Abstract
The genus Helicobacter is a group of Gram-negative, helical-shaped pathogens consisting of at least 36 bacterial species. Helicobacter pylori (H. pylori), infecting more than 50% of the human population, is considered as the major cause of gastritis, peptic ulcer, and gastric cancer. However, the genetic underpinnings of H. pylori that are responsible for its large scale epidemic and gastrointestinal environment adaption within human beings remain unclear. Core-pan genome analysis was performed among 75 representative H. pylori and 24 non-pylori Helicobacter genomes. There were 1173 conserved protein families of H. pylori and 673 of all 99 Helicobacter genus strains. We found 79 genome unique regions, a total of 202,359bp, shared by at least 80% of the H. pylori but lacked in non-pylori Helicobacter species. The operons, genes, and sRNAs within the H. pylori unique regions were considered as potential ones associated with its pathogenicity and adaptability, and the relativity among them has been partially confirmed by functional annotation analysis. However, functions of at least 54 genes and 10 sRNAs were still unclear. Our analysis of protein-protein interaction showed that 30 genes within them may have the cooperation relationship.
1. Introduction
H. pylori is a Gram-negative, spiral-shaped epsilon-proteobacterium. It colonizes 50% of the world's human population, even as high as 80% in developing countries, making it one of the most successful pathogens [1, 2]. This bacterium can cause gastrointestinal disease, such as gastritis, peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma [3–5]. As research continues, a great number of non-pylori Helicobacter species (NPHS) inhabiting in a wide variety of human beings, mammals, and birds have been found [6]. Until now, there are at least 36 species of the Helicobacter genus that have been studied (http://www.bacterio.net/helicobacter.html).
The Helicobacter genus strains have been detected in more than 142 vertebrate species [7]. Among them, H. pylori is the major pathogenic bacterium in human beings. Besides H. pylori, some NPHS were also found to associate with human body function disorders [8]. For instance, H. heilmannii, H. winghamensis, H. pullorum, and H. canis were considered as causative agent of stomach and intestinal diseases [9–11].
Many genome regions of H. pylori, involved in the mechanism of pathogenesis and adaption to the host environment, have been identified and studied. The well-known Cag-pathogenicity island, an approximately 40 kb DNA region that encodes type IV secretion system (T4SS) and effector molecule cancer-associated gene toxin (cagA), has been proved to play a significant role in pathogenicity [12, 13]. The urea enzymes encoded by urease gene cluster can catalyze the hydrolysis of urea to ammonium and carbon dioxide. It is an influential colonization factor and contributes to gastric acid resistance [14]. Vacuolating cytotoxin (VacA) is a pore-forming toxin that implicates in altering host cell biology, including autophagy, apoptosis, cell vacuolation, and inhibition of T-cell proliferation [15–17].
In the past two decades, the whole genome of H. pylori and NHPS have been widely sequenced, which give us a more open field of version to study its pathogenicity and adaption mechanism. Previous studies indicated that H. pylori has a high rate of gene recombination and unusual genetic flexibility, and those traits were considered to be helpful for the adaption to the dynamic environment [18, 19]. Even though massive virulence factors of them have been studied, the mechanisms that the essential genome components of H. pylori lead to its large scale epidemic and gastrointestinal environment adaptation within human beings remain to be further elucidated.
In this study, comparative analysis of whole genome was made to reveal general character and characteristics of Helicobacter genus [20]. H. pylori and NHPS genomes that are available on public databases were used in the analysis. We intended to identify potential regions of H. pylori genomes that are responsible for its epidemicity and adaptability. In addition, comparative genome analysis among Helicobacter genus species can give a comprehensive insight into the genomic diversity in each species and help us to understand the relationship well among them.
2. Materials and Methods
2.1. Data Selection and Management
Helicobacter genus involves at least 36 species, while H. pylori is given more prominence for medicine. There are multiple complete genomes of them available on public databases, and the genomic data was acquired from NCBI FTP site (ftp://ftp.ncbi.nlm.nih.gov/genomes/) in this study. 99 genomes were selected, including 75 complete H. pylori genomes and 24 NPHS genomes, which belong to 19 species (released at the analysis time). To ensure the accuracy and consistency of initial data, chromosome, plasmids, and scaffolds of each candidate strain were concatenated by sequence “NNNNNCATTCCATTCATTAATTAATTAATGAATGAATGNNNNN” to establish a pseudochromosome for further analysis [21].
In order to get the accordance dataset and avoid contradiction that was caused by difference of the gene prediction method applied in different projects, a single gene finding program, Glimmer version 3.02 [22], was used to predict open reading frames (ORFs). The ORFs were removed while their start or end position was inside the sideward sequence. The predicted results and raw databases information were corroborated to one another. And the program RNAmmer-1.2 [23] was used to predict full length of rRNA gene sequences. The size, GC content, number of genes, source, and other characteristics of all selected genomes were listed in Table 1.
Table 1.
Helicobacter species genomic information used in the present study.
| Organism | Size (bp) | GC (%) | Scaffolds | Plasmids | CDS | rRNA | tRNA | Natural host |
|---|---|---|---|---|---|---|---|---|
| H. acinonychis str. Sheeba | 1557588 | 38.17 | 1 | 1 | 1706 | 6 | 36 | Cheetah, tiger |
| H. ailurogastricus | 1578404 | 47.05 | 9 | 0 | 1633 | 3 | 36 | Feline |
| H. bilis ATCC 43879 | 2530521 | 34.7 | 9 | 0 | 2728 | 3 | 36 | Mice |
| H. bilis WiWa | 2559659 | 34.68 | 17 | 0 | 2751 | 9 | 40 | Mice |
| H. bizzozeronii CIII-1 ∗ | 1807534 | 45.66 | 1 | 1 | 1998 | 6 | 36 | Dog, cat |
| H. canadensis MIT 98-5491 ∗ | 1623845 | 33.69 | 1 | 0 | 1624 | 9 | 40 | Barnacle, geese, rodent |
| H. canis NCTC 12740 ∗ | 1932823 | 44.82 | 1 | 0 | 1914 | 6 | 39 | Dogs |
| H. cetorum MIT 00-7128 | 1960111 | 34.53 | 1 | 1 | 1897 | 6 | 38 | dolphin, whale |
