Abstract
Repetitive acute intermittent hypoxia (rAIH) increases growth/trophic factor expression in respiratory motor neurons, thereby eliciting spinal respiratory motor plasticity and/or neuroprotection. Here we demonstrate that rAIH effects are not unique to respiratory motor neurons, but are also expressed in non-respiratory, spinal alpha motor neurons and upper motor neurons of the motor cortex. In specific, we used immunohistochemistry and immunofluorescence to assess growth/trophic factor protein expression in spinal sections from rats exposed to AIH three times per week for 10 weeks (3 × wAIH). 3 × wAIH increased brain-derived neurotrophic factor (BDNF), its high-affinity receptor, tropomyosin receptor kinase B (TrkB), and phosphorylated TrkB (pTrkB) immunoreactivity in putative alpha motor neurons of spinal cervical 7 (C7) and lumbar 3 (L3) segments, as well as in upper motor neurons of the primary motor cortex (M1). 3 × wAIH also increased immunoreactivity of vascular endothelial growth factor A (VEGFA), the high-affinity VEGFA receptor (VEGFR-2) and an important VEGF gene regulator, hypoxia-inducible factor-1α (HIF-1α). Thus, rAIH effects on growth/trophic factors are characteristic of non-respiratory as well as respiratory motor neurons. rAIH may be a useful tool in the treatment of disorders causing paralysis, such as spinal injury and motor neuron disease, as a pretreatment to enhance motor neuron survival during disease, or as preconditioning for cell-transplant therapies.
Keywords: intermittent hypoxia, BDNF, VEGF, TrkB, HIF-1, motor neuron
INTRODUCTION
System and cellular adaptations to hypoxia are crucial in many physiological and pathophysiological states. At the systems level, intermittent hypoxia (IH) elicits respiratory plasticity, potentially minimizing future recurrence of IH (Mitchell et al., 2001; Feldman et al., 2003; Mitchell and Johnson, 2003; Mahamed and Mitchell, 2007; Devinney et al., 2013). IH-induced plasticity occurs at multiple sites in the neural system controlling breathing, including peripheral chemoreceptors (Prabhakar, 2001, 2011), brainstem integrating neurons (Ling et al., 2001; Kline et al., 2007; Kline, 2010) and respiratory motor nuclei (Baker-Herman and Mitchell, 2002; Baker-Herman et al., 2004).
On a cellular level, IH alters the expression of key molecules associated with both respiratory plasticity and neuroprotection. The most widely studied model of IH-induced respiratory plasticity, phrenic long-term facilitation (pLTF) following acute intermittent hypoxia (AIH), requires spinal serotonin receptor activation (Bach and Mitchell, 1996; Baker-Herman and Mitchell, 2002) and serotonin-dependent synthesis of brain-derived neurotrophic factor (BDNF; Baker-Herman et al., 2004). Repetitive AIH (rAIH) elicits long-lasting increases in the expression of many molecules necessary for pLTF within the phrenic motor nucleus, including BDNF and its high-affinity receptor, tropomyosin receptor kinase B (TrkB) (Wilkerson and Mitchell, 2009; Lovett-Barr et al., 2012; Satriotomo et al., 2012). Apart from its key role in neuroplasticity, BDNF is neuroprotective for neurons stressed by ischemia (Duncan et al., 2004; Ferrer et al., 2004). The transcription factor hypoxia inducible factor 1α (HIF-1α; Semenza, 2007) regulates expression of other growth/trophic factors, such as vascular endothelial growth factor (VEGF) and its high-affinity receptor, VEGFR-2 (Calvani et al., 2012). VEGF and VEGFR-2 are expressed in motor neurons (Yang et al., 2003), elicit respiratory motor plasticity (Dale-Nagle et al., 2011), and are neuroprotective against ischemic injury (van Bruggen et al., 1999; Jin et al., 2000). Thus, BDNF and VEGF are hypoxia-regulated genes that elicit both spinal plasticity and neuroprotection.
IH elicits plasticity in neural systems not directly linked to breathing. For example, a single presentation of AIH elicits transient increases in sympathetic nerve activity (Dick et al., 2007; Xing and Pilowsky, 2010) and daily AIH (dAIH) for one week elicits prolonged improvement in forelimb function of rats with cervical spinal injuries, an effect that lasts weeks following treatment (Lovett-Barr et al., 2012; Prosser-Loose et al., 2015). A single AIH exposure (15, 1-min hypoxic episodes, 9% inspired O2; 1-min intervals) improves leg strength in persons with chronic spinal injuries (Trumbower et al., 2012), and dAIH (15, 1.5 min episodes per day, 9% O2; 1.5 min intervals) and dAIH paired with 30-min of overground walking practice improved walking speed and endurance in patients with chronic incomplete spinal cord injuries (Hayes et al., 2014). Thus, IH may elicit similar plasticity in respiratory and non-respiratory motor systems. Fundamental mechanisms giving rise to such similar functional plasticity have not been adequately explored.
We previously demonstrated that a distinct protocol of repetitive AIH consisting of AIH (10, 5-min episodes of 10.5% O2 per day; 5-min normoxic intervals) three times per week for 10 weeks (3 × wAIH) elicits neurochemical plasticity in phrenic motor neurons (Satriotomo et al., 2012). Here, we tested the hypothesis that 3 × wAIH also increases the BDNF, TrkB, p-TrkB, HIF-1α, VEGF and VEGFR-2 expression in non-respiratory motor neurons. Immunohistochemical techniques were utilized to localize the expression of these growth/trophic factors and their main receptors in alpha motor neurons innervating upper and lower limbs in C7 and L3 ventral gray matter, and in the primary motor cortex (M1).
An understanding of rAIH-induced growth/trophic factor expression may be useful as we develop therapeutic strategies to treat motor deficits in patients, including those with cervical spinal injuries or motor neuron disease (Dale et al., 2014; Navarrete-Opazo and Mitchell, 2014).
EXPERIMENTAL PROCEDURES
Animals and experimental treatments
Twenty male adult Sprague–Dawley (SD) rats weighing 300–330 g were randomly exposed to normoxia (n = 10) or 3 × wAIH for 10 weeks (n = 10). AIH was accomplished by placing unrestrained rats in Plexiglass chambers (one rat per chamber, dimensions 12 in × 4.5 in × 4.5 in) while gases flushing through the chambers (4 L/min) were alternated between 21% and 10.5% O2 at 5-min intervals. One day prior to treatment onset, rats were acclimated to the exposure chambers before beginning the 3 × wAIH protocol: 10, 5-min hypoxic episodes (FIO2 = 0.105), separated by 5-min normoxic intervals (FIO2 = 0.21), three times per week for 10 weeks as described previously (Satriotomo et al., 2012). Sham rats were in chambers for an equivalent period of time, but did not receive hypoxia. Chamber oxygen levels were continuously monitored (AX300-1, Teledyne Analytical Instruments, City of Industry, CA, USA). Both 3 × wAIH and normoxia-treated rats rested quietly or slept during exposure periods. All procedures in this study were carried out in accordance with the National Institutes of Health (NIH) guidelines for care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at the School of Veterinary Medicine, University of Wisconsin-Madison.
