Abstract
Background
Drug interactions, particularly those involving warfarin, are a major clinical and public health problem. Minimizing serious bleeding caused by anticoagulants is a recent major focus of the United States (US) Department of Health and Human Services. This study quantified the risk of gastrointestinal bleeding (GIB) and intracranial hemorrhage (ICH) among concomitant users of warfarin and individual antihyperlipidemics.
Methods
The authors conducted a high-dimensional propensity score-adjusted cohort study of new concomitant users of warfarin and an antihyperlipidemic, among US Medicaid beneficiaries from five states during 1999–2011. Exposure was defined by concomitant use of warfarin plus one of eight antihyperlipidemics. The primary outcome measure was a composite of GIB/ICH within the first 30 days of concomitant use. As a secondary outcome measure, GIB/ICH was examined within the first 180 days of concomitant use.
Results
Among 236,691 persons newly-exposed to warfarin and an antihyperlipidemic, the crude incidence of GIB/ICH was 13.2 (95% confidence interval 12.7 to 13.8) per 100 person-years. Users were predominantly older, female, and Caucasian. Adjusted hazard ratios (aHRs) for warfarin and individual statins were consistent with no association. Warfarin + gemfibrozil was associated with an 80% increased risk of GIB/ICH within the first month of concomitant use (aHR = 1.8, 1.4 to 2.4). Warfarin + fenofibrate was associated with a similar increased risk (aHR = 1.8, 1.2 to 2.7), yet with an onset during the second month of concomitant use.
Conclusions
Among warfarin-treated persons, the use of fibrates—but not statins—increases the risk of hospital presentation for GIB/ICH.
Keywords: cohort studies, drug interactions, hemorrhage, Medicaid, pharmacoepidemiology, warfarin
1. INTRODUCTION
Drug-drug interactions (DDIs) are a large and growing clinical and public health problem, especially in older adults, of whom >76% take two or more drugs[1] and >50% take five or more drugs in a given month.[2] Given the high prevalence of polypharmacy in older adults, it is not surprising that known DDIs are responsible for 13% of all adverse drug events[3] and 4.8% of hospital admissions[4] in this population. Relatively few studies have examined the health effects of specific potential DDI pairs in populations, which leave critical knowledge gaps for clinicians, patients, editors and users of DDI compendia, and those who manage and use clinical decision support systems. Recognizing these gaps, attendees of a 2009 stakeholder meeting on DDIs made the conduct of additional research on the health effects of DDIs its principal recommendation.[5]
Anticoagulants have been consistently identified as among the most common causes of serious adverse drug events.[6] Underscoring this, the United States (US) Department of Health and Human Services issued a National Action Plan for Adverse Drug Event Prevention that identified serious bleeding caused by anticoagulants as one of only three adverse drug events specifically targeted because they are common, serious, and potentially preventable.[6] Despite the rapid market uptake of the direct oral anticoagulants, warfarin remains the most widely-prescribed anticoagulant.[7] DDIs involving warfarin are of major concern, since the drug: is commonly-used;[7,8] has a narrow therapeutic index; may interact with almost every therapeutic class;[9] and is the leading cause of adverse drug event-related hospitalizations in older adults.[10] Further, clinical sequelae resulting from over-anticoagulation—particularly gastrointestinal bleeding (GIB) and intracranial hemorrhage (ICH), the vast majority of serious bleeds in warfarin-treated persons[11]—are common and can be fatal.[12] Given that most persons treated with warfarin are older adults,[8] and dyslipidemia is a prevalent comorbidity in older individuals, many such persons are concomitantly-treated with an antihyperlipidemic to manage cardiovascular disease risk. In fact, publically-available data from the US Centers for Disease Control and Prevention[13] suggest that 46% of warfarin-treated older adults take a statin or fibrate.
Drug interactions with warfarin may potentiate bleeding risk via inhibition of hepatic cytochrome P450 (CYP) enzymes responsible for its metabolism. In particular, antihyperlipidemic drugs may inhibit CYP2C9, CYP3A, and CYP1A2,[14,15] each of which is partially responsible for the inactivation of warfarin.[16] Antihyperlipidemics may also displace warfarin from plasma proteins, thereby increasing free plasma concentrations of warfarin;[17-19] these displacement effects may be transient and are of debated clinical relevance. Such mechanisms might result in over-anticoagulation in concomitant users of warfarin and certain antihyperlipidemics. Further, there is some suggestion that statins and fibrates themselves may have antiplatelet and anticoagulant properties.[20-22] Yet, statins alone have not been associated with GIB[23] or ICH.[24,25] Corresponding outcome data on fibrates alone are scant.
We therefore quantified and compared the rates of GIB/ICH among concomitant users of warfarin and individual antihyperlipidemics.
2. METHODS
2.1. Overview and study population
We conducted a high-dimensional propensity score (hdPS)-adjusted cohort study of adult users of warfarin. The cohort consisted exclusively of person-time concomitantly-exposed to warfarin plus one of the following antihyperlipidemics: atorvastatin; fenofibrate (including fenofibric acid[26]); fluvastatin; gemfibrozil; lovastatin; pravastatin; rosuvastatin; or simvastatin. Study data included demographic, enrollment, and healthcare claims from the Medicaid programs of California, Florida, New York, Ohio, and Pennsylvania from 1999–2011.[27] These states comprise ~38% of the US Medicaid population,[28] with the 13-year dataset recording the experience of more than 69 million cumulative enrollees and nearly 222 million person-years (p-y) of observation. Because a substantive proportion of Medicaid beneficiaries are co-enrolled in Medicare,[29-31] we also obtained Medicare claims to ascertain a more complete picture of enrollees’ healthcare.[32] Findings from 1999–2003 data, for a subset of the drug pairs examined herein and using different methods, were reported previously.[33]
2.2. Defining the study cohort
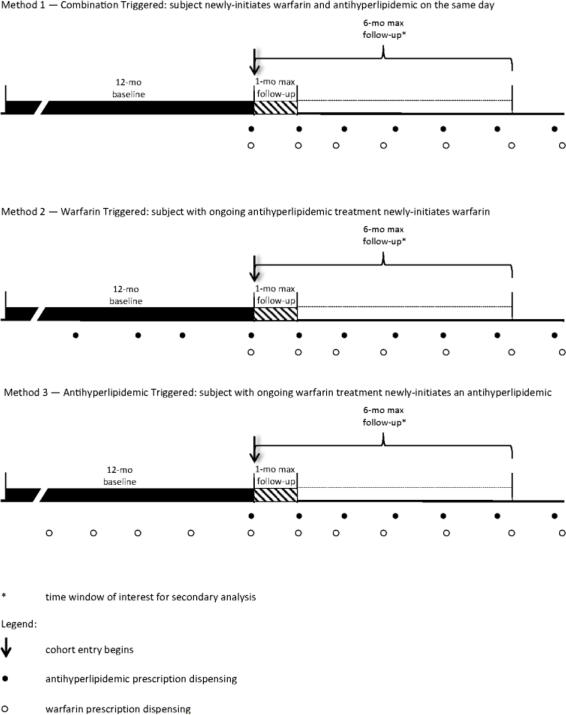
Persons under study were apparent new users of concomitant therapy, i.e., having ≥12 months of Medicaid enrollment before their first overlapping use of warfarin and an antihyperlipidemic. The day on which users were first co-exposed defined cohort entry. The 12-month period immediately preceding cohort entry served as the baseline period. Use of a fixed baseline period is standard in studies utilizing hdPS methods.[34] Persons entered the cohort without regard to the initiation order of warfarin and the antihyperlipidemic (Figure 1).[35]
Figure 1.
