Abstract
The connotation of “nausea” has changed across several millennia. The medical term ‘nausea’ is derived from the classical Greek terms ναυτια and ναυσια, which designated the signs and symptoms of seasickness. In classical texts, nausea referred to a wide range of perceptions and actions, including lethargy and disengagement, headache (migraine), and anorexia, with an awareness that vomiting was imminent only when the condition was severe. However, some recent articles have limited the definition to the sensations that immediately precede emesis. Defining nausea is complicated by the fact that it has many triggers, and can build-up slowly or rapidly, such that the prodromal signs and symptoms can vary. In particular, disengagement responses referred to as the “sopite syndrome” are typically present only when emetic stimuli are moderately provocative, and do not quickly culminate in vomiting or disengagement from the triggering event. This review considers how the definition of “nausea” has evolved over time, and summarizes the physiological changes that occur prior to vomiting that may be indicative of nausea. Also described are differences in the perception of nausea, as well as the accompanying physiological responses, that occur with varying stimuli. This information is synthesized to provide an operational definition of nausea.
Keywords: nausea, vomiting, emesis, sopite syndrome, seasickness
1. Introduction
Although the expulsion of gastric contents through vomiting is easy to recognize, the sequelae of physiological responses that precede this response are less clear-cut. Money divided the physiological events associated with vomiting into two categories: stomach-emptying responses and stress responses (Money, 1970; Money et al., 1996). The latter term has mainly been used to describe responses such as cold-sweating (Cheung et al., 2011; Hemingway, 1944; Nobel et al., 2012; Sclocco et al., 2015) and pallor (Cassano et al., 1989; Kolev et al., 1997) that often occur following exposure to emetic stimuli, which are mediated through the sympathetic nervous system (Hammam et al., 2012). However, other physiological changes may be associated with the pre-emetic stress response, including alterations in heart rate variability (Doweck et al., 1997; Kim et al., 2011; Kim et al., 2005) and a release of vasopressin from the posterior pituitary (Fisher et al., 1982; Robertson, 1976; Rowe et al., 1979; Sorensen et al., 1985). Along with these physiological responses are a number of perceptions with accompanying behavioral changes (Graybiel et al., 1968; Muth et al., 1996), including an awareness that stomach emptying is imminent, loss of appetite (Farmer et al., 2015; Heer et al., 2006; Hiura et al., 2012; Lackner, 2014; Sanger et al., 2013), anxiety and foreboding (Coelho et al., 2015; Fox et al., 1988; Lackner, 2014; Tarbell et al., 2014), as well as lethargy and disinterest in engaging in routine activities (Graybiel et al., 1976; Lackner, 2014; Lawson et al., 1998; Matsangas et al., 2014; Van Ombergen et al., 2015). The latter disengagement responses were first documented by Graybiel (Graybiel et al., 1976), and later described by others, who referred to them as the “sopite syndrome” (Graybiel et al., 1976; Lackner, 2014; Lawson et al., 1998; Matsangas et al., 2014; Van Ombergen et al., 2015).
Nausea is the term that is commonly-used to describe an awareness that vomiting is imminent. However, individuals often experience nausea without vomiting (Horn, 2014; Lackner, 2014). Moreover, individuals describe nausea in various ways (Graybiel et al., 1968; Muth et al., 1996), in accordance with the wide range of perceptions that can occur prior to vomiting. For example, over 30 definitions of nausea were provided in a recent book on the topic (Stern et al., 2011).
Nausea and emesis have a number of etiologies, including motion-related signals that deviate from those expected, administration of toxins, exposure to radiation, migraine, gastrointestinal disease, pregnancy (hormonal changes), and even psychological stimuli (e.g., stress and extreme emotional reactions, as well as classically conditioned smell and taste aversions) (Feyer et al., 2014; Hederos, 1992; Heer et al., 2006; Horn et al., 2014; Kenward et al., 2015; Matthews et al., 2014; Olden et al., 2005; Singh et al., 2016; Sugino et al., 2015; Wiesmann et al., 2015; Yates et al., 2014). There is some evidence that the prodromal signs and symptoms preceding vomiting can vary depending on the emetic trigger. For example, the sopite syndrome has mainly been associated with motion sickness (Graybiel et al., 1976; Lackner, 2014; Lawson et al., 1998; Matsangas et al., 2014; Van Ombergen et al., 2015). In some cases, prodromal signs and symptoms build-up slowly and have a long duration; in others, vomiting occurs suddenly after an emetic stimulus, such that prodromal signs and symptoms are of short duration (Katelaris et al., 1989; Kovacic et al., 2014; Lackner, 2014; Metz et al., 2007; Olden et al., 2005). These complexities have confounded the description of nausea in the literature.
Since nausea is a perception, a verbal report of a patient is required to ascertain if they are nauseated. However, it is often desirable to establish whether nausea is present, or if vomiting is likely, in individuals who cannot report their sensations (e.g., pediatric and postoperative patients) as well as animal subjects used in experiments to develop anti-nausea agents. This review provides historical perspectives on the definition of “nausea,” as well as a summary of the physiological changes that occur prior to vomiting that may be indicative of nausea. Also described are differences in the description of nausea, as well as the accompanying physiological responses, that occur with varying stimuli. This information is synthesized to provide an operational definition of nausea.
2. Historical Perspectives on Nausea: Cross-Cultural Insights into a Definition1
The definition of the sensation (or symptom) of nausea has its roots in ancient medicine. Historical perspectives on “nausea” are broader than contemporary definitions, which often classify nausea as a precursor or predisposing factor for vomiting. For example, Dorland’s Illustrated Medical Dictionary (1988) defines nausea as: “an unpleasant sensation, vaguely referred to the epigastrium and abdomen, and often culminating in vomiting.” Similarly, an earlier dictionary defined nausea simply as “tendency to vomit; sickness at the stomach” (Dorland, 1929). There are more expansive original meanings of “nausea,” though, that provide a conceptually richer context for understanding this sensation, its perceptual dimensions, its time course, and potential underlying biological mechanisms.
After experiencing a bout of seasickness, the American classics scholar John Carew Rolfe published a comprehensive guide to Greek and Latin references about seasickness (Rolfe, 1904). His publication provides a useful and comprehensive summary of the usage of the word ‘nausea’ and related terms in classical texts. The medical term ‘nausea’ is derived from the classical Greek terms ναυτια and ναυσια, which designated the signs and symptoms of seasickness. The term was adopted in Latin as the loan words nausea, nausia and nautea, which designate manifestations of seasickness or, more figuratively, mean “a qualm” (Lewis and Short, 1879) or “a sense of queasiness.” In fact, the definition encompassed the symptom clusters for early motion sickness that have been termed the ‘sopite syndrome’ (Graybiel et al., 1976; Lackner, 2014; Lawson et al., 1998; Matsangas et al., 2014): excessive drowsiness, lassitude, lethargy, degraded ability to concentrate on tasks, disinclination for mental or physical work and signs of mild depression.
The contextual richness of this definition can be appreciated from a passage from a letter (Epistle 53, 1st century CE) written by the Roman philosopher Lucius Seneca (Seneca, 1917) about a bout of seasickness:
“Peius autem vexabar, quam ut mihi periculum succurreret. Nausia enim me segnis haec et sine exitu torquebat, quae bilem movet nec effundit.”
I was suffering too grievously to think of the danger, since a sluggish seasickness [Nausia] which brought no relief was racking me, the sort that upsets the liver without clearing it. (p 353–354)
The meaning of the reference to ‘upsetting the liver [bilem]’, the seat of the bile, can be appreciated in the context of a passage from Aretæus of Cappadocia (1st–2nd century CE) regarding treatment of cholera (Adams, 1856):
“But if all the remains of the food have been discharged downwards, and if bile be evacuated, and if there still be bilious vomiting, retchings, and nausea, uneasiness and loss of strength, we must give two or three cupfuls (cyathi) of cold water, as an astringent of the belly, to stop the reflux, and in order to cool the burning stomach; and this is to be repeatedly done when what even has been drunk is vomited.”
The descriptions of the development of seasickness in monographs from the latter half of the 19th and early 20th centuries (Beard, 1881; Byrne, 1912; Chapman, 1864; Liveing, 1873; Skinner, 1894) provide a context for understanding the evolution of the demarcation of symptom clusters associated with the original term ‘nausea’. Skinner’s (Skinner, 1894) description is reasonably comprehensive: “extreme prostration of the patient; asthenia and feeling of great weakness, which render the seasick incapable of making the least exertion and which oblige them to keep abed days and even weeks; the vertigo, the terrible feeling of instability, as if all were about to disappear into a great abyss; the cephalagia, mostly frontal, often temporal, sometimes in general; the sensation of heaviness in the head, the constriction of the temples; the feeling of malaise, of indefinable torture; and lastly, the insomnia…” He added digestive system features that included anorexia (“one of the earliest symptoms that announce the approach of seasickness”), nausea (which could progress to vomiting) and constipation, which he attributed to gastrointestinal paresis. These features appear all to have been encompassed by the Greek terms ναυτια and ναυσια.
An understanding of the descriptive domain of the perceptual phenomenon of ‘nausea’ can be inferred directly from the consideration of usage and context of associated words in classical and more recent texts. The term ‘nausea’ has been used to denote both a disorder (diagnosis of seasickness) and a symptom (or symptom cluster) associated with other disorders. This pattern also appeared for terms for vertigo, such as ‘vertigine’, dinos, scotomatikos and skotodininos (Balaban et al., 2001). Hence, one must ask whether the term nausea denoted seasickness explicitly or whether it denoted the appearance of a ‘cluster of signs and symptoms of seasickness’ as a component of other primary disorders.
Three of Spencer’s translated short passages from the 1st-century CE medical treatise De Medicina (Celsus, 1935) are representative of the use of nausea to denote “the symptoms and signs associated with seasickness” in classical texts. Noteworthy features include the relationship to digestive status of the stomach contents and the therapeutic value of either spontaneous or induced vomiting.
“He too who on a voyage is troubled by seasickness [nausea], if he has vomited out a quantity of bile, should fast or take very little food. If he has spewed out sour phlegm, he may take food notwithstanding, but lighter than usual; if he has nausea [more properly, seasickness] without vomiting, he should either fast, or after food excite a vomit.”2
“For if the meal has been larger than can be digested, it is not well to risk its corruption; and if it has already become corrupted, nothing is more to the purpose than to eject it by whatever way its expulsion is first possible. When, therefore, there are bitter eructations, with pain and weight over the heart, recourse should be had at once to a vomit, which is likewise of service to anyone who has heartburn and copious salivation or nausea [the symptoms and signs associated with seasickness], or ringing in the ears, or watering of the eyes, or a bitter taste in the mouth; similarly in the case of one who is making a change of climate or locality; as well as in the case of those who become troubled by pain over the heart when they have not vomited for several days…”3
“Weakness of the stomach is indicated by pallor, wasting, pain over the heart, nausea [the symptoms and signs associated with seasickness], and involuntary vomiting, headache when the stomach is empty; where these symptoms are absent, the stomach is sound. Nor must one absolutely trust those of our patients who when very unwell have conceived a longing for wine or cold water, and in backing up their desires, lay the blame on their perfectly innocent stomach.” 4
The works of the 1st -2nd century CE Greek physician Aretæus of Cappadocia (Adams, 1856) further illustrate the associations between the term nausea and the signs and symptoms of seasickness in a variety of disorders that today are within the realm of neurology, otology and internal medicine. Nausea is mentioned in On Acute Diseases among symptoms of epileptic paroxysms (Book 1 Chapter 5), bringing up of blood (Book 2 Chapter 2), syncope (Book 2 Chapter 3), cholera (Book 2, Chapter 5), acute liver affectations (Book 2 Chapter 7), vena cava acute disease (Book 2 Chapter 8), and acute kidney disease (Book 2 Chapter 9). In On Chronic Diseases, nausea is also cited as a symptom of Cephaleae (with migraine like symptoms and vertigo Book 1 Chapter 2), vertigo or scotoma (Book 1 Chapter 3), spleen/schirrus (Book 1 Chapter 4), diabetes (Book 2 Chapter 2), stomach affectations (Book 2 Chapter 6), and dysentery (Book 2 Chapter 9). This comorbidity with vertigo and migraine-like symptoms of Cephaleae is a recurring component of descriptions of sea-sickness.
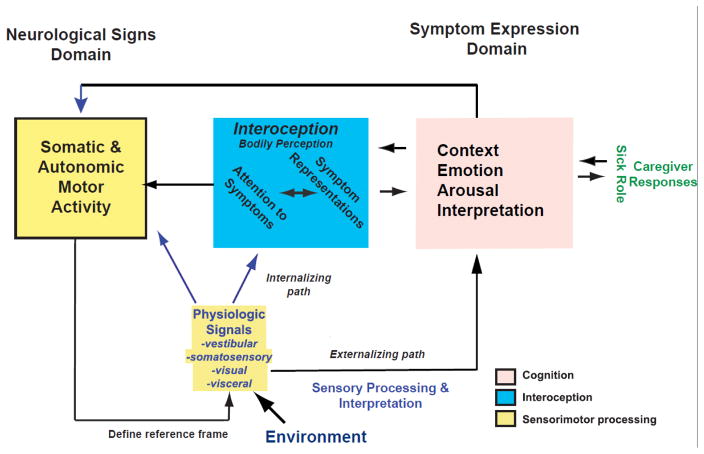
To place these historical perspectives into biological context, Figure 1 provides a conceptual guide for considering the features associated with broad and narrow constructs for nausea. This schema also incorporates co-morbid balance, migraine and anxiety manifestations, due to the integral nature of vestibular, somatic, cardiovascular and visceral information in brain stem pathways (review: (Balaban et al., 2004; Staab et al., 2013)). This heuristic schema depicts the expression of signs and symptoms of nausea as an interaction between (a) a primary neurologic sensorimotor performance component that produces signs of nausea (neurologic signs domain), (b) a cognitive-behavioral component for expression of symptoms of nausea (symptom expression domain), and (c) an interoceptive or ‘bodily perception’ domain providing an interface between the two. The ‘behavioral medicine’ perspective of this schema is a synthesis of concepts for co-morbid features of mental disorders with literature regarding symptom presentations (Barsky et al., 1999; Barsky et al., 2002; Mayou et al., 2005) and the development of chronic medically unexplained symptoms (Brown, 2004). The cognitive-behavioral domain components are shown in light red boxes. The interoceptive domain is represented by cyan boxes; it provides an interface for ‘gut level’ inferences to influence both signs (sensorimotor performance) and symptoms (cognizance) of seasickness and nausea. It is the cognitive attribution and ‘symptom story formulation process’ that shapes the description of the symptoms. It also recognizes contribution of social interactions with caregivers with the sick role assumed by affected individuals.
