Abstract
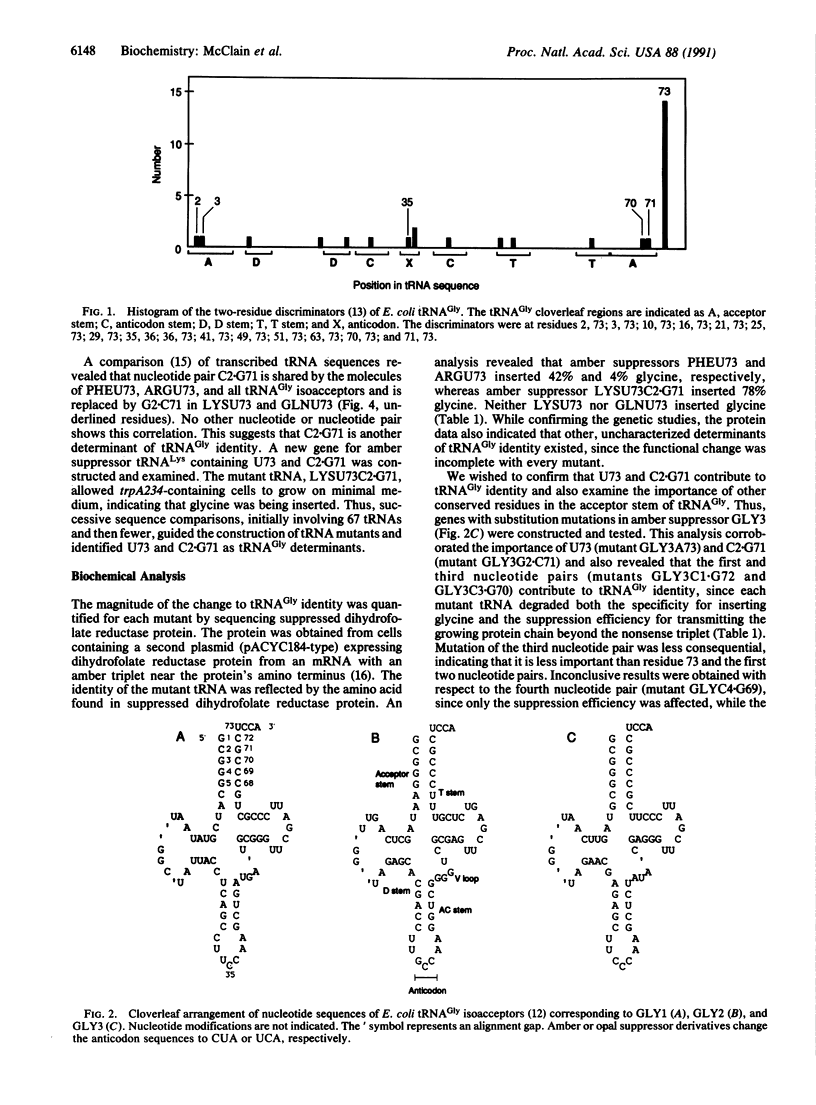
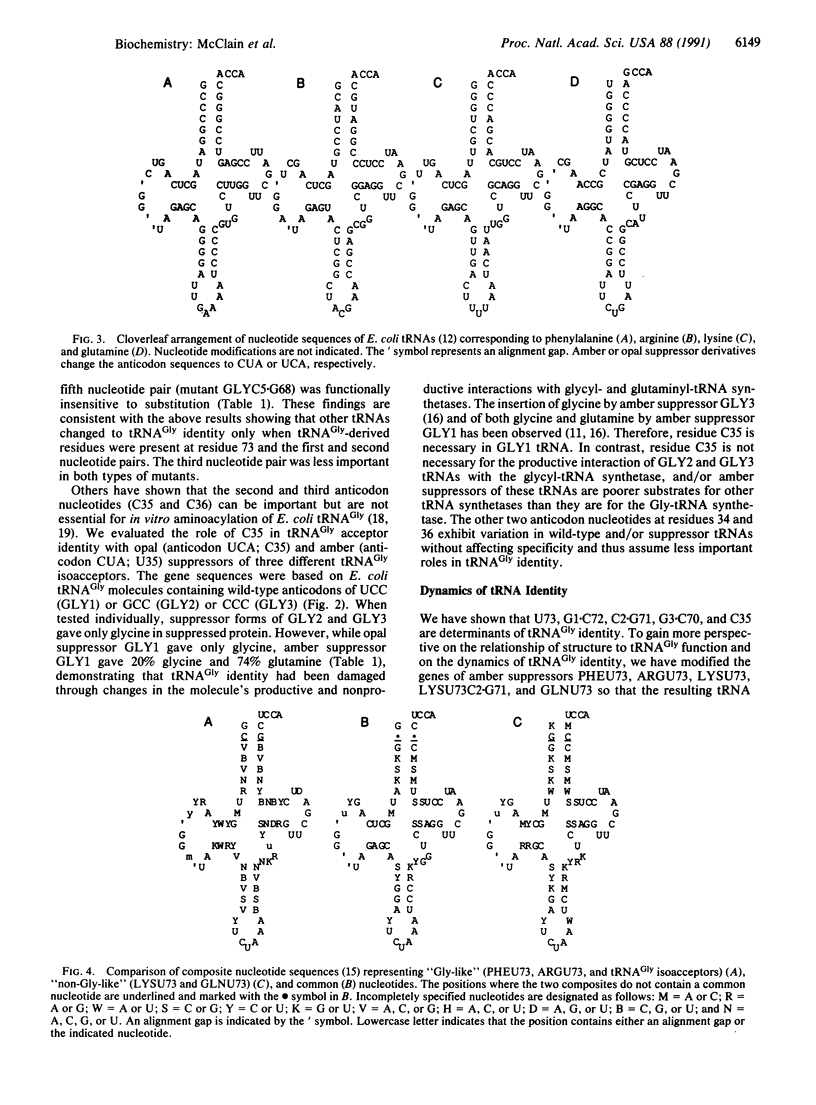
Expression of the genetic code depends on the recognition of specific tRNAs by the enzymes that aminoacylate them. A computer comparison of tRNA sequences coupled with analysis of mutant nonsense-suppressor tRNAs has revealed the structural features that distinguish the acceptor identity of Escherichia coli tRNA(Gly) from tRNAs that accept phenylalanine, arginine, lysine, and glutamine. On replacement of several nucleotides in the acceptor stem and anticodon of the latter tRNAs with tRNA(Gly)-derived residues, the resulting molecules acquired a tRNA(Gly) identity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hill C. W., Combriato G., Dolph W. Three different missense suppressor mutations affecting the tRNA GGG Gly species of Escherichia coli. J Bacteriol. 1974 Feb;117(2):351–359. doi: 10.1128/jb.117.2.351-359.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno H., Hasegawa T., Ueda T., Watanabe K., Shimizu M. Conversion of aminoacylation specificity from tRNA(Tyr) to tRNA(Ser) in vitro. Nucleic Acids Res. 1990 Dec 11;18(23):6815–6819. doi: 10.1093/nar/18.23.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y. M., Schimmel P. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature. 1988 May 12;333(6169):140–145. doi: 10.1038/333140a0. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Inada T., Nakamura Y. Conditionally lethal and recessive UGA-suppressor mutations in the prfB gene encoding peptide chain release factor 2 of Escherichia coli. J Bacteriol. 1988 Nov;170(11):5378–5381. doi: 10.1128/jb.170.11.5378-5381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain W. H., Foss K. Changing the acceptor identity of a transfer RNA by altering nucleotides in a "variable pocket". Science. 1988 Sep 30;241(4874):1804–1807. doi: 10.1126/science.2459773. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Foss K. Changing the identity of a tRNA by introducing a G-U wobble pair near the 3' acceptor end. Science. 1988 May 6;240(4853):793–796. doi: 10.1126/science.2452483. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Foss K., Jenkins R. A., Schneider J. Nucleotides that determine Escherichia coli tRNA(Arg) and tRNA(Lys) acceptor identities revealed by analyses of mutant opal and amber suppressor tRNAs. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9260–9264. doi: 10.1073/pnas.87.23.9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain W. H., Foss K. Nucleotides that contribute to the identity of Escherichia coli tRNA(Phe). J Mol Biol. 1988 Aug 20;202(4):697–709. doi: 10.1016/0022-2836(88)90551-7. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Nicholas H. B., Jr Differences between transfer RNA molecules. J Mol Biol. 1987 Apr 20;194(4):635–642. doi: 10.1016/0022-2836(87)90240-3. [DOI] [PubMed] [Google Scholar]