| H. cetorum MIT 99-5656 | 1847790 | 35.54 | 1 | 1 | 1852 | 6 | 36 | dolphin, whale |
| H. cinaedi CCUG 18818 ATCC BAA-847 ∗ | 2240130 | 38.34 | 1 | 0 | 2510 | 6 | 39 | Human |
| H. cinaedi PAGU611 ∗ | 2101402 | 38.55 | 1 | 1 | 2329 | 6 | 39 | Human |
| H. felis ATCC 49179 ∗ | 1672681 | 44.51 | 1 | 0 | 1776 | 5 | 36 | Cat, dog, rabbit, cheetah |
| H. fennelliae MRY12-0050 ∗ | 2155647 | 37.9 | 49 | 0 | 2503 | 3 | 38 | Human |
| H. heilmannii ASB1.4 ∗ | 1804601 | 47.38 | 1 | 0 | 2113 | 7 | 41 | Human |
| H. hepaticus ATCC 51449 | 1799146 | 35.93 | 1 | 0 | 1863 | 3 | 37 | Mice |
| H. himalayensis strain YS1 | 1829936 | 39.89 | 1 | 0 | 1896 | 6 | 39 | Marmota himalayana |
| H. macacae MIT 99-5501 | 2369528 | 40.41 | 4 | 0 | 2669 | 6 | 39 | Macaques |
| H. mustelae 12198 | 1578097 | 42.47 | 1 | 0 | 1745 | 6 | 38 | Ferret |
| H. pametensis ATCC 51478 | 1435066 | 40.08 | 11 | 0 | 1432 | 8 | 38 | Pig, bird |
| H. pullorum 229313-12 ∗ | 1691799 | 34.56 | 60 | 0 | 1754 | 3 | 36 | Poultry |
| H. pullorum MIT 98-5489 ∗ | 1951667 | 33.58 | 44 | 0 | 2105 | 3 | 36 | Poultry |
| H. suis HS1 ∗ | 1635292 | 39.91 | 136 | 0 | 1814 | 5 | 38 | Pig, macaque |
| H. typhlonius | 1920832 | 38.85 | 1 | 0 | 2109 | 6 | 39 | Mouse |
| H. winghamensis ATCC BAA-430 ∗ | 1690216 | 34.74 | 21 | 0 | 1742 | 3 | 36 | human liver |
| H. pylori 2017 | 1548238 | 39.3 | 1 | 0 | 1595 | 3 | 36 | Human |
| H. pylori 2018 | 1562832 | 39.29 | 1 | 0 | 1604 | 3 | 36 | Human |
| H. pylori 26695-1CH | 1667302 | 38.87 | 1 | 0 | 1667 | 7 | 36 | Human |
| H. pylori 26695-1CL | 1667239 | 38.87 | 1 | 0 | 1667 | 7 | 36 | Human |
| H. pylori 26695-1 | 1667638 | 38.87 | 1 | 0 | 1667 | 7 | 36 | Human |
| H. pylori 26695-1MET | 1667303 | 38.87 | 1 | 0 | 1669 | 7 | 36 | Human |
| H. pylori 26695 | 1667867 | 38.87 | 1 | 0 | 1681 | 7 | 36 | Human |
| H. pylori 29CaP | 1667159 | 38.81 | 1 | 0 | 1704 | 7 | 36 | Human |
| H. pylori 35A | 1566655 | 38.87 | 1 | 0 | 1583 | 6 | 36 | Human |
| H. pylori 51 | 1589954 | 38.77 | 1 | 0 | 1606 | 6 | 36 | Human |
| H. pylori 52 | 1568826 | 38.94 | 1 | 0 | 1578 | 6 | 36 | Human |
| H. pylori 7C | 1631276 | 39.01 | 1 | 1 | 1627 | 7 | 36 | Human |
| H. pylori 83 | 1617426 | 38.72 | 1 | 0 | 1634 | 6 | 36 | Human |
| H. pylori 908 | 1549666 | 39.3 | 1 | 0 | 1605 | 3 | 36 | Human |
| H. pylori Aklavik117 | 1636125 | 38.73 | 1 | 2 | 1607 | 6 | 36 | Human |
| H. pylori Aklavik86 | 1507930 | 39.21 | 1 | 2 | 1487 | 6 | 36 | Human |
| H. pylori B38 | 1576758 | 39.16 | 1 | 0 | 1582 | 7 | 36 | Human |
| H. pylori B8 | 1680029 | 38.78 | 1 | 1 | 1673 | 6 | 36 | Human |
| H. pylori BM012A | 1660425 | 38.88 | 1 | 0 | 1679 | 7 | 36 | Human |
| H. pylori BM012B | 1659060 | 38.88 | 1 | 0 | 1676 | 7 | 36 | Human |
| H. pylori BM012S | 1660469 | 38.88 | 1 | 0 | 1683 | 7 | 36 | Human |
| H. pylori BM013A | 1604233 | 38.96 | 1 | 0 | 1584 | 7 | 36 | Human |
| H. pylori BM013B | 1604212 | 38.96 | 1 | 0 | 1586 | 7 | 36 | Human |
| H. pylori Cuz20 | 1635449 | 38.86 | 1 | 0 | 1616 | 6 | 36 | Human |
| H. pylori ELS37 | 1669876 | 38.88 | 1 | 1 | 1676 | 6 | 36 | Human |
| H. pylori F16 | 1575399 | 38.88 | 1 | 0 | 1593 | 6 | 36 | Human |
| H. pylori F30 | 1579693 | 38.8 | 1 | 1 | 1582 | 6 | 36 | Human |
| H. pylori F32 | 1581461 | 38.86 | 1 | 1 | 1587 | 6 | 36 | Human |
| H. pylori F57 | 1609006 | 38.73 | 1 | 0 | 1619 | 6 | 36 | Human |
| H. pylori G27 | 1663013 | 38.87 | 1 | 1 | 1672 | 7 | 36 | Human |
| H. pylori Gambia9424 | 1712468 | 39.12 | 1 | 1 | 1694 | 6 | 36 | Human |
| H. pylori Hp238 | 1586473 | 38.7 | 1 | 0 | 1616 | 5 | 36 | Human |
| H. pylori HPAG1 | 1605736 | 39.07 | 1 | 1 | 1595 | 6 | 36 | Human |
| H. pylori HUP-B14 | 1607584 | 39.04 | 1 | 1 | 1597 | 6 | 36 | Human |
| H. pylori India7 | 1675918 | 38.9 | 1 | 0 | 1664 | 6 | 36 | Human |
| H. pylori J166 | 1650561 | 38.93 | 1 | 0 | 1630 | 6 | 36 | Human |
| H. pylori J99 | 1643831 | 39.19 | 1 | 0 | 1629 | 6 | 36 | Human |
| H. pylori Lithuania75 | 1640673 | 38.87 | 1 | 1 | 1659 | 6 | 36 | Human |
| H. pylori ML1 | 1629815 | 38.69 | 1 | 0 | 1701 | 6 | 36 | Human |
| H. pylori ML2 | 1562125 | 38.92 | 1 | 0 | 1764 | 6 | 36 | Human |
| H. pylori ML3 | 1635334 | 38.64 | 1 | 1 | 1744 | 4 | 36 | Human |
| H. pylori NY40 | 1696917 | 38.81 | 1 | 0 | 1751 | 6 | 36 | Human |
| H. pylori OK113 | 1616617 | 38.73 | 1 | 0 | 1649 | 6 | 36 | Human |
| H. pylori OK310 | 1595436 | 38.77 | 1 | 1 | 1595 | 6 | 36 | Human |
| H. pylori oki102 | 1633212 | 38.81 | 1 | 0 | 1630 | 6 | 36 | Human |
| H. pylori oki112 | 1637925 | 38.81 | 1 | 0 | 1635 | 6 | 36 | Human |
| H. pylori oki128 | 1553826 | 38.97 | 1 | 0 | 1565 | 6 | 36 | Human |
| H. pylori oki154 | 1599700 | 38.8 | 1 | 0 | 1626 | 6 | 36 | Human |
| H. pylori oki422 | 1634852 | 38.83 | 1 | 0 | 1641 | 6 | 36 | Human |
| H. pylori oki673 | 1595058 | 38.82 | 1 | 0 | 1623 | 6 | 36 | Human |
| H. pylori oki828 | 1600345 | 38.8 | 1 | 0 | 1618 | 6 | 36 | Human |
| H. pylori oki898 | 1634875 | 38.83 | 1 | 0 | 1612 | 6 | 36 | Human |
| H. pylori P12 | 1684038 | 38.79 | 1 | 1 | 1688 | 6 | 36 | Human |
| H. pylori PeCan18 | 1660685 | 39.02 | 1 | 0 | 1629 | 6 | 36 | Human |
| H. pylori PeCan4 | 1638269 | 38.91 | 1 | 1 | 1622 | 6 | 36 | Human |
| H. pylori Puno120 | 1637762 | 38.9 | 1 | 1 | 1617 | 6 | 36 | Human |
| H. pylori Puno135 | 1646139 | 38.82 | 1 | 0 | 1616 | 6 | 36 | Human |
| H. pylori Rif1 | 1667883 | 38.87 | 1 | 0 | 1678 | 7 | 36 | Human |
| H. pylori Rif2 | 1667890 | 38.87 | 1 | 0 | 1674 | 7 | 36 | Human |
| H. pylori Sat464 | 1567570 | 39.09 | 1 | 1 | 1553 | 6 | 36 | Human |
| H. pylori Shi112 | 1663456 | 38.77 | 1 | 0 | 1651 | 6 | 36 | Human |
| H. pylori Shi169 | 1616909 | 38.86 | 1 | 0 | 1593 | 6 | 36 | Human |
| H. pylori Shi417 | 1665719 | 38.77 | 1 | 0 | 1623 | 6 | 36 | Human |
| H. pylori Shi470 | 1608548 | 38.91 | 1 | 0 | 1612 | 6 | 36 | Human |
| H. pylori SJM180 | 1658051 | 38.9 | 1 | 0 | 1640 | 6 | 36 | Human |
| H. pylori SNT49 | 1610830 | 39 | 1 | 1 | 1599 | 6 | 36 | Human |
| H. pylori SouthAfrica20 | 1622903 | 38.57 | 1 | 0 | 1701 | 6 | 36 | Human |
| H. pylori SouthAfrica7 | 1679829 | 38.42 | 1 | 1 | 1689 | 6 | 36 | Human |
| H. pylori UM032 | 1593537 | 38.82 | 1 | 0 | 1613 | 6 | 36 | Human |
| H. pylori UM037 | 1692794 | 38.89 | 1 | 0 | 1708 | 6 | 36 | Human |
| H. pylori UM066 | 1658047 | 38.62 | 1 | 0 | 1651 | 6 | 36 | Human |
| H. pylori UM298 | 1594544 | 38.82 | 1 | 0 | 1618 | 6 | 36 | Human |
| H. pylori UM299 | 1594569 | 38.82 | 1 | 0 | 1617 | 6 | 36 | Human |
| H. pylori v225d | 1595604 | 38.94 | 1 | 1 | 1608 | 6 | 36 | Human |
| H. pylori XZ274 | 1656544 | 38.57 | 1 | 1 | 1798 | 7 | 36 | Human |
Note: (1) ∗NPHS associated with gastric disease in humans.
