Abstract
Taenia solium (the pork tapeworm) is present in most developing countries, where it is a frequent cause of seizures and other neurological disease. Parasitic larvae invade the human brain, establish, and eventually resolve, leaving a calcified scar. While these lesions are common in endemic regions and most of these are clinically silent, a proportion of individuals with calcified cysticerci develop seizures from these lesions and in from 30–65% of the time associated with perilesional edema, likely due to host inflammation. This manuscript summarizes the importance, characteristics, natural history and potential prevention and treatments of symptomatic calcified neurocysticercosis.
Keywords: Neurocysticercosis, calcified granuloma, perilesional edema, epilepsy, seizures
Neurocysticercosis: A Leading Cause of Seizures Worldwide
Taenia solium neurocysticercosis (NCC) is a major contributor to seizures and other neurological morbidity worldwide and thus an important global health problem [1, 2]. Cysticercosis is also increasingly diagnosed in industrialized countries, mainly due to migration from endemic areas, with approximately 2 000 new cases diagnosed in the United States each year [3, 4]. In endemic countries, nearly 30% of neurologic seizures are attributable to NCC [5]. Neurocysticercosis is endemic in most of the developing world where pigs are raised in non-industrialized conditions as food source, such as Latin America, most of Asia including the Indian subcontinent, and regions of China, Sub-Saharan Africa, and parts of Oceania (Figure 1) [1, 6, http://www.who.int/mediacentre/factsheets/fs376/en/].
Figure 1. Endemicity of Taenia solium.
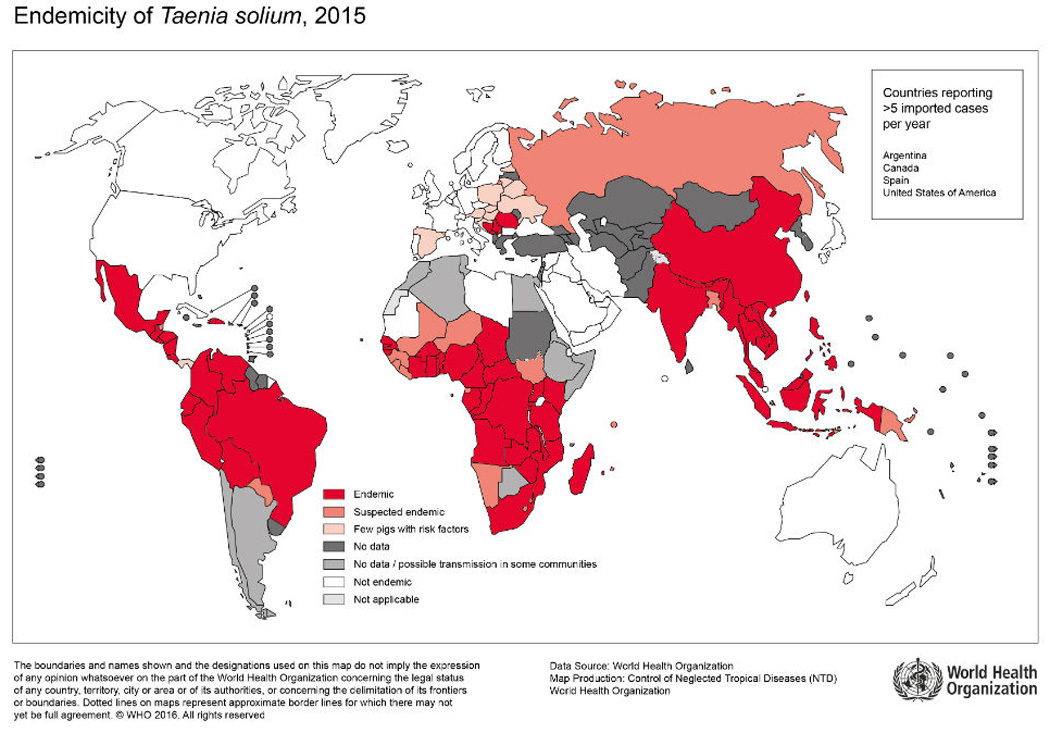
Reproduced, with permission, from http://www.who.int/mediacentre/factsheets/Endemicity_Taenia_Solium_2015-1000x706.jpg?ua=1. Accessed September 7th, 2016.
Calcified Taenia solium brain granulomas (see Glossary) are usually, but not always, small round lesions that develop from degenerating larvae mostly in the parenchyma of the brain. In endemic populations, these are the most common radiologic finding, detected in 10–20% of unselected persons by computed tomography (CT) [7–12]. In these settings, a large majority of individuals with calcifications are asymptomatic and most have never had symptoms related to NCC. In fact, calcifications were designated as “inactive” neurocysticercosis and thought to play no role in the pathophysiology of disease and seizure activation. However, several studies over the past 15 years point to a subset of calcifications that are the foci for intermittent seizure activity and are often associated with the temporary presence of surrounding perilesional edema, which is likely due to host inflammation [13–15]. Here we review the importance, characteristics, natural history and potential prevention and treatments of symptomatic calcified neurocysticercosis.
Characteristics of Calcified Neurocysticercosis
Calcifications are frequently found in CT images of individuals with and without a history of seizure and/or epilepsy in T. solium endemic regions [7, 9–11, 16–19] (Table 1). They appear to be more frequent in endemic regions but no official comparison has been reported [7–10]. Although these calcifications cannot usually be differentiated from similar calcified lesions due to other etiologies, there is little question that most are derived from degenerating granulomas. The most direct evidence comes from serial observations using computerized tomography (CT) and magnetic resonance imaging (MRI) of patients with characteristic T. solium cysts that degenerate naturally or following cysticidal treatment [20, 21]. In a sizable proportion, these lesions gradually become calcified, typically evolving into characteristic dense calcifications. The proportion of cysts that calcify vary, ranging from approximately 10–60% following cysticidal treatment [20, 22]. In addition, characteristic scolices (anlage of the head of tapeworm in the cyst) are frequently detected in calcified lesions by phase corrected gradient echo MRI. Gupta and colleagues reported scolices in 14 of out 21 (67%) of patients with a single brain calcification [23]. Definitive histopathological studies of calcifications are non-existent or possibly are lost in the early literature; only a few examples of the histopathology of calcifications are described in available older literature or in recent publications [24–27] (Figure 2).
Table 1.