Figure 1.
An overview of a framework for co-morbid balance, migraine and anxiety manifestations, which provides a conceptual guide for considering the comorbid features associated with broad and narrow constructs for nausea. An interaction between automatic sensorimotor processing, interoceptive processing and cognitive/behavioral processing is proposed to explain the progression of nonspecific symptoms to a precept of nausea, in response to external and internal triggers and modulators.
The framework in Figure 1 provides a heuristic understanding of the interactions between sensorimotor, interoceptive and cognitive factors in dynamics of the perception of nausea. The prodromal signs and symptoms are vague and neither specific nor localizing, and certainty experiencing nausea increases as one proceeds along a trajectory toward having an urge to vomit. Hence, the identification of nausea is essentially a hypothesis-driven search to resolve ambiguity about a pattern of nonspecific symptoms. The sensory inputs are often subliminal; for example, triggers for anticipatory nausea during chemotherapy may include classical conditioning and anxiety (Kamen et al., 2014). Awareness of progressive visceral and somatic motor responses such as pallor, perspiration, saliva secretion and altered respiratory patterns contribute to this process. The ‘gut-level’ percept of being nauseated is the product of this interactive process.
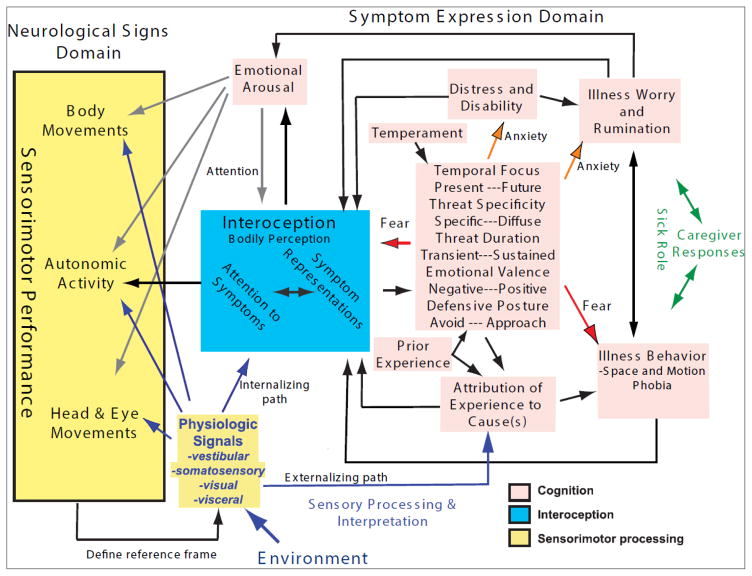
The schema is illustrated in more detail in Figure 2. The neurological signs component includes normal and pathological operations of postural and ocular control pathways that are responsible for maintaining stable control of head and body orientation during normal activities. The underlying postural control mechanisms include vestibulo-ocular, vestibulospinal and vestibulocollic pathways that maintain gaze orientations (and movement velocities) within a relatively limited range. For example, the mean head position is controlled to maintain the utricular macula and the horizontal semicircular canals near an earth horizontal orientation in humans (Grossman et al.; Mulavara et al., 2002; Pozzo et al., 1990; Pozzo et al., 1995) and non-human primates (Dunbar et al., 1998; Dunbar et al., 2004) during even vigorous physical activity. This generates a stable platform to provide context for interpreting vestibular, autonomic, visual and somatosensory information in terms of head orientation relative to gravity.
Figure 2.
A detailed schema of a framework for understanding the interaction of sensory information, sensorimotor responses, interoceptive mechanisms and cognitive/behavioral processes in the expression of a perception of nausea. See text for details.
Interpretation of orthostatic, visceral, visual, proprioceptive and motion sensory inputs requires a fundamental reconciliation of physiological signals in the context of internal models of wellness, egocentric (or self-referenced) and allocentric (or world-referenced) coordinate frames. Visual, vestibular, somatosensory and visceral physiological channels are fundamentally egocentric because sensors are anchored within body tissues. As a consequence, they rely implicitly on appropriate motor control of the head and body to establish their relationship to the allocentric framework of the external environment. Sensations of visual motion, blood pooling and inertial motion are interpreted within the assumed context of stable head control relative to gravity. On the other hand, as reviewed previously (Balaban et al., 2004), perception of post-cranial body orientation (relative to gravity) also involves processing of proprioceptive, mechanoreceptive, cardiovascular and visceral sensory information. The assumption of head stability in an internal model provides a basis for interpreting head-fixed vestibular, visual, auditory, gustatory and olfactory information in terms of the outside world (allocentric frame of reference).
The interoceptive domain occupies a nexus between sensory processes, representations of the physiological condition of the body (Craig, 2002), and their translation into subjective awareness and feelings, i.e., the “sentient self” (Craig, 2009). Interoception is defined broadly as any ‘molar organic behavior’ initiated by visceral sensory activity (Cameron, 2002), which encompasses not only generation of vague feelings of malaise or motion sickness, but also physiological signals that produce defensive responses such as altered postural control, situational discomfort and avoidance behaviors. Because all tissues in the body have mass, the orientation of gravity creates an implicit load for controlling automatic functions such as respiration and vascular tone to maintain blood distribution. Sensations may be viewed as benign or may be given the conceptual status of “symptom” heralding a dangerous or uncomfortable event. In this sense, interoception is collection of processes that defines and maintains the most current situational representation of ‘how we feel’ from an egocentric perspective.
The symptom expression (or cognitive) domain includes a rational interpretation component and an affective interpretation component. The rational interpretation component is engaged for meaning attribution and for effortful regulation of the affective state. The affective interpretation component governs involuntary or homeostatic regulation of affective responses, including conditioning by previous experience. The affective component generates responses along dimensions of anxiety (future temporal focus, diffuse and sustained threat profile, and approach responses) and fear (present temporal focus, specific and acute threat profile, and avoidance responses). The association of the “signs and symptoms of seasickness” with multiple diagnoses provide a substantial domain for symptom expression and interoceptive interpretation. For example, the cognitive, symptom expression domain appears to play a major role in chemotherapy-related anticipatory nausea and vomiting (Kamen et al., 2014), nausea in bulimic women presented with palatable food (Broberg et al., 1990), and the increased likelihood of pregnancy-related nausea and vomiting in women with purging subtype bulimia nervosa (Torgersen et al., 2008).
Table 1 summarizes the progression of seasickness from detailed accounts published between the mid-19th and early 20th centuries (Chapman 1864, Liveing 1873, Beard 1881, Skinner 1894, Byrne 1912). The symptoms and signs have been grouped and color-coded to correspond to the framework in Figure 1. For example, Joseph Byrne (1912) presented an analytical view of the progression from mild to more severe seasickness:
The chief symptoms experienced by the subject were a slight headache, and the familiar “queer feeling all over” which was present only at times. In addition to these there were slight disturbances of the nervous system, as manifested by disinclination for work, irritability of temper, and a consciousness of the respiratory movements. Long, deep inspirations were a matter of frequent occurrence. (p.339)
Even in the fresh breeze, there were some subjective symptoms, such as “lump-sensation” in the stomach, and a “queer sick feeling” in the head, as well as deep, long drawn breaths and eructations. These symptoms were present in spite of the fact that the subject was not feeling badly. (p.343)
Table 1.
The progression of seasickness described in accounts published between the mid-19th and early 20th centuries (Beard, 1881; Byrne, 1912; Chapman, 1864; Liveing, 1873; Skinner, 1894). The symptoms and signs have been grouped and color-coded to correspond to the framework in Figure 1.
| Earliest | Early mild | Milder phases | More severe | Severe |
|---|---|---|---|---|
| Consciousness of respiratory movements | → | Abdomen and thorax movements | ||
| Long, deep breaths | ||||
| ”Lump-sensation” in the throat and/or stomach | burning sensation in the stomach | ”swallowing to keep it down.” | Retch Emesis | |
| nausea increased salivation | ||||
| Eructations | → | |||
| Intermittent familiar “queer feeling all over” | ”queer sick feeling” in the head | → | ||
| that dreadful “dolor cerebri” familiar to all who have suffered from sea sickness | ||||
| Disinclination for work | → | → | ||
| Irritability of temper with tendency to worry about trifles | → | → | ||
| Fullness and lightness in the head | ||||
| Headache (Slight) | → | Occipital headache especially a sense of tension in the occipital region | Headache | |
| Scalp paraesthesias | → | Olfactory sensitivity/aversion Fullness or ringing in the ears Flushing of the face |
“Earlier and milder phases” of seasickness were characterized by:
…headache, with fulness and lightness in the head, paraesthesias of the scalp, especially a sense of tension in the occipital region, psychic and motor depression, irritability of temper, disinclination for work, perversion of sensory function, whereby the respiratory and gastro-intestinal movements were registered in consciousness, perversion of the sense of smell, whereby the odour of tobacco-smoke, agreeable under ordinary circumstances, became obnoxious, photophobia, and annoyance from the use of the eyes, especially in looking at the moving water (fatigue of the oculo-motor apparatus)…Digestive disturbances appeared early. The “lump sensation” in the stomach i.e., a feeling as if a foreign body were in that organ, is one of the early symptoms of seasickness…
More severe seasickness during a rough Atlantic crossing was described as follows:
The ‘lump-sensation’ in the stomach and throat was so marked, that he constantly kept swallowing to keep it down. He experienced, in this position, slight ache in the muscles attached to the occiput upon the right side. Any concentration or mental effort was disagreeable, and tended to make him sick. The subject lay upon either side to see if the position would have any effect in alleviating or aggravating his condition. It was noted that although lying upon the side made him feel worse than lying on the back, it did not make any material difference upon which side he lay. By 8.15 a.m., the subject was suffering from all the effects of fully developed seasickness. The “lump-sensation’ in the stomach was much in evidence... During this time the ship continued to roll and pitch excessively. The subject experienced all the subjective phenomena of seasickness, with lump-sensation in the stomach, headache, nausea, increased salivation, psychic and motor depression, and that dreadful “dolor cerebri” familiar to all who have suffered from sea sickness…
The mid-19th to early 20th century texts described the earliest supraliminal symptoms of seasickness as an intermittent, familiar “queer feeling all over,” accompanied by a disinclination to work, mood changes (increased fastidiousness, somatization and irritability), and a consciousness of respiratory movements leading to long, deep breaths. The thoraco-abdominal symptoms would then proceed to ‘a lump sensation in the thoat’ and the gastrointestinal sensations leading to retching and emesis. It is also important to note that the earliest symptoms could include allodynia that resembled symptoms of prodromal migraine, such as mild headache and scalp paraesthesia, which could develop into symptoms of migraine headache, and the general recognition of the resemblance of the sick-headache to seasickness (Liveing 1873). The description of these migraine symptoms is not surprising; more recent studies that have confirmed augmented motion sickness susceptibility in migraineurs (Drummond 2002, Drummond and Granston 2004; Drummond 2005; Furman et al. 2010). However, it raises the more general question of whether multimodal central migraine circuits (Ho, Edvinsson et al. 2010; Furman, Marcus et al. 2013) are engaged in the production of classically defined nausea, the complex of symptoms, and signs of seasickness.
Examination of British and Continental disease classification and dictionaries from the late seventeenth through the early nineteenth centuries (Blancard, 1726; Blankaart, 1690; Boissier de Sauvages, 1771; Boyer, 1729; Darwin, 1797; Quincy, 1787; Rädlein, 1711; Tommaséo, 1838; Willis, 1692) provides further insight into the vocabulary that evolved to describe symptom clusters for perceptions included in the categories of seasickness and ‘nausea.’ English, French, German and Italian terms encompass sensations associated with (1) sea-sickness (original meaning), (2) cephalalgea or migraine, (3) a sense of weariness or fatigue, (4) gastric discomfort (‘seething’), (5) a bothersome, cloying sense of over-satiety or bloating, and (6) conditioned aversion to food, drink and odors. The progression of sea-sickness (Table 1) encompasses all of these sensations. The latter three terms describe dimensions that encompass perceptions of impending emesis, heartburn, belching, digestive discomfort, disgust, aversion and anorexia, which are later stages of sea-sickness. The definition in the Blankaart’s Lexicon Novum Medicum Graeco-Latinum (1690, 44a) is illustrative:
Nausea is a sense of disgust or loathing (fastidiousness) coupled with an impulse to vomit, which may or may not ensue: the lower lip trembles and a considerable amount of liquid [saliva] flows from the mouth. It is not infrequent that nausea even progresses to a completion by vomiting. Moreover, along with its loathing for food & drink, Nausea is often so great that not even the swallowing of medicines can be accomplished… All food is treated as if for anorexia or distaste and dislike.5
Thus, through the early twentieth century, nausea was equated with seasickness, a condition associated with a number of signs and symptoms that extend well beyond the perception that vomiting may be imminent. However, it is essential to recognize that the operational definitions of nausea have always been embedded implicitly within the context of causal and prognostic narratives (or story lines). The classical literature presented several narratives, the scenario of seasickness, the scenario of overeating, the scenario of intoxication, the scenario of migraine and the scenario of digestive disease. It is unclear how (or why) the meaning was subsequently constrained by some authors to encompass only the scenario of prodroma of vomiting.