(2) Latin name, genome size, GC-content, scaffolds number, plasmid number, information of genes, and natural host are listed.
2.2. Phylogenetic Analysis of 16S rRNA
In order to better understand the phylogenetic relationships among Helicobacter species, a phylogenetic tree was constructed using the 16S rRNA genes obtained from the 99 genomes. In addition, Campylobacter jejuni and Campylobacter fetus were used as outgroup. Multiple sequence alignment of 101 16S rRNA genes was performed using MAFFT version 7.123b [24]. The phylogenetic tree was inferred by the Neighbor-Joining method [25] using MEGA7 [26]. To estimate the consensus tree, 1000-bootstrap resampling was done.
2.3. Cluster Analysis of Core and Pan Genome
Orthologous group analyses were performed with software OrthoMCL version 2.0.9 [27], which could generate a similarity matrix normalized by species representation relationship of sequences, and it was then grouped using the Markov Clustering Algorithm (MCL) [28]. All-against-all BLASTP comparisons were used to get pair sequences of protein dataset in OrthoMCL at start. An E-value cutoff of 1e − 5 and the aligned sequence length longer than the coverage of 50% of a query sequence was chosen to perform OrthoMCL.
A family matrix, which was generated from the genome pairwise comparison of the gene contents of any two genomes, was visualized. The gene families obtained from the OrthoMCL were used to get core and pan genome datasets. The number of unique genes and gene families for each individual species relative to other 98 genomes was calculated and visualized with bar graph.
2.4. Functional Classification of the Core and Accessory Genome
The dataset was combined into three groups: 75 H. pylori genomes alone, 24 NPHS genomes alone, and all the tested 99 Helicobacter genomes. For core and accessory genome of three groups, functional annotation and category were analyzed by performing BLASTP program against database Clusters of Orthologous Groups (COGs, 2014 update, https://www.ncbi.nlm.nih.gov/COG/), respectively [29, 30]. The percentage of each function category was illustrated by bar chart. All the heatmap and bar were plotted by R (https://www.r-project.org/).
2.5. Unique Regions Analysis of H. pylori
Each of the genomes was aligned to H. pylori 26695 using BLASTN program. Then, the genome regions shared by at least 80% of the H. pylori meanwhile lacked in NHPS were detected by a Perl script. The genomic lengths of unique regions only greater than 200 bp were considered. If the genomic length between each adjacent unique regions is less than 300 bp, it was regarded as a part of unique region. DOOR (Database for prOkaryotic OpeRons) [31] was used to predicate operons of H. pylori 26695 genome. Virulence factor database (VFDB) [32], COG database [29], InterProScan [33], and nonredundant (NR) protein database [34] were used to annotate and predict the functions of these genes within the target region. Furthermore, pfam [35], KEGG [36], GO [37], and TrEMBL [38] were used to discover more about the putative function of the hypothetical proteins of them.
Small noncoding RNAs (sRNAs) are ubiquitous regulators existing in all living organisms. They can impact various biological processes via interacting with mRNA targets or binding to regulatory proteins [39, 40]. RNAspace.org (http://RNAspace.org/), which is a comprehensive prediction and annotation tool of ncRNA [41], was used to predict ncRNA of H. pylori. Then, the particular ones contained by unique regions of H. pylori (URHP) were detected.
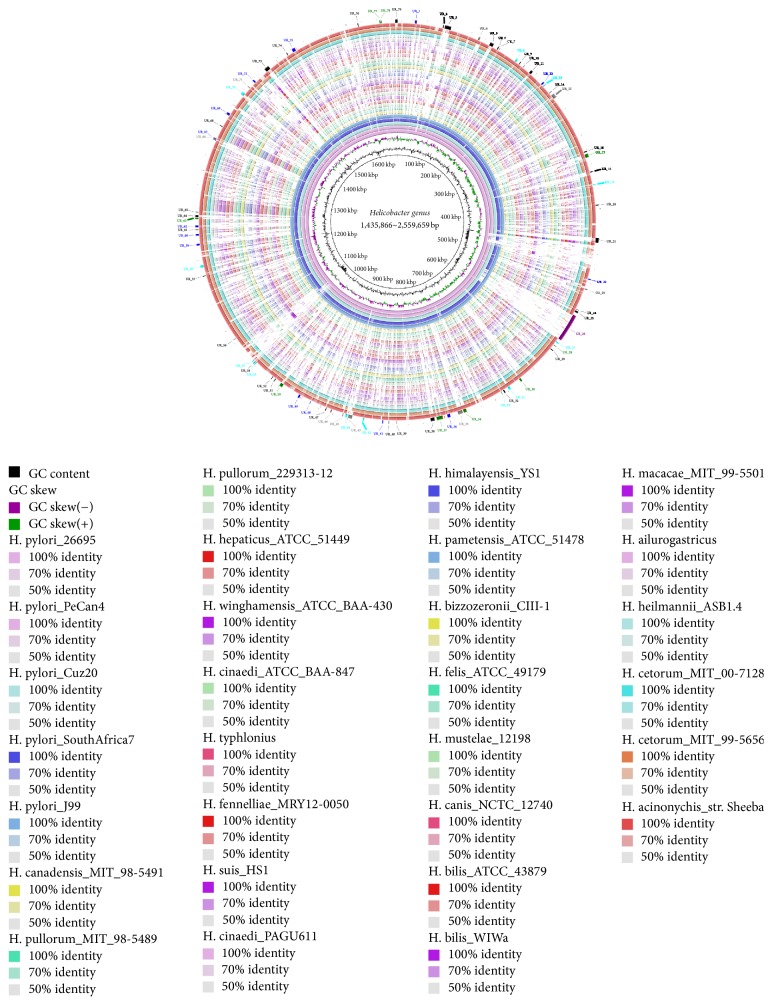
The analysis results were virtualized by BLAST ring image generator (BRIG) [42]. Five H. pylori strains, 26695, Cuz-20, J99, PeCan4, and SouthAfrica7, were drawn on the inner rings to represent the H. pylori species. URHP were drawn on the outer ring and twenty-four NHPS were drawn between them.
2.6. Protein-Protein Interaction Network Analysis of URHP Proteins
To better understand the role of URHP proteins in the H. pylori adaption and pathogenicity, protein-protein interaction network analysis of URHP proteins was carried out using Search Tool for the Retrieval of Interacting Genes/Proteins (STRING version 10.0) [43]. The STRING database (http://string-db.org/) is a comprehensive database that could provide a strict assessment and integration of protein-protein interactions, including physical as well as functional interrelationships.
3. Results and Discussion
3.1. Genome Statistics and Features
H. pylori was discovered by Warren and Marshall in 1983 and proved to be the pathogen that caused gastritis [44]. Then, the important pathogen strain H. pylori 26695 genome was completely sequenced in 1997 [45]. Altogether, ninety-nine genomes were used in this study and listed in Table 1, including 75 complete H. pylori genomes and 24 NPHS genomes, and plasmids were identified within 27 genomes (Table 1). The NPHS, which can be classified into 20 Helicobacter species, includes 11 completed genomes. Average genome size of all strains is 1,689,380 bp, ranging from 1,435,066 bp (H. pametensis ATCC 51478) to 2,559,659 bp (H. bilis WiWa). The genomes are relatively small and compact compared with other bacteria, which may indicate a specific adaptation for their obligate pathogenic lifestyles [46, 47]. This genus has a low GC content, whose average GC content is 38.91%, ranging from 33.58% (H. pullorum MIT 98-5489) to 47.38% (H. heilmannii ASB1.4). The average number of protein coding sequences predicted is 1,730, ranging from 1,432 (H. pametensis ATCC 51478) to 2,751 (H. bilis WiWa).
