated by PNGase F release of N-glycans such as InstantABTM [72, 73] may be suitable for reversed-phase separation of glycans yet these analyses have still to be developed. Recently isotopic aminoxyTMT labels were introduced by Afiuni-Zadeh et al. [74], and these can be used for quantitation of peptides as well as glycans in MS. Zhou et al. [75] used these labels for glycan analysis on a PGC column, and stated that the label was not hydrophobic enough to obtain effective separation on a reversed-phase column.
Fig. 1.
Structures of labels used in reversed-phase chromatography of oligosaccharides. AA anthranilic acid, AA-Ac 3-(acetylamino)-6-aminoacridine, AB 2-aminobenzamide, ABBE 4-aminobenzoic acid butyl ester, ABEE 4-aminobenzoic acid ethyl ester, ABME >4-aminobenzoic acid methyl ester, ABP 2-amino-5-bromopyridine, AMAC 2-aminoacridone, ANTS 2-aminonapthalene trisulfone, HOA 4-n-heptyloxyaniline, INLIGHT individuality normalization when labeling with isotopic glycan hydrazide tags, PA 2-aminopyridine, PMP 1-phenyl-3-methyl-5-pyrazolone
Most labels described in Fig. 1 can be coupled to glycans by reductive amination, which is the most commonly used glycan derivatization technique. In this reaction, first a Schiff-base intermediate is formed. This Schiff base is subsequently reduced with sodium cyanoborohydride or 2-picoline borane to form a stable secondary amine [76, 77]. This derivatization reaction is often performed in methanol or dimethyl sulfoxide with acetic acid added to the organic solvent [78]. An important feature of this method is the stoichiometric coupling of one label per glycan, which together with the usually high labeling efficacies makes quantitation by fluorescence or UV detection possible [29]. The reaction mechanism for Schiff-base formation was described by Anumula [79], who showed that the labeling reaction is initiated by the attack of the lone pair of the amino group of the label on the carbon of the aldehyde of the reducing end of the glycan.
Various molecules containing an amino group can be coupled to glycans by reductive amination. The most commonly used label for glycan analysis is AB, which is used for the analysis of N-glycans [61, 80–84] as well as O-glycans [60, 84]. This label can be combined with various glycoanalytical methods, including several chromatographic phases (e.g., HILIC and reversed phase) and detection methods (e.g. UV detectors, fluorescence, and MS) [85, 86]. AA is a label that is similar to AB. This label contains a carboxylic acid moiety instead of the amide group. Labeling with AA provides at least two times more sensitivity in fluorescence detection as compared with labeling with AB [79]. In addition, AB and AA can both be used in positive and negative ion mode MS [32, 38, 81]. The labeling procedures for AA and AB labels were optimized by Bigge et al. [87] in the 1990s. They measured a labeling efficiency of more than 80 % for AB and approximately 80 % for AA. These labeling efficiencies were also investigated by Ruhaak et al. [77], who reported that, with sodium cyanoborohydride and 2-picoline borane as reducing agents, in most cases an almost complete labeling of glucose polymers was obtained.
Another label that is often used for labeling at the reducing end by reductive amination is PA [70, 88–95]. Hase et al. [92] were the first to use PA labeling for HPLC analysis of glycans. PA has a relatively low hydrophobicity compared with the other labels mentioned, which results in a rapid desorption from the stationary phase, providing sharp elution peaks [96]. In many of the methods using PA, a buffer was added to the solvents to maintain a low pH. As the pK a of PA is 6.8, the molecule is protonated at low pH [97]. Testa and Wild [98] showed that this protonation results in increased fluorescence. This is beneficial for fluorescence detection, which is often used in combination with this label (Table 1). As a consequence of the low hydrophobicity of the label, low concentrations of organic solvents are used in the eluents, which makes direct coupling with ESI-MS less convenient [70]. ABP is PA with an additional bromine moiety. This bromine is advantageous when MS analysis is used, because of the natural equal abundances of 79Br and 81Br. In high-resolution MS, signals resulting from labeled species can easily be recognized by the typical isotopic pattern [39].
ABME, ABEE, ABBE, and HOA are less commonly used glycan derivatization reagents. These labels were compared by Schmid et al. [59], who showed that the retention times increased with a longer alkyl chain at the 4-position of the label. The polarities of the glycans tend to be very similar after derivatization with ABME, resulting in rapid elution and poor resolution for large glycans. Longer retention and better separation were observed for the other three labels [59, 99]. Sensitivity increases for positive and negative mode ESI-MS were tested by Pabst et al. [70], who showed that in positive mode, ABEE increased sensitivity by a factor of 2 compared with the native glycans. In negative mode a slight increase in sensitivity was observed with ABEE and ABBE.
AMAC is a labeling reagent with pronounced hydrophobic properties [100]. The molecule has fluorescent properties and a strong UV absorbance, which can be used for fluorescence analysis [101, 102]. Nevertheless, the fluorescence sensitivity of AMAC was somewhat less than that of AB and around four times than that of AA [32]. Charlwood et al. [103] reported AA-Ac based on AMAC as a new derivatization reagent. This label has higher fluorescence intensity than AMAC [103]. In positive ion mode ESI-MS, an intensity gain of a factor 2 was observed for AA-Ac compared with AA, whereas for AMAC the intensity was considerably lower [70].
ANTS is a charged molecule, which means that the label is hardly retained in reversed-phase chromatography [104]. Although it might not seem a natural choice to use this charged label in reversed-phase chromatography, Gennaro et al. [104] used it to separate glycans with a sensitivity in the low femtomole range. An ion-pairing reagent, triethylammonium acetate, was added to the mobile phase to create neutral complexes with the label, which then could be retained by the reversed-phase stationary phase. Like ANTS, 8-aminopyrene-1,3,6-trisulfonic acid, which is similar to ANTS, has been used for glycan separation on a reverse-phase stationary phase [105]. Glycans derivatized with ANTS were reported to have increased sensitivity in ESI-MS as compared with native glycans [104]. However, this increase was not observed by Pabst et al. [70].
ANTS was first used as a fluorophore in gel electrophoresis. In addition, it is used as a derivatizing reagent for CE and HILIC separations [29, 106]. Analysis of ANTS derivatives by reversed-phase chromatography can provide information on isomers and can be used as a technique complementary to CE and HILIC [104]. Reversed-phase separation of ANTS-derivatized glycans was obtained with an ion-pairing reagent. Without this ion-pairing reagent, ANTS-labeled analytes are found in the flow-through [104]. Various labels coupled to the reducing end by reductive amination were compared by Pabst et al. [70]. Unfortunately the derivatization protocols were not optimized, and thus no labeling efficiencies were shown.
PMP is a label that is added with a stoichiometry of two labels per glycan [107]. This addition of two labels might be a disadvantage, as it leads to a rather bulky reducing-end modification that may dominate separation. The labeling reaction with PMP is a Michael addition, which is performed under alkaline conditions [29]. In these alkaline conditions, loss of the sialic acids during derivatization is prevented [108]. PMP is a UV-absorbing molecule, but does not have fluorescent properties [29, 78]. In positive ion mode in ESI-MS, the sensitivity is approximately double that of native glycans, whereas in negative mode this gain was not observed [70]. PMP has been used for CE, HILIC and reversed-phase chromatography separations of glycans [109–111]. Saba et al. [110] observed in reversed-phase chromatography an elution range of only 6 min for the N-glycans of ovalbumin, whereas for HILIC the glycans were eluted over a range of 35 min. In addition, in-source fragments were observed in reversed phase LC–MS, whereas these were not observed with HILIC coupling. For these reasons, it was concluded that reversed-phase conditions were more useful for desalting PMP-derivatized samples than as a separation method [110, 112].
Derivatization of glycans can also be performed with hydrazine labels [40, 112–114]. The carbonyl group of the reducing-end aldehyde reacts with the hydrazine moiety to form a hydrazone bond [78]. This reaction is relatively clean, because no salts are used or produced during the reaction, which makes sample cleanup after the reaction often not necessary [40]. High labeling efficiencies for phenylhydrazines were observed for this reaction by Lattova and Perrault [40]. Dependent on the analysis technique, various hydrazine-containing molecules can be coupled to glycans; for example, dansylhydrazine, which has fluorescent properties and thus can be used in fluorescence detection [114]. Another hydrazine label for glycan analysis, the INLIGHT label, was introduced by Walker et al. [115] in 2011. There are two variants of this label, one of which contains six 13C atoms and the other one does not. The use of both labels makes quantification by MS possible. These labels can be used in combination with HILIC separation [116, 117], but reversed-phase separations are also performed [118, 119]. Unfortunately, no information was given on the separation efficiency of glycans with these labels.