3. Prodromal Physiological Changes: Potential Physiological Markers for Nausea
3.1 Mechanisms of nausea and vomiting
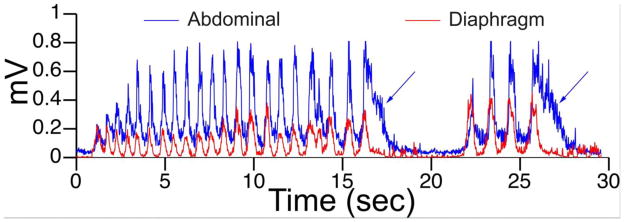
As described in many previous reviews (Andrews, 1992; Andrews et al., 1988; Grelot et al., 1994; Grélot et al., 1996; Miller, 1990; Yates et al., 2014; Yates et al., 1998), the motor act of vomiting is accomplished through the synchronized contractions of muscles that also are engaged in breathing. During retching and expulsion, there is a coordinated co-contraction of the diaphragm and abdominal muscles, contraction of the airway muscles to open the upper esophageal sphincter and close the glottis, and changes in posture needed to facilitate stomach emptying. The contractions of the diaphragm and abdominal muscles during retching and expulsion are stereotyped, as indicated in Figure 3, such that the responses can be readily detected in animal studies through recording of electromyographic activity from the muscles.
Figure 3.
Recordings of electrical activity from the diaphragm (red line) and abdominal muscles (blue line) of a cat during retching and vomiting elicited by bilateral galvanic vestibular stimulation. Two episodes are shown, consisting of co-contraction of the diaphragm and abdominal muscles during retching followed by expulsion (indicated by arrows), when the contraction of the abdominal muscles persists longer than diaphragm activity. Adapted from (Yates et al., 2014); used with permission of Springer.
Although the neural pathways that mediate vomiting have not been conclusively established, recent work has provided a number of insights regarding these circuits (Balaban et al., 2014; Yates et al., 2014). The notion that a “vomiting center” coordinates the activity of the respiratory muscles that produce emesis has been largely abandoned (Balaban et al., 2014; Miller, 1999; Miller et al., 1994a; Miller et al., 1994b; Yates et al., 1998). Instead, it is now believed that a network of neurons distributed throughout the brainstem controls the emetic response. However, the key neural circuits for producing vomiting are located in the medulla, since emesis can be evoked in animals following the removal of the remainder of the brain (Fukuda et al., 1991; Miller et al., 1994a). In contrast, nausea is a perception, and the neural circuits that generate this perception include supratentorial regions (Farmer et al., 2015; Kim et al., 2011; Napadow et al., 2013). Thus, although nausea and vomiting can be triggered by the same stimuli, they are mediated in part through different neural circuits. This dichotomy may be similar to that for responses to painful stimuli, which include a motor component (nociceptive flexion reflex) that is mainly mediate through spinal cord circuits and separate perceptual/affective components that are mediated through the limbic system and other telencephalic brain areas (Moayedi et al., 2013; Neugebauer et al., 2009; Skljarevski et al., 2002; Willis et al., 1997).
We reported recently that principal component analysis of Fos activation patterns revealed five brainstem circuit networks in cats expressing signs of nausea during galvanic vestibular stimulation (Balaban et al., 2014). These interconnected components explained a large proportion of the total variance in brainstem Fos labeling. However, to date a comparable principal component analysis has not been completed for supratentorial Fos labeling evoked by emetic stimuli, which constrains the assessment of which brain areas mediate particular signs of nausea.
In our study of brainstem Fos labeling patterns induced by galvanic vestibular stimulation (Balaban et al., 2014), Component 1 reflected strong Fos co-activation within locus coeruleus, vestibular nuclei, lateral nucleus tractus solitarius (NTS), medial parabrachial nucleus and periaqueductal gray, and a more moderate relationship with the Kölliker-Fuse nucleus. Component 2 incorporated a push-pull interaction between interconnected networks related to nucleus raphe magnus, lateral parabrachial nucleus, medial subnucleus of NTS, medial parabrachial nucleus, Kölliker-Fuse nucleus, inferior vestibular nucleus, and lateral subnucleus of NTS and the serotonergic cells in the dorsal raphe nucleus (e.g., medial aspect of the superior vestibular nucleus). Component 3 reflected Fos activation in the Kölliker-Fuse nucleus and concurrent Fos inhibition in a network that included serotonergic and non-serotonergic cells in the nuclei raphe pallidus et obscurus, which is possibly related to respiratory components of aversive stimulation. Component 4, like Component 1, identified correlated Fos labeling in a network involving interconnections between the periaqueductal gray and medial parabrachial nucleus. However, this labeling was combined with Fos activation in the lateral aspect of the superior vestibular nucleus and the subtrigeminal and external cuneate nuclei. Component 5 encompassed Fos labeling in the commissural nucleus of NTS, non-serotonergic dorsal raphe neurons, dorsal periaqueductal gray cells, serotonergic neurons in the nucleus raphe magnus and the medial aspect of the superior vestibular nucleus. Thus, a wide variety of brain areas can be engaged during a nauseogenic stimulus, depending on the signs and symptoms expressed by an individual.
3.2 Prodromal responses: general considerations
The physiological responses that precede emesis in humans are well-documented from case studies in patients as well as experimental studies in research subjects. Often the stimulus used in these experiments was a moving visual field (Farmer et al., 2015; Koch, 1999; Sclocco et al., 2015; Sclocco et al., 2016), which is regularly (but erroneously) referred to as “vection.” Vection is an illusion of self-motion, and studies that utilize moving visual fields to induce motion sickness rarely attempt to validate if the stimulus evokes a perception of self-movement (Lawson et al., 2015). Although some experimental studies in humans used agents such as apomorphine or ipecacuanha to induce nausea (Axelsson et al., 2006; Nussey et al., 1988; Proctor et al., 1978; Rowe et al., 1979), employing moving visual fields has two principal advantages: it is not necessary to administer agents that could produce extraneous side effects and the stimulus can be rapidly terminated at the subject’s request.
Moving visual fields used in the laboratory as an emetic stimulus, such as an optokinetic drug, do not mimic the “real world” conditions that generate motion sickness. The effectiveness of the stimulus may be related to its novelty. Susceptible individuals typically develop motion sickness within a few minutes of exposure to the moving visual field inside an optokinetic drum (Bos et al., 2004; Bubka et al., 2006; Dahlman et al., 2009; Matchock et al., 2008). Motion sickness during vehicular travel, such as seasickness, can evolve slowly over time (Lackner, 2014; Lawther et al., 1988; Wertheim, 1998), resulting in different physiological responses and sensations than those during an acute response to a highly provocative stimulus. Similarly, slowly-evolving prodromal signs and symptoms resulting from progressive exposure to a toxic agent, as well as conditions associated with chronic nausea such as the cyclic vomiting syndrome (Abell et al., 2008; Li et al., 2000), could differ from those that develop within a few minutes.
Another confounding factor in the literature is inconsistency in classifying whether responses that accompany emetic stimuli are prodromal responses. For example, the sopite syndrome has sometimes been classified as a separate response from motion sickness (Graybiel et al., 1976; Lackner, 2014; Lawson et al., 1998; Matsangas et al., 2014; Van Ombergen et al., 2015). Interestingly, lethargy is a typical antecedent to emesis in patients with the cyclic vomiting syndrome (Abell et al., 2008; Li et al., 2000), suggesting that disengagement may be a common prodromal response in conditions where the emetic response builds slowly over time. There is also a lack of clarity in the literature about the relationship between comorbid conditions and the primary trigger for emetic responses. For example, there is an association between susceptibility to migraine and motion sickness (Cuomo-Granston et al., 2010; Eggers et al., 2014; Furman et al., 2012; Marcus et al., 2005), as well as migraine and the cyclic vomiting syndrome (Adamiak et al., 2015; Hejazi et al., 2014; Li et al., 2000; Spiri et al., 2014). However, such relationships are rarely considered when investigating the mechanisms that evoke emetic responses.
3.3 Prodromal responses: gastrointestinal system
As noted above, the motor act of vomiting is accomplished by the coordinated contraction of respiratory muscles, and denervation or inactivation of the gastrointestinal system does not prevent vomiting (Eggleston et al., 1912; Lang et al., 1989; Magendie, 1824), indicating that changes in gastrointestinal motility are not essential to empty the stomach. However, a variety of studies have shown that gastrointestinal responses routinely occur prior to emesis.
One such classically-defined response is a change in salivation and salivary amylase levels (Gordon et al., 1994; Igarashi et al., 1989; Igarashi et al., 1993). Although most studies described increased salivation prior to emesis, others reported a decrease in salivation (Gordon et al., 1988; Hatcher et al., 1923). The disparity could be related to the time that salivation was measured relative to emesis. It is possible that salivation occurs just prior to vomiting, in order to protect the teeth against stomach acid, and is not an early prodromal indicator. Another complexity is that swallowing serves to remove saliva, and the frequency of swallowing may change prior to emesis (Furukawa et al., 1998; Furukawa et al., 1994; Lang et al., 1999; Money et al., 1996). Thus, further studies are needed to ascertain the reliability of salivation as a prodromal indicator of nausea.
Lang and colleagues conducted a thorough analysis in dogs and cats of the changes in gastrointestinal motility that precede, accompany, and follow vomiting (Lang, 2015; Lang et al., 1986; Lang et al., 1989; Lang et al., 1999). They demonstrated that motion sickness and administration of peripherally or centrally-acting emetic agents results in a decrease in gastrointestinal motility, relaxation of the proximal stomach, and a retrograde giant contraction in the small intestine. The latter may serve to either buffer the stomach contents or to remove toxic agents from the small intestine. Both the enteric and central nervous systems participate in eliciting these changes in gut motility (Lang, 2015). In some (but not all) humans, decreases in gastric fundus and lower esophageal pressure have been shown to occur during nauseogenic stimuli (Schaub et al., 2014), supporting Lang’s findings about gastric relaxation prior to emesis.
Studies by Koch, Stern and their colleagues noninvasively recorded gastric myoelectric activity (electrogastrograms) in humans during nauseogenic stimuli (for reviews see: (Koch, 1989; Koch, 1999; Koch, 2014; Stern et al., 1985)). They concluded that either an increase (tachygastria) or decrease (bradygastria) in the frequency of this electrical activity occurs during nausea, and that electrogastrograms can provide an “objective measure of nausea” (Koch, 2014). However, others failed to confirm this assertion (Cheung et al., 1998; DiBaise et al., 2001; Kiernan et al., 1997; Lackner et al., 2006), but instead showed that changes in electrogastrogram frequency may not be temporally related to the onset of nausea, and may occur in the absence of nausea. The reason why studies in different laboratories produced distinct results is unclear. It is noteworthy that the studies of Koch and Stern typically used an optokinetic drum, a novel stimulus not experienced in the real world that often leads to a rapid onset of nausea, which could be one factor leading to disparity of findings between studies. Perhaps changes in the electrogastrogram are not obvious when stimuli are provided that cause slowly-evolving nausea. A recent study in ferrets confirmed that gastric myoelectric activity is disrupted by the administration of emetic agents, but that the effects “were subtle and not detectable by a simple DF (dominant frequency) analysis” (Percie du Sert et al., 2009). In addition, disruptions in gastric myogenic activity were most evident during and following emetic episodes, and not before (Percie du Sert et al., 2010). These findings suggest that at least in animal studies, reliance on recordings of gastric myographic activity may not be adequate to define if nausea is present.
3.4 Prodromal responses: endocrine system
Studies in humans (Edwards et al., 1989; Feldman et al., 1988; Koch et al., 1990; Miaskiewicz et al., 1989; Rowe et al., 1979), ferrets (Billig et al., 2001; Hawthorn et al., 1988; Wilkens et al., 2005), dogs (Cubeddu et al., 1990), and monkeys (Verbalis et al., 1987) showed that a 20- to 30-fold increase in plasma vasopressin levels occur prior to emesis, without an increase in the levels of the other posterior pituitary hormone, oxytocin. Neither the mechanism producing nor the physiological significance of the vasopressin increase prior to emesis is currently known. It has been postulated that the increase in vasopressin levels serves to induce vomiting and nausea, and triggers changes in gastrointestinal motility (Caras et al., 1997; Cheung et al., 1994; Ikegaya et al., 2002; Kim et al., 1997; Song et al., 2006). However, removal of the posterior pituitary does not delay the onset of motion induced-emesis (Money et al., 1970), and patients with familial cranial diabetes insipidus experience apomorphine-induced nausea without an appreciable increase in plasma vasopressin (Baylis et al., 1981). Thus, while an increase in vasopressin may be correlated with nausea, the hormone is not critical to cause the sensation. Vasopressin is an antidiuretic agent (Nielsen et al., 1995), and increases in the release of the hormone would serve to conserve water when vomiting is likely. In addition, the levels of vasopressin reach sufficient levels to cause vasoconstriction of gastrointestinal blood vessels (Lote et al., 1981; Skarstein, 1978), to protect against absorption of toxins.