Three methods by which concomitant warfarin and antihyperlipidemic users could enter the study cohort
Persons were excluded if <18 or ≥100 years of age. Persons with exposure to a nonwarfarin oral anticoagulant (e.g., dabigatran) during the baseline period were not excluded from the cohort, as patients can switch anticoagulants; however, baseline use of a non-warfarin oral anticoagulant was a prespecified covariate included in the PS. Baseline use of a subcutaneous or injectable anticoagulant was treated in the same manner. Neither baseline GIB nor ICH served as an exclusion criterion, as bleeding events can recur;[36] as above, prior GIB/ICH was a prespecified variable included in the PS. Further, we conducted a prespecified secondary analysis limited to incident outcomes.
Follow-up began upon cohort entry and continued until the first occurrence of the following: a) outcome of interest (defined below); b) death, as ascertained from linkage to the Social Security Administration Death Master File (National Technical Information Service: Alexandria, VA); c) the 31st day of follow-up (relaxed to the 181st day in a secondary analysis); d) >15-day gap in either warfarin or antihyperlipidemic therapy; e) prescription for a non-warfarin oral anticoagulant or antihyperlipidemic different than that upon cohort entry (i.e., indicative of switching to an alternate therapy); f) disenrollment from Medicaid; or g) the end of the dataset. Follow-up time occurring during a period of hospitalization was excluded, although hospitalization did not serve as a censoring event. This exclusion served to minimize immeasurable time bias.[37]
2.3. Exposure and covariate ascertainment
Exposure was defined by the antihyperlipidemic active on the day of cohort entry. The following antihyperlipidemics were excluded because of scant use: cerivastatin; clofibrate; and pitavastatin. Pravastatin served as the reference exposure, as it is a negligible inhibitor of CYP isozymes[38] involved in the metabolism of warfarin.[16] Therefore, pravastatin would not be expected to interact pharmacokinetically with warfarin. Further, statins alone (including pravastatin[39]) have not been shown to, themselves, impact bleeding risk.[23,25,39,40]
Potential confounders included prespecified variables and those identified via empiric methods, both of which informed the PS. Prespecified variables included demographics, baseline measures of intensity of healthcare utilization, baseline drug exposures, and baseline comorbidities. Empiric covariates included those identified during baseline via a high-dimensional approach,[41,42] which ranks and selects potential confounders (or proxies thereof) based on their empirical associations with exposure and outcome (see specifications in Appendix Table 1).
2.4. Outcome ascertainment
The outcome of interest was a composite of GIB or ICH within 30 days of cohort entry (relaxed to within 180 days in a secondary analysis). Operational definitions, including quantitative measures of algorithm performance,[33,43-45] are presented in Table 1. GIB and ICH are the most common types of serious bleeding attributed to anticoagulants (capturing the vast majority of all events and nearly all serious events) and outcomes that can be fatal[11,12]—this was the rationale for studying a composite outcome of bleeding from these sites.
Table 1.
Operational definition of composite outcome of interest
| Outcome component | Diagnosis | ICD-9-CM discharge diagnosis code(s) | Discharge diagnosis position and claim type | PPV |
|---|---|---|---|---|
| Gastrointestinal bleeding | esophageal ulcer, with hemorrhage | 530.21 | Any-position discharge diagnosis on an inpatient hospitalization claim | ~81%[33] |
| gastric ulcer, with hemorrhage | 531.0X, 531.2X, 531.4X, 531.6X | |||
| duodenal ulcer, with hemorrhage | 532.0X, 532.2X, 532.4X, 532.6X | |||
| peptic ulcer, with hemorrhage | 533.0X, 533.2X, 533.4X, 533.6X | |||
| gastrojejunal ulcer, with hemorrhage | 534.0X, 534.2X, 534.4X, 534.6X | |||
| gastritis and duodenitis, with hemorrhage | 535.01, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 535.71 | |||
| other specified disorder of stomach and duodenum, with hemorrhage | 537.83, 537.84 | |||
| diverticula of intestine, with hemorrhage | 562.02, 562.03, 562.12, 562.13 | |||
| other disorders of intestine, with hemorrhage | 569.85, 569.86 | |||
| gastrointestinal hemorrhage | 578.X | |||
| Intracranial hemorrhage | subarachnoid hemorrhage | 430 | Any-position discharge diagnosis on an emergency department or inpatient hospitalization claim | ~77-94%[43-45] |
| intracerebral hemorrhage | 431 |
ICD-9-CM = International Classification of Diseases 9th Revision Clinical Modification; PPV = positive predictive value
2.5. Statistical analysis
We calculated descriptive statistics for baseline variables and calculated incidence and unadjusted association measures, the latter via Cox proportional-hazards models. We utilized the hdPS approach to reduce the impact of measured potential confounders. However, as we wished to compare multiple antihyperlipidemic drugs to a common active comparator, matching on PS was impractical, and the hdPS algorithm has so far been developed only for pairwise comparisons.[42,46] As described below, we therefore used pairwise hdPS to identify potential confounders for each antihyperlipidemic drug versus pravastatin and included all such empirically-identified variables (plus prespecified variables) in a multinomial PS model. We first used the hdPS program[42,46] to identify the 200 most prevalent diagnosis, procedure and drug codes (excluding drug codes indicative of warfarin or antihyperlipidemic prescribing) in each of nine data dimensions, to assess their associations with the antihyperlipidemic of interest versus pravastatin, and to assess their associations with the outcome. We then used these associations to select the top 500 codes with the largest potential for causing confounding. Because of the large number of variables in the final multinomial PS model, empirically-identified covariates did not include measures of frequency (i.e., sporadic, frequent) as generated by the hdPS program. Then, the union of all confounders arising from the seven sets of 500 hdPS-identified variables (one for each antihyperlipidemic versus pravastatin) were included in the multinomial PS. Prespecified covariates included in the multinomial PS model are presented in Appendix Table 2. The multinomial PSs were modeled using multinomial logistic regression,[47] generating for each subject the predicted probability of receiving each antihyperlipidemic drug. These PSs were then included in the outcome model as continuous covariates.[48] PS-adjusted HRs and 95% CIs were calculated via Cox proportional-hazards regression. Association measures were examined overall within the first 30 days of follow-up and also stratified as three prespecified, mutually exclusive time periods (i.e., 1–10 days, 11–20 days, and 21–30 days).
Numerous prespecified secondary analyses (Table 2) were conducted to assess the robustness of our primary findings. Primary and secondary analyses were conducted using SAS v9.4 (SAS Institute Inc.: Cary, NC). The research described herein was approved by the institutional review board of the University of Pennsylvania.
Table 2.