The hosts of this genus species have great variety. All the H. pylori strains and H. cinaedi, H. fennelliae, H. heilmannii, and H. winghamensis were originally isolated from humans. The natural hosts of H. canis, H. bizzozeronii, H. Canadensis, H. felis, H. pullorum, and H. suis are mammals or birds, including pig, cat, dog, and geese. At the same time, the above six NHPS were also found to associate with gastric disease in humans [48–51]. H. acinonychis, H. ailurogastricus, H. bilis, H. cetorum, H. hepaticus, H. himalayensis, H. macacae, H. mustelae, H. pametensis, and H. typhlonius were isolated from nonhuman sources only, which had not been reported in human infection before [52–54].
3.2. Phylogenetic Analysis of 16S rRNA
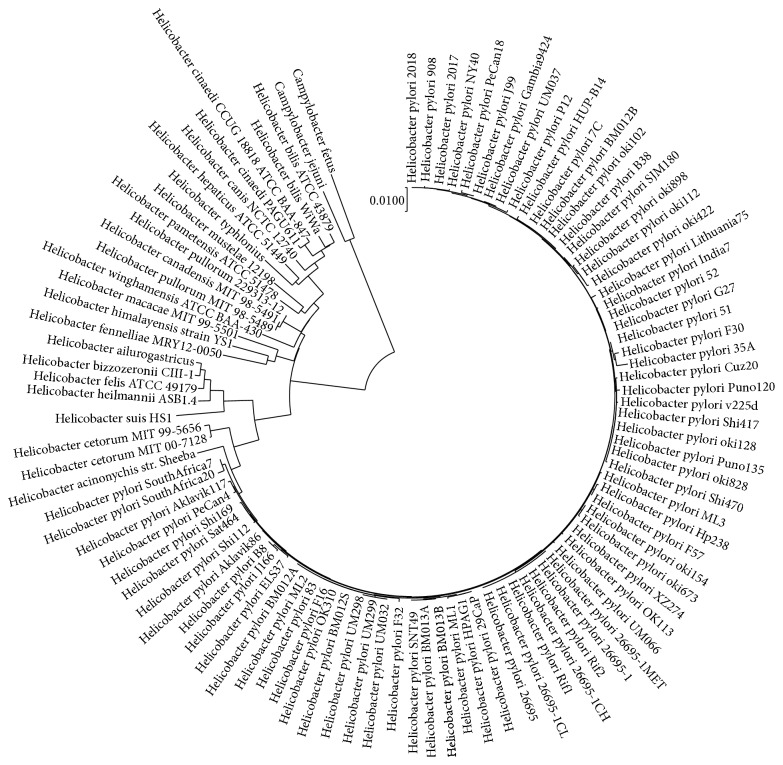
Helicobacter genus species have a wide range of hosts. However, H. pylori is one of the most prevalent pathogenic bacteria that comigrated and evolved with human beings all around the world [55]. Each Helicobacter species has its own specific or broad hosts or even only survives in several host's organs [56], suggesting that each one of them has developed a balance of adaption with its hosts. In order to better understand the pattern of evolution in this genus, a phylogenetic tree based on 16S rRNA has been constructed for 99 Helicobacter species with Campylobacter fetus and Campylobacter jejuni as outgroup. After multiple alignments, the common gaps and missing data were masked. In the final dataset, there were 1,489 bp of each aligned sequence. As shown in Figure 1, H. acinonychis and H. cetorum, whose nature hosts are cats and aquatic mammals, respectively, are the closest species to H. pylori, and H. pylori strains have a very close relationship among them.
Figure 1.
16S rRNA phylogenetic tree of 99 Helicobacter genus strains and 2 Campylobacter species was constructed by Neighbor-Joining (NJ) algorithms. The sum of branch length of the optimal tree is 0.47957369. The evolutionary distances were computed using the p-distance method.
3.3. Homologous Proteome Analysis by Pairwise Comparisons
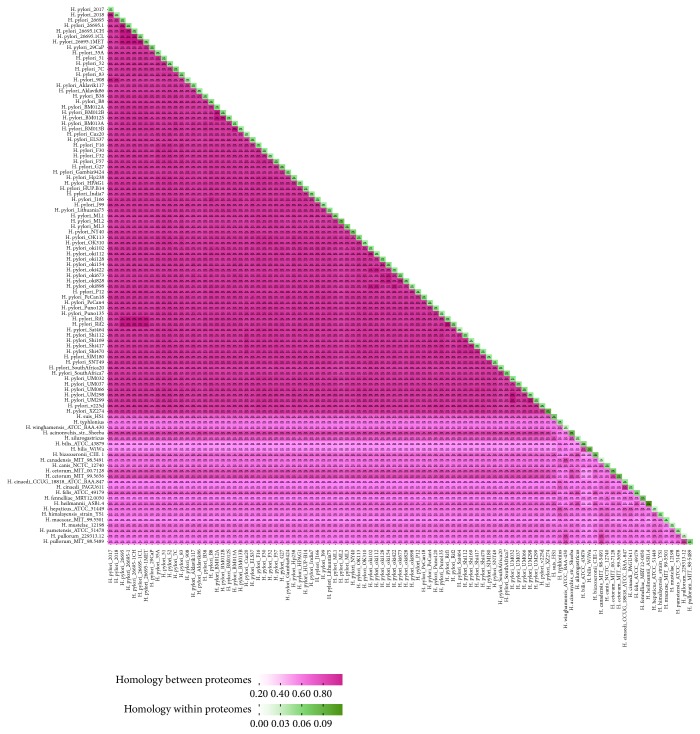
The whole predicted proteins (proteome) of each strain used in this study were compared to estimate the amount of proteins they shared. The homolog between any two different proteomes ranged from 43.71% (H. heilmannii ASB 1.4 versus H. bilis ATCC 43879) to 99.87% (H. pylori BM013A versus H. pylori BM013B), while it is generally to be above 80% within the H. pylori strains (Figure 2). The results also showed that H. acinonychis (average 81.7%) and H. cetorum (average 75.59%) had the highest similarity with H. pylori. The relationships shown by the homologous analysis are consistent when compared with the phylogenetic tree. The internal homology against its own proteome ranged from 1.45% (H. pullorum 229313-12) to 9.52% (H. heilmannii ASB1.4) with average 3.50%, which indicates that this genus's strains have a low redundancy in their genome composition.
Figure 2.
Homologous proteins analysis among proteomes (orthologous) and internal proteomes (paralogous) in the Helicobacter genus species. The blocks on the diagonal represent paralogous data and the others represent orthologous data. The percentage of orthologous and paralogous proteins are represented by red and green, respectively. The similarity is indicated by depth of color. The number of homologs and percentage of similarities between/within proteomes are shown in corresponding block.
3.4. Core-Pan Genome Analysis
The core genome, which is responsible for the basic life processes and major phenotypic characteristics, is composed of the gene families that are shared by all the Helicobacter species strains. The pan genome is the overall gene families existing in any Helicobacter species strain. The pan genome size of 75 H. pylori genomes is 4,409 with an average of about 39 new gene families extended with followed addition of genome. The increasing speed of pan genome size is almost the same with previous analysis of Ali et al., and their sample size is 39 genomes [57]. For 24 NPHS genomes along, the pan genome size is 12,010, including 4,412 singleton genes. When all NPHS and H. pylori genomes were used, the pan genome size was rapidly increased to 14,686, including 8,243 singleton genes. It is more than thrice the size of 75 H. pylori pan genome size. The above pan genome analytic results suggest that the genomes of Helicobacter genus species are open and have diversity. Nevertheless, the core genome size is relatively stable. There are 1,173 gene families shared by all the H. pylori genomes, which represent more than 74% of their average gene family contents (~1,565). For all the NPHS genomes along, the core genome size is 682, which is almost the same with the size (673) for all H. pylori with NPHS genomes together. It is interesting that there is an obvious difference between the core genome size of H. pylori and NPHS. This may indicate that those unique gene families shared by H. pylori strains are very relevant to their adaption to unique living environment, pathogenicity, and epidemic.