Baseline levels of vasopressin vary between individuals and physiological states (Beardwell et al., 1975), and the assays to detect vasopressin cannot be performed instantaneously. Thus, monitoring of vasopressin levels has not proven to be a convenient or reliable method to ascertain the presence of nausea (Billig et al., 2001; Wilkens et al., 2005).
There is also evidence that levels of adrenocorticotrophic hormone (ACTH) and cortisol secretion triggered by this hormone increase during motion sickness (Eversmann et al., 1978; Grigoriev et al., 1988; Klosterhalfen et al., 2000; Kohl, 1985; Otto et al., 2006). Most of these studies indicated that increased hormonal levels were generalized, and not correlated with the intensity of the nausea sensation. However, it is not clear whether changes in these stress hormone levels were directly related to the percept of stomach distress, or to the anxiety accompanying the sensation.
3.5 Prodromal responses: central nervous system and autonomic nervous system
A number of studies have assumed that emetic prodromal responses are due to a change in the balance of sympathetic and parasympathetic nervous system activity (Hejazi et al., 2011; LaCount et al., 2011; Morrow et al., 1992; Sclocco et al., 2015). This assumption rests on the outmoded notion that changes in autonomic nervous system activity, particularly sympathetic activity, are “all or none” (Cannon, 1963). A large recent literature shows that activity of sympathetic efferent fibers innervating different tissue types (Deuchars, 2015; Janig et al., 2003; Janig et al., 1992) and body regions (Kerman et al., 2000) can be differentially controlled. For example, the activity of sympathetic nerve fibers innervating blood vessels in skin is mainly dependent on body temperature (Janig et al., 1983), whereas baroreceptor signals play a major role in regulating the activity of sympathetic nerve fibers controlling vasoconstriction of visceral blood vessels (Bahr et al., 1986). Lack of attention to current information on control of autonomic function is a major limitation in the literature on the nausea and vomiting.
Nonetheless, several prodromal responses are mediated by changes in sympathetic nervous system activity. The most evident of these responses are cold-sweating (Cheung et al., 2011; Hemingway, 1944; Nobel et al., 2012; Sclocco et al., 2015), changes in cutaneous thermoregulatory vascular tone (Nalivaiko et al., 2015), and pallor (Cassano et al., 1989; Kolev et al., 1997). Changes in skin blood flow that result in pallor are not accompanied by alterations in blood pressure (Sunahara et al., 1987; Sunahara et al., 1964), showing that they are not due to a global increase in sympathetic nervous system activity. In fact, there is evidence of increased blood flow to muscle and decreased blood flow to the skin during motion sickness (Cheung et al., 2001). The physiological significance of facial pallor and cold sweating during emetic stimuli is unknown, although they may be related to impaired thermoregulation induced by emetic stimuli (Del Vecchio et al., 2014; Nalivaiko et al., 2015). There is evidence from studies in both humans and animals that both provocative motion (Cheung et al., 2011; Del Vecchio et al., 2014; Ngampramuan et al., 2014; Nobel et al., 2006; Nobel et al., 2012) and administration of lithium chloride (Guimaraes et al., 2015) trigger a drop in body temperature. It has been suggested that disrupted thermoregulatory responses during nausea could contribute to the development of the sopite syndrome (Del Vecchio et al., 2014).
Several studies have also indicated that heart rate variability changes during motion sickness (Doweck et al., 1997; Farmer et al., 2014; Kim et al., 2011; Kim et al., 2005; Lacount et al., 2009; Malinska et al., 2015; Ohyama et al., 2007). The general consensus is that heart rate becomes more consistent (variability is reduced) during exposure to provocative motion stimuli, although there are inconsistencies in results between studies. Stress and anxiety also produce changes in heart rate variability (Dimitriev et al., 2016; Dishman et al., 2000; Ramirez et al., 2015; Sheps et al., 2001; Tonello et al., 2014), such that it is unclear whether alterations during provocative motion are due to the perception of nausea or stress and anxiety accompanying the condition. Less work has been done to examine heart rate variability during exposure to emetic stimuli other than provocative motion, but anxiety during such stimuli would likely limit the utility of this measure to indicate that emesis is imminent.
Some studies reported variations in the electroencephalogram (EEG) during motion sickness (Arsalan Naqvi et al., 2015; Chelen et al., 1993; Chen et al., 2010; Chuang et al., 2016; Hu et al., 1999; Lin et al., 2007; Lutz, 1979; Park et al., 2008). A variety of changes in cognitive state are associated with alterations in the EEG, including fatigue and reduced alertness (Clayton et al., 2015; Huang et al., 2016; Wascher et al., 2014). Thus, the EEG changes reported in the studies could have been an early marker of the sopite syndrome. Unfortunately, the EEG changes were inconsistent between studies, limiting the utility of altered EEG activity to distinguish the presence of nausea. In addition, no studies have considered whether stimuli other than provocative motion or moving visual scenes induce EEG changes. Thus, it is presently unknown whether EEG changes, or other alterations in brain electrical activity, could serve as a marker for nausea elicited by any triggering mechanism. Further research in this area is needed.
3.6 Prodromal responses: non-emetic animals
A large number of studies have examined behavioral changes and physiological responses to emetic stimuli in animals that lack the capacity to vomit (Horn et al., 2013), particularly rodents. Behaviors such as eating non-nutritive substances like clay (Mitchell et al., 1977; Morita et al., 1988; Rudd et al., 2002; Takeda et al., 1993; Yamamoto et al., 2002a; Yamamoto et al., 2002b) and gaping (Limebeer et al., 2006; Parker et al., 2006) can accompany or follow emetic stimuli in non-emetic animals. Furthermore, rodents develop powerful conditioned taste aversions or disgust responses to agents that produce vomiting in emetic species (Parker, 2003; Parker, 2006; Parker, 2014; Parker et al., 2008). Curiously, plasma levels of oxytocin (but not vasopressin) increase in rodents following such stimuli (Carter et al., 1987; Verbalis et al., 1986), while in emetic animals increases in vasopressin (but not oxytocin) occur (Billig et al., 2001; Cubeddu et al., 1990; Edwards et al., 1989; Feldman et al., 1988; Hawthorn et al., 1988; Koch et al., 1990; Miaskiewicz et al., 1989; Rowe et al., 1979; Verbalis et al., 1987; Wilkens et al., 2005).
While there is controversy about the reliability of rodent models for developing antiemetic drugs (Holmes et al., 2009; Rudd et al., 2002), it is clear that motion stimuli and toxins can produce distinct behavioral changes in these species. Presumably, these behavioral changes are accompanied by alterations in interoception, which the animals perceive. However, it is debated whether the perception of nausea is similar in rodents and humans (Andrews et al., 2014; Stern et al., 2011). If the definition of nausea is restricted to a sensation that vomiting is imminent, then non-emetic animals cannot experience nausea, although some do classify the responses of rodents to emetic stimuli as indicators of nausea (Alhadeff et al., 2015; Cui et al., 2015; Parker, 2014; Parker et al., 2006; Parker et al., 2015; Rock et al., 2015). This clouds the definition of “nausea” in the literature.
4. An Operational Definition for Nausea
Despite assertions by some that there are distinct physiological markers for nausea, the discussion above shows that there are individual differences that may be affected by confounding factors such as anxiety or comorbid conditions. For example, perspiration (e.g., cold sweating) can be produced by emetic stimuli (Cheung et al., 2011; Hemingway, 1944; Nobel et al., 2012; Sclocco et al., 2015) or anxiety (Harker, 2013), and differentiating the etiology of the response is complicated. In addition, physiological responses vary depending on how provocative an emetic stimulus is. For example, it is unlikely that a stimulus like a rapidly-spinning optokinetic drum would result in the sopite syndrome, since individuals will either discontinue engaging in the stimulus or will vomit within a few minutes.
Nausea is a perception, and variability in the articulation of that perception is not unexpected (Graybiel et al., 1968; Muth et al., 1996). Moreover, one must also recognize that the perception and perceived progression of nausea is intertwined with personalized contextual: causal and prognostic narratives (or story lines) to explain the evolving, but otherwise nonspecific symptoms and signs (Figure 2). Examples of these narratives include seasickness, motion sickness (if in a car or virtual immersive environment), overeating, alcohol or drug intoxication, migraine and a gastrointestinal disorder. The trigger can also be internal, such as classically conditioned taste and odor aversions and anticipation of a nauseogenic stimulus (Pratt et al., 1984; Roscoe et al., 2011; Stockhorst et al., 2006). The 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013) descriptions of separation anxiety, generalized anxiety disorder, and panic disorder also include nausea as a symptom.
While most will recognize the onset of retching and gastric reflux just prior to expulsion, differentiating prodromal perceptions triggered by an emetic stimulus is more difficult, and may be influenced by context. However, recognizing early indicators is important, so an individual can disengage in the activity evoking the response or take an anti-emetic medication before symptoms become severe.
On the basis of historical considerations and analysis of the prodromal signs that occur before emesis, we would like to offer a new working definition of nausea that is applicable to many situations, but unfortunately not all. Nausea is present during or following exposure to a stimulus that can generate vomiting (e.g., toxins, provocative motion, and gastrointestinal disease), which produces stomach awareness, anxiety, anorexia, or causes an individual to lose interest in ongoing events (sopite syndrome). The presence and intensity of nausea increases the probability that prodromal indicators are also observed, particularly cold sweating, pallor, or overt indicators of anxiety (e.g., increase in respiration rate or heart rate), albeit these indicators can be difficult to recognize or differentiate from those produced by other causes. In this sense, ‘nausea’ describes time-varying processes in sensorimotor, interoceptive and higher order cognitive processing pathways that have a potential to produce an urge to vomit.
However, it is difficult to identify nausea in all individuals, since it is a percept that is attributed to a variety of nonspecific signs and symptoms. Nausea can be evoked by triggers that are not readily apparent, such as hormonal changes, migraine, stress and extreme emotional reactions, and can be conditioned to particular situations (anticipatory nausea) (Pratt et al., 1984; Roscoe et al., 2011; Stockhorst et al., 2006). In such cases, a context may not be present to associate the signs and symptoms elicited by these triggers with nausea. It will also be difficult to identify patients who remain in a chronic nauseated state, but fail to vomit. Caregivers should be cognizant about the multifaceted causes and indicators of nausea, and should be alert to changes in these indicators, as the percept of nausea is more evident immediately before stomach emptying.
Monitoring of physiological activity can be used in experimental studies to determine whether an exogenous stimulus (e.g., administration of an emetic compound) has activated neural pathways that can trigger nausea. Changes in respiration and heart rate, blood pressure, motor activity, skin blood flow, body temperature, hormone levels, and salivation that are linked to the administration of the emetic stimulus show that it has been detected and processed by the brain (Balaban et al., 2014; Ngampramuan et al., 2014). For example, a previous study considered an abrupt change in blood pressure upon intragastric administration of copper sulfate as an indicator that the compound activated vagal afferents (Sugiyama et al., 2011). Since no physiological marker is uniquely associated with nausea, confidence that an emetic stimulus activated neural pathways is stronger if a constellation of indicators is present. Although physiological monitoring can never definitively reveal the presence of nausea, overt changes in physiological markers after the delivery of a provocative stimulus at least suggests that nausea is likely. However, determining if an animal or nonverbal human is nauseated is much more difficult if it unknown whether a nauseogenic stimulus was recently provided.
Highlights.
Nausea was historically equated with seasickness, and was associated with many signs and symptoms.
The prodromal signs and symptoms preceding vomiting can vary depending on the emetic trigger, particularly whether the trigger rapidly evokes vomiting.
Triggering stimuli used in laboratory studies of nausea and vomiting are usually highly provocative, and may not elicit the same prodromal indicators of nausea as “real world” stimuli.
An operational definition of nausea must consider that prodromal signs and symptoms of emesis can vary between individuals and triggering mechanisms.
Accordingly, a variety of neural pathways can be activated by emetic stimuli, depending on the responses evoked in an individual.
Acknowledgments
Support for this work was provided by NIH grants R01-DC003732 and R01-DC013788.
Footnotes
Classical references to nausea were obtained from classical medical texts used in earlier publications (e.g, Balaban and Jacob,2001; Balaban, Erlen and Siderits (eds), The Skilful Physician, Harwood Academic 1997; Balaban, Erlen and Siderits (eds), The Ladies’ Dispensatory, Routedge:New York, 2003); from a keyword search of Perseus Project database (http://www.perseus.tufts.edu/hopper/), from bibliographic references cited by recent secondary sources in the humanities (e.g., Rolfe, 1904), reference works such as R.J. Cunliffe’s A Lexicon of the Homeric Dialect (1963) and from citations in references obtained from key word search of The Index-Catalogue of the Library of the Surgeon-General’s Office through the IndexCat database (https://www.nlm.nih.gov/hmd/indexcat/).