Prespecified secondary analyses
| Analysis | Rationale |
|---|---|
| Minimizing the role of chance, bias, and/or confounding | |
| Exclusion of persons with baseline history of GIB/ICH | Restricts study to incident outcomes; analysis minimizes potential residual confounding in studying incident and recurrent outcomes, as incident events may predispose an individual for a second event |
| Exclusion of persons with baseline enrollment in Medicaid managed care | Restricts study to enrollees thought to have complete data capture, as there may be incomplete capture of hospitalizations among Medicaid managed care enrollees; analysis minimizes potential misclassification of outcomes and covariates |
| Exclusion of ICH outcomes with a co-occurring intracranial injury diagnosis* | Restricts study to outcomes with a higher likelihood of DDI as etiology, as injury may cause ICH; analysis strengthens causality of associations |
| Exclusion of empirical covariates from the PS thought to be strong correlates of exposure but not associated with the outcome | Restricts PS covariates to non-instruments, as inclusion of an instrumental variable in a PS can increase standard error and bias; analysis minimizes standard error and bias |
| Exclusion of persons with a pre-cohort entry to on-or-after cohort entry increase in warfarin dose | Restricts underlying cohort to enrollees on a stable dose of warfarin, as outcomes in persons with a warfarin dose increase may be attributable to an exaggerated pharmacodynamic effect of warfarin alone and not a DDI between warfarin and an antihyperlipidemic; analysis strengthens causality of associations |
| Further elucidating the association between exposure and outcome | |
| Increasing maximum follow-up time to 180 days, including examining the following discreet time windows: 1–29 days; 30–59 days; 60–119 days; and 120–180 days | Allows for the elucidation of a potential delayed-onset DDI between warfarin and an antihyperlipidemic |
| Examining effect modification by presumed indication for warfarin† | Allows for the elucidation of the impact of level of anticoagulation; indication serves as an indirect surrogate for clinician-desired level of anticoagulation |
| Examining effect modification by laboratory monitoring for level of anticoagulation | Allows for the elucidation of the impact of clinician vigilance in managing level of anticoagulation; clinicians may increase monitoring when adding drugs that putatively interact with warfarin |
| Examining effect modification by duration of prior warfarin use‡ | Allows for elucidation of effect of depletion of susceptibles |
DDI = drug-drug interaction; GIB = gastrointestinal bleeding; ICH = intracranial hemorrhage; PS = propensity score
events with an International Classification of Diseases 9th Revision Clinical Modification discharge diagnosis code indicative of intracranial injury (with or without skull fracture) during the same hospitalization as the putative outcome, defined by 800.X–804.X (fractures: fracture of skull) or 850.X–854.X (intracranial injury excluding those with skull fracture)
venous thromboembolism vs. atrial fibrillation/flutter vs. valvular heart disease vs. other indication
new warfarin initiator (i.e., first warfarin dispensing on cohort entry) vs. recent warfarin initiator (i.e., first warfarin dispensing within 45 days prior to cohort entry) vs. chronic warfarin user (i.e., first warfarin dispensing greater than 45 days prior to cohort entry)
3. RESULTS
3.1 Cohort characteristics and outcome frequency
We identified 236,691 concomitant users of warfarin and an antihyperlipidemic of interest; their baseline characteristics are presented in Table 3. Overall, users were predominantly female (63.0%) and Caucasian (54.1%), with a median age of 71.0 years. In the primary analysis examining events within the first 30 days of concomitant use, these individuals contributed 18,135p-y of concomitant exposure, during which we identified 2,401 GIB/ICH outcomes (unadjusted incidence rate = 13.2 per 100p-y [95% confidence interval 12.7 to 13.8]). Findings stratified by individual antihyperlipidemics are presented in Table 3. In the secondary analysis examining events within the first 180 days of concomitant use, we identified 4,621 outcomes during 51,905p-y of concomitant exposure (unadjusted incidence rate = 8.9 per 100p-y [8.6 to 9.2]). These incidence rates are similar to prior findings, particularly within older adult populations.[49-51]
Table 3.
Characteristics of warfarin users, by antihyperlipidemic exposure group, and measures of association for the primary analysis
| Statins | Fibrates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pravastatin | atorvastatin | fluvastatin | lovastatin | rosuvastatin | simvastatin | fenofibrate | gemfibrozil | ||
| Ns (unless otherwise noted) | |||||||||
| Users, concomitant with warfarin | 20,238 | 79,391 | 2,793 | 14,316 | 11,763 | 94,738 | 7,807 | 5,645 | |
| Person-years of follow-up | 1,555 | 6,119 | 215 | 1,104 | 896 | 7,268 | 564 | 415 | |
| GIB/ICH outcomes within 30 days of cohort entry | 192 | 778 | 23 | 104 | 100 | 1,073 | 58 | 73 | |
| GIB outcomes within 30 days of cohort entry | 166 | 685 | * | 91 | 87 | 955 | 51 | * | |
| ICH outcomes within 30 days of cohort entry | 26 | * | * | * | * | * | * | * | |
| Both outcomes (contemporaneously) within 30 days of cohort entry | 0 | * | * | * | * | * | * | * | |
| Unadjusted incidence rate of GIB/ICH, per 100 person-years | 12.3 | 12.7 | 10.7 | 9.4 | 11.2 | 14.8 | 10.3 | 17.6 | |
| Measures of Association | |||||||||
| Unadjusted hazard ratio for GIB/ICH within 30 days of cohort entry (95% CI) | 1.00 (reference) | 1.03 (0.88-1.21) | 0.87 (0.56-1.34) | 0.76 (0.60-0.97) | 0.90 (0.71-1.15) | 1.20 (1.03-1.39) | 0.83 (0.62-1.12) | 1.42 (1.09-1.86) | |
| Adjusted hazard ratio for GIB/ICH within 30 days of cohort entry (95% CI); see • in Figure 2 | 1.00 (reference) | 0.99 (0.84-1.16) | 1.13 (0.73-1.75) | 0.99 (0.78-1.27) | 0.87 (0.68-1.11) | 1.02 (0.87-1.19) | 1.03 (0.76-1.38) | 1.79 (1.36-2.35) | |
| Demographics | Group | % (unless otherwise noted) | |||||||
| Event triggering concomitant use (see Figure 1) | combination triggered | 6.2 | 7.0 | 5.2 | 7.3 | 4.0 | 8.4 | 2.6 | 4.5 |
| warfarin triggered | 59.7 | 61.1 | 63.5 | 60.3 | 64.5 | 63.2 | 60.1 | 52.1 | |
| antihyperlipidemic triggered | 34.1 | 31.9 | 31.3 | 32.4 | 31.5 | 28.4 | 37.3 | 43.4 | |
| Age in years at cohort entry, continuous | Median (Q1-Q3) | 71.4 (61.1-79.0) | 70.7 (60.3-78.6) | 71.4 (61.8-79.1) | 73.7 (64.7-80.9) | 70.8 (60.4-78.4) | 71.2 (61.0-79.4) | 67.0 (54.5-76.6) | 66.1 (53.8-75.4) |
