Abstract
This study tested the effects of long-term estradiol (E2) replacement on social behavior and gene expression in brain nuclei involved in the regulation of these social behaviors in adult female rats. We developed an ultrasonic vocalization (USV) test and a sociability test to examine communications, social interactions, and social preference, using young adult female cagemates. All rats were ovariectomized (OVX) and implanted with a Silastic capsule containing E2 or vehicle, and housed in same-treatment pairs for a 3-month period. Then, rats were behaviorally tested, euthanized, and 5 nuclei in the brain’s social decision-making circuit were selected for neuromolecular profiling by a multiplex qPCR method. Our novel USV test proved to be a robust tool to measure numbers and types of calls emitted by cagemates that had been reintroduced after a 1-week separation. Results also showed that E2-treated OVX rats had profoundly decreased numbers of USV calls compared to vehicle-treated OVX rats. In a test of sociability, in which a female was allowed to choose between her cagemate or a same-treatment novel rat, we found few effects of E2 compared to vehicle, although interestingly, rats chose the cagemate over an unfamiliar conspecific. Gene expression results revealed that the supraoptic nucleus had the greatest number of gene changes caused by E2: Oxt, Oxtr and Avp were increased, and Drd2, Htr1a, Grin2b, and Gabbr1 were decreased, by E2. No genes were affected in the prefrontal cortex, and 1–4 genes were changed in paraventricular nucleus (Pgr), bed nucleus of the stria terminalis (Oxtr, Esr2, Dnmt3a), and medial amygdala (Oxtr, Ar, Foxp1, Tac3). Thus, E2 changes communicative interactions between adult female rats, together with selected expression of genes in the brain, especially in the supraoptic nucleus.
Keywords: Estradiol
Topic: Ultrasonic vocalization, Menopause, Hypothalamus, Social behavior, Gene expression, Oxytocin, Vasopressin, Dopamine, Serotonin, Epigenetics
1. Introduction
The loss of ovarian estrogens during natural or surgical menopause results in a variety of symptoms that can impair quality of life. Among the neurological and neurobehavioral symptoms reported by perimenopausal women are increased depression and anxiety (Freeman et al., 2004; Schmidt et al., 2004; Bromberger et al., 2011). Estrogens, especially estradiol (E2), regulate these behaviors in animals as well as women (De Kloet et al., 2005; Klenerova et al., 2009; Meyer-Lindenberg et al., 2011; Rubinow et al., 1998). In clinical studies, women given estradiol have decreased anxiety and depressive symptoms compared to women given placebo (De Novaes Soares et al., 2001; Zweifel and O’Brien, 1997; Schmidt et al., 2000). However, there is controversy about the risks and benefits of hormone replacement therapy (HRT) following the publication of the results from the Women’s Health Initiative, which suggested a small but significant increase in adverse cardiovascular and breast cancer incidents in women taking HRT (Rossouw et al., 2002; Manson et al., 2013). Although those findings have been partially discredited (Klaiber et al., 2005; Bhupathiraju and Manson, 2014), to this day the question of whether, when, and for how long HRT should be used is still debated.
Along with its effects on affective behavior are estradiol’s effects on social behavior. In rodents, ovariectomy causes deficits in social interaction and social memory that are improved with estradiol replacement (Hlinák, 1993; Tang et al., 2005). Mice with knockouts for the estrogen receptor α (ERα) or ERβ genes also exhibit social behavioral deficits (Choleris et al., 2006; Imwalle et al., 2002). This appears to translate to humans, as women with menopausal depression or anxiety report problems with interpersonal relationships and a decreased desire to engage in social interactions (Uguz et al., 2011; Deeks and McCabe, 2004; Lanza di Scalea et al., 2012; Schmidt et al., 2000). Schmidt et al. (2000 & 2015) have reported that estradiol decreased social isolation during perimenopause compared to placebo, whereas another study found that hormone replacement therapy was associated with an increase in social isolation (Achat et al., 1998). Therefore, the effect of HRT on social behavior requires further investigation.
The neurobiology of social behavior involves a complex network of brain regions that signal via diverse cellular and molecular pathways. The prefrontal cortex (PFC), medial amygdala (MeA), bed nucleus of the stria terminalis (BNST) and the hypothalamus are among the estrogen-sensitive nodes of this neural circuit (Arimatsu and Hatanaka, 1986; Kugaya et al., 2003; Han and De Vries, 2003; Garcia et al., 2016). Considering their key roles and abundant expression of ERs (Kugaya et al., 2003; Walker et al., 2003; Saper et al., 2005), they are important targets of analysis for understanding potential neuromolecular substrates involved in estrogens’ regulation of social behavior.
Thus, this study was designed in order to gain insight into how long-term estradiol treatment affects social and communicative interactions in female rats, using novel behavioral tasks designed to assess sociality in cagemates. In addition, to gain insight into the molecular changes that occur with estradiol deprivation and replacement, we systematically profiled the expression of genes that are implicated in the regulation of social behavior, and that are estrogen-sensitive.
2. Materials and methods
2.1. Animals and husbandry
Animal procedures were conducted in accordance with The Guide for the Care and Use of Laboratory Animals following protocols approved by The University of Austin IACUC committee and NIH standards. Reproductively mature adult female (MAT, 3–4 months, sexually naïve, n = 40) Sprague Dawley rats (Harlan) were purchased. Upon arrival, rats were pair housed in a 12:12 light:dark cycle (lights on at 0700), and given water and rat chow ad libitum. Prior to surgery they were allowed to acclimate to the room for two weeks. During this period estrous cyclicity was monitored daily by vaginal lavage with sterile saline. Only females with regular 4–5 day cycles were used. Ovariectomy (OVX) surgery was performed on all rats under isofluorane inhalation anesthesia, with cagemates assigned to the same treatment group. A single injection of Rimadyl (5 mg/kg) was given at the start of surgery. Bilateral dorsolateral incisions were made through the skin, muscle, and peritoneum, and the ovaries were ligated and removed. Muscles were sutured and wound clips used to close the skin. At the time of surgery animals were implanted subcutaneously between the shoulder blades with Silastic capsules containing either 100% cholesterol (Veh) or 5% 17β-estradiol/95% cholesterol (E2). Animals were housed separately for 5 days post-OVX, then returned to pair-housing with their original partner. The final number of experimental rats was n = 20 (Veh) and n = 20 (E2).
2.2. Behavioral paradigms
Behavioral testing began 2.5 months after OVX and hormone treatment, when rats were ~6.5 months of age and had been housed in pairs for 3 months. At that time, all animals were separated from their cagemates and individually housed for one week. Testing began with an ultrasonic vocalization test (2 consecutive days) followed the next day by sociability testing (2 consecutive days). All behavior testing took place during the lights-on period, from 0900 to 1200 h. Rats were euthanized one week after the completion of testing at ~7 months of age.
2.2.1. Ultrasonic vocalization test (USV)
USV testing took place over 2 consecutive days in a Plexiglas tank (23L × 29W × 40H cm, Fig. 1) held within a sound-attenuating chamber equipped with a microphone. On Day 1, each rat was placed in recording chamber for 5 min alone, during which USVs were recorded for each individual (Trial 1, habituation). Day 2 consisted of a sequence of three 5-minute trials, referred to as Trials 2, 3 and 4, in which the cagemates were re-introduced, allowed to interact, and subsequently separated. More specifically, in Trial 2 (re-introduction across a barrier) prior to placement of the rats, a removable perforated plastic grid was placed across the center of the apparatus to bisect it; it allowed for nose touching, auditory and visual contact but not gross physical contact. The cagemates were re-introduced during this trial but were separated by the grid. USVs were recorded from the pair. For Trial 3 (physical interactions), the grid was removed and the rats were able to freely interact with one another. Videotaping was performed during this trial in order to quantify activity, time interacting, and anogenital investigation. Before the start of Trial 4 (separation), one of the cagemates was randomly removed from the testing chamber and placed into an identical chamber in a separate sound-attenuated chamber. Each animal was immediately recorded for a final 5-minute trial before being individually rehoused.
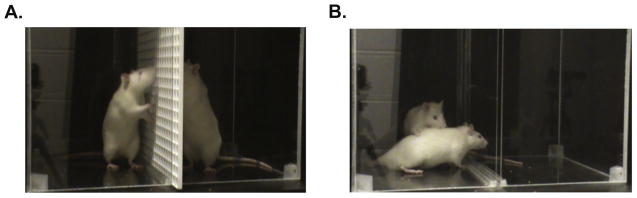
Fig. 1.
The ultrasonic vocalization apparatus and set-up are shown for Trial 2 (reintroduction across a barrier) and Trial 3 (physical interaction). A) In Trial 2 the cagemates were reintroduced across a perforated plastic grid that allowed sniffing and nose-touching but no other physical interactions. B) In Trial 3 the grid was removed from the apparatus and the cagemates were allowed to freely interact, and USVs recorded. Trial 3 was also videotaped and scored for activity, time interacting, and time engaged in anogenital investigation. During Trials 1 (habituation of each rat separately) and 4 (separation at the end of the test) the animals were alone in the apparati and recorded separately (images not shown).