In native glycans, the monosaccharide at the reducing-end terminus equilibrates between an open-ring conformation and a closed-ring conformation [2]. In the closed-ring conformation the C-1 atom is a chiral center with α and β anomers. In chromatography, small differences or double peaks could be observed for saccharides with a degree of polymerization of three monomers or more, because of these α and β anomers in the molecules. To overcome this without further labeling of the glycan, the reducing-end aldehyde in the open-ring conformation can be reduced with a reducing agent such as sodium borohydride, to form an alditol [29, 44, 120]. Other methods to eliminate double peaks (e.g., increasing the column temperature) exist but they will not be discussed in this review [10].
Glycans can also be derivatized by permethylation [43, 44, 48, 121, 122]. In contrast to the other derivatization methods discussed in this review, this derivatization does not occur at the reducing end. The hydrogens on the oxygen and nitrogen atoms in the glycans are replaced by methyl moieties, resulting in a largely hydrophobic molecule [122, 123]. This change in polarity and hydrophobicity is beneficial for glycan analysis with reversed-phase chromatography. In addition, permethylated compounds show an increase in detection sensitivity in ESI-MS, but detection in the femtomole range is challenging [48, 49, 122]. Notably, sialic acids are stabilized by permethylation via esterification, thereby preventing the ready loss of sialic acids observed in matrix-assisted laser desorption/ionization time-of-flight MS analysis of native glycans [124].
The influence of reducing-end labels on glycan retention
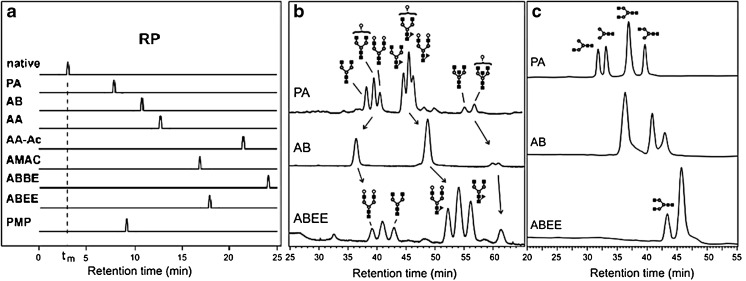
Glycans are hydrophilic molecules that tend to be hardly retained on many reversed-phase materials. Various labeling techniques may help to achieve glycan retention reversed-phase separation. An overview of the retention of native glycans as well as some labeled glycans is given in Fig. 2a. PA is the least hydrophobic of the commonly used labels and thus has a relatively low interaction with the stationary phase. The weak interactions of this label with the stationary phase results in the contribution of the glycan itself to the retention being relatively high with the PA tag compared with more hydrophobic tags [70]; therefore structural information can be derived from the glycans derivatized with this label. With the small, low-hydrophobicity PA label, specific glycan structural details tend to have relatively large influences on separation, which results in better separation of different species, as was shown by Pabst et al. [70]. They compared the separation of PA-, AB- and ABEE-labeled immunoglobulin G glycans, which is shown in Fig. 2b and c. In the case of PA-labeled glycans, small complex glycans were eluted first, whereas for ABEE-labeled glycans, the larger complex glycans were eluted first. This can be explained by the aforementioned contribution of glycans to the retention. In the case of PA, the retention is for a large part dependent on the interaction of the glycan with the stationary phase. Thus if the glycan has a higher degree of polymerization, it may engage in more interactions, resulting in longer retention. In the case of ABEE, the retention is mainly based on the hydrophobic label and the size of the hydrophilic glycan. One may speculate that the glycan interferes with the interaction of ABEE and the stationary phase, thereby leading to earlier elution of glycans with increasing degree of polymerization. For AB-labeled glycans, the glycan hardly modulates the retention contribution of the tag, resulting in a relatively poor reversed-phase separation of glycans [70]. Nevertheless, the separation of AB-labeled glycans can still be accomplished, with use of long gradients [80, 84, 125]. In this separation a negative correlation between the retention and the size of the high-mannose glycans was observed, whereas for complex glycans this correlation was positive [61, 126].
Fig. 2.
a Overview of the chromatograms of various reducing end derivatized N-glycans and a native glycan that is eluted at the void volume (dashed line). b Reversed-phase chromatograms of desialylated immunoglobulin G N-glycans derivatized with PA, AB, or ABEE. c Chromatograms of the separation of a mixture of four glycans: (GlcNAc)2(Man)3(GlcNAc)2, (GlcNAc)2(Man)3, and two isomers of (GlcNAc)2(Man)3(GlcNAc), where GlcNAc is N-acetylglucosamine and Man is mannose. RP reversed phase. (Reproduced and modified from Pabst et al. [70] with permission)
Schmid et al. [59] investigated on the separation of glycans labeled with ABME, ABEE, ABBE, and HOA. The first three labels mentioned differ by the length of the alkyl chain at the para position from the amine, and HOA contains an n-heptoxyl group at the para position. It was shown that this small difference in chain length had a substantial influence on the separation of the glycans and the run time per sample. The retention times are longer for ABEE and ABBE than for ABME because these labels are more hydrophobic. The longest retention times were measured for HOA. Better separations, including baseline-separated peaks, were obtained for ABEE, ABBE, and HOA.
Besides the different labels, Schmid et al. also tested different C18 stationary phases (i.e., differences in end-capping, pore size, etc.) for the separation of ABBE-labeled glycans, and showed differences in separation and retention times. One of these stationary phases was, for example, coated with a reactive polymeric silicone film that chemically binds to the silica gel and is alkylated afterward. For this encapsulated stationary phase, baseline separation of most of the analytes was observed, whereas for the partially trimethylsilyl end-capped stationary phase, overlap between all analytes was observed [59]. Furthermore, Gillmeister et al. [88] compared columns from various manufacturers, and showed major differences in retention and separation of glycans. The choice of the C18 column for separation can thus have a substantial influence on the separation efficiency.
Separation of permethylated glycans
Permethylation of glycans has also regularly been used as a derivatization technique followed by reversed-phase LC–MS (Table 1). In this case the glycan itself is made less hydrophilic by the substitution of hydrogen atoms for methyl groups. This separation is thus based on the properties of the glycan and results in the smallest glycans being eluted first. Coelution of permethylated glycans is observed for the more complex samples, but because of MS detection these overlapping glycan species can still be identified [43, 51, 52]. Ritamo et al. [44] and Zhou et al. [50] observed separation of isomers, although in some cases the sample complexity was limited. The latter researchers performed the separation of permethylated glycans at higher temperature, which increased the chromatographic resolution of isomers and improved the peak shape and decreased the peak width. In addition, it was concluded that the influence of the three-dimensional structure of the glycans was reduced because of the higher-temperature separations, resulting in more predictable retention times, which could be beneficial for the identification of glycans.
Glycan structural features influencing retention
AB labeling is often used in LC–MS methods. The separation of glycans labeled with AB was studied by Prater et al. [83] and Higel et al. [126], who showed that oligomannose glycans are eluted first. The main separation of the complex and hybrid glycans is caused by the core fucose: glycans containing a core fucose are eluted later than glycans without core fucosylation. Within these two groups, the acidic hybrid and complex glycans are eluted before the neutral hybrid and complex glycans, with the hybrid glycans being eluted before the complex ones. For AA labeling this elution order is almost the same [126]. Unfortunately, no information on isomers, bisecting variants, or triantennary variants was presented. Notably, when an ion-pairing reagent is used in combination with AB-labeled glycans the retention time of the sialylated glycans increases, which results in elution of sialylated glycans after neutral glycans [61]. Chen and Flynn [80] also performed reversed-phase separation of AB-labeled N-glycans, and found a slightly different elution order of the different types of glycans. They described that fucosylated sialylated glycans are eluted first from the column, followed by high-mannose glycans and neutral complex glycans [80, 81]. The difference between the elution order described by Higel et al. and this elution order might be due to differences in the columns, eluents, and gradients used, but this was not investigated. In addition Chen and Flynn [80] also described the elution of fucosylated triantennary and tetraantennary glycans. With their method these triantennary and tetraantennary glycans are eluted after the nonfucosylated glycans and before fucosylated diantennary species.
