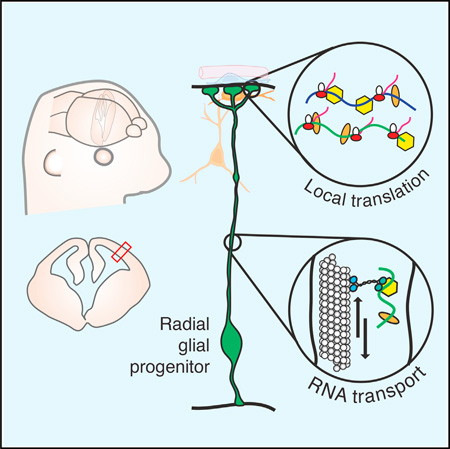
SUMMARY
In the developing brain, neurons are produced from neural stem cells termed radial glia [1, 2]. Radial glial progenitors span the neuroepithelium, extending long basal processes to form endfeet hundreds of micrometers away from the soma. Basal structures influence neuronal migration, tissue integrity, and proliferation [3–7]. Yet, despite the significance of these distal structures, their cell biology remains poorly characterized, impeding our understanding of how basal processes and endfeet influence neurogenesis. Here we use live imaging of embryonic brain tissue to visualize, for the first time, rapid mRNA transport in radial glia, revealing that the basal process is a highway for directed molecular transport. RNA- and mRNA-binding proteins, including the syndromic autism protein FMRP, move in basal processes at velocities consistent with microtubule-based transport, accumulating in endfeet. We develop an ex vivo tissue preparation to mechanically isolate radial glia endfeet from the soma, and we use photoconvertible proteins to demonstrate that mRNA is locally translated. Using RNA immunoprecipitation and microarray analyses of endfeet, we discover FMRP-bound transcripts, which encode signaling and cytoskeletal regulators, including many implicated in autism and neurogenesis. We show FMRP controls transport and localization of one target, Kif26a. These discoveries reveal a rich, regulated local transcriptome in radial glia, far from the soma, and establish a tractable mammalian model for studying mRNA transport and local translation in vivo. We conclude that cytoskeletal and signaling events at endfeet may be controlled through translation of specific mRNAs transported from the soma, exposing new mechanistic layers within stem cells of the developing brain.
Graphical abstract
RESULTS
In the developing cerebral cortex, neurons are generated by neural stem cells termed radial glial cells (RGCs) [1, 2]. RGCs are polarized with a cell body adjacent to the ventricle, a basal process spanning 150–450 µm in the mouse, and a basal endfoot, which is surrounded by basal lamina, meninges, Cajal-Retzius cells, and neurons (Figure 1A). The basal process is a scaffold for migrating excitatory neurons, and its asymmetric inheritance following cell division correlates with progenitor cell fate [3, 8]. Basal endfeet interactions with the overlying lamina define the pial border for migrating neurons [9, 10]. Extrinsic cues from the basal niche cells influence RGCs [4, 5, 11, 12], suggesting that endfeet also may promote signaling back to the cell body [13, 14]. Consistent with this notion, endfeet dynamically interact with the basal lamina via filopodia-like protrusions [6, 15]. These findings underscore the functional significance of the RGC basal process and endfoot. Yet, we know strikingly little about molecular and cell biological mechanisms within these cellular compartments, limiting our understanding of how RGCs impact cortical development.
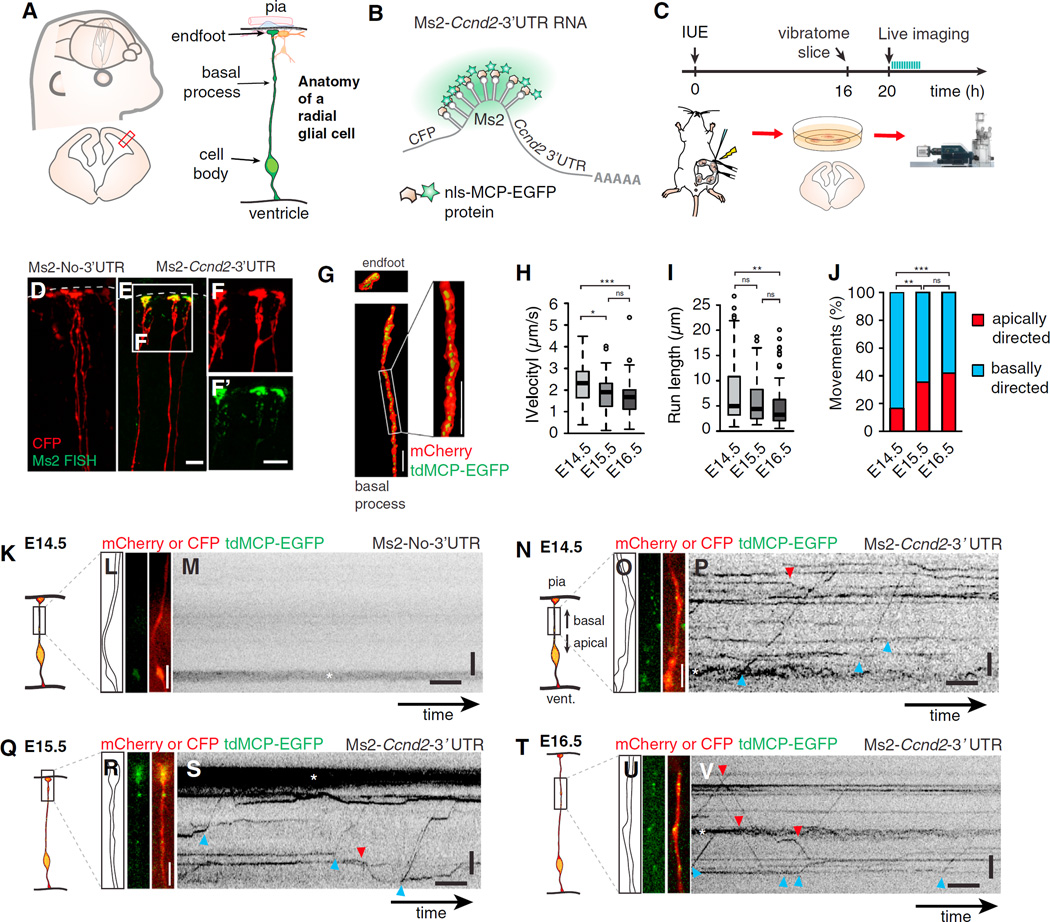
Figure 1. Live Imaging of Embryonic Organotypic Brain Slices Shows Directed mRNA Movement in Radial Glia Basal Processes.