USVs were recorded using UltraSoundGate hardware and software and analyzed with Saslab Pro (all Avisoft, Germany). We used the Saslab Pro software to automatically detect and quantify calls, between 45 and 70 kHz, and to differentiate them as frequency modulated (FM) or non-frequency modulated (NFM). NFM calls are thought to be a form of social communication or coordination, whereas FM USVs likely represent a positive or hedonic affective state (Burgdorf et al., 2011; Knutson et al., 2002). We did not observe any calls under 45 kHz, which are associated with a negative affective state. In this study frequency modulated calls were defined as having more than a 9 kHz change in frequency. Examples of representative NFM and FM USV calls are shown in Fig. 2. Each pair of cagemates was analyzed as a unit, because during Trials 2 and 3 when both animals were present in the same chamber, it was impossible to distinguish calls from the individuals. Therefore, for Trials 1 and 4, when the animals were recorded separately, their calls were summed for statistical analysis. During Trial 3, while rats were allowed to physically interact, the behavioral test was simultaneously recorded using a digital video camera. Recordings were subsequently analyzed by a blind observer for overall activity, interactions, and anogenital investigation.
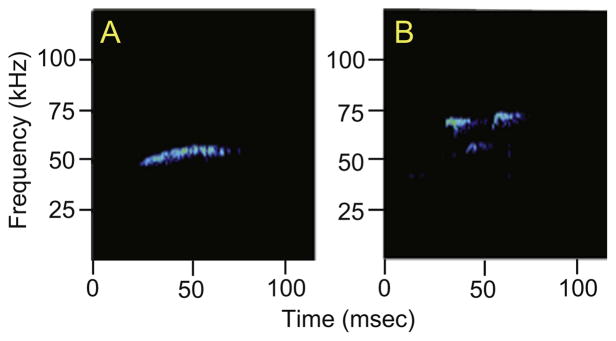
Fig. 2.
Representative examples of a non-frequency modulated (flat) USV (A) and a frequency-modulated USV (B) are shown for an experimental rat.
Statistical analyses were conducted using SPSS. Due to the non-homogeneity of these data sets all data were transformed using either a square root or log transformation. One pair of animals from the vehicle treated group was removed as an outlier, due to calls being > 2 SD above the mean of the group. The final number of pairs used for USV analysis was 9 and 10 for E2 vs. Veh, respectively. A repeated measures test was used to analyze the effects of treatment (E2 vs. Veh) and trial (Trials 1 through 4) for numbers of total calls, non-frequency modulated calls, and frequency-modulated calls. For all of these analyses, alpha was set at 0.05 and significant main or interaction effects were followed by two-tailed independent sample t-tests. Independent sample t-test was also used to analyze the difference between treatments on overall activity, interaction and anogenital investigation. We calculated the effect size for main effects and interactions identified by repeated-measures ANOVAs, as indicated by eta-squared (η2). An η2 of 0.02 is considered small, η2 of 0.13 medium, and η2 of 0.26 large. We used the effect size calculator from http://www.uccs.edu/~lbecker/ to obtain the Cohen’s d (d) effect size for t-tests. An effect size of 0.2 represents a small effect size, 0.5 medium, and 0.8 or above a large effect size. Correlations between USV behavior and calls made during trial 3, while the animals were freely interacting, were analyzed using a Spearman’s rank correlation.
2.2.2. Sociability test
On day 1, one of the cagemates was randomly chosen to be the experimental rat and was habituated to a Stoelting three-chamber apparatus (100L × 100W × 34.5H cm total) containing two holding cages in each corner of the side chambers (Fig. 3), for 5 min prior to testing. After the habituation trial the experimental animal was placed back in its home cage, during which time its cagemate was placed in one of the two holding cages [small cage in one of the far corners (lower-left and lower-right, Fig. 3)]. Holding cages have vertical bars that are spaced to allow for nose touching and anogenital investigation between the bars. The second holding cage was used to house a novel (unfamiliar) rat of the same sex, age, and treatment. The experimental rat was then placed back into the center chamber and was allowed to roam freely for the 5-minute sociability test, during which her behaviors were tracked by Any-Maze software (Stoelting Co., Wood Dale, IL). Rats were re-housed separately overnight. On day 2 the cagemate that was previously used as a stimulus rat was used as the experimental rat. That rat’s partner was now placed into a holding cage, and the other holding cage contained a new unfamiliar rat of the same sex, age, and treatment. Again, the experimental rat’s movements were tracked by Any-Maze. The software was subsequently used to quantify time immobile, time spent in each chamber, and time spent in a smaller 19 × 19 cm zone (approximately one body length distance) designated around the stimulus rats (Fig. 3). After the end of day 2 of sociability testing, animals were rehoused with their cagemate for one week, after which they were euthanized.
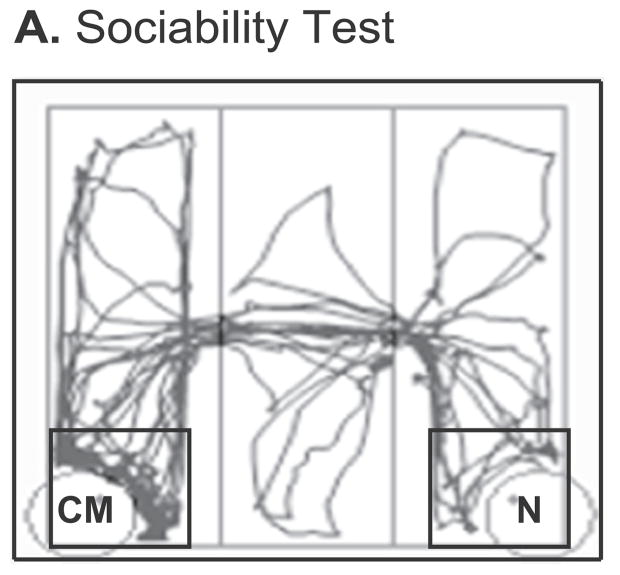
Fig. 3.
The 3-chamber sociability test apparatus is shown, with a trace of a representative rat’s path as tracked by ANY-maze. The black square around each of the two corner holding cages represents the area in proximity to the stimulus rats. For the sociability test the experimental rat’s cagemate (CM) was placed in one of the two corner holding cages while a novel (N) rat of the same age, sex and treatment was placed in the other. The experimental rat was placed in the center chamber and allowed to explore the apparatus for 5 min.
Statistics were conducted using SPSS. Due to the violation of assumptions of normality, total time freezing and time spent near stimulus rats were transformed using a log and square root transformation, respectively. A repeated measures ANOVA was used to analyze time spent near the stimulus rats and an independent sample t-test was used for total time immobile. Data for time immobile near the stimulus rat, time spent in chambers, time immobile in chambers, and visit duration to stimulus rat did not meet assumptions even after transformation. Therefore, a Friedman’s test was performed to analyze the effect of chambers while Wilcoxon signed-rank test was performed to analyze the effect of treatment. Effect sizes were calculated and reported as eta-squared (η2) and Cohen’s d (d) as described above for USV tests.
2.3. Brain tissue processing
Rats were euthanized at ~7 months of age, one week after completion of behavioral testing. All animals were weighed and euthanized during the lights-on period between 1330 and 1600 h, by rapid decapitation. The brain was removed and sectioned using an ice-cold stainless steel brain matrix to collect 1-mm coronal brain sections. A glass vial containing 1.5 ml of RNAlater (Life technologies, Grand Island, NY) was used to store each section overnight at 4 °C. Sections were then mounted onto chilled slides and placed in a −20 °C freezer until brain punches were taken within a month of storage. Bilateral punches of brain tissue were taken using Palkovits punches and the Paxinos and Watson (2009) rat brain atlas (all coordinates are based on that atlas) under a dissecting microscope. Rostral borders of each region relative to Bregma were (punch diameter given parenthetically) were: paraventricular nucleus of the hypothalamus (PVN, 1.22 mm diameter) −0.84 mm; supraoptic nucleus (SON, 0.96 mm diameter) −0.60 mm; MeA (1.22 mm diameter) −1.56 mm; BNST (0.96 mm diameter) 0.00 mm; and PFC (1.22 mm diameter) 4.20 mm. Punched tissue was placed in a frozen Eppendorf tube and stored at −80 °C until time of PCR. A trunk blood sample was collected at euthanasia, allowed to clot, and centrifuged at 2300 ×g for 5 min. Serum was collected and stored at −80 °C in Eppendorf tubes until time of hormone assays.