- Murgola E. J. tRNA, suppression, and the code. Annu Rev Genet. 1985;19:57–80. doi: 10.1146/annurev.ge.19.120185.000421. [DOI] [PubMed] [Google Scholar]
- Nicholas H. B., Chen Y. M., McClain W. H. Comparison of tRNA sequences. Comput Appl Biosci. 1987 Mar;3(1):53–53. doi: 10.1093/bioinformatics/3.1.53. [DOI] [PubMed] [Google Scholar]
- Normanly J., Kleina L. G., Masson J. M., Abelson J., Miller J. H. Construction of Escherichia coli amber suppressor tRNA genes. III. Determination of tRNA specificity. J Mol Biol. 1990 Jun 20;213(4):719–726. doi: 10.1016/S0022-2836(05)80258-X. [DOI] [PubMed] [Google Scholar]
- Normanly J., Ogden R. C., Horvath S. J., Abelson J. Changing the identity of a transfer RNA. Nature. 1986 May 15;321(6067):213–219. doi: 10.1038/321213a0. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Sabban E. L., Bhanot O. S. The effect of bisulfite-induced C to U transitions on aminoacylation of Escherichia coli glycine tRNA. J Biol Chem. 1982 May 10;257(9):4796–4805. [PubMed] [Google Scholar]
- Sampson J. R., DiRenzo A. B., Behlen L. S., Uhlenbeck O. C. Nucleotides in yeast tRNAPhe required for the specific recognition by its cognate synthetase. Science. 1989 Mar 10;243(4896):1363–1366. doi: 10.1126/science.2646717. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon switching changes the identity of methionine and valine transfer RNAs. Science. 1988 Nov 4;242(4879):765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. The anticodon contains a major element of the identity of arginine transfer RNAs. Science. 1989 Dec 22;246(4937):1595–1597. doi: 10.1126/science.2688091. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Hoben P., Sumner-Smith M., Uemura H., Watson L., Söll D. Accuracy of in vivo aminoacylation requires proper balance of tRNA and aminoacyl-tRNA synthetase. Science. 1988 Dec 16;242(4885):1548–1551. doi: 10.1126/science.3144042. [DOI] [PubMed] [Google Scholar]
- Yarus M. Intrinsic precision of aminoacyl-tRNA synthesis enhanced through parallel systems of ligands. Nat New Biol. 1972 Sep 27;239(91):106–108. doi: 10.1038/newbio239106a0. [DOI] [PubMed] [Google Scholar]