| Sex | female | 63.7 | 63.4 | 70.1 | 64.9 | 63.9 | 62.7 | 59.2 | 55.2 |
| Race | white | 54.8 | 55.0 | 58.1 | 51.4 | 52.3 | 52.4 | 66.9 | 56.7 |
| black | 15.0 | 15.2 | 13.4 | 14.5 | 12.8 | 17.3 | 6.4 | 8.6 | |
| hispanic/latino- | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 | 0.4 | |
| other/unknown | 29.9 | 29.5 | 28.2 | 33.8 | 34.7 | 30.1 | 26.4 | 34.3 | |
| State of residence | CA | 40.9 | 39.3 | 45.1 | 49.7 | 27.0 | 31.7 | 27.5 | 48.1 |
| FL | 12.9 | 8.9 | 13.8 | 14.3 | 24.9 | 15.2 | 18.4 | 15.0 | |
| NY | 23.9 | 27.9 | 17.5 | 19.2 | 32.8 | 28.6 | 22.8 | 17.8 | |
| OH | 9.4 | 12.0 | 8.7 | 6.7 | 7.8 | 11.5 | 16.6 | 9.7 | |
| PA | 12.8 | 11.9 | 14.9 | 10.2 | 7.6 | 12.9 | 14.7 | 9.4 | |
| Calendar year of cohort entry | 2000 | 7.4 | 5.4 | 11.3 | 1.3 | 0.0 | 2.2 | 1.9 | 7.0 |
| 2001 | 8.9 | 7.2 | 11.5 | 1.1 | 0.0 | 3.3 | 3.3 | 8.7 | |
| 2002 | 9.1 | 8.1 | 14.7 | 1.6 | 0.0 | 3.8 | 4.5 | 9.2 | |
| 2003 | 10.0 | 8.8 | 13.9 | 4.4 | 0.7 | 4.3 | 6.0 | 9.1 | |
| 2004 | 8.3 | 9.4 | 11.1 | 5.8 | 4.4 | 3.9 | 6.0 | 7.3 | |
| 2005 | 7.3 | 11.2 | 10.1 | 7.8 | 6.2 | 5.5 | 8.1 | 7.5 | |
| 2006 | 7.8 | 15.2 | 13.9 | 25.8 | 13.1 | 9.1 | 12.6 | 11.5 | |
| 2007 | 5.2 | 10.0 | 5.2 | 13.7 | 10.6 | 10.6 | 10.3 | 8.5 | |
| 2008 | 5.7 | 7.4 | 3.7 | 9.2 | 8.8 | 10.8 | 8.8 | 6.4 | |
| 2009 | 8.4 | 6.1 | 2.6 | 10.4 | 15.8 | 14.4 | 11.6 | 8.5 | |
| 2010 | 10.4 | 5.7 | 1.1 | 10.0 | 20.5 | 17.0 | 14.5 | 8.6 | |
| 2011 | 11.3 | 5.4 | 0.9 | 8.7 | 20.0 | 15.1 | 12.5 | 7.7 | |
| Medicare enrolled | Yes | 80.1 | 78.6 | 79.3 | 86.6 | 78.1 | 79.2 | 77.1 | 71.4 |
| Nursing home residence, ever during baseline | 16.5 | 20.3 | 14.5 | 21.8 | 12.7 | 24.7 | 18.9 | 17.9 | |
| Healthcare utilization intensity measures, in baseline period† | Group | Measures of Central Tendency | |||||||
| # prescriptions dispensed | Median (Q1-Q3) | 61.0 (33.0-98.0) | 63.0 (33.0-102) | 52.0 (25.0-85.0) | 47.0 (20.0-85.0) | 69.0 (39.0-109) | 64.0 (34.0-104) | 80.0 (45.0-126) | 66.0 (33.0-108) |
| # unique drugs dispensed | 15.0 (10.0-22.0) | 16.0 (10.0-23.0) | 13.0 (8.0-20.0) | 13.0 (7.0-19.0) | 17.0 (10.0-24.0) | 16.0 (10.0-23.0) | 17.0 (11.0-25.0) | 16.0 (10.0-23.0) | |
| # inpatient diagnosis codes | 6.0 (0.0-15.0) | 7.0 (0.0-15.0) | 3.0 (0.0-9.0) | 1.0 (0.0-9.0) | 7.0 (0.0-16.0) | 9.0 (0.0-18.0) | 6.0 (0.0-14.0) | 4.0 (0.0-11.0) | |
| # unique inpatient diagnosis codes | 6.0 (0.0-11.0) | 6.0 (0.0-11.0) | 3.0 (0.0-9.0) | 1.0 (0.0-9.0) | 7.0 (0.0-12.0) | 8.0 (0.0-14.0) | 6.0 (0.0-11.0) | 3.0 (0.0-9.0) | |
| # outpatient diagnosis codes | 84.0 (39.0-161) | 90.0 (45.0-171) | 68.0 (30.0-122) | 50.0 (3.0-120) | 88.0 (40.0-177) | 89.0 (36.0-182) | 91.0 (44.0-171) | 74.0 (33.0-150) | |
| # unique outpatient diagnosis codes | 25.0 (14.0-38.0) | 26.0 (16.0-38.0) | 22.0 (12.0-32.0) | 17.0 (2.0-31.0) | 26.0 (14.0-40.0) | 26.0 (13.0-40.0) | 26.0 (15.0-38.0) | 22.0 (12.0-35.0) | |
| # outpatient CPT-4/HCPCS procedure codes | 95.0 (44.0-174) | 101 (51.0-183) | 77.0 (38.0-140) | 61.0 (11.0-136) | 100 (46.0-188) | 97.0 (40.0-187) | 104 (49.0-184) | 87.0 (39.0-166) | |
| # unique outpatient CPT-4/HCPCS procedure codes | 45.0 (24.0-69.0) | 47.0 (27.0-70.0) | 38.0 (20.0-60.0) | 30.0 (4.0-55.0) | 48.0 (25.0-74.0) | 45.0 (21.0-71.0) | 47.0 (25.0-70.0) | 41.0 (20.0-64.0) | |
| Other investigator predefined covariates, in baseline period | Group | % | |||||||
| GIB/ICH | Yes | 2.8 | 3.1 | 2.2 | 1.9 | 2.5 | 3.5 | 2.3 | 2.8 |
| Coagulation defect | 10.9 | 11.0 | 10.2 | 7.5 | 10.8 | 9.7 | 12.6 | 11.5 | |
| Liver disease | 13.2 | 13.1 | 9.7 | 8.6 | 14.4 | 13.1 | 15.1 | 14.5 | |
| Kidney disease | 33.9 | 35.1 | 25.3 | 25.9 | 35.7 | 37.7 | 36.3 | 31.7 | |
| Obesity | 13.7 | 14.4 | 12.0 | 11.4 | 17.2 | 16.4 | 18.5 | 15.2 | |
| Ulcer | 4.8 | 4.9 | 4.4 | 2.9 | 4.4 | 4.5 | 4.5 | 4.9 | |
| Esophagitis/gastritis/duodenitis | 28.7 | 29.0 | 24.7 | 21.5 | 34.9 | 30.9 | 33.3 | 25.6 | |
| Esophageal varices | 0.2 | 0.1 | 0.0 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | |
| Diabetes mellitus | 50.0 | 53.4 | 46.4 | 41.5 | 52.7 | 52.9 | 58.5 | 55.7 | |
| Non-warfarin anticoagulant, oral | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | |
| Non-warfarin anticoagulant, subcutaneous/injectable | 6.1 | 6.1 | 3.9 | 6.2 | 7.9 | 7.6 | 9.0 | 6.9 | |
| Antiplatelet agent, oral | 17.5 | 19.1 | 13.1 | 13.8 | 25.4 | 20.9 | 16.7 | 11.6 | |
| ASA/NSAID, oral | 40.5 | 42.4 | 35.8 | 33.0 | 43.9 | 40.6 | 37.4 | 38.2 | |
| Gastroprotective agent, oral | 51.1 | 51.7 | 43.6 | 43.9 | 56.7 | 53.0 | 56.9 | 50.7 | |
| Selective COX-2 inhibitor | 17.5 | 17.5 | 20.0 | 6.2 | 10.5 | 10.4 | 13.0 | 14.0 | |
| Drug that can interact with warfarin, oral | 72.3 | 72.3 | 68.0 | 63.3 | 73.9 | 71.2 | 89.9 | 74.2 | |
| Clinically relevant CYP2C9/3A4/1A2 inhibitor | 56.7 | 54.9 | 50.9 | 47.5 | 54.7 | 53.4 | 58.4 | 56.6 | |
| Clinically relevant CYP2C9/3A4/1A2 inducer | 11.8 | 13.0 | 11.4 | 9.7 | 12.3 | 11.5 | 14.3 | 13.6 | |
ASA = aspirin; CI = confidence interval; CPT-4 = Current Procedural Terminology-4; COX = cyclooxygenase; CYP = cytochrome P450; GIB = gastrointestinal bleeding; ICH = intracranial hemorrhage; NSAID = nonsteroidal anti-inflammatory drug; Q = quartile
cell suppressed to comply with Centers for Medicare and Medicaid Services privacy policy
the following healthcare utilization covariates were excluded from presentation the table, as their median values were zero for the majority of antihyperlipidemics: # inpatient ICD-9 procedure codes; # unique inpatient ICD-9 procedure codes; # inpatient CPT-4/HCPCS procedure codes; # unique inpatient CPT-4/HCPCS procedure codes; # outpatient ICD-9 procedure codes; # unique outpatient ICD-9 procedure codes; # other setting ICD-9 diagnosis codes; # unique other setting ICD-9 diagnosis codes; # other setting ICD-9 procedure codes; # unique other setting ICD-9 procedure codes
3.2 Measures of association: primary analysis
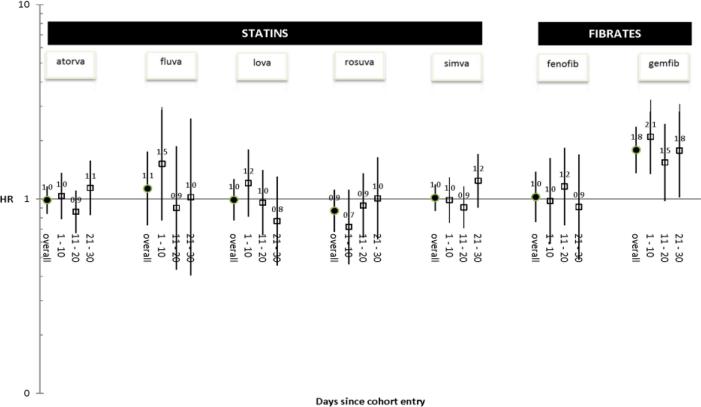
The hdPS algorithm identified 688 covariates for inclusion in the PS model (Appendix Table 3). Among these, 34 variables occurred very infrequently (N < 10 for at least one antihyperlipidemic exposure group) and were excluded to avoid model instability. Therefore, the multinomial PS model included 654 empirically-identified covariates; each model also included 24 predefined covariates (Appendix Table 2). Crude hazard ratios (HRs) are presented in Table 3; PS-adjusted HRs are presented in Table 3 and Figure 2. Notably, concomitant use of warfarin with gemfibrozil (vs. pravastatin) was associated with an elevated rate of GIB/ICH within 30 days (adjusted HR = 1.79, 1.36 to 2.35)—an association most pronounced within the first 10 days of concomitant use (adjusted HR = 2.09, 1.35 to 3.24).