2.4. Real-time PCR assays and analysis
Extraction of RNA from frozen PVN, SON, MeA, BNST, and PFC punches was performed using an Allprep RNeasy mini kit (Qiagen, Valencia, California), according to the manufacturer’s protocol. The quality of the RNA was verified using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). A GloMax-Multi Detection System (Promega, Madison, WI) was used to assess the quantity of RNA. After RNA extraction mRNA (200 ng) was converted to single-stranded cDNA using a high-capacity cDNA reverse transcription kit (Life Technologies, Grand Island, NY), and samples stored at −20 °C until use. Samples from the 5 brain regions (n = 11–18 per group) were run on a customized rat Taqman low-density array (TLDA) Microfluidic 48-gene real-time PCR cards (Applied Biosystems). Quantitative real-time PCR was performed as published in Walker et al., 2009 using Taqman universal mastermix (Life Technologies, Grand Island, NY) and detected on a ViiA7 Real time PCR machine (Applied Biosystems, Life Technologies, Grand Island, NY) with the following run parameters: 0 °C for 2 min, 95 °C for 10 min, 45 cycles of 95 °C for 15 s, and 60 °C for 1 min. Each TLDA card allowed for 8 samples to be run on a single card, for a total of 19 cards. No samples were pooled for gene expression analysis. The genes for the assay were chosen based on their roles in social behavior and their regulation by steroid hormones (45 genes of interest and three normalizing genes; Table 1).
Table 1.
List of genes quantified using Taqman low-density qPCR arrays. Genes with a significant effect of treatment that survived false-discovery rate correction are shown by up-arrows (E2 > vehicle) or down-arrows (Vehicle > E2).
| Gene | Name | PVN | BNST | MeA | SON | PFC |
|---|---|---|---|---|---|---|
| Steroid hormone receptors | ||||||
| Esr1 | Estrogen receptor alpha | – | – | – | – | – |
| Esr2 | Estrogen receptor beta | – | ↓ | – | – | – |
| Ar | Androgen receptor | – | – | ↑ | – | – |
| Pgr | Progesterone receptor | ↑ | – | – | – | – |
| Nr3c1 | Nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor) | – | – | – | ↓ | – |
| Oxytocin and vasopressin signaling | ||||||
| Avp | Vasopressin | – | – | – | ↑ | – |
| Avpr1a | Vasopressin receptor 1a | – | – | – | – | – |
| Oxt | Oxytocin | – | – | – | ↑ | – |
| Oxtr | Oxytocin receptor | – | ↑ | ↑ | ↑ | – |
| Neurotransmission | ||||||
| Drd1a | Dopamine receptor D1A | – | – | – | – | – |
| Drd2 | Dopamine receptor D2 | – | – | – | ↓ | – |
| Htr1a | 5-Hydroxytryptamine (serotonin) receptor 1A, G protein-coupled | – | – | – | ↓ | – |
| Htr2a | 5-Hydroxytryptamine (serotonin) receptor 2A, G protein-coupled | – | – | – | – | – |
| Htr2c | 5-Hydroxytryptamine (serotonin) receptor 2C, G protein-coupled | – | – | – | – | – |
| Oprm1 | Opioid receptor, Mu 1 | – | – | – | – | – |
| Oprk1 | Opioid receptor, kappa 1 | – | – | – | – | – |
| Oprd1 | Opioid receptor, delta 1 | – | – | – | – | – |
| Grin1 | Glutamate receptor, ionotropic, N-methyl D-aspartate 1 | – | – | – | – | – |
| Grin2a | Glutamate receptor, ionotropic, N-methyl D-aspartate 2A | – | – | – | – | – |
| Grin2b | Glutamate receptor, ionotropic, N-methyl D-aspartate 2B | – | – | – | ↓ | – |
| Grin2c | Glutamate receptor, ionotropic, N-methyl D-aspartate 2C | – | – | – | – | – |
| Grin2d | Glutamate receptor, ionotropic, N-methyl D-aspartate 2D | – | – | – | – | – |
| Gria1 | Glutamate receptor, ionotropic, AMPA 1 | – | – | – | – | – |
| Gria2 | Glutamate receptor, ionotropic, AMPA 2 | – | – | – | – | – |
| Gabbr1 | GABA B receptor 1 | – | – | – | ↓ | – |
| Gabbr2 | GABA B receptor 2 | – | – | – | – | – |
| Growth factor signaling | ||||||
| Bdnf | Brain-derived neurotrophic factor | – | – | – | – | – |
| Igf1 | Insulin-like growth factor 1 | – | – | – | – | – |
| Igf1r | Insulin-like growth factor 1 receptor | – | – | – | – | – |
| Epigenetic signaling | ||||||
| Hdac1 | Histone deacetylase 1 | – | – | – | – | – |
| Hdac2 | Histone deacetylase 2 | – | – | – | – | – |
| Hdac4 | Histone deacetylase 4 | – | – | – | ↓ | – |
| Dnmt1 | DNA (cytosine-5-)-methyltransferase 1 | – | – | – | – | – |
| Dnmt3a | DNA (cytosine-5-)-methyltransferase 3 alpha | – | ↓ | – | – | – |
| Dnmt3b | DNA (cytosine-5-)-methyltransferase 3 alpha | – | – | – | – | – |
| Other | ||||||
| Crh | Corticotropin releasing hormone | – | – | – | – | – |
| Egr1 | Early growth response protein 1 | – | – | – | – | – |
| Foxp2 | Forkhead box P2 | – | – | – | ↓ | – |
| Foxp1 | Forkhead box P1 | – | – | ↑ | – | – |
| Nlgn3 | Neuroligin 3 | – | – | – | – | – |
| Shank1 | SH3 and multiple ankyrin repeat domains 1 | – | – | – | – | – |
| Tac3 | Tachykinin 3 | – | – | ↓ | – | – |
Relative expression was determined for each sample using the comparative cycle threshold method (Pfaffl, 2001; Schmittgen and Livak, 2008). All samples were normalized to the geometric mean of the housekeeping genes Gapdh, Rpl13a, and 18s and then calibrated to the median δ-cycle threshold of the vehicle treated group. Samples that amplified at or above 35ct were excluded from analysis and any samples 2 SD above the mean of the group were removed as outliers.
The effect of treatment (E2 vs. Veh) on gene expression was analyzed using independent sample t-test using SPSS. Cohen’s d (d) effect sizes were calculated for each significant comparison. Those data that did not pass the assumptions of normality and/or variance were transformed using either a square root or log transformation. Data that did not meet assumptions even after transformation were analyzed using a Kruskal-Wallis test. For all of these analyses, alpha was set at 0.05 and the Benjamini-Hochberg false discovery rate (FDR) method (Benjamini and Hochberg, 1995) was used to correct for multiple comparisons.
2.5. Estradiol hormone assay
Levels of serum estradiol (E2) were determined by radioimmunoassay (Ultrasensitive Estradiol RIA, Cat No DSL4800, Lot # 150622C, Beckman Coulter, Pasadena, CA), according to the manufacturer’s directions. A single assay was used for all the samples. Samples were run in duplicates with volumes of 100 μl of serum. Assay sensitivity was 2.2 pg/ml and intrassay C.V. was 1.30%. As expected, the estradiol treated animals had significantly higher concentrations of E2 (37 ± 3 pg/ml) than did the vehicle (14 ± 1 pg/ml) treated animals (p < 0.01).
3. Results
3.1. Ultrasonic vocalizations
3.1.1. USV calls
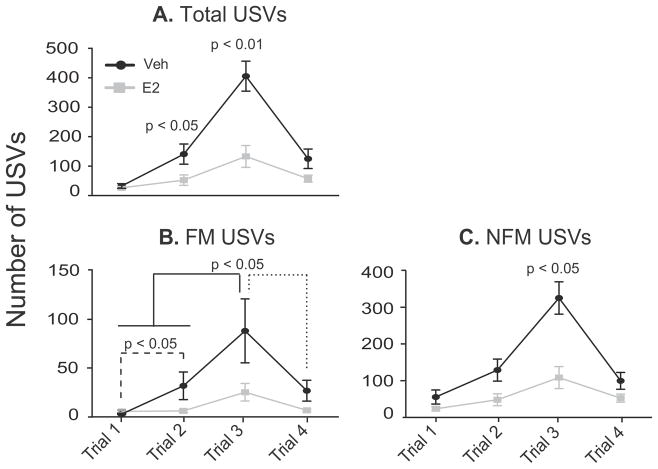
Analysis of total numbers of USV calls showed a significant interaction between trial and treatment [F(3, 51) = 11.54, η2 = 0.19, p < 0.05]. Vehicle animals called significantly more than E2 animals on Trials 2 [t(17) = 2.29, d = 1.07, p < 0.05] and 3 [t(17) = 4.01, d = 2.00, p < 0.01; Fig. 4A]. When differentiated into frequency modulated (FM) and non-frequency modulated (NFM) calls, analysis of FM calls yielded a significant main effect of trial [F(3, 51) = 8.57, η2 = 0.30, p < 0.01], with more calls being emitted on Trial 3 (physical interaction) than Trials 1 [habituation; t(18) = 4.17, d = 1.05, p < 0.01; Fig. 4B] and 4 [separation; t(18) = 3.85, d = 0.77, p < 0.01; Fig. 4B]. For NFM calls, a significant interaction between trial and treatment was found [F(3, 51) = 9.49, η2 = 0.18, p < 0.01] with the vehicle rats emitting significantly more NFM calls than E2 rats on Trial 3 [t(17) = 3.79, d = 1.69, p < 0.01, Fig. 4C].
