Abstract
Rationale
Synthetic cathinones have emerged as the newest class of abused monoamine transporter substrates. Structurally, these compounds are all beta-ketone amphetamine (cathinone) analogs. Whether synthetic cathinone analogs produce differential behavioral effects from their amphetamine analog counterparts has not been systematically examined. Preclinical drug discrimination procedures have been useful for determining the structure activity relationships (SAR) of abused drugs; however, direct comparisons between amphetamine and cathinone analogs are lacking and, in particular, in nonhuman primate models.
Objectives
The study aim was to determine the potency and time course of (±)-amphetamine, (±)-cathinone, (±)-methamphetamine, and their 3,4-methylenedioxy analogs (±)-MDA, (±)-MDC, and (±)-MDMA, respectively to produce cocaine-like discriminative stimulus effects. If cathinone analogs have similar behavioral pharmacological properties to their amphetamine counterparts, then we would predict similar potencies and efficacies to produce cocaine-like discriminative stimulus effects.
Methods
Male rhesus monkeys (n=4) were trained to discriminate intramuscular cocaine (0.32 mg/kg) from saline in a two-key food-reinforced discrimination procedure.
Results
Racemic amphetamine, cathinone, and methamphetamine produced dose-dependent and full, ≥90% cocaine-appropriate responding, in all monkeys. Addition of 3,4-methylenedioxy moiety attenuated both the potency and efficacy of amphetamine (MDA), cathinone (MDC), and methamphetamine (MDMA) to produce full cocaine-like effects. Moreover, the cocaine-like effects of amphetamine and cathinone were attenuated to a greater extent than methamphetamine or previously published methcathinone (Smith et al. 2016).
Conclusion
The presence of an N-methyl group blunted both the potency and efficacy shift of the 3,4-methylenedioxy addition for both amphetamine and cathinone analogs.
Keywords: discrimination, amphetamine, cathinone, methamphetamine, MDMA, rhesus monkey
INTRODUCTION
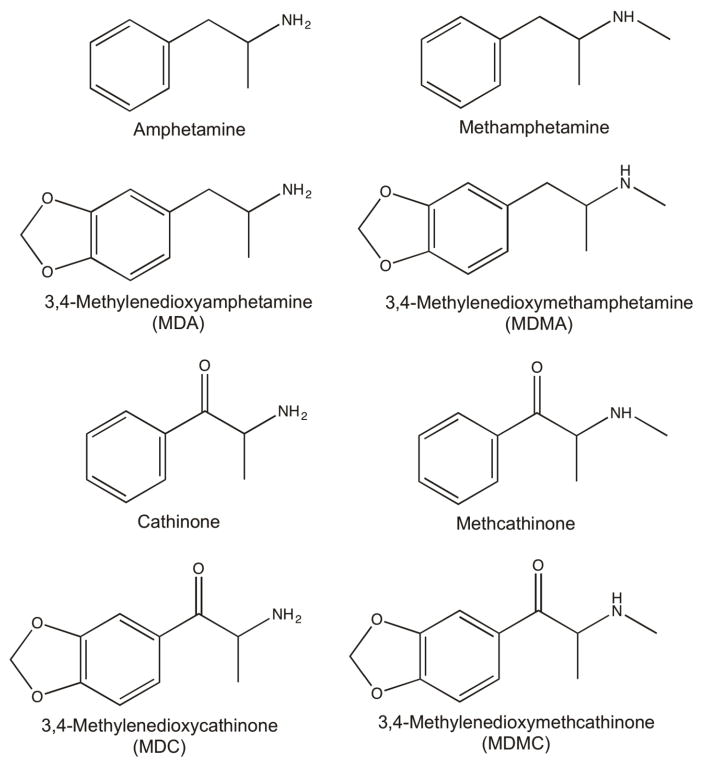
Chemical structure modifications to the prototypic monoamine transporter substrate amphetamine (Figure 1) alter the relative selectivity of the compound to bind to the dopamine (DAT) versus serotonin (SERT) transporter. These modifications may alter the neurochemical or behavioral effects of the compound. For example, the addition of an N-methyl to amphetamine (methamphetamine) slightly attenuated the relative selectivity to release dopamine (DA) vs. serotonin (5-HT) in vitro (Rothman et al. 2001; Simmler et al. 2013) and also attenuated the potency to increase extracellular DA levels and enhanced the potency to increase extracellular 5-HT levels in the nucleus accumbens (Baumann et al. 2012; Baumann et al. 2011). However, in drug discrimination studies, both (+)-amphetamine and (+)-methamphetamine produce overlapping discriminative stimulus effects in monkeys (Banks et al. 2016; Woolverton and English 1997) and humans (Lamb and Henningfield 1994; Sevak et al. 2009) suggesting these neurochemical differences produced by an N-methyl addition minimally impact the discriminative stimulus of these drugs. Similar to the N-methyl substitution, addition of a 3,4-methylenedioxy moiety to amphetamine (3,4-methylenedioxyamphetamine; MDA) also attenuated the DA vs. 5-HT selectivity to increase extracellular monoamine levels (KankaanpÄÄ et al. 1998; Nash and Nichols 1991). In rats and pigeons, (±)-MDA produced complete and full substitution for (+)-amphetamine in a discrimination procedure (Evans and Johanson 1986; Glennon and Young 1984a). However, in contrast to these rat and pigeon amphetamine discrimination results, (±)-MDA produced full (+)-amphetamine-like discriminative stimulus effects in 2 out of 3 monkeys (Kamien et al. 1986). Overall, these data suggest that chemical structure modifications may differentially impact the discriminative stimulus effects of monoamine transporter substrates depending upon where the chemical structure modification occurs and what type of moiety is being attached. These results also suggest potential species differences in the abuse-related behavioral effects of amphetamine analogs.