Figure 2.
Propensity score-adjusted hazard ratios (HRs) for association between warfarin + antihyperlipidemic drug (vs. pravastatin) and gastrointestinal bleeding/intracranial hemorrhage within 30 days of concomitant use—overall and within three prespecified time windows
3.3 Measures of association: secondary analyses
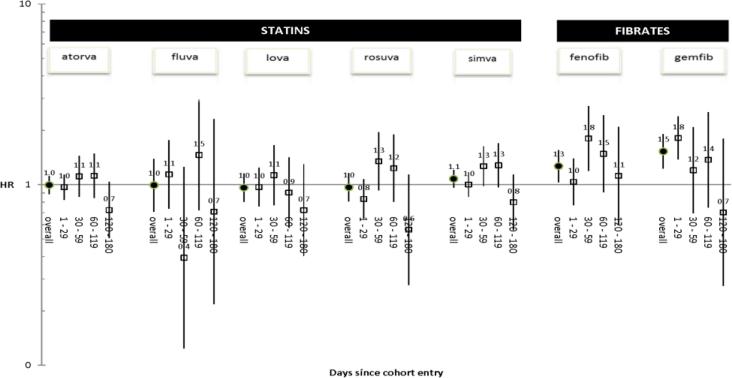
For the secondary analysis examining events within the first 180 days of concomitant use, warfarin + gemfibrozil and warfarin + fenofibrate (each vs. pravastatin) were associated with an elevated rate of GIB/ICH (adjusted HRs 1.53, 1.23 to 1.90 and 1.27, 1.03 to 1.55, respectively). Crude and PS-adjusted HRs for all antihyperlipidemics are presented in Appendix Table 4 and Figure 3, respectively. Results from the remaining secondary analyses (detailed in Table 2) are presented in Table 4.
Figure 3.
Propensity score-adjusted hazard ratios (HRs) for association between warfarin + antihyperlipidemic drug (vs. pravastatin) and gastrointestinal bleeding/intracranial hemorrhage within 180 days of concomitant use—overall and within four prespecified time windows
Table 4.
Summary of findings from prespecified secondary analyses
| Analysis | Relevant results*,† | |
|---|---|---|
| Minimizing the role of chance, bias, and/or confounding | ||
| Exclusion of persons with baseline history of GIB/ICH | Excluded N = 7,259 users (3.1% of cohort) gemfibrozil: aHR within 30 days of cohort entry = 1.76, 1.31 to 2.37 gemfibrozil: aHR within 180 days of cohort entry = 1.51, 1.20 to 1.91 fenofibrate: aHR within 180 days of cohort entry = 1.32, 1.06 to 1.63 |
|
| Exclusion of persons with baseline enrollment in Medicaid managed care | Excluded N = 77,251 users (32.6% of cohort) gemfibrozil: aHR within 30 days of cohort entry = 1.37, 0.95 to 1.97 Excluded N = 80,923 users (34.2% of cohort) gemfibrozil: aHR within 180 days of cohort entry = 1.25, 0.94 to 1.66 fenofibrate: aHR within 180 days of cohort entry = 1.25, 0.97 to 1.59 |
|
| Exclusion of ICH outcomes with a co-occurring intracranial injury diagnosis | Excluded N = 21 users (<0.01% of cohort) gemfibrozil: aHR within 30 days of cohort entry = 1.81, 1.37 to 2.37 Excluded N = 54 users (0.02% of cohort) gemfibrozil: aHR within 180 days of cohort entry = 1.55, 1.25 to 1.93 fenofibrate: aHR within 180 days of cohort entry = 1.27, 1.04 to 1.56 |
|
| Exclusion of empirical covariates from the PS thought to be strong correlates of exposure but not associated with the outcome | Excluded N = 149 covariates (22.0% of covariates included in final model) gemfibrozil: aHR within 30 days of cohort entry = 1.78, 1.36 to 2.34 gemfibrozil: aHR within 180 days of cohort entry = 1.51, 1.21 to 1.87 fenofibrate: aHR within 180 days of cohort entry = 1.24, 1.01 to 1.52 |
|
| Exclusion of persons with a pre-cohort entry to on-or-after cohort entry increase in warfarin dose | Excluded N = 13,709 users (5.8% of cohort) gemfibrozil: aHR within 30 days of cohort entry = 1.82, 1.38 to 2.41 gemfibrozil: aHR within 180 days of cohort entry = 1.57, 1.26 to 1.97 fenofibrate: aHR within 180 days of cohort entry = 1.23, 1.00 to 1.53‡ |
|
| Further elucidating the association between exposure and outcome | ||
| Increasing maximum follow-up time to 180 days, including examining the following discreet time windows: 1–29 days; 30–59 days; 60–119 days; and 120–180 days | See Figure 3 and Appendix Table 4 | |
| Examining effect modification by presumed indication for warfarin | atrial fibrillation/flutter | P-value for interaction term (antihyperlipidemic × atrial fibrillation/flutter) = 0.026 Among users with atrial fibrillation/flutter: gemfibrozil: aHR within 30 days of cohort entry = 2.29, 1.61 to 3.25 Among users without atrial fibrillation flutter: gemfibrozil: aHR within 30 days of cohort entry = 1.32, 0.86 to 2.03 |
| valvular heart disease | As the p-value for the interaction term (antihyperlipidemic × valvular heart disease) was non-significant (p = 0.211), stratified results are not presented | |
| venous thromboembolism | As the p-value for the interaction term (antihyperlipidemic × venous thromboembolism) was non-significant (p = 0.681), stratified results are not presented | |
| other indication | As the p-value for the interaction term (antihyperlipidemic × other indication) was non-significant (p = 0.560), stratified results are not presented | |
| Examining effect modification by laboratory monitoring for level of anticoagulation | As the p-value for the interaction term (antihyperlipidemic × laboratory monitoring) was non-significant (p = 0.921), stratified results are not presented | |
| Examining effect modification by duration of prior warfarin use | As the p-value for the interaction term (antihyperlipidemic × duration of prior warfarin use) was non-significant (p = 0.964), stratified results are not presented | |
aHR = propensity score-adjusted hazard ratio; GIB = gastrointestinal bleeding; ICH = intracranial hemorrhage; PS = propensity score
most non-significant findings not shown; data available from the authors
comparison vs. pravastatin
p = 0.053
4. DISCUSSION
We examined serious bleeding associated with potential drug-drug interactions between warfarin and individual antihyperlipidemics. The incidence of GIB/ICH among concomitant warfarin and antihyperlipidemic users was about 13 per 100p-y. While this rate is consistent with some prior findings, [49-51] it is generally many-fold greater than that reported in other cohorts.[52-57] This is likely explained by: a) the left-skewed age distribution of our study population (Appendix Figure 1); b) our study of economically-disadvantaged individuals with a generally poor health status and more barriers to timely care;[58] c) our inclusion of persons with dual enrollment in Medicaid and Medicare, a group enriched with the sickest and poorest individuals served by the US Centers for Medicare and Medicaid Services;[59] d) the large proportion of our population newly-initiating warfarin upon cohort entry (Table 3), as the period immediately following warfarin initiation carries the highest bleeding risk;[60] and e) differences in outcome definitions across studies. As only 3.1% of warfarin users had a history of GIB/ICH during the baseline period (Table 3), this seems unlikely to contribute to our high incidence rate.