Fig. 4.
Numbers of USV calls are shown. Note that the y-axis varies across graphs. A) The total number of USVs was significantly higher in Veh than E2 treated animals on Trials 2 and 3. B) For FM calls both Veh and E2 rats called significantly more on Trial 3 compared to all other trials, and had more FM calls on Trial 2 than on Trial 1. However, there were no significant E2 effects on FM USVs. C) Non-frequency modulated (NFM) calls were higher in Veh than E2 rats on Trial 3. Data are shown as mean ± SEM.
3.1.2. Behaviors during USV Trial 3 (physical interaction)
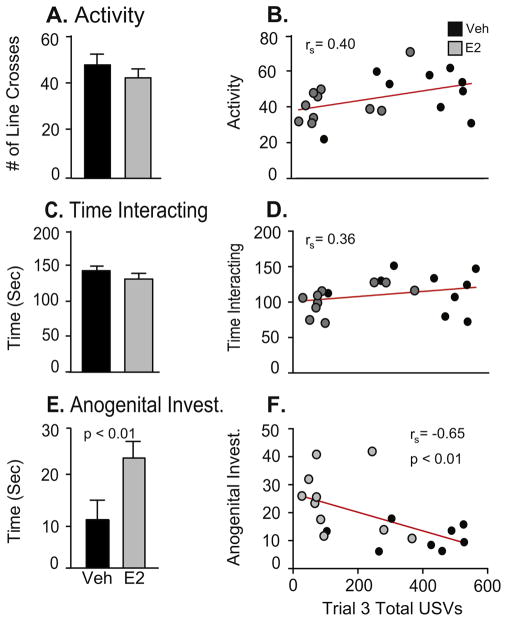
Video recordings during Trial 3 were scored for overall activity, interaction and anogenital investigation. Independent-sample t-tests showed that there was a significant effect of treatment on anogenital investigation [t(18) = 2.99, d = 1.34, p < 0.01; Fig. 5E], greater in E2 than vehicle rats. There were no treatment differences for activity or time interacting (Fig. 5A, C).
Fig. 5.
Behavior data are shown from videotapes taken during Trial 3 (physical interaction) of the USV test. Note that the y-axis varies across graphs. A) Activity, measured by the number of line crosses, and C) Time interacting, were not affected by E2 treatment. E) Time spent participating in anogenital investigation was significantly higher in the E2 rats. Correlation analyses were performed for these 3 measures with the total number of USVs measured in Trial 3. B) Activity and D) Time interacting were not correlated with total USV calls, whereas F) Time spent engaged in anogenital investigation was negatively correlated with total USVs. A–C: Data are shown as mean + SEM. D–F: Datapoints for individual rats are denoted with black (Veh) or gray (E2) dots.
3.1.3. Correlations between total USVs and behaviors in trial 3 (physical interaction)
Correlation analysis of total numbers of USV calls emitted during Trial 3 and the behaviors videotaped during that trial showed that call numbers were not correlated with activity (Fig. 5B) or time interacting (Fig. 5D). However, anogenital investigation was significantly negatively correlated with total calls [rs = −0.65, p < 0.01; Fig. 5F].
3.2. Sociability test
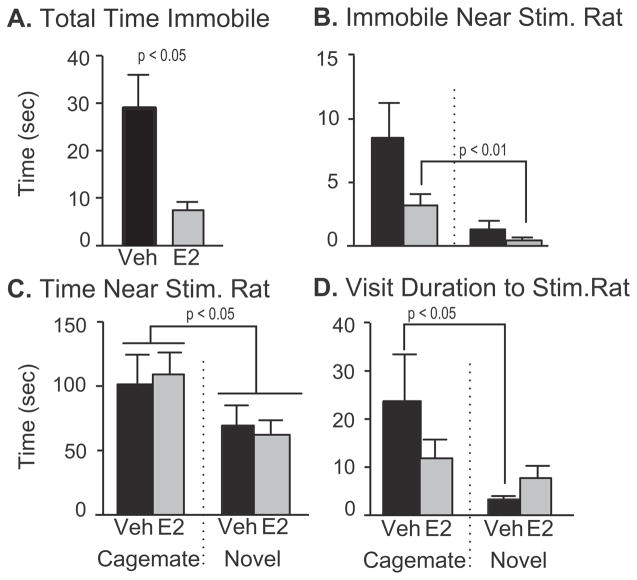
E2 treated rats spent significantly less time immobile during the sociability test than Veh treated animals [t(28) = 3.10, d = 1.13, p < 0.01; Fig. 6A]. The E2 animals also spent more time immobile in close proximity to their cagemate than they did with the novel rat [z = −2.55, d = 1.13, p < 0.01; Fig. 6B]. Rats of both treatment groups spent more time in proximity to their cagemate than the novel rat [F(1, 29) = 4.68, η2 = 0.14, p < 0.05; Fig. 6C]. The vehicle treated animals had longer mean visit durations to their cagemate than they did with the novel rat (z = −2.22, d = 0.64, p < 0.05; Fig. 6D). For time spent in chambers and time immobile in chambers there were no differences between the treatment groups.
Fig. 6.
Sociability data are shown for those behaviors that were significantly affected by treatment. Note that the axes vary across graphs. A) Total time spent immobile was significantly greater in Veh than E2 rats. B) When this behavior was further analyzed in proximity to the cagemate or novel stimulus rat, the E2 group spent more time immobile near the familiar (cagemate) rat than near the novel rat. There was also a treatment effect for time spent immobile near the novel stimulus rat, which was significantly greater in Veh than E2 animals. C) Total time spent near the cagemate was greater than that near the novel rat, irrespective of hormone treatment. D) Vehicle animals had on average longer visit durations to the cagemate. Data are shown as mean + SEM.
3.3. Gene expression
Gene expression results are summarized for PVN, BNST, MeA, SON, and PFC in Table 1. Data are graphed only for genes surviving the false discovery rate test.
3.3.1. Paraventricular nucleus
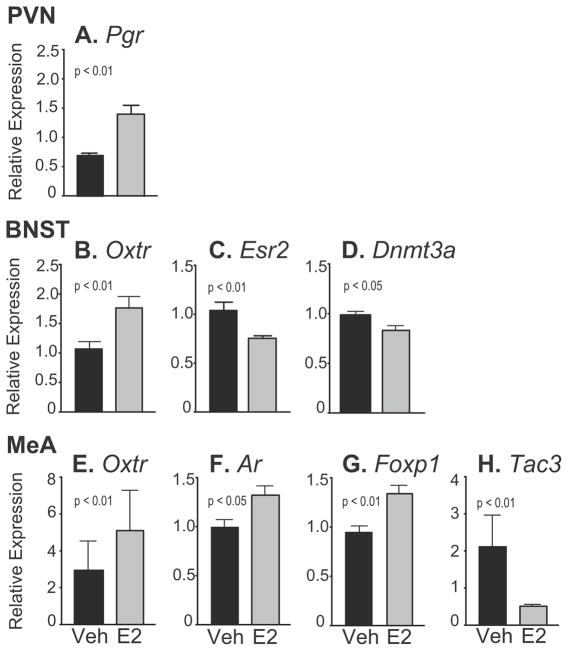
Only one gene, Pgr (progesterone receptor) was significantly affected by treatment in the PVN, which was upregulated by E2 compared to Veh [t(24) = 4.20, d = 1.72, p < 0.01; Fig. 7A].
Fig. 7.
Relative gene expression data are shown for the PVN, BNST and MeA. Note that the scale of the y-axis varies depending on the gene. A) In the PVN, Pgr was significantly higher in E2 than Veh rats. B–D) In the BNST, Oxtr was upregulated, and Esr2 and Dnmt3a downregulated, by E2 compared to Veh. E–H) In the MeA, Oxtr, Ar and Foxp1 were upregulated, and Tac3 downregulated, by E2 compared to Veh. Data are shown as mean + SEM.
3.3.2. Bed nucleus of the stria terminalis
Three genes showed a significant effect of treatment in the BNST. Oxtr (Oxytocin receptor) was upregulated by E2 [t(31) = 3.06, d = 1.07, p < 0.01; Fig. 7B) while Esr2 (estrogen receptor beta, Fig. 7C) and Dnmt3a (DNA methyltransferase 3 alpha, Fig. 7D) were downregulated by E2 [Esr2: z = −3.12, d = 1.13, p < 0.01; Dnmt3a: t(28) = 2.67, d = 0.99, p < 0.05].
3.3.3. Medial amygdala
In the MeA three genes were significantly upregulated by E2: Oxtr [t(24) = 3.14, d = 1.29, p < 0.01; Fig. 7E], Ar [androgen receptor; t(23) = 2.48, d = 0.44, p < 0.05; Fig. 7F] and Foxp1 [forkhead box P1; t(21) = 3.30, d = 1.50, p < 0.01; Fig. 7G]. Tac3 (Tachykinin 3) was downregulated by E2 in the MeA [t(22) = 3.66, d = 1.41, p < 0.01; Fig. 7H].