Figure 1.
Chemical structure of amphetamine, methamphetamine, cathinone, methcathinone, and their 3,4-methylenedioxy analogs.
Recently, synthetic cathinones have emerged as a heterogeneous class of abused compounds that function as either monoamine transport substrates or monoamine transport inhibitors (Baumann et al. 2012; Cozzi et al. 2013; Simmler et al. 2013). Cathinone is the beta-ketone analog of amphetamine (Figure 1) that serves as the pharmacophore for these synthetic analogs, and like amphetamine, functions as a monoamine transporter substrate (Rothman et al. 2003; Simmler et al. 2013) to increase extracellular nucleus accumbens DA levels (Pehek et al. 1990). However, there are a paucity of in vivo studies that have determined the behavioral consequences of different chemical structure modifications to cathinone. Furthermore, most studies have focused on phenyl ring modifications to N-methylcathinone (methcathinone) (Bonano et al. 2015; Sakloth et al. 2015; Suyama et al. 2016), given these analogs have been most prevalent in Drug Enforcement Administration seizures and forensic toxicology reports (DEA 2014). Moreover, only one study has examined the effects of a 3,4-methylenedixoy moiety addition to cathinone (3,4-methylenedioxycathinone; MDC) in rats (Dal Cason et al. 1997). In contrast to the full substitution of (±)-MDA in (+)-amphetamine-trained rats, (±)-MDC produced partial substitution in (+)amphetamine-trained rats. Overall, this literature suggests that amphetamine and cathinone may be differentially sensitive to the same chemical structure modifications. However, direct comparisons of the same chemical structure modifications on the abuse-related effects of amphetamine and cathinone analogs in nonhuman primate models are lacking.
Given the potential for species differences noted above for MDA, the aim was to conduct structure activity relationship (SAR) studies to directly compare the effects of an N-methyl and 3,4-methylenedioxy addition on the cocaine-like discriminative stimulus effects of (±)-amphetamine and (±)-cathinone in rhesus monkeys. Preclinical drug discrimination procedures have been especially useful to improve our understanding of the pharmacological mechanisms and SARs of central nervous system active compounds (Glennon and Young 2011). Cocaine was chosen as the training drug because its discriminative stimulus effects have been extensively characterized in nonhuman primates (Banks et al. 2014; Garza and Johanson 1983; Kleven et al. 1990; Spealman 1995) and these results suggest that the cocaine training dose used in the present study is primarily mediated by dopaminergic mechanisms. Moreover, cocaine has also been used as the training stimulus in rodent drug discrimination studies examining some of the amphetamine and cathinone analogs examined in the present study (Gatch et al. 2015; Gatch et al. 2013; Glennon and Young 1984b), thus allowing for a direct translational comparison between rodents and nonhuman primates. For comparison, we also determined the effects of a 3,4-methylenedioxy addition on the cocaine-like effects of methamphetamine (3,4-methylenedioxymethamphetamine; MDMA). We have previously published (±)-methcathinone and (±)-3,4-methylenedioxymethcathinone (MDMC) results in these same monkeys under the same cocaine discrimination procedure (Smith et al. 2016). These previously published results suggested a 3,4-methylenedioxy addition to methcathinone did not significantly alter the potency or efficacy of methcathinone to produce cocaine-like effects, but did prolong the time course. We hypothesized that the cocaine-like discriminative stimulus effects of amphetamine and cathinone would be attenuated by a 3,4-methylenedioxy addition. Furthermore, we hypothesized the cocaine-like discriminative stimulus effects of methamphetamine would not be altered by a 3,4-methylenedioxy addition, similar to our previous methcathinone and MDMC results (Smith et al. 2016).