PA-labeled glycans show an increased relative retention of glycans containing a higher number of sialic acids [127, 128]. In contrast, for PMP-derivatized glycans the sialylated glycans are eluted earlier than (GlcNAc)2(Man)3, where GlcNAc is N-acetylglucosamine and Man is mannose, but in this case it cannot be said if this was due to the size of the glycans or the sialic acids present [108]. Tomiya and Takahashi [128] determined the elution positions of various neutral and sialylated PA-derivatized glycans. They calculated the contributions of the different monosaccharides at various positions in the N-glycan, taking linkages into account. These contributions were expressed in glucose units, which can be calculated by comparison of the retention times of the glycans with the retention times of the standard PA-isomaltooligosaccharides or a standard dextran ladder. The advantage of using glucose units instead of retention times is that glucose units are largely independent of the system and column used, which makes glucose units highly reproducible [60, 128].
The contribution of sialic acids to the retention on a C18 stationary phase strongly depends on the linkage (e.g. α2,3 linkage vs α2,6 linkage) and also on the type of sialic acid present (e.g., N-acetylneuraminic acid or N-glycolylneuraminic acid). In addition, the retention is also highly dependent on which antenna is sialylated [128]. For glycopeptides the elution order of neutral and sialylated species is the opposite: neutral glycan chains are eluted earlier than sialylated glycans with the same peptide moiety. Glycopeptides containing two sialic acid moieties on their glycans are eluted even later [129]. It appears that the different contributions of sialic acids to the retention of reducing-end labeled glycans as well as glycopeptides is still incompletely understood.
Besides the presence of sialic acids, also bisection of the glycan can influence the retention on the reversed-phase stationary phase. The addition of a bisecting GlcNAc to PA-labeled glycans results in a strong positive contribution to the retention [128]. The retention time increases even more if the bisecting GlcNAc contains a β1,4-linked galactose, which is a glycan structure that was discovered in immunoglobulin G by Takegawa et al. [130].
The separation of AMAC-labeled glycans was described by Okafo et al. [100]. The elution order of glycans was comparable to the elution order of the other relatively more hydrophobic labels, thus with the tetraantennary glycans being eluted before the triantennary glycans, which are eluted before the diantennary glycans. Okafo et al. [100] also found that antenna fucosylated glycans were eluted earlier than their nonfucosylated analogues, whereas the core-fucosylated glycans showed more retention than their nonfucosylated analogues.
This elution order of fucosylated glycans was observed not only for AMAC-labeled glycans but also for AB- and PA-labeled glycans [47, 128]. Tomiya and Takahashi [128] showed with their calculations on PA-labeled glycans that a core fucose on a N-glycan can have a major effect on the retention of the glycan on a reversed-phase stationary phase. This effect again depends on the linkage of this fucose (e.g., α1,6-linked fucose or α1,3-linked fucose). In vertebrate N-glycans, mainly α1,6-linked core fucoses are present, and these fucoses have a large positive contribution to the retention on a C18 stationary phase. Furthermore, the contribution of antenna fucosylation was also calculated. This contribution again depends on on which antenna the fucose is located and the linkage to this antenna. It was calculated that all antenna fucoses have no or a negative contribution to the retention in a C18 stationary phase [128]. This difference in retention of core and antenna fucoses might be explained by the polarity of a fucose. Fucoses are relatively apolar monosaccharides compared with the other monosaccharides present in N-glycans, because of their methyl group. In core-fucosylated glycans, the fucose is located relatively close to the label that interacts with the stationary phase, and both may interact concertedly with the stationary phase, leading to increased retention. In contrast, when the fucose is positioned on one of the antennae such a concerted interaction is not likely, which may explain the slightly lower retention of antenna-fucosylated glycans as compared with core-fucosylated glycans [47, 100, 128]. In PGC analysis of fucosylated glycans it was similarly found that antenna-fucosylated glycans are eluted before core-fucosylated glycans [131]. The different contribution of antenna and core fucoses to the retention is minor when a HILIC stationary phase (e.g., an amide column) is used because both types of fucosylation have a similar positive contribution to the retention [71, 128]. Notably, in glycopeptides also an effect in reversed-phase retention is observed when a core fucose is present. This effect again depends on the linkage of the fucose: when the fucose is α1,6-linked, it has a positive contribution to the retention and when the fucose is α1,3-linked, it has a negative contribution to the retention [132].
Toward full resolution of glycan isomers
As can be seen from Table 1, the ability to separate glycans depends highly on the complexity of the sample. When the complexity of the sample is high, separation of all structures is often not obtained. However, for glycan standards containing fewer glycan species and small saccharides, separation of the different glycan species and isomers was observed.
A low peak capacity is observed in many of the methods mentioned. This clustering of peaks can be expected because in most cases the label has a major influence on the retention. In addition, the structural difference between the glycans is often small and has only a minor influence on the retention, resulting in low selectivity. For these reasons, high-resolution glycan separations are often not obtained. To spread the signals, longer run times can be used for separation. However, this will also increase the turnaround time of the method. In addition, resolution can be increased by use of ultra-high-performance LC, where separation is performed at pressures above 400 bar [83, 133]. Another option to obtain full separation of glycans is the addition of a second-dimension separation, as mentioned before [38, 45, 47]. A protocol for the separation of AB-labeled glycans with HILIC separation in the first dimension and reversed-phase separation in the second dimension has been described [38]. An advantage of this approach is that with these two HPLC dimensions, isobaric and isomeric structures can often be separated, which is beneficial for the characterization of individual glycans.
Notably, reversed-phase chromatography is sometimes used to desalt the sample or to create a rough separation of highly complex samples and not to obtain fully separated glycans [49, 110, 113, 121, 134]. This full separation of the various glycans is often not necessary when MS detection is used, because ions of a specific m/z can be selected for further analysis or to obtain an extracted ion chromatogram. However, the separation of isomeric and isobaric species is of particular relevance for glycan analysis. With use of ANTS, a (Glc)3210 maltooligosaccharide ladder was successfully separated by Gennaro et al. [104]. In addition, high-mannose N-glycan structures (GlcNAc)2(Man)5–9 from ribonuclease B and three isomers of Man7 and two isomers of Man8 were separated. However, the separation of the high-mannose structures was only visible with MS detection, because the different high-mannose species (Man5–9) are eluted with an overlap within 4 min. Unfortunately, in UV or fluorescence detection a distinction between overlapping peaks cannot be made. Full separation of glycan species and isomers is thus desired, which can be a challenge for complex samples containing many glycan species.
Other stationary phases might also be of use for the separation of glycan isomers. Similarly to reversed-phase chromatography, PGC chromatography is based on hydrophobic interactions with the analytes. However, in PGC chromatography polar and ionic interactions are also involved in the retention [34, 37]. It should be noted that the exact retention mechanisms of PGC chromatography are debatable [127]. The polar and ionic interactions with the analytes mean that native glycans can be separated by PGC chromatography [131]. This separation can be performed on the basis of the branching, sequence, and linkage of the glycans and therefore PGC chromatography is highly suitable for the separation of isomers [13, 135]. For example, Thaysen-Andersen et al. [127] reported on the linkage-specific retention of α2,3- and α2,6-linked sialylated glycans, showing later retention of α2,3-linked sialylated diantennary and triantennary glycans. Nevertheless, the robustness and reproducibility of PGC chromatography is limited [61]. PGC chromatography and HILIC have been found to be more powerful in separating glycan structural isomers than reversed-phase chromatography of the AB-labeled variants [127]. However, reversed-phase separation of N-glycans is generally more efficient than HILIC separation, as was discussed by Walker et al. [136]. The different stationary phases might be used for different purposes, as was mentioned by Melmer et al. [61]. HILIC was described as a useful method for the analysis of complex samples, because of its high peak capacity and PGC chromatography was described as a useful method for separation of isomers. Reversed-phase chromatography was described as a useful method for quality control purposes, because of its reproducibility.
