Abstract
Rationale
Regulator of G protein signaling (RGS) proteins act as negative modulators of G protein signaling. RGS4 has been shown to negatively modulate G protein signaling mediated by the delta opioid receptor (DOPr) in vitro. However, the role of RGS4 in modulating DOPr-mediated behaviors in vivo has not been elucidated.
Objective
The aim of this study was to compare the ability of the DOPr agonist SNC80 to induce DOPr-mediated antinociception, antihyperalgesia, antidepressant-like effects, and convulsions in wildtype and RGS4 knockout mice.
Methods
Antinociception was assessed in the acetic acid stretch assay. Antihyperalgesia was measured in a nitroglycerin-induced thermal hyperalgesia assay. Antidepressant-like effects were evaluated in the forced swim and tail suspension tests. Mice were also observed for convulsive activity post-SNC80 treatment. SNC80-induced phosphorylation of MAP kinase in striatal tissue from RGS4 wild-type and knockout mice was quantified by Western blot. DOPr number from forebrain tissue was measured using [3H]DPDPE saturation binding.
Results
Elimination of RGS4 potentiated SNC80-induced antinociception and antihyperalgesia. SNC80-induced antidepressant-like effects were potentiated in RGS4 knockout mice in the forced swim test but not the tail suspension test. Additionally, RGS4 knockout did not alter SNC80-induced convulsions. SNC80-induced phosphorylation of MAP kinase was potentiated in striatum from RGS4 knockout mice. Loss of RGS4 did not affect total DOPr number.
Conclusions
Overall, these findings demonstrate that reduction of RGS4 functionally increases the therapeutic index of SNC80. These results provide the first evidence of differential regulation of DOPr-mediated behaviors by RGS proteins and G protein signaling pathways.
Keywords: Delta opioid receptor, Regulator of G protein signaling 4, Mice, Antinociception, Antidepressant, Convulsion
Introduction
Regulator of G protein signaling 4 (RGS4) is a member of the R4 subfamily of RGS proteins and interacts with Gαi/o proteins (Hollinger and Hepler 2002). Like other RGS proteins, RGS4 inhibits G protein signaling by binding Gα and accelerating Gα-mediated GTP hydrolysis. This acceleration of GTP hydrolysis shortens the lifetime of the active Gα-GTP complex and limits signal transduction to downstream effectors. RGS4 is highly expressed in multiple brain regions including the cerebral cortex, amygdala, hippocampus and striatum (Nomoto et al. 1997) and negatively regulates signaling at multiple G protein-coupled receptor (GPCR) types including 5-HT1A (Gu et al. 2007), M3 muscarinic (Blazer et al. 2015), and delta (DOPr) and mu (MOPr) opioid receptors (Wang et al. 2009; Leontiadis et al. 2009).
Opioid receptors are class A GPCRs that couple to Gαi/o proteins. In rodents, activation of DOPr induces antinociception, antihyperalgesia, and antidepressant-like effects without the constipation, respiratory depression, and abuse liability associated with mu opioid receptor agonists (for review see Chu Sin Chung and Kieffer 2013). Some DOPr agonists also produce convulsions, hindering their development as therapeutics (Comer et al. 1993; Hong et al. 1998). Little is known about the signaling molecules and pathways involved in DOPr-mediated behaviors and potential therapeutic effects. DOPr is abundantly expressed in brain regions with high RGS4 expression (Lutz and Kieffer 2013). There are multiple, albeit somewhat discrepant, reports on the role of RGS4 in modulating opioid receptor function. In transfected HEK293 cells, RGS4 is recruited to the plasma membrane upon agonist-induced activation of DOPr or MOPr where it associates with either receptor (Leontiadis et al. 2009). RGS4 also attenuated DOPr- and MOPr-mediated phosphorylation of ERK in those cells. However, in a SH-SY5Y neuroblastoma cell line endogenously expressing RGS4, DOPr, and MOPr, siRNA-induced inhibition of RGS4 potentiated ERK phosphorylation and inhibition of cAMP accumulation mediated by DOPr, but not MOPr (Wang et al. 2009). In vivo, a single dose of the DOPr agonist SNC80 (5 mg/kg) in the mouse forced swim test (FST) decreased immobility to a greater degree in RGS4 knockout mice relative to wild-type mice (Stratinaki et al. 2013), suggesting that RGS4 may also regulate DOPr signaling in vivo; however, its function is not clear based on this single dose experiment. The role of RGS proteins in modulating other DOPr-mediated behaviors has not been fully elucidated. Determining the intracellular signaling pathways that give rise to DOPr-mediated behaviors, and DOPr-mediated convulsions in particular, is critical for the development of DOPr drugs with improved safety and clinical utility. Therefore, to better understand the downstream signaling mechanisms, specifically the role of RGS4, contributing to DOPr-mediated behaviors, we evaluated the effects of the DOPr selective agonist SNC80 to induce antinociception, antihyperalgesia, antidepressant-like effects, and convulsions in wildtype and RGS4 knockout mice.
Materials and Methods
Subjects
The Rgs4tm1Dgen/J mouse strain was obtained from The Jackson Laboratory (Bar Harbor, Maine, http://jaxmice.jax.org/strain/ 005833.html; Cifelli et al. 2008). Mice were backcrossed at least six generations into a C57BL/6 background and maintained in-house as heterozygote harem (1 male, 2 female) breeding groups. Wild-type littermates (+/+) were used as controls in all experiments involving C57RGS4 heterozygote (+/R4) and homozygote (R4/R4) knockout mice. For studies in which transgenic mice were not required, C57BL/6N mice (17-30g) were obtained from Envigo (formerly Harlan, Indianapolis, IN). Mice were housed in groups of two to four animals per cage. Animals were used between 8 and 15 weeks of age at time of experiment and weighed 15-32 g. Mice had free access to standard lab chow and water and were maintained in a temperature- and humidity-controlled environment on a 12-h dark/light cycle with lights on at 7:00 AM. All animal use procedures complied with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health, and were approved by the University of Michigan Institutional Committee on the Use and Care of Animals. Mice were tested only once, and all analyses are between-subject.