(A) Cartoon representations show embryonic neocortex, coronal section (left), and radial glial cell (RGC) with the indicated features.
(B) Approach used for imaging mRNA, in which mCherry or CFP is followed by a 3′ UTR with binding sites for the MCP-EGFP protein, as shown.
(C) Timeline and overview of the live-imaging experiment (IUE, in utero electroporation).
(D–F) Confocal images show smFISH against Ms2 (green; D, E, and F’) in Ms2-No-3′ UTR (D) and Ms2-Ccnd2–3′ UTR (E and F) electroporated RGCs (red).
(G) 3D reconstructions show an RGC endfoot and basal process (red) electroporated with mCherry-Ms2-Ccnd2–3′ UTR and tdMCP-EGFP (green).
(H and I) Boxplots showing velocities (H) and run lengths (I) at the indicated ages. Velocity was calculated from kymographs and absolute value of average velocities for apical and basal movements.
(J) Graph depicts apically (red) and basally (blue) directed movements at the indicated ages. E14.5, n = 153 movements, n = 28 cells, three experiments; E15.5, n = 98 movements, n = 18 cells, three experiments; E16.5, n = 100 movements, n = 62 cells, three experiments.
(K–V) Examples of live imaging of mRNAs in RGCs of brain slices from E14.5 (K–P), E15.5 (Q–S), and E16.5 (T–V). Shown are schematic representations of the regions imaged (K, N, Q, and T), outlines and still images acquired prior to live imaging (L, O, R, and U), and kymographs of Ms2-Ccnd2–3′ UTR (P, S, and V) or negative control (no 3′ UTR) (M). RNAs were actively moving apically (red arrowhead) or basally (blue arrowhead) and were evident within varicosities (*).
Scale bars, 10 (D–F) and 4 µm, (L, M, O, P, R, S, U, and V) y axis, length, 5 µm; x axis, time, 5 s. Boxplots represent median; bottoms and tops of boxes represent 25th and 75th percentiles, and whiskers represent median ± 1.5 × interquartile range. *p < 0.05, **p < 0.01, and ***p < 0.001. See also Figure S1 and Movie S1.
One highly conserved mechanism for controlling distal functions of polarized cells, including neurons, is RNA localization and local translation [16, 17], prompting us to consider its role in RGCs. In RGCs, in situ hybridization of candidate mRNAs and fixed analysis of CyclinD2 (Ccnd2) mRNA reporters have revealed a handful of transcripts that localize to endfeet, suggesting they could be locally translated [18–20]. However, without live imaging, it remains unknown if RNAs move actively or passively and whether local translation occurs in endfeet. Here we visualize directed and rapid mRNA transport in embryonic RGCs, demonstrating for the first time that the basal process acts as a highway for directed molecular transport. We show that basal endfeet contain a local transcriptome, which can be locally translated far from the soma. We further discover that the autism-associated RNA-binding protein FMRP regulates this local transcriptome. Our findings establish a new model of mRNA transport and local translation in mammalian tissue and demonstrate novel dynamic cellular mechanisms within neural stem cells of the developing brain.
Live Imaging of Embryonic Organotypic Brain Slices Shows Directed mRNA Movement in Radial Glia Basal Processes
We performed single-molecule fluorescence in situ hybridization (smFISH) of several transcripts predicted to localize to endfeet based on online databases [21]. Consistent with previous findings [18, 19], Ccnd2 and Nestin (Nes) transcripts localized to embryonic day (E)16.5 EGFP+ RGC endfeet labeled by in utero electroporation (Figures S1A and S1B). In contrast, Gapdh and Vimentin (Vim) were not enriched in endfeet and primarily localized to the cortical plate and meninges, respectively (Figures S1C and S1D). These results demonstrate specific mRNAs localize to RGC endfeet.
To determine if mRNAs are actively transported in RGCs in vivo, we employed the Ms2 system, in which reporter mRNAs containing Ms2 stem loops are co-expressed with EGFP-MCP (Ms2 coat protein) (Figure 1B) [22, 23]. For proof of principle, we used a Ccnd2–3′ UTR reporter, previously shown by Tsunekawa et al. to localize to RGC endfeet [18]. Brains were co-electroporated in utero with fluorescently tagged Ms2-Ccnd2–3′ UTR or Ms2-No-3′ UTR (control) and nls-MCP-EGFP constructs (Figure 1C). Similar expression levels were achieved with both reporters (Figures S1E and S1F). 3D reconstruction of smFISH images revealed Ms2-tagged mRNAs, but not tagged-control mRNAs, localized within RGC endfeet and basal processes (Figures 1D–1G).
Using high-speed imaging of live organotypic brain slices, we observed rapid directed motility of Ms2-Ccnd2–3′ UTR reporters, but not of control (Figures 1H–1V and S1G–S1R; Movie S1). Motility of RNA granules was evident at three developmental stages, E14.5, E15.5, and E16.5, shown in kymographs (Figures 1K–1V and S1G–S1L). At E14.5, mRNAs averaged velocities of 2.4 µm/s and run lengths of 5 µm (Figures 1H, 1I, S1M, and S1P). In comparison, at E15.5 and E16.5, velocity and processivity (run lengths) were significantly reduced (Figures 1H, 1I, S1N, S1O, S1Q, and S1R). At E14.5, 85% of movements were basally directed, whereas at later stages movements were less biased (Figures 1J and S1M–S1R). All velocities were comparable to microtubule-mediated mRNA transport in neurons [22, 24]. Altogether these data indicate that mRNAs actively move in RGCs and this may be developmentally regulated. This provides the first evidence of rapid directed RNA transport in RGCs.
Live Imaging in Ex Vivo Tissue Preparations Reveals Local Translation in Radial Glia Basal Endfeet
We next developed assays to assess local translation in RGC endfeet. Intact RGC endfeet and the surrounding niche were microdissected in ex vivo endfoot preparations (Figure 2A). Transmitted electron microscopy (EM) analyses revealed intact cytoarchitecture of endfoot preparations comparable to brain sections, showing basal lamina associated with RGC endfeet (Figures 2B and 2C). Immunofluorescence confirmed the presence of PECAM+ vasculature, LAMININ+ basal lamina, and cytoplasmic neuronal processes (CALRETININ and TUJ1+) but the absence of neuronal nuclei (Figures S2A–S2F). Markers of RGCs, Cajal-Retzius and excitatory neurons were detectable by western analyses (Figure S2G). The majority (85%–90%) of EGFP+ endfeet contained Ccnd2 transcript and protein, consistent with previous observations in mice and humans (Figures S2H–S2M) [18]. Most endfeet contained the translation markers, 5.8 s rRNA and phosphorylated ribosomal protein S6 (PS6) (Figures S2N–S2S). These data indicate that RGC endfeet ex vivo preparations reconstitute much of the pial niche and contain translation machinery.