Immunohistochemistry
All rats treated with normoxia or 3 × wAIH were euthanized and perfused transcardially with cold 0.01 M phosphate-buffered saline (PBS, pH 7.4), followed by 4% buffered paraformaldehyde. The brain and spinal cords were immediately removed, and cryoprotected in 30% sucrose at 4 °C until they sank. Transverse sections of the cortical area of the primary motor cortex (M1), cervical spinal (C7) and lumbar spinal segments (L3) were processed for immunohistochemistry. Transverse sections (40 µm) were cut using a freezing microtome (Leica SM 200R, Germany). For immunostaining, free-floating sections were washed in 0.1 M Tris-buffered saline with 0.1% Triton-X100 (TBS-Tx; 3 × 5 min) and incubated (30 min) in TBS containing 1% H2O2. After washing (3 × 5 min) in TBS-Tx, tissues were blocked (60 min) with 5% of normal goat serum or normal rabbit serum and then tissue was incubated at 4 °C overnight in primary antibodies: rabbit polyclonal anti-BDNF (N-20, 1/1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit polyclonal anti-TrkB (1/500, Santa Cruz Biotechnology, Santa Cruz, CA); rabbit serum anti phospo-TrkB (1/1000, courtesy of Dr. Moses Chao, NYU); rabbit polyclonal anti-VEGF (A-20, 1/1000, Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-VEGFR-2 or KDR (Kinase insert Domain Receptor) (V3003, 1/500, Sigma–Aldrich, St. Louis, MO, USA) and rabbit polyclonal anti-HIF-1α (1/500, Santa Cruz Biotechnology, Santa Cruz, CA). Following overnight incubation, sections were washed and incubated in either biotinylated secondary goat anti-rabbit antibody (1:1,000, Vector Laboratories, Burlingame, CA, USA) for BDNF, TrkB, phospho-TrkB, and VEGF, or biotinylated secondary goat anti-mouse antibody for VEGFR-2 (1:1000, Vector Laboratories, Burlingame, CA). Conjugation with avidin–biotin complex (Vecstatin Elite ABC kit, Vector Laboratories, Burlingame, CA) was followed by visualization with 3,′-diaminobenzidine-hydrogen peroxidase (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. Sections were then washed in TBS, placed in gelatin-coated slides, dried, dehydrated in a graded alcohol series, and then cleared with xylenes and mounted with Eukitt mounting medium (Electron microscope sciences, Hatfield, PA, USA).
All images were captured and analyzed with a digital camera (SPOT II; Diagnostic Instruments, Sterling Heights, MI, USA). Final photomicrographs were created with Adobe Photoshop software (Adobe System, San Jose, CA, USA). All images received equivalent adjustments to tone scale, gamma and sharpness. Sections incubated without primary or secondary antibodies served as negative controls. In addition we pre-absorbed the primary BDNF and VEGF antibodies with a fivefold (by concentration) excess of specific blocking peptides (sc-546 P and sc-152 P; both from Santa Cruz Biotechnology). A parallel set of TrkB-stained sections was subjected to cresyl-violet staining to identify the morphology of the cells.
Immunofluorescence
To identify the expression of the neurotrophic/growth- and transcription-factor proteins in somatic motor neurons, tissues were incubated at 4 °C overnight with either rabbit polyclonal anti-BDNF (N-20; 1/500, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-VEGF (A-20, 1/200, Sigma– Aldrich, St. Louis, MO) or rabbit polyclonal anti-HIF-1α (1/200, Santa Cruz Biotechnology, Santa Cruz, CA), and a second primary antibody for the neuronal marker mouse monoclonal anti-NeuN (1/200, Chemicon, Temecula, CA). After washing with TBS-Tx (3 × 5 min), tissues were incubated in a mixture of conjugated goat anti-rabbit red fluorescent Alexa 495 and conjugated goat anti-mouse green fluorescent Alexa 488 (1:200, Molecular Probes, Eugene, Oregon) at room temperature for 60 min. Stained tissues were mounted on glass using anti-fade solution (Prolong Gold anti fade reagent, Invitrogen, Oregon) and examined using an epifluorescence microscope (Nikon, Japan).
Quantification and statistical analysis
The expression of BDNF, VEGF and HIF-1α in lumbar L3 spinal segments was quantified as previously described (Satriotomo et al., 2012). The expression of growth/neurotrophic factors in C7 spinal segment and M1 upper motor neurons was not quantified because the C7 segment may contribute to intercostal muscle (C7–T13s segments) but the L3 segment does not innervate the abdominal respiratory motor neuron (i.e. T8–L2 segment; Giraudin et al., 2008). Sections of the L3 spinal segment were numbered sequentially, and every 8th section was selected for immunohistochemistry using, 30-diaminobenzidine-hydrogen peroxidase (DAB) methods. Approximately five sections from each segmental level were used in this study. Putative alpha motor neurons were identified as large (> ~295 µm2) NeuN-positive cells (Friese et al., 2009) in lamina IX of the lumbar L3 ventral horn (Paxinos and Watson, 1997); the investigator was blinded to the experimental treatment in all analyses.
Analysis of BDNF, VEGF and HIF-1α immunoreactive alpha motor neurons was performed on images taken at 40× (SPOT II; Diagnostic Instruments, Sterling Heights, MI). Raw images were analyzed using Image J software (NIH, Bethesda, MD; http://rsb.info.nih.gov/ij). All images were converted to eight-bit resolution, and threshold was set between 120 and 160 during all analyses; a threshold was chosen for each group in which all motor neurons were visible but not saturated in ImageJ (i.e. both normoxia and 3 × wAIH images were treated identically within each group). Densitometry was performed by circumscribing individual motor neurons in lamina IX and the fractional area occupied by BDNF, VEGF or HIF-1α label was computed by Image J (i.e. the percentage of immuno-positive pixels within each motor neuron that was above threshold). Area fraction for individual motor neurons were then averaged for each section, and then further averaged per animal and group for each treatment. Data were compared between the 3 × wAIH and normoxia-treated groups for BDNF (n = 7 for normoxia and n = 10 for 3 × wAIH), VEGF (each n = 6) and HIF-1α (each n = 4) using a T-test. Differences were considered significant if p < 0.05. All values are expressed as mean±1 SEM.
