Abstract
Objectives
Our previous studies indicated that highly selective kappa opioid receptor (KOR) agonists could protect the brain, indicating an important role of KOR agonist in brain ischemia. In this study, we investigated the role and related mechanisms of KOR agonists in brain ischemia in a middle cerebral artery occlusion (MCAO) mouse model.
Subjects and Interventions
The MCAO model was established by 120 minutes of ischemia followed by 24 h reperfusion in male adult mice. Various doses of salvinorin A (SA), a highly selective and potent KOR agonist, were administered intranasally 10 min after initiation of reperfusion. Norbinaltorphimine (2.5 mg/kg, i.p.) as a KOR antagonist was administered in one group before administration of SA (50 µg/kg) to investigate the specific role of KOR. After 24 h reperfusion, neurobehavioral outcome was determined. Infarct volume, KOR expression, and Evans blue extravasation in the brain were determined. Immunohistochemistry and western blot were performed to detect the activated caspase-3, IL-10 and TNF-alpha levels to investigate the role of apoptosis and inflammation.
Main Results
KOR expression was elevated significantly in the ischemic penumbral area compared to that in the non-ischemic area. SA reduced infarct volume and improved neurological deficits dose-dependently. SA at the dose of 50 µg/kg reduced Evans blue extravasation, suggesting reduced impairment of the blood-brain barrier, and decreased the expression of cleaved casepase-3, IL-10 and TNF-alpha in the penumbral areas. All these changes were blocked or alleviated by norbinaltorphimine.
Conclusions
KORs were up-regulated and played a critical role in brain ischemia and reperfusion. KOR activation could potentially protect the brain and improve neurological outcome via blood brain barrier protection, apoptosis reduction and inflammation inhibition.
Keywords: Kappa opioid receptor, brain, ischemia, salvinorin A, stroke, middle cerebral artery occlusion
Cerebral ischemia and reperfusion (IR) injury, one of the major components of stroke, as well as cardiac arrest and many other vascular disease processes, could cause irreversible motor and sensory deficits. Complex mechanisms are involved during brain IR injury. Using salvinorin A (SA), a highly selective and potent kappa opioid receptor (KOR) agonist, we demonstrated that SA could dose-dependently dilate the pial artery, preserve artery auto-regulation and protect the brain in a piglet global cerebral ischemia model. (1–3) The potential neuroprotective effects of SA have not been well described in models of focal ischemia. One of the major pharmacological properties of SA is that it is a fast-onset and short-acting agent that additionally can pass the blood brain barrier easily. Thus, SA could be a powerful tool to investigate the role of KOR in these pathological conditions with very narrow therapeutics windows like brain ischemia. Although SA has limited potential for use in humans because of its psychotropic effects, effectiveness in a mouse focal ischemia model could provide a basis for developing kappa opioid agonists with less prominent side effects. In the present study, we aimed to use SA to investigate the role of KOR agonist in a mouse focal cerebral ischemia model.
In this study, we investigated whether administration of KOR agonist SA could reduce cerebral infarction, protect blood brain barrier and improve neurological outcomes via specific KOR activation in a mouse middle cerebral artery occlusion (MCAO) model. The expression of KOR and activated caspase-3 protein as an apoptotic marker, and the levels of IL-10 and TNF-alpha as the markers of inflammation were determined to elucidate their relationships. This study has significant clinical relevance for the following reasons: 1) opioids are commonly used in critically ill patients; 2) There is no neuro-protectant available in clinical practice for brain ischemia; 3) opioid receptors are widely expressed in the central nervous system, their role in modulating brain ischemia has to be elucidated.
Materials and methods
Drugs
SA (purity ≥98%) was obtained from Apple Pharms (Asheville, NC). Norbinaltorphimine (purity≥99.8%) was obtained from Tocris Bioscience (Minneapolis, MN). The pharmaceutical grade medications for animal studies were obtained from the pharmacy of the University of Pennsylvania. The remaining compounds were obtained from Sigma-Aldrich (St. Louis, MO).
Experimental protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. C57BL/6J mice (male) of 8–9 weeks (20–25 g) were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed with free access to water and food. Animals were assigned to groups randomly and all the tests were conducted in a blinded manner. The protocol consisted of two experiments.
Experiment 1
This experiment aimed to determine the relationship between dose and effect. Intranasal SA was administered with various doses (0 µg/kg, 12.5 µg/kg, 25 µg/kg and 50 µg/kg in DMSO) 10 min after initiation of reperfusion. The animals were randomly divided into the following 5 groups (n=6 per group) (sham, MCAO, SA12.5, SA25 and SA50 groups). The infarct size and neurological score were determined in a blinded manner.
Experiment 2
This portion of the study aimed to study the specific role of KOR receptor and potential involvement of other pathological process in brain ischemia. The reasonable dose of SA used in experiment 2 was determined to be 50µg/kg based on the data from experiment 1. Mice were randomly divided into 4 groups (n=18 per group) : (1) sham group: mice in this group had the carotid arteries exposed without MCAO; (2) MCAO group: MCAO mice received intranasal administration of 25% DMSO 10 min after reperfusion; (3) SA50 group: MCAO mice received intranasal administration of SA (50 µg/kg) in DMSO 10min after reperfusion; (4) nor-BNI+SA50 group: MCAO mice received an i.p. injection of the KOR antagonist (norbinaltorphimine, nor-BNI) (0.05 mg/kg) 30 min before reperfusion and then intranasal administration of SA (50 µg/kg) in DMSO 10 min after reperfusion.
Mouse MCAO model
The MCAO model was established using filament insertion as reported previously.(4) Mice were anesthetized with ketamine, xylazine and acepromazine. After local infiltration with bupivacaine, the right common carotid artery was isolated and its branches were electrocoagulated. The proximal middle cerebral artery was occluded for 120 minutes by a 6-0 rounded tip nylon suture with length of 2-cm (Doccol corporation, Sharon, MA, USA) and blood flow was restored by removing the suture. During the procedure, the animals were placed over a feedback-controlled heating pad (Model TC-1000 Temperature Controller, CWE Inc.) to maintain the body temperature. After the surgery, mice were allowed to recover for 24 hours with adequate post-operative analgesia with meloxicam (5 mg/kg). In sham control animals, the carotid arteries were exposed without occlusion of the middle cerebral artery.