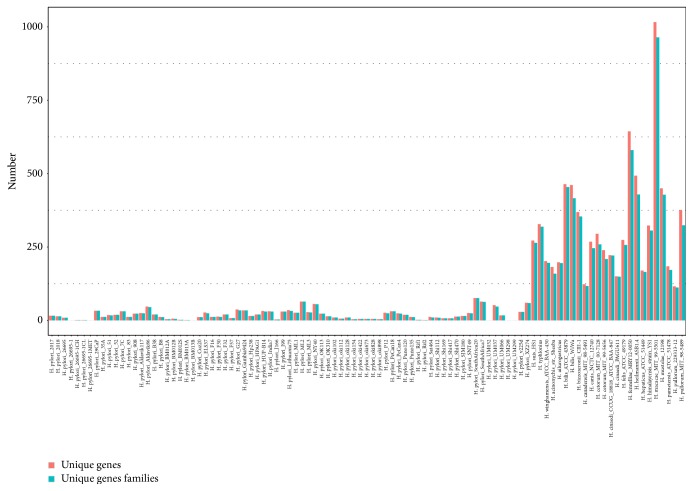
Estimation of the size of unique genes and gene families for each individual species relative to all 99 genomes was simultaneously carried out (Figure 3). H. macacae MIT 99-5501 has the largest number of unique genes and gene families, which are 1,016 and 964, respectively. It accounts for 38.07 percent of its gene contents. The number of unique genes of H. pylori is relatively few. This may be due to the fact that too many H. pylori genomes were compared with each other. For example, H. pylori BM013A genome and H. pylori BM013B genome exhibit a high degree of similarity, so only few unique genes exist between them. For all the NHPS, the average number of unique genes and gene families are 325 and 303. It once again implies the obvious genomic plasticity among Helicobacter species living in different habits and possessing diverse lifestyles.
Figure 3.
The number of unique genes and gene families for each individual species relative to all 99 genomes. Orange and turquoise bar graphs represent unique genes and gene families for each individual species, respectively.
3.5. COG Category of Core Genome and Accessory Genome
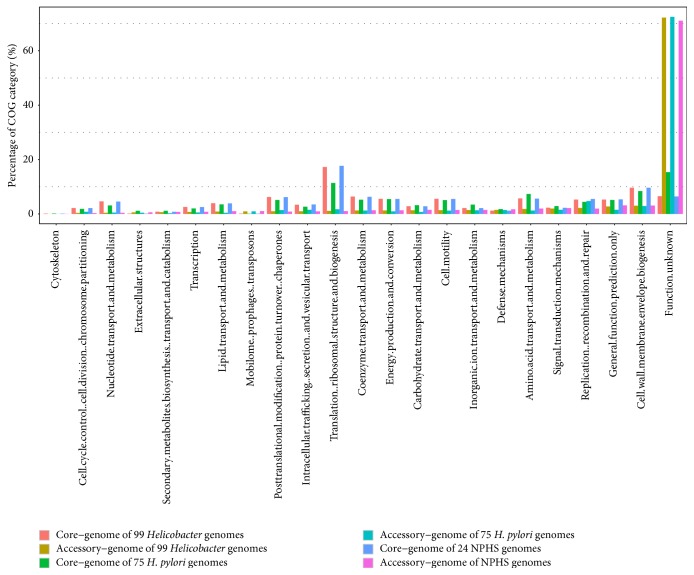
The core genome and accessory genome of 99 Helicobacter strains were composed of 673 and 14,013 protein families, separately. For 75 H. pylori genomes along, the core genome and accessory genome sizes were 1,173 and 3,236, as well as 682 and 11,328 for 24 NPHS genomes along. According to COG category analysis of the above six datasets, possible functions of their gene clusters were identified and subdivided into 23 subcategories. The unassigned gene clusters were put into the same class with function unknown (Figure 4). For three core genome datasets, more than 90% protein clusters were assigned to COG function category. Nevertheless, average 28.1% protein clusters were assigned for three accessory datasets, suggesting that there are still a plenty of proteins without clear biological functions that need to be studied.
Figure 4.
Functional classification of core genome and accessory genome by COG database. Core genome and accessory genome of 99 Helicobacter genomes and core genome and accessory genome of 75 H. pylori genomes, along with core genome and accessory genome of 24 NPHS genomes are shown using different colors, respectively.
In line with what we expected, the significant protein clusters belonging to core genome were assigned to the groups of housekeeping functions. For core genome of 99 Helicobacter strains, translation, ribosomal structure, biogenesis (category J), and cell wall/membrane/envelope biogenesis (category M) take up 17.26% and 9.65%, respectively, and the percentages are far more than accessory genome. On the contrary, for functional subcategories extracellular structures (category W), mobilome, prophages, transposons (category X), and defense mechanisms (category V), the proportion of accessory genome is greater than core genome. Most of these protein clusters closely related to the interaction of strains and their living environment [58–60]. For instance, type IV pilus (TFP) assembly proteins (category W) are important components of TFP pilus which help H. pylori colonization [61]; multiple transposase genes (category X) which can cause antibiotic resistance and transposition are also important to create genetic diversity within species and adaptability to dynamic living conditions [62]; ABC-type multidrug transport system proteins (category V) are used to drug resistance [63] and so on. In addition, the poorly characterized part accounting for more than 70% may be involved in specific adaptations that help Helicobacter species survive in novel environments.
3.6. Identification of H. pylori Unique Regions
A reasonable hypothesis often made in studying bacteria evolution is that the numerous host specific adaptation that a bacterial species displays will be correlated with its specific regions and genes [64]. In this study, seventy-nine sequence segments, total length of 202,359 bp, about 12.4% of the H. pylori genome, were identified as unique regions. These regions are shared by H. pylori strains but absent from NHPS. The lengths of the unique regions range from 211 bp to 27,269 bp and median length is 1,502. A total of 155 genes are contained in them. Functional annotation of the above genes was performed by VFDB, COG database, InterProScan, and NR database, respectively. Furthermore, the results were integrated (Table S1, in Supplementary Material available online at http://dx.doi.org/10.1155/2016/6106029) and classified into different function categories (Figure 5). Besides, a total of 28 sRNAs within the URHP were identified (Table S2).
Figure 5.
Regions conserved in H. pylori and absent from NHPS. From inside to outside, rings 1 and 2 are GC content and GC skew of reference genome H. pylori 26695, respectively; rings 3–7 represent H. pylori strains while rings 8–31 represent NHPS. The depth of color of rings 3–31 indicates the sequence similarity. Outer ring is the unique regions of H. pylori and absent from NHPS. Inside the outer ring, different colors represent different function categories: purple: Cag-PAI; blue: membrane genes; green: transport and metabolism genes; gray: cell growth, division, and basic metabolism; aqua: other functional genes; black: hypothetical genes; red: sRNAs.
In the circular graph, the largest H. pylori unique region named UR_26 containing 28 genes can be observed obviously. Average about two genes were contained in each unique region. However, about 82.3% unique regions contain two genes or less. Operons, as the basic units of transcription and cellular functions, have been proved that they are extensively existing in H. pylori genome [65]. Within H. pylori, sixty unique genes, more than three quarters, are contained in nineteen unique polycistrons. Twenty-three polycistrons are located partly in URHP, in addition to seventy-one monocistrons (Table S1). The known acid induction of H. pylori adaptability and virulence operons, such as cag-pathogenicity island, transcriptional regulator (tenA), catalase, and membrane protein (hopT), are included in them [65–67]. These results indicate that H. pylori can regulate the expression of those unique genes by control of operons depending on environmental conditions.