Drugs
All drugs were injected at a volume of 10 ml/kg unless otherwise noted. SNC80 ((+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide) was dissolved in 1 M HCl and diluted in sterile water to a concentration of 3% HCl. Naltrindole was dissolved in sterile water. N-methylnaltrexone (Sigma-Aldrich, St. Louis, MO) was dissolved in saline. The RGS4-selective RGS inhibitor CCG-203769 (2-ethyl-4-butyl-1,2,4-thiadiazolidine-3,5-dione) was synthesized as previously described and dissolved in saline (Turner et al. 2012). Nitroglycerin (NTG) was provided by Dr. Adam Lauver (Department of Pharmacology, University of Michigan) and was diluted in saline. Glacial acetic acid (Mallinckrodt Specialty Chemicals, Paris, KY) was diluted in sterile water to a concentration of 0.6% and given as a standard 0.4 ml similar to published methods (Broom et al. 2000, 2002b). All drugs were administered subcutaneously (sc) except for NTG and acetic acid which were given by intraperitoneal (ip) injection.
Acetic Acid Stretch Assay
The acetic acid stretch assay protocol was performed as previously described (Hong et al. 1998; Broom et al. 2000; Broom et al. 2002). Male and female mice were injected with SNC80 (1, 3.2, 10, 32 mg/kg), morphine (0.1, 1 mg/kg), or vehicle and immediately placed in separate cages (18 × 28 × 13 cm). 30 minutes following drug injection, 0.4 ml of 0.6% acetic acid was administered ip. Animals were observed for abdominal stretches for 30 min beginning 5 min after acetic acid administration. Abdominal stretches were characterized by contraction of the abdominal musculature and extension of the hind limbs. CCG-203769 (0.01, 0.1, 1 mg/kg) was administered 3 min after injection of acetic acid (2 min before observation of stretching). Naltrindole (3.2 mg/kg) was injected 30 min prior to, and naltrexone (3.2 mg/kg) and N-methylnaltrexone (10 mg/kg) were injected 10 min prior to SNC80 administration.
Tail Suspension Test
The procedure for the tail suspension test was adapted from Steru et al (1985). Male C57RGS4 mice were given SNC80 (0.32, 1, 3.2, 10 mg/kg) or vehicle. After 30 min, mice were suspended by their tail from a height of ~35cm using self-sticking tape and their behavior was recorded for 6 minutes using a Sony HDR-CX220 digital camcorder. In one experiment for comparison with the forced swim test (described below), mice were given SNC80 (1, 3.2, 10 mg/kg) 60 min prior to the tail suspension test. Videos were analyzed by individuals blind to the experimental conditions, and the time (out of the total 6 min) which the animals spent immobile was quantified. Immobility was defined as the animal remaining motionless or making only minor, non-escape related movements.
Forced Swim Test
The forced swim test was adapted from Porsolt et al (1977). Sixty min after SNC80 (0.1, 0.32, 1, 3.2, 10, 32 mg/kg) or vehicle injection, each mouse was placed in a 4L beaker filled with 15 cm of 25±1°C water and its behavior was recorded for 6 min using a Sony HDR-CX220 digital camcorder. Videos were analyzed by individuals blind to the experimental conditions and the amount of time the animals spent immobile was quantified. Immobility was defined as the mouse not actively traveling through the water and making only movements necessary to stay afloat. The time the mouse spends immobile after the first 30 sec of the assay was recorded.
Nitroglycerin-Induced Hyperalgesia
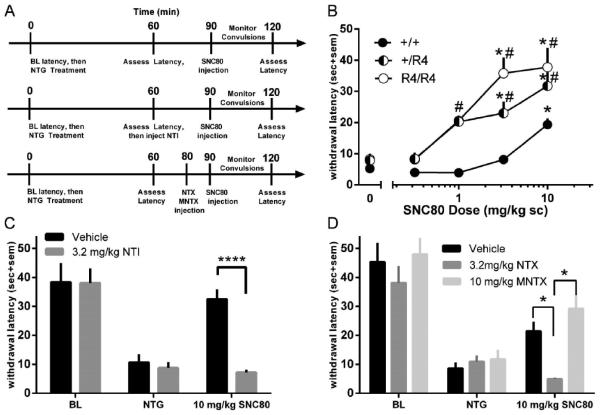
The NTG-induced hyperalgesia assay was adapted from Bates et al (2010) using modifications described in Pradhan et al (2014). Male C57BL6 or male and female C57RGS4 mice were used to evaluate NTG-induced hyperalgesia. Hyperalgesia was assessed by immersing the tail (~5cm from the tip) in a 46°C water bath and determining the latency for the animal to withdraw its tail with a cut-off time of 60 sec. After determining baseline withdrawal latencies, 10 mg/kg NTG (ip) was administered to each animal. Tail withdrawal latency was assessed again 1 hr after NTG administration. At 90 min post-NTG, animals received an injection of SNC80 (0.32, 1, 3.2, 10 mg/kg) or vehicle, and mice were observed continuously in individual cages for 20 min to observe for convulsions (see section below). Tail withdrawal latencies were assessed again 30 min after SNC80 administration. Naltrindole (3.2 mg/kg) was injected 30 min prior to SNC80 administration. Naltrexone (3.2 mg/kg) and N-methylnaltrexone (10 mg/kg) were injected 10 min prior to SNC80 administration (see Fig. 4A).
Figure 4.
(A) Diagrams of NTG-induced hyperalgesia test schedule, depending on antagonist treatment. (B) Effect of different doses of SNC80 on tail withdrawal latency in NTG treated RGS4 wild-type (●) and mutant (◐ +/R4 ; ○ R4/R4) mice. (C) Effect of the DOPr antagonist NTI (gray bar) or vehicle (black bar) on SNC80-induced antihyperalgesia in C57BL6 mice. (D) Effect of pretreatment with vehicle (black bar), 3.2 mg/kg NTX (gray bar), or10 mg/kg MNTX (light gray bar) on SNC80-induced antihyperalgesia in C57BL6 mice. n = 6-10 mice per group for all experiments and data are shown as average per treatment condition with standard error of the mean (sem). * p < 0.05 compared to vehicle or NTX treatment in the same genotype, # p < 0.05 compared to wild-type mice with same drug dose.