Prevalence of Intraparenchymal Brain Calcifications Detected by CT in Seizure-Free Individuals in Endemic Regions.
| Country | Year | Prevalence | Comments | Refs |
|---|---|---|---|---|
| Honduras | 1999 | 18.9% (14/74) | Seronegative | [12] |
| Ecuador | 1999 | 14.4% (17/118) | ------ | [16] |
| Guatemala | 2001 | 19.6% (10/51) | ------ | [7] |
| Mexico | 2003 | 9.1% (14/154) | ------ | [10] |
| Ecuador | 2005 | 5.3% (1/19) | ------ | [11] |
| Peru | 2005 | 13.8% (8/58) | Seronegative | [9] |
| India | 2011 | 11.3% (67/595) | Pig farming community | [17] |
| Ecuador | 2015 | 11.3% (28/248) | >60 years | [18] |
| Brazil | 2015 | 11.3% (12/106) | Headache patients | [19] |
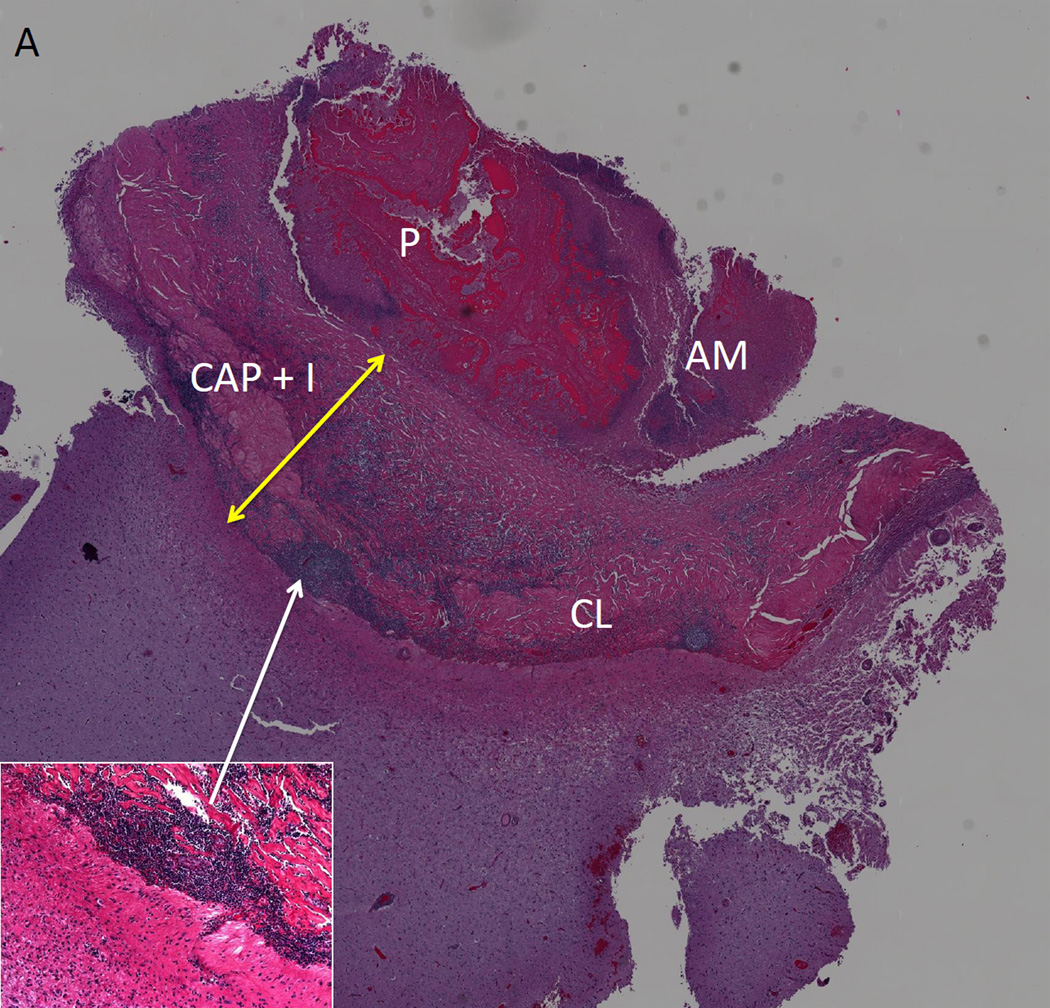
Figure 2. Histopathology of a Calcified Taenia solium Lesion.
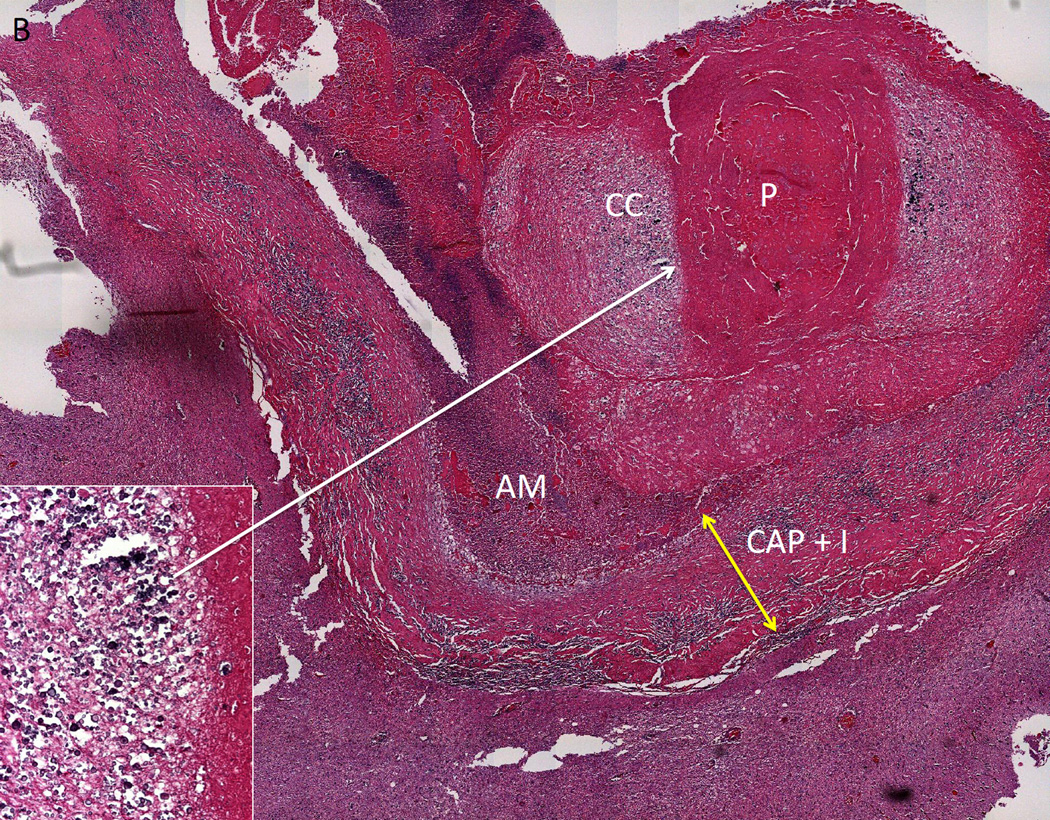
Two histological sections of excised calcified lesion that was causing multiple episodes of perilesional edema. In A and B, parasite remnants (P) are surrounded by a capsule that contains inflammation (CAP+I, yellow arrow) and in A, an increase in collagen (CL). Amorphous material (AM) is closely associated with P. The adjacent brain has increased glial fibrillary acid protein presence (not shown), perivascular infiltrates and eosinophilic material. In B, aggregates of calcareous corpuscles (CC) surround P, and are likely responsible for the calcification. The inset in (A) shows a higher magnification of one area of inflammation; the inset in B shows a higher magnification of a dense aggregation of calcareous corpuses. Adapted from [25], with permission.