Neurological evaluation
Neurologic outcome was evaluated with the modified Grip test (5,6,7), which uses a scale of 0–5 points, and Garcia scoring.(8) The Garcia scoring system assigned 3–18 points and included the following six tests: spontaneous activity; forelimb walking; limb symmetry; climbing; side stroking and vibris touch. Higher points indicated an improved neurological outcome.
TTC staining and infarct area measuring
24h after MCAO, TTC (2, 3, 7-Triphenyltetrazolium chloride) (Sigma Inc.) staining (n=6) was used to determine the infarct area.(9) The brain was cut into 2 mm thick coronal sections and then immersed in 2% TTC solution for half an hour at 37 °C. The healthy tissue stained red as the tetrazolium reacted with the dehydrogenases in the viable cells, and the infarct tissue stained white because it lacked the enzymes with which the TTC reacts. The infarct area of each section was measured using an Image J analysis system (1.49v, http://rsb.info.nih.gov/ij/). The infarction rate was calculated as followed: infarct area in the right hemisphere / total area of the right hemisphere.
Immunohistochemistry staining
For the histological analysis, animals of each group were deeply anesthetized 24h after reperfusion. The brains were perfused with 10 ml of PBS and 20 ml of 4% paraformaldehyde, and then post-fixed with formalin, and embedded with paraffin. Brain sections were cut on a microtome (Leitz 1512) into width of 5 µm thick. Immunohistochemistry labeling was performed as in a previous study with the primary antibody of rabbit anti cleaved caspase-3 (#9661).(9) The staining was observed under a microscope (Olympus BX41).
Evans blue extravasation
Evans blue extravasation observation was used to determine the blood–brain barrier permeability. 4% Evans blue solution (0.1 ml, Sigma) was injected into the tail vein 1 h after reperfusion. Mice were perfused with PBS 24 h after reperfusion and brains were cut into coronal sections of 2 mm thick using a mouse coronal brain matrix (World Precision Instruments, Inc., FL, USA) for imaging purposes. The concentration of the Evans blue in the ischemic brain tissue was determined (expressed as µg/g of brain tissue) using the method previously reported.(10)
Protein extraction and western blotting analysis
The penumbra area was separated from the ischemic hemisphere and homogenized as reported previously.(11) The protein concentration was determined by the Pierce™ BCA Protein Assay Kit (Thermo Scientific, IL). 50–100 µg of protein samples of each group was loaded onto 10% polyacrylamide gel, electrophoresed, and transferred to a nitrocellulose membrane. The nitrocellulose membranes were blocked and incubated with the primary antibody of rabbit anti cleaved caspase-3 (#9661), rabbit anti TNF-α (#3707) and rabbit anti IL-10 (#12163) overnight at 4 °C (Cell Signaling Technology, Inc.). The nitrocellulose membranes were then processed with secondary antibodies (Santa Cruz, Inc., USA) for 1 h at room temperature. Immuno-blots were detected using ECL Western Blotting Substrate (Thermo Fisher Scientific Inc., IL) and the membrane was detected by Carestream Molecular Imaging Software (Standard Edition).
Fluorescent labeling
KOR expression in the ischemic and non-ischemic hemisphere of the brain was determined and compared using fluorescent labeling with the mouse monoclonal antibody against KOR (MO15098, Neuromics, Inc.). Double fluorescence labeling was conducted based on previous study.(9) Coronal sections were double stained with TUNEL (Terminal dexynucleotidyl transferase(TdT)-mediated dUTP Nick End Labeling) and rabbit polyclonal antibody (1:200, Santa Cruz, Biotechnology) of NeuN (Neuronal Nuclear antigen, Neuronal marker), iba1 (Ionized calcium-binding adapter molecule 1, microglia marker) and GFAP (Glial Fibrillary Acidic Protein, Astrocyte marker) and goat anti-rabbit IgG-tetramethylrhodamine isothiocyanate (TRITC) (red, fluorescein). The sections were cover-slipped and observed under a confocal microscope (Leica SP5). The images were processed with Image J analysis system (1.49v).
Data analysis
Data were expressed as mean ± SEM. The neurological behavior test was compared by Kryskal–Wallis one-way ANOVA followed by multiple comparison procedures. Statistical significance was assured by ANOVA using a one-way ANOVA followed by the Tukey test. A probability value of p< 0.05 was considered statistically significant.