METHODS
Subjects
Drug discrimination studies were conducted in 4 adult male rhesus monkeys (Macaca mulatta) weighing between 9–13 kg. All monkeys had a cocaine discrimination experimental history (Banks 2014; Banks et al. 2013; Banks et al. 2015; Smith et al. 2016) and were maintained on a diet of biscuits (Lab Diet High Protein Monkey Biscuits, PMI Feeds, Inc., St. Louis, MO) and fresh fruit provided after the behavioral session. Experimental sessions were conducted in the monkey’s home chamber and water was available throughout behavioral sessions. Additionally, monkeys could earn 1-gm banana-flavored pellets (5TUR grain-based precision primate tablets, Test Diets, Richmond, IN) during daily experimental sessions (described below). A 12 h light-dark cycle was in effect (lights on from 6AM to 6PM). Environmental enrichment consisting of various food puzzles, TV, or radio was provided daily at the conclusion of the behavioral sessions. Facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Council 2011) and under Institutional Animal Care and Use Committee-approved research and enrichment protocols.
Cocaine Discrimination Procedure
Experimental sessions were conducted in each monkey’s home chamber as described previously in detail (Banks et al. 2013). On the front wall of each chamber was a custom operant response panel with three horizontally arranged square response keys and only the left and right keys were used in the present studies. Attached to each panel was a pellet dispenser (Med Associates, ENV-203-1000, St. Albans, VT). Equipment operation and data collection were accomplished with a Windows-based computer and MED-PC software (Med Associates).
Monkeys were trained to discriminate 0.32 mg/kg cocaine intramuscularly (IM) from saline in a two-key, food-reinforced drug discrimination procedure as previously described (Banks et al. 2013). Discrimination training was conducted 5 days per week during daily sessions composed of multiple components. Each component consisted of a 5-minute response period, during which the right and left response keys were transilluminated red and green, respectively, and monkeys could earn up to 10 food pellets by responding under a fixed-ratio (FR) 30 schedule of food presentation. Key color and position were not counterbalanced between monkeys. Training sessions were composed of three components presented at 2-h intervals, and either saline or cocaine (0.32 mg/kg) was administered IM approximately 15 min prior to the start of each component. Thus, on training days, monkeys would receive a sequence of saline (S) and cocaine (C) injections in the order SSS, SSC, SCS, CSS, SCC, CSC, CCS, or CCC. These training sequences were randomly presented to engender daily experience with randomized sequences of saline- and cocaine-appropriate components. The 2h duration of inter-component intervals was selected to exceed the time course of discriminative stimulus effects produced by the cocaine-training dose in rhesus monkeys (Lamas et al. 1995) and to thereby minimize effects of cocaine administered in earlier trials on performance during later trials on the same day. Following administration of saline, only responding on the green key (the saline-appropriate key) produced food, whereas following administration of 0.32 mg/kg cocaine, only responding on the red key (the cocaine-appropriate key) produced food. Responses on the inappropriate key reset the FR requirement on the appropriate key. The criterion for accurate discrimination was ≥85% injection-appropriate responding before delivery of the first reinforcer, ≥90% injection-appropriate responding for the entire component, and response rates ≥0.1 responses/s (sufficient to earn at least one pellet) for all components during 7 of 8 consecutive sessions.
Test sessions were identical to training sessions except that (a) responding on either the left or right key produced food, (b) monkeys received only one injection of vehicle or a test drug dose at the start of the session, and (c) 5-min response components began 10, 30, 56, 100, 180, 300, and 560 min after the injection to assess the time course of drug effects. The drugs and dose ranges tested were: (±)amphetamine (0.1–1.0 mg/kg), (±)-3,4-methylenedioxyamphetamine (MDA; 0.32–3.2 mg/kg), (±)-cathinone (0.032–1.0 mg/kg), (±)-3,4-methylenedioxycathinone (MDC; 1.0–10.0 mg/kg), (±)-methamphetamine (0.032–0.32 mg/kg), and (±)-3,4-methylenedioxymethamphetamine (MDMA; 0.1–3.2 mg/kg). Test sessions were generally conducted on Tuesdays and Fridays with training sessions conducted on Mondays, Wednesdays, and Thursdays. Test sessions were conducted only if performance during the previous two training sessions met the criteria for accurate discrimination (described above). All test drug doses were determined once in each monkey. All doses of a given test drug were evaluated in a given monkey before testing the next drug, and vehicle (saline) test sessions were conducted before or after evaluation of each test drug. The order of drug doses and drugs was counterbalanced across monkeys.