Brain tissue calcifications form as a result of infectious and non-infectious processes including tumors, degenerative diseases, inflammatory diseases, and a large number of genetic abnormalities. Why some T. solium cysts calcify and why calcifications commonly occur in some tissue dwelling helminthes or their ova is unclear. For instance, Trichinella, Schistosoma hematobium, Schistosoma japonicum and cysts of Echinoccocus granulosus all tend to calcify in tissues. T. solium, as well as all other cestodes, contains calcareous corpuscles [28], which are small calcified granules mostly in the neck of the invaginated scolex that may act as a nidus for calcium deposition and at times form visible aggregates detected in CT examinations as typical small round calcifications [25, 29].
Factors associated with seizures in calcified cysticerci
In persons who present with seizures caused by viable cysts or by a single T. solium degenerating cyst (enhanced on MRI or CT scan after contrast injection), the development of calcification is a risk factor for recurrent seizures or epilepsy [30]. Although the propensity to develop seizures is enhanced in symptomatic NCC patients whose lesions eventually calcify, in endemic settings, most individuals with calcifications do not have a history of seizures [7–12]. Furthermore, persons with numerous calcifications and seizures usually have only one or a few calcifications that are foci of seizure activation and cause perilesional edema [31]. Therefore, the mechanism(s) that result in seizures associated with a specific calcification must center on past or present characteristics or features associated with that particular lesion. Whether there is something special about a calcification that leads to seizures, the inability of some calcified lesions to fully consolidate and still maintain regions of active inflammation, the persistence of parasite antigen intermingled within the calcification chronically inciting an inflammatory response with intermittent exacerbations, or the prior presence of a particularly injurious inflammatory response during the degenerative process resulting in a propensity of seizures, is unclear. The contribution of the host is obviously important and essential; it appears to be necessary but not sufficient.
In patients from endemic regions that present seizures or in immigrant communities originating from endemic countries, the proportion of persons with calcifications is consistently higher than those without seizures [9, 29], which suggests that individuals with calcifications have an increased propensity to have seizures. A number of more exacting studies implicated specific calcifications as seizure foci in 50–73% of those with seizures and calcifications [32–34]. Patients presenting with calcified NCC may have as high seizure recurrence rates compared to other stages of NCC. Sharma et al. [35] reported a not statistically significant greater proportion of recurrent seizures within 6 months in those who presented with an incident seizure due to a calcification compared those who presented with an incident seizures due to a single enhancing granuloma (34.3 % vs. 25.0%) [35] In a cohort of patients followed prospectively who had only calcified NCC, a history of seizures (in comparison with individuals with calcified NCC and no seizures, e.g. headache only), and a positive cysticercosis serology had a predicted recurrent seizure rate of 36% at 5 years [13]. In the same study, those who developed perilesional edema did not differ in age, sex, cysticidal treatment, location of the calcification or duration of disease, when compared to a matched control group.
Certain calcifications are more likely to be foci of seizures. These include calcifications with a visible scolex detected by MRI gradient echo imaging giving rise to perilesional edema episodes [23], those with presence of perilesional gliosis (that can be demonstrated using magnetization transfer MRI) [36, 37], increased enhancement (also better determined by dynamic contrast-enhanced MRI), and in the same study, in patients with increased serum matrix metalloproteinase-9 (MMP-9) levels and MMP-9 gene polymorphisms [38]. In addition, there may be a link to seizures and calcifications leading to mesial temporal sclerosis resulting in a secondary cause of epilepsy in this group [39,40].
Perilesional Edema in Areas of Seizure Activity
Besides semiological and electroencephalogram (EEG) localization of repetitive seizures around a calcification, the most compelling evidence implicating calcifications as areas of seizure activity is the presence of perilesional edema (PE) around a calcification at the time of clinical seizure or focal neurological finding [31]. PE (Box 1) is defined as the episodic appearance of edema and enhancement surrounding a calcification by MRI, most intense closest to the calcified lesion, a typical pattern of edema caused by a leaky blood brain barrier (BBB). In early studies, there was an almost perfect association of the presence of PE with a clinical event, usually a seizure but sometimes headaches or other focal neurological complaints [14]. With a more liberal use of MRI, later studies also showed that PE may less commonly occur without any symptoms. Edema usually subsides within 6 weeks although it may persist for longer sometimes associated with waxing and waning edema and symptoms [31]. The presence of perilesional edema around calcifications was first reported by Del Brutto in 1992 [41] in 5/16 patients with recurring seizures after stopping anti-seizure medication and subsequently briefly noted in reports by White et al [42] and Garg et al. [43]. The first full report of a patient with perilesional edema was by Sheth et al in 1999 [44]. In the same year Nash and Patronas reported 3 patients with recurring perilesional edema episode, one over 10 years, which suggested these may be an important mechanism of epilepsy associated with neurocysticercosis [45]. Subsequently, a number of series documented the perilesional edema around calcifications in the United States [13, 14,45], Brazil [46], Peru [14], Germany [47], and India [23, 38,43] indicating this is a general phenomenon and not limited to a specific geographic region [48–55]. These and additional studies of the cause of seizures in patients with calcified NCC found that roughly between 30–65% of patients were found to have PE [13, 14, 45, 46, 56, 57]. Only one prospective study has been performed that suggests PE episodes are a common cause of seizures and/or epilepsy in a well-defined endemic urban population [13]. This study followed a cohort of patients with calcified NCC residing in urban Lima, Peru. All had a history of seizures, the presence of calcified neurocysticercosis and absence of other brain lesions, and a positive cysticercosis serology.. Of the 29 persons who incurred recurrent seizures, 24 could be studied of which 50% showed perilesional edema. The study also showed a 9% (2/23) presence of asymptomatic perilesional edema in the matched controls, indicating that silent perilesional edema is not a rare event. The presence of edema around a calcification in a subset of cases suggests the existence of at least two different mechanisms causing seizures, one associated with PE and the other not associated with PE. Alternatively, seizures in all individuals with calcified NCC may involve similar mechanisms but differ in the presence of overt edema, or the magnitude of the edema may be lower than the levels detectable by imaging.