A total of 101 genes could get the certain functional annotation within the URHP, compared to the above 4 databases. Unique region UR_26 represents the T4SS, which can deliver effector protein cancer-associated gene toxin (cagA) into gastric epithelial cells. It is reported that T4SS plays a crucial role in the pathogenesis of gastric cancer [12, 60]. Besides T4SS, a plenty of genes, which have been proved strongly to correlate to pathogenicity and adaption, are contained in the unique regions. For instance, membrane proteins babB/hopT, sabB/hopO, and sabA/hopP, and so forth are involved in cell adhesion. These genes facilitate colonization of H. pylori and increase immune response, resulting in enhanced mucosal inflammation [68–70]; abundant restriction-modification (RM) system proteins have large effects on gene expression and genome maintenance. They give H. pylori the ability to adapt to dynamic environmental conditions during long-term colonization [71]; ABC transporters, MFS transporter, sugar efflux transporter, short-chain fatty acids transporter, and so forth, which are important virulence factors because they play roles in nutrient uptake and secretion of toxins and antimicrobial agents, are important for their interactions with complicated and changeable environments [72–74]. Even though pfam, KEGG, GO, and TrEMBL databases were used for functional annotation, the other 54 genes still cannot get the clear function information, accounting for nearly a third of all URHP genes.
Noncoding small RNAs act as posttranscriptional regulators that fine-tune important physiological processes in pathogens to adapt dynamic, intricate environment [75, 76]. To investigate the regulatory roles of the putative unique sRNAs, we mapped them to the genome of H. pylori 26695 [76]. Eighteen of them have matches with genes, unexpectedly (Table S2). Ten sRNAs (SR1, SR2, SR6, SR15, SR20, SR21, SR22, SR23, SR13, and SR25) match perfectly with the known acid induction genes, including eight membrane proteins, DNA polymerase III subunits gamma, tau, and adenine-specific DNA methyltransferase [67, 77]. Besides, SR5 matches with HcpA, which is considered as a virulence factor to trigger the release of a concerted set of cytokines to active the inflammatory response [78]. The small CRISPR RNAs SR7 and SR18 are guides of the CRISPR-Cas system, which was reported as potential participants in bacteria stress responses and virulence [79].
Altogether, it has been proved that the close associations exist between most of the operons, genes, or sRNAs within URHP and adaptability or virulence of H. pylori. However, some of them cannot get the certain functional information via current databases, which indicates that our genetic knowledge is still incomplete to explain pathogenicity and adaption mechanism of H. pylori fully and these function unknown genes need to be further studied.
3.7. Protein-Protein Interaction Network Analysis
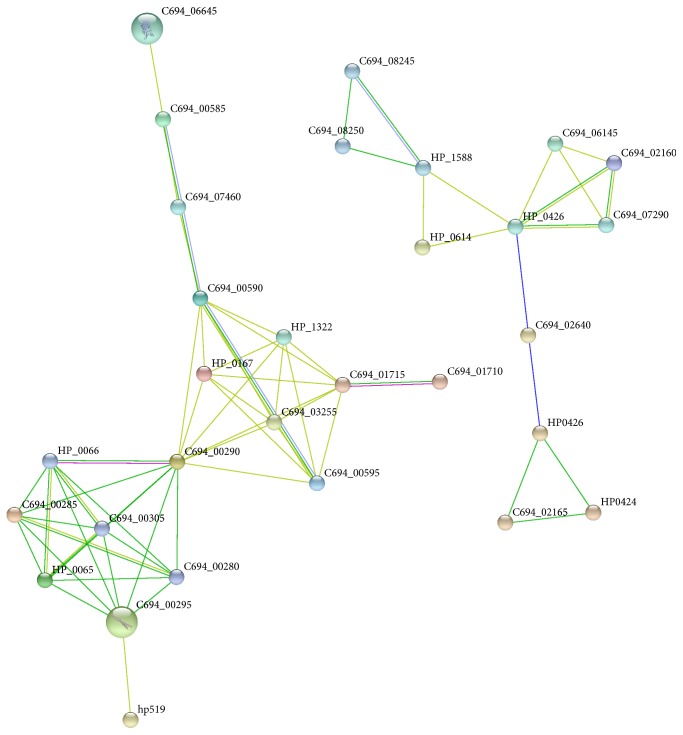
The 155 URHP genes and 54 genes with unknown functions of H. pylori were analyzed using STRING to build protein-protein interaction map, respectively. As shown in Figure S1, a total of 125 genes were assigned into an independent interaction network. It is easy to find two main protein-protein interaction groups: one is well-known cag-pathogenicity island, and the other takes succinyl-CoA-3-ketoacid CoA transferase (encoded by scoA and scoB of operon UO_54), acetone carboxylase (encoded by C694_03570, C694_03590, and C694_03595 of operon UO_55), and acetyl-CoA acetyltransferase (encoded by C694_03555 of operon UO_54) as the center of the interaction map. The second main protein-protein interaction group genes are involved in acetone metabolism. Brahmachary et al. proved that those genes play an important role in survival and colonization of the H. pylori in gastric mucosa [80, 81]. Figure 6 shows a possible protein-protein interaction map of the 54 URHP function unknown genes. Thirty proteins were targeted to two divided interaction maps. One includes 18 proteins; the other includes 12 ones. These genes may have synergistic effect on surviving characteristics of H. pylori. They could be used as the most possible proteins to further explore the common pathogenic behavior of this pathogen.
Figure 6.
Protein-protein interaction networks of URHP function unknown genes. Thirty proteins are shown in two interaction networks. Network nodes represent proteins and edges represent protein-protein associations. Different colors represent the types of evidence for the interaction.
4. Conclusions
H. pylori is an age-old pathogenic microorganism that has infected more than half of the population with strong adaptability. In this study, we presented a comparative genomics analysis of 75 representative H. pylori complete genomes and 24 NHPS ones. Pan genome analysis showed that both all Helicobacter genus strains and only H. pylori species had an open and diverse genome, which may be the result of the different strains that cope with their specific living conditions. However, the core genome is conserved relatively higher. We found 1173 conserved protein families for 75 H. pylori strains and 673 for all the 99 Helicobacter genus strains. The regions and genes, which are conserved among H. pylori genomes but absent from NHPS genomes, were considered as potential targets that were associated with H. pylori pathogenicity and adaptation. Functional annotation of 155 genes within 79 URHP indicated that most of them are well-known pathogenic and adaptive associated ones, such as cag-pathogenicity island, babB, sabB, and ABC transporter, whereas there are still 54 genes of which the biological functions remain unclear. Protein-protein interaction network analysis showed that 30 of them could be assigned to two different interaction networks. Besides, the functional analysis of the operons and sRNAs which were unique to H. pylori also showed the intimate association between these genomic structures and its pathogenicity and adaptation. All the URHP, especially those components whose functions remain unclear, could be as potential candidates for further studying and deeply understanding the mechanism of widespread epidemics and pathogenicity in H. pylori. In addition, the analysis tools and pipeline used in this study could be as a reference applied to other species.
Supplementary Material
Supplemental Information includes: Table S1, Unique regions of H. pylori (URHP) and function annotations of relative genes; Table S2, sRNAs shared by all H. pylori but absent from NHPS; Fig. S1 Protein-protein interaction networks of 155 URHP genes.
Acknowledgments
This work was supported by Key Laboratory of Microbial Infection Research in Western Guangxi (Youjiang Medical University for Nationalities), Guangxi Key Discipline Fund of Pathogenic Microbiology (no. [2013]16), Key Laboratory Fund of Colleges and Universities in Guangxi (no. Gui Jiao Ke Yan [2014]6), National Natural Science Foundation of China (no. 31460023), Natural Science Foundation of Guangxi (no. 2014GXNSFAA118206), and Special Fund for Public Welfare Research and Capacity Building in Guangdong Province (no. 2014A020212288).
Abbreviations
- MALT:
Mucosa-associated lymphoid tissue
- NPHS:
Non-pylori Helicobacter species
- T4SS:
Type IV secretion system
- cagA:
Cancer-associated gene toxin
- VacA:
Vacuolating cytotoxin
- ORFs:
Open reading frames
- COGs:
Clusters of Orthologous Groups
- VFDB:
Virulence factor database
- NR:
Nonredundant
- URHP:
Unique regions of H. pylori
- DOOR:
Database for prokaryotic operons
- sRNAs:
Small noncoding RNAs
- BRIG:
BLAST ring image generator.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
The authors consider that De-Min Cao and Qun-Feng Lu contributed equally to this work. De-Min Cao, Yan-Qiang Huang, and Hong-Kai Bi conceived and designed the study. De-Min Cao and Qun-Feng Lu collected the data and performed the analysis. De-Min Cao, Ju-Ping Wang, Song-Bo Li, and Yu-Li Chen wrote the manuscript with the assist of all authors. All authors read and approved the final paper.