SNC80-Induced Convulsions
Mice were observed continuously in individual cages for convulsions. NTG treatment had no effect on the frequency or nature of SNC80-induced convulsions (data not shown). Convulsions were comprised of a tonic phase characterized by sudden tensing of the musculature and extension of the forepaws followed by clonic contractions that extended the length of the body. Convulsions were followed by a period of catalepsy that lasted 2-5 min after which the animals were indistinguishable from untreated controls. Post-convulsion catalepsy was assessed by placing a horizontal rod under the forepaws of the mouse and a positive catalepsy score was assigned if the mouse did not remove its forepaws after 30 sec.
DOPr Saturation Binding and Signaling Assay
RGS4 protein was identified in whole striatum of R4/R4 mice and wild-type littermates by Western blot as compared to purified RGS4 protein with a specific RGS4 antibody as previously described (Wang et al. 2009). α-Tubulin was used as a loading control. For saturation binding assays, mice were decapitated and whole brain or striatum was removed and membranes were freshly prepared as previously described (Broom et al. 2002b). Protein concentrations were determined with a BCA assay kit (Thermo Scientific, Rockford, IL). Specific binding of the DOPr agonist [3H]DPDPE was determined as described using 10μM of the opioid antagonist naloxone to define non-specific binding as described (Broom et al. 2002b). Reactions were incubated for 60 min at 26ºC and stopped by rapid filtration through GF/C filter mats using a MLR-24 harvester (Brandel, Gaithersburg, MD). Bound [3H]DPDPE was determined by scintillation counting and Bmax and Kd values calculated using nonlinear regression analysis with GraphPad Prism version 6.02 (GraphPad, San Diego, CA).
Whole striatal tissues from R4/R4 mice or wild-type littermates were incubated with or without 10 μM SNC80 for 5 min at 37 °C. Samples were prepared and subjected to gel electrophoresis as previously described (Wang et al. 2009). The level of activation of the MAPK pathway was determined by Western blotting with anti-phospho-p44/42MAPK (Thr202/Tyr204) antibody or anti-p44/42 MAPK antibody (Cell Signaling Technology, Danvers, MA). Images were acquired and quantified using an Odyssey FC imaging system (Li-COR Biosciences, Lincoln, NE). MAPK activity was calculated as the ratio of normalized arbitrary units (a.u.) of phosphorylated ERK1/2 over total ERK1/2 and presented as percent of vehicle-treated control.
Data Analysis
Three-way ANOVA was performed using SPSS Statistics 22 (IBM, Armonk, NY). All other data analysis was performed using GraphPad Prism version 6.02 (GraphPad, San Diego, CA). Post hoc analysis was conducted using the Tukey’s post hoc test to correct for multiple comparisons. For all tests, level of significance (α) was set to 0.05. ED50 values were calculated using GraphPad Prism version 6.02 by extrapolating the 50% maximum effect from the straight line analysis of the averaged treatment group data used to generate each dose effect function.
Results
Effects of RGS4 on DOPr-mediated antinociception
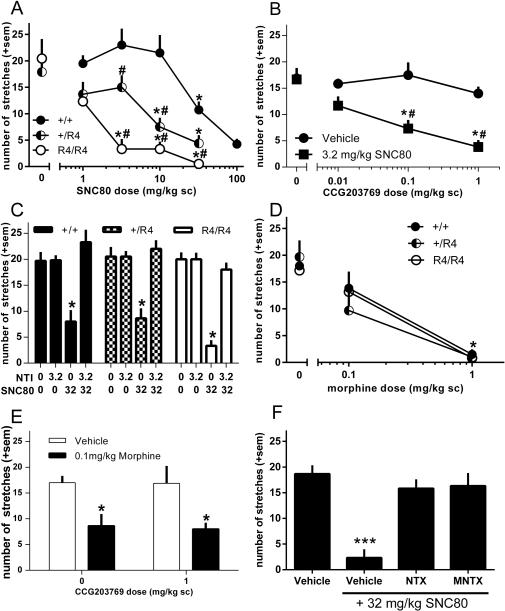
To determine whether RGS4 plays a role in DOPr-mediated antinociception, the effects of the DOPr agonist SNC80 were evaluated in RGS4 wild-type (+/+), heterozygous (+/R4), and homozygous (R4/R4) mutant mice in the acetic acid stretch assay (Figure 1A). Two-way ANOVA revealed a significant interaction (SNC80 dose [1-32 mg/kg only] X genotype, F(8,86) = 3.2, p = 0.0034), as well as significant main effects of SNC80 dose (F(4,86) = 15.4, p < 0.0001) and genotype (F(2,86) = 25.07, p < 0.0001). Overall, the potency of SNC80 to reduce stretching was significantly increased in the RGS4 mutant mice as evidenced by an approximate 6-fold leftward shift in the +/R4 dose effect curve and 27-fold leftward shift in the dose effect curve of the R4/R4 mice (ED50 values: +/+: 41 mg/kg; +/R4: 6.9 mg/kg; R4/R4: 1.5 mg/kg).
Figure 1.
Effects of RGS4 on opioid-mediated antinociception in the mouse acetic acid stretch assay. Number of acetic acid-induced stretches after treatment with (A) different doses of SNC80 in RGS4 wild-type (●) and mutant (◐ +/R4 ; ○ R4/R4) mice, (B) different doses of the RGS4 inhibitor CCG-203769 in combination with an inactive SNC80 dose (3.2 mg/kg, ■) or SNC80 vehicle (●) (in C57BL6 wild-type mice, (C) SNC80 following pretreatment with 3.2 mg/kg of the DOPr antagonist naltrindole in RGS4 wild-type (filled bar) and mutant mice (checked bar +/R4, open bar R4/R4), (D) the MOPr agonist morphine in RGS4 wild-type (●) and mutant (◐ +/R4 ; ○ R4/R4) mice, (E) different doses of the RGS4 inhibitor CCG-203769 in combination with a low dose of morphine (filled bar) or vehicle (open bar), (F) SNC80 following pretreatment with 3.2 mg/kg of the opioid antagonist naltrexone or 10 mg/kg of the peripherally-restricted opioid antagonist N-methylnaltrexone. n = 6-9 mice per group for all experiments and data are shown as average per treatment condition with standard error of the mean (sem). * p < 0.05 compared to vehicle treatment in the same genotype, *** p < 0.001 compared to vehicle treatment, # p < 0.05 compared to wild-type mice or control condition with same drug dose.