We found an increased hazard of GIB/ICH during the first month of concomitant use of warfarin and gemfibrozil. The highest risk period was within the first 10 days, during which the adjusted HR was more than 2-fold as large as that for warfarin + pravastatin. Interestingly, we found an increased hazard of GIB/ICH during the second month of concomitant use of warfarin and fenofibrate, during which the adjusted HR was 1.8-fold as large as that for warfarin + pravastatin. One could hypothesize that the difference in onset of these interactions is related to the disparate half-lives of gemfibrozil (1.5 hours) and fenofibrate (19.6–26.6 hours).[61] There was no corresponding increased hazard in these months (and up through the first six months) among concomitant users of warfarin and individual statins. Importantly, these findings were broadly consistent across all secondary analyses.
The null associations for statins are consistent with case-control findings by Douketis et al in which recent statin use (considered collectively) among warfarin users was not associated with GIB/ICH (adjusted odds ratio [OR] = 1.04, 0.74 to 1.48). Those investigators did not examine fibrates. Our positive association for gemfibrozil is consistent with case-control findings by Schelleman et al (adjusted OR for GIB within the first 30 days of concomitant use = 1.96, 1.19 to 3.24). That earlier study using a subset of the data reported here was too small to generate a precise estimate for fenofibrate, reporting an adjusted OR of 2.07 (0.91 to 4.69) during days 31–60—consistent with our fenofibrate result. Interestingly, and discordant from our findings, Schelleman et al further reported an increased GIB risk within the first 30 days of concomitant use for simvastatin and atorvastatin (adjusted ORs = 1.33 [1.00 to 1.78] and 1.29 [1.04 to 1.61], respectively). Yet, this prior study adjusted for a small number of potential confounders and ORs were noticeably attenuated when comparing minimally- to more fully-adjusted models. This may suggest that the more complete control of potential confounders would have rendered their statin findings null.
A potential explanation for the positive association with fibrates (but not statins) could be a healthy user effect among our pravastatin-exposed reference group; statin users may be healthier than fibrate users and, by virtue of their health, have an apparent lower GIB/ICH risk.[62,63] In fact, compared to fibrate users, we found that statin users were less likely to have an obesity diagnosis and to have preexisting diabetes, coagulation defects, and liver disease. Yet, we found that statin users were older, more likely to reside in a nursing home, and more likely to be dually-enrolled in Medicaid and Medicare. Regardless, we adjusted for baseline differences by including these and many other covariates in our PS. This minimizes the likelihood that a healthy user effect explains our findings.
Evidence supporting the positive association with fibrates includes clinical observations of international normalized ratio elevations and serious bleeding among concomitant users of warfarin and fibrates.[19,61,64-67] These events have occurred within the first few days of concomitant use through a month thereafter, a time frame broadly consistent with our findings. A number of mechanisms underlying this interaction, while neither fully elucidated nor agreed upon, have been proposed—including, as examples: inhibition of warfarin metabolism via CYP2C9; displacement of warfarin from protein binding sites; and inherent anticoagulant and antiplatelet effects of fibrates.[20,64,66]
Our results suggest that the apparent drug interaction between warfarin and fibrates is unlikely to be mediated primarily by CYP2C9 inhibition. Support for this conclusion is as follows. First, neither gemfibrozil nor fenofibrate are thought to be clinically-important inhibitors of CYP2C9.[68] Second, concomitant use of warfarin with rosuvastatin or fluvastatin, important competitive inhibitors of CYP2C9,[14,69] was not associated with an increased rate of GIB/ICH. Third, the increase in GIB/ICH rate with concomitant use of warfarin and fenofibrate was delayed, and interactions involving enzymatic inhibition are usually rapid-onset interactions.[70] This apparent drug interaction is also unlikely mediated by protein binding displacement. Such interactions are generally considered transient because the increased amount of unbound drug is readily metabolized or eliminated.[19] Further, concomitant use of warfarin with simvastatin or fluvastatin, examples of highly protein bound statins,[71] was not associated with an increased rate of GIB/ICH. Finally, the potential mechanism of fibrates’ inherent anticoagulant and antiplatelet effects is intriguing, but these effects have also been reported with statins.[20-22] Future work should elucidate the mechanism(s) underlying this apparent drug interaction.
Our study has notable strengths. It is the largest to date to examine the association between warfarin + antihyperlipidemics and GIB. Further, it is the first pharmacoepidemiologic study of this interaction to: a) include ICH as a clinical endpoint, while elucidating antihyperlipidemic agent-specific rates; b) consider a warfarin-triggered drug interaction (Figure 1); c) include Medicare Part D prescription data; d) quantify agent-specific rates of GIB/ICH in time windows within the first 30 days of use; and e) fully quantify the rate of GIB/ICH among concomitant users of warfarin + fenofibrate and warfarin + fluvastatin. Our use of an active comparator, hdPS methods, and sensitivity analyses serves to mitigate confounding. Further, our large sample sizes allow for the examination of the time-course of the interactions. Finally, our algorithms to identify GIB and ICH have very good positive predictive values.
Our study also has limitations. First, we did not have access to biosamples and were therefore unable to examine genetic CYP polymorphisms. Second, we lacked data on adherence to prescribed warfarin and antihyperlipidemic therapies. Third, although we were able to identify and consider orders for laboratory monitoring (e.g., international normalized ratio), Medicaid and Medicare claims do not include laboratory results. Fourth, administrative databases may poorly capture some lifestyle behaviors and nonprescription therapies that may modify bleeding risk. Regardless, such factors seem unlikely to differ substantially by antihyperlipidemic exposure. Finally, our results may not be generalizable beyond a US Medicaid population. Nevertheless, this population was specifically chosen because of its inherent vulnerability and inclusion of large numbers of women and minorities—groups typically understudied. Biological associations identified in Medicaid populations are often replicated in commercially insured populations and vice versa.[27]
Warfarin is highly prone to drug interactions via numerous complex mechanisms.[72] By far the most important consequence of such interactions is serious bleeding—an outcome of significant clinical and public health concern that is feared by clinicians, patients, and their relatives. We found that concomitant therapy with warfarin and a fibrate, but not statin, is associated with an increased rate of GIB/ICH. The mechanism underlying this apparent drug-drug interaction needs further elucidation, but is unlikely to solely involve a pharmacokinetic interaction mediated by CYP inhibition or displacement of binding from plasma proteins. Clinicians should be attuned to both immediate- and delayed-onset bleeding in their patients on this drug combination.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank: Dr. Adam Cuker and Dr. Todd Miano from the University of Pennsylvania for their clinical input; Ms. Min Du and Ms. Qing Liu from the University of Pennsylvania for their statistical programming support; and Ms. Geralyn Barosso from the University of Minnesota's Research Data Assistance Center for her Centers for Medicare and Medicaid Services data support.