3.3.4. Supraoptic nucleus
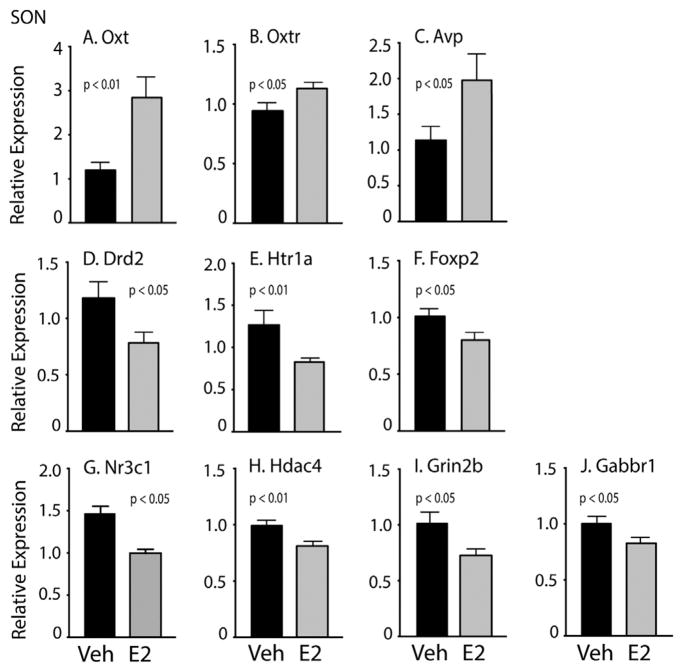
A total of 10 genes were significantly altered by E2 treatment. Of these, three were upregulated by E2, all related to social behavior (Fig. 8A–C): Oxt [oxytocin; t(29) = 3.53, d = 1.22, p < 0.01], Oxtr [t(27) = 2.10, d = 0.79, p < 0.05], and Avp [vasopressin; t(23) = 2.11, d = 0.74, p < 0.05]. Seven genes were significantly downregulated by E2 in the SON (Fig. 8D–J), mainly involved in neurotransmission and epigenetic regulation: Drd2 [dopamine receptor D2; t(28) = 2.16, d = 0.83, p < 0.05], Nr3c1 [glucocorticoid receptor; t(29) = 2.05, d = 0.75, p < 0.05], Htr1a [serotonin receptor 1A; z = −2.87, d = 0.85, p < 0.01], Foxp2 [forkhead box P2; t(28) = 2.14, d = 0.81, p < 0.05], Hdac4 [histone deacetylase 4; t(23) = 2.78, d = 1.02, p < 0.01], Grin2b [NMDA receptor subunit 2b; t(29) = 2.26, d = 0.82, p < 0.05], and Gabbr1 [GABA-B receptor 1; t(29) = 2.03, d = 0.72, p < 0.05].
Fig. 8.
Relative gene expression data are shown for the SON. Note that the scale of the y-axis varies depending on the gene. A–C) Oxt, Oxtr, and Avp were higher in E2 than Veh animals. D–J) Drd2, Htr1a, Foxp2, Nr3c1, Hdac4, Grin2b, and Gabbr1 were lower in E2 than Veh treated animals. Data are shown mean + SEM.
3.3.5. Prefrontal cortex
Although two genes were initially identified as upregulated by E2 in the PFC, Drd2 and Oxtr, they did not survive false discovery rate correction for multiple comparisons.
4. Discussion
This study assessed effects of estradiol on social behavior, and on the underlying neuromolecular circuits involved in the regulation of these behaviors. We designed and utilized novel tests of USV communications and social novelty and preference between same-sex cagemates to assess the influence of E2 on these interactions. Our focus on USVs emitted in the context of familiar non-intruder female-to-female interactions differs from the literature that typically focuses on opposite-sex interactions (Matochik et al., 1992b; Bialy et al., 2000; Harding and McGinnis, 2003). Similarly, our sociability test of preference for a cagemate vs. an unfamiliar rat of the same treatment extends research on sociability, most of which utilizes unfamiliar stimulus rats of the same or opposite sex in tests of social novelty and memory.
Our key results were that 3 months of E2 replacement to OVX rats, roughly estimated as ~5 years in humans (Sengupta, 2013; Quinn, 2005), substantially changed behavior in several surprising ways. In the USV test, compared to E2 rats, vehicle rats engaged in USV calling at significantly higher levels and had lower anogenital investigation. All rats, regardless of hormone status, emitted more NFM calls than FM calls indicating that they were using these calls for primarily communicative purposes. This contrasts to USVs emitted in a sociosexual situation, in which FM calls are more frequent (Burgdorf et al., 2008; White et al., 1990; Wöhr et al., 2008). Our result may be attributable to using same-sex as opposed to opposite sex animals in a social context. In the sociability test, the expected preference of a rat for a novel compared to a familiar conspecific was not seen in the context of our female cagemate model, in which rats spent more time near the cagemate. Finally, assessment of gene expression in 5 brain regions revealed that the SON was most responsive to hormone treatment, and that across brain regions, the oxytocin and vasopressin signaling systems were most commonly affected, a result that likely ties back to the behavioral results.
4.1. Effects of estradiol treatment on social behaviors
4.1.1. Estradiol decreased USVs calls between female cagemates
Studies conducted in rodents of both sexes show an increased preference for a novel over a familiar conspecific (Markham and Juraska, 2007; Carr et al., 1976; Berlyne, 1950; Bevins and Besheer, 2006). We expected to observe such a preference, and further predicted that it would be enhanced in the E2 group relative to the vehicle rats based on work conducted in other social contexts. For example, the number of USVs emitted by cycling, intact female rats is highest during proestrus, when serum E2 concentrations are high (Matochik et al., 1992b; Matochik et al., 1992a). Other studies in ovariectomized female rats reported that short-term treatment with E2 plus progesterone increased USV calling (Matochik et al., 1992a; McGinnis and Vakulenko, 2003).
Contrary to this prediction, our E2-treated rats emitted significantly fewer total USV calls than vehicle rats on Trials 2 and 3, when the cagemates were first re-introduced following a 1-week period of separation across a barrier, and subsequently allowed to freely interact. A possible explanation for these seemingly disparate outcomes is the role of E2 in social memory. Studies using a model of resident intruder have shown that if a female rat or mouse is exposed to the same female intruder several times separated by intervals the resident emitted fewer calls each time, interpreted as indication of social memory (D’Amato and Moles, 2001; Moles et al., 2007; Haney and Miczek, 1993). Thus the fewer calls being emitted by the E2 compared to vehicle rats in the current study suggest that recognition of a cagemate is enhanced by hormone treatment. This is in line with previous studies that have found that E2 treatment after OVX led to enhanced social recognition compared to their OVX non-treated counterparts (Hlinák, 1993; Tang et al., 2005). Beyond the resident intruder test, female USVs have been examined in tests of mating behavior, when E2 enhanced calling (Thomas and Barfield, 1985; Matochik et al., 1992b). Again, we interpret differences with current work to the context of an opposite-sex potential sexual partner, vs. a same-sex familiar cagemate. Thus, our current findings warrant further investigation of USVs as an index of social memory, and application of this type of behavioral analysis to same-sex interactions.
4.1.2. Estradiol had little effect on social preference in a sociability test
The literature on social novelty shows that rats typically spend more time with novel than familiar animals (Markham and Juraska, 2007; Carr et al., 1976; Berlyne, 1950; Bevins and Besheer, 2006), although female rats have been shown to exhibit a lower novelty preference than males (Cyrenne and Brown, 2011). In our model using cagemates separated for one week, we found the opposite effect, namely, that the preference was for the cagemate over a novel (same-sex, same-treatment) rat. We saw only relatively modest effects of E2 treatment in the test for social preference and social interaction. This was also contrary to our original hypothesis that E2 would enhance an animal’s preference for the novel rat, based on research using OVX rodents given acute injections of estradiol, and on results from studies using receptor knockout mice (ERα, ERβ) on social behaviors (Hlinák, 1993; Choleris et al., 2003; Vetter-O’Hagen and Spear, 2012). The lack of novelty preference in our paradigm may be due to its setup: it did not enable physical contact between the experimental and stimulus rats. In support of this, Vetter-O’Hagen and Spear (2012) demonstrated that in a social interaction test where the experimental animal was able to freely interact with novel animals, OVX rats spent less time interacting with a novel rat than did their intact counterparts. The preference for a familiar animal over a novel animal is in line with the nonhuman primate literature, which reported a preference for spending time with a familiar over a novel conspecific (Fredrickson and Sackett, 1984; Sackett and Fredrickson, 1987; Southwick et al., 1974). In addition, the human literature has also found that as people age their social networks begin to narrow and their established relationships become more meaningful (Charles and Carstensen, 2010; Fung et al., 2001; Birditt and Fingerman, 2003).