Box 1. Characteristics of Perilesional Edema around T. solium Calcifications.
Episodic edema and enhancement.
Found in 30–60% of persons with recurrent seizures and only calcifications.
Usually 3–6 weeks in duration, can be longer.
Involves single calcifications usually, multiple calcifications sometimes.
Mostly symptomatic usually causing seizures, asymptomatic at times.
Variable frequency, may be frequent and disabling.
Likely inflammatory.
No proven treatment, but symptomatic treatment and anti-seizure medication may work.
Abrupt corticosteroid withdrawal may precipitate perilesional edema in calcifications previously involved and uninvolved in seizures.
The proportion of seizures or epilepsy due to PE in rural endemic regions has not been determined but the aforementioned study suggests that the proportion will be sizable due to the association between seizures and/or epilepsy and the presence of calcified NCC, conjointly with the absence of viable or degenerating cysts. On the other hand, a factor that might lower the proportion of seizures or epilepsy due to PE in rural endemic regions is the frequent negative serology in patients with calcifications [58,59]. Our initial study in Peru required a positive serology, which may have selected a group with more ‘active’ lesions, increasing the proportion with PE [14]. Older lesions could be less frequently associated with perilesional edema.
Evolution of Perilesional Edema
The natural history of perilesional edema episodes has not been well described. In particular, long-term follow-up of a large group of patients is lacking. An example of serial observations in a single patient is demonstrated in Figure 3. This patient presented four separate episodes over 7 years beginning about 1.6 years after treatment for NCC.
Figure 3. Patient with Multiple Episodes of Perilesional Edema Around Calcifications.
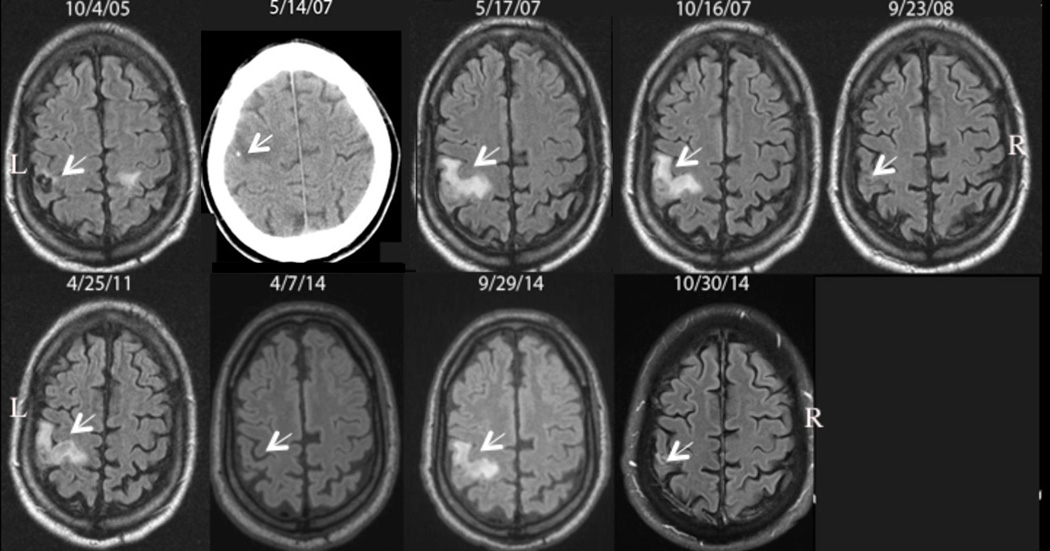
Serial fluid-attenuated inversion-recovery (FLAIR) images over time from left to right demonstrating appearance and resolution of perilesional edema around a Taenia solium calcification in the right frontal lobe. The patient was diagnosed and treated on 10/4/05; a viable cyst is noted by the arrow that subsequently calcifies as documented by a computed tomography examination a few days before (5/14/07) the first episode on 5/17/07. Perilesional edema develops on 10/16/07, 4/25/11, and 9/29/14 with interim resolution on 9/23/08, 4/7/14 and 10/30 14. The associated edema of a left sided cyst is partially seen on 10/4/05. R and L denote right and left sides of the brain. Arrows point to the site of the lesion with or without the presence of edema. The date the images were taken is shown.
PEs are commonly recurring usually involving one or several calcifications in the same patient undergoing PE concurrently or at different times. The number of events can vary from one to many leading to a severe disability. Serial imaging in a number of patients showed that in many, PE resolved within 6 weeks, although PE may be surprisingly prolonged often associated with waxing and waning symptoms associated with the involved calcification [14, 45–47, 60].