Conclusion
Reversed-phase chromatography is an technique often used for the separation of glycans, and consequently a wide variety of methods have been described. Many different columns and eluent mixtures have been used, which results in variation in separation efficiency. In addition, various labeling reagents have been used to increase hydrophobicity and detectability of the glycans. It was found that the hydrophobicity of the tag has a major influence on the separation and elution order of the different glycans. Moreover, this hydrophobicity also influences the contribution of the glycan itself to the retention.
Because of the relatively low hydrophobicity of PA, more structural information on the glycan can be derived. However, because of this low hydrophobicity, direct coupling with ESI-MS was less convenient. The more hydrophobic labels show a different elution order of glycans compared with PA-labeled glycans, but a uniform order could not be identified. Among other factors, the presence of an ion-pairing agent in the eluent, the type of column, the gradient, and the composition of the eluent have a substantial influence on the elution of glycans. Moreover, the presence of sialic acids or fucoses on the glycan also has a major influence on the retention. This influence is linkage specific, and in case of the fucose the influence also depends on whether the fucose it is located on the core or on the antenna of the N-glycan. In addition, the influence of sialic acids on the retention of glycopeptides is contradictory to the findings in released glycans. It appears that the exact influence of these monosaccharides is still incompletely understood, and additional research should be performed on this subject.
The quality of separation of the glycans is highly dependent on the complexity of the sample. In addition, a clustering of peaks is caused by the major contribution of the label to the retention, resulting in a low peak capacity. In complex samples, overlap between peaks of different glycan species has been observed. In fluorescence and UV-detection this causes difficulties with the identification of glycans, but for MS analysis this was often not a problem, as long as isomeric and isobaric species were separated from each other. The choice of the column, run time, and eluents influences the ability to separate isomers. In less complex samples, isomers were separated by reversed-phase chromatography. Unfortunately, no information on the elution order of these isomers was provided, which could have given insights into the retention of glycans on a reversed-phase stationary phase. The separation of isomers in complex samples could also be obtained by reversed-phase chromatography in a second dimension after HILIC separation. In addition, PGC chromatography, an emerging method for glycan separation, also has the capability to separate isomeric and isobaric glycans, also without derivatization. HILIC and PGC chromatography were compared with reversed-phase chromatography and from the data obtained it was concluded that both were better able to separate glycan species and their isomers. However, these methods have been proven to be less robust and reproducible than reversed-phase methods. Improvement of these properties might eliminate the need for reversed-phase glycan analyses in the future.
Further research should provide more information on the influence of the sialic acids and fucoses on the retention of the glycans and also of the glycopeptides. Especially the linkage and structure specificity of the fucoses should be investigated. In addition, the analysis of samples containing triantennary and tetraantennary glycans could give more insights into the influence of the sialic acid moieties.
Acknowledgement
The authors acknowledge support by the European Union (Seventh Framework Programme HighGlycan project, grant number 278535).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Varki A, Esko JD, Colley KJ, et al. Cellular organization of glycosylation. In: Varki A, Cummings R, Esko J, Freeze HH, Goldsmith HW, Bertozzi CR, et al., editors. Essentials of glycobiology. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. [Google Scholar]
- 2.Bertozzi C, Rabuka D, et al. Structural basis of glycan diversity. In: Varki A, Cummings R, Esko J, Freeze HH, Goldsmith HW, Bertozzi CR, et al., editors. Essentials of glycobiology. 2. New York: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 3.Geyer H, Geyer R. Strategies for analysis of glycoprotein glycosylation. Biochim Biophys Acta. 1764;2006:1853–1869. doi: 10.1016/j.bbapap.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Eklund EA, Freeze HH. Essentials of glycosylation. Semin Pediatr Neurol. 2005;12:134–143. doi: 10.1016/j.spen.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bondt A, Selman MHJ, Deelder AM, Hazes JMW, Willemsen SP, Wuhrer M, et al. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J Proteome Res. 2013;12:4522–4531. doi: 10.1021/pr400589m. [DOI] [PubMed] [Google Scholar]
- 7.Haselberg R, de Jong GJ, Somsen GW. CE-MS for the analysis of intact proteins 2010-2012. Electrophoresis. 2013;34:99–112. doi: 10.1002/elps.201200439. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs JFM, Wevers RA, Lefeber DJ, van Scherpenzeel M. Fast, robust and high-resolution glycosylation profiling of intact monoclonal IgG antibodies using nanoLC-chip-QTOF. Clin Chim Acta. 2016;461:90–97. doi: 10.1016/j.cca.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Desaire H. Glycopeptide analysis, recent developments and applications. Mol Cell Proteomics. 2013;12:893–901. doi: 10.1074/mcp.R112.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Rassi Z. Carbohydrate analysis: high performance liquid chromatography and capillary electrophoresis. Amsterdam: Elsevier; 1994. [Google Scholar]
- 11.Wuhrer M, Catalina MI, Deelder AM, Hokke CH. Glycoproteomics based on tandem mass spectrometry of glycopeptides. J Chromatogr B. 2007;849:115–128. doi: 10.1016/j.jchromb.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Kolli V, Schumacher KN, Dodds ED. Engaging challenges in glycoproteomics: recent advances in MS-based glycopeptide analysis. Bioanalysis. 2015;7:113–131. doi: 10.4155/bio.14.272. [DOI] [PubMed] [Google Scholar]
- 13.Pabst M, Altmann F. Glycan analysis by modern instrumental methods. Proteomics. 2011;11:631–643. doi: 10.1002/pmic.201000517. [DOI] [PubMed] [Google Scholar]
- 14.Tharmalingam T, Adamczyk B, Doherty MA, Royle L, Rudd PM. Strategies for the profiling, characterisation and detailed structural analysis of N-linked oligosaccharides. Glycoconj J. 2013;30:137–146. doi: 10.1007/s10719-012-9443-9. [DOI] [PubMed] [Google Scholar]
- 15.Domann PJ, Pardos-Pardos AC, Fernandes DL, Spencer DIR, Radcliffe CM, Royle L, et al. Separation-based glycoprofiling approaches using fluorescent labels. Proteomics. 2007;7(Suppl 1):70–76. doi: 10.1002/pmic.200700640. [DOI] [PubMed] [Google Scholar]
- 16.Dalpathado DS, Desaire H. Glycopeptide analysis by mass spectrometry. Analyst. 2008;133:731–738. doi: 10.1039/b713816d. [DOI] [PubMed] [Google Scholar]
- 17.Merry T, Astrautsova S. Chemical and enzymatic release of glycans from glycoproteins. Methods Mol Biol. 2003;213:27–40. doi: 10.1385/1-59259-294-5:27. [DOI] [PubMed] [Google Scholar]