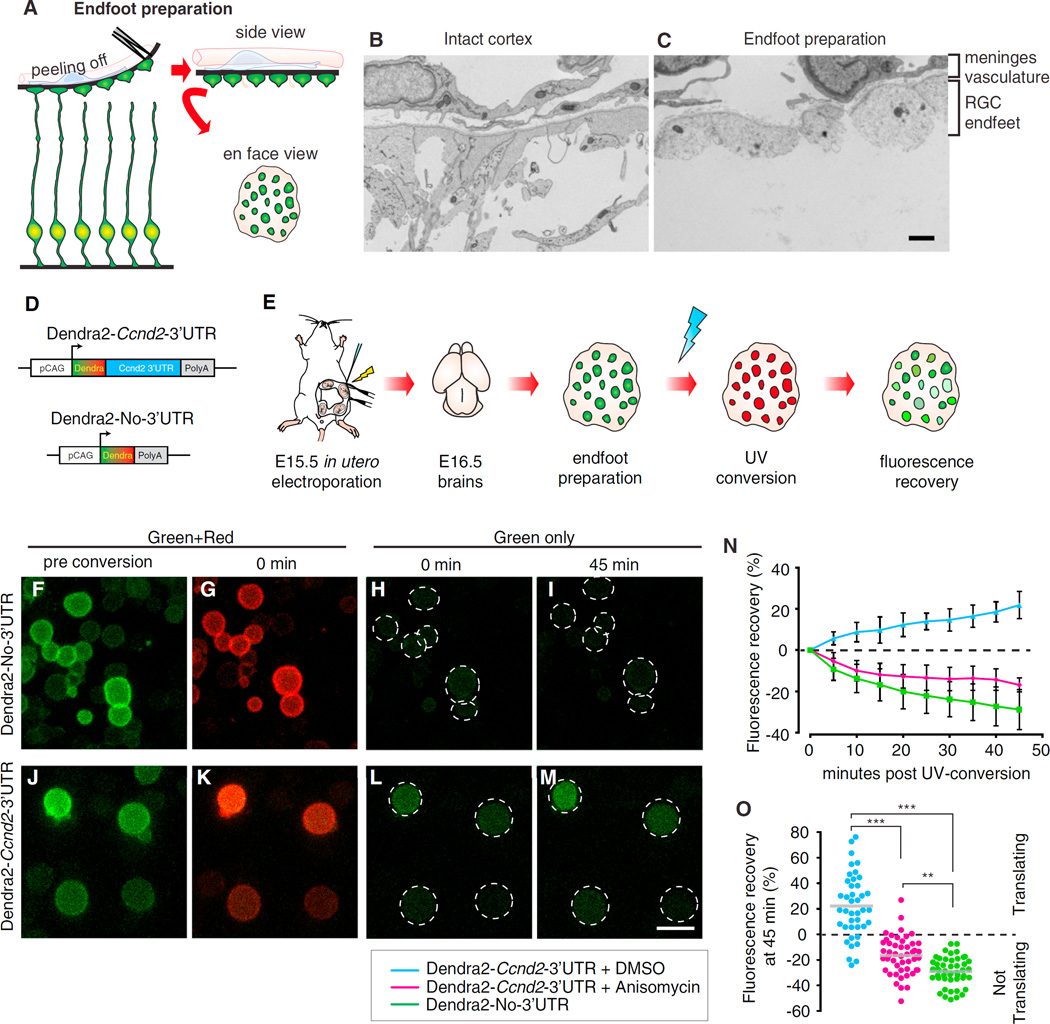
Figure 2. Live Imaging in Ex Vivo Tissue Preparations Reveals Local Translation in Radial Glia Basal Endfeet.
(A) Schematic overview shows endfoot preparation from embryonic brain slices.
(B and C) Electron micrographs of E16.5 intact cortex (B) and endfoot preparation (C), with the indicated putative cellular populations.
(D and E) Constructs used (D) and schematic overview (E) of DENDRA2 translation assay.
(F–M) Time-lapse panels show pre-UV conversion (F and J) and post-UV conversion (G–I and K–M).
(N) Green fluorescence recovery following UV conversion across time is shown (Anisomycin treatment, 40 µM, 20 min).
(O) Green fluorescence recovery at 45 min plotted for individual endfeet (circles) shows translation (>0) or no translation (<0). n = 12–19 endfeet/replicate, n = 3 replicates each.
Scale bars, 2 (B and C) and 5 µm (F–M). Error bars, SD. **p < 0.01 and ***p < 0.001. See also Figure S2.
We then used photoconvertible Dendra2 reporters to test the hypothesis that localized mRNAs are translated in RGC endfeet [17, 25]. Dendra2-Ccnd2–3′ UTR or Dendra2-No-3′ UTR (control) constructs were in utero electroporated into E15.5 brains (Figures 2D and 2E). At E16.5, smFISH against Dendra mRNA confirmed that the 3′ UTR reporter, but not control, localized to RGC endfeet in tissue sections and endfoot preparations (Figures S2T–S2AA). Following UV conversion of endfoot preparations, green Dendra2 was irreversibly photoconverted to red in both reporters (Figures 2F, 2G, 2J, and 2K). Time-lapse imaging over the subsequent 45 min revealed a steady increase in green fluorescence recovery in RGC endfeet expressing Dendra2-Ccnd2–3′ UTR, indicative of de novo protein synthesis (Figures 2L–2O). In contrast, negative green fluorescence recovery was observed in control-expressing or Dendra2-Ccnd2–3′ UTR-expressing endfeet pre-treated with the translational inhibitor Anisomycin (Figures 2H, 2I, 2N, and 2O). This suggests no new Dendra2 protein is produced in control conditions and may be photobleached. Importantly, no correlation was evident between conversion efficiency and fluorescence recovery (Figures S2BB–S2EE). By 45 min, 80% of Dendra2-Ccnd2–3′ UTR endfeet showed active translation (fluorescence recovery greater than 0) (Figure 2O). In contrast, de novo translation was evident in only 11% and 0% of Anisomycin-treated and control endfeet, respectively (Figure 2O). The fraction of actively translating endfeet is similar to those expressing rRNA and PS6 (Figures S2N–S2S), suggesting the majority of endfeet can undergo local translation, while a small fraction are translationally dormant.