There are a number of characteristics and findings that point to an inflammatory pathophysiology as a cause of PE. First, some calcifications enhance, suggesting a perturbation of the BBB, which in the case of neurocysticercosis is most likely due to continuing inflammation. The presence of enhancement around a subset of calcifications is consistent with the idea that calcifications differ in important ways. For unclear reasons, some calcifications maintain varying amounts of inflammation while most others do not. Only two full histopathological descriptions of calcifications associated with PE episodes have been adequately examined following surgical removal [25, 26]. Both show degenerating but recognizable cestode larval structures with a pronounced inflammation consisting of mononuclear lymphocytes, macrophages and some eosinophils with astrogliosis and microgliosis in the surrounding brain parenchyma. Sizable aggregates of calcareous corpuscles are the only foci of calcifications detected on CT examination in these lesions. This histopathology contrasts with the few photographs and accounts in the older literature describing mostly amorphous calcifications, little if any recognizable cystic elements, and modest capsular inflammation [24, 61, 62]. Again, this suggests the histopathology of calcifications are not uniform and based on the limited number of examples, point to histopathological differences between individual calcifications. Thirdly, positron emission tomography (PET) studies employing a ligand to the translocator protein, (TSPO), which is upregulated in activated microglia, astrocytes and macrophages, revealed increased uptake of the ligand in the regions surrounding affected calcifications [15]. In a few patients studied multiple times and in others who experienced prior PE episodes with calcifications that were unaffected at the time of study, increased uptake was detected around some calcifications that had undergone documented earlier episodes. Since increased uptake of this ligand is mostly due to inflammatory processes, these results suggest that perilesional edema is due to inflammation. Persistent increase in uptake of ligand in calcifications that had undergone prior PE indicates either persisting activation after a PE episode or ongoing activation with periodic acute recurrent exacerbations of inflammation leading to PE [31].
Other pathophysiologic mechanisms have also been suggested, such as edema due to seizure activity or intermittent ionic calcium release [45]. However, the edema associated with seizures is usually diffuse [63–65]. The pattern of edema in focal status is complex and may involve both cytotoxic and vasogenic edema [66]. Continued subclinical seizure activity cannot be ruled out [31].
There is no established treatment for symptomatic perilesional edema. Anti-seizure medication for prophylaxis and treatment, similar to other causes of epilepsy, appears to be effective. Fortunately, most clinical episodes resolve spontaneously. [45,46] A common practice is to treat brain edema due to diverse causes with high dose corticosteroids, which was the clinical approach employed initially. Although there seemed to be initial clinical improvement, as the dose is lowered or shortly after stopping corticosteroids, symptomatic perilesional episodes may reoccur, sometimes involving previously involved calcifications [31, 67]. Abrupt cessation of corticosteroids may trigger PE episodes around calcifications [47, 68]. Despite the possibility of exacerbation of signs and symptoms with acute and serious neurological deficits [69, 70], there may be a role for high dose corticosteroids. However, a slow deliberate corticosteroid taper might prevent rebound edema and recurrent symptoms [47, 68, 71]. The treatment options for patients with multiple recurrences and disabling symptoms who develop PE episodes upon a corticosteroid taper are limited. One patient with many prior disabling PE episodes ceased having them for years coincident with administration of methotrexate, an antifolate drug used in the cancer therapy and to treat several autoimmune disorders [72]. Although the experience in this setting is limited, the clinical effect was dramatic and reinforced the concept that the pathophysiology of PE is due to inflammation. The effectiveness of corticosteroids and deleterious effects upon abrupt withdrawal suggest an immunologically regulated cellular inflammatory response and perhaps the development of an imbalance favoring activating over repressing cellular responses, upon corticosteroid withdrawal. This type of mechanism might occur spontaneously in calcified perilesional edema lesions as well as in degenerating cysts, which also undergo rebound edema upon abruptly stopping corticosteroids [67].
A unifying hypothesis is that PE is similar mechanistically to what is seen in degenerating cysts. Degenerating cysts at any stage may spontaneously undergo an exacerbated inflammatory response with increased enhancement and perilesional edema frequently resulting in seizures [73]. The presence of calcification neither excludes the presence of inflammation nor the continuation of the same mechanism responsible for inflammation, edema and resulting seizures caused by degenerating cysts. The available evidence no longer supports the view that all calcifications are inert monolithic lesions [13, 14, 23, 29, 44–47, 60]. We suggest, as proposed by others earlier, that the origin of the inflammation in degenerating cysts with and without calcifications and specific calcified cysts is driven by the presence or episodic availability of parasite antigen, and/or an imbalance between activating and repressing elements that occur naturally within the lesion, or by abrupt corticosteroid withdrawal or by abrupt corticosteroid withdrawal [31, 56]. Calcifications may limit complete parasite degeneration and allow continued availability of antigen to the host. In this view, interventions aimed to avoid the process of calcifications could result in improved prognosis. Further research is necessary to elucidate and modulate immunological mechanisms associated to calcified T. solium granuloma (see Outstanding Questions).
Outstanding Questions.
What is the natural history and prognosis of patients who undergo perilesional episodes?
Do repeated perilesional episodes lead to gliosis and propensity to develop seizures caused by non-perilesional edema?
Which factors determine which calcifications undergo perilesional edema?
What is the nature of the inflammation associated with perilesional edema and how is it controlled or induced?
Does the systemic immune state influence promote/control perilesional edema episodes?
Is the presence of chronic inflammation required for subsequent perilesional edema events?
Can modified treatment regimens prevent perilesional edema from occurring?
What is the role of calcification in pathogenesis of perilesional edema?
Can immunosuppressive measures prevent and/or treat perilesional edema?
How does abrupt cessation of corticosteroids induce perilesional edema in previously uninvolved steroids?
Concluding Remarks
In endemic communities, calcified T. solium brain granulomas or calcifications are a common finding and may be present in up to 19% of the general population [7, 9–11, 16–19]. Although the propensity to cause seizures is obviously decreased compared to degenerating viable cysts, in most endemic villages where transmission is not unusually high, calcified lesions predominate [7–10, 29]. Because calcifications are so common and do not disappear over time, there is a large number of persons at risk, even though the risk is low. In a subset of patients with more severe symptoms due to relapsing perilesional edema, anti-inflammatory and/or immunosuppressive therapy may be of clinical benefit.. In T. solium-endemic regions there is a substantial increase in seizure activity and epilepsy. About 30% of seizure activity in these communities is attributed to T. solium infection of the brain. In contrast to hospitalized patients with seizures that are more frequently caused by degenerating viable cysts, most patients in endemic regions show calcified lesions typical of end stage degenerated T. solium granulomas. Recent studies implicate these calcifications as loci of seizure activity and about 30–50% are associated with surrounding edema that is likely due to an inflammatory process. Much is not known about how this process occurs and the long-term consequences in this affected population. Recurring episodes of overt pericalcification inflammation, which can be frequent and disabling, are common in NCC and either rare or not recognized in other causes of epilepsy. Future understanding of these mechanisms offers potential novel methods to prevent and control seizure activity in these patients, in addition to the use of anti-seizure medications. Can anti-inflammatory medications be used to dampen injurious seizure causing inflammation or circumvent the mechanisms that initiate perilesional edema episodes? Can methods to inhibit the formation of calcifications prevent seizures? These are a just of a few of the questions that need to be answered and helpful to the many patients that have seizures due to this mechanism.