RESULTS
3 × wAIH increases BDNF, TrkB and p-TrkB immunoreactivity in motor neurons
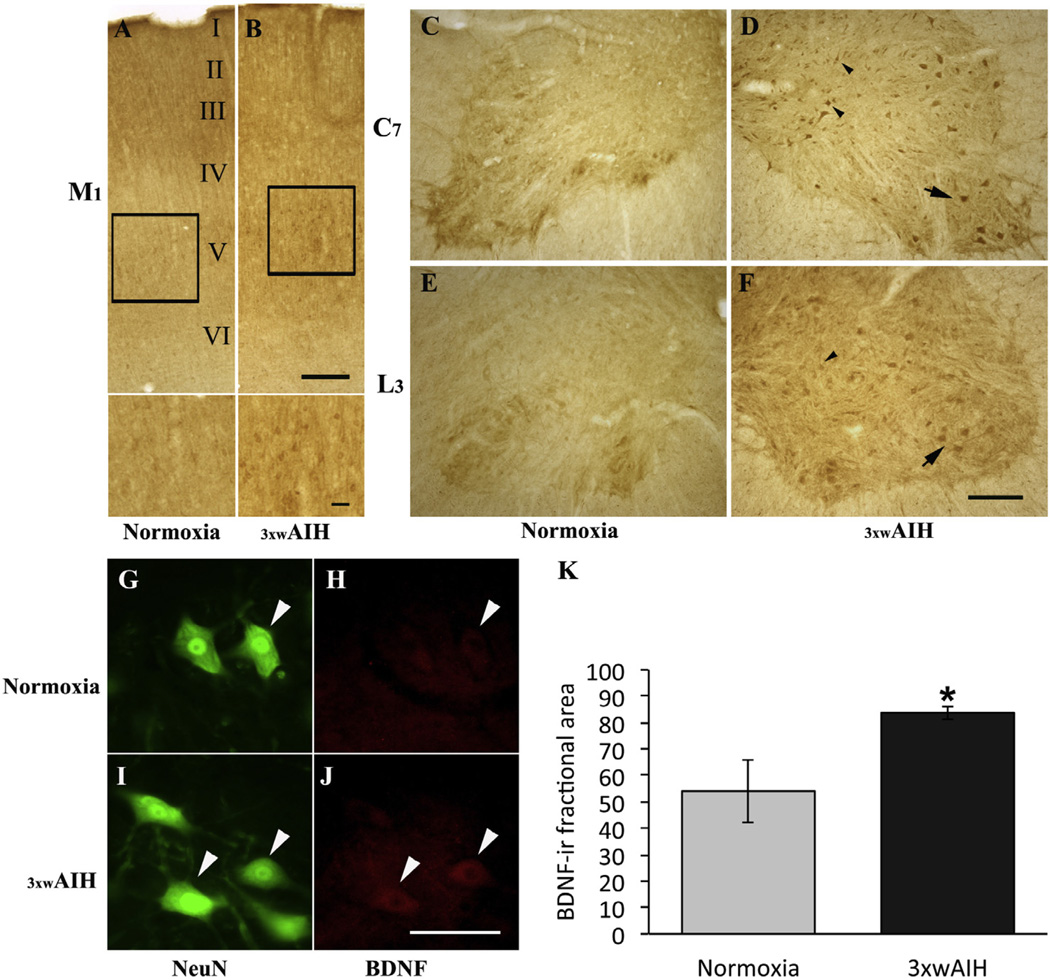
The primary motor cortex (M1), including layers I–V (Miller, 1987; Fig. 1A), sends long axons down the spinal cord that synapse on spinal interneurons and alpha motor neurons. Photomicrographs of coronal sections of the cerebral cortex showed only faint BDNF staining in any layer in normoxic rats (Fig. 1A). 3 × wAIH enhanced BDNF immunoreactivity in M1 motor cortex, especially in Betz cells of layer V as revealed at higher magnification (Fig. 1B).
Fig. 1.
Representative images of BDNF immunostaining in primary motor cortex (M1), cervical (C7) and lumbar (L3) ventral horn spinal cord. 3 × wAIH increased BDNF protein expression in upper motor neurons in M1, especially in layer V (Boxes) (B) versus normoxic control (A). Higher magnification images from layer V are in the bottom of the images. Immunostaining also confirmed BDNF expression in presumptive alpha motor neurons (big arrow) and interneurons (small arrow heads) in cervical (C7) and lumbar (L3) segments, and its immunoreactivity is enhanced following 3 × wAIH (C–F). BDNF and NeuN (Neuronal marker)-positive neurons (white arrow head) in L3 in lamina nine ventral horn, where BDNF immunoreactivity was increased by 3 × wAIH (G–J), as confirmed by densitometry (K). Scale bars: A, B 400 µm, higher-magnification on figures A, B is 100 µm, C–F is 200 µm, G–J is 100 µm. Data are mean±1 SEM. *p < 0.05 versus normoxia.
3 × wAIH also increases BDNF immunostaining in somatic motor neurons of the C7 and L3 ventral horns (Fig. 1C–F); increased BDNF immunoreactivity was observed in putative alpha motor neurons (larger group of neurons in lamina IX, arrows) and presumptive interneurons (group of small neurons in lamina VII and VIII, arrow head) in C7 (Fig. 1C, D) and L3 (Fig. 1E, F) ventral horns, respectively. Immunofluorescence also revealed increased BDNF expression after 3 × wAIH in L3 putative alpha-motor neurons (larger cells stained with NeuN; Fig. 1G–J); increased BDNF following 3 × wAIH was confirmed by densitometry in L3 ventral horn (83.8±2.5 vs. 54.1±11.8, 3 × wAIH vs. normoxia respectively, p < 0.05; Fig. 1K).
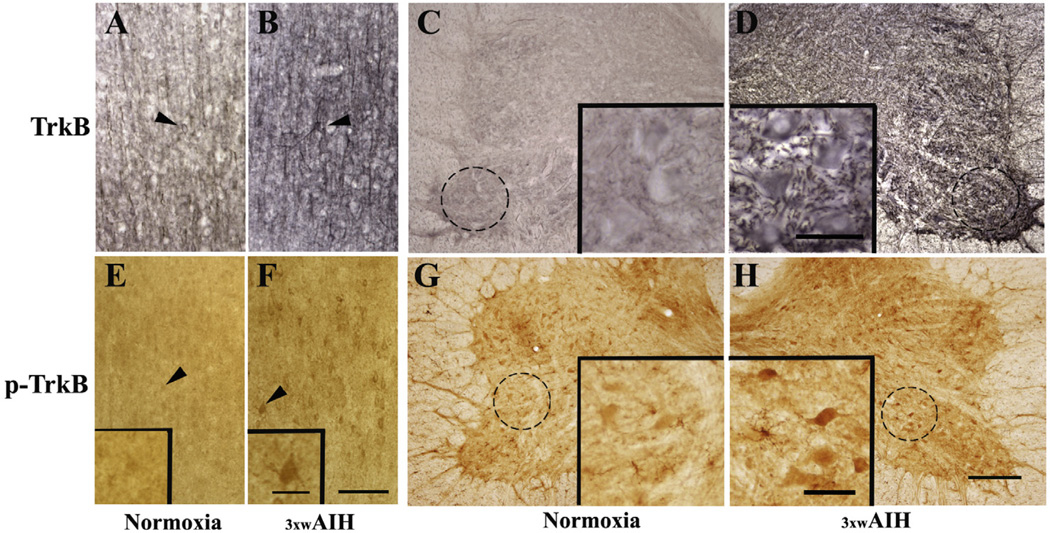
In normoxic rats, TrkB immunoreactivity in layer V of motor cortex was faint in neurons and axonal fibers (Fig. 2A). 3 × wAIH increased TrkB immunostaining in pyramidal neurons (Fig. 2B). Increased TrkB expression was observed in the cell soma, and surrounding regions that presumably reflect labeling in neuronal processes. 3 × wAIH also increased TrkB immunostaining within non-respiratory motor neurons of the C7 ventral horn (Fig. 2C, D). 3 × wAIH also increased phospho-TrkB immunoreactivity in neurons of layer V of cortical M1, and in the C7 spinal ventral horn (Fig. 2E–H). Increased TrkB phosphorylation suggests greater receptor activation following 3 × wAIH.