4.2. Effects of estradiol treatment on gene expression in the brain
Five brain regions selected for their importance in the social decision-making network were used for qPCR profiling of a suite of genes involved in steroid hormone signaling, the vasopressin and oxytocin systems, other neuropeptides and neurotransmitters, and epigenetic modifiers. After correction for false discovery rate, no significant effects of E2 were found in the PFC. Only one gene (Pgr) was affected in the PVN. The BNST and MeA had 3 and 4 significant genes each, with one gene in common (Oxtr) which was up-regulated in both by E2. The region with greatest change was the SON. Because of the interconnected-ness of the selected brain regions, we have framed the following discussion around 3 functional gene families: steroid hormone signaling, vasopressin and oxytocin signaling, and neurotransmitters involved in social behavior.
4.2.1. Steroid hormone receptors
Three steroid hormone receptor genes were significantly affected by long-term E2 in our study: Pgr in the PVN (increased by E2), Esr2 in the BNST (decreased by E2), and Ar in the MeA (increased by E2). The increase in Pgr in the PVN of E2 compared to vehicle rats is consistent with the literature that shows this gene’s high estrogen responsiveness (Tetel and Lange, 2009; Mani and Oyola, 2012). Ar is abundantly expressed in the MeA (Simerly et al., 1990; Stanić et al., 2014), and the AR is sensitive to the changes in sex steroid hormone levels during the estrous cycle in the amygdala (Feng et al., 2010). There is a high density of ERβ expressing cells throughout the BNST (Shughrue et al., 1997; Shughrue and Merchenthaler, 2001) so the down-regulation of Esr2 by E2 was not surprising. The literature on the regulatory effects of estradiol on ERβ in the BNST is mixed, with some studies reporting downregulation (Brown et al., 1996; Gréco et al., 2001) and others no change after E2 treatment (Patisaul, Whitten & Young, 1999; Shima et al., 2003). E2 regulates expression of both estrogen receptors in a region specific manner (Patisaul, Whitten & Young, 1999; Gréco et al., 2001; Zhou et al., 1995); our lack of effect of E2 on Esr1 in any region, and only one effect on Esr2 in the BNST, may be attributable to the longer-term E2 treatment to our OVX rats relative to prior work.
4.2.2. Vasopressin and oxytocin signaling
Oxytocin (Oxt) and vasopressin (Avp), as well as their receptors, are involved in a variety of social behaviors (for reviews, see Young, 1999; Neumann and Landgraf, 2012). Mice whose genes for either Oxt (Choleris et al., 2003; Winslow and Insel, 2002) or Avp1ra (Bielsky et al., 2004) have been knocked out exhibit deficits in social memory and social interaction. Interestingly, similar deficits have been seen in rats with knockouts of either estrogen receptor (Choleris et al., 2006; Imwalle et al., 2002). Removal of steroid hormones by OVX led to deficits in social interaction and social memory that are mitigated with administration of estradiol replacement (Hlinák, 1993; Tang et al., 2005).
In our gene expression work, Oxtr was upregulated by E2 in all 5 brain regions, but this only survived false discovery rate correction in the BNST, MeA, and SON, the latter previously shown to express this receptor (Adan et al., 1995; Elands et al., 1988; Vaccari et al., 1998). Research on Oxtr reported that elevated estrogen levels during the latter phases of pregnancy and partition were associated with increased Oxtr expression in the BNST and SON (Meddle et al., 2007; Young et al., 1997). Also, E2 treatment increased Oxtr expression in the MeA after 48 h (Quiñones-Jenab et al., 1997) and 9 days (Patisaul et al., 2003) of treatment. In the SON, one study reported no changes in Oxtr across the different stages of the estrous cycle or pregnancy (Young et al., 1997), whereas Oxtr was increased during late pregnancy in rats (Bealer et al., 2006) and prairie voles (Ophir et al., 2013). Bealer et al. (2006) also reported that E2 treatment after OVX increased Oxtr expression in the SON compared to vehicle.
In the SON, along with Oxtr, both vasopressin (Avp) and oxytocin (Oxt) gene expression were upregulated by E2 treatment. Estrogens regulate Avp and Oxt in the SON predominantly through the ERβ (Winslow and Insel, 2004; Hrabovszky et al., 1998), and ERβ is also colocalized with both nonapeptides in this region (Hrabovszky et al., 1998; Alves et al., 1998; Patisaul et al., 2003). However, the literature on E2 regulation of Avp and Oxt in the SON is mixed. Some studies showed that E2 upregulated (Roy et al., 1999), downregulated (Shughrue et al., 2002; Van Tol et al., 1988), or had no effect on expression (Peter et al., 1990; Rhodes et al., 1981). Our data add to this literature on the SON by showing that long-term E2 treatment upregulates expression of genes involved in nonapeptide signaling.
4.2.3. Neurotransmitters involved in social and affective behavior
Depression and anxiety are twice as common in women than in men (Wong and Licinio, 2001), and menopause is associated with increases in affective dysfunctions (Freeman et al., 2004; Schmidt et al., 2004; Bromberger et al., 2011). Estrogens are believed to play a role in anxiety and depressive behavior by modulating serotoninergic and dopaminergic neural systems (Morissette and Di Paolo, 1993; Van De Kar et al., 2002; Bazzett and Becker, 1994; Lammers et al., 1999). GABA and glutamate neurotransmission, also implicated in these behaviors, are also estrogen-sensitive (Petty, 1995; Brambilla et al., 2003; Hashimoto et al., 2013; Herbison, 1997; Micevych and Mermelstein, 2008).
In our study, expression of the dopamine receptor D2 (Drd2), serotonin receptor 1a (Htr1a), NMDA receptor subunit 2b (Grin2b), and the GABA-B receptor 1 (Gabbr1) were downregulated by E2, a result that was specific to the SON. Although there is a strong literature on effects of E2 on serotonin receptors and transporters, and for roles of E2 in modulating affective behaviors (McQueen et al., 1997; Sumner and Fink, 1993, 1995; Biegon and McEwen, 1982; Raap et al., 2000; Charoenphandhu et al., 2011; Mize et al., 2001, Klemenhagen et al., 2006; Parks et al., 1998; Ramboz et al., 1998, Lerer et al., 1999), little work has been conducted in the SON. It has been postulated that E2 regulation of serotonin receptors may impact a women’s responses to serotonin modulating drugs (Fischette et al., 1983; Kendall et al., 1981; Rubinow et al., 1998) and that combining both estrogens with SSRIs could lead to better therapeutic effects (Xu et al., 2009).
The dopamine receptor D2 is the most abundant subtype in the central nervous system (Dailly et al., 2004), including the hypothalamus (Bouthenet et al., 1991; Mansour et al., 1990; Meador-Woodruff et al., 1991). However, most research on E2 regulation of dopaminergic signaling has focused on the mesolimbic system (Roy et al., 1990; Bédard et al., 1983; Gordon and Perry, 1983). In addition, in striatum, E2 decreased expression of the D2 receptor (Lammers et al., 1999). Our result that expression of Drd2 in the SON was downregulated by long-term E2 treatment adds to this literature.
4.3. Conclusions
Our results that E2 had strong effects on genes involved in social behavioral regulation, especially in the SON, taken together with our results on USV communications, lead us to speculate that the molecular changes caused by long-term E2 are associated with, or may even cause, the behavioral outcomes. It is interesting that the nonapeptide signaling pathways involved in social interaction and social memory, are all upregulated by E2, whereas neurotransmitter pathways are all downregulated by E2. Clearly more work would be needed to prove a causal relationship between E2 treatment and its effects on neural pathways, and behavior, but our work is an important first step in identifying potential candidates.
The novel USV test developed for this study represents an emerging technique that can be used to measure social memory or behavior in other contexts (Ciucci et al., 2008; Johnson et al., 2015; Lee et al., 2015). Translation of studies in rats may help inform research in women, especially those undergoing surgical or natural menopause. While E2 has benefits in the treatment of depression and anxiety (Zweifel and O’Brien, 1997; Schmidt et al., 2000; Gambacciani et al., 2003) there is little research on the importance of the social context. In our future work, we will apply these behavioral and molecular measures to a preclinical model of menopause in aging rats. Future studies will extend this behavioral test to non-cagemates, and use more complex social environments, to better understand communicative and social behaviors and underlying neuromolecular mechanisms.
Acknowledgments
Grant support
NIH PO1 AG16765
We thank Krittika Krishnan for assistance in preparing the sonogram shown in Fig. 2.