Trends Box.
Calcified brain cysticercosis lesions can be found in a sizable (10–20%) proportion of people living in endemic regions
Calcified neurocysticercosis is strongly associated with epilepsy: lesions are more prevalent in symptomatic compared to asymptomatic participants, and seizure semiology is frequently concordant with the localization of the calcified lesion
Seizures in individuals with calcified disease are frequently associated with perilesional brain edema around one or more calcified lesions.
Gliosis surrounding calcified lesions seems to be associated with refractory epilepsy.
Calcified NCC has been associated with hippocampal sclerosis, both for lesions in the temporal lobe as well as for distant lesions.
Acknowledgments
The support for this study was in part received from the Intramural National Institutes of Allergy and Infectious diseases at the National Institutes of Health and NIH-FIC Training Grant D43TW001140. HG is supported by a Wellcome Trust Senior International Research Fellowship in Public Health and Tropical Medicine. JB is supported by NIAID-NIH Grant 1R01AI116456.
Glossary
- Blood brain barrier (BBB)
A highly selective permeability barrier separating the circulating blood from the extracellular fluid of the brain, composed of specialized endothelial cells preventing many substances from entering the brain.
- Calcareous corpuscles
naturally inorganic deposits composed of concentric layers of calcium carbonate, located in the scolex of the adults and vesicular stages of T. solium.
- Calcified T. solium brain granulomas
The calcified end stage in the involutive process of cysts due to antiparasitic treatment or natural death.
- Cellular edema
Edema caused by the entry of water into the cells, causing them to swell (cellular swelling).
- Corticosteroids
Man-made drugs used for a wide range of conditions mostly employed to inhibit diseases or processes involving immune mechanisms, inflammation and edema.
- Epilepsy
a disease characterized by an enduring predisposition to generate epileptic seizures and by the neurobiological, cognitive, psychological, and social consequences of this condition.
- Gliosis
is the reactive change of glial cells in response to trauma or injury to the central nervous system (CNS). In most cases, reactive gliosis involves the proliferation and/or hypertrophy of several types of glial cells, including astrocytes, microglia, and oligodendrocytes.
- Matrix metalloproteinase-9 (MMP-9)
Enzyme that belongs to the zinc-metalloproteinases involved in the degradation of the extracellular matrix (collagens IV and V, gelatins I and V, and fibronectin). Also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB).
- Mesial temporal sclerosis
Also known as hippocampal sclerosis, is the loss of neurons and scarring of the deepest portion of the temporal lobe and is associated with certain brain injuries such as chronic oxygen starvation to the brain, head trauma, or brain infection, but can also occur without an apparent cause. In this process, neurons die and scar tissue tends to form within the hippocampus and amygdala. Mesial temporal sclerosis can cause a form of temporal lobe epilepsy with partial seizures that can spread or secondarily generalize.
- Neurocysticercosis (NCC)
Infection of the central nervous system by the larval stage of the pork tapeworm T. solium.
- Perilesional edema (PE)
Accumulation of excess extracellular fluid around the calcified T. solium brain granuloma detected by neuroimaging (CT scan or MRI). The most plausible hypothesis is that edema represents an inflammatory response to calcified granuloma and is frequently and associated with episodic seizure activity or other neurological signs and symptoms.
- Scolex
Cephalic structure of a T. solium tapeworm, having suckers, hooks, for attachment to the intestine wall of the definitive host. It is invaginated inside the cyst stage.
- Seizure
Transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.
- Semiology
Is the body of knowledge that deals with the identification of symptoms (referred by patient such as headache or nauseas) and signs (identified by examination such as fever or arterial hypertension) of the various pathological manifestations or medical condition. Semiology as far as seizures or epilepsy is concerned relates to the temporal symptoms and signs that points to a particular locus of seizure activity. For instance, the seizures begin with a heaviness of a right arm that progress to clonic –tonic movements of the arm implicating the left motor strip.
- Taenia solium
Cestode helminth with a life cycle consisting of a tapeworm in the intestines of humans host alternating with infection in the pig, the natural intermediate host. When cysts formed by the infection grow within the brain causing neurologic syndromes such as epileptic seizures, the infection is called neurocysticercosis.
- Translocator protein (TSPO)
A mitochondrial protein (18 kDa) usually located in the external mitochondrial membrane. In the brain, it is found in monocytes, astrocytes and microglial and is upregulated in a number of conditions, most notably those involving inflammation or infections.
- Vasogenic edema
Edema caused by increment in extracellular fluid volume due to increased permeability of capillary endothelial cells to macromolecular serum proteins such as albumin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. The Lancet. Neurology. 2005;4:653–661. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 2.Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet. 2012;380:1193–1201. doi: 10.1016/S0140-6736(12)61381-6. [DOI] [PubMed] [Google Scholar]
- 3.Schantz PM. Taenia solium cysticercosis: an overview of global distribution and transmission. In: Singh G, Prabhakar S, editors. Taenia solium cysticercosis : from basic to clinical science. CABI Pub; 2002. pp. 63–73. [Google Scholar]
- 4.O'Neal SE, Flecker RH. Hospitalization frequency and charges for neurocysticercosis, United States, 2003–2012. Emerging infectious diseases. 2015;21:969–976. doi: 10.3201/eid2106.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ndimubanzi PC, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS neglected tropical diseases. 2010;4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coral-Almeida M, et al. Taenia solium Human Cysticercosis: A Systematic Review of Sero-epidemiological Data from Endemic Zones around the World. PLoS neglected tropical diseases. 2015;9:e0003919. doi: 10.1371/journal.pntd.0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Noval J, et al. An epidemiological study of epilepsy and epileptic seizures in two rural Guatemalan communities. Ann Trop Med Parasitol. 2001;95:167–175. doi: 10.1080/00034980120050260. [DOI] [PubMed] [Google Scholar]