Fig. 2.
Photomicrographs of TrkB (A–D) and phospho-TrkB (E, F) in primary motor cortex (layer V of M1) and C7 ventral horn. TrkB immunoreactivity (dark brown) was counterstained with cresyl-violet (blue) to determine the localization of Betz cells in layer V of the primary motor cortex (A, B) and in alpha motoneurons of C7 ventral horn (C, D). TrkB receptor protein expression and phospho-TrkB were upregulated in motor neurons of M1 and C7 following 3 × wAIH treatment (B, D, F, H) compared to normoxic controls (A, C, E, G). Higher-magnification images (100 µm; insets) from C7 (C, D, G, H) and M1 (E, F) are shown in enlarged boxes. Scale bars: A–F 200 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
VEGF and VEGFR-2 immunoreactivity are upregulated by 3 × wAIH
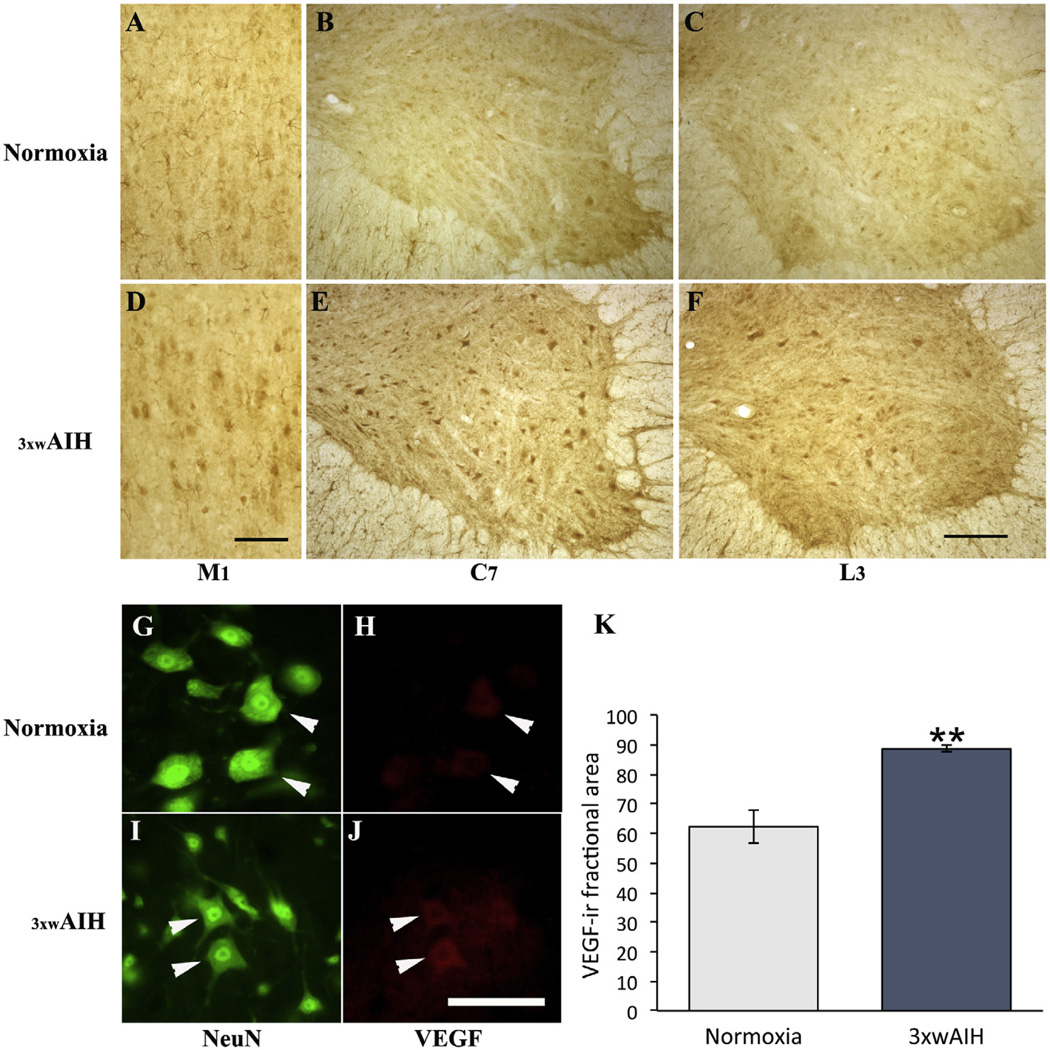
As shown in Fig. 3(A–C), VEGF expression in cortical M1, and C7 and L3 spinal ventral horns is low in normoxic rats. However, 3 × wAIH induced VEGF immunoreactivity in neurons within each region, including putative alpha motor neurons and inter-neurons (Fig. 3D–F). When visualized with immunofluorescence, VEGF protein was localized in presumptive alpha motor neurons (large NeuN-positive cells); 3 × wAIH enhanced VEGF expression in these cells (Fig. 3G–J). Using densitometry, 3 × wAIH increased VEGF expression in the L3 ventral horn (88.6±1.1 vs. 62.3±5.6; 3 × wAIH vs. normoxia respectively; p < 0.001; Fig. 3K).
Fig. 3.
Representative images of VEGF immunostaining in layer V of M1 and cervical (C7) and lumbar (L3) ventral horn. VEGF immunostaining is increased in M1, C7 and L3 ventral horn after 3 × wAIH treatment (D–F) versus normoxic control (A–C). VEGF and NeuN-positive neurons (white arrow head), where VEGF immunoreactivity was increased following 3 × wAIH (G–J), as confirmed by densitometry (K). Scale bar for A–F: 200 µm; G–J is 100 µm. Data are mean±1 SEM. **p < 0.001 versus normoxia.