Because elimination or reduced levels of RGS4 enhanced SNC80-induced antinociception, we evaluated the effects of pharmacological inhibition of RGS4 with CCG-203769 (Blazer et al. 2015) on SNC80-induced antinociception in C57BL6 wild-type mice (Figure 1B). Two-way ANOVA revealed a significant interaction (SNC80 dose X CCG-203769 dose, F(3,42) = 3.9, p = 0.02) and significant main effects of SNC80 dose (F(1,42) = 24.3, p < 0.0001) and CCG-203769 dose (F(3,42) = 6.9, p = 0.0007). Administration of either an ineffective SNC80 dose (3.2 mg/kg) or various doses of CCG-203769 alone did not alter stretching behavior; however, CCG-203769 dose-dependently enhanced antinociception produced by 3.2 mg/kg SNC80, such that the combination of 3.2 mg/kg SNC80 with either 0.1 mg/kg or 1 mg/kg CCG-203769 significantly reduced stretching.
To assess the role of DOPr in SNC80-induced antinociception in the acetic acid stretch assay, RGS4 knockout mice were pretreated with the DOPr-selective antagonist naltrindole (Figure 1C). Three-way ANOVA revealed a significant main effect of SNC80 (F(1,63) = 38.7, p < 0.0001) and a trend towards a significant SNC80 X genotype interaction (F(2,63) = 2.5, p = 0.087). SNC80-induced reduction of stretching was completely blocked upon pretreatment with 3.2 mg/kg naltrindole (SNC80 dose X naltrindole dose, F(1,63) = 52.2, p < 0.0001), indicating a DOPr-mediated effect. This effect of naltrindole did not differ between RGS4 wild-type and mutant mice.
To investigate whether the effects of SNC80 in the acetic acid stretch assay were peripherally- or centrally-mediated, C57BL6 wild-type mice were pretreated with the nonspecific opioid antagonist naltrexone or its peripherally-limited analog N-methylnaltrexone in the acetic acid stretch assay (Figure 1D). Administration of 32 mg/kg SNC80 significantly reduced stretching in vehicle-pretreated animals, but pretreatment with either 3.2 mg/kg naltrexone or 10 mg/kg N-methylnaltrexone significantly attenuated the SNC80-induced reduction in stretching (one-way ANOVA: F(3,20) = 14.6, p < 0.0001).
To determine whether the role of RGS4 in opioid-induced antinociception was limited to DOPr-mediated antinociception, the mu opioid receptor agonist actions of morphine were evaluated in RGS4 transgenic mice in the acetic acid stretch assay (Figure 1E). The main effect of morphine dose was significant (F(2,45) = 28.5, p < 0.0001) but there was no main effect of genotype and no morphine dose X genotype interaction. Increasing doses of morphine produced similar decreases in stretching in RGS4 +/+, +/R4, and R4/R4 mice. In addition, pharmacological inhibition of RGS4 by CCG-203769 did not alter the effects of morphine in the acetic acid stretch assay (Figure 1F). Treatment of C57BL6 wild-type mice with 0.1 mg/kg morphine significantly reduced stretching relative to vehicle treated controls (F(1,26) = 13.2, p = 0.001) and this decrease was not enhanced by administration of 1 mg/kg CCG-203769.
Effects of RGS4 on DOPr-mediated antidepressant-like effects
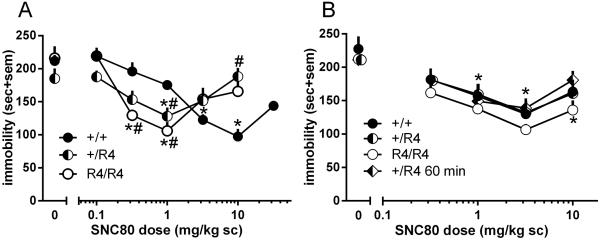
The effects of SNC80 on immobility time in the forced swim test were evaluated in RGS4 +/+, +/R4, and R4/R4 mice (Figure 2A). Two-way ANOVA revealed a significant interaction (SNC80 dose [0.1-10 mg/kg only] X genotype, F(10,102) = 6,1, p < 0.0001) and a significant main effect of SNC80 dose (F(5,102) = 17.1, p < 0.0001), but no effect of genotype. In all three genotypes, SNC80 produced a U-shaped dose effect curve with similar magnitude of maximum effect, albeit at different doses (+/+: 10 mg/kg, +/R4 and R4/R4: 1 mg/kg). Both the descending and ascending limbs of this U-shaped curve were shifted to the left in both the +/R4 and R4/R4 mice, indicating an increase in the potency, but not efficacy, of SNC80.
Figure 2.
Effects of RGS4 on DOPr-mediated antidepressant-like effects. Immobility scores of RGS4 wild-type (●) and mutant (◐ +/R4 ; ○ R4/R4) mice following treatment with different doses of SNC80 in the (A) forced swim test, and (B) tail suspension test (with ◩ indicating SNC80 given as 60 min prior to TST). n = 6-10 mice per group and data are shown as average per treatment condition with standard error of the mean (sem). * p < 0.05 compared to vehicle treatment in the same genotype, # p < 0.05 compared to wild-type mice with same drug dose.
To further probe the role of RGS4 in DOPr-mediated antidepressant-like effects, the effects of SNC80 were evaluated in the tail suspension test (TST) in RGS4 +/+, +/R4, and R4/R4 mice (Figure 2B). Doses up to 3.2 mg/kg SNC80 produced dose-dependent decreases in immobility in RGS4 +/+, +/R4, and R4/R4 mice; however, the largest dose tested failed to further decrease immobility. The effect of SNC80 dose was significant (F(4,98) = 18.2, p < 0.0001) but there was only a non-significant trend for genotype (F(2,98) = 2.8, p = 0.07) and a non-significant SNC80 dose X genotype interaction. Although the magnitude of effect of SNC80 on immobility reduction was slightly greater in the R4/R4 mice, this effect was not statistically significant. There was no difference in the magnitude of effect or shape of the dose effect curve in RGS4 heterozygous mutant mice treated with SNC80 30 or 60 minutes prior to the tail suspension test.