Is vero, qui navigavit et nausea pressus est, si multam bilem evomuit, vel abstinere a cibo debet vel paulum aliquid adsumere. Si pituitam acidam effudit, utique sumere cibum, sed adsueto leviorem: si sine vomitu nausea fuerit, vel abstinere vel post cibum vomere. Lib 1 3. 11
Vomitus utilior est hieme quam aestate: nam tunc et pituitae et capitis gravitas maior subest. Inutilis est gracilibus et inbecillum stomachum habentibus: utilis plenis, biliosis omnibus, si vel nimium se replerunt, vel parum concoxerunt. Nam sive plus est quam quod concoqui possit, periclitari ne conrumpatur non oportet: si vero corruptum est, nihil commodius est quam id, qua via primum expelli potest, eicere. Itaque ubi amari ructus cum dolore et gravitate praecordiorum sunt, ad hunc protinus confugiendum est. Item prodest ei, cui pectus aestuat et frequens saliva vel nausea est, aut sonant aures, aut madent oculi, aut os amarum est; similiterque ei, qui vel caelum vel locum mutat; isque, quibus, si per plures dies non vomuerunt, dolor praecordia infestat. Lib 1 3. 19–20
Stomachum autem infirmum indicant pallor, macies, praecordiorum dolor, nausea, et nolentium vomitus, ieiuno dolor capitis; quae in quo non sunt, is firmi stomachi est. Neque credendum utique nostris est, qui cum in adversa valetudine vinum aut frigidam aquam concupiverunt, deliciarum patrocinium in accusationem non merentis stomachi habet. Liber 1 8:2
Nausea est Fastidii species & ad vomitum conatus qui aut subsequitur aut non: labiis inferíoribus tremore actis & plerumque humore ex ore fluente. Est etiam nausea non infrequens qua à vomitu peracto aliquandíu remanet. Nausea insuper secum trahit Fastidíum cibi potus & medicamentorum quae tanta est saepe ut nequidem deglutitio fieri possit…cSumitur & pro Anorexia sive Omni ciborum fastidio & aversatione
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dorland’s Illustrated Medical Dictionary. 27. W.B. Saunders Company, Harcourt Brace Jovanovich Inc; Philadelphia: 1988. 1888. [Google Scholar]
- Abell TL, Adams KA, Boles RG, Bousvaros A, Chong SK, Fleisher DR, Hasler WL, Hyman PE, Issenman RM, Li BU, Linder SL, Mayer EA, McCallum RW, Olden K, Parkman HP, Rudolph CD, Tache Y, Tarbell S, Vakil N. Cyclic vomiting syndrome in adults. Neurogastroenterol Motility. 2008;20:269–284. doi: 10.1111/j.1365-2982.2008.01113.x. [DOI] [PubMed] [Google Scholar]
- Adamiak TR, Jensen MJ. Cyclic vomiting syndrome. SD Med. 2015;68:9–11. 13. [PubMed] [Google Scholar]
- Adams F. The Extant Works of Aretæus, the Cappodocian. The Sydenham Society; London: 1856. [Google Scholar]
- Alhadeff AL, Holland RA, Nelson A, Grill HJ, De Jonghe BC. Glutamate receptors in the central nucleus of the amygdala mediate cisplatin-induced malaise and energy balance dysregulation through direct hindbrain projections. J Neurosci. 2015;35:11094–11104. doi: 10.1523/JNEUROSCI.0440-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Andrews PL. Physiology of nausea and vomiting. British J Anaesthesia. 1992;69:2S–19S. doi: 10.1093/bja/69.supplement_1.2s. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Hawthorn J. The neurophysiology of vomiting. Baillieres Clinical Gastroenterol. 1988;2:141–168. doi: 10.1016/0950-3528(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Sanger GJ. Nausea and the quest for the perfect anti-emetic. Eur J Pharmacol. 2014;722:108–121. doi: 10.1016/j.ejphar.2013.09.072. [DOI] [PubMed] [Google Scholar]
- Arsalan Naqvi SA, Badruddin N, Jatoi MA, Malik AS, Hazabbah W, Abdullah B. EEG based time and frequency dynamics analysis of visually induced motion sickness (VIMS) Australas Phys Eng Sci Med. 2015;38:721–729. doi: 10.1007/s13246-015-0379-9. [DOI] [PubMed] [Google Scholar]
- Axelsson P, Thorn SE, Lovqvist A, Wattwil L, Wattwil M. Betamethasone does not prevent nausea and vomiting induced by the dopamine-agonist apomorphine. Can J Anaesth. 2006;53:370–374. doi: 10.1007/BF03022501. [DOI] [PubMed] [Google Scholar]
- Bahr R, Bartel B, Blumberg H, Janig W. Functional characterization of preganglionic neurons projecting in the lumbar splanchnic nerves: vasoconstrictor neurons. J Auton Nerv Syst. 1986;15:131–140. doi: 10.1016/0165-1838(86)90009-3. [DOI] [PubMed] [Google Scholar]
- Baillou (Ballonii) G. Opera Omnia. In: Baillou (Ballonii) G, editor. De vertigine. I. Fratres de Tournes; Geneva: 1762. p. 291. [Google Scholar]
- Balaban CD, Jacob RG. Background and history of the interface between anxiety and vertigo. J Anxiety Disord. 2001;15:27–51. doi: 10.1016/s0887-6185(00)00041-4. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Ogburn SW, Warshafsky SG, Ahmed A, Yates BJ. Identification of neural networks that contribute to motion sickness through principal components analysis of fos labeling induced by galvanic vestibular stimulation. PloS one. 2014;9:e86730. doi: 10.1371/journal.pone.0086730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban CD, Yates BJ. Vestibulo-autonomic interactions: a teleologic perspective. In: Highstein SM, Fay RR, Popper AN, editors. Anatomy and Physiology of the Central and Peripheral Vestibular System. Springer; Heidelberg: 2004. [Google Scholar]
- Barsky AJ, Boras JF. Functional somatic syndromes. Annals Internal Med. 1999;130:910–921. doi: 10.7326/0003-4819-130-11-199906010-00016. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Saintfort R, Rogers MP. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- Baylis PH, Robertson GL. Vasopressin function in familial cranial diabetes insipidus. Postgrad Med J. 1981;57:36–40. doi: 10.1136/pgmj.57.663.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard GM. A Treatise on Sea-Sickness: Its Symptoms, Nature and Treatment. E. B. Treat; New York: 1881. p. 104. [Google Scholar]
- Beardwell CG, Geelen G, Palmer HM, Roberts D, Salamonson L. Radioimmunoassay of plasma vasopressin in physiological and pathological states in man. J Endocrinol. 1975;67:189–202. doi: 10.1677/joe.0.0670189. [DOI] [PubMed] [Google Scholar]
- Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am J Physiol Reg Integrative Comp Physiol. 2001;281:R1243–1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- Blancard S. The Physical Dictionary, Wherein the Terms of Anatomy, the Names and Causes of Diseases, Chirurgical Instruments, and Their Use, Are Accurately Described. 7. John and Benjamin Sprint; London: 1726. [Google Scholar]
- Blankaart S. Lexicon Novum Medicum Græco-Latinum Cornelium Boutesteyn. Jordaanum Luchtmans, Lugduni Batavorum. 1690:806. [Google Scholar]
- Boissier de Sauvages F. Ouvrage augmenté de quelques Notes en forme de Commentaire, par M. Nicholas, Chirurgien gradué. Hérissant le Fils; Paris: 1771. Nosologie Méthodique, dans laquelle les maladies sont rangées par classes, suivant le systême de Sydenham, & l’ordre des botanistes. Traduites du Latin de M. François Boissier de Sauvages, Docteur en Médicine & Professeur Royal en l’Université de Montpellier; de l’Academie des Sciences de la même ville; de celles de Londres, d’Upsal, de Berlin; del lat Societé Physico-Botanique de Suède, des Curieux de la Nature, & de l’Institut de Bologne. [Google Scholar]
- Bos JE, Bles W. Motion sickness induced by optokinetic drums. Aviat Space Environ Med. 2004;75:172–174. [PubMed] [Google Scholar]
- Boyer A. The Royal Dictionary, French and English, and English and French: Extracted from the Writings of the Best Authours in Both Languages Knapton, Darey, Midwinter,Bettesworth, Innys, Fayram, Pemberton, Osborn and Longman, Ford, Rivington, Robinson, Motte, Hooke, Clay, Bailly, Ward, Simon, Browne, Lacy, Osborne, Lyon and Wellington. London: 1729. p. 1189. [Google Scholar]
- Broberg DJ, Dorsa DM, Bernstein IL. Nausea in bullimic wormen in response to a palatable food. J Abnormal Psychol. 1990;99:183–188. doi: 10.1037//0021-843x.99.2.183. [DOI] [PubMed] [Google Scholar]
- Brown RJ. Psychological mechanisms of medically unexplained symptoms: an integrative conceptual approach. Psychological Bull. 2004;130:793–812. doi: 10.1037/0033-2909.130.5.793. [DOI] [PubMed] [Google Scholar]
- Bubka A, Bonato F, Urmey S, Mycewicz D. Rotation velocity change and motion sickness in an optokinetic drum. Aviat Space Environ Med. 2006;77:811–815. [PubMed] [Google Scholar]
- Byrne J. On the Physiology of the Semicircular Canals and Their Relation to Seasickness. J.T. Dougherty; New York: 1912. [Google Scholar]
- Cameron OG. Visceral Sensory Neuroscience. Oxford University Press; New York: 2002. [Google Scholar]
- Cannon WB. The Wisdom of the Body. W. W. Norton; New York: 1963. [Google Scholar]
- Caras SD, Soykan I, Beverly V, Lin Z, McCallum RW. The effect of intravenous vasopressin on gastric myoelectrical activity in human subjects. Neurogastroenterol Motility. 1997;9:151–156. doi: 10.1046/j.1365-2982.1997.d01-37.x. [DOI] [PubMed] [Google Scholar]
- Carter DA, Lightman SL. A role for the area postrema in mediating cholecystokinin-stimulated oxytocin secretion. Brain Res. 1987;435:327–330. doi: 10.1016/0006-8993(87)91617-9. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Petracca A, Perugi G, Toni C, Tundo A, Roth M. Derealization and panic attacks: a clinical evaluation on 150 patients with panic disorder/agoraphobia. Compr Psychiatry. 1989;30:5–12. doi: 10.1016/0010-440x(89)90112-0. [DOI] [PubMed] [Google Scholar]
- Celsus. De Medicina. In: Spencer WG, editor. De Medicina. Harvard University Press; Cambridge: 1935. [Google Scholar]
- Chapman J. Functional Diseases of the Stomach. Part I. Sea-Sickness: its Nature and Treatment. Trübner and Company; London: 1864. [Google Scholar]
- Chelen WE, Kabrisky M, Rogers SK. Spectral analysis of the electroencephalographic response to motion sickness. Aviat Space Environ Med. 1993;64:24–29. [PubMed] [Google Scholar]
- Chen YC, Duann JR, Chuang SW, Lin CL, Ko LW, Jung TP, Lin CT. Spatial and temporal EEG dynamics of motion sickness. Neuroimage. 2010;49:2862–2870. doi: 10.1016/j.neuroimage.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Cheung B, Hofer K. Coriolis-induced cutaneous blood flow increase in the forearm and calf. Brain Res Bull. 2001;54:609–618. doi: 10.1016/s0361-9230(01)00463-4. [DOI] [PubMed] [Google Scholar]
- Cheung B, Nakashima AM, Hofer KD. Various anti-motion sickness drugs and core body temperature changes. Aviat Space Environ Med. 2011;82:409–415. doi: 10.3357/asem.2903.2011. [DOI] [PubMed] [Google Scholar]
- Cheung B, Vaitkus P. Perspectives of electrogastrography and motion sickness. Brain Res Bull. 1998;47:421–431. doi: 10.1016/s0361-9230(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Cheung BS, Kohl RL, Money KE, Kinter LB. Etiologic significance of arginine vasopressin in motion sickness. J Clin Pharmacol. 1994;34:664–670. doi: 10.1002/j.1552-4604.1994.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Chuang SW, Chuang CH, Yu YH, King JT, Lin CT. EEG alpha and gamma modulators mediate motion sickness-related spectral responses. Int J Neural Syst. 2016;26:1650007. doi: 10.1142/S0129065716500076. [DOI] [PubMed] [Google Scholar]
- Clayton MS, Yeung N, Cohen Kadosh R. The roles of cortical oscillations in sustained attention. Trends Cogn Sci. 2015;19:188–195. doi: 10.1016/j.tics.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Coelho CM, Balaban CD. Visuo-vestibular contributions to anxiety and fear. Neurosci Biobehav Rev. 2015;48:148–159. doi: 10.1016/j.neubiorev.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Craig A. How do you feel--now? The anterior insula and human awareness. Nature Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Cubeddu LX, Lindley CM, Wetsel W, Carl PL, Negro-Vilar A. Role of angiotensin II and vasopressin in cisplatin-induced emesis. Life Sci. 1990;46:699–705. doi: 10.1016/0024-3205(90)90075-3. [DOI] [PubMed] [Google Scholar]
- Cui Y, Wang L, Shi G, Liu L, Pei P, Guo J. Electroacupuncture alleviates cisplatin-induced nausea in rats. Acupunct Med. 2015;34:120–6. doi: 10.1136/acupmed-2015-010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo-Granston A, Drummond PD. Migraine and motion sickness: what is the link? Prog Neurobiol. 2010;91:300–312. doi: 10.1016/j.pneurobio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Dahlman J, Sjors A, Lindstrom J, Ledin T, Falkmer T. Performance and autonomic responses during motion sickness. Hum Factors. 2009;51:56–66. doi: 10.1177/0018720809332848. [DOI] [PubMed] [Google Scholar]
- Darwin E. Zoonomia; or The Laws of Organic Life. T. Dobson; Philadelphia: 1797. [Google Scholar]
- Del Vecchio F, Nalivaiko E, Cerri M, Luppi M, Amici R. Provocative motion causes fall in brain temperature and affects sleep in rats. Exp Brain Res. 2014;232:2591–2599. doi: 10.1007/s00221-014-3899-8. [DOI] [PubMed] [Google Scholar]