The RNA-Binding Protein FMRP Actively Moves in Radial Glia to Basal Endfeet
We evaluated RNA-binding proteins that could influence RNA dynamics. Endogenous Staufen and Pumillio, previously implicated in neurogenesis and RNA localization [26–28], both localized to neurons and RGC basal processes and endfeet (Figures S3A–S3J). Exogenous Staufen also localized to RGC endfeet (Figures S3K–S3M). We assessed FMRP, another RNA-binding protein implicated in neurogenesis and RNA localization, and predicted it to localize to RGC endfeet [29–32]. Compared to Staufen and Pumillio, endogenous and exogenous FMRP (EGFP-FMRP) showed the strongest enrichment in RGC basal processes and endfeet (Figures 3A–3F and S3N–S3P).
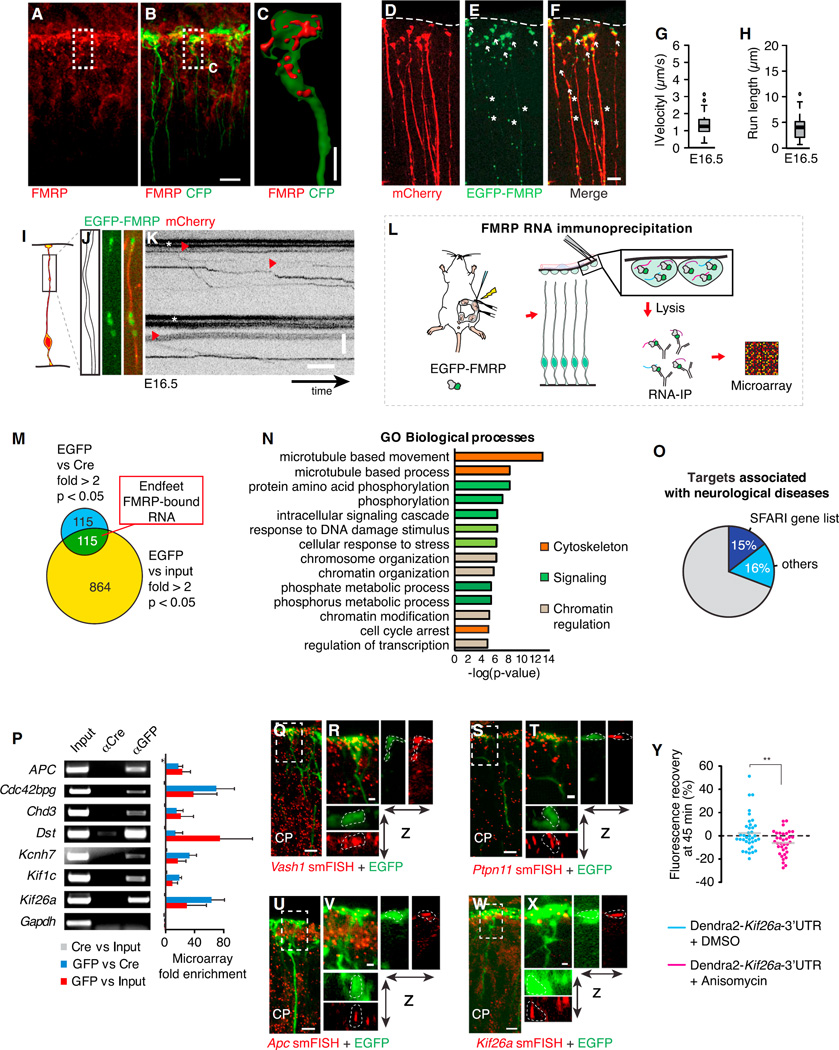
Figure 3. The RNA-Binding Protein FMRP Actively Moves in Radial Glia to Basal Endfeet, Where It Is Bound to a Local Transcriptome.
(A–C) Localization of FMRP protein (red) in E16.5 CFP+ RGC endfeet (A and B), with 3D reconstruction of endfoot (C), as shown.
(D–F) Electroporated mCherry (D; cytoplasmic filler), EGFP-FMRP (E) localization in basal processes (asterisks) and endfeet (arrows), and overlay of mCherry and EGFP-FMRP signal (F).
(G–K) Live imaging shows EGFP-FMRP in E16.5 RGCs in brain slices, with boxplots showing velocity (G) and run length (H) quantification (n = 57 granules, 155 cells, two experiments), schematic representation of the region imaged (I), outline and still images (J), and kymographs (K).
(L) Schematic overview shows RNA immunoprecipitation-microarray (RIP-chip) experiment.
(M) Venn diagram represents criteria for identifying endfeet FMRP-bound RNAs.
(N) Gene ontology (GO) analyses of FMRP-bound RNAs in RGC endfeet.
(O) Graph depicts the fraction of FMRP endfoot target genes associated with neurological disorders, including those genes identified in the SFARI database as autism associated.
(P) The RT-PCR validation of select identified RIP-chip transcripts showing input, and immunoprecipitation by Cre and GFP. Gapdh is a negative control.
(Q–X) The smFISH (red) validation of Vash1 (Q and R), Ptpn11 (S and T), Apc (U and V), and Kif26a (W and X) in EGFP+ RGCs (green) in E16.5 cortices (CP, cortical plate). (R, T, V, and X) Close-up views show regions highlighted in (Q), (S), (U), and (W) with x-z and y-z projections.
(Y) Green fluorescence recovery at 45 min plotted for individual endfeet (circles), showing translation (>0) or no translation (<0) of an exogenous Dendra2-Kif26a-3′ UTR mRNA ± Anisomycin treatment (40 µM, 20 min) (n = 10–15 endfeet/replicate, n = 3 replicates each). Boxplots represent median; bottoms and tops of boxes represent 25th and 75th percentiles, and whiskers represent median ± 1.5 × interquartile range. **p < 0.01.
Scale bars, 10 (A, B, D–F, Q, S, U, and W), 1.25 (R, T, V, and X), and 2.5 µm (C). (J and K) y axis, 5 µm; x axis, 5 s. Error bars, SD. See also Figure S3, Movies S2 and S3, and Table S1.
We next asked if FMRP actively moves in RGCs, using live imaging of in utero-electroporated EGFP-FMRP in E16.5 organotypic slices. Of RGC processes, 12% showed directed motility of FMRP granules, with velocities and run lengths averaging 1.3 µm/s and 4 µm, respectively (Figures 3G–3K, S3Q, and S3R; Movie S2). This is comparable to microtubule-based FMRP movement in neurons [22, 33] and to velocities and behaviors of mRNA reporters in RGCs. Altogether, these data demonstrate that, similar to mRNA granules, FMRP is rapidly transported in RGCs.