Results
SA reduced infarct volume and improved the neurological score dose-dependently
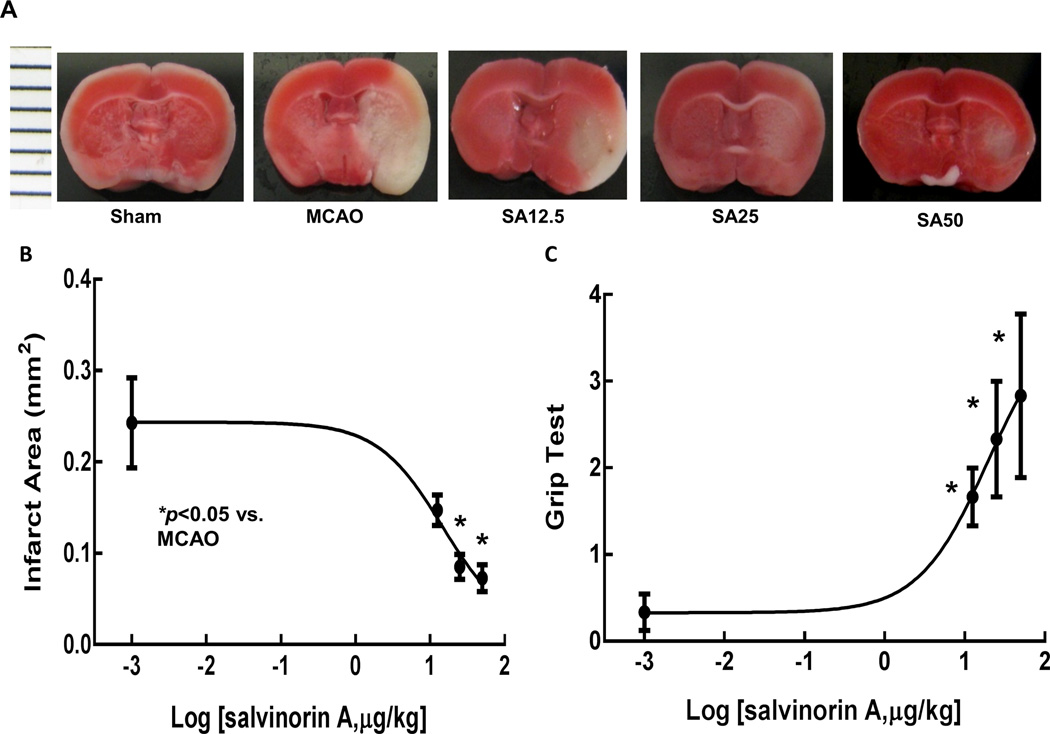
Figure 1A shows representative samples of brains from mice that received different doses of SA. SA administration reduced infarction area dose-dependently as showed in Figures 1B. The neurological outcome evaluation as shown in Figure 1C (Grip test) indicated that SA improved the neurological outcome significantly in a dose-dependent manner. The Garcia score was 12.2 ± 5.3, 13.5 ± 3.7, 13 ± 3.4, and 8.5 ± 0.4 respectively in SA12.5, SA25, SA50, and MCAO groups (p<0.05 SA groups compared to MCAO group). Both tests indicated significant improvement in neurological outcome after SA administration.
Fig. 1.
TTC (2,3,5-triphenyltetrazolium chloride) staining analysis of brain slices. Infarction (white area) is shown in MCAO group. Infarct regions, especially in cortex decreased in SA treated animals at 2 mm, 4 mm, 6 mm and 8 mm from the anterior pole (only 4 mm one showed in this figure) with dose of 12.5, 25 or 50 µg/kg in Figure 1A. Figure 1B showed that SA treatment reduced infarct size dose-dependently. (*p<0.05 vs. MCAO group) Neurological outcome 24h after reperfusion improved dose dependently as indicated in Figure 1C. (*p<0.05 vs. MCAO group). MCAO, middel cerebral artery occlusion; SA, salvinorin A.
SA preserved the blood brain barrier
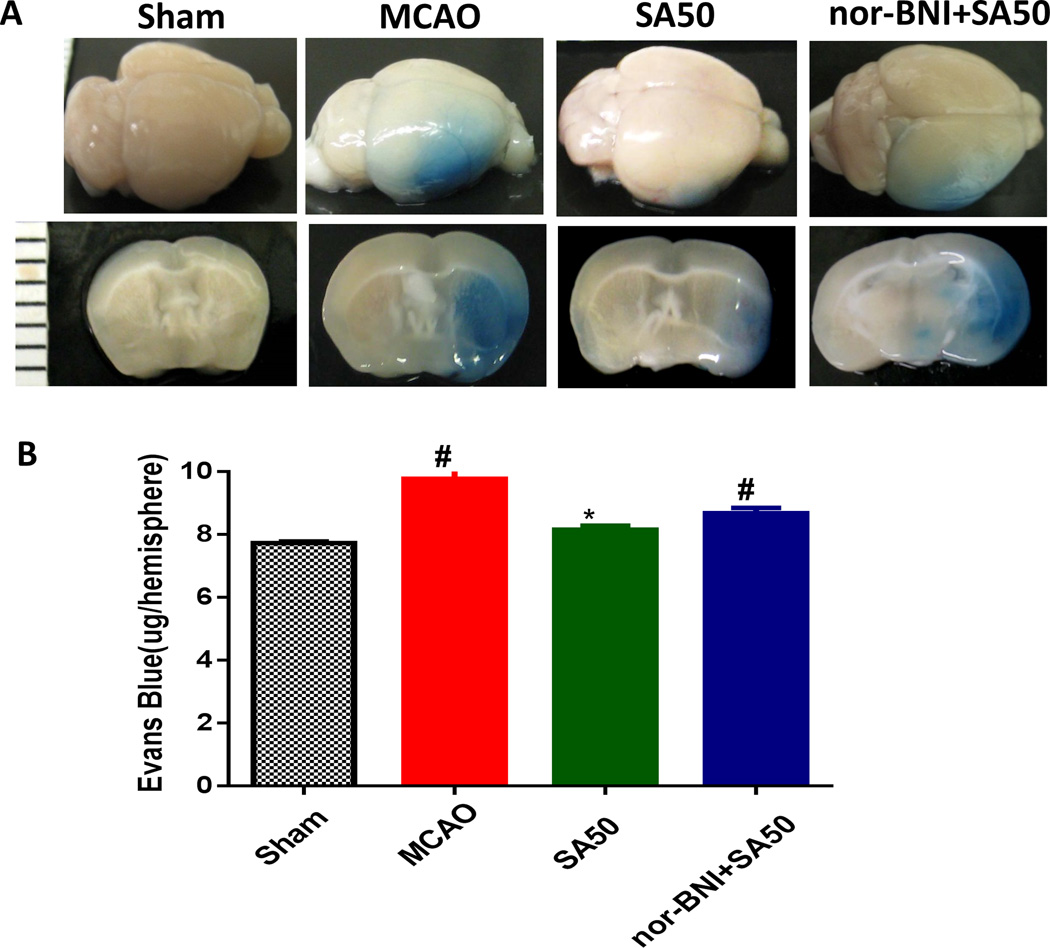
Figure 2A shows the Evans blue-stained brain section, reflecting the severity of the blood-brain barrier damage. In the MCAO and KOR antagonist nor-BNI groups, intensive staining was seen in the cortex and striate areas in the ischemic area. SA treatment without the KOR antagonist showed significant less Evans blue staining in the ischemic area. SA administration significantly reduced Evans blue content in the SA50 group as compared with that in the MCAO and nor-BNI+SA50 groups (Figure 2B). Nor-BNI given before the SA administration abolished the protective effects of SA.