- 18.Tarentino AL, Gómez CM, Plummer TH. Deglycosylation of asparagine-linked glycans by peptide: N-glycosidase F. Biochemistry. 1985;24:4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- 19.Lingg N, Zhang P, Song Z, Bardor M. The sweet tooth of biopharmaceuticals: importance of recombinant protein glycosylation analysis. Biotechnol J. 2012;7:1462–1472. doi: 10.1002/biot.201200078. [DOI] [PubMed] [Google Scholar]
- 20.Bereman MS, Williams TI, Muddiman DC. Development of a nanoLC LTQ Orbitrap mass spectrometric method for profiling glycans derived from plasma from healthy, benign tumor control, and epithelial ovarian cancer patients. Anal Chem. 2009;81:1130–1136. doi: 10.1021/ac802262w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruhaak LR, Miyamoto S, Kelly K, Lebrilla CB. N-Glycan profiling of dried blood spots. Anal Chem. 2012;84:396–402. doi: 10.1021/ac202775t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selman MHJ, Hemayatkar M, Deelder AM, Wuhrer M. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal Chem. 2011;83:2492–2499. doi: 10.1021/ac1027116. [DOI] [PubMed] [Google Scholar]
- 23.An HJ, Peavy TR, Hedrick JL, Lebrilla CB. Determination of N-glycosylation sites and site heterogeneity in glycoproteins. Anal Chem. 2003;75:5628–5637. doi: 10.1021/ac034414x. [DOI] [PubMed] [Google Scholar]
- 24.Morelle W, Michalski J-C. Analysis of protein glycosylation by mass spectrometry. Nat Protoc. 2007;2:1585–1602. doi: 10.1038/nprot.2007.227. [DOI] [PubMed] [Google Scholar]
- 25.Clark GF, Grassi P, Pang P-C, Panico M, Lafrenz D, Drobnis EZ, et al. Tumor biomarker glycoproteins in the seminal plasma of healthy human males are endogenous ligands for DC-SIGN. Mol Cell Proteomics. 2012;11:1–12. doi: 10.1074/mcp.M111.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao L, Zhang Y, Chen L, Shen A, Zhang X, Ren S, et al. Sample preparation for mass spectrometric analysis of human serum N-glycans using hydrophilic interaction chromatography-based solid phase extraction. Analyst. 2014;139:4538–4546. doi: 10.1039/C4AN00660G. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Han H, Bai H, Tong W, Zhang Y, Ying W, et al. A highly efficient and visualized method for glycan enrichment by self-assembling pyrene derivative functionalized free graphene oxide. Anal Chem. 2013;85:2703–2709. doi: 10.1021/ac303101t. [DOI] [PubMed] [Google Scholar]
- 28.Sun N, Deng C, Li Y, Zhang X. Highly selective enrichment of N-linked glycan by carbon-functionalized ordered graphene/mesoporous silica composites. Anal Chem. 2014;86:2246–2250. doi: 10.1021/ac404103r. [DOI] [PubMed] [Google Scholar]
- 29.Ruhaak LR, Zauner G, Huhn C, Bruggink C, Deelder AM, Wuhrer M. Glycan labeling strategies and their use in identification and quantification. Anal Bioanal Chem. 2010;397:3457–3481. doi: 10.1007/s00216-010-3532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohrer JS, Thayer J, Weitzhandler M, Avdalovic N. Analysis of the N-acetylneuraminic acid and N-glycolylneuraminic acid contents of glycoproteins by high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC/PAD) Glycobiology. 1998;8:35–43. doi: 10.1093/glycob/8.1.35. [DOI] [PubMed] [Google Scholar]
- 31.Alpert AJ. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr. 1990;499:177–196. doi: 10.1016/S0021-9673(00)96972-3. [DOI] [PubMed] [Google Scholar]
- 32.Anumula KR. Advances in fluorescence derivatization methods for high-performance liquid chromatographic analysis of glycoprotein carbohydrates. Anal Biochem. 2006;350:1–23. doi: 10.1016/j.ab.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 33.Wuhrer M, De Boer AR, Deelder AM. Structural glycomics using hydrophilic interaction chromatography (HILIC) with mass spectrometry. Mass Spectrom Rev. 2009;28:192–206. doi: 10.1002/mas.20195. [DOI] [PubMed] [Google Scholar]
- 34.Ruhaak LR, Deelder AM, Wuhrer M. Oligosaccharide analysis by graphitized carbon liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2009;394:163–174. doi: 10.1007/s00216-009-2664-5. [DOI] [PubMed] [Google Scholar]
- 35.Stavenhagen K, Kolarich D, Wuhrer M. Clinical glycomics employing graphitized carbon liquid chromatography–mass spectrometry. Chromatographia. 2014;78:307–320. doi: 10.1007/s10337-014-2813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolarich D, Windwarder M, Alagesan K, Altmann F. Isomer-specific analysis of released N-glycans by LC-ESI MS/MS with porous graphitized carbon. Methods Mol Biol. 2015;1321:427–435. doi: 10.1007/978-1-4939-2760-9_29. [DOI] [PubMed] [Google Scholar]
- 37.Fan JQ, Kondo A, Kato I, Lee YC. High-performance liquid chromatography of glycopeptides and oligosaccharides on graphitized carbon columns. Anal Biochem. 1994;219:224–229. doi: 10.1006/abio.1994.1261. [DOI] [PubMed] [Google Scholar]
- 38.Wuhrer M, Koeleman CAM, Deelder AM. Two-dimensional HPLC separation with reverse-phase-nano-LC-MS/MS for the characterization of glycan pools after labeling with 2-aminobenzamide. In: Packer NH, Karlsson NG, editors. Glycomics. New York: Humana; 2009. pp. 79–91. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Kinzer JA. Structural analysis of oligosaccharides by a combination of electrospray mass spectrometry and bromine isotope tagging of reducing-end sugars with 2-amino-5-bromopyridine. Rapid Commun Mass Spectrom. 2003;17:1462–1466. doi: 10.1002/rcm.1064. [DOI] [PubMed] [Google Scholar]
- 40.Lattova E, Perreault H. Labelling saccharides with phenylhydrazine for electrospray and matrix-assisted laser desorption-ionization mass spectrometry. J Chromatogr B. 2003;793:167–179. doi: 10.1016/S1570-0232(03)00374-X. [DOI] [PubMed] [Google Scholar]
- 41.Snyder LR, Kirkland JJ. The column. In: Dolan JW, editor. Introduction to modern liquid chromatography. 3. Hoboken: Wiley; 2010. pp. 199–252. [Google Scholar]
- 42.Claessens H, van Straten M, Cramers C, Jezierska M, Buszewski B. Comparative study of test methods for reversed-phase columns for high-performance liquid chromatography. J Chromatogr A. 1998;826:135–156. doi: 10.1016/S0021-9673(98)00749-3. [DOI] [Google Scholar]
- 43.Hu Y, Mechref Y. Comparing MALDI-MS, RP-LC-MALDI-MS and RP-LC-ESI-MS glycomic profiles of permethylated N-glycans derived from model glycoproteins and human blood serum. Electrophoresis. 2012;33:1768–1777. doi: 10.1002/elps.201100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritamo I, Räbinä J, Natunen S, Valmu L. Nanoscale reversed-phase liquid chromatography-mass spectrometry of permethylated N-glycans. Anal Bioanal Chem. 2013;405:2469–2480. doi: 10.1007/s00216-012-6680-5. [DOI] [PubMed] [Google Scholar]
- 45.Wuhrer M, Koeleman CAM, Fitzpatrick JM, Hoffmann KF, Deelder AM, Hokke CH. Gender-specific expression of complex-type N-glycans in schistosomes. Glycobiology. 2006;16:991–1006. doi: 10.1093/glycob/cwl020. [DOI] [PubMed] [Google Scholar]
- 46.Robijn MLM, Koeleman CAM, Hokke CH, Deelder AM. Schistosoma mansoni eggs excrete specific free oligosaccharides that are detectable in the urine of the human host. Mol Biochem Parasitol. 2007;151:162–172. doi: 10.1016/j.molbiopara.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Wuhrer M, Koeleman CAM, Deelder AM, Hokke CH. Repeats of LacdiNAc and fucosylated LacdiNAc on N-glycans of the human parasite Schistosoma mansoni. FEBS J. 2006;273:347–361. doi: 10.1111/j.1742-4658.2005.05068.x. [DOI] [PubMed] [Google Scholar]