Transcriptome-wide Discovery of FMRP-Bound Transcripts in Radial Glia Endfeet
As both RNA and FMRP accumulate in RGC endfeet, we postulated that FMRP regulates a local transcriptome. To identify FMRP-bound mRNAs in RGC endfeet, EGFP-FMRP was in utero electroporated at E14.5, and at E15.5 endfoot preparations were isolated (Figure 3L). Importantly, with this timing paradigm, we specifically targeted only RGCs, as neuronal progeny had not yet migrated to upper layers. We performed RNA immunoprecipitations (RIPs) coupled with microarray (RIP-chip) using anti-EGFP and anti-Cre (control) (Figure S3S) [34]. As proof of principle, Ccnd2 mRNA was enriched in anti-EGFP RIPs relative to control (Figure S3S). Consistent with this, FMRP and Ccnd2 mRNA co-localized in basal processes and endfeet (Figures S3T–S3W; Movie S3). Microarray of EGFP-RIP, Cre-RIP, and input samples identified 115 transcripts with >2-fold enrichment and p < 0.05 in both EGFP/Cre and EGFP/input (Figure 3M; Table S1). The endfoot FMRP interactome significantly overlapped with known FMRP targets in adult brains [30], immortalized cells [35], and transcripts enriched in astrocyte glial protrusions [36] (Figures S3X and S3Y). FMRP-associated mRNAs in RGC endfeet were disproportionately enriched for microtubule-associated and signaling molecules, including five kinesins, five Actomyosin regulators, six GTPase regulators, five tyrosine kinases, and four MAP kinases (Figure 3N; Table S1). FMRP-bound transcripts encoding nuclear proteins also were identified. Of FMRP-bound endfoot mRNAs, 31% were associated with neurological diseases, with 17 found in the SFARI autism gene database, a 5-fold enrichment above random (Figures 3O, S3AA, and S3Z; Table S1). Together this provides evidence of a local RGC endfoot transcriptome with transcripts that could influence neurological pathology.
We validated FMRP endfeet targets by several approaches. The qPCR confirmed seven transcripts enriched in FMRP-EGFP IP, but not control IP (Figure 3P). In contrast, Gapdh was neither enriched in the microarray nor by qPCR. The smFISH against six FMRP targets demonstrated localization in basal endfeet (Figures 3Q–3X and S3BB–S3EE). This included Vash1 and Camsap2, two of seven autism-associated novel FMRP targets [30, 35]. FMRP co-localized with three transcripts associated with microtubule-based movement, Dst, Apc, and Kif26a, to similar extents within RGC basal processes and endfeet (Figures S4A–S4N). As further validation, we identified the Kif26a sequence sufficient for endfoot localization, and then we used this in a translation assay (Figures S3FF and S3GG). By 45 min, a significant fraction of Dendra2-Kif26a-3′ UTR-expressing endfeet showed increased fluorescence recovery, compared to endfeet exposed to Anisomycin (Figure 3Y). Along with the Ccnd2 reporter, this suggests that translation may be a common yet regulated mechanism in endfeet (Figure 3Y). Taken together, the RIP-chip approach successfully identified bona fide FMRP endogenous targets in RGC endfeet, expanding the repertoire of transcripts known to localize and be locally translated in these structures.
FMRP Controls RNA Transport to RGC Basal Endfeet
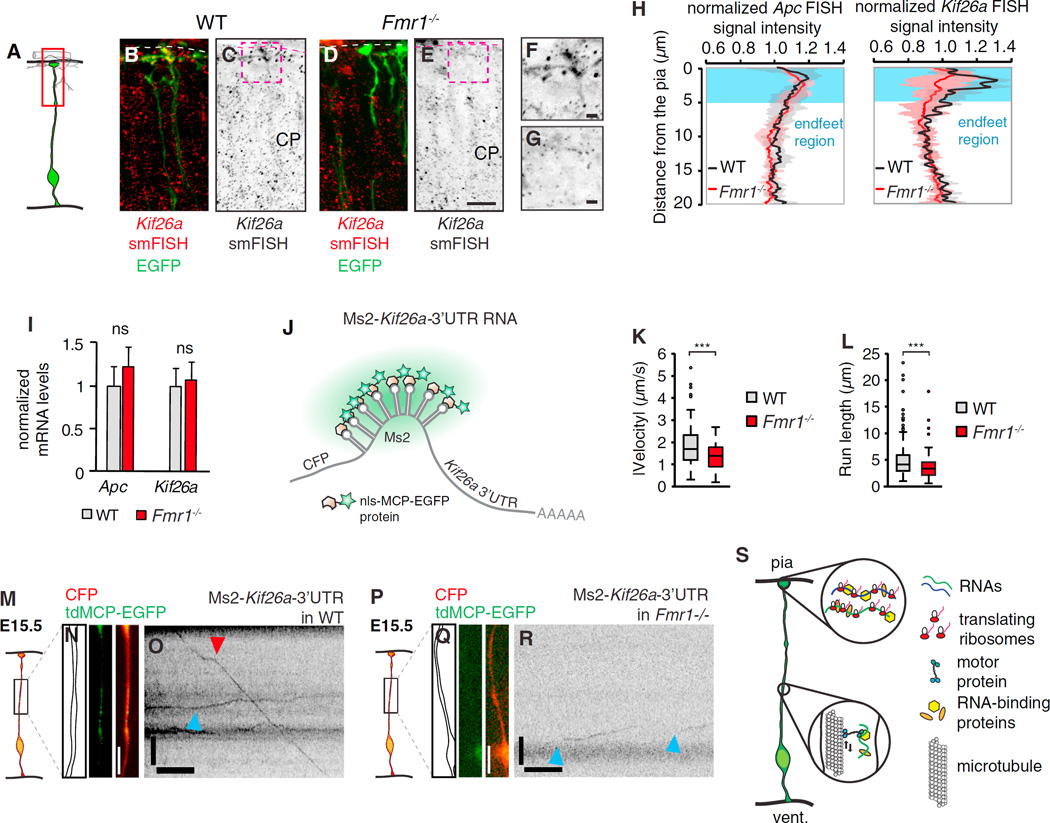
FMRP is implicated in mRNA transport and localization [33, 37] and RGC endfeet are intact in Fmr1−/− brains [31]. To determine if Kif26a localization depends on FMRP, we performed smFISH in control and Fmr1−/− brains (Figures 4A–4G). Relative to control, Kif26a localization was significantly reduced in Fmr1−/− endfeet, as evidenced by quantification of total signal intensity and Kif26a+ granules per endfeet (Figures 4H, S4P, and S4Q). Notably, localization of Dst, but not Apc, was also FMRP dependent (Figures 4H and S4O). Importantly, Dst, Apc, and Kif26a transcript levels were normal in Fmr1−/− brains (Figures 4I and S4R). Together this suggests that FMRP can differentially influence mRNA localization in RGCs. To further elucidate how FMRP influences Kif26a endfoot localization, we quantified Kif26a transport in wild-type (WT) and Fmr1−/− RGCs by imaging an Ms2-Kif26a-3′ UTR reporter (Figures 4J–4R and S4S–S4U). In control E15.5 brains, the Kif26a reporter moved with similar velocity and behaviors as Ccnd2. However, in Fmr1−/− brain slices, Ms2-Kif26a-3′ UTR moved significantly slower and with lower processivity. Thus, defective Kif26a transport may help explain its reduced localization in FMRP-deficient endfeet. Together this supports a functional role for the RNA-binding protein, FMRP, in the transport and localization of mRNA targets to basal structures of RGCs.