Fig. 2.
Evans blue extravasation evaluation. Images in Panal A are representative samples of Evans blue-stained brain and brain sections. Evans blue staining was seen in the ischemic area in MCAO and KOR antagonist nor-BNI group. SA treatment without the KOR antagonist showed weak Evans blue staining in the striate area. Panel B indicates that SA mitigated blood brain barrier disruption. Vascular leakage was determined by measuring the amount of brain-extracted Evans blue by spectrophotometry at 610 nm and expressed as µg/hemisphere of brain tissue. SA administration reduced Evans blue leakage. However, nor-BNI given before the SA administration eliminated such protectgive effect. (#p<0.05 vs. sham; *p<0.05 vs. MCAO & nor-BNI+SA50). MCAO, middel cerebral artery occlusion; SA, salvinorin A; KOR, kappa opioid receptor; nor-BNI, norbinaltorphimine.
SA decreased IL-10 and TNF-alpha in the penumbra area
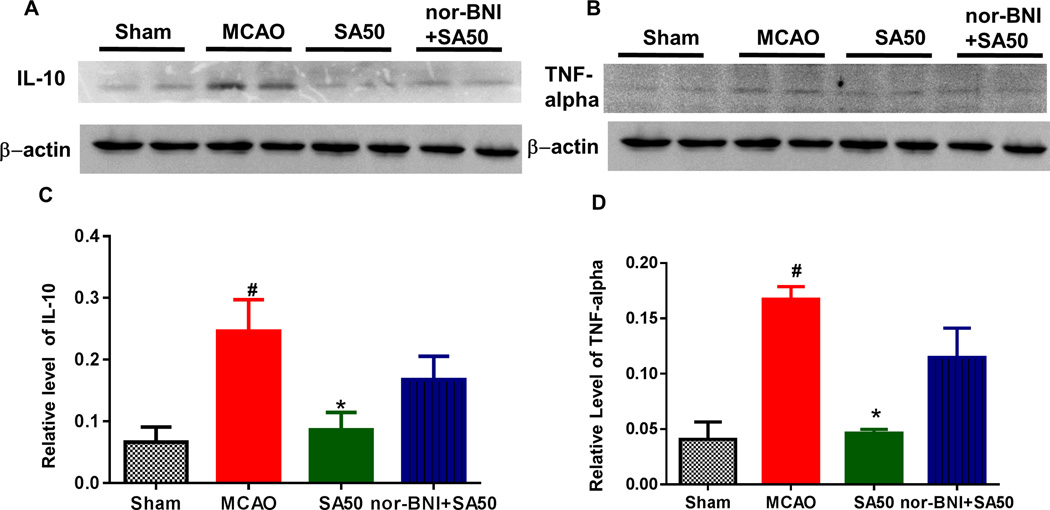
SA significantly reduced the production of IL-10. SA also reduced the levels of TNF-alpha, a cytokine that plays an important role in the inflammatory response in the hippocampus and cortex after ischemic injury. As shown in Figure 3A and 3B, expression of both IL-10 and TNF-alpha proteins increased significantly in the MCAO group compared to that in the sham group (#p<0.05) at 24 h, but declined after treatment with SA (Figure 3C, D) and such reduction was blocked by nor-BNI (*p<0.05).
Fig. 3.
The western blot study of inflammatory markers of IL-10 and TNF-alpha. Both IL-10 and TNF-alpha protein expression increased significantly in MCAO group as compared to that in sham group at 24 h after reperfusion (#p<0.05). Such elevations were reduced with SA (Figure 3A–B, Figure 3C, Figure 3D). MCAO, middel cerebral artery occlusion; SA, salvinorin A; KOR, kappa opioid receptor.