References
- 1.Suerbaum S., Josenhans C. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nature Reviews Microbiology. 2007;5(6):441–452. doi: 10.1038/nrmicro1658. [DOI] [PubMed] [Google Scholar]
- 2.Kennemann L., Didelot X., Aebischer T., et al. Helicobacter pylori genome evolution during human infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):5033–5038. doi: 10.1073/pnas.1018444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusters J. G., Van Vliet A. H. M., Kuipers E. J. Pathogenesis of Helicobacter pylori infection. Clinical Microbiology Reviews. 2006;19(3):449–490. doi: 10.1128/cmr.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S., Moise L., Moss S. F. H. pylori vaccines: why we still don't have any. Human Vaccines. 2011;7(11):1153–1157. doi: 10.4161/hv.7.11.17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsonnet J., Friedman G. D., Vandersteen D. P., et al. Helicobacter pylori infection and the risk of gastric carcinoma. New England Journal of Medicine. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 6.Gueneau P., Loiseaux-De Goër S. Helicobacter: molecular phylogeny and the origin of gastric colonization in the genus. Infection, Genetics & Evolution. 2002;1(3):215–223. doi: 10.1016/s1567-1348(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 7.Schrenzel M. D., Witte C. L., Bahl J., et al. Genetic characterization and epidemiology of helicobacters in non-domestic animals. Helicobacter. 2010;15(2):126–142. doi: 10.1111/j.1523-5378.2009.00744.x. [DOI] [PubMed] [Google Scholar]
- 8.Smet A., Flahou B., Mukhopadhya I., et al. The other helicobacters. Helicobacter. 2011;16(1):70–75. doi: 10.1111/j.1523-5378.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 9.Nikin Z., Bogdanovic B., Kukic B., Nikolic I., Vukojevic J. Helicobacter heilmannii associated gastritis: case report. Archive of Oncology. 2011;19(3-4):73–75. doi: 10.2298/AOO1104073V. [DOI] [Google Scholar]
- 10.Van Den Bulck K., Decostere A., Baele M., et al. Identification of non-Helicobacter pylori spiral organisms in gastric samples from humans, dogs, and cats. Journal of Clinical Microbiology. 2005;43(5):2256–2260. doi: 10.1128/jcm.43.5.2256-2260.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melito P. L., Munro C., Chipman P. R., Woodward D. L., Booth T. F., Rodgers F. G. Helicobacter winghamensis sp. nov., a novel Helicobacter sp. isolated from patients with gastroenteritis. Journal of Clinical Microbiology. 2001;39(7):2412–2417. doi: 10.1128/JCM.39.7.2412-2417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frick-Cheng A. E., Pyburn T. M., Voss B. J., Mcdonald W. H., Ohi M. D., Cover T. L. Molecular and structural analysis of the Helicobacter pylori cag Type IV secretion system core complex. mBio. 2016;7(1):77–80. doi: 10.1128/mBio.02001-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tohidpour A. CagA-mediated pathogenesis of Helicobacter pylori. Microbial Pathogenesis. 2016;93:44–55. doi: 10.1016/j.micpath.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Mora D., Arioli S. Microbial urease in health and disease. PLoS Pathogens. 2014;10(12) doi: 10.1371/journal.ppat.1004472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cover T. L., Blanke S. R. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nature Reviews Microbiology. 2005;3(4):320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 16.Yahiro K., Satoh M., Nakano M., et al. Low-density lipoprotein receptor-related protein-1 (LRP1) mediates autophagy and apoptosis caused by Helicobacter pylori VacA. Journal of Biological Chemistry. 2012;287(37):31104–31115. doi: 10.1074/jbc.M112.387498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerat E., Ochman H. Recognizing the pseudogenes in bacterial genomes. Nucleic Acids Research. 2005;33(10):3125–3132. doi: 10.1093/nar/gki631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer W., Breithaupt U., Kern B., Smith S. I., Spicher C., Haas R. A comprehensive analysis of Helicobacter pylori plasticity zones reveals that they are integrating conjugative elements with intermediate integration specificity. BMC Genomics. 2014;15(1, article 310) doi: 10.1186/1471-2164-15-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haley K. P., Gaddy J. A. Helicobacter pylori: genomic insight into the host-pathogen interaction. International Journal of Genomics. 2015;2015:8. doi: 10.1155/2015/386905.386905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medini D., Donati C., Tettelin H., Masignani V., Rappuoli R. The microbial pan-genome. Current Opinion in Genetics & Development. 2005;15(6):589–594. doi: 10.1016/j.gde.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Tettelin H., Masignani V., Cieslewicz M. J., et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial ‘pan-genome’. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delcher A. L., Harmon D., Kasif S., White O., Salzberg S. L. Improved microbial gene identification with GLIMMER. Nucleic Acids Research. 1999;27(23):4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagesen K., Hallin P., Rødland E. A., Stærfeldt H.-H., Rognes T., Ussery D. W. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Research. 2007;35(9):3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katoh K., Standley D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular biology and evolution. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L., Stoeckert C. J., Jr., Roos D. S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Research. 2003;13(9):2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dongen S. Information Systems [INS] 2000. A cluster algorithm for graphs; pp. 1–40. [Google Scholar]
- 29.Galperin M. Y., Makarova K. S., Wolf Y. I., Koonin E. V. Expanded Microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Research. 2015;43(1):D261–D269. doi: 10.1093/nar/gku1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatusov R. L., Galperin M. Y., Natale D. A., Koonin E. V. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Research. 2000;28(1):33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao F., Dam P., Chou J., Olman V., Xu Y. DOOR: a database for prokaryotic operons. Nucleic Acids Research. 2009;37(supplement 1):D459–D463. doi: 10.1093/nar/gkn757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerat E., Ochman H. Ψ-Φ: Exploring the outer limits of bacterial pseudogenes. Genome Research. 2004;14(11):2273–2278. doi: 10.1101/gr.2925604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones P., Binns D., Chang H.-Y., et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30(9):1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruitt K. D., Tatusova T., Maglott D. R. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Research. 2006;35(1):D61–D65. doi: 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finn R. D., Bateman A., Clements J., et al. Pfam: The protein families database. Nucleic Acids Research. 2014;42(1):D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogata H., Goto S., Sato K., Fujibuchi W., Bono H., Kanehisa M. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Research. 1999;27(1):29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashburner M., Ball C. A., Blake J. A., et al. Gene ontology: tool for the unification of biology. Nature Genetics. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camon E., Magrane M., Barrell D., et al. The Gene Ontology Annotation (GOA) Project: implementation of GO in SWISS-PROT, TrEMBL, and InterPro. Genome Research. 2003;13(4):662–672. doi: 10.1101/gr.461403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westhof E. The amazing world of bacterial structured RNAs. Genome Biology. 2010;11(11):79–82. doi: 10.1186/gb-2010-11-7-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storz G., Haas D. A guide to small RNAs in microorganisms. Current Opinion in Microbiology. 2007;10(2):93–95. doi: 10.1016/j.mib.2007.03.017. [DOI] [Google Scholar]
- 41.Cros M.-J., De Monte A., Mariette J., et al. RNAspace.org: an integrated environment for the prediction, annotation, and analysis of ncRNA. RNA. 2011;17(11):1947–1956. doi: 10.1261/rna.2844911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alikhan N.-F., Petty N. K., Ben Zakour N. L., Beatson S. A. BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12, article no. 402 doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szklarczyk D., Franceschini A., Wyder S., et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Research. 2015;43(1):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warren J. R., Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. The Lancet. 1983;321(8336):1273–1275. doi: 10.1016/s0140-6736(83)92719-8. [DOI] [PubMed] [Google Scholar]
- 45.Tomb J.-F., White O., Kerlavage A. R., et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388(6642):539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 46.Bryant J., Chewapreecha C., Bentley S. D. Developing insights into the mechanisms of evolution of bacterial pathogens from whole-genome sequences. Future Microbiology. 2012;7(11):1283–1296. doi: 10.2217/fmb.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moran N. A. Microbial minimalism: genome reduction in bacterial pathogens. Cell. 2002;108(5):583–586. doi: 10.1016/s0092-8674(02)00665-7. [DOI] [PubMed] [Google Scholar]