- Deuchars SA. How sympathetic are your spinal cord circuits? Exp Physiol. 2015;100:365–371. doi: 10.1113/EP085031. [DOI] [PubMed] [Google Scholar]
- DiBaise JK, Brand RE, Lyden E, Tarantolo SR, Quigley EM. Gastric myoelectrical activity and its relationship to the development of nausea and vomiting after intensive chemotherapy and autologous stem cell transplantation. Am J Gastroenterol. 2001;96:2873–2881. doi: 10.1111/j.1572-0241.2001.04241.x. [DOI] [PubMed] [Google Scholar]
- Dimitriev DA, Saperova EV, Dimitriev AD. State anxiety and nonlinear dynamics of heart rate variability in students. PloS one. 2016;11:e0146131. doi: 10.1371/journal.pone.0146131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Inter J Psychophysiol. 2000;37:121–133. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Dorland WAN. The American Illustrated Medical Dictionary. 15. W.B. Saunders Company; Philadephia: 1929. p. 1427. [Google Scholar]
- Doweck I, Gordon CR, Shlitner A, Spitzer O, Gonen A, Binah O, Melamed Y, Shupak A. Alterations in R-R variability associated with experimental motion sickness. J Auton Nerv Syst. 1997;67:31–37. doi: 10.1016/s0165-1838(97)00090-8. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Motion sickness and migraine: optokinetic stimulation increases scalp tenderness, pain sensitivity in the fingers and photophobia. Cephalagia. 2002;22:117–124. doi: 10.1046/j.1468-2982.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Triggers of motion sickness in migraine sufferers. Headache. 2005;45:653–656. doi: 10.1111/j.1526-4610.2005.05132.x. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Granston A. Facial pain increases nausea and headache during motion sickness in migraine sufferers. Brain. 2004;127:526–534. doi: 10.1093/brain/awh061. [DOI] [PubMed] [Google Scholar]
- Dunbar DC, Badam GL. Development of posture and locomotion in free-ranging primates. Neurosci Biobehav Rev. 1998;22:541–546. doi: 10.1016/s0149-7634(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Dunbar DC, Badam GL, Hallgrimsson B, Vieilledent S. Stabilization and mobility of the head and trunk in wild monkeys duing terrestrial and flat-surface walks and gallops. J Exp Biol. 2004;207:1027–1042. doi: 10.1242/jeb.00863. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Carmichael J, Baylis PH, Harris AL. Arginine vasopressin--a mediator of chemotherapy induced emesis? Br J Cancer. 1989;59:467–470. doi: 10.1038/bjc.1989.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vest Res. 2014;24:387–395. doi: 10.3233/VES-140525. [DOI] [PubMed] [Google Scholar]
- Eggleston C, Hatcher RA. The site of the emetic action of various drugs. J Pharmacol Exp Ther. 1912;7:225. [Google Scholar]
- Eversmann T, Gottsmann M, Uhlich E, Ulbrecht G, von Werder K, Scriba PC. Increased secretion of growth hormone, prolactin, antidiuretic hormone, and cortisol induced by the stress of motion sickness. Aviat Space Environ Med. 1978;49:53–57. [PubMed] [Google Scholar]
- Farmer AD, Al Omran Y, Aziz Q, Andrews PL. The role of the parasympathetic nervous system in visually induced motion sickness: systematic review and meta-analysis. Exp Brain Res. 2014;232:2665–2673. doi: 10.1007/s00221-014-3964-3. [DOI] [PubMed] [Google Scholar]
- Farmer AD, Ban VF, Coen SJ, Sanger GJ, Barker GJ, Gresty MA, Giampietro VP, Williams SC, Webb DL, Hellstrom PM, Andrews PL, Aziz Q. Visually induced nausea causes characteristic changes in cerebral, autonomic and endocrine function in humans. J Physiol. 2015;593:1183–1196. doi: 10.1113/jphysiol.2014.284240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M, Samson WK, O’Dorisio TM. Apomorphine-induced nausea in humans: release of vasopressin and pancreatic polypeptide. Gastroenterology. 1988;95:721–726. doi: 10.1016/s0016-5085(88)80020-9. [DOI] [PubMed] [Google Scholar]
- Feyer P, Jahn F, Jordan K. Radiation induced nausea and vomiting. European J Pharmacol. 2014;722:165–171. doi: 10.1016/j.ejphar.2013.09.069. [DOI] [PubMed] [Google Scholar]
- Fisher RD, Rentschler RE, Nelson JC, Godfrey TE, Wilbur DW. Elevation of plasma antidiuretic hormones (ADH) associated with chemotherapy-induced emesis in man. Cancer Treat Rep. 1982;66:25–29. [PubMed] [Google Scholar]
- Fox S, Arnon I. Motion sickness and anxiety. Aviat Space Environ Med. 1988;59:728–733. [PubMed] [Google Scholar]
- Fukuda H, Koga T. The Botzinger complex as the pattern generator for retching and vomiting in the dog. Neurosci Res. 1991;12:471–485. doi: 10.1016/s0168-0102(09)80001-1. [DOI] [PubMed] [Google Scholar]
- Furman JM, Marcus DA. Migraine and motion sensitivity. Continuum. 2012;18:1102–1117. doi: 10.1212/01.CON.0000421621.18407.96. [DOI] [PubMed] [Google Scholar]
- Furman JM, Marcus DA, Balaban CD. Rizatriptan reduces vestibular-induced motion sickness in migraineurs. J Headache Pain. 2010;12:81–88. doi: 10.1007/s10194-010-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706–715. doi: 10.1016/S1474-4422(13)70107-8. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Fukuda H, Hatano M, Koga T, Shiroshita Y. A neurokinin-1 receptor antagonist reduced hypersalivation and gastric contractility related to emesis in dogs. Am J Physiol. 1998;275:G1193–1201. doi: 10.1152/ajpgi.1998.275.5.G1193. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Okada H. Canine salivary secretion from the submaxillary glands before and during retching. Am J Physiol. 1994;267:G810–817. doi: 10.1152/ajpgi.1994.267.5.G810. [DOI] [PubMed] [Google Scholar]
- Gordon CR, Ben-Aryeh H, Spitzer O, Doweck I, Gonen A, Melamed Y, Shupak A. Seasickness susceptibility, personality factors, and salivation. Aviat Space Environ Med. 1994;65:610–614. [PubMed] [Google Scholar]
- Gordon CR, Ben-Aryeh H, Szargel R, Attias J, Rolnick A, Laufer D. Salivary changes associated with experimental motion sickness condition in man. J Auton Nerv Syst. 1988;22:91–96. doi: 10.1016/0165-1838(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Graybiel A, Knepton J. Sopite syndrome: a sometimes sole manifestation of motion sickness. Aviat Space Environ Med. 1976;47:873–882. [PubMed] [Google Scholar]
- Graybiel A, Wood CD, Miller EF, Cramer DB. Diagnostic criteria for grading the severity of acute motion sickness. Aerosp Med. 1968;39:453–455. [PubMed] [Google Scholar]
- Grelot L, Miller A. Vomiting - Its Ins and Outs. News Physiol Sci. 1994;9:142–147. [Google Scholar]
- Grélot L, Miller AD. Neural control of respiratory muscle activity during vomiting. In: Miller AD, Bianchi AL, Bishop BP, editors. Neural Control of the Respiratory Muscles. CRC Press; Boca Raton, FL: 1996. pp. 239–248. [Google Scholar]
- Grigoriev AI, Nichiporuk IA, Yasnetsov VV, Shashkov VS. Hormonal status and fluid electrolyte metabolism in motion sickness. Aviat Space Environ Med. 1988;59:301–305. [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Guimaraes DD, Andrews PL, Rudd JA, Braga VA, Nalivaiko E. Ondansetron and promethazine have differential effects on hypothermic responses to lithium chloride administration and to provocative motion in rats. Temperature (Austin) 2015;2:543–553. doi: 10.1080/23328940.2015.1071700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammam E, Dawood T, Macefield VG. Low-frequency galvanic vestibular stimulation evokes two peaks of modulation in skin sympathetic nerve activity. Exp Brain Res. 2012;219:441–446. doi: 10.1007/s00221-012-3090-z. [DOI] [PubMed] [Google Scholar]
- Harker M. Psychological sweating: a systematic review focused on aetiology and cutaneous response. Skin Pharmacol Physiol. 2013;26:92–100. doi: 10.1159/000346930. [DOI] [PubMed] [Google Scholar]
- Hatcher RA, Weiss S. Studies on vomiting. J Pharmacol Exp Therapeutics. 1923;22:139–193. [Google Scholar]
- Hawthorn J, Andrews PL, Ang VT, Jenkins JS. Differential release of vasopressin and oxytocin in response to abdominal vagal afferent stimulation or apomorphine in the ferret. Brain Res. 1988;438:193–198. doi: 10.1016/0006-8993(88)91338-8. [DOI] [PubMed] [Google Scholar]
- Hederos CA. Psychogenic vomiting. Lancet. 1992;339:1228. doi: 10.1016/0140-6736(92)91163-3. [DOI] [PubMed] [Google Scholar]
- Heer M, Paloski WH. Space motion sickness: incidence, etiology, and countermeasures. Auton Neurosci: Basic and Clinical. 2006;129:77–79. doi: 10.1016/j.autneu.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Hejazi RA, Lavenbarg TH, Pasnoor M, Dimachkie M, Foran P, Herbelin L, McCallum RW. Autonomic nerve function in adult patients with cyclic vomiting syndrome. Neurogastroenterol Motility. 2011;23:439–443. doi: 10.1111/j.1365-2982.2011.01679.x. [DOI] [PubMed] [Google Scholar]
- Hejazi RA, McCallum RW. Cyclic vomiting syndrome: treatment options. Exp Brain Res. 2014;232:2549–2552. doi: 10.1007/s00221-014-3989-7. [DOI] [PubMed] [Google Scholar]
- Hemingway A. Cold sweating in motion sickness. Am J Physiol. 1944;141:172–175. [Google Scholar]
- Hiura Y, Takiguchi S, Yamamoto K, Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Fujiwara Y, Mori M, Doki Y. Fall in plasma ghrelin concentrations after cisplatin-based chemotherapy in esophageal cancer patients. Int J Clin Oncol. 2012;17:316–323. doi: 10.1007/s10147-011-0289-0. [DOI] [PubMed] [Google Scholar]
- Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights in migraine pathophysiology. Nature Reviews Neurol. 2010;6:573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- Holmes AM, Rudd JA, Tattersall FD, Aziz Q, Andrews PL. Opportunities for the replacement of animals in the study of nausea and vomiting. British J Pharmacol. 2009;157:865–880. doi: 10.1111/j.1476-5381.2009.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC. Measuring the nausea-to-emesis continuum in non-human animals: refocusing on gastrointestinal vagal signaling. Exp Brain Res. 2014;232:2471–2481. doi: 10.1007/s00221-014-3985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PLR. Why Can’t Rodents Vomit? A Comparative Behavioral, Anatomical, and Physiological Study. PloS one. 2013;8:e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Wallisch WJ, Homanics GE, Williams JP. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. European J Pharmacol. 2014;722:55–66. doi: 10.1016/j.ejphar.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, McChesney KA, Player KA, Bahl AM, Buchanan JB, Scozzafava JE. Systematic investigation of physiological correlates of motion sickness induced by viewing an optokinetic rotating drum. Aviat Space Environ Med. 1999;70:759–765. [PubMed] [Google Scholar]
- Huang KC, Huang TY, Chuang CH, King JT, Wang YK, Lin CT, Jung TP. An EEG-Based Fatigue Detection and Mitigation System. Int J Neural Syst. 2016;26:1650018. doi: 10.1142/S0129065716500180. [DOI] [PubMed] [Google Scholar]
- Igarashi M, MacDonald S, Chae SY, Plishker GA, Kohl RL. Sodium concentration in saliva along the time course of experimental Coriolis sickness. Acta Otolaryngol. 1989;107:485–488. doi: 10.3109/00016488909127545. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Reschke MF, Henley C, MacDonald S, Kohl R, Mizukoshi K. Salivary total protein and experimental Coriolis sickness. Acta Otolaryngol Suppl. 1993;504:38–40. doi: 10.3109/00016489309128119. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Matsuki N. Vasopressin induces emesis in Suncus murinus. Jpn J Pharmacol. 2002;89:324–326. doi: 10.1254/jjp.89.324. [DOI] [PubMed] [Google Scholar]
- Janig W, Habler HJ. Neurophysiological analysis of target-related sympathetic pathways--from animal to human: similarities and differences. Acta Physiol Scand. 2003;177:255–274. doi: 10.1046/j.1365-201X.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- Janig W, McLachlan EM. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 1992;15:475–481. doi: 10.1016/0166-2236(92)90092-m. [DOI] [PubMed] [Google Scholar]
- Janig W, Sundlof G, Wallin BG. Discharge patterns of sympathetic neurons supplying skeletal muscle and skin in man and cat. J Auton Nerv Syst. 1983;7:239–256. doi: 10.1016/0165-1838(83)90077-2. [DOI] [PubMed] [Google Scholar]
- Kamen C, Tejani MA, Chandwani K, Janelsins M, Peoples AR, Roscoe JA, Morrow GR. Anticipatory nausea and vomiting due to chemotherapy. Eur J Pharmacology. 2014;722:172–1799. doi: 10.1016/j.ejphar.2013.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katelaris PH, Jones DB. Chronic nausea and vomiting. Digestive Dis. 1989;7:324–333. doi: 10.1159/000171232. [DOI] [PubMed] [Google Scholar]