Effects of RGS4 on DOPr-mediated convulsions
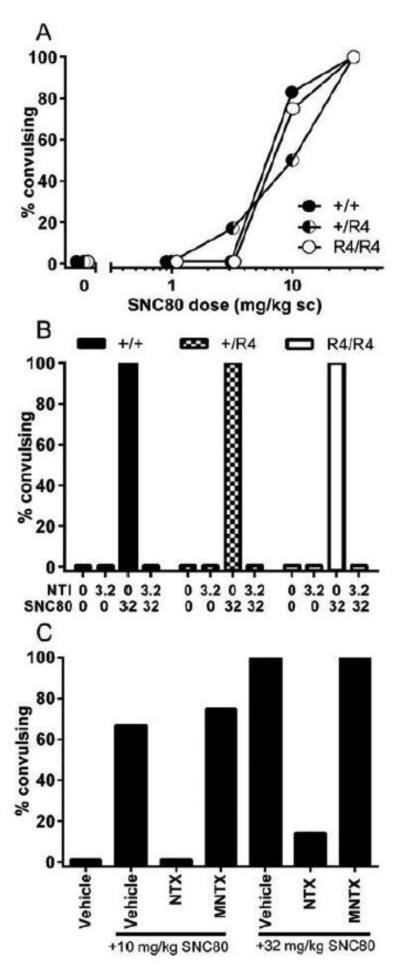
SNC80 produced dose-dependent increases in convulsion frequency in RGS4 +/+, +/R4, and R4/R4 mice (Figure 3A). These convulsions were blocked by pretreatment with 3.2 mg/kg naltrindole in all genotypes (Figure 3B). There were no significant differences in the frequency, time of onset, and duration (data not shown) of convulsions in the +/R4 or R4/R4 animals relative to wild-type littermates for a given dose of SNC80. There were also no differences between genotypes in the frequency of convulsions produced by the chemical convulsant pentylenetetrazol (data not shown).
Figure 3.
Frequency of SNC80-induced convulsions (A) in RGS4 wild-type (●) and mutant (◐ +/R4 ; ○ R4/R4) mice, (B) following pretreatment with 3.2 mg/kg of the DOPr antagonist naltrindole in RGS4 wild-type (filled bar) and mutant (checked bar +/R4, open bar R4/R4) mice, (C), following pretreatment with 3.2 mg/kg of the opioid antagonist naltrexone or 10 mg/kg of the peripherally-restricted opioid antagonist N-methylnaltrexone in C57BL6 wild-type mice. n = 6-12 mice per group for all experiments and data are shown as average per treatment condition with standard error of the mean (sem).
To evaluate whether SNC80-induced convulsions are centrally-mediated, C57BL6 wild-type mice were pretreated with the nonspecific opioid antagonist naltrexone or its peripherally-restricted analog N-methylnaltrexone (Figure 3C). 10 mg/kg and 32 mg/kg SNC80 produced convulsions that were blocked by pretreatment with naltrexone but not N-methylnaltrexone.
Effects of RGS4 on DOPr-mediated antihyperalgesia
In order to determine if RGS4 can modulate DOPr-mediated antihyperalgesia, the potency of SNC80 to reverse NTG-induced thermal hyperalgesia in RGS4 +/+, +/R4, and R4/R4 mice was assessed (Figure 4B). There were no differences between genotypes in the baseline tail withdrawal latencies prior to NTG treatment (+/+: 39.1 ± 2.4 s, +/R4: 43.3 ± 2.2 s, R4/R4: 42.4 ± 2.3 s). Administration of 10 mg/kg NTG (ip) significantly decreased tail withdrawal latency to a similar degree in all genotypes (+/+: 7.4 ± 1.2 s, +/R4: 9.0 ± 1.1 s, R4/R4: 8.3 ± 0.6 s). Reduction of RGS4 enhanced the ability of SNC80 to increase tail withdrawal latency. Two-way ANOVA revealed a significant interaction (SNC80 dose X genotype, F(8,92) = 2.6, p = 0.01), as well as significant main effects of SNC80 dose (F(4,92) = 25.3, p < 0.0001) and genotype (F(2,92) = 20.9, p < 0.0001). This enhanced effect of SNC80 resulted in a pronounced leftward shift (~10-fold) in the dose response curve, indicating a significant increase in the potency of SNC80.
To evaluate the role of DOPr in SNC80-induced antihyperalgesia, C57BL6 wild-type mice were pretreated with the DOPr selective antagonist naltrindole (Figure 4C). Two-way repeated measure ANOVA revealed a significant interaction (NTI dose X time point, F(2,20) = 8.7, p = 0.01), and a significant main effect of NTI dose (F(1,10) = 5.0, p = 0.05), indicating a DOPr-mediated effect. To evaluate the relative contributions of central and peripheral opioid receptors to SNC80-induced antihyperalgesia, C57BL6 wild-type mice were pretreated with the nonselective opioid antagonist naltrexone or the peripherally-limited analog N-methylnaltrexone (Figure 4D). Two-way repeated measure ANOVA revealed a significant interaction (pretreatment X time point, F(4,32) = 3.0, p = 0.03) and a significant main effect of pretreatment (F(2,16) = 4.4, p = 0.03). Post-hoc analysis further revealed that SNC80-induced increases in tail withdrawal latency were blocked by naltrexone (p < 0.01) but not by N-methylnaltrexone.
Elimination of RGS4 enhances DOPr signaling but does not affect DOPr number
To evaluate the effect of RGS4 on DOPr signaling in brain, whole striatum of R4/R4 mice or their wild-type littermate controls were treated with the DOPr agonist SNC80 (1.0 μM) and phosphorylation of ERK1/2 was examined. This concentration of SNC80 did not increase ERK1/2 phosphorylation in striatum as compared with vehicle treatment in RGS4 +/+ mice but caused a marked increase in ERK1/2 phosphorylation as compared with vehicle in striatal tissue from R4/R4 knockout mice (125 ± 67%) (Fig. 5B).
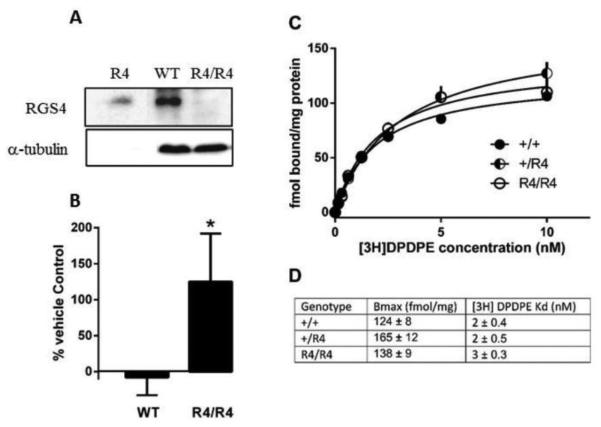
Figure 5.