Figure 4. FMRP Controls RNA Transport to Radial Glia Basal Endfeet.
(A) Cartoon representation shows an EGFP+ RGC, with a red box highlighting the regions shown in (B)–(G).
(B–G) Kif26a smFISH in EGFP+ RGCs of control (B, C, and F) and Fmr1−/− brain sections with (F) and (G) showing close-up views of regions highlighted in (C) and (E), respectively. Dotted line, basement membrane. CP, cortical plate.
(H) The smFISH signal intensity analyses of Apc and Kif26a show the decrease in pial Kif26a, but not Apc, smFISH signal in Fmr1−/− (red) versus WT (black) E15.5 neocortices (n = 3).
(I) qPCR analyses of Apc and Kif26a transcripts in WT (black) and Fmr1−/− (red) E15.5 neocortices (n = 4).
(J) Approach used for imaging mRNA, in which CFP is followed by a Kif26a-3′ UTR with Ms2-binding sites for the MCP-EGFP protein, as shown.
(K and L) Boxplots show velocities (K) and run lengths (L) quantified for the indicated genotypes. WT, n = 171 RNA movements from n = 10 embryos; Fmr1−/−, n = 119 movements from n = 6 embryos.
(M–R) Examples of live imaging of mRNAs in RGCs of brain slices from WT (M–O) and Fmr1−/− (P–R), E15.5 cortices. Shown are the schematic representations of the regions imaged (N and Q), outlines and still images acquired prior to live imaging (N and Q), and kymographs of Ms2-Kif26a-3′ UTR (O and R) RNAs moving apically (red arrowhead) or basally (blue arrowhead).
(S) Cartoon model represents the study findings. RNA is transported bi-directionally within RGC basal processes and translated in RGC endfeet.
Scale bars, 20 (B–E) and 5 µm (F and G). (N, O, Q, and R) y axis, length, 5 µm; x axis, time, 5 s. Boxplots represent median; bottoms and tops of boxes represent 25th and 75th percentiles, and whiskers represent median ± 1.5 × interquartile range. ***p < 0.001; ns, non-significant. Error bars, SD. See also Figure S4.
DISCUSSION
RGCs are precursors for neurons and glia of the developing brain, yet our understanding of dynamic cellular events within these stem cells has been limited. For the first time, events in RGCs at a temporal scale of <100 ms are described. We demonstrate that the basal process serves as a highway for mRNAs and their binding proteins, enabling local translation in endfeet far from the cell body. Altogether, our findings indicate cytoskeletal and signaling events at endfeet may be controlled through the translation of specific mRNAs transported from the soma, exposing new mechanisms relevant for stem cell regulation, cortical development, and disease.
Our study provides new insights regarding developmental functions of FMRP, which has been more extensively studied in the adult brain. FMRP mutations cause fragile X syndrome, postulated to result in part from aberrant prenatal development [29, 31, 32, 38–41]. We discover many FMRP targets in the embryonic and adult brain are conserved, as evidenced by overlap between our dataset and others [30, 35]. The FMRP interactome in RGC endfeet is enriched for known autism genes, including novel FMRP targets. Finally, we demonstrate that FMRP controls mRNA transport and localization in RGCs, reinforcing its known function in neurons [33, 37]. Altogether this fits with a building literature connecting prenatal defects with autism [42–45], suggesting that FMRP-mediated RNA regulation in RGCs may be relevant for disease pathology.
Our study establishes a novel tractable paradigm for studying mRNA transport and local translation in neural stem cells of mammalian tissue. To date most studies of RNA localization and translation have used cultured cells or mechanical axotomy, limiting our understanding of in vivo events. Recent development of a β-actin mRNA reporter mouse exposed dynamic movements of this transcript in post-mitotic neurons in vivo [24]. Our model system is advantageous for monitoring any mRNA within radial glia. Moreover, the endfoot preparation enables measurement of local translation within an intact tissue, which has been technically challenging due to soma contributions. With this experimental paradigm, one can now ask many exciting questions, including what RNAs and RNA-binding proteins move in the brain and how is their transport and translation controlled. In doing so, we can begin to understand why RNA is transported and localized far from the cell body.
We discovered one key function of mRNA transport is to enable local protein synthesis in endfeet. Notably, there were developmental stage differences in RNA velocity, movement, and directionality, suggesting that RNA transport may influence cortical development. How might this occur? We propose locally generated proteins in RGC endfeet promote intra- and extracellular signaling, modulate endfoot growth, and influence intracellular transport (Figures 4S and S4V). During development, local synthesis of cytoskeletal and signaling molecules would be particularly advantageous as the cortex undergoes radial expansion [46], necessitating remodeling and growth of RGC endfeet. Local translation of fate determinants also could impact progenitor symmetric and asymmetric divisions and specify distinct neuronal sub-types [18, 47]. Indeed, we discovered endfoot transcripts encoding nuclear factors, which could function locally or shuttle back to the cell body, as has been observed in neuronal axons [48, 49].
What features influence RNA transport and translation? Developmental control of RNA movement may be due to inherent differences in RGC microtubule density and orientation and/or abundance of molecular motors and RNA-binding proteins. FMRP exemplifies one such regulatory mechanism. Translation also may be differentially regulated, as two mRNA reporters showed distinct translational magnitudes. Translation is likely regulated intrinsically within endfeet and/or extrinsically by signals from the surrounding niche. Future experiments aimed at defining how RNA transport and translation are controlled in RGCs will provide important tools to further assess function.