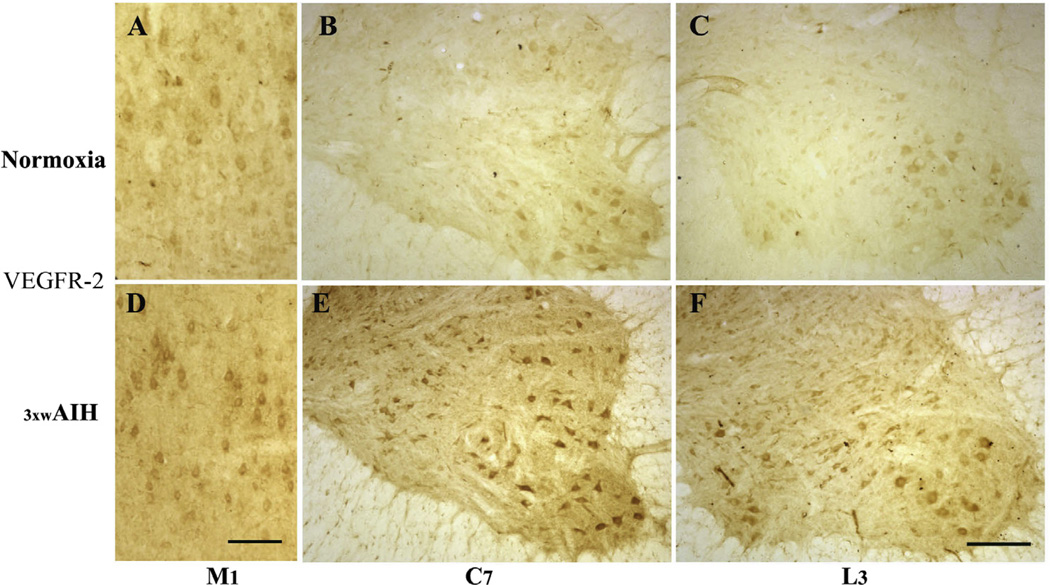
VEGFR-2 protein is also expressed in neurons of cortical M1 and in the C7 and L3 spinal ventral horns (Fig. 4A–C); 3 × wAIH increased VEGFR-2 expression in each of these regions (Fig. 4D–F).
Fig. 4.
Photomicrographs of VEGF receptor-2 (VEGFR-2/KDR) immunostaining in layer V of M1 and in C7 and L3 ventral horn. 3 × wAIH increased VEGF receptor protein expression in layer V of primary motor cortex (M1) and in alpha-motor neurons of C7 and L3 ventral horn (D–F) versus normoxic control (A–C). Scale bars for A–F: 200 µm.
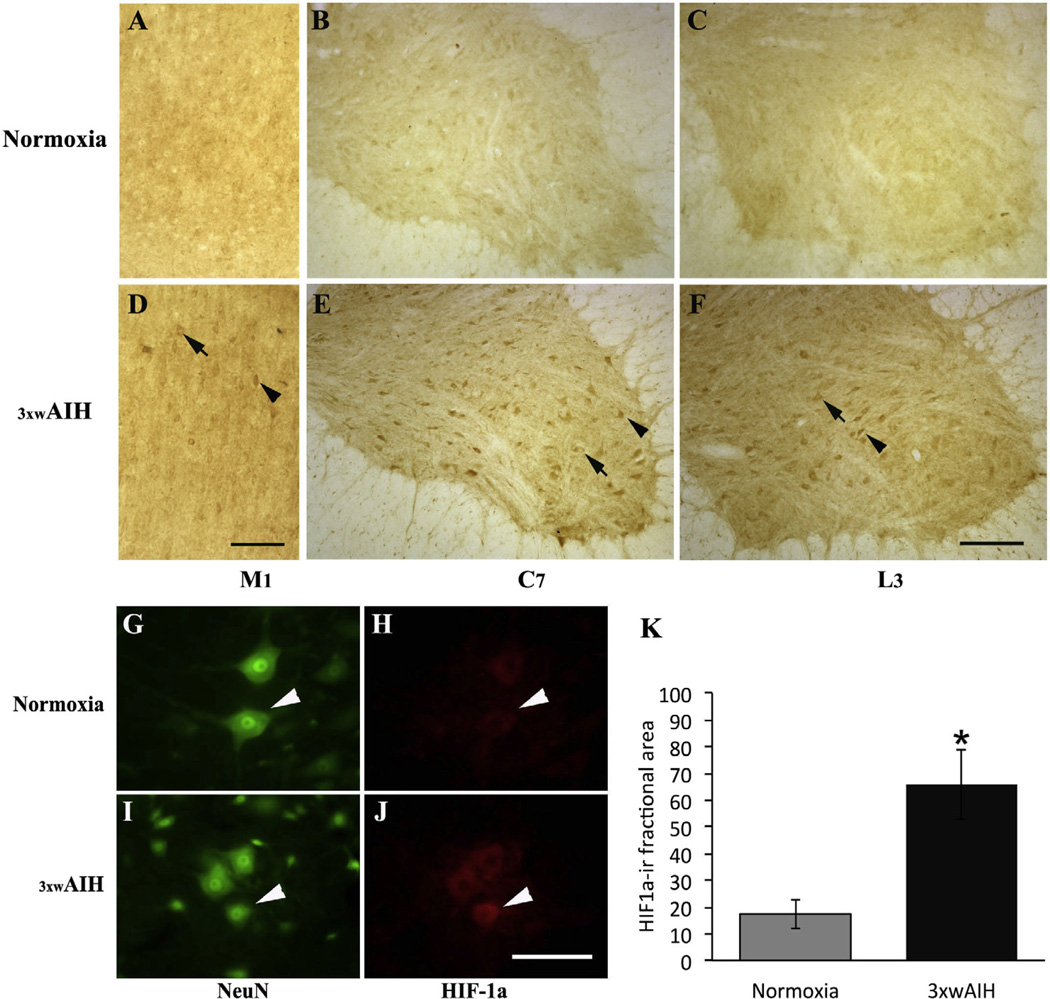
HIF1α is upregulated following 3 × wAIH
Because of its role in regulating gene expression in response to hypoxia, we evaluated HIF-1α expression after 3 × wAIH. In normoxia, HIF-1α is expressed in neurons of M1 cortex, and in the C7 and L3 spinal ventral horns (Fig. 5A–C). 3 × wAIH increased HIF-1α immunostaining versus normoxia (Fig. 5D–F). Increased HIF-1α was observed in the cytoplasm (arrow) and cell nucleus (arrow head). With immunofluorescence, HIF-1α immunostaining in the C7 spinal ventral horn is faint in normoxia, and predominantly in the cytoplasm (Fig. 5G, H). After 3 × wAIH, cytoplasmic staining increased, and was now also evident in the cell nucleus (arrow head; Fig. 5I, J). Densitometry confirmed that HIF-1α protein expression increased after 3 × wAIH (65.9±13.1 vs. 17.5±5.4; 3 × wAIH vs. normoxia respectively; p < 0.05; Fig. 5K). Thus, HIF-1α may induce transcriptional regulation of hypoxia-sensitive genes in spinal motor neurons following 3 × wAIH.
Fig. 5.