- Kenward H, Pelligand L, Savary-Bataille K, Elliott J. Nausea: current knowledge of mechanisms, measurement and clinical impact. Veterinary J. 2015;203:36–43. doi: 10.1016/j.tvjl.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Kerman IA, McAllen RM, Yates BJ. Patterning of sympathetic nerve activity in response to vestibular stimulation. Brain Res Bull. 2000;53:11–16. doi: 10.1016/s0361-9230(00)00303-8. [DOI] [PubMed] [Google Scholar]
- Kiernan BD, Soykan I, Lin Z, Dale A, McCallum RW. A new nausea model in humans produces mild nausea without electrogastrogram and vasopressin changes. Neurogastroenterol Motility. 1997;9:257–263. doi: 10.1046/j.1365-2982.1997.d01-61.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Napadow V, Kuo B, Barbieri R. A combined HRV-fMRI approach to assess cortical control of cardiovagal modulation by motion sickness. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2011;2011:2825–2828. doi: 10.1109/IEMBS.2011.6090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Chey WD, Owyang C, Hasler WL. Role of plasma vasopressin as a mediator of nausea and gastric slow wave dysrhythmias in motion sickness. Am J Physiol. 1997;272:G853–862. doi: 10.1152/ajpgi.1997.272.4.G853. [DOI] [PubMed] [Google Scholar]
- Kim YY, Kim HJ, Kim EN, Ko HD, Kim HT. Characteristic changes in the physiological components of cybersickness. Psychophysiology. 2005;42:616–625. doi: 10.1111/j.1469-8986.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- Klosterhalfen S, Ruttgers A, Krumrey E, Otto B, Stockhorst U, Riepl RL, Probst T, Enck P. Pavlovian conditioning of taste aversion using a motion sickness paradigm. Psychosomatic Med. 2000;62:671–677. doi: 10.1097/00006842-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Koch KL. Gastric dysrhythmias and the current status of electrogastrography. Pract Gastroenterol. 1989;13:37, 41–44. [PubMed] [Google Scholar]
- Koch KL. Illusory self-motion and motion sickness - a model for brain-gut interactions and nausea. Digestive Dis Sci. 1999;44:53S–57S. [PubMed] [Google Scholar]
- Koch KL. Gastric dysrhythmias: a potential objective measure of nausea. Exp Brain Res. 2014;232:2553–2561. doi: 10.1007/s00221-014-4007-9. [DOI] [PubMed] [Google Scholar]
- Koch KL, Summy-Long J, Bingaman S, Sperry N, Stern RM. Vasopressin and oxytocin responses to illusory self-motion and nausea in man. J Clin Endocrin Metab. 1990;71:1269–1275. doi: 10.1210/jcem-71-5-1269. [DOI] [PubMed] [Google Scholar]
- Kohl RL. Endocrine correlates of susceptibility to motion sickness. Aviat Space Environ Med. 1985;56:1158–1165. [PubMed] [Google Scholar]
- Kolev OI, Moller C, Nilsson G, Tibbling L. Responses in skin microcirculation to vestibular stimulation before and during motion sickness. Can J Neurol Sci. 1997;24:53–57. doi: 10.1017/s0317167100021090. [DOI] [PubMed] [Google Scholar]
- Kovacic K, Chelimsky G. Chronic idiopathic nausea. Pediatric Annals. 2014;43:e89–94. doi: 10.3928/00904481-20140325-10. [DOI] [PubMed] [Google Scholar]
- Lackner JR. Motion sickness: more than nausea and vomiting. Exp Brain Res. 2014;232:2493–2510. doi: 10.1007/s00221-014-4008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, Dizio P. Space motion sickness. Exp Brain Res. 2006;175:377–399. doi: 10.1007/s00221-006-0697-y. [DOI] [PubMed] [Google Scholar]
- Lacount L, Napadow V, Kuo B, Park K, Kim J, Brown E, Barbieri R. Dynamic cardiovagal response to motion sickness: a point-process heart rate variability study. Comput Cardiol. 2009;36:49–52. [PMC free article] [PubMed] [Google Scholar]
- LaCount LT, Barbieri R, Park K, Kim J, Brown EN, Kuo B, Napadow V. Static and dynamic autonomic response with increasing nausea perception. Aviat Space Environ Med. 2011;82:424–433. doi: 10.3357/asem.2932.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IM. The role of central and enteric nervous systems in the control of the retrograde giant contraction. J Neurogastroenterol Motil. 2015;22:321–32. doi: 10.5056/jnm15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IM, Marvig J, Sarna SK, Condon RE. Gastrointestinal myoelectric correlates of vomiting in the dog. Am J Physiol. 1986;251:G830–838. doi: 10.1152/ajpgi.1986.251.6.G830. [DOI] [PubMed] [Google Scholar]
- Lang IM, Sarna SK. Motor and myoelectric activity associated with vomiting, regurgitation, and nausea. In: Wood JD, editor. Handbook of Physiology, the Gastrointestinal System, vol. 1, Motility and Circulation. American Physiological Society; Bethesda, MD: 1989. pp. 1179–1198. [Google Scholar]
- Lang IM, Sarna SK, Shaker R. Gastrointestinal motor and myoelectric correlates of motion sickness. Am J Physiol. 1999;277:G642–652. doi: 10.1152/ajpgi.1999.277.3.G642. [DOI] [PubMed] [Google Scholar]
- Lawson BD, Mead AM. The sopite syndrome revisited: drowsiness and mood changes during real or apparent motion. Acta Astronautica. 1998;43:181–192. doi: 10.1016/s0094-5765(98)00153-2. [DOI] [PubMed] [Google Scholar]
- Lawson BD, Rieche BE. Perception of body motion. In: Hale KS, Stanney KM, editors. Handbook of Virtual Environments: Design, Implementation, and Applications. 2. CRC Press; Boca Raton, FL: 2015. pp. 163–196. [Google Scholar]
- Lawther A, Griffin MJ. Motion sickness and motion characteristics of vessels at sea. Ergonomics. 1988;31:1373–1394. doi: 10.1080/00140138808966783. [DOI] [PubMed] [Google Scholar]
- Lewis CT, Short C. 1879 A Latin Dictionary. Clarendon Press; Oxford: 2019. [Google Scholar]
- Li BU, Balint JP. Cyclic vomiting syndrome: evolution in our understanding of a brain-gut disorder. Adv Pediatr. 2000;47:117–160. [PubMed] [Google Scholar]
- Limebeer CL, Hall G, Parker LA. Exposure to a lithium-paired context elicits gaping in rats: A model of anticipatory nausea. Physiol Behav. 2006;88:398–403. doi: 10.1016/j.physbeh.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Lin CT, Chuang SW, Chen YC, Ko LW, Liang SF, Jung TP. EEG effects of motion sickness induced in a dynamic virtual reality environment. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:3872–3875. doi: 10.1109/IEMBS.2007.4353178. [DOI] [PubMed] [Google Scholar]
- Liveing E. On Megrim, Sick-Headache, and Some Allied Disorders. J. and A. Churchill; London: 1873. [Google Scholar]
- Lote K, Folling M, Lekven J, Rosengren B. Mesenteric arterial vasopressin in cats: local and systemic effects. Am J Roentgenology. 1981;136:969–975. doi: 10.2214/ajr.136.5.969. [DOI] [PubMed] [Google Scholar]
- Lutz EG. Motion sickness and occipital spiking in the electroencephalogram. J Clin Psychiatry. 1979;40:365. [PubMed] [Google Scholar]
- Magendie F. A Summary of Physiology. 2. E. J. Coale; Baltimore: 1824. p. 444. [Google Scholar]
- Malinska M, Zuzewicz K, Bugajska J, Grabowski A. Heart rate variability (HRV) during virtual reality immersion. Int J Occup Saf Ergon. 2015;21:47–54. doi: 10.1080/10803548.2015.1017964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DA, Furman JM, Balaban CD. Motion sickness in migraine sufferers. Expert Opin Pharmacother. 2005;6:2691–2697. doi: 10.1517/14656566.6.15.2691. [DOI] [PubMed] [Google Scholar]
- Matchock RL, Levine ME, Gianaros PJ, Stern RM. Susceptibility to nausea and motion sickness as a function of the menstrual cycle. Womens Health Issues. 2008;18:328–335. doi: 10.1016/j.whi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsangas P, McCauley ME. Sopite syndrome: a revised definition. Aviat Space Environ Med. 2014;85:672–673. doi: 10.3357/asem.3891.2014. [DOI] [PubMed] [Google Scholar]
- Matthews A, Haas DM, O’Mathuna DP, Dowswell T, Doyle M. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Systematic Rev. 2014;3:CD007575. doi: 10.1002/14651858.CD007575.pub3. [DOI] [PubMed] [Google Scholar]
- Mayou R, Kirmayer LJ, Simon G, Kroenke K, Sharpe M. Somatoform disorders: time for a new approach in DSM-V. Am J Psychiat. 2005;162:847–855. doi: 10.1176/appi.ajp.162.5.847. [DOI] [PubMed] [Google Scholar]
- Metz A, Hebbard G. Nausea and vomiting in adults--a diagnostic approach. Australian Fam Physic. 2007;36:688–692. [PubMed] [Google Scholar]
- Miaskiewicz SL, Stricker EM, Verbalis JG. Neurohypophyseal secretion in response to cholecystokinin but not meal-induced gastric distention in humans. J Clinical Endocrin Metab. 1989;68:837–843. doi: 10.1210/jcem-68-4-837. [DOI] [PubMed] [Google Scholar]
- Miller AD. Respiratory muscle control during vomiting. Canadian J Physiol Pharmacol. 1990;68:237–241. doi: 10.1139/y90-037. [DOI] [PubMed] [Google Scholar]
- Miller AD. Central mechanisms of vomiting. Digestive Dis Sci. 1999;44:39S–43S. [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res. 1994a;647:255–264. doi: 10.1016/0006-8993(94)91325-0. [DOI] [PubMed] [Google Scholar]
- Miller AD, Ruggiero DA. Emetic reflex are revealed by expression of the Immediate-Early gene C-Fos in the cat. J Neurosci. 1994b;14:871–888. doi: 10.1523/JNEUROSCI.14-02-00871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D, Laycock JD, Stephens WF. Motion sickness-induced pica in the rat. Am J Clinical Nutrition. 1977;30:147–150. doi: 10.1093/ajcn/30.2.147. [DOI] [PubMed] [Google Scholar]
- Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109:5–12. doi: 10.1152/jn.00457.2012. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Money KE. Motion sickness. Physiological Rev. 1970;50:1–39. doi: 10.1152/physrev.1970.50.1.1. [DOI] [PubMed] [Google Scholar]
- Money KE, Lackner JR, Cheung RSK. The autonomic nervous system and motion sickness. In: Yates BJ, Miller AD, editors. Vestibular Autonomic Regulation. CRC Press; Boca Raton: 1996. pp. 147–173. [Google Scholar]
- Money KE, Wood JD. Neural mechanisms underlying the symptomatology of motion sickness. Fourth Symposium on the Role of the Vestibular Organs in Space Exploration; Houston: NASA; 1970. pp. 35–44. NASA Special Publication no. 187. [Google Scholar]
- Morita M, Takeda N, Kubo T, Matsunaga T. Pica as an index of motion sickness in rats. ORL J Otorhinolaryngol Relat Spec. 1988;50:188–192. doi: 10.1159/000275989. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Angel C, Dubeshter B. Autonomic changes during cancer chemotherapy induced nausea and emesis. Br J Cancer Suppl. 1992;19:S42–45. [PMC free article] [PubMed] [Google Scholar]
- Mulavara AP, Verstraete MC, Bloomberg JJ. Modulation of head movement control in humans during treadmill walking. Gait Posture. 2002;16:271–282. doi: 10.1016/s0966-6362(02)00016-4. [DOI] [PubMed] [Google Scholar]
- Muth ER, Stern RM, Thayer JF, Koch KL. Assessment of the multiple dimensions of nausea: the Nausea Profile (NP) J Psychosomatic Res. 1996;40:511–520. doi: 10.1016/0022-3999(95)00638-9. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Davis SL, Blackmore KL, Vakulin A, Nesbitt KV. Cybersickness provoked by head-mounted display affects cutaneous vascular tone, heart rate and reaction time. Physiol Behav. 2015;151:583–590. doi: 10.1016/j.physbeh.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Napadow V, Sheehan JD, Kim J, Lacount LT, Park K, Kaptchuk TJ, Rosen BR, Kuo B. The brain circuitry underlying the temporal evolution of nausea in humans. Cerebral Cortex. 2013;23:806–813. doi: 10.1093/cercor/bhs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel G, Eiken O, Tribukait A, Kolegard R, Mekjavic IB. Motion sickness increases the risk of accidental hypothermia. Eur J Appl Physiol. 2006;98:48–55. doi: 10.1007/s00421-006-0217-6. [DOI] [PubMed] [Google Scholar]
- Nobel G, Tribukait A, Mekjavic IB, Eiken O. Effects of motion sickness on thermoregulatory responses in a thermoneutral air environment. Eur J Appl Physiol. 2012;112:1717–1723. doi: 10.1007/s00421-011-2142-6. [DOI] [PubMed] [Google Scholar]
- Nussey SS, Hawthorn J, Page SR, Ang VT, Jenkins JS. Responses of plasma oxytocin and arginine vasopressin to nausea induced by apomorphine and ipecacuanha. Clin Endocrinol (Oxf) 1988;28:297–304. doi: 10.1111/j.1365-2265.1988.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Ohyama S, Nishiike S, Watanabe H, Matsuoka K, Akizuki H, Takeda N, Harada T. Autonomic responses during motion sickness induced by virtual reality. Auris Nasus Larynx. 2007;34:303–306. doi: 10.1016/j.anl.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Olden KW, Crowell MD. Chronic nausea and vomiting: new insights and approach to treatment. Curr Treat Options Gastroenterol. 2005;8:305–310. doi: 10.1007/s11938-005-0023-y. [DOI] [PubMed] [Google Scholar]