- 48.Alley WR, Madera M, Mechref Y, Novotny MV. Chip-based reversed-phase liquid chromatography-mass spectrometry of permethylated N-linked glycans: a potential methodology for cancer-biomarker discovery. Anal Chem. 2010;82:5095–5106. doi: 10.1021/ac100131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desantos-Garcia JL, Khalil SI, Hussein A, Hu Y, Mechref Y. Enhanced sensitivity of LC-MS analysis of permethylated N-glycans through online purification. Electrophoresis. 2011;32:3516–3525. doi: 10.1002/elps.201100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou S, Hu Y, Mechref Y. High-temperature LC-MS/MS of permethylated glycans derived from glycoproteins. Electrophoresis. 2016;37:1506–1513. doi: 10.1002/elps.201500568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong X, Zhou S, Mechref Y. LC-MS/MS analysis of permethylated free oligosaccharides and N-glycans derived from human, bovine, and goat milk samples. Electrophoresis. 2016;37:1532–1548. doi: 10.1002/elps.201500561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Y, Shihab T, Zhou S, Wooding K, Mechref Y. LC-MS/MS of permethylated N-glycans derived from model and human blood serum glycoproteins. Electrophoresis. 2016;37:1498–1505. doi: 10.1002/elps.201500560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirsch S, Bindila L. Nano-LC and HPLC-chip-ESI-MS: an emerging technique for glycobioanalysis. Bioanalysis. 2009;1:1307–1327. doi: 10.4155/bio.09.110. [DOI] [PubMed] [Google Scholar]
- 54.Hua S, Lebrilla C, An HJ. Application of nano-LC-based glycomics towards biomarker discovery. Bioanalysis. 2011;3:2573–2585. doi: 10.4155/bio.11.263. [DOI] [PubMed] [Google Scholar]
- 55.Meiring HD, Van Der Heeft E, Ten Hove GJ, De Jong APJM. Nanoscale LC-MSn: technical design and applications to peptide and protein analysis. J Sep Sci. 2002;25:557–568. doi: 10.1002/1615-9314(20020601)25:9<557::AID-JSSC557>3.0.CO;2-F. [DOI] [Google Scholar]
- 56.Hernández-Borges J, Aturki Z, Rocco A, Fanali S. Recent applications in nanoliquid chromatography. J Sep Sci. 2007;30:1589–1610. doi: 10.1002/jssc.200700061. [DOI] [PubMed] [Google Scholar]
- 57.Wuhrer M, Deelder AM, Hokke CH. Protein glycosylation analysis by liquid chromatography-mass spectrometry. J Chromatogr B. 2005;825:124–133. doi: 10.1016/j.jchromb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 58.Wuhrer M, Koeleman CAM, Hokke CH, Deelder AM. Nano-scale liquid chromatography-mass spectrometry of 2-aminobenzamide- labeled oligosaccharides at low femtomole sensitivity. Int J Mass Spectrom. 2004;232:51–57. doi: 10.1016/j.ijms.2003.11.009. [DOI] [Google Scholar]
- 59.Schmid D, Behnke B, Metzger J, Kuhn R. Nano-HPLC-mass spectrometry and MEKC for the analysis of oligosaccharides from human milk. Biomed Chromatogr. 2002;16:151–156. doi: 10.1002/bmc.152. [DOI] [PubMed] [Google Scholar]
- 60.Royle L, Mattu TS, Hart E, Langridge JI, Merry AH, Murphy N, et al. An analytical and structural database provides a strategy for sequencing O-glycans from microgram quantities of glycoproteins. Anal Biochem. 2002;304:70–90. doi: 10.1006/abio.2002.5619. [DOI] [PubMed] [Google Scholar]
- 61.Melmer M, Stangler T, Premstaller A, Lindner W. Comparison of hydrophilic-interaction, reversed-phase and porous graphitic carbon chromatography for glycan analysis. J Chromatogr A. 2011;1218:118–123. doi: 10.1016/j.chroma.2010.10.122. [DOI] [PubMed] [Google Scholar]
- 62.Lareau NM, May JC, McLean JA. Non-derivatized glycan analysis by reverse phase liquid chromatography and ion mobility-mass spectrometry. Analyst. 2015;140:3335–3338. doi: 10.1039/C5AN00152H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams DK, Meadows CW, Bori ID, Hawkridge AM, Comins DL, Muddiman DC. Synthesis, characterization, and application of iodoacetamide derivatives utilized for the ALiPHAT strategy. J Am Chem Soc. 2008;130:2122–2123. doi: 10.1021/ja076849y. [DOI] [PubMed] [Google Scholar]
- 64.Shuford CM, Muddiman DC. Capitalizing on the hydrophobic bias of electrospray ionization through chemical modification in mass spectrometry-based proteomics. Expert Rev Proteomics. 2011;8:317–323. doi: 10.1586/epr.11.24. [DOI] [PubMed] [Google Scholar]
- 65.Fenn JB. Ion formation from charged droplets: roles of geometry, energy, and time. J Am Soc Mass Spectrom. 1993;4:524–535. doi: 10.1016/1044-0305(93)85014-O. [DOI] [PubMed] [Google Scholar]
- 66.Null AP, Benson LM, Muddiman DC. Enzymatic strategies for the characterization of nucleic acids by electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:2699–2706. doi: 10.1002/rcm.1255. [DOI] [PubMed] [Google Scholar]
- 67.Bleicher K, Bayer E. Various factors influencing the signal intensity of oligonucleotides in electrospray mass-spectrometry. Biol Mass Spectrom. 1994;23:320–322. doi: 10.1002/bms.1200230604. [DOI] [PubMed] [Google Scholar]
- 68.Muddiman DC, Cheng X, Udseth HR, Smith RD. Charge-state reduction with improved signal intensity of oligonucleotides in electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 1996;7:697–706. doi: 10.1016/1044-0305(96)80516-2. [DOI] [PubMed] [Google Scholar]
- 69.Shubhakar A, Reiding KR, Gardner RA, Spencer DIR, Fernandes DL, Wuhrer M. High-throughput analysis and automation for glycomics studies. Chromatographia. 2015;78:321–333. doi: 10.1007/s10337-014-2803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pabst M, Kolarich D, Pöltl G, Dalik T, Lubec G, Hofinger A, et al. Comparison of fluorescent labels for oligosaccharides and introduction of a new postlabeling purification method. Anal Biochem. 2009;384:263–273. doi: 10.1016/j.ab.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 71.Nwosu C, Yau HK, Becht S. Assignment of core versus antenna fucosylation types in protein N-glycosylation via procainamide labeling and tandem mass spectrometry. Anal Chem. 2015;87:5905–5913. doi: 10.1021/ac5040743. [DOI] [PubMed] [Google Scholar]
- 72.Albanese J, Lee R. A unique workflow for glycoprotein characterization – from sample preparation to MS/MS spectral interpretation. J Biomol Tech. 2011;22(Suppl):S57. [Google Scholar]
- 73.Cook KS, Bullock K, Sullivan T. Development and qualification of an antibody rapid deglycosylation method. Biologicals. 2012;40:109–117. doi: 10.1016/j.biologicals.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Afiuni-Zadeh S, Rogers JC, Snovida SI, Bomgarden RD, Griffin TJ. AminoxyTMT: a novel multi-functional reagent for characterization of protein carbonylation. Biotechniques. 2016;60:186–196. doi: 10.2144/000114402. [DOI] [PubMed] [Google Scholar]
- 75.Zhou S, Hu Y, Veillon L, Snovida SI, Rogers JC, Saba J, et al. Quantitative LC–MS/MS glycomic analysis of biological samples using aminoxyTMT. Anal Chem. 2016;88:7515–7522. doi: 10.1021/acs.analchem.6b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harvey DJ. Electrospray mass spectrometry and fragmentation of N-linked carbohydrates derivatized at the reducing terminus. J Am Soc Mass Spectrom. 2000;11:900–915. doi: 10.1016/S1044-0305(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 77.Ruhaak LR, Steenvoorden E, Koeleman CAM, Deelder AM, Wuhrer M. 2-Picoline-borane: a non-toxic reducing agent for oligosaccharide labeling by reductive amination. Proteomics. 2010;10:2330–2336. doi: 10.1002/pmic.200900804. [DOI] [PubMed] [Google Scholar]
- 78.Lamari FN, Kuhn R, Karamanos NK. Derivatization of carbohydrates for chromatographic, electrophoretic and mass spectrometric structure analysis. J Chromatogr B. 2003;793:15–36. doi: 10.1016/S1570-0232(03)00362-3. [DOI] [PubMed] [Google Scholar]