(A) RGS4 protein expression by Western blot. Lysates were prepared from striatal brain tissue from wildtype and R4/R4 mice (middle and right lanes, respectively) as compared with RGS4 purified protein (left lane). (B) SNC80-mediated stimulation of ERK1/2 phosphorylation over vehicle control in striatal tissue from wild-type and R4/R4 mice (t(5.4)=1.9, p=0.05). (C) Saturation binding of [3H]DPDPE to membranes prepared from forebrains of RGS4 wild-type (●) or mutant (◐ +/R4 ; ○ R4/R4) mice. Points represent data averaged (shown with standard error of the mean) from 3-4 mice, each assayed in triplicate. (D) Calculated Bmax and Kd values.
Western blot analysis confirmed a lack of RGS4 protein expression in the RGS4 knockout mice (Fig. 5A). However, it is possible that the enhanced behavioral effects of SNC80 in RGS4 mutant mice are due to elevated density or agonist affinity of DOPr relative to their wild-type littermates. Saturation binding with the radiolabeled DOPr agonist [3H]DPDPE was performed with brain tissue from RGS4 +/+, +/R4, and R4/R4 mice to assess potential changes in DOPr density and agonist affinity (Fig. 5C). There were no significant differences in total receptor number between the three genotypes (wild-type: 124 ± 8 fmol/mg, +/R4: 165 ± 12 fmol/mg, R4/R4: 138±9 fmol/mg). There were also no changes in the Kd of [3H]DPDPE for DOPr in the RGS4 mutant mice (Fig. 5D).
Discussion
In this report, we demonstrate that RGS4 differentially regulates DOPr-mediated behaviors acting as a negative regulator of some, but not all, behavioral outcomes. In the acetic acid stretch assay (antinociception), SNC80 alleviated acid-induced stretches at a relatively large dose (32 mg/kg) consistent with previous reports (Broom et al. 2002b; Gallantine and Meert 2005; Negus et al. 2012). Large doses of SNC80 (10 and 32 mg/kg) were also required to reverse NTG-induced thermal hyperalgesia, consistent with the findings of Pradhan et al. (2014). SNC80-induced antinociception, but not hyperalgesia, was blocked by pretreatment with the peripherally-restricted opioid antagonist N-methylnaltrexone, suggesting peripheral DOPr likely mediate the antinociceptive actions of SNC80 in the acetic acid stretch assay while central DOPr mediated SNC80-induced antihyperalgesia. Genetic loss of RGS4 or acute pharmacological inhibition of RGS4 with CCG-203769 increased the potency of SNC80 to produce antinociception and antihyperalgesia. The fact that significant changes are seen in both the heterozygote (+/R4) and homozygote (R4/R4) knockout mice suggests that DOPr-mediated signaling is exquisitely sensitive to the actions of RGS4. Overall, these observations suggest that central and peripheral DOPr within various pain pathways and neurocircuits are likely colocalized with RGS4 proteins to modulate DOPr signaling and DOPr-induced pain relief in vivo.
Interestingly, pharmacological inhibition or genetic alterations of RGS4 did not alter the potency of the MOPr agonist morphine to produce antinociception in the acetic acid stretch assay, similar to other reports in vivo (Han et al. 2010) and in SHSY5Y cells endogenously expressing opioid receptors and RGS4 proteins (Wang et al. 2009). However, RGS4 proteins have been shown to alter MOPr signaling in vitro and in vivo, depending on the cell type, brain region, or MOPr agonist used. For example, RGS4 was shown to regulate the rewarding effects of morphine and the antinociceptive effects of the MOPr agonists methadone and fentanyl but not morphine (Han et al. 2010). In addition, in heterologous expression systems, RGS4 can regulate MOPr signaling (Leonidas et al. 2009; Talbot et al. 2010). Together, these data suggest that RGS4 proteins may have the capacity to regulate MOPr-mediated signaling and behaviors as long as the appropriate proteins are co-expressed in the necessary cells and/or circuits.
It has previously been shown that loss of RGS4 altered SNC80 action in the mouse forced swim test (Stratinaki et al. 2013); however, this report only examined a single dose of SNC80 (5 mg/kg) in one behavioral assay making it difficult to fully assess the role of RGS4 in SNC80-induced antidepressant-like effects. This report sought to expand on those findings and shows that RGS4 plays a complex role in regulating DOPr-mediated antidepressant-like effects, depending on the type of assay employed. In wild-type mice, SNC80 produced decreases in immobility in the forced swim and tail suspension tests, consistent with previous work showing that DOPr agonists produce antidepressant-like effects in these assays (Broom et al. 2002a; Naidu et al. 2007; Saitoh et al. 2011). In both assays, SNC80 produced U-shaped dose effect curves, such that larger doses of SNC80 (10 mg/kg in TST, 32 mg/kg in FST) failed to produce significant antidepressant-like effects. The lack of effect observed with large SNC80 doses is possibly due to competing behaviors, such as possible recovery from recent seizure events or excessive locomotor stimulation (Chu Sin Chung et al. 2015). In the forced swim test, elimination of RGS4 shifted the entire U-shaped function to the left, indicating that RGS4 acts as a negative regulator of DOPr-mediated antidepressant-like effects (as well as any possible competing behaviors) in this assay. However, loss of RGS4 activity had no significant effect on SNC80-induced antidepressant-like effects in the tail suspension test. It is unlikely that different SNC80 pretreatment times were responsible for the different effects of RGS4 in the TST and FST because even longer pretreatments (60 min) in the TST did not alter the effects of SNC80 in RGS4 heterozygous knockout mice. Also, relatively similar doses of SNC80 produced antidepressant-like effects in both assays, so it is unlikely that differences in receptor occupancy or efficacy requirement could account for this disparity between the TST and FST. Differences in drug responses between these two behavioral assays have been reported previously (for review see, Cryan et al. 2005), and it has been argued that the biological substrates underlying these behaviors may be distinct. Therefore, it is possible that the behavioral effects of SNC80 in the two assays may be governed by separate brain regions, behavioral mechanisms, and/or signaling pathways that are differentially dependent on RGS4 signaling.