Apoptosis happens mostly on neurons in the ischemic hemisphere
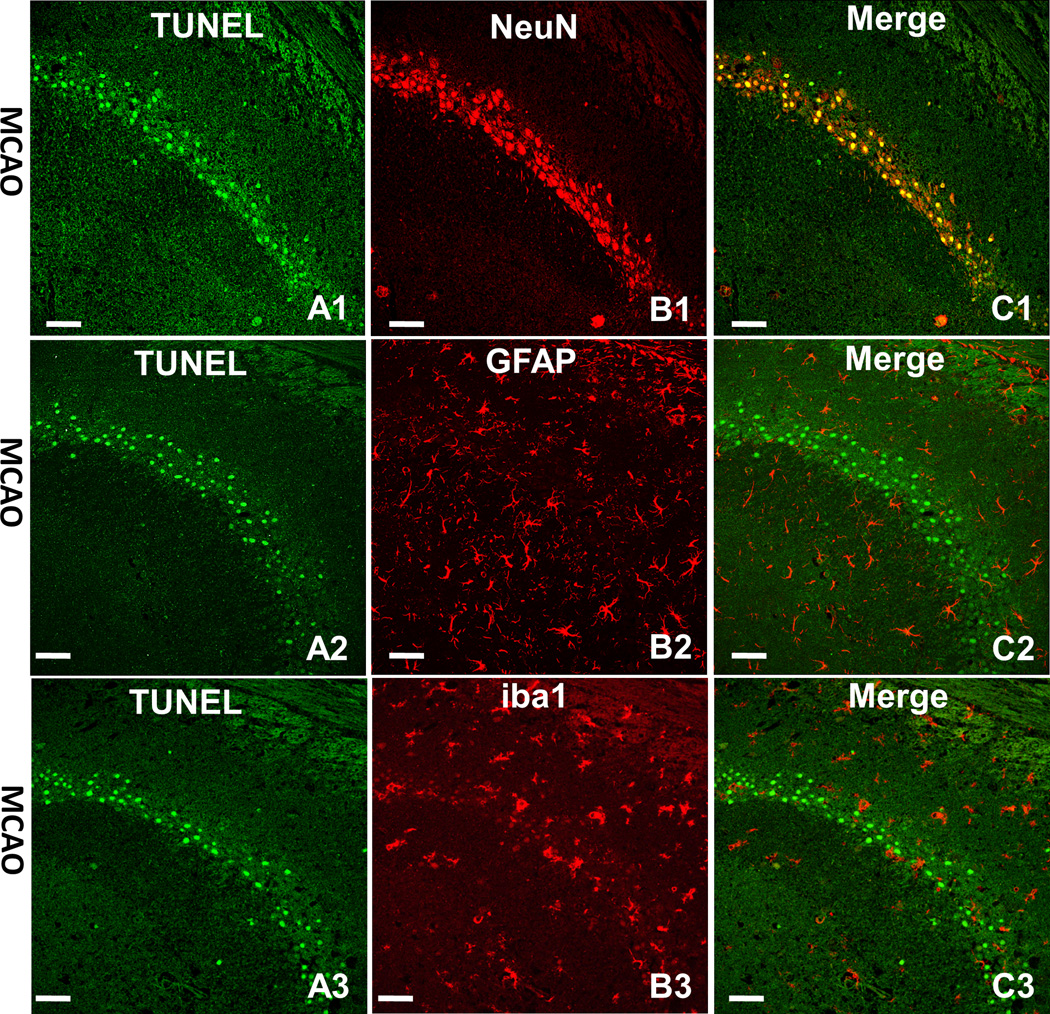
As shown in Figure 4, double immunofluorescence staining was conducted to determine which kind of cell undergoes apoptosis in ischemic hemisphere. Apoptotic cells (green) were shown as TUNEL positive cells (Figure 4A1, A2, A3). Neurons (red) were shown as NeuN positive staining cells (Figure 4B1), astrocytes (red) were shown as GFAP positive staining cells (Figure 4B2) and microglia (red) were shown as iba1 positive staining cells (Figure 4B3). From the merged images, we found that the apoptotic cells in the hippocampus co-localized with neurons (Figure 4C1) but rarely with astrocytes (Figure 4C2) and microglia (Figure 4C3). The same co-localization happened in the cortex as well (data not shown).
Fig. 4.
The double immunofluorescence staining. Neurons, astrocytes and microglia (red) expressed by TRITC. The apoptotic cell (green) shown as TUNEL positive staining (Figure 4A1, 4A2 and 4A3) in hippocampus co-localized with neurons (red) shown as NeuN positive staining (Figure 4B1). However, the apoptotic positive cells in hippocampus rarely co-localized with astrocyte (red) shown as GFAP positive staining (Figure 4B2) and also rarely co-localized with microglia (red) shown as iba1 positive staining (Figure 4B3). Image C was the merger of A and B. The merged images indicated that the apoptotic cells in the hippocampus co-localized with neurons (yellow, Figure 4C1) but rarely with astrocytes (Figure 4C2) and microglia (Figure 4C3). Scale bars indicated 50µm.
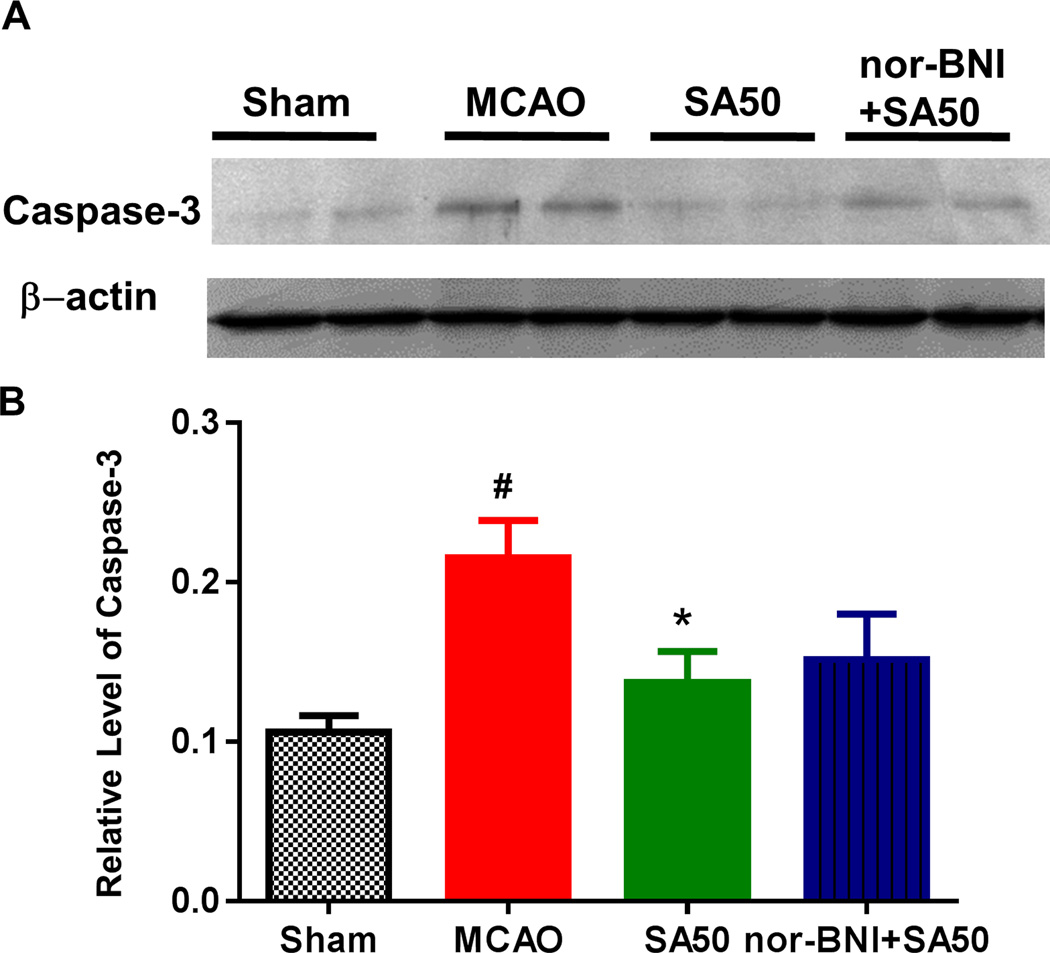
SA inhibited the apoptotic process
The positive staining of activated caspase-3 was elevated in the border of the infarct area, which could also be referred as the penumbral area after MCAO injury. The penumbra is the area of the hypoperfused brain supplied by the collateral circulation that is still viable, but at risk for irreversible infarction if blood flow is not restored. The expression of activated caspase-3 was significantly inhibited after administration of SA in various regions of hippocampus such as CA1 and CA2/3 and Dentate Gyrus (DG), as well as in the cortex area (data not shown). Meanwhile the inhibition was prominent both in immunohistochemistry staining and in western blot analysis (Figure 5). The quantitative study showed that the expression of caspase-3 was significantly decreased by SA, and such effect was blocked by KOR antagonist nor-BNI.