- 8.Medina MT, et al. Prevalence, incidence, and etiology of epilepsies in rural honduras: The Salama study. Epilepsia. 2005;46:124–131. doi: 10.1111/j.0013-9580.2005.11704.x. [DOI] [PubMed] [Google Scholar]
- 9.Montano SM, et al. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–233. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 10.Fleury A, et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22:139–145. doi: 10.1159/000068748. [DOI] [PubMed] [Google Scholar]
- 11.Del Brutto OH, et al. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–587. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez AL, et al. A population-based, case-control study of Taenia solium taeniasis and cysticercosis. Ann Trop Med Parasitol. 1999;93:247–258. [PubMed] [Google Scholar]
- 13.Nash TE, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurology. 2008;7:1099–1105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash TE, et al. Calcified cysticerci provoke perilesional edema and seizures. Clinical Infectious Diseases. 2001;33:1649–1653. doi: 10.1086/323670. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M, et al. PET Reveals Inflammation around Calcified Taenia solium Granulomas with Perilesional Edema. PloS one. 2013;8 doi: 10.1371/journal.pone.0074052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz ME, et al. Epilepsy and neurocysticercosis in an Andean community. International journal of epidemiology. 1999;28:799–803. doi: 10.1093/ije/28.4.799. [DOI] [PubMed] [Google Scholar]
- 17.Prasad KN, et al. An epidemiological study of asymptomatic neurocysticercosis in a pig farming community in northern India. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105:531–536. doi: 10.1016/j.trstmh.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Del Brutto OH, et al. Calcified Neurocysticercosis Associates with Hippocampal Atrophy: A Population-Based Study. American Journal of Tropical Medicine and Hygiene. 2015;92:64–68. doi: 10.4269/ajtmh.14-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oliveira Taveira M, et al. Neurocysticercotic Calcifications and Hippocampal Sclerosis: A Case-Control Study. PloS one. 2015;10:e0131180. doi: 10.1371/journal.pone.0131180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia HH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350:249–258. doi: 10.1056/NEJMoa031294. [DOI] [PubMed] [Google Scholar]
- 21.Otte WM, et al. Drug therapy for solitary cysticercus granuloma: a systematic review and meta-analysis. Neurology. 2013;80:152–162. doi: 10.1212/WNL.0b013e31827b90a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das K, et al. Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8 year randomised study. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2007;14:1172–1177. doi: 10.1016/j.jocn.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Gupta RK, et al. Demonstration of scolex within calcified cysticercus cyst: its possible role in the pathogenesis of perilesional edema. Epilepsia. 2002;43:1502–1508. doi: 10.1046/j.1528-1157.2002.21302.x. [DOI] [PubMed] [Google Scholar]
- 24.Henneberg R. Die tierischen parasiten des zentralnerven-systems. In. In: Lewandowsky M, editor. Handbuch Der Neurologie. Verlag Von Julius Springer; 1912. pp. 643–712. [Google Scholar]
- 25.Ooi WW, et al. Short Report: A Calcified Taenia solium Granuloma Associated with Recurrent Perilesional Edema Causing Refractory Seizures: Histopathological Features. American Journal of Tropical Medicine and Hygiene. 2011;85:460–463. doi: 10.4269/ajtmh.2011.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nash TE, et al. Case Report: Calcified Neurocysticercus, Perilesional Edema, and Histologic Inflammation. American Journal of Tropical Medicine and Hygiene. 2014;90:318–321. doi: 10.4269/ajtmh.13-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grau E, et al. Calcification of the cysticerci of Taenia solium in the human brain. In: Flisser A, et al., editors. Cysticercosis. Present state of knowledge and perspectives. Academic Press; 1982. pp. 499–516. [Google Scholar]
- 28.Vargas-Parada L, Laclette JP. Role of the calcareous corpuscles in cestode physiology: a review. Revista latinoamericana de microbiologia. 1999;41:303–307. [PubMed] [Google Scholar]
- 29.Nash TE, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–1938. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delbrutto OH. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology. 1994;44:1706–1709. doi: 10.1212/wnl.44.9.1706. [DOI] [PubMed] [Google Scholar]
- 31.Nash T. Edema surrounding calcified intracranial cysticerci: clinical manifestations, natural history, and treatment. Pathogens and Global Health. 2012;106:275–279. doi: 10.1179/2047773212Y.0000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murthy JM, Reddy VS. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure : the journal of the British Epilepsy Association. 1998;7:153–157. doi: 10.1016/s1059-1311(98)80072-1. [DOI] [PubMed] [Google Scholar]
- 33.Cukiert A, et al. Congruence of the topography of intracranial calcifications and epileptic foci. Arquivos de neuro-psiquiatria. 1994;52:289–294. doi: 10.1590/s0004-282x1994000300001. [DOI] [PubMed] [Google Scholar]
- 34.Singh G, et al. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia. 2000;41:718–726. doi: 10.1111/j.1528-1157.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 35.Sharma LN, et al. Seizure recurrence in patients with solitary cystic granuloma or single parenchymal cerebral calcification: a comparative evaluation. Seizure : the journal of the British Epilepsy Association. 2013;22:840–845. doi: 10.1016/j.seizure.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RK, et al. Magnetization transfer magnetic resonance imaging demonstration of perilesional gliosis-relation with epilepsy in treated or healed neurocyticercosis. The Lancet. 1999;354:44–45. doi: 10.1016/s0140-6736(99)00881-8. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal A, et al. Epilepsy with focal cerebral calcification: role of magnetization transfer MR imaging. Neurology India. 2004;52:197–199. [PubMed] [Google Scholar]
- 38.Gupta RK, et al. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology. 2012;78:618–625. doi: 10.1212/WNL.0b013e318248deae. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira MC, et al. Small calcified lesions suggestive of neurocysticercosis are associated with mesial temporal sclerosis. Arquivos de neuropsiquiatria. 2014;72:510–516. doi: 10.1590/0004-282x20140080. [DOI] [PubMed] [Google Scholar]
- 40.Singh G, et al. From seizures to epilepsy and its substrates: neurocysticercosis. Epilepsia. 2013;54:783–792. doi: 10.1111/epi.12159. [DOI] [PubMed] [Google Scholar]