Photomicrographs of HIF-1α immunostaining in layer V of cortical M1 and in C7 and L3 ventral horns. Repetitive AIH increased HIF-1α protein expression in motor neurons in layer V of the primary motor cortex (M1) and in alpha motor neurons of C7 and L3 ventral horns (D–F) versus normoxic control (A–C). HIF-1α and NeuN-positive neurons (white arrow head), where HIF-1α immunoreactivity was increased following 3 × wAIH (G–J), as confirmed by densitometry (K). Scale bars for A–F: 200 µm; G–J is 100 µm. Data are mean±1 SEM. *p < 0.05 versus normoxia.
DISCUSSION
Here, we demonstrate increased expression of growth/trophic factors known to mediate neuroprotection and neuroplasticity in non-respiratory spinal motor neurons and the primary motor cortex following a modest protocol of repetitive AIH. In specific, we demonstrate that 3 × wAIH increases BDNF and VEGF protein levels (and their receptors) in putative alpha motor neurons of the C7 and L3 spinal ventral horn, and in neurons of layer V of the motor cortex.
AIH and rAIH elicit motor plasticity in non-respiratory motor systems following spinal injury (Lovett-Barr et al., 2012; Trumbower et al., 2012; Hayes et al., 2014; Prosser-Loose et al., 2015), and we demonstrated previously increased growth/trophic factor expression associated with dAIH-induced motor plasticity in both respiratory (C4) and non-respiratory (C7) motor nuclei (Lovett-Barr et al., 2012). We now demonstrate similar changes in key growth/trophic factors at other CNS sites associated with non-respiratory motor behaviors, including the motor cortex and non-respiratory (C7 and L3) spinal motor neurons. Each of these changes may contribute to motor plasticity (Dale et al., 2014).
Previous attempts to harness exogenous growth/trophic factor delivery for therapeutic benefit have met with limited success, at least in part because their high-affinity receptors down regulate (Wada et al., 2006; Jia et al., 2008), undermining therapeutic efficacy. Repetitive AIH may represent a safe, simple and effective means of endogenous growth factor ‘‘delivery” to the CNS since it increases expression of growth/trophic factors and their high-affinity receptors.
rAIH regulation of BDNF and TrkB expression
New cervical spinal BDNF synthesis is necessary and sufficient for AIH-induced spinal respiratory motor plasticity (Baker-Herman et al., 2004). Following AIH, new BDNF synthesis requires spinal serotonin receptor activation (Baker-Herman et al., 2004), suggesting that BDNF synthesis is regulated by intermittent serotonin receptor activation versus hypoxia per se (Baker-Herman and Mitchell, 2002; MacFarlane and Mitchell, 2009; MacFarlane et al., 2011). In this short time domain, new BDNF synthesis most likely involves translational regulation from existing mRNA (Baker-Herman and Mitchell, 2002).
Although we do not yet know if rAIH-induced BDNF expression in non-respiratory motor neurons is serotonin-dependent, we speculate that similar, serotonin-dependent mechanisms increase BDNF synthesis following AIH in respiratory and nonrespiratory motor neurons (Lovett-Barr et al., 2012). The most likely cellular mechanism of BDNF upregulation is referred as the “Q pathway” to phrenic motor facilitation (pMF; Dale-Nagle et al., 2010a,b; Dale et al., 2014), since multiple metabotropic Gq protein-coupled receptors elicit pMF by a similar BDNF synthesis-dependent mechanism. Metabotropic receptors known to elicit the Q pathway to pMF include serotonin 2A and 2B (MacFarlane and Mitchell, 2009; MacFarlane et al., 2011), and α1-adrenergic receptors (Huxtable et al., 2014).
With multi-day, repetitive AIH exposures, additional mechanisms involving hypoxia-induced transcriptional regulation are likely involved. Our observation that 3 × wAIH upregulates HIF-1α in motor neurons is consistent with a role in transcriptional regulation of BDNF and TrkB since both are either directly or indirectly regulated by HIF-1α (Martens et al., 2007). Although the BDNF gene is not reported to have a hypoxia response element in its promoter (i.e. the promoter site binding HIF-1α), hypoxia response elements are found on the promoter regions of other transcription factors that directly regulate BDNF (e.g. DEC1/BHLHB2; Miyazaki et al., 2002; Jiang et al., 2008). Further, Neurotrophic Tyrosine Kinase Receptor 2 (NTRK2), which regulates TrkB, is in turn regulated by HIF-1α (Martens et al., 2007). The respective roles of serotonin versus HIF-1α-dependent BDNF and TrkB regulation after 3 × wAIH require further investigation.
BDNF and TrkB roles in rAIH-induced motor plasticity
Modest rAIH protocols induce respiratory motor plasticity and metaplasticity (Wilkerson and Mitchell, 2009; Vinit et al., 2010; Lovett-Barr et al., 2012). For example, dAIH (10 hypoxic episodes per day, 7 days) increases ventral spinal BDNF (Wilkerson and Mitchell, 2009), enhances phrenic motor facilitation after a single (three episode) AIH exposure (Wilkerson and Mitchell, 2009), and improves breathing capacity in rats with chronic cervical spinal injuries (Lovett-Barr et al., 2012). Similarly, 3 × wAIH (4 or 10 weeks) enhances phrenic motor facilitation (Vinit et al., 2010) and increases BDNF protein expression in the ventral C4 spinal cord (Satriotomo et al., 2012). Although dAIH elicits a lesser increase in BDNF expression versus 3 × wAIH, there is no apparent difference in BDNF upregulation with 3 × wAIH for 4 versus 10 weeks (Satriotomo et al., 2010, 2012).
In addition to its effects on respiratory function, dAIH improves forelimb function in rats with chronic cervical spinal injuries (Lovett-Barr et al., 2012). Even a single AIH exposure increases leg strength (plantar flexion torque) (Trumbower et al., 2012), and daily AIH for 5 days improves walking ability in patients with chronic, incomplete spinal injuries (Hayes et al., 2014). Thus, rAIH represents a novel therapeutic approach to improve non-respiratory somatic motor function following chronic spinal injury. These findings also suggest that common mechanisms may be at play, with the specific motor enhancement arising from the same cellular mechanisms arising in different motor neuron pools (Dale et al., 2014).
BDNF is a powerful modulator of neuronal excitability and synaptic transmission (Causing et al., 1997; Lu and Figurov, 1997; Kafitz et al., 1999). Physical activity also increases brain and spinal BDNF expression (Neeper et al., 1995; Gomez-Pinilla et al., 2001) and, in turn, spinal BDNF enhances locomotion (Jakeman et al., 1998). Repetitive AIH represents a novel means of inducing spinal BDNF and TrkB function to trigger motor plasticity without the difficulties of over-ground walking in paralyzed individuals, complications due to exogenous BDNF administration (Jia et al., 2008; Zhang et al., 2011), or deleterious effects characteristic of severe/prolonged intermittent hypoxia protocols (e.g. hypertension, hippocampal inflammation or neuronal cell death; for review see Navarrete-Opazo and Mitchell, 2014). Thus, BDNF (and TrkB) may play important roles in non-respiratory motor plasticity following intermittent hypoxia (Dale-Nagle et al., 2010a,b; Lovett-Barr et al., 2012; Trumbower et al., 2012; Dale et al., 2014; Hayes et al., 2014; Navarrete-Opazo and Mitchell, 2014).