- 79.Anumula KR. Single tag for total carbohydrate analysis. Anal Biochem. 2014;457:31–37. doi: 10.1016/j.ab.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 80.Chen X, Flynn GC. Analysis of N-glycans from recombinant immunoglobulin G by on-line reversed-phase high-performance liquid chromatography/mass spectrometry. Anal Biochem. 2007;370:147–161. doi: 10.1016/j.ab.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 81.Chen X, Flynn GC. Gas-phase oligosaccharide nonreducing end (GONE) sequencing and structural analysis by reversed phase HPLC/mass spectrometry with polarity switching. J Am Soc Mass Spectrom. 2009;20:1821–1833. doi: 10.1016/j.jasms.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 82.Adamczyk B, Tharmalingam-Jaikaran T, Schomberg M, Szekrényes Á, Kelly RM, Karlsson NG, et al. Comparison of separation techniques for the elucidation of IgG N-glycans pooled from healthy mammalian species. Carbohydr Res. 2014;389:174–185. doi: 10.1016/j.carres.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 83.Prater BD, Connelly HM, Qin Q, Cockrill SL. High-throughput immunoglobulin G N-glycan characterization using rapid resolution reverse-phase chromatography tandem mass spectrometry. Anal Biochem. 2009;385:69–79. doi: 10.1016/j.ab.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 84.Garner B, Merry AH, Royle L, Harvey DJ, Rudd PM, Thillet J. Structural elucidation of the N- and O-glycans of human apolipoprotein(a). Role of O-glycans in conferring protease resistance. J Biol Chem. 2001;276:22200–22208. doi: 10.1074/jbc.M102150200. [DOI] [PubMed] [Google Scholar]
- 85.Mariño K, Lane JA, Abrahams JL, Struwe WB, Harvey DJ, Marotta M, et al. Method for milk oligosaccharide profiling by 2-aminobenzamide labeling and hydrophilic interaction chromatography. Glycobiology. 2011;21:1317–1330. doi: 10.1093/glycob/cwr067. [DOI] [PubMed] [Google Scholar]
- 86.Mariño K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6:713–723. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 87.Bigge J, Patel T, Bruce J. Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal Biochem. 1995;230:229–238. doi: 10.1006/abio.1995.1468. [DOI] [PubMed] [Google Scholar]
- 88.Gillmeister MP, Tomiya N, Jacobia SJ, Lee YC, Gorfien SF, Betenbaugh MJ. An HPLC-MALDI MS method for N-glycan analyses using smaller size samples: application to monitor glycan modulation by medium conditions. Glycoconj J. 2009;26:1135–1149. doi: 10.1007/s10719-009-9235-z. [DOI] [PubMed] [Google Scholar]
- 89.Kita Y, Miura Y, Furukawa J, Nakano M, Shinohara Y, Ohno M, et al. Quantitative glycomics of human whole serum glycoproteins based on the standardized protocol for liberating N-glycans. Mol Cell Proteomics. 2007;6:1437–1445. doi: 10.1074/mcp.T600063-MCP200. [DOI] [PubMed] [Google Scholar]
- 90.Hase S, Sugimoto T, Takemoto H, Ikenaka T, Schmid K. The structure of sugar chains of Japanese quail ovomucoid. The occurrence of oligosaccharides not expected from the classical biosynthetic pathway for N-glycans; a method for the assessment of the structure of glycans present in picomolar amounts. J Biochem. 1986;99:1725–1733. doi: 10.1093/oxfordjournals.jbchem.a135649. [DOI] [PubMed] [Google Scholar]
- 91.Hase S, Koyama S, Daiyasu H, Takemoto H, Hara S, Kobayashi Y, et al. Structure of a sugar chain of a protease inhibitor isolated from barbados pride (Caesalpinia pulcherrima Sw.) seeds. J Biochem. 1986;100:1–10. doi: 10.1093/oxfordjournals.jbchem.a121681. [DOI] [PubMed] [Google Scholar]
- 92.Hase S, Ikenaka T, Matsushima Y. A highly sensitive method for analyses of sugar moieties of glycoproteins by fluorescence labeling. J Biochem. 1981;90:407–414. doi: 10.1093/oxfordjournals.jbchem.a133487. [DOI] [PubMed] [Google Scholar]
- 93.Yanagida K, Ogawa H, Omichi K, Hase S. Introduction of a new scale into reversed-phase high-performance liquid chromatography of pyridylamino sugar chains for structural assignment. J Chromatogr A. 1998;800:187–198. doi: 10.1016/S0021-9673(97)01109-6. [DOI] [PubMed] [Google Scholar]
- 94.Homma H, Tozawa K, Yasui T, Itoh Y, Hayashi Y, Kohri K. Abnormal glycosylation of serum IgG in patients with IgA nephropathy. Clin Exp Nephrol. 2006;10:180–185. doi: 10.1007/s10157-006-0422-y. [DOI] [PubMed] [Google Scholar]
- 95.Hase S, Ikenaka T. Estimation of elution times on reverse-phase high-performance liquid chromatography of pyridylamino derivatives of sugar chains from glycoproteins. Anal Biochem. 1990;184:135–138. doi: 10.1016/0003-2697(90)90025-5. [DOI] [PubMed] [Google Scholar]
- 96.Hirabayashi J. Glycan profiling. In: Taniguchi N, Suzuki A, Ito Y, Narimatsu H, Kawasaki T, Hase S, editors. Experimental glycoscience. Glycochemistry. Tokyo: Springer; 2008. p. 58. [Google Scholar]
- 97.Chen DL, McLaughlin LW. Use of pKa differences to enhance the formation of base triplets involving C-G and G-C base pairs. J Org Chem. 2000;65:7468–7474. doi: 10.1021/jo000754w. [DOI] [PubMed] [Google Scholar]
- 98.Testa AC, Wild UP. Inversion of close-lying 1.pi.* and 1.pi.pi.* states in 2-aminopyridine by protonation. A CNDO study. J Phys Chem. 1981;85:2637–2639. doi: 10.1021/j150618a014. [DOI] [Google Scholar]
- 99.Kratschmar D, Wallner S, Florenski M, Schmid D, Kuhn R. Analysis of oligosaccharides by MEKC with aminobenzoic alkyl esters as derivatization agents. Chromatographia. 1999;50:596–600. doi: 10.1007/BF02493666. [DOI] [Google Scholar]
- 100.Okafo G, Langridge J, North S, Organ A, West A, Morris M, et al. High-performance liquid chromatographic analysis of complex N-linked glycans derivatized with 2-aminoacridone. Anal Chem. 1997;69:4985–4993. doi: 10.1021/ac9707139. [DOI] [PubMed] [Google Scholar]
- 101.Volpi N, Galeotti F, Yang B, Linhardt RJ. Analysis of glycosaminoglycan-derived, precolumn, 2-aminoacridone-labeled disaccharides with LC-fluorescence and LC-MS detection. Nat Protoc. 2014;9:541–558. doi: 10.1038/nprot.2014.026. [DOI] [PubMed] [Google Scholar]
- 102.Deakin JA, Lyon M. A simplified and sensitive fluorescent method for disaccharide analysis of both heparan sulfate and chondroitin/dermatan sulfates from biological samples. Glycobiology. 2008;18:483–491. doi: 10.1093/glycob/cwn028. [DOI] [PubMed] [Google Scholar]
- 103.Charlwood J, Birrell H, Gribble A, Burdes V, Tolson D, Camilleri P. A probe for the versatile analysis and characterization of N-linked oligosaccharides. Anal Chem. 2000;72:1453–1461. doi: 10.1021/ac991268f. [DOI] [PubMed] [Google Scholar]
- 104.Gennaro LA, Harvey DJ, Vouros P. Reversed-phase ion-pairing liquid chromatography/ion trap mass spectrometry for the analysis of negatively charged, derivatized glycans. Rapid Commun Mass Spectrom. 2003;17:1528–1534. doi: 10.1002/rcm.1079. [DOI] [PubMed] [Google Scholar]
- 105.Yoshinaka Y, Ueda Y, Suzuki S. Ion-pair chromatographic separation of glycoprotein derived oligosaccharides as their 8-aminopyrene-1,3,6-trisulfonic acid derivatives. J Chromatogr A. 2007;1143:83–87. doi: 10.1016/j.chroma.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 106.Gennaro LA, Delaney J, Vouros P, Harvey DJ, Domon B. Capillary electrophoresis/electrospray ion trap mass spectrometry for the analysis of negatively charged derivatized and underivatized glycans. Rapid Commun Mass Spectrom. 2002;16:192–200. doi: 10.1002/rcm.564. [DOI] [PubMed] [Google Scholar]
- 107.Mason KE, Meikle PJ, Hopwood JJ, Fuller M. Characterization of sulfated oligosaccharides in mucopolysaccharidosis type IIIA by electrospray ionization mass spectrometry. Anal Chem. 2006;78:4534–4542. doi: 10.1021/ac052083d. [DOI] [PubMed] [Google Scholar]