- Otto B, Riepl RL, Otto C, Klose J, Enck P, Klosterhalfen S. mu-Opiate receptor agonists -- a new pharmacological approach to prevent motion sickness? British J Clinical Pharmacol. 2006;61:27–30. doi: 10.1111/j.1365-2125.2005.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JR, Lim DW, Lee SY, Lee HW, Choi MH, Chung SC. Long-term study of simulator sickness: differences in EEG response due to individual sensitivity. Int J Neurosci. 2008;118:857–865. doi: 10.1080/00207450701239459. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Parker LA. The role of nausea in taste avoidance learning in rats and shrews. Autonomic Neurosci: Basic and Clinical. 2006;125:34–41. doi: 10.1016/j.autneu.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Parker LA. Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. Europ J Pharmacol. 2014;722:122–133. doi: 10.1016/j.ejphar.2013.09.070. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL. Conditioned gaping in rats: a selective measure of nausea. Autonomic Neurosci: Basic and Clinical. 2006;129:36–41. doi: 10.1016/j.autneu.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Canadian J Exper Psychology. 2008;62:198–209. doi: 10.1037/a0012531. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rock EM, Sticht MA, Wills KL, Limebeer CL. Cannabinoids suppress acute and anticipatory nausea in preclinical rat models of conditioned gaping. Clinical Pharmacol Therapeut. 2015;97:559–561. doi: 10.1002/cpt.98. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Reduced normogastric electrical activity associated with emesis: a telemetric study in ferrets. World J Gastroenterol. 2009;15:6034–6043. doi: 10.3748/wjg.15.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Telemetry in a motion-sickness model implicates the abdominal vagus in motion-induced gastric dysrhythmia. Exp Physiol. 2010;95:768–773. doi: 10.1113/expphysiol.2009.052001. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans. Exp Brain Res. 1990;82:97–102. doi: 10.1007/BF00230842. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Levik Y, Berthoz A. Head and trunk movements in the frontal plane during complex dynamic equilibrium tasks in humans. Exp Brain Res. 1995;106:327–338. doi: 10.1007/BF00241128. [DOI] [PubMed] [Google Scholar]
- Pratt A, Lazar RM, Penman D, Holland JC. Psychological parameters of chemotherapy-induced conditioned nausea and vomiting: a review. Cancer Nurs. 1984;7:483–490. [PubMed] [Google Scholar]
- Proctor JD, Chremos AN, Evans EF, Wasserman AJ. An apomorphine-induced vomiting model for antiemetic studies in man. J Clin Pharmacol. 1978;18:95–99. doi: 10.1002/j.1552-4604.1978.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Quincy J. Lexicon Physico-Medicum; or, a New Medicinal Dictionary, Explaining the Difficult Terms Used in the Several Branches of the Profession, and in such Parts of Natural Philosophy, As are Introductory thereunto. T. Longman; London: 1787. [Google Scholar]
- Rädlein J. Europäischer Sprach-Schatz Oder An Wörtern so wol als auserlesenen und der Sprachen Eigenschafft gemässen Redens-Arten reiches und vollkommenes Wörter-Buch Der vornehmsten Sprachen in Europa, In Drey Theile verfasset, in sich begreiffende I. Das Teutsche, Italiänische und Frantzösische, II. Das Frantzösische, Teutsche und Italiänische, III. Das Italiänische, Teutsche und Frantzösische. Johann Friedrich Braun; Leipzig: 1711. p. 1127. [Google Scholar]
- Ramirez E, Ortega AR, Reyes Del Paso GA. Anxiety, attention, and decision making: The moderating role of heart rate variability. International J Psychophysiol. 2015;98:490–496. doi: 10.1016/j.ijpsycho.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Robertson GL. The regulation of vasopressin function in health and disease. Recent Prog Horm Res. 1976;33:333–385. doi: 10.1016/b978-0-12-571133-3.50015-5. [DOI] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Parker LA. Effect of combined doses of Delta(9)-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea using rat (Sprague- Dawley) models of conditioned gaping. Psychopharmacol (Berl) 2015;232:4445–4454. doi: 10.1007/s00213-015-4080-1. [DOI] [PubMed] [Google Scholar]
- Rolfe JC. Some references to seasickness in the Greek and Latin writers. American Journal Philology. 1904;25:192–200. [Google Scholar]
- Roscoe JA, Morrow GR, Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer. 2011;19:1533–1538. doi: 10.1007/s00520-010-0980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Shelton RL, Helderman JH, Vestal RE, Robertson GL. Influence of the emetic reflex on vasopressin release in man. Kidney International. 1979;16:729–735. doi: 10.1038/ki.1979.189. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Yamamoto K, Yamatodani A, Takeda N. Differential action of ondansetron and dexamethasone to modify cisplatin-induced acute and delayed kaolin consumption (“pica”) in rats. European J Pharmacol. 2002;454:47–52. doi: 10.1016/s0014-2999(02)02472-x. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Broad J, Andrews PL. The relationship between gastric motility and nausea: gastric prokinetic agents as treatments. European J Pharmacol. 2013;715:10–14. doi: 10.1016/j.ejphar.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Schaub N, Ng K, Kuo P, Aziz Q, Sifrim D. Gastric and lower esophageal sphincter pressures during nausea: a study using visual motion-induced nausea and high-resolution manometry. Am J Physiol Gastro Liver Physiol. 2014;306:G741–747. doi: 10.1152/ajpgi.00412.2013. [DOI] [PubMed] [Google Scholar]
- Sclocco R, Citi L, Garcia RG, Cerutti S, Bianchi AM, Kuo B, Napadow V, Barbieri R. Combining sudomotor nerve impulse estimation with fMRI to investigate the central sympathetic response to nausea. IEEE Engineering in Medicine and Biology Society Conference. 2015;2015:4683–4686. doi: 10.1109/EMBC.2015.7319439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclocco R, Kim J, Garcia RG, Sheehan JD, Beissner F, Bianchi AM, Cerutti S, Kuo B, Barbieri R, Napadow V. Brain circuitry supporting multi-organ autonomic outflow in response to nausea. Cerebral Cortex. 2016;26:485–497. doi: 10.1093/cercor/bhu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneca L. Ad Lucilium Epistulae Morales. Vol. 1. Harvard University Press; Cambridge, MA: 1917. [Google Scholar]
- Sheps DS, Sheffield D. Depression, anxiety, and the cardiovascular system: the cardiologist’s perspective. J Clinical Psychiatry. 2001;62(Suppl 8):12–18. [PubMed] [Google Scholar]
- Singh P, Yoon SS, Kuo B. Nausea: a review of pathophysiology and therapeutics. Therap Adv Gastroenterol. 2016;9:98–112. doi: 10.1177/1756283X15618131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstein A. Effect of vasopressin on blood flow distribution in the stomach of cats with gastric ulcer. Scand J Gastroenterol. 1978;13:783–788. doi: 10.3109/00365527809182191. [DOI] [PubMed] [Google Scholar]
- Skinner WW. Recent Studies in Naupathia, or Seasickness: Symptomatology, Diagnosis, Pathogenesis and Treatment by a new and Efficacious Method. D. Appleton and Company; New York, NY: 1894. p. 48. [Google Scholar]
- Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans -- review article. Pain. 2002;96:3–8. doi: 10.1016/s0304-3959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- Song G, Hou X, Yang B, Sun Y, Liu J, Qian W, Chen JD. Efficacy and efficiency of gastric electrical stimulation with short pulses in the treatment of vasopressin-induced emetic responses in dogs. Neurogastro Motility. 2006;18:385–391. doi: 10.1111/j.1365-2982.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- Sorensen PS, Hammer M. Vasopressin in plasma and ventricular cerebrospinal fluid during dehydration, postural changes, and nausea. Am J Physiol. 1985;248:R78–83. doi: 10.1152/ajpregu.1985.248.1.R78. [DOI] [PubMed] [Google Scholar]
- Spiri D, Rinaldi VE, Titomanlio L. Pediatric migraine and episodic syndromes that may be associated with migraine. Ital J Pediatr. 2014;40:92. doi: 10.1186/s13052-014-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JP, Balaban CD, Furman JM. Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol. 2013;33:297–306. doi: 10.1055/s-0033-1356462. [DOI] [PubMed] [Google Scholar]
- Stern RM, Koch KL, Andrews PLR. Nausea: Mechanisms and Management. Oxford University Press; New York: 2011. [Google Scholar]
- Stern RM, Koch KL, Leibowitz HW, Lindblad IM, Shupert CL, Stewart WR. Tachygastria and motion sickness. Aviation Space Environ Med. 1985;56:1074–1077. [PubMed] [Google Scholar]
- Stockhorst U, Steingrueber HJ, Enck P, Klosterhalfen S. Pavlovian conditioning of nausea and vomiting. Auton Neurosci. 2006;129:50–57. doi: 10.1016/j.autneu.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Sugino S, Janicki PK. Pharmacogenetics of chemotherapy-induced nausea and vomiting. Pharmacogenomics. 2015;16:149–160. doi: 10.2217/pgs.14.168. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki T, DeStefino VJ, Yates BJ. Integrative responses of neurons in nucleus tractus solitarius to visceral afferent stimulation and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1380–1390. doi: 10.1152/ajpregu.00361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara FA, Farewell J, Mintz L, Johnson WH. Pharmacological interventions for motion sickness: cardiovascular effects. Aviat Space Environ Med. 1987;58:A270–276. [PubMed] [Google Scholar]
- Sunahara FA, Johnson WH, Taylor NB. Vestibular stimulation and forearm blood flow. Can J Physiol Pharmacol. 1964;42:199–207. doi: 10.1139/y64-023. [DOI] [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- Tarbell SE, Shaltout HA, Wagoner AL, Diz DI, Fortunato JE. Relationship among nausea, anxiety, and orthostatic symptoms in pediatric patients with chronic unexplained nausea. Exp Brain Res. 2014;232:2645–2650. doi: 10.1007/s00221-014-3981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommaséo N. Nuovo Dizionario dei Sinomimi della Lingua Italiana Presso Gio. Pietro Vieusseux; Firenze: 1838. [Google Scholar]
- Tonello L, Rodrigues FB, Souza JW, Campbell CS, Leicht AS, Boullosa DA. The role of physical activity and heart rate variability for the control of work related stress. Front Physiol. 2014;5:67. doi: 10.3389/fphys.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen L, Von Holle A, Reichborn-Kjennerud T, Knoph-berg C, Hamer R, Sullivan P, Bulik CM. Nausea and vomiting of pregnancy in women with Bullimia Nervosa and eating disorders not otherwise specified. Int J Eat Disord. 2008;41:722–727. doi: 10.1002/eat.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ombergen A, Lawson BD, Wuyts FL. Motion sickness and sopite syndrome associated with parabolic flights: a case report. Int J Audiol. 2015;55:189–94. doi: 10.3109/14992027.2015.1111526. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–1419. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–1419. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Richardson DW, Stricker EM. Vasopressin release in response to nausea-producing agents and cholecystokinin in monkeys. Am J Physiol. 1987;252:R749–753. doi: 10.1152/ajpregu.1987.252.4.R749. [DOI] [PubMed] [Google Scholar]
- Wertheim AH. Working in a moving environment. Ergonomics. 1998;41:1845–1858. doi: 10.1080/001401398186018. [DOI] [PubMed] [Google Scholar]
- Wiesmann T, Kranke P, Eberhart L. Postoperative nausea and vomiting - a narrative review of pathophysiology, pharmacotherapy and clinical management strategies. Expert Opin Pharmacother. 2015;16:1069–1077. doi: 10.1517/14656566.2015.1033398. [DOI] [PubMed] [Google Scholar]
- Wilkens EP, Yates BJ. Pretreatment with ondansetron blunts plasma vasopressin increases associated with morphine administration in ferrets. Anesthes Analges. 2005;101:1029–1033. doi: 10.1213/01.ane.0000166954.61498.2c. [DOI] [PubMed] [Google Scholar]
- Willis T. In: The London Practice of Physick, being the Practical Part of Physick Contain’d in the Works of the Famous. Basset Willis T, Dring T, Harper C, Crook W., editors. London: 1692. [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Matsunaga S, Matsui M, Takeda N, Yamatodani A. Pica in mice as a new model for the study of emesis. Methods Find Exp Clin Pharmacol. 2002a;24:135–138. doi: 10.1358/mf.2002.24.3.802297. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takeda N, Yamatodani A. Establishment of an animal model for radiation-induced vomiting in rats using pica. J Radiat Res. 2002b;43:135–141. doi: 10.1269/jrr.43.135. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Catanzaro MF, Miller DJ, McCall AA. Integration of vestibular and emetic gastrointestinal signals that produce nausea and vomiting: potential contributions to motion sickness. Exp Brain Res. 2014;232:2455–2469. doi: 10.1007/s00221-014-3937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998;47:395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]