In contrast to the role of RGS4 in antinociception, antihyperalegsia, and antidepressant-like effects in the FST (but not the TST), reductions in RGS4 did not alter the potency of SNC80 to induce convulsions. The ability of RGS4 to alter a behavioral endpoint does not appear to be correlated with the potency of SNC80, since similar doses of SNC80 were required to produce convulsions, antinociception, and antihyperalgesia in wild-type mice. Alternatively, if we measured more subtle electroencephalographic activity rather than overt convulsive behavior (Jutkiewicz et al. 2006), we may have been able to observe an effect of RGS4 on this endpoint and future studies will evaluate this. In the present study, convulsions were evaluated in mice that received injections of NTG to induce hyperalgesia in an attempt to reduce the number of animals used. While NTG did not alter the frequency or nature of SNC80-induced convulsions in wild-type mice, it is possible that NTG masked or altered the dependency of convulsions on RGS4. Nevertheless, these results suggest that RGS4 selectively regulates signaling pathways mediating different behavioral outcomes of DOPr activation and is likely not involved in the signaling mechanisms mediating convulsions.
RGS proteins function as negative regulators of G protein signaling by binding Gα-GTP and accelerating Gα-mediated GTP hydrolysis which returns Gα to an inactive state. Loss of RGS function should prolong the lifetime of active Gα and increase downstream signaling. Consistent with this theory, the present study demonstrated that loss of RGS4 potentiated DOPr-mediated: 1) phosphorylation of ERK1/2 in mouse striatal tissue, 2) peripheral antinociception, 3) central antihyperalgesia, and 4) antidepressant-like effects measured in the FST, likely due to prolongation of DOPr-mediated G protein signaling and amplification of downstream effectors. These findings identify some specific DOPr-mediated behaviors and downstream signaling molecules in certain brain regions that are regulated by RGS4. However, DOPr-mediated convulsions and behaviors in the TST were not significantly altered by reductions in RGS4, suggesting that different signaling pathways may underlie these behaviors. DOPr and RGS4 may not be expressed in the same neurons within circuits mediating these behavioral outcomes. For example, while DOPr and RGS4 are both highly expressed in the hippocampus, the proposed origin site of convulsions (Simmons and Chavkin 1996; Chung et al. 2015), they may not be co-expressed in the same cells and, therefore, may not functionally interact. It is also possible that RGS proteins other than RGS4 modulate the DOPr-induced convulsive effects and behaviors in the TST. Alternatively, these specific DOPr-mediated behaviors may be generated by a G protein-independent, arrestin-mediated signaling mechanism (Violin 2014). Previous studies demonstrated that DOPr activation leads to signaling through G protein-dependent and - independent pathways (Bradbury et al. 2009; Charfi et al. 2014; Charfi et al. 2015); however, there are few reports connecting these distinct signaling mechanisms to specific behavioral outputs (Chiang et al. 2016; Pradhan et al. 2016). In conclusion, this study demonstrates that RGS4 differentially regulates SNC80-induced behaviors, suggesting that different molecular or cellular signaling pathways or neurocircuitry mediate these behavioral outcomes. Future work will investigate the role of other RGS proteins in DOPr-mediated convulsions and the underlying signaling mechanism and pathways mediating the convulsive and other behavioral effects of DOPr agonists.
Acknowledgments
Funding and Disclosure
Part of this work was funded by a PhRMA Foundation Research Starter Grant awarded to EMJ and funds from the University of Michigan Medical School. She has consulted for Trevena, Inc in 2011-2012 with compensation. JRT has no conflicts and is supported by National Institute on Drug Abuse grant DA 035316. This study was also supported in part by the Intramural Research Program of the National Institute of Alcohol Abuse and Alcoholism and by the National Institute on Drug Abuse (KCR). RRN is founder and owner of Argessin LLC which holds a license for small molecule RGS inhibitors and is supported by NIH DA RO1 023252.
References
- Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptáček Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30:170–178. doi: 10.1111/j.1468-2982.2009.01864.x. doi: 10.1111/j.1468-2982.2009.01864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer LL, Storaska AJ, Jutkiewicz EM, Turner EM, Calcagno M, Wade SM, Wang Q, Huang XP, Traynor JR, Husbands SM, Morari M, Neubig RR. Selectivity and anti-Parkinson’s potential of thiadiazolidinone RGS4 inhibitors. ACS Chem Neurosci. 2015;6:911–919. doi: 10.1021/acschemneuro.5b00063. doi: 10.1021/acschemneuro.5b00063. [DOI] [PubMed] [Google Scholar]
- Bradbury FA, Zelnik JC, Traynor JR. G protein independent phosphorylation and internalization of the delta-opioid receptor. J Neurochem. 2009;109:1526–1535. doi: 10.1111/j.1471-4159.2009.06082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom DC, Guo L, Copp A, Husbands SM, Lewis JW, Woods JH, Traynor JR. BU:48 a novel buprenorphine analog that exhibits δ-opioid-mediated convulsions but not δ-opioid-mediated antinociception in mice. J Pharmacol Exp Ther. 2000;294:1195–1200. [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002a;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. doi:10.1038/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002b;303:723–729. doi: 10.1124/jpet.102.036525. doi:10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Charfi I, Nagi K, Mnie-Filali O, Thibault D, Balboni G, Schiller PW, Trudeau LE, Pineyro G. Ligand- and cell-dependent determinants of internalization and cAMP modulation by delta opioid receptor (DOR) agonists. Cell Mol Life Sci. 2014;71:1529–1546. doi: 10.1007/s00018-013-1461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charfi L, Audet N, Bagheri Tudashki H, Pineyro G. Identifying ligand-specific signaling within biased responses: focus on d opioid receptor ligands. Br J Pharmacol. 2015;172:435–448. doi: 10.1111/bph.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, De Costa BR, Mosberg HI, Woods JH. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267:888–895. [PubMed] [Google Scholar]