- 108.Saba JA, Shen X, Jamieson JC, Perreault H. Effect of 1-phenyl-3-methyl-5-pyrazolone labeling on the fragmentation behavior of asialo and sialylated N-linked glycans under electrospray ionization conditions. Rapid Commun Mass Spectrom. 1999;13:704–711. doi: 10.1002/(SICI)1097-0231(19990430)13:8<704::AID-RCM543>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 109.Honda S. Separation of neutral carbohydrates by capillary electrophoresis. J Chromatogr A. 1996;720:337–351. doi: 10.1016/0021-9673(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 110.Saba JA, Shen X, Jamieson JC, Perreault H. Investigation of different combinations of derivatization, separation methods and electrospray ionization mass spectrometry for standard oligosaccharides and glycans from ovalbumin. J Mass Spectrom. 2001;36:563–574. doi: 10.1002/jms.158. [DOI] [PubMed] [Google Scholar]
- 111.Bardelmeijer HA, Lingeman H, de Ruiter C, Underberg WJ. Derivatization in capillary electrophoresis. J Chromatogr A. 1998;807:3–26. doi: 10.1016/S0021-9673(98)00230-1. [DOI] [PubMed] [Google Scholar]
- 112.Lattova E, Perreault H. Profiling of N-linked oligosaccharides using phenylhydrazine derivatization and mass spectrometry. J Chromatogr A. 2003;1016:71–78. doi: 10.1016/S0021-9673(03)01297-4. [DOI] [PubMed] [Google Scholar]
- 113.Lattová E, Varma S, Bezabeh T, Petrus L, Perreault H. Mass spectrometric profiling of N-linked oligosaccharides and uncommon glycoform in mouse serum with head and neck tumor. J Am Soc Mass Spectrom. 2008;19:671–685. doi: 10.1016/j.jasms.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 114.Alpenfels W. A rapid and sensitive method for the determination of monosaccharides as their dansyl hydrazones by high-performance liquid chromatography. Anal Biochem 1981;157:153–7. [DOI] [PubMed]
- 115.Walker SH, Budhathoki-Uprety J, Novak BM, Muddiman DC. Stable-isotope labeled hydrophobic hydrazide reagents for the relative quantification of n-linked glycans by electrospray ionization mass spectrometry. Anal Chem. 2011;83:6738–6745. doi: 10.1021/ac201376q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walker SH, Lilley LM, Enamorado MF, Comins DL, Muddiman DC. Hydrophobic derivatization of N-linked glycans for increased ion abundance in electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2011;22:1309–1317. doi: 10.1007/s13361-011-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walker SH, Papas BN, Comins DL, Muddiman DC. Interplay of permanent charge and hydrophobicity in the electrospray ionization of glycans. Anal Chem. 2010;82:6636–6642. doi: 10.1021/ac101227a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Walker SH, Taylor AD, Muddiman DC. Individuality normalization when labeling with isotopic glycan hydrazide tags (INLIGHT): a novel glycan-relative quantification strategy. J Am Soc Mass Spectrom. 2013;24:1376–1384. doi: 10.1007/s13361-013-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loziuk PL, Hecht ES, Muddiman DC. N-linked glycosite profiling and use of Skyline as a platform for characterization and relative quantification of glycans in differentiating xylem of Populus trichocarpa. Anal Bioanal Chem. 2016 doi: 10.1007/s00216-016-9776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jensen PH, Karlsson NG, Kolarich D, Packer NH. Structural analysis of N- and O-glycans released from glycoproteins. Nat Protoc. 2012;7:1299–1310. doi: 10.1038/nprot.2012.063. [DOI] [PubMed] [Google Scholar]
- 121.Viseux N, de Hoffmann E, Domon B. Structural analysis of permethylated oligosaccharides by electrospray tandem mass spectrometry. Anal Chem. 1997;69:3193–3198. doi: 10.1021/ac961285u. [DOI] [PubMed] [Google Scholar]
- 122.Delaney J, Vouros P. Liquid chromatography ion trap mass spectrometric analysis of oligosaccharides using permethylated derivatives. Rapid Commun Mass Spectrom. 2001;15:325–334. doi: 10.1002/rcm.230. [DOI] [PubMed] [Google Scholar]
- 123.Botelho JC, Atwood JA, Cheng L, Alvarez-Manilla G, York WS, Orlando R. Quantification by isobaric labeling (QUIBL) for the comparative glycomic study of O-linked glycans. Int J Mass Spectrom. 2008;278:137–142. doi: 10.1016/j.ijms.2008.04.003. [DOI] [Google Scholar]
- 124.Sekiya S, Wada Y, Tanaka K. Derivatization for stabilizing sialic acids in MALDI-MS. Anal Chem. 2005;77:4962–4968. doi: 10.1021/ac050287o. [DOI] [PubMed] [Google Scholar]
- 125.Morelle W, Page A, Michalski JC. Electrospray ionization ion trap mass spectrometry for structural characterization of oligosaccharides derivatized with 2-aminobenzamide. Rapid Commun Mass Spectrom. 2005;19:1145–1158. doi: 10.1002/rcm.1900. [DOI] [PubMed] [Google Scholar]
- 126.Higel F, Demelbauer U, Seidl A, Friess W, Sörgel F. Reversed-phase liquid-chromatographic mass spectrometric N-glycan analysis of biopharmaceuticals. Anal Bioanal Chem. 2013;405:2481–2493. doi: 10.1007/s00216-012-6690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thaysen-Andersen M, Larsen MR, Packer NH, Palmisano G. Structural analysis of glycoprotein sialylation – part I: pre-LC-MS analytical strategies. RSC Adv. 2013;3:22706–22726. doi: 10.1039/c3ra42960a. [DOI] [Google Scholar]
- 128.Tomiya N, Takahashi N. Contribution of component monosaccharides to the coordinates of neutral and sialyl pyridylaminated N-glycans on a two-dimensional sugar map. Anal Biochem. 1998;264:204–210. doi: 10.1006/abio.1998.2849. [DOI] [PubMed] [Google Scholar]
- 129.Wuhrer M, Stam JC, Van De Geijn FE, Koeleman CAM, Verrips CT, Dolhain RJEM, et al. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007;7:4070–4081. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- 130.Takegawa Y, Deguchi K, Nakagawa H, Nishimura SI. Structural analysis of an N-glycan with “B1-4 bisecting branch” from human serum IgG by negative-ion MSn spectral matching and exoglycosidase digestion. Anal Chem. 2005;77:6062–6068. doi: 10.1021/ac050843e. [DOI] [PubMed] [Google Scholar]
- 131.Nwosu CC, Aldredge DL, Lee H, Lerno LA, Zivkovic AM, German JB, et al. Comparison of the human and bovine milk N-glycome via high-performance microfluidic chip liquid chromatography and tandem mass spectrometry. J Proteome Res. 2012;11:2912–2924. doi: 10.1021/pr300008u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roitinger A, Leiter H, Staudacher E, Altmann F. HPLC method for the determination of Fuc to Asn-linked GlcNAc fucosyltransferases. Glycoconj J. 1998;15:89–91. doi: 10.1023/A:1006951802623. [DOI] [PubMed] [Google Scholar]
- 133.Prien JM, Prater BD, Qin Q, Cockrill SL. Mass spectrometric-based stable isotopic 2-aminobenzoic acid glycan mapping for rapid glycan screening of biotherapeutics. Anal Chem. 2010;82:1498–1508. doi: 10.1021/ac902617t. [DOI] [PubMed] [Google Scholar]
- 134.Shen X, Perreault H. Electrospray ionization mass spectrometry of 1-phenyl-3-methyl-5-pyrazolone derivatives of neutral and N-acetylated oligosaccharides. J Mass Spectrom. 1999;34:502–510. doi: 10.1002/(SICI)1096-9888(199905)34:5<502::AID-JMS800>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 135.Itoh S, Kawasaki N, Hashii N, Harazono A, Matsuishi Y, Hayakawa T, et al. N-linked oligosaccharide analysis of rat brain Thy-1 by liquid chromatography with graphitized carbon column/ion trap-Fourier transform ion cyclotron resonance mass spectrometry in positive and negative ion modes. J Chromatogr A. 2006;1103:296–306. doi: 10.1016/j.chroma.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 136.Walker SH, Carlisle BC, Muddiman DC. Systematic comparison of reverse phase and hydrophilic interaction liquid chromatography platforms for the analysis of N-linked glycans. Anal Chem. 2012;84:8198–8206. doi: 10.1021/ac3012494. [DOI] [PMC free article] [PubMed] [Google Scholar]