VEGF and VEGF receptor 2 expression following rAIH
VEGF and VEGF receptors are expressed in phrenic motor neurons (Dale-Nagle et al., 2011) and are upregulated by 3 × wAIH (Satriotomo et al., 2012; Dale and Mitchell, 2013). Here we confirm that VEGF and VEGF receptor 2 are expressed in non-respiratory spinal motor neurons (Sato et al., 2012), and demonstrate that they are also upregulated by 3 × wAIH. Mechanisms increasing VEGF and VEGFR-2 expression are unknown, but likely result from HIF1 transcriptional regulation.
Many long-term cellular adaptations to hypoxia are mediated by HIF-1 (Semenza, 2007; Sharp et al., 2001; Yamakawa et al., 2003; Zhou et al., 2003). During normoxia, HIF-1α subunits are rapidly degraded in the proteasome, but are stabilized by hypoxia (Maxwell et al., 1999); HIF-1α then translocates to the cell nucleus where it binds with HIF-1β subunits, and initiates transcriptional activity. HIF-1 is a major regulator of the VEGF gene (Yamakawa et al., 2003). On other hand, HIF-2α shares 80% sequence homology to HIF-1α and interacts with HIF-1β (Ema et al., 1997). Some HIF-1 target genes, including VEGF, EPO, GLUT1 and EGLN3, can be activated either by HIF-1 or by HIF-2 (Elvidge et al., 2006). Nanduri et al. (2009) demonstrated that HIF-1α was up-regulated and HIF-2α was down-regulated by chronic intermittent hypoxia. An understanding of the complex interactions between these HIF isoforms and their respective contributions to growth/trophic factor regulation in somatic motor neurons awaits further investigation.
BDNF also regulates VEGF by a TrkB and HIF-1-dependent mechanism (Nakamura et al., 2006). Conversely, transcriptional regulation of the NTRK2 gene, which encodes the TrkB receptor tyrosine kinase, increases during hypoxia by a HIF-1 dependent mechanism (Martens et al., 2007). Thus, rAIH may increase VEGF and VEGF receptor expression indirectly via HIF-1 effects on the BDNF/TrkB system.
Roles of VEGF and VEGF receptor 2 in rAIH-induced motor plasticity
Although originally known for its roles in angiogenesis and cell permeability, VEGF is also an important trophic factor that is neuroprotective in motor neurons (Storkebaum et al., 2004; Zachary, 2005). Cervical spinal VEGF injections elicit phrenic motor plasticity, similar to BDNF (Dale-Nagle et al., 2011; Dale and Mitchell, 2013). Although 3 × wAIH upregulates VEGF and VEGF receptor 2 expression in phrenic motor neurons, we found no clear evidence that this upregulation affects the magnitude of VEGF-induced phrenic motor facilitation (Dale and Mitchell, 2013). It is not currently known if VEGF and VEGF receptor activation elicit similar motor plasticity in non-respiratory motor neurons. Thus, additional work is needed to determine what role VEGF upregulation following 3 × wAIH plays in intermittent hypoxia-induced plasticity.
Significance
Increased expression of growth/trophic factors that confer neuroprotection and neuroplasticity may have considerable advantage to an animal faced with repetitive exposure to intermittent hypoxia. These responses to “low-dose” intermittent hypoxia (well below those experienced during sleep apnea) may represent a form of pre-conditioning, protecting against future (and more severe) hypoxic insults. The ability of these same growth factors to elicit respiratory and non-respiratory motor plasticity is also of considerable interest, and may present selective advantages by: (1) stabilizing breathing, thereby the recurrence of the hypoxic events; and/or (2) enhancing non-respiratory somatic motor function, enabling movement away from regions of environmental hypoxia, at least in aquatic organisms (Dale et al., 2014).
From another perspective, our findings may have considerable clinical significance, presenting a novel means of increasing endogenous levels of growth/trophic factors that elicit motor plasticity in respiratory and non-respiratory motor neurons. For example, in disorders such as spinal injury and motor neuron disease that cause paralysis, rAIH may be a simple, safe and effective means of restoring lost motor function (Mitchell, 2007). Proof of principle that these concepts apply to non-respiratory (limb) motor systems has already been provided in rodent models (Lovett-Barr et al., 2012) and humans with chronic incomplete spinal injuries (Trumbower et al., 2012; Hayes et al., 2014), as well as in rodent models of ALS (Nichols et al., 2013). “Low-dose” intermittent hypoxia may be used in combination with other therapeutic strategies, including preconditioning for stem cell therapies (Nichols et al., 2013), or traditional physical rehabilitation.
Acknowledgments
Supported by grants from the National Institutes of Health (HL080209 and HL69064) and the ALS Association. N.L.N. was supported by NIH grant K99 HL119606. The authors thank Daniel Harrigan for technical assistance.
Abbreviations
- AIH
acute intermittent hypoxia
- BDNF
brain-derived neurotrophic factor
- dAIH
daily AIH
- HIF-1α
hypoxia-inducible factor-1α
- IH
intermittent hypoxia
- NTRK2
Neurotrophic Tyrosine Kinase Receptor 2
- pLTF
phrenic long-term facilitation
- pMF
phrenic motor facilitation
- pTrkB
phosphorylated TrkB
- rAIH
repetitive acute intermittent hypoxia
- TrkB
tropomyosin receptor kinase B
- VEGF
vascular endothelial growth factor.