Our findings argue that molecular transport in RGCs may be broadly important for neurogenesis. Feedback signaling from upper layers via RGCs has been observed from excitatory upper layer neurons, Cajal-Retzius cells, and overlying meningeal cells [4, 5, 12]. Yet the precise mechanisms of these signaling events are enigmatic. Given our discoveries, we favor a model in which the basal process helps communicate events from the pial surface back to the cell body, by enabling transport of signaling molecules or locally produced proteins. Going forward, our study lays the groundwork for considering how molecular transport and local regulation within RGCs influence brain development.
EXPERIMENTAL PROCEDURES
Ms2-EGFP and EGFP-FMRP Live Imaging in Brain Slices
Ms2-tagged RNA and the MCP-EGFP coat-protein constructs were introduced into C57BL/6J brains using in utero electroporation, and slices were prepared 16 hr later, as previously reported [50]. Live imaging was performed using an Andor XD revolution spinning-disk confocal microscope, and images were collected every 50–100 ms for 60–120 s in the EGFP channel only. Prior to live imaging, static images of the basal processes were systematically acquired in both the fluorescent-filler (mCherry or CFP) and EGFP channels to optimize the subsequent generation of kymographs. Analyses of particle movements were performed in ImageJ. See the Supplemental Experimental Procedures for additional details.
Dendra Live Imaging of Basal Endfeet
Dendra2 constructs were introduced into C57BL/6J brains by in utero electroporation. Basal endfeet were microdissected away from the cortex by peeling away the meninges and basal lamina. Fluorescent tissue samples were mounted on 35-mm glass-bottom dishes (MatTek) in a 1.5 mg/mL collagen/DMEM solution, and they were imaged on a Zeiss 780 confocal microscope. Both green and red laser power were set to the minimum level required to visualize the fluorescence. Dendra2 was photoconverted from green to red fluorescence using the 405-nm laser line to scan through the z stack at a reduced speed (longer pixel dwell time). Immediately after conversion, the 488- and 561-nm laser lines were used to acquire a time-lapse image series of the endfeet every 5 min after conversion. The 40 µM Anisomycin or DMSO (vehicle control) was added to the media 20 min prior to photoconversion. The z stack time-lapse images were analyzed using Imaris software (Bitplane). See the Supplemental Experimental Procedures for additional details.
RNA Immunoprecipitation/Microarray
Brains were in utero electroporated with CAG-EGFP-FMRP plasmids, and endfoot preparations were harvested about 16 hr later. Pooled endfoot preparations (16–50) were subjected to RNA immunoprecipitation following the protocol from Jack Keene’s group with modifications [34]. Importantly, this protocol is efficient at preventing redistribution of RNA-binding proteins onto different RNAs in vitro. For microarray, two pooled replicates were used for each condition (EGFP-RIP, Cre-RIP, and input). Hybridization targets were prepared with the Affymetrix WT Pico Kit from total RNA, hybridized to GeneChip mouse transcriptome array 1.0. See the Supplemental Experimental Procedures for additional details.
Supplementary Material
In Brief.
Pilaz et al. use live imaging in mouse embryonic brain tissue to visualize active RNA movement and local translation in radial glia basal processes and distal endfeet. They identify an endfoot FMRP-bound transcriptome enriched for signaling and cytoskeletal regulators. This study exposes dynamic RNA mechanisms in stem cells of the developing brain.
Highlights.
The radial glia basal process is a highway for active directed transport of RNA
mRNA is locally translated in radial glia endfeet hundreds of micrometers from the soma
Endfeet FMRP-bound RNAs encode autism-related signaling and cytoskeletal regulators
FMRP controls RNA localization and active mRNA transport in radial glia
Acknowledgments
We thank members of the D.L.S. lab, Dr. Jack Keene, Dr. Matthew Friedersdorf, Dr. Cagla Eroglu, Dr. Paola Arlotta, Dr. Jeremy Kay, Dr. Dong Yan, and Dr. Christopher Nicchitta for helpful discussions and reading the manuscript. This work was supported by a Fay/Frank Seed grant BRFSG-2013-10 from the Brain Research Foundation (to D.L.S.). We thank the Duke light microscopy, Duke electron microscopy, and Duke Microarray Core facility (funded by NIH grant P30 CA014236) for technical support. D.L.S. is funded by NIH grant R01NS083897.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, four figures, one table, and three movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.10.040.
AUTHOR CONTRIBUTIONS
L.-J.P. and D.L.S. conceived the study, designed the study, and wrote the manuscript. L.-J.P., A.L.L., J.P.R., and D.L.S. performed experiments.
REFERENCES
- 1.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 2.Malatesta P, Hartfuss E, Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- 3.Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J. Neurosci. 2011;31:3683–3695. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seuntjens E, Nityanandam A, Miquelajáuregui A, Debruyn J, Stryjewska A, Goebbels S, Nave K-A, Huylebroeck D, Tarabykin V. Sip1 regulates sequential fate decisions by feedback signaling from postmitotic neurons to progenitors. Nat. Neurosci. 2009;12:1373–1380. doi: 10.1038/nn.2409. [DOI] [PubMed] [Google Scholar]
- 6.Radakovits R, Barros CS, Belvindrah R, Patton B, Müller U. Regulation of radial glial survival by signals from the meninges. J. Neurosci. 2009;29:7694–7705. doi: 10.1523/JNEUROSCI.5537-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Götz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- 8.Kosodo Y, Toida K, Dubreuil V, Alexandre P, Schenk J, Kiyokage E, Attardo A, Mora-Bermúdez F, Arii T, Clarke JDW, Huttner WB. Cytokinesis of neuroepithelial cells can divide their basal process before anaphase. EMBO J. 2008;27:3151–3163. doi: 10.1038/emboj.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Jin Z, Koirala S, Bu L, Xu L, Hynes RO, Walsh CA, Corfas G, Piao X. GPR56 regulates pial basement membrane integrity and cortical lamination. J. Neurosci. 2008;28:5817–5826. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myshrall TD, Moore SA, Ostendorf AP, Satz JS, Kowalczyk T, Nguyen H, Daza RA, Lau C, Campbell KP, Hevner RF. Dystroglycan on radial glia end feet is required for pial basement membrane integrity and columnar organization of the developing cerebral cortex. J. Neuropathol. Exp. Neurol. 2012;71:1047–1063. doi: 10.1097/NEN.0b013e318274a128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griveau A, Borello U, Causeret F, Tissir F, Boggetto N, Karaz S, Pierani A. A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortical neuroepithelium. PLoS Biol. 2010;8:e1000440. doi: 10.1371/journal.pbio.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartfuss E, Förster E, Bock HH, Hack MA, Leprince P, Luque JM, Herz J, Frotscher M, Götz M. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development. 2003;130:4597–4609. doi: 10.1242/dev.00654. [DOI] [PubMed] [Google Scholar]