References
- Achat H, Kawachi I, Levine S, Berkey C, Coakley E, Colditz G. Social networks, stress and health-related quality of life. Qual Life Res. 1998;7(8):735–750. doi: 10.1023/a:1008837002431. [DOI] [PubMed] [Google Scholar]
- Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG, Burbach JP. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: Partial sequence and immunocytochemical localization. Endocrinology. 1995;136(9):4022–4028. doi: 10.1210/endo.136.9.7649111. [DOI] [PubMed] [Google Scholar]
- Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc Nat Acad Sci U S A. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimatsu Y, Hatanaka H. Estrogen treatment enhances survival of cultured fetal rat amygdala neurons in a defined medium. Dev Brain Res. 1986;26:151–159. doi: 10.1016/0165-3806(86)90017-9. [DOI] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637(1–2):163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. Am J Phys Regul Integr Comp Phys. 2006;291(1):R53–R58. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- Bédard P, Boucher R, Di Paolo T, Labrie F. Biphasic effect of estradiol and domperidone on lingual dyskinesia in monkeys. Exp Neurol. 1983;82(1):172–182. doi: 10.1016/0014-4886(83)90252-2. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- Berlyne DE. Novelty and curiosity as determinants of exploratory behavior. Br J Psychol. 1950;41:68–80. [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1(3):1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bhupathiraju SN, Manson JE. Menopausal hormone therapy and chronic disease risk in the Women’s Health Initiative: is timing everything? Endocr Pract. 2014;20(11):1201–1213. doi: 10.4158/EP14205.RA. [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114(5):983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Biegon A, McEwen BS. Modulation by estradiol of serotonin receptors in brain. J Neurosci. 1982;2(2):199–205. doi: 10.1523/JNEUROSCI.02-02-00199.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29(3):483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Birditt KS, Fingerman KL. Age and gender differences in adults: description of emotional reactions to interpersonal problems. J Gerontol B Psychol Sci Soc Sci. 2003;58(4):237–245. doi: 10.1093/geronb/58.4.p237. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564(2):203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8(8):721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: study of Women’s Health Across the Nation (SWAN) Psychol Med. 2011;41:1879–1888. doi: 10.1017/S003329171100016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, Scherz B, Hochberg RB, MacLusky NJ. Regulation of estrogen receptor concentrations in the rat brain - effects of sustained androgen and estrogen exposure. Neuroendocrinology. 1996;63(1):53–60. doi: 10.1159/000126935. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122(4):357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Weiss C, Oh MM, Disterhoft JF, Brudzynski SM, Panksepp J, Moskal JR. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience. 2011;192:515–523. doi: 10.1016/j.neuroscience.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr WJ, Yee L, Gable D, Marasco E. Olfactory recognition of conspecifics by domestic Norway rats. J Comp Physiol Psychol. 1976;90(9):821–828. doi: 10.1037/h0077266. [DOI] [PubMed] [Google Scholar]
- Charles ST, Carstensen LL. Social and emotional aging. Annu Rev Psychol. 2010;61:383–409. doi: 10.1146/annurev.psych.093008.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenphandhu J, Teerapornpuntakit J, Nuntapornsak A, Krishnamra N, Charoenphandhu N. Anxiety-life behaviors and expression of SERT and TPH in the dorsal raphé of estrogen and fluoxetine treated ovariectomized rats. Pharmacol Biochem Behav. 2011;98(4):503–510. doi: 10.1016/j.pbb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100(10):6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5(7):528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T. Limb use and complex ultrasonic vocalization in a rat model of Parkinson’s disease: deficit-targeted training. Parkinsonism Relat Disord. 2008;14(2):S172–S175. doi: 10.1016/j.parkreldis.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrenne DL, Brown GR. Ontogeny of sex differences in response to novel objects from adolescence to adulthood in lister-hooded rats. Dev Psychobiol. 2011;53(7):670–676. doi: 10.1002/dev.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailly E, Chenu F, Renard CE, Bourin M. Dopamine, depression and antidepressants. Fundam Clin Pharmacol. 2004;18(6):601–607. doi: 10.1111/j.1472-8206.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Moles A. Ultrasonic vocalizations as an index of social memory in female mice. Behav Neurosci. 2001;115(4):834–840. doi: 10.1037//0735-7044.115.4.834. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Novaes Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women. A double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- Deeks AA, McCabe MP. Well-being and menopause: an investigation of purpose in life, self-acceptance and social role in premenopausal, perimenopausal, and post-menopausal women. Qual Life Res. 2004;13(2):389–398. doi: 10.1023/B:QURE.0000018506.33706.05. [DOI] [PubMed] [Google Scholar]
- Elands J, Beetsma A, Barberis C, de Kloet ER. Topography of the oxytocin receptor system in rat brain: an autoradiographical study with a selective radioiodinated oxytocin antagonist. J Chem Neuroanat. 1988;1(6):293–302. [PubMed] [Google Scholar]
- Feng Y, Weijdegård B, Wang T, Egecioglu E, Fernadez-Rodriguez J, Huhtaniemi I, Stener-Victorin E, Biling H, Shao R. Spatiotemporal expression of androgen receptors in the female rat brain during the oestrous cycle and the impact of exogenous androgen administration: a comparison with gonadally intact males. Mol Cell Endocrinol. 2010;321(2):161–174. doi: 10.1016/j.mce.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Fischette CT, Biegon A, McEwen BS. Sex differences in serotonin 1 receptor binding in rat brain. Science. 1983;222(4621):333–335. doi: 10.1126/science.6623080. [DOI] [PubMed] [Google Scholar]
- Fredrickson WT, Sackett GP. Kin preferences in primates (Macaca nemestrina): relatedness or familiarity? J Comp Psychol. 1984;98(1):29–34. [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61(1):62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- Fung HH, Carstensen LL, Lang FR. Age-related patterns in social networks among European Americans and African Americans: implications for sociemotional selectivity across the life span. Int J Aging Hum Dev. 2001;52:185–206. doi: 10.2190/1ABL-9BE5-M0X2-LR9V. [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Monteleone P, Benussi C, Bevilacqua G, Genazzani AR. Effects of low-dose, continuous combined estradiol and noretisterone acetate on menopausal quality of life in early postmenopausal women. Maturitas. 2003;44(2):157–163. doi: 10.1016/s0378-5122(02)00327-4. [DOI] [PubMed] [Google Scholar]
- Garcia AN, Depena CK, Yin W, Gore AC. Testing the critical window of estradiol replacement on gene expression of vasopressin, oxytocin, and their receptors in the hypothalamus of aging female rats. Mol Cell Endocrinol. 2016;419:102–112. doi: 10.1016/j.mce.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JH, Perry KO. Pre- and postsynaptic neurochemical alterations following estrogen-induced striatal dopamine hypo- and hypersensitivity. Brain Res Bull. 1983;10(4):425–428. doi: 10.1016/0361-9230(83)90137-5. [DOI] [PubMed] [Google Scholar]
- Gréco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ERβ with ERα and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142(12):5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54(3):502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Haney M, Miczek KA. Ultrasounds during agonistic interactions between female rats (Rattus norvegicus) J Comp Psychol. 1993;107(4):373–379. doi: 10.1037/0735-7036.107.4.373. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Effects of testosterone in the VMN on copulation, partner preference, and vocalizations in male rats. Horm Behav. 2003;43(2):327–335. doi: 10.1016/s0018-506x(02)00049-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Malchow B, Falkai P, Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci. 2013;263(5):367–377. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull. 1997;44(4):321–326. doi: 10.1016/s0361-9230(97)00210-4. [DOI] [PubMed] [Google Scholar]
- Hlinák Z. Social recognition in ovariectomized and estradiol-treated female rats. Horm Behav. 1993;27(2):159–166. doi: 10.1006/hbeh.1993.1012. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kalló I, Hajszán T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139(5):2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Scordakales EM, Rissman EF. Estrogen receptor a influences socially motivated behaviors. Horm Behav. 2002;42:484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Johnson AM, Grant LM, Schallert T, Ciucci MR. Changes in rat 50-kHz ultrasonic vocalizations during dopamine denervation and aging: relevance to neurodegerneration. Curr Neuropharmacol. 2015;13(2):211–219. doi: 10.2174/1570159X1302150525122416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DA, Stancel GM, Enna SJ. Imipramine: effect of ovarian steroids on modifications in serotonin receptor binding. Science. 1981;211(4487):1183–1185. doi: 10.1126/science.6258229. [DOI] [PubMed] [Google Scholar]