Fig. 5.
Western blotting analysis of the infarcted brain tissue. Both the original data (Figure 5A) and statitistal analysis (Figure 5B) showed that the expression levels of cleaved casepase-3 in the MCAO group significantly increased as compared to that in sham group (#p < 0.05). Administration of SA reduced the levels of cleaved casepase-3 proteins (*p < 0.05 vs MCAO), such effect was blocked by kappa opioid receptor antagonist nor-BNI. MCAO, middel cerebral artery occlusion; SA, salvinorin A;
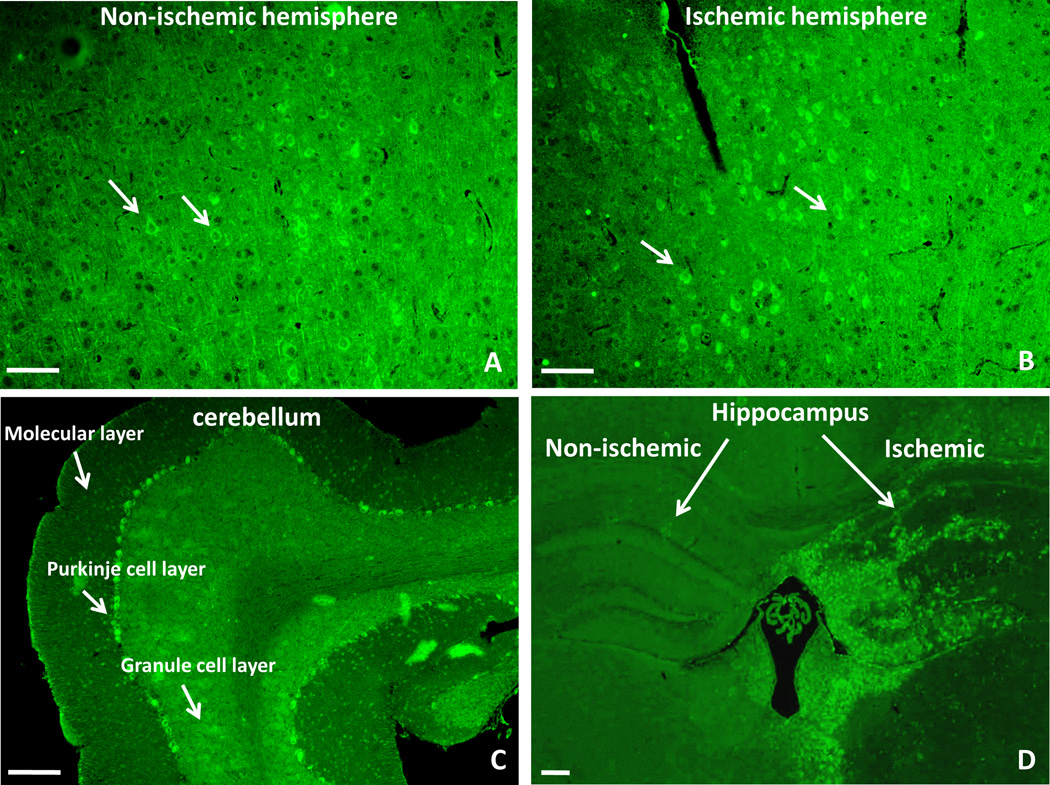
KOR over-expressed in the ischemic penumbral area after MCAO
The KOR expression increased in the ischemic penumbra cortex compared to that in the non-ischemic area after MCAO (Figure 6A and 6B). In particular, a high level of KOR expression was detected in the Purkinje cell layer of cerebellum (Figure 6C). An abundant expression in this region was described in guinea pigs,(12) whereas it was completely absent in rats.(13) Meanwhile, the KOR expression was also elevated in the ischemic hippocampus than that in the non-ischemic area (Figure 6D).
Fig. 6.
The immunofluorescent staining of the kappa opioid receptor (KOR) after MCAO. The positive staining of KOR was shown as bright green cells against to the background. After MCAO, the number of KOR increased in the ischemic cortex as indicated in Figure 6A as compared to that in the contralateral cortex (penumbra area) (Figure 6B). A high level of KOR expression was detected in the Purkinje cell layer of the cerebellum instead of molecular layer and granule cell layer (Figure 6C). In the hippocampus, the KOR expression also elevated in the ischemic side than that in the non-ischemic side (Figure 6D). Small white arrows indicate examples of the positive cells. Scale bars indicated 50µm. MCAO, middel cerebral artery occlusion.
Discussion
The following new findings were identified in this study: (1) significant KOR upregulation was observed in the ischemic penumbra area after MCAO. (2) SA as a non-opioid and highly-selective KOR agonist reduced infarct volume dose-dependently, protected the vascular integrity and improved the neurological outcome. (3) The underlying mechanism involved in the activation of KOR, inhibition of apoptosis and reduction of inflammation factors such as IL-10 and TNF-alpha.
KOR agonists had a neuroprotective effect for ischemic injury in multiple animal models.(14–16) Rat, mouse, rabbit or piglet hypoxia and ischemia models with pretreatment or post-treatment of KOR agonists were used for the observational studies.(17) Our previous study showed that administration of SA 30 min before, immediately and 30 min after reperfusion preserved cerebrovascular autoregulation and was protective to the neurovascular unit in a piglet HI model.(1–3) Thus, KOR agonist must play a critical role in brain ischemic injury in both animal models. The present study not only further confirms the critical role of KOR agonist, but also serves as an important study to elucidate the related mechanisms.
Our previous study indicated that KOR agonist dilates pial arteries via activation of nitric oxide synthase, adenosine triphosphate-sensitive potassium channels, and opioid receptors.(2) SA administration after global cerebral HI in piglet preserves autoregulation of pial artery via KOR activation and the extracellular-signal-regulated kinases (ERK) activity inhibition.(3) However, activation of ERK (increased ratio of pERK/ERK) may be related to protective effects of preconditioning in the pretreatment of SA on the pial artery autoregulation to hypotension and hypercapnia after HI in a piglet model.(1)
SA was traditionally used as an anti-inflammatory ingredient and previous studies showed that SA could reduce lipopolysaccharide-induced paw edema and formalin-induced inflammation.(18, 19) Here we investigated the possible anti-inflammatory effect of SA in a well-established animal MCAO model. Microglia in the central nervous system can be activated after focal ischemia to serve as a key mediator in immune defense, which was also observed in this study (data not shown). Previous studies have shown that TNF-alpha protein, released from microglia under hypoxic-ischemia,(20) was related to the blood brain barrier (BBB) disruption as it can damage the tight junction.(21) Therefore the protection of BBB shown from Evans blue extravasation observation might be attributed to the inhibition of the production of TNF-alpha released by microglia. In this study, ischemia increased the levels of IL-10, which is a regulatory cytokine limiting the inflammatory response. SA restored the level of IL-10 to levels similar to those seen in the sham group. Based on this observation, SA might intercept the inflammatory initiation at a converging point downstream to serve as an anti-inflammation molecule.
In contrast to necrosis, apoptosis occurs in the ischemic penumbra zone, where reperfused blood flow reduces the degree of hypoxia after several hours or days. Apoptotic induction might be mediated by increasing the pro-apoptotic factors or decreasing the anti-apoptotic factors.(22, 23) In ischemic stroke, the caspase family is important in the brain ischemic injury-induced apoptosis of neuronal cells. Apoptosis happens mostly in neuronal cells instead of in microglia and astrocytes, which is shown by our double immunofluorescence staining. Caspase-3 can be activated through extrinsic and intrinsic signal pathway, and is the main executioner in ischemic stroke animal models.(24, 25) Both immune-staining and western blot showed that administration of SA decreased caspase-3 activation after the brain ischemia. Thus, it is likely that KOR agonist reduced the ischemic injury-induced apoptosis through inactivation of initiator of caspase. The neuroprotection of SA was induced by inhibition of apoptosis to prevent some neurons from transitioning into infarct and by decreasing the overall infarct volume.
Behavioral improvement is the key end point and judgment of the potential of a drug in pre-clinical stroke studies. In this study, animals after MCAO injury showed impaired neurological behavior. After the administration of SA, the neuronal outcome improved dose-dependently.
KOR expression increased in the ischemic penumbra area instead of the core area, suggesting that the KOR played important roles in the process of ischemic brain injury and repair. The activation of KOR might also be related to the survival of the brain tissue in the penumbra instead of core area after MCAO. However, the duration for this endogenous upregulation of KOR and the subsequent pathway needs to be investigated in future studies.
It has been demonstrated that the neurological protective effects of KOR agonists are sex-specific.(26) In this study only male mice were used to avoid the effects of estrogen. Sex-specific effects will be investigated in future studies. Previous studies demonstrated that BRL 52537 (a selective KOR agonist) at certain dosage showed neuroprotection in male but not female rats, which highlighted the importance of using both sexes in animal models of experimental ischemia.(27) Most of the compounds that proved effective in the short term failed in long term clinical observation.(28) We only used short term (24 h) observation for the neurological outcome evaluation. Thus, for validation and potential for clinical translation, long term study is necessary to confirm the neuro-protective effect of KOR agonist. Also, we did not differentiate whether it is protective to ischemia or to the reperfusion injury, which needs a permanent ischemic animal model to induce only ischemic injury.
Conclusion
KORs were up-regulated and played a critical role in regulating brain ischemia and reperfusion. KOR activation could potentially protect and improve neurological outcome in a mouse MCAO model via blood brain barrier protection, apoptosis reduction and inflammation inhibition.
Acknowledgments
Disclosure of funding: This research was supported by NIH K08-GM-093115 (PI: RL), NIH 1R01GM111421 (PI: RL) and funding from The Institute for the Translational Medicine and Therapeutics at the University of Pennsylvania (PI, RL and TA).
Dr. Liu received support for article research from the National Institutes of Health (NIH). Dr. Chen received support for article research from the NIH. Dr. Xi received support for article research from the NIH. Dr. Abel received funding from Elsevier.
Footnotes
Copyright form disclosures: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Su D, Riley J, Armstead WM, et al. Salvinorin A pretreatment preserves cerebrovascular autoregulation after brain hypoxic/ischemic injury via extracellular signal-regulated kinase/mitogen-activated protein kinase in piglets. AnesthAnalg. 2012;114:200–204. doi: 10.1213/ANE.0b013e31823a5d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su D, Riley J, Kiessling WJ, et al. Salvinorin A produces cerebrovasodilation through activation of nitric oxide synthase, kappa receptor, and adenosine triphosphate-sensitive potassium channel. Anesthesiology. 2011;114(2):374–379. doi: 10.1097/ALN.0b013e318204e029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Ma N, Riley J, et al. Salvinorin A administration after global cerebral hypoxia/ischemia preserves cerebrovascular autoregulation via kappa opioid receptor in piglets. PLOSONE. 2012;7:e41724. doi: 10.1371/journal.pone.0041724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 5.Peled-Kamar M, Lotem J, Wirguin I, et al. Oxidative stress mediates impairment of muscle function in transgenic mice with elevated level of wild-type Cu/Zn superoxide dismutase. P Natl A Sci USA. 1997;94:3883–3887. doi: 10.1073/pnas.94.8.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran PM, Higgins LS, Cordell B, et al. Age-related learning deficits in transgenic mice expressing the 751-amino acid isoform of human beta-amyloid precursor protein. P Natl A Sci USA. 1995;92(12):5341–5345. doi: 10.1073/pnas.92.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara A, El Bejaoui S, Seyen S, et al. The usefulness of operant conditioning procedures to assess long-lasting deficits following transient focal ischemia in mice. Behav Brain Res. 2009;205(2):525–534. doi: 10.1016/j.bbr.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Garcia JH, Wagner S, Liu KF, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Hu Q, Yan J, et al. Early inhibition of HIF-1alpha with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobio Dis. 2009;33(3):509–517. doi: 10.1016/j.nbd.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Gursoy-Ozdemir Y, Bolay H, Saribas O, et al. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31(8):1974–1980. doi: 10.1161/01.str.31.8.1974. discussion 1981. [DOI] [PubMed] [Google Scholar]
- 11.Ashwal S, Tone B, Tian HR, et al. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke. 1998;29(5):1037–1046. [PubMed] [Google Scholar]
- 12.Xie GX, Meng F, Mansour A, et al. Primary structure and functional expression of a guinea pig kappa opioid (dynorphin) receptor. P Natl A Sci USA. 1994;91(9):3779–3783. doi: 10.1073/pnas.91.9.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng F, Xie GX, Thompson RC, et al. Cloning and pharmacological characterization of a rat kappa opioid receptor. P Natl A Sci USA. 1993;90(21):9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusumoto K, Mackay KB, McCulloch J. The effect of the kappa-opioid receptor agonist CI-977 in a rat model of focal cerebral ischaemia. Brain Res. 1992;576(1):147–151. doi: 10.1016/0006-8993(92)90621-f. [DOI] [PubMed] [Google Scholar]
- 15.Gueniau C, Oberlander C. The kappa opioid agonist niravoline decreases brain edema in the mouse middle cerebral artery occlusion model of stroke. J Pharmacol Exper Ther. 1997;282(1):1–6. [PubMed] [Google Scholar]
- 16.Zhang Z, Chen TY, Kirsch JR, et al. Kappa-opioid receptor selectivity for ischemic neuroprotection with BRL 52537 in rats. AnesthAnalg. 2003;97(6):1776–1783. doi: 10.1213/01.ANE.0000087800.56290.2E. [DOI] [PubMed] [Google Scholar]
- 17.Chunhua C, Chunhua X, Megumi S, et al. Kappa Opioid Receptor Agonist and Brain Ischemia. Transl Perioper Pain Med. 2014;1(2):27–34. [PMC free article] [PubMed] [Google Scholar]
- 18.Aviello G, Borrelli F, Guida F, et al. Ultrapotent effects of salvinorin A, a hallucinogenic compound from Salvia divinorum, on LPS-stimulated murine macrophages and its anti-inflammatory action in vivo. J Mol Med. 2011;89(9):891–902. doi: 10.1007/s00109-011-0752-4. [DOI] [PubMed] [Google Scholar]
- 19.Capasso R, Borrelli F, Zjawiony J, et al. The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A reduce inflammation-induced hypermotility in mice. Neurogastroen Motil. 2008;20(2):142–148. doi: 10.1111/j.1365-2982.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 20.Deng Y, Lu J, Sivakumar V, et al. Amoeboid microglia in the periventricular white matter induce oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway in hypoxic neonatal rats. Brain Path. 2008;18(3):387–400. doi: 10.1111/j.1750-3639.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries HE, Blom-Roosemalen MC, van Oosten M, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64(1):37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 22.Schwartzman RA, Cidlowski JA. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993;14(2):133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- 23.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nature Rev Mol Cell Bio. 2008;9(3):231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 24.Asahi M, Hoshimaru M, Uemura Y, et al. Expression of interleukin-1 beta converting enzyme gene family and bcl-2 gene family in the rat brain following permanent occlusion of the middle cerebral artery. J Cereb Blood Flow Metab. 1997;17(1):11–18. doi: 10.1097/00004647-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 26.Zeynalov E, Nemoto M, Hurn PD, et al. Neuroprotective effect of selective kappa opioid receptor agonist is gender specific and linked to reduced neuronal nitric oxide. J Cereb Blood Flow Metab. 2006;26(3):414–420. doi: 10.1038/sj.jcbfm.9600196. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, Toung TJ, Hurn PD, et al. Ischemic neuroprotection with selective kappa-opioid receptor agonist is gender specific. Stroke. 2005;36(7):1557–1561. doi: 10.1161/01.STR.0000169928.76321.3d. [DOI] [PubMed] [Google Scholar]
- 28.Warner DS, James ML, Laskowitz DT, et al. Translational Research in Acute Central Nervous System Injury: Lessons Learned and the Future. JAMA Neuro. 2014 doi: 10.1001/jamaneurol.2014.1238. [DOI] [PubMed] [Google Scholar]