- Chiang T, Sansuk K, van Rijn RM. β-Arrestin 2 dependence of δ opioid receptor agonists is correlated with alcohol intake. Br J Pharmacol. 2016;173:332–343. doi: 10.1111/bph.13374. doi: 10.1111/bph.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Sin Chung P, Keyworth HL, Martin-Garcia E, Charbogne P, Darcq E, Bailey A, Filliol D, Matifas A, Scherrer G, Ouagazzal AM, Gaveriaux-Ruff C, Befort K, Maldonado R, Kitchen I, Kieffer BL. A novel anxiogenic role for the delta opioid receptor expressed in GABAergic forebrain neurons. Biol Psychiatry. 2015;77:404–415. doi: 10.1016/j.biopsych.2014.07.033. doi: 10.1016/j.biopsych.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Sin Chung P, Kieffer BL. Delta opioid receptors in brain function and disease. Pharmacol Ther. 2013;140:112–120. doi: 10.1016/j.pharmthera.2013.06.003. doi: 10.1016/j.pharmthera.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung PC, Boehrer A, Stephan A, Matifas A, Scherrer G, Darcq E, Befort K, Kieffer BL. Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures. Behav Brain Res. 2015;278:429–434. doi: 10.1016/j.bbr.2014.10.029. doi: 10.1016/j.bbr.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifelli C, Rose RA, Zhang H, Voigtlaender-Bolz J, Bolz SS, Backx PH, Heximer SP. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103:527–35. doi: 10.1161/CIRCRESAHA.108.180984. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci and Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of mu- opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. DOI: 10.1111/j.1742-7843.2005.pto_97107. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Yan Z. RGS4 modulates serotonin signaling in prefrontal cortex and links to serotonin dysfunction in a rat model of schizophrenia. Mol Pharmacol. 2007;71:1030–1039. doi: 10.1124/mol.106.032490. doi:10.1124/mol.106.032490. [DOI] [PubMed] [Google Scholar]
- Han MH, Renthal W, Ring RH, Rahman Z, Psifogeorgou K, Howland D, Birnbaum S, Young K, Neve R, Nestler EJ, Zachariou V. Brain region specific actions of regulator G protein signaling 4 oppose morphine reward and dependence but promote analgesia. Biol Psychiatry. 2010;67:761–769. doi: 10.1016/j.biopsych.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Rice KC, Calderon SN, Woods JH, Traynor JR. Convulsive behavior of nonpeptide δ-opioid ligands: comparison of SNC80 and BW373U86 in mice. Analgesia. 1998;3:269–276. [Google Scholar]
- Jutkiewicz EM, Baladi MG, Folk JE, Rice KC, Woods JH. The convulsive and electroencephalographic changes produced by nonpeptidic δ-opioid agonists in rats: comparison with pentylenetetrazol. J Pharmacol Exp Ther. 2006;317:1337–1348. doi: 10.1124/jpet.105.095810. [DOI] [PubMed] [Google Scholar]
- Leontiadas LJ, Papakonstantinou MP, Georgoussi Z. Regulator of G protein signaling 4 confers selectivity to specific G proteins to modulate mu- and delta-opioid receptor signaling. Cell Signal. 2009;21:1218–1228. doi: 10.1016/j.cellsig.2009.03.013. doi: 10.1016/j.cellsig.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Lichtman AH, Archer CC, May EL, Harris LS, Aceto MD. NIH 11082 produces antidepressant-like activity in the mouse tail-suspension through a delta opioid receptor mechanism of action. Eur J Pharmacol. 2007;566:132–136. doi: 10.1016/j.ejphar.2007.03.031. doi:10.1016/j.ejphar.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rosenberg MB, Altarifi AA, O’Connell RH, Folk JE, Rice KC. Effects of the δ opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain. 2012;13:317–327. doi: 10.1016/j.jpain.2011.12.003. doi: 10.1016/j.jpain.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto S, Adachi K, Yang LX, Hirata Y, Muragachi S, Kiuchi K. Distribution of RGS4 mRNA in mouse brain shown by in situ hybridization. Biochem Biophys Res Commun. 1997;241:281–287. doi: 10.1006/bbrc.1997.7802. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Zyuzin J, Charles A. δ-opioid receptor agonists inhibit migraine related hyperalgesia, aversive state, and cortical spreading depression in mice. Br J Pharmacol. 2014;171:2375–2384. doi: 10.1111/bph.12591. doi: 10.1111/bph.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Perro J, Walwyn WM, Smith ML, Vicente-Sanchez A, Segura L, Bana A, Keiffer BL, Evans CJ. Agonist-specific recruitment of arrestin isforms differentially modify delta opioid receptor function. J Neurosci. 2016;36:3541–3551. doi: 10.1523/JNEUROSCI.4124-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, Nagase H, Yamada M. The novel δ opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011;223:271–279. doi: 10.1016/j.bbr.2011.04.041. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. Endogenous opioid regulation of hippocampal function. Int Rev Neurobiol. 1996;39:145–196. doi: 10.1016/s0074-7742(08)60666-2. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stratinaki M, Varidaki A, Mitsi V, Ghose S, Magida J, Dias C, Russo SJ, Vialou V, Caldarone BJ, Tamminga CA, Nestler EJ, Zachariou V. RGS4 is a crucial modulator of antidepressant drug action in depression and neuropathic pain models. Proc Natl Acad Sci USA. 2013;110:8254–8259. doi: 10.1073/pnas.1214696110. doi: 10.1073/pnas.1214696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JN, Roman DL, Clark MJ, Roof RA, Tesmer JJ, Neubig RR, Traynor JR. Differential modulation of my-oioid receptor signaling to adenylyl cyclase by regulators of G proteins 4 or 8 and 7 in permeabilised C6 cells is Galpha subtype dependent. J Neurochem. 2010;112:1026–1034. doi: 10.1111/j.1471-4159.2009.06519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner EM, Blazer LL, Neubig RR, Husbands SM. Small molecule inhibitors of regulator of G protein signalling (RGS) proteins. ACS Med Chem Lett. 2012;9:146–150. doi: 10.1021/ml200263y. DOI: 10.1021/ml200263y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin JD. Biased ligands at mu and delta opioid receptors: targeting selective signalling to develop improved therapeutics; International narcotic research conference.2014. 2014. p. 22. [Google Scholar]
- Wang Q, Liu-Chen LY, Traynor JR. Differential modulation of mu- and delta-opioid receptor agonists by endogenous RGS4 protein in SH-SY5Y cells. J Biol Chem. 2009;284:18357–18367. doi: 10.1074/jbc.M109.015453. doi: 10.1074/jbc.M109.015453. [DOI] [PMC free article] [PubMed] [Google Scholar]