Grant Support:
The authors declare support from the following organizations: United States National Institutes of Health's (US NIH's) National Institute on Aging (R01 AG025152), US NIH's National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK102694), and US NIH's National Center for Advancing Translational Sciences (UL1 TR000138). These organizations had no role in the study design other than through comments made during the grant's peer review process. These organizations had no role in data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit this manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
Author Contributions:
Wrote manuscript: Dr. Leonard
Revised manuscript: Ms. Brensinger, Dr. Bilker, Dr. Kimmel, Dr. Han, Dr. Nam, Dr. Gagne, Ms. Mangaali, Dr. Hennessy
Designed research: Dr. Leonard, Dr. Bilker, Dr. Kimmel, Dr. Hennessy
Performed research: Dr. Leonard, Ms. Brensinger, Dr. Bilker, Dr. Han, Dr. Nam, Ms. Mangaali, Dr. Hennessy
Analyzed data: Ms. Brensinger, Dr. Bilker
Contributed analytic tools: Ms. Brensinger, Dr. Bilker, Dr. Gagne
Potential Conflicts of Interest:
The following authors declare no financial relationships with any organization that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work: Dr. Leonard; Ms. Brensinger; Dr. Han; Dr. Nam; and Ms. Mangaali. The following authors declare relationships, as stated: Dr. Bilker reports personal fees from Janssen and grant funding from Novartis; Dr. Kimmel reports personal fees from Pfizer, Bayer, and Merck, outside the submitted work; Dr. Gagne is principal investigator of an investigator-initiated research grant from Novartis to the Brigham and Women's Hospital for unrelated work and he is a consultant to Aetion, Inc. and to Optum, Inc.; Dr. Hennessy reports personal fees from Merck Sharp & Dohme Corp, grants and personal fees from AstraZeneca Pharmaceuticals, and grants and personal fees from Bristol-Myers Squibb, unrelated to the submitted work.
REFERENCES
- 1.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007-2008. NCHS Data Brief. 2010;(42):1–8. [PubMed] [Google Scholar]
- 2.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 4.Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641–651. doi: 10.1002/pds.1351. [DOI] [PubMed] [Google Scholar]
- 5.Hines LE, Malone DC, Murphy JE. Recommendations for generating, evaluating, and implementing drug-drug interaction evidence. Pharmacotherapy. 2012;32:304–313. doi: 10.1002/j.1875-9114.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services' Office of Disease Prevention and Health Promotion [11/07/2016];National action plan for adverse drug event prevention. 2014 https://health.gov/hcq/ade-action-plan.asp. Last updated: 11/07/2016.
- 7.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med. 2015;128:1300–1305. e2. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin PJ. Reviewing the reality: why we need to change. Eur Heart J Suppl. 2005;7:E15–E20. [Google Scholar]
- 10.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 11.Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest. 2005;127:1515–1522. doi: 10.1378/chest.127.5.1515. [DOI] [PubMed] [Google Scholar]
- 12.Wallvik J, Sjalander A, Johansson L, Bjuhr O, Jansson JH. Bleeding complications during warfarin treatment in primary healthcare centres compared with anticoagulation clinics. Scand J Prim Health Care. 2007;25:123–128. doi: 10.1080/02813430601183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention [11/07/2016];About the ambulatory health care surveys. 2009 http://www.cdc.gov/nchs/ahcd/about_ahcd.htm. Last updated: 11/06/2015.
- 14.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Wen X, Wang JS, Backman JT, Kivisto KT, Neuvonen PJ. Gemfibrozil is a potent inhibitor of human cytochrome P450 2C9. Drug Metab Dispos. 2001;29:1359–1361. [PubMed] [Google Scholar]
- 16.Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67–74. doi: 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 17.Shaik AN, Bohnert T, Williams DA, Gan LL, LeDuc BW. Mechanism of Drug-Drug Interactions Between Warfarin and Statins. J Pharm Sci. 2016 doi: 10.1016/j.xphs.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Bjornsson TD, Meffin PJ, Swezey S, Blaschke TF. Clofibrate displaces warfarin from plasma proteins in man: an example of a pure displacement interaction. J Pharmacol Exp Ther. 1979;210:316–321. [PubMed] [Google Scholar]
- 19.Kim KY, Mancano MA. Fenofibrate potentiates warfarin effects. Ann Pharmacother. 2003;37:212–215. doi: 10.1177/106002800303700210. [DOI] [PubMed] [Google Scholar]
- 20.Ali FY, Armstrong PC, Dhanji AR, et al. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler Thromb Vasc Biol. 2009;29:706–711. doi: 10.1161/ATVBAHA.108.183160. [DOI] [PubMed] [Google Scholar]
- 21.Undas A, Brummel-Ziedins KE, Mann KG. Anticoagulant effects of statins and their clinical implications. Thromb Haemost. 2014;111:392–400. doi: 10.1160/TH13-08-0720. [DOI] [PubMed] [Google Scholar]
- 22.Owens AP, 3rd, Mackman N. The antithrombotic effects of statins. Annu Rev Med. 2014;65:433–445. doi: 10.1146/annurev-med-051812-145304. [DOI] [PubMed] [Google Scholar]
- 23.Badillo R, Schmidt R, Mortensen EM, Frei CR, Mansi I. Statin therapy and gastrointestinal hemorrhage: a retrospective cohort study with propensity score-matching. Pharmacoepidemiol Drug Saf. 2015;24:849–857. doi: 10.1002/pds.3817. [DOI] [PubMed] [Google Scholar]
- 24.McKinney JS, Kostis WJ. Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke. 2012;43:2149–2156. doi: 10.1161/STROKEAHA.112.655894. [DOI] [PubMed] [Google Scholar]
- 25.Hackam DG, Woodward M, Newby LK, et al. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011;124:2233–2242. doi: 10.1161/CIRCULATIONAHA.111.055269. [DOI] [PubMed] [Google Scholar]
- 26.Ling H, Luoma JT, Hilleman D. A review of currently available fenofibrate and fenofibric acid formulations. Cardiol Res. 2013;4:47–55. doi: 10.4021/cr270w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennessy S, Freeman CP, Cunningham F. US Government Claims Databases. In: Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology. Wiley-Blackwell; 2012. p. 209. [Google Scholar]
- 28.Kaiser Family Foundation [11/07/2016];Medicaid enrollment: June 2010 data snapshot. 2011 Report #8050-03. Available at: https://kaiserfamilyfoundation.files.wordpress.com/2013/01/8050-03.pdf.
- 29.Holahan J, Ghosh A. [11/07/2016];Dual eligibles: Medicaid enrollment and spending for Medicare beneficiaries in 2003. 2005 Report #7346. Available at: http://kff.org/medicaid/issue-brief/dual-eligiblesmedicaid-enrollment-and-spending-for-medicare/.