REFERENCES
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Calvani M, Comito G, Giannoni E, Chiarugi P. Time-dependent stabilization of hypoxia inducible factor-1 by different intracellular sources of reactive oxygen species. PLoS One. 2012;7(10):e38388. doi: 10.1371/journal.pone.0038388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causing CG, Gloster A, Aloyz R, Bamji SX, Chang E, Fawcett J, Kuchel G, Miller FD. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- Dale EA, Mitchell GS. Spinal vascular endothelial growth factor (VEGF) and erythropoietin (EPO) induced phrenic motor facilitation after repetitive acute intermittent hypoxia. Respir Physiol Neurobiol. 2013;185:481–488. doi: 10.1016/j.resp.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology. 2014;29:39–48. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol. 2010a;669:225–230. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit A, Mitchell GS. Spinal plasticity following intermittent hypoxia: implication for spinal injury. Ann NY Acad Sci. 2010b;1198:252–259. doi: 10.1111/j.1749-6632.2010.05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci. 2011;31:7682–7690. doi: 10.1523/JNEUROSCI.0239-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci. 2013;1279:143–153. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Cock ML, Harding R, Rees SM. Neurotrophin expression in the hippocampus and cerebellum is affected by chronic placental insufficiency in the late gestational ovine fetus. Dev Brain Res. 2004;153:243–250. doi: 10.1016/j.devbrainres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Elvidge GP, Glenny L, Applehoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1, HIF-2, and other pathways. J Biol Chem. 2006;281:15215–15226. doi: 10.1074/jbc.M511408200. [DOI] [PubMed] [Google Scholar]
- Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Krupinski J, Goutan E, Marti E, Ambrosio S, Arenas E. Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 2004;101:229–238. doi: 10.1007/s004010000268. [DOI] [PubMed] [Google Scholar]
- Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S. Gamma and alpha motor neurons distinguished by expression of transcription factor Err3. PNAS. 2009;106:13588–13593. doi: 10.1073/pnas.0906809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudin A, Cabirol-Pol MJ, Simmers J, Morin D. Intercostal and abdominal respiratory motor neurons in the neonatal rat spinal cord: spatiotemporal organization and responses to limb afferent stimulation. J Neurophysiol. 2008;99:2626–2640. doi: 10.1152/jn.01298.2007. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82:104–113. doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, MacFarlane PM, Vinit S, Nichols NL, Dale EA, Mitchell GS. Adrenergic α1 receptor activation is sufficient, but not necessary for phrenic long-term facilitation. J Appl Physiol. 2014;116:1345–1352. doi: 10.1152/japplphysiol.00904.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol. 1998;154:170–184. doi: 10.1006/exnr.1998.6924. [DOI] [PubMed] [Google Scholar]
- Jia JM, Chen Q, Zhou Y, Miao S, Zheng J, Zhang C, Xiong ZQ. Brain-derived neurotropic factor-tropomyosin-related kinase B signaling contributes to activity-dependent changes in synaptic proteins. J Biol Chem. 2008;283:21242–21250. doi: 10.1074/jbc.M800282200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Tian F, Du Y, Copeland NG, Jenkins NA, Tessarollo Wu X, Hongna P, Hu XZ, Xu K, Kenney H, Egan SE, Turley H, Harris AL, Marini AM, Lipsky RH. BHLHB2 control Bdnf promoter activity and neuronal excitability. J Neurosci. 2008;28:1118–1130. doi: 10.1523/JNEUROSCI.2262-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Kline DD. Chronic intermittent hypoxia affects integration of sensory input by neurons in the nucleus tractus solitarii. Respir Physiol Neurobiol. 2010;174:29–36. doi: 10.1016/j.resp.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical injury. J Neurosci. 2012;32:3591–3600. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Figurov A. Role of neurotrophins in synapse development and plasticity. Rev Neurosci. 1997;8:1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol (London) 2009;587:5469–5481. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Satriotomo I, Mitchell GS. Serotonin 2A and 2B receptor induced phrenic motor facilitation: differential requirement for NADPH oxidase activity. Neuroscience. 2011;178:45–55. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Martens LK, Kirschner KM, Warnacke C, Scholz H. Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activators of the TrkB neurotrophin receptor gene. J Biol Chem. 2007;282:14379–14388. doi: 10.1074/jbc.M609857200. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Miller MW. The origin of corticospinal projection neurons in the rat. J Comp Neurol. 1987;257:372–387. [Google Scholar]
- Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Gaultier C, editor. Genetic basis for respiratory control disorders. New York: Springer Publishing Company; 2007. pp. 291–311. [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:354–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited reviewer: intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Kawamoto T, Tanimoto K, Nishiyama M, Honda H, Kato Y. Identification of functional hypoxia response elements in the promoter region of the DEC1 and DEC2 genes. J Biol Chem. 2002;277:47014–47021. doi: 10.1074/jbc.M204938200. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1 in neuroblastoma cells. Cancer Res. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1181–R1197. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone P, McHugh J, Svendsen CN, Mitchell GS. Phrenic motor output is restored by intermittent hypoxia-induced plasticity and neural progenitor cell transplants in a rat model of ALS. Am J Respir Crit Care Med. 2013;187:535–542. doi: 10.1164/rccm.201206-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain. San Diego, CA: Stereotaxic Coordinates Academic Press Inc; 1997. [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Sensory plasticity of the carotid body: role of reactive oxygen species and physiological significance. Respir Physiol Neurobiol. 2011;178:375–380. doi: 10.1016/j.resp.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser-Loose EJ, Hassan A, Mitchell GS, Muir GD. Delayed intervention with intermittent hypoxia and training task improves forelimb function in a rat model of cervical spinal injury. J Neurotrauma. 2015;32:1–10. doi: 10.1089/neu.2014.3789. [DOI] [PubMed] [Google Scholar]
- Sato K, Morimoto N, Kurata T, Mimoto T, Miyazaki K, Ikeda Y, Abe K. Impaired response of hypoxic sensor protein HIF-1α and its downstream proteins in the spinal motor neurons of ALS model mice. Brain Res. 2012;1473:55–62. doi: 10.1016/j.brainres.2012.07.040. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Vinit S, Flom AL, Mitchell GS. Repetitive acute intermittent hypoxia increases BDNF and TrkB expression in respiratory motor neurons: dose effects. FASEB J. 2010;24:799.8. [Google Scholar]
- Satriotomo I, Dale EA, Dahlberg J, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol. 2012;237:103–115. doi: 10.1016/j.expneurol.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;407 doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Bergeron M, Bernaudin M. Hypoxia-inducible factor in brain. Adv Exp Med Biol. 2001;502:273–291. doi: 10.1007/978-1-4757-3401-0_18. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. BioEssays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, MacFarlane PM, Satriotomo I, Mitchell GS. Enhanced phrenic long-term facilitation (pLTF) following repetitive acute intermittent hypoxia. FASEB J. 2010;24:799.14. [Google Scholar]
- Wada T, Haigh JJ, Ema M, Hitoshi S, Chaddah R, Rossant J, Nagy A, van der Kooy D. Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J Neurosci. 2006;26:6803–6812. doi: 10.1523/JNEUROSCI.0526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009;217:116–123. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol. 2010;588:3075–3088. doi: 10.1113/jphysiol.2010.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- Yang SZ, Zhang LM, Huang YL, Sun FY. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:851–856. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- Zachary I. Neuroprotective role of vascular endothelial growth factor: signaling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang J, Zhou Q, Xu Y, Pu S, Wu J, Xue Y, Tian Y, Lu J, Jiang W, Du D. Brain-derived neurotrophic factor-activated astrocytes produce mechanical allodynia in neuropathic pain. Neuroscience. 2011;199:452–460. doi: 10.1016/j.neuroscience.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Zhou J, Schmid T, Brune B. Tumor necrosis factor-alpha causes accumulation of a ubiquitinated form of hypoxia inducible factor-1alpha through a nuclear factor-kappaB-dependent pathway. Mol Biol Cell. 2003;14:2216–2225. doi: 10.1091/mbc.E02-09-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]