- 13.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Rash BG, Ackman JB, Rakic P. Bidirectional radial Ca(2+) activity regulates neurogenesis and migration during early cortical column formation. Sci. Adv. 2016;2:e1501733. doi: 10.1126/sciadv.1501733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokota Y, Eom T-Y, Stanco A, Kim W-Y, Rao S, Snider WD, Anton ES. Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development. 2010;137:4101–4110. doi: 10.1242/dev.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buxbaum AR, Wu B, Singer RH. Single β-actin mRNA detection in neurons reveals a mechanism for regulating its translatability. Science. 2014;343:419–422. doi: 10.1126/science.1242939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt CE, Schuman EM. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron. 2013;80:648–657. doi: 10.1016/j.neuron.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsunekawa Y, Britto JM, Takahashi M, Polleux F, Tan S-S, Osumi N. Cyclin D2 in the basal process of neural progenitors is linked to non-equivalent cell fates. EMBO J. 2012;31:1879–1892. doi: 10.1038/emboj.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res. Dev. Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 20.Saarikangas J, Hakanen J, Mattila PK, Grumet M, Salminen M, Lappalainen P. ABBA regulates plasma-membrane and actin dynamics to promote radial glia extension. J. Cell Sci. 2008;121:1444–1454. doi: 10.1242/jcs.027466. [DOI] [PubMed] [Google Scholar]
- 21.Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–D556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buxbaum AR, Haimovich G, Singer RH. In the right place at the right time: visualizing and understanding mRNA localization. Nat. Rev. Mol. Cell Biol. 2015;16:95–109. doi: 10.1038/nrm3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol. Cell. 1998;2:437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- 24.Park HY, Lim H, Yoon YJ, Follenzi A, Nwokafor C, Lopez-Jones M, Meng X, Singer RH. Visualization of dynamics of single endogenous mRNA labeled in live mouse. Science. 2014;343:422–424. doi: 10.1126/science.1239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung K-M, van Horck FPG, Lin AC, Allison R, Standart N, Holt CE. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vessey JP, Amadei G, Burns SE, Kiebler MA, Kaplan DR, Miller FD. An asymmetrically localized Staufen2-dependent RNA complex regulates maintenance of mammalian neural stem cells. Cell Stem Cell. 2012;11:517–528. doi: 10.1016/j.stem.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Kusek G, Campbell M, Doyle F, Tenenbaum SA, Kiebler M, Temple S. Asymmetric segregation of the double-stranded RNA binding protein Staufen2 during mammalian neural stem cell divisions promotes lineage progression. Cell Stem Cell. 2012;11:505–516. doi: 10.1016/j.stem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiebler MA, Hemraj I, Verkade P, Köhrmann M, Fortes P, Marión RM, Ortín J, Dotti CG. The mammalian staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J. Neurosci. 1999;19:288–297. doi: 10.1523/JNEUROSCI.19-01-00288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saffary R, Xie Z. FMRP regulates the transition from radial glial cells to intermediate progenitor cells during neocortical development. J. Neurosci. 2011;31:1427–1439. doi: 10.1523/JNEUROSCI.4854-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Fata G, Gärtner A, Domínguez-Iturza N, Dresselaers T, Dawitz J, Poorthuis RB, Averna M, Himmelreich U, Meredith RM, Achsel T, et al. FMRP regulates multipolar to bipolar transition affecting neuronal migration and cortical circuitry. Nat. Neurosci. 2014;17:1693–1700. doi: 10.1038/nn.3870. [DOI] [PubMed] [Google Scholar]
- 32.Castrén M, Tervonen T, Kärkkäinen V, Heinonen S, Castrén E, Larsson K, Bakker CE, Oostra BA, Akerman K. Altered differentiation of neural stem cells in fragile X syndrome. Proc. Natl. Acad. Sci. USA. 2005;102:17834–17839. doi: 10.1073/pnas.0508995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev. Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keene JD, Komisarow JM, Friedersdorf MB. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nat. Protoc. 2006;1:302–307. doi: 10.1038/nprot.2006.47. [DOI] [PubMed] [Google Scholar]
- 35.Ascano M, Jr, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492:382–386. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomsen R, Pallesen J, Daugaard TF, Børglum AD, Nielsen AL. Genome wide assessment of mRNA in astrocyte protrusions by direct RNA sequencing reveals mRNA localization for the intermediate filament protein nestin. Glia. 2013;61:1922–1937. doi: 10.1002/glia.22569. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Dictenberg JB, Ku L, Li W, Bassell GJ, Feng Y. Dynamic association of the fragile X mental retardation protein as a messenger ribonucleoprotein between microtubules and polyribosomes. Mol. Biol. Cell. 2008;19:105–114. doi: 10.1091/mbc.E07-06-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 40.Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 41.Kwan KY, Lam MMS, Johnson MB, Dube U, Shim S, Rašin M-RR, Sousa AMM, Fertuzinhos S, Chen J-G, Arellano JI, et al. Species-dependent posttranscriptional regulation of NOS1 by FMRP in the developing cerebral cortex. Cell. 2012;149:899–911. doi: 10.1016/j.cell.2012.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 43.Kaushik G, Zarbalis KS. Prenatal neurogenesis in autism spectrum disorders. Front Chem. 2016;4:12. doi: 10.3389/fchem.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang W-Q, Chen W-W, Jiang L, Liu K, Yung W-H, Fu AKY, Ip NY. Overproduction of upper-layer neurons in the neocortex leads to autism-like features in mice. Cell Rep. 2014;9:1635–1643. doi: 10.1016/j.celrep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J. Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsunekawa Y, Kikkawa T, Osumi N. Asymmetric inheritance of Cyclin D2 maintains proliferative neural stem/progenitor cells: a critical event in brain development and evolution. Dev. Growth Differ. 2014;56:349–357. doi: 10.1111/dgd.12135. [DOI] [PubMed] [Google Scholar]
- 48.Jung H, Gkogkas CG, Sonenberg N, Holt CE. Remote control of gene function by local translation. Cell. 2014;157:26–40. doi: 10.1016/j.cell.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cosker KE, Fenstermacher SJ, Pazyra-Murphy MF, Elliott HL, Segal RA. The RNA-binding protein SFPQ orchestrates an RNA regulon to promote axon viability. Nat. Neurosci. 2016;19:690–696. doi: 10.1038/nn.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilaz L-J, McMahon JJ, Miller EE, Lennox AL, Suzuki A, Salmon E, Silver DL. Prolonged mitosis of neural progenitors alters cell fate in the developing brain. Neuron. 2016;89:83–99. doi: 10.1016/j.neuron.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.