- 30.Holahan J, Miller DM, Rousseau D. [11/07/2016];Dual eligibles: Medicaid enrollment and spending for Medicare beneficiaries in 2005. 2009 Report #7846. Available at: http://web.archive.org/web/20110608201215/ http://www.kff.org/medicaid/upload/7846.pdf.
- 31.Rousseau D, Clemans-Cope L, Lawton E, Langston J, et al. [11/07/2016];Dual eligibles: Medicaid enrollment and spending for Medicare beneficiaries in 2007. 2010 Report #7846-02. Available at: http://www.ncbi.nlm.nih.gov/nlmcatalog/101560987.
- 32.Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid Services (CMS). Med Care. 2007;45:1216–1220. doi: 10.1097/MLR.0b013e318148435a. [DOI] [PubMed] [Google Scholar]
- 33.Schelleman H, Bilker WB, Brensinger CM, Wan F, Yang YX, Hennessy S. Fibrate/Statin initiation in warfarin users and gastrointestinal bleeding risk. Am J Med. 2010;123:151–157. doi: 10.1016/j.amjmed.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garbe E, Kloss S, Suling M, Pigeot I, Schneeweiss S. High-dimensional versus conventional propensity scores in a comparative effectiveness study of coxibs and reduced upper gastrointestinal complications. Eur J Clin Pharmacol. 2013;69:549–557. doi: 10.1007/s00228-012-1334-2. [DOI] [PubMed] [Google Scholar]
- 35.Hennessy S, Leonard CE, Gagne JJ, et al. Pharmacoepidemiologic Methods for Studying the Health Effects of Drug-Drug Interactions. Clin Pharmacol Ther. 2016;99:92–100. doi: 10.1002/cpt.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen WC, Chen YH, Hsu PI, et al. Gastrointestinal hemorrhage in warfarin anticoagulated patients: incidence, risk factor, management, and outcome. Biomed Res Int. 2014;2014:463767. doi: 10.1155/2014/463767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am J Epidemiol. 2008;168:329–335. doi: 10.1093/aje/kwn135. [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb RA. Cytochrome P450: major player in reperfusion injury. Arch Biochem Biophys. 2003;420:262–267. doi: 10.1016/j.abb.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Gulmez SE, Lassen AT, Aalykke C, et al. Do statins protect against upper gastrointestinal bleeding? Br J Clin Pharmacol. 2009;67:460–465. doi: 10.1111/j.1365-2125.2009.03362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosendaal FR. Statins and venous thrombosis: a story too good to be true? PLoS Med. 2012;9:e1001311. doi: 10.1371/journal.pmed.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rassen JA, Doherty M, Huang W, Schneeweiss S. Pharmacoepidemiology toolbox. Boston, MA: [11/07/2016]. Available at: http://www.drugepi.org/dope-downloads/#Pharmacoepidemiology%20Toolbox. [Google Scholar]
- 43.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 45.Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):100–128. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rassen JA, Glynn RJ, Brookhart MA, Schneeweiss S. Covariate selection in high-dimensional propensity score analyses of treatment effects in small samples. Am J Epidemiol. 2011;173:1404–1413. doi: 10.1093/aje/kwr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983:41–55. [Google Scholar]
- 49.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 50.Johnson CE, Lim WK, Workman BS. People aged over 75 in atrial fibrillation on warfarin: the rate of major hemorrhage and stroke in more than 500 patient-years of follow-up. J Am Geriatr Soc. 2005;53:655–659. doi: 10.1111/j.1532-5415.2005.53215.x. [DOI] [PubMed] [Google Scholar]
- 51.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 52.Fihn SD, Callahan CM, Martin DC, McDonell MB, Henikoff JG, White RH. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics. Ann Intern Med. 1996;124:970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 53.Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 54.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. doi: 10.1136/bmj.h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med. 2006;166:853–859. doi: 10.1001/archinte.166.8.853. [DOI] [PubMed] [Google Scholar]
- 56.Fang MC, Go AS, Hylek EM, et al. Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006;54:1231–1236. doi: 10.1111/j.1532-5415.2006.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang HY, Zhou M, Tang W, Alexander GC, Singh S. Risk of gastrointestinal bleeding associated with oral anticoagulants: population based retrospective cohort study. BMJ. 2015;350:h1585. doi: 10.1136/bmj.h1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheung PT, Wiler JL, Lowe RA, Ginde AA. National study of barriers to timely primary care and emergency department utilization among Medicaid beneficiaries. Ann Emerg Med. 2012;60:4–10. e2. doi: 10.1016/j.annemergmed.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 59.Musumeci M. [11/07/2016];Dual Eligibles. Available at: http://kff.org/interactive/dual-eligibles-tutorial/.
- 60.Garcia DA, Lopes RD, Hylek EM. New-onset atrial fibrillation and warfarin initiation: high risk periods and implications for new antithrombotic drugs. Thromb Haemost. 2010;104:1099–1105. doi: 10.1160/TH10-07-0491. [DOI] [PubMed] [Google Scholar]
- 61.Aldridge MA, Ito MK. Fenofibrate and warfarin interaction. Pharmacotherapy. 2001;21:886–889. doi: 10.1592/phco.21.9.886.34556. [DOI] [PubMed] [Google Scholar]
- 62.Douketis JD, Melo M, Bell CM, Mamdani MM. Does statin therapy decrease the risk for bleeding in patients who are receiving warfarin? Am J Med. 2007;120:369.e9–369.e14. doi: 10.1016/j.amjmed.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 63.van Rein N, Cannegieter SC, le Cessie S, et al. Statins and Risk of Bleeding: An Analysis to Evaluate Possible Bias Due to Prevalent Users and Healthy User Aspects. Am J Epidemiol. 2016;183:930–936. doi: 10.1093/aje/kwv255. [DOI] [PubMed] [Google Scholar]
- 64.Ascah KJ, Rock GA, Wells PS. Interaction between fenofibrate and warfarin. Ann Pharmacother. 1998;32:765–768. doi: 10.1345/aph.17310. [DOI] [PubMed] [Google Scholar]
- 65.Rindone JP, Keng HC. Gemfibrozil-warfarin drug interaction resulting in profound hypoprothrombinemia. Chest. 1998;114:641–642. doi: 10.1378/chest.114.2.641. [DOI] [PubMed] [Google Scholar]
- 66.Dixon DL, Williams VG. Interaction between gemfibrozil and warfarin: case report and review of the literature. Pharmacotherapy. 2009;29:744–748. doi: 10.1592/phco.29.6.744. [DOI] [PubMed] [Google Scholar]
- 67.Ahmad S. Gemfibrozil: interaction with glyburide. South Med J. 1991;84:102. [PubMed] [Google Scholar]
- 68.Schelleman H, Han X, Brensinger CM, et al. Pharmacoepidemiologic and in vitro evaluation of potential drug-drug interactions of sulfonylureas with fibrates and statins. Br J Clin Pharmacol. 2014;78:639–648. doi: 10.1111/bcp.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhelyazkova-Savova M, Gancheva S, Sirakova V. Potential statin-drug interactions: prevalence and clinical significance. Springerplus 2014; 3:168-1801-3-168. eCollection. 2014 doi: 10.1186/2193-1801-3-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amin M, Suksomboon N. Pharmacotherapy of type 2 diabetes mellitus: an update on drug-drug interactions. Drug Saf. 2014;37:903–919. doi: 10.1007/s40264-014-0223-2. [DOI] [PubMed] [Google Scholar]
- 71.Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109:III50–7. doi: 10.1161/01.CIR.0000131519.15067.1f. [DOI] [PubMed] [Google Scholar]
- 72.Juurlink DN. Drug interactions with warfarin: what clinicians need to know. CMAJ. 2007;177:369–371. doi: 10.1503/cmaj.070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.