- Klaiber EL, Vogel W, Rako S. A critique of the Women’s Health Initiative hormone therapy study. Fertil Steril. 2005;84(6):1589–1601. doi: 10.1016/j.fertnstert.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31(1):101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Modulatory effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. J Physiol Pharmacol. 2009;60(2):57–62. [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Epperson CN, Zoghbi S, Van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Selbyl JP, Innis RB. Increase in prefrontal cortex serotonin2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatr. 2003;160(8):1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- Lammers CH, D’Souza U, Qin ZH, Lee SH, Yajima S, Mouradian MM. Regulation of striatal dopamine receptors by estrogen. Synapse. 1999;34(3):222–227. doi: 10.1002/(SICI)1098-2396(19991201)34:3<222::AID-SYN6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lanza di Scalea T, Matthews KA, Avis NE, Thurston RC, Brown C, Harlow S, Bromberger JT. Role stress, role reward and mental health in a multiethnic sample of midlife women: results from the study of Woman’s Health Across the Nation (SWAN) J Women’s Health. 2012;21(5):481–489. doi: 10.1089/jwh.2011.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kwak M, Lee PC. Impairment of social behavior and communication in mice lacking the Uba6-dependent ubiquitin activation system. Behav Brain Res. 2015;281:78–85. doi: 10.1016/j.bbr.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Lerer B, Gelfin Y, Gorfine M, Allolio B, Lesch KP, Newman ME. 5-HT(1A) receptor function in normal subjects on clinical doses of fluoxetine: blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacology. 1999;20(6):628–639. doi: 10.1016/S0893-133X(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Mani SK, Oyola MG. Progesterone signaling mechanisms in brain and behavior. Front Endocrinol (Lausanne) 2012;3:7. doi: 10.3389/fendo.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, … Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended potstopping phase of the Women’s Health Initiative randomized trials. J Am Med Assoc. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10(8):2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Social recognition memory: influence of age, sex, and ovarian hormonal status. Physiol Behav. 2007;92(5):881–888. doi: 10.1016/j.physbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, Barfield RJ, Nyby J. Regulation of sociosexual communication in female Long–Evans rats by ovarian hormones. Horm Behav. 1992a;26:545. doi: 10.1016/0018-506x(92)90021-m. [DOI] [PubMed] [Google Scholar]
- Matochik JA, White NR, Barfield RJ. Variations in scent marking and ultrasonic vocalizations by Long-Evans rats across the estrous cycle. Physiol Behav. 1992b;51(4):783–786. doi: 10.1016/0031-9384(92)90116-j. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiol Behav. 2003;80(1):81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17 beta increases serotonin transporter (SERT) mRNA levels and the density of the SERT-binding sites in female rat brain. Brain Res Mol Brain Res. 1997;45(1):13–23. doi: 10.1016/s0169-328x(96)00233-1. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civello O, Watson SJ., Jr Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5(4):231–242. [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, Van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148(10):5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38(1):66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mize AL, Poisner AM, Alper RH. Estrogens act in rat hippocampus and frontal cortex to produce rapid, receptor-mediated decreases in serotonin 5-HT(1A) receptor function. Neuroendocrinology. 2001;73(3):166–174. doi: 10.1159/000054633. [DOI] [PubMed] [Google Scholar]
- Moles A, Costantini F, Garbugino L, Zanettini C, D’Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav Brain Res. 2007;182(2):223–230. doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem. 1993;60(5):1876–1883. doi: 10.1111/j.1471-4159.1993.tb13415.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Sorochman G, Evans BL, Prounis GS. Stability and dynamics of forebrain vasopressin receptor and oxytocin receptor during pregnancy in prairie voles. J Neuroendocrinol. 2013;25(8):719–728. doi: 10.1111/jne.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Nat Acad Sci U S A. 1998;95(18):10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Whitten PL, Young LJ. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17beta-estradiol and the phytoestrogen, coumestrol. Brain Res Mol Brain Res. 1999;67(1):165–171. doi: 10.1016/s0169-328x(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor β in the female mouse hypothalamus. J Neuroendocrinol. 2003;15(16):787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Compact. 6. Elsevier Inc; San Diego, CA: 2009. [Google Scholar]
- Peter J, Burbach H, Adan RA, Tol HH, Verbeeck MA, Axelson JF, Leeuwen FW, Beekman JM, Ab G. Regulation of the rat oxytocin gene by estradiol. J Neuroendocrinol. 1990;2(5):633–639. doi: 10.1111/j.1365-2826.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Petty F. GABA and mood disorders: a brief review and hypothesis. J Affect Disord. 1995;34(4):275–281. doi: 10.1016/0165-0327(95)00025-i. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21(6):775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Jenab S, Ogawa S, Adan RA, Burbach JP, Pfaff DW. Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology. 1997;65(1):9–17. doi: 10.1159/000127160. [DOI] [PubMed] [Google Scholar]
- Raap DK, Doncarlos L, Garcia F, Muma NA, Wolf WA, Battaglia G, Van De Kar LD. Estrogen desensitizes 5-HT(1A) receptors and reduces levels of G(z), G(i1) and G(i3) proteins in the hypothalamus. Neuropharmacology. 2000;39(10):1823–1832. doi: 10.1016/s0028-3908(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Nat Acad Sci U S A. 1998;95(24):14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW. Changes in oxytocin content in the magnocellular neurons of the rat hypothalamus following water deprivation or estrogen treatment. Quantitative immunohistological studies. Cell Tissue Res. 1981;216(1):47–55. doi: 10.1007/BF00234544. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risk and benefits of estrogen plus progestin in healthy postmenopausal women: principle results from the Women’s Health Initiative randomized controlled trial. J Am Med Assoc. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Roy EJ, Buyer DR, Licari VA. Estradiol in the striatum: effects on behavior and dopamine receptors but no evidence for membrane steroid receptors. Brain Res Bull. 1990;25(2):221–227. doi: 10.1016/0361-9230(90)90064-7. [DOI] [PubMed] [Google Scholar]
- Roy BN, Reid RL, Van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140(5):2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Sackett GP, Fredrickson WT. Social preference by pigtail macaques: familiarity versus degree and type of kinship. Anim Behav. 1987;35:603–607. [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183(2):414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004;161(12):2238–2244. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, Rubinow DR. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714–726. doi: 10.1001/jamapsychiatry.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- Shima N, Yamaguchi Y, Yuri K. Distribution of estrogen receptor β mRNA-containing cells in ovariectomized and estrogen-treated female rat brain. Anat Sci Int. 2003;78(2):85–97. doi: 10.1046/j.0022-7722.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunore-activity in the rat central nervous system. J Comp Neurol. 2001;436(1):64–81. [PubMed] [Google Scholar]
- Shughrue PL, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor α and β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-β. Prog Brain Res. 2002;139:15–29. doi: 10.1016/s0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Southwick CH, Siddiqi MF, Farooqui MY, Pal BC. Xemophobia Among Free-ranging Rhesus Groups in India. Academic Press; New York, NY: 1974. pp. 185–209. [Google Scholar]
- Stanić D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC. Characterization of aromatase expression in the adult male and female mouse brain. I Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS One. 2014;9(3):e90451. doi: 10.1371/journal.pone.0090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner BE, Fink G. Effects of acute estradiol on 5-hydroxytryptamine and dopamine receptor subtype mRNA expression in female rat brain. Mol Cell Neurosci. 1993;4(1):83–92. doi: 10.1006/mcne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Fink G. Estrogen increases the density of 5-hydroxytryptamine(2A) receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Mol Biol. 1995;54(1–2):15–20. doi: 10.1016/0960-0760(95)00075-b. [DOI] [PubMed] [Google Scholar]
- Tang AC, Nakazawa M, Romeo RD, Reeb BC, Sisti H, McEwen BS. Effects of long-term estrogen replacement on social investigation and social memory in ovariectomized C57BL/6 mice. Horm Behav. 2005;47(3):350–357. doi: 10.1016/j.yhbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Lange CA. Molecular genomics of progestin actions. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin R, editors. Hormones, Brain and Behavior. Vol. 3. Academic Press; San Diego: 2009. pp. 1439–1465. [Google Scholar]
- Thomas DA, Barfield RJ. Ultrasonic vocalization of the female rat (Rattus norvegicus) during mating. Anim Behav. 1985;33(3):720–725. [Google Scholar]
- Uguz F, Sahingoz M, Gezhinc K, Ayhan MG. Quality of life in postmenopausal women: the impact of depressive and anxiety disorders. Int J Psychiatry Med. 2011;41(3):281–292. doi: 10.2190/PM.41.3.g. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait S, Ostrowski N. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139(12):5015–5033. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- Van De Kar LD, Raap DK, Battaglia G, Muma NA, Garcia F, DonCarlos LL. Treatment of cycling female rats with fluoxetine induces desensitization of hypothalamic 5-HT1A receptors with no change in 5-HT2A receptors. Neuropharmacology. 2002;43(1):45–54. doi: 10.1016/s0028-3908(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Bolwerk EL, Liu B, Burbach JP. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypopshyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988;122(3):945–951. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2012;227(1):224–232. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1–3):199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker DM, Juenger TE, Gore AC. Developmental profiles of neuroendocrine gene expression in the preoptic area of male rats. Endocrinology. 2009;150:2308–2316. doi: 10.1210/en.2008-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NR, Cagiano R, Moises AU, Barfield RJ. Changes in mating vocalizations over the ejaculatory series in rats (Rattus norvegicus) J Comp Psychol. 1990;104(3):255–262. doi: 10.1037/0735-7036.104.3.255. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36(2–3):221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 2004;14(2):248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Houx B, Schwarting RKW, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008;93(4–5):766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2(5):343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158(4):1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J Neuroendocrinol. 1997;9(11):859–865. doi: 10.1046/j.1365-2826.1997.00654.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shughrue PJ, Dorsa DM. Estrogen receptor protein is differentially regulated in the preoptic area of the brain and in the uterus during the rat estrous cycle. Neuroendocrinology. 1995;61(3):276–283. doi: 10.1159/000126849. [DOI] [PubMed] [Google Scholar]
- Zweifel JE, O’Brien WH. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22(3):189–212. doi: 10.1016/s0306-4530(96)00034-0. [DOI] [PubMed] [Google Scholar]