- 48.Dewhirst F. E., Seymour C., Fraser G. J., Paster B. J., Fox J. G. Phylogeny of Helicobacter isolates from bird and swine feces and description of Helicobacter pametensis sp. nov. International Journal of Systematic Bacteriology. 1994;44(3):553–560. doi: 10.1099/00207713-44-3-553. [DOI] [PubMed] [Google Scholar]
- 49.Waldenström J., On S. L. W., Ottvall R., Hasselquist D., Harrington C. S., Olsen B. Avian reservoirs and zoonotic potential of the emerging human pathogen helicobacter canadensis. Applied & Environmental Microbiology. 2003;69(12):7523–7526. doi: 10.1128/aem.69.12.7523-7526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young V. B., Chien C.-C., Knox K. A., Taylor N. S., Schauer D. B., Fox J. G. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum . Journal of Infectious Diseases. 2000;182(2):620–623. doi: 10.1086/315705. [DOI] [PubMed] [Google Scholar]
- 51.Kersulyte D., Rossi M., Berg D. E. Sequence divergence and conservation in genomes of Helicobacter cetorum strains from a dolphin and a whale. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0083177.e83177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marini R. P., Muthupalani S., Shen Z., et al. Persistent infection of rhesus monkeys with ‘Helicobacter macacae’ and its isolation from an animal with intestinal adenocarcinoma. Journal of Medical Microbiology. 2010;59(8):961–969. doi: 10.1099/jmm.0.019117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank J., Dingemanse C., Schmitz A. M., et al. The complete genome sequence of the murine pathobiont helicobacter typhlonius. Frontiers in Microbiology. 2016;6, article 1549 doi: 10.3389/fmicb.2015.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haesebrouck F., Pasmans F., Flahou B., et al. Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clinical Microbiology Reviews. 2009;22(2):202–223. doi: 10.1128/CMR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falush D., Wirth T., Linz B., et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299(5612):1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 56.Mikkonen T. P., Kärenlampi R. I., Hänninen M.-L. Phylogenetic analysis of gastric and enterohepatic Helicobacter species based on partial HSP60 gene sequences. International Journal of Systematic & Evolutionary Microbiology. 2004;54(3):753–758. doi: 10.1099/ijs.0.02839-0. [DOI] [PubMed] [Google Scholar]
- 57.Ali A., Naz A., Soares S. C., et al. Pan-genome analysis of human gastric pathogen H. pylori: comparative genomics and pathogenomics approaches to identify regions associated with pathogenicity and prediction of potential core therapeutic targets. BioMed Research International. 2015;2015:17. doi: 10.1155/2015/139580.139580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schilling C. H., Covert M. W., Famili I., Church G. M., Edwards J. S., Palsson B. O. Genome-scale metabolic model of Helicobacter pylori 26695. Journal of Bacteriology. 2002;184(16):4582–4593. doi: 10.1128/JB.184.16.4582-4593.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guillemin K. J., Salama N. R. Helicobacter pylori functional genomics. Methods in Microbiology. 2002;33:291–319. doi: 10.1016/S0580-9517(02)33017-4. [DOI] [Google Scholar]
- 60.Sgouras D. N., Trang T. T. H., Yamaoka Y. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2015;20:8–16. doi: 10.1111/hel.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.So M. Pilus retraction powers bacterial twitching motility. Nature. 2000;407(6800):p. 98. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 62.Aziz R. K., Breitbart M., Edwards R. A. Transposases are the most abundant, most ubiquitous genes in nature. Nucleic Acids Research. 2010;38(13):4207–4217. doi: 10.1093/nar/gkq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lubelski J., Konings W. N., Driessen A. J. M. Distribution and physiology of ABC-type transporters contributing to multidrug resistance in bacteria. Microbiology and Molecular Biology Reviews. 2007;71(3):463–476. doi: 10.1128/MMBR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lefébure T., Stanhope M. J. Evolution of the core and pan-genome of Streptococcus: positive selection, recombination, and genome composition. Genome Biology. 2007;8(5, article R71) doi: 10.1186/gb-2007-8-5-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma C. M., Hoffmann S., Darfeuille F., et al. The primary transcriptome of the major human pathogen Helicobacter pylori . Nature. 2010;464(7286):250–255. doi: 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- 66.Merrell D. S., Goodrich M. L., Otto G., Tompkins L. S., Falkow S. pH-regulated gene expression of the gastric pathogen Helicobacter pylori . Infection and Immunity. 2003;71(6):3529–3539. doi: 10.1128/iai.71.6.3529-3539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen Y., Marcus E. A., Matrubutham U., Gleeson M. A., Scott D. R., Sachs G. Acid-adaptive genes of Helicobacter pylori. Infection and Immunity. 2003;71(10):5921–5939. doi: 10.1128/IAI.71.10.5921-5939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pride D. T., Meinersmann R. J., Blaser M. J. Allelic variation within Helicobacter pylori babA and babB . Infection & Immunity. 2001;69(2):1160–1171. doi: 10.1128/iai.69.2.1160-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahdavi J., Sondén B., Hurtig M., et al. Helicobacter pylori sabA adhesin in persistent infection and chronic inflammation. Science. 2002;297(5581):573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rad R., Gerhard M., Lang R., et al. The Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a nonspecific immune response. Journal of Immunology. 2002;168(6):3033–3041. doi: 10.4049/jimmunol.168.6.3033. [DOI] [PubMed] [Google Scholar]
- 71.Furuta Y., Namba-Fukuyo H., Shibata T. F., et al. Methylome diversification through changes in DNA methyltransferase sequence specificity. PLoS Genetics. 2014;10(4) doi: 10.1371/journal.pgen.1004272.e1004272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hendricks J. K., Mobley H. L. T. Helicobacter pylori ABC transporter: effect of allelic exchange mutagenesis on urease activity. Journal of Bacteriology. 1997;179(18):5892–5902. doi: 10.1128/jb.179.18.5892-5902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan N. Structural advances for the major facilitator superfamily (MFS) transporters. Trends in Biochemical Sciences. 2013;38(3):151–159. doi: 10.1016/j.tibs.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Davidson A. L., Chen J. ATP-binding cassette transporters in bacteria. Annual Review of Biochemistry. 2004;73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 75.Ortega Á. D., Quereda J. J., Graciela Pucciarelli M., García-del Portillo F. Non-coding RNA regulation in pathogenic bacteria located inside eukaryotic cells. Frontiers in Cellular and Infection Microbiology. 2014;4, article no. 162 doi: 10.3389/fcimb.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao B., Li W., Guo G., et al. Identification of small noncoding RNAs in Helicobacter pylori by a bioinformatics-based approach. Current Microbiology. 2009;58(3):258–263. doi: 10.1007/s00284-008-9318-2. [DOI] [PubMed] [Google Scholar]
- 77.Bury-Moné S., Thiberge J.-M., Contreras M., Maitournam A., Labigne A., De Reuse H. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori . Molecular Microbiology. 2004;53(2):623–638. doi: 10.1111/j.1365-2958.2004.04137.x. [DOI] [PubMed] [Google Scholar]
- 78.Deml L., Aigner M., Decker J., et al. Characterization of the Helicobacter pylori cysteine-rich protein A as a T-helper cell type 1 polarizing agent. Infection and Immunity. 2005;73(8):4732–4742. doi: 10.1128/IAI.73.8.4732-4742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Louwen R., Staals R. H. J., Endtz H. P., Van Baarlen P., Van Der Oost J. The role of CRISPR-cas systems in virulence of pathogenic bacteria. Microbiology and Molecular Biology Reviews. 2014;78(1):74–88. doi: 10.1128/MMBR.00039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brahmachary P., Wang G., Benoit S. L., Weinberg M. V., Maier R. J., Hoover T. R. The human gastric pathogen Helicobacter pylori has a potential acetone carboxylase that enhances its ability to colonize mice. BMC Microbiology. 2008;8(1, article 14):1–8. doi: 10.1186/1471-2180-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang M.-J., Zhao F., Xiao D., et al. Comparative proteomic analysis of passaged Helicobacter pylori. Journal of Basic Microbiology. 2009;49(5):482–490. doi: 10.1002/jobm.200800372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information includes: Table S1, Unique regions of H. pylori (URHP) and function annotations of relative genes; Table S2, sRNAs shared by all H. pylori but absent from NHPS; Fig. S1 Protein-protein interaction networks of 155 URHP genes.