- 41.Del Brutto OH, et al. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology. 1992;42:389–392. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 42.White AC., Jr Neurocysticercosis: a major cause of neurological disease worldwide. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997;24:101–113. doi: 10.1093/clinids/24.2.101. quiz 114-105. [DOI] [PubMed] [Google Scholar]
- 43.Garg RK, et al. Neuroimaging abnormalities in Indian patients with uncontrolled partial seizures. Seizure : the journal of the British Epilepsy Association. 1998;7:497–500. doi: 10.1016/s1059-1311(98)80009-5. [DOI] [PubMed] [Google Scholar]
- 44.Sheth TN, et al. Reactivation of neurocysticercosis: case report. The American journal of tropical medicine and hygiene. 1999;60:664–667. doi: 10.4269/ajtmh.1999.60.664. [DOI] [PubMed] [Google Scholar]
- 45.Nash TE, Patronas NJ. Edema associated with calcified lesions in neurocysticercosis. Neurology. 1999;53:777–781. doi: 10.1212/wnl.53.4.777. [DOI] [PubMed] [Google Scholar]
- 46.Antoniuk SA, et al. Seizures associated with calcifications and edema in neurocysticercosis. Pediatr Neurol. 2001;25:309–311. doi: 10.1016/s0887-8994(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 47.Poeschl P, et al. Calcified neurocysticercosis lesions trigger symptomatic inflammation during antiparasitic therapy. American Journal of Neuroradiology. 2006;27:653–655. [PMC free article] [PubMed] [Google Scholar]
- 48.Debroy P, et al. A 25 year Experience with Neurocysticercosis in a Tropical Medicine Clinic in New York City. 65th Annual Meeting, American Society of Tropical Medicine & Hygiene. 2015 [Google Scholar]
- 49.Oselkin M, et al. Intermittent enhancement in chronic nodular calcified neurocysticercosis. Radiology case reports. 2016;10:1102. doi: 10.2484/rcr.v10i2.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.do Amaral LL, et al. Neurocysticercosis: evaluation with advanced magnetic resonance techniques and atypical forms. Topics in magnetic resonance imaging : TMRI. 2005;16:127–144. doi: 10.1097/01.rmr.0000189106.78146.98. [DOI] [PubMed] [Google Scholar]
- 51.Mahajan SK, et al. Neurocysticercosis presenting with psychosis. The Journal of the Association of Physicians of India. 2004;52:663–665. [PubMed] [Google Scholar]
- 52.Amaral L, et al. Ununsual manifestations of neurocysticercosis in MR imaging: analysis of 172 cases. Arquivos de neuro-psiquiatria. 2003;61:533–541. doi: 10.1590/s0004-282x2003000400002. [DOI] [PubMed] [Google Scholar]
- 53.Agapejev S, et al. Chronic brain edema in neurocysticercosis. Arquivos de neuro-psiquiatria. 1998;56:569–576. doi: 10.1590/s0004-282x1998000400009. [DOI] [PubMed] [Google Scholar]
- 54.Litt AW, Mohuchy T. Case 10: neurocysticercosis. Radiology. 1999;211:472–476. doi: 10.1148/radiology.211.2.r99ma38472. [DOI] [PubMed] [Google Scholar]
- 55.Lachuriya G, et al. Toll-like Receptor-4 Polymorphisms and Serum Matrix Metalloproteinase-9 in Newly Diagnosed Patients With Calcified Neurocysticercosis and Seizures. Medicine. 2016;95:e3288. doi: 10.1097/MD.0000000000003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Brutto OH. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology. 1994;44:1706–1709. doi: 10.1212/wnl.44.9.1706. [DOI] [PubMed] [Google Scholar]
- 57.Sheth TN, et al. Persistent MR contrast enhancement of calcified neurocysticercosis lesions. AJNR. American journal of neuroradiology. 1998;19:79–82. [PMC free article] [PubMed] [Google Scholar]
- 58.Moyano LM, et al. Neurocysticercosis as a Cause of Epilepsy and Seizures in Two Community-Based Studies in a Cysticercosis-Endemic Region in Peru. PLoS neglected tropical diseases. 2014;8 doi: 10.1371/journal.pntd.0002692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia HH, et al. Discrepancies Between Cerebral Computed Tomography and Western Blot in the Diagnosis of Neurocysticercosis. The American journal of tropical medicine and hygiene. 1994;50:152–157. doi: 10.4269/ajtmh.1994.50.152. [DOI] [PubMed] [Google Scholar]
- 60.Park SY, et al. Clinical mplications of calcified lesions of neurocysticercosis. The Pediatric Infectious Disease Journal. 2000;19:581–583. doi: 10.1097/00006454-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 61.Escobar A. Pathology of the Nervous System. In: Palacios E, et al., editors. Cysticercosis of the Central Nervous System. Charles Thomas; 1983. pp. 27–54. [Google Scholar]
- 62.Marquez-Monter H. Cysticercosis. In: Marcial-Rojas RA, editor. Pathology of Protozoal and Helminthic Diseases. The Williams and Wilkens Company; 1971. pp. 592–617. [Google Scholar]
- 63.Cole AJ. Status epilepticus and periictal imaging. Epilepsia. 2004;45(Suppl 4):72–77. doi: 10.1111/j.0013-9580.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 64.Kim JA, et al. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR. American journal of neuroradiology. 2001;22:1149–1160. [PMC free article] [PubMed] [Google Scholar]
- 65.Milligan TA, et al. Frequency and patterns of MRI abnormalities due to status epilepticus. Seizure : the journal of the British Epilepsy Association. 2009;18:104–108. doi: 10.1016/j.seizure.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Mendes A, Sampaio L. Brain magnetic resonance in status epilepticus: A focused review. Seizure : the journal of the British Epilepsy Association. 2016;38:63–67. doi: 10.1016/j.seizure.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Nash TE, et al. Corticosteroid use in neurocysticercosis. Expert Review of Neurotherapeutics. 2011;11:1175–1183. doi: 10.1586/ern.11.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mejia R, Nash TE. Corticosteroid withdrawal precipitates perilesional edema around calcified Taenia solium cysts. The American journal of tropical medicine and hygiene. 2013;89:919–923. doi: 10.4269/ajtmh.13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran ND, et al. Aquaporin-1-mediated cerebral edema following traumatic brain injury: effects of acidosis and corticosteroid administration. Journal of neurosurgery. 2010;112:1095–1104. doi: 10.3171/2009.8.JNS081704. [DOI] [PubMed] [Google Scholar]
- 70.Duffy BA, et al. Dexamethasone exacerbates cerebral edema and brain injury following lithium-pilocarpine induced status epilepticus. Neurobiology of disease. 2014;63:229–236. doi: 10.1016/j.nbd.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia HH, et al. Enhanced steroid dosing reduces seizures during antiparasitic treatment for cysticercosis and early after. Epilepsia. 2014;55:1452–1459. doi: 10.1111/epi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitre E, et al. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44:549–553. doi: 10.1086/511040. [DOI] [PubMed] [Google Scholar]
- 73.Garg RK, Malhotra HS. Solitary cysticercus granuloma. Expert Review of Anti-Infective Therapy. 2012;10:597–612. doi: 10.1586/eri.12.35. [DOI] [PubMed] [Google Scholar]