Data Analysis
The primary dependent measures were (1) percent cocaine-appropriate responding (%CAR) {defined as (number of responses on the cocaine-associated key divided by the total number of responses on both the cocaine-and saline-associated keys)*100}, and (2) response rates during each component. These dependent measures were then plotted as a function of time after drug or saline administration. Percent CAR and response rates were analyzed using linear mixed effect analysis with drug dose and time as the main fixed effects and subjects as the random effect (JMP Pro 11.1.1, SAS, Cary, NC). A significant drug×time interaction was followed by the Dunnett’s post-hoc test for comparison to vehicle (saline) conditions within a given time point. Drug doses that produced ≥90% cocaine-appropriate responding at any time point were considered full substitution. In addition, ED50 values were defined as the test drug dose that produced 50% cocaine-appropriate responding at the 30-min time point. ED50 values were determined by linear regression when three data points were available or by interpolation when only two data points were available (one data point below and one data point above 50% cocaine-appropriate responding) in each individual monkey, and individual values were averaged to yield mean ED50 values and 95% confidence limits. Duration of action comparisons between equivalent drug doses was inferred by the number of time points significantly different from saline.
Drugs
(−)-Cocaine HCl, (±)-cathinone HCl, and (±)-methamphetamine HCl were supplied by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Bruce Blough (RTI) synthesized (±)-amphetamine fumarate, (±)-MDA HCl and (±)-MDC HCl. David E. Nichols synthesized and generously donated (±)-MDMA HCl. Drug doses were calculated and expressed using the salt forms listed above.
Results
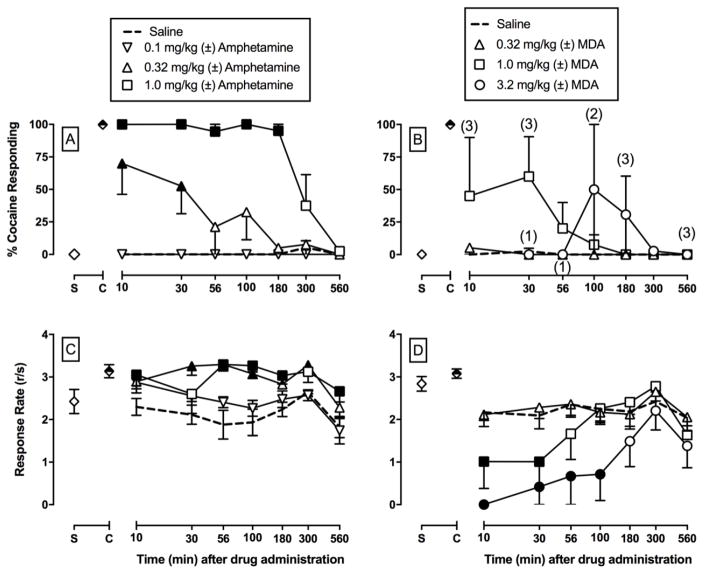
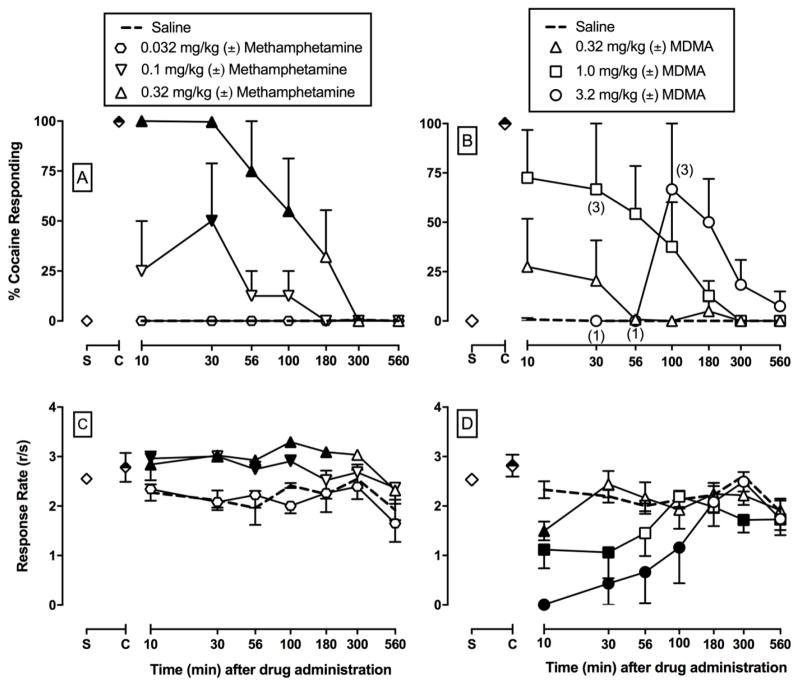
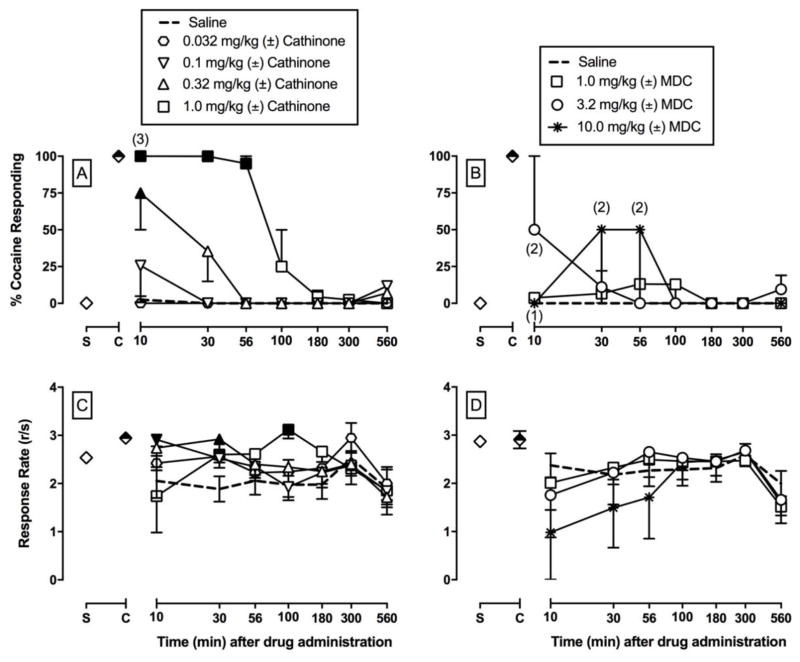
On all training days that preceded test days, percent injection-appropriate responding was 99.9% ± 0.1% and 99.9% ± 0.1% during cocaine- and saline-appropriate components, respectively. Rates of operant responding during cocaine and saline training components were 2.9 ± 0.2 and 2.6 ± 0.2 responses/s, respectively. Saline administration engendered <10% cocaine-appropriate responding at all time points (Figures 2–4) and had no significant effect on rates of responding in all time course test sessions (Figures 2–4). (±)-Amphetamine, (±)-cathinone, and (±)-methamphetamine produced dose-dependent and full substitution, ≥ 90% cocaine-appropriate responding, in all four monkeys (Figures 2–4). In contrast, (±)-MDA produced full substitution in 2 out of 4 monkeys, (±)-MDC produced full substitution in 1 out of 3 monkeys, and (±)-MDMA produced full substitution in 3 out of 4 monkeys. Table 1 shows the ED50 values to produce cocaine-like discriminative stimulus effects at the 30 min time point as this time point represented peak effects for the majority of the compounds. Amphetamine and cathinone were equipotent. Methamphetamine and previously published (Smith et al. 2016) methcathinone results in these same monkeys were also equipotent. Amphetamine and methamphetamine were also equipotent; however, both methamphetamine and methcathinone were significantly more potent than cathinone to produce cocaine-like effects as denoted by the non-overlapping confidence limits for methamphetamine or methcathinone and cathinone (Table 1). In general, the addition of a 3,4-methylenedioxy moiety attenuated the potency of the parent compound to produce cocaine-like effects. The one exception was (±)-3,4-methylenedioxymethcathinone (MDMC; Smith et al. 2016). However, exact potency shifts for all 3,4-methylenedioxy analogs were complicated due to the inability to calculate individual ED50 values in all monkeys for all compounds. Limited MDC supply also precluded testing in all 4 monkeys and complicated potency comparisons.
Figure 2.
Potency and time course of (±)-amphetamine (A, C; 0.1 – 1.0 mg/kg, IM) and (±)-3,4-methylenedioxyamphetmaine (MDA) (B, D; 0.32 – 3.2 mg/kg, IM) in male rhesus monkeys (n=4) trained to discriminate cocaine (0.32 mg/kg, IM) vs. saline. Abscissae: time in min after injection (log scale). Top panel ordinates: percent cocaine-appropriate responding. Bottom panel ordinates: operant response rates in responses per second. Symbols above “S” and “C” represent the group averages for all saline- and cocaine-training sessions preceding test sessions, respectively. Filled symbols indicate statistical significance compared to saline at a given time point (p < 0.05). Number in parentheses indicate the number of subjects contributing to that data point if less than the total number of subjects tested an indicative of a time point where a monkey failed to complete at least one ratio requirement.
Figure 4.
Potency and time course of (±)-methamphetamine (A, C; 0.032 – 0.32 mg/kg, IM) and (±)-3,4-methylenedioxymethamphetamine (MDMA) (B, D; 0.32 – 3.2 mg/kg, IM) in male rhesus monkeys (n=4) trained to discriminate cocaine (0.32 mg/kg, IM) vs. saline. Abscissae: time in min after injection (log scale). Top panel ordinates: percent cocaine-appropriate responding. Bottom panel ordinates: operant response rates in responses per second. Symbols above “S” and “C” represent the group averages for all saline- and cocaine-training sessions preceding test sessions, respectively. Filled symbols indicate statistical significance compared to saline at a given time point (p < 0.05). Number in parentheses indicate the number of subjects contributing to that data point if less than the total number of subjects tested an indicative of a time point where a monkey failed to complete at least one ratio requirement.
Table 1.
Mean ED50 values for test drugs in rhesus monkeys trained to discriminate 0.32 mg/kg intramuscular cocaine from saline. The fraction of monkeys in which an ED50 value could be determined is also shown under “Number.” Note that the mean ED50 values show results only in those monkeys in which an ED50 value could be calculated.
| Compound | Number | ED50 in mg/kg (95% confidence limits) |
|---|---|---|
| (±)-Amphetamine | 4/4 | 0.26 (0.15–0.45) |
| (±)-3,4-Methylenedioxyamphetamine (MDA) | 2/4 | 0.61 (0.53–0.70) |
| (±)-Cathinone | 4/4 | 0.39 (0.26–0.59) |
| (±)-3,4-Methylenedioxycathinone (MDC) | 1/3 | 5.66 |
| (±)-Methamphetamine | 4/4 | 0.10 (0.05–0.19) |
| (±)-3,4-Methylenedioxymethamphetamine (MDMA) | 3/4 | 0.40 (0.21–0.78) |
| (±)-Methcathinonea | 4/4 | 0.15 (0.11–0.20) |
| (±)-3,4-Methylenedioxymethcathinone (MDMC)a | 4/4 | 0.31 (0.11–0.89) |
Determined from previously published results (Smith et al. 2016)
Cocaine-like effects of amphetamine and its 3,4-methylenedioxy analog MDA
Figure 2 shows the potency and time course of (±)-amphetamine and (±)-MDA to produce cocaine-like effects (2A, 2B) and alter rates of responding (2C, 2D). 0.32 mg/kg amphetamine produced full substitution in 2 monkeys and 1.0 mg/kg amphetamine produced full substitution in all 4 monkeys. Amphetamine produced significant dose- and time-dependent cocaine-like effects, and 1.0 mg/kg amphetamine produced cocaine-like effects that were significant from saline for 180 min (amphetamine dose × time: F18,80 = 5.0, p<0.0001). For MDA, 1.0 mg/kg produced full substitution in 2 monkeys, and 3.2 mg/kg produced full substitution in 1 monkey. In contrast to amphetamine, there was only a significant effect of MDA dose (MDA dose: F3,44 = 4.8, p=0.0053). Amphetamine (dose: F3,81 = 2.9, p=0.0417) significantly increased rates of responding and MDA (MDA dose: F3,81 = 10.3, p<0.0001) significantly decreased rates of responding (2C and 2D).
Cocaine-like effects of cathinone and its 3,4-methylenedioxy analog MDC
Figure 3 shows the potency and time course of (±)-cathinone and (±)-MDC to produce cocaine-like effects (3A, 3B) and alter rates of operant responding (3C, 3D). 0.1 mg/kg cathinone produced full substitution in 1 monkey, 0.32 mg/kg cathinone produced full substitution in 3 monkeys, and 1.0 mg/kg cathinone produced full substitution in all 4 monkeys. Cathinone produced significant dose- and time-dependent cocaine-like effects, and 1.0 mg/kg cathinone produced cocaine-like effects that were significant from saline for 56 min (cathinone dose × time: F24,101 = 7.5, p<0.0001). For MDC, both 3.2 and 10 mg/kg produced full substitution in a single monkey. In contrast to cathinone, there was only a significant effect of MDC dose (MDC dose: F3,49.3 = 3.7, p=0.0177). Larger MDC doses could not be tested because of limited drug supply. Cathinone significantly increased rates of responding (dose: F4,102 = 4.1, p=0.0037; dose × time: F24,102 = 1.9, p=0.0181) (3C). MDC did not significantly alter rates of responding up to 10 mg/kg, although 2 out of the 3 monkeys failed to complete at least one response requirement during the 10 min component after administration of 10 mg/kg MDC (3D).
Figure 3.
Potency and time course of (±)-cathinone (A, C; 0.032 – 1.0 mg/kg, IM) and (±)-3,4-methylenedioxycathinone (MDC) (B, D; 1.0 – 10.0 mg/kg, IM) in male rhesus monkeys (n=3–4) trained to discriminate cocaine (0.32 mg/kg, IM) vs. saline. Cathinone was tested in 4 monkeys whereas MDC was tested in 3 monkeys. Abscissae: time in min after injection (log scale). Top panel ordinates: percent cocaine-appropriate responding. Bottom panel ordinates: operant response rates in responses per second. Symbols above “S” and “C” represent the group averages for all saline- and cocaine-training sessions preceding test sessions, respectively. Filled symbols indicate statistical significance compared to saline at a given time point (p < 0.05). Number in parentheses indicate the number of subjects contributing to that data point if less than the total number of subjects tested an indicative of a time point where a monkey failed to complete at least one ratio requirement.
Cocaine-like effects of methamphetamine and its 3,4-methylenedioxy analog MDMA
Figure 4 shows the potency and time course of (±)-methamphetamine and (±)-MDMA to produce cocaine-like effects (4A, 4B) and alter rates of operant responding (4C, 4D). 0.1 mg/kg methamphetamine produced full substitution in 2 monkeys, and 0.32 mg/kg methamphetamine produced full substitution in all 4 monkeys. Methamphetamine produced dose- and time-dependent cocaine-like effects, and 0.32 mg/kg methamphetamine produced cocaine-like effects that were significant from saline for 100 min (methamphetamine dose × time: F18,81 = 3.8, p<0.0001). In addition, methamphetamine produced longer lasting cocaine-like effects compared to previously published methcathinone results at equivalent doses (Smith et al. 2016). For MDMA, 0.32 mg/kg produced full substitution in 1 monkey, 1.0 mg/kg produced full substitution in 3 monkeys, and 3.2 mg/kg produced full substitution in 2 monkeys. In contrast to methamphetamine, there was only a significant effect of MDMA dose (dose: F3,53.2 = 6.1, p=0.0013). Methamphetamine significantly increased rates of responding (dose: F3,81 = 4.1, p=0.0094). In contrast, MDMA dose-dependently decreased rates of responding (dose: F3,81 = 12, p<0.0001; dose × time: F18,81 = 2.8, p=0.0007).
DISCUSSION
The present study aim was to compare and contrast the effects of a 3,4-methylenedioxy addition on (±)-amphetamine, (±)-cathinone, and (±)-methamphetamine cocaine-like discriminative stimulus effects in rhesus monkeys. There were two main findings. First, amphetamine, cathinone and methamphetamine produced full, ≥ 90%, cocaine-like discriminative stimulus effects. At equivalent doses, methamphetamine produced longer lasting cocaine-like effects than amphetamine or cathinone. However, comparisons between equally efficacious doses revealed a time course rank order of amphetamine>methamphetamine>cathinone from longest to shortest. These findings demonstrate that neither an N-methyl nor a β-ketone addition attenuates the potency or efficacy of (±)-amphetamine to produce cocaine-like discriminative stimulus effects. Second, a 3,4-methylenedioxy addition attenuated both the potency and efficacy of amphetamine, cathinone, and methamphetamine to produce cocaine-like effects and the rank order of sensitivity was cathinone ≥ amphetamine > methamphetamine. Previously, we reported a 3,4-methylenedioxy addition to methcathinone (MDMC) did not alter the potency or efficacy to produce cocaine-like effects (Smith et al. 2016). These results suggest an N-methyl group attenuated the impact of a 3,4-methylenedioxy addition on amphetamine- and cathinone-induced discriminative stimulus effects. Overall, these behavioral results in nonhuman primates suggest the impact of chemical structure modifications on the subjective-like discriminative stimulus effects of cathinone analogs cannot be predicted based on results from similar structural modifications to its amphetamine analog.
Effects of amphetamine, cathinone, and their 3,4-methylenedioxy analogs
To the best of our knowledge, this is the first report of (±)-amphetamine to produce cocaine-like discriminative stimulus effects. Racemic amphetamine was approximately 3-fold less potent than (+)amphetamine to produce cocaine-like effects (Banks et al. 2014; Banks et al. 2015). The dose-dependent and complete substitution profile of (±)-cathinone for a cocaine discriminative stimulus in the present study was consistent with previous studies in both rats (Huang and Wilson 1986; Young and Glennon 1993) and monkeys (Garza and Johanson 1983). The present results extend upon these previous findings by reporting the time course of (±)-amphetamine and (±)-cathinone discriminative stimulus effects. At equivalent doses, (±)-amphetamine produced a longer duration of cocaine-like stimulus effects than (±)-cathinone. However, (±)-cathinone and (±)-amphetamine were equipotent to produce cocaine-like effects in monkeys based on ED50 values. The overall consistency of the present results with the scientific literature provide an empirical foundation upon which to determine the effects of a 3,4-methylenedioxy addition.
The 3,4-methylenedioxy addition attenuated the cocaine-like discriminative stimulus effects of both (±)-cathinone and (±)-amphetamine in the present study. The (±)-MDC substitution profile for cocaine in the present study is generally consistent with a previous (±)-MDC study in rats trained to discriminative (+)-amphetamine (Dal Cason et al. 1997) and extends upon this finding by determining the time course of MDC effects and by testing in nonhuman primates. Moreover, the present (±)-MDA results are also consistent with and extend upon previous MDA results in monkeys trained to discriminate (+)amphetamine (Kamien et al. 1986) by reporting the time course of MDA effects. In rats, (±)-MDA completely cross-generalizes with cocaine (Glennon and Young 1984b; Glennon et al. 1984) and (+)amphetamine (Glennon and Young 1984a). Direct potency and time course comparisons between the present (±)-MDC and (±)-MDA results are complicated by the lower than expected (±)-MDC potency and limited drug supply that prohibited testing in all 4 monkeys and the assessment of larger doses. However, in the 3 monkeys that did receive both (±)-MDC and (±)-MDA, both (±)-MDC and (±)-MDA produced full substitution in one monkey, only (±)-MDA produced full substitution in another monkey, and neither (±)-MDC nor (±)-MDA produced full substitution in the last monkey. In summary, the present results are generally consistent with and extend upon the range of experimental conditions upon which a 3,4-methylendioxy addition attenuates the abuse-related subjective-like effects of amphetamine and cathinone and highlight the importance of individual subject differences.
Effects of methamphetamine and its 3,4-methylenedioxy analog
The dose-dependent and complete substitution profile of (±)-methamphetamine in the present study extends upon results in rats trained to discriminate (+)-amphetamine (Glennon et al. 1987) in two ways. First, the present results extend these previous findings to nonhuman primates trained to discriminate cocaine. Furthermore, racemic methamphetamine and (+)-methamphetamine were equipotent to produce cocaine-like stimulus effects in monkeys (Negus et al. 2007). Second, the present results determined the time course of (±)-methamphetamine discriminative stimulus effects, which had not been previously reported to the best of our knowledge. (±)-Methamphetamine produced longer lasting cocaine-like effects compared to our previously published (±)-methcathinone results (Smith et al. 2016) at equivalent doses. Also similar was that (±)-methamphetamine and (±)-methcathinone were equipotent to produce cocaine-like effects in monkeys, and this result was consistent with the equipotency of both compounds to produce (+)-amphetamine-like discriminative stimulus effects in rats (Glennon 1986; Glennon et al. 1987). The overall consistency of the present racemic methamphetamines results with the scientific literature provided an empirical foundation upon which to determine the effects of a 3,4-methylenedioxy addition.
The substitution profile of (±)-MDMA for a cocaine discriminative stimulus in nonhuman primates has not been previously reported to the best of our knowledge. The present (±)-MDMA results demonstrating full cocaine-like effects in three out of four monkeys are somewhat consistent with previous cocaine discrimination studies in rats reporting that (±)-MDMA produced significant, but partial substitution (Khorana et al. 2004; Kueh and Baker 2007). In (±)-MDMA-trained rats, cocaine produced full substitution in one study (Khorana et al. 2004), but only partial substitution in another study (Kueh and Baker 2007). Whether these inconsistent MDMA results are the consequence of species differences or cocaine training dose differences remains to be fully elucidated. Furthermore, the present (±)-MDMA results are also generally consistent with previous (±)-MDMA results in monkeys trained to discriminate (+)-amphetamine (Kamien et al. 1986), although (±)-MDMA produced full substitution for (+)amphetamine in all monkeys. One potential reason for the differential MDMA substitution profile between cocaine and (+)-amphetamine in nonhuman primates could be related to the noradrenergic component of the training drug discriminative stimulus. For example, the selective norepinephrine transporter (NET) inhibitor nisoxetine produced full substitution in an (+)-amphetamine (Kamien and Woolverton 1989), but not cocaine (Kleven et al. 1990) discrimination procedure in rhesus monkeys. However, the noradrenergic component of the cocaine discriminative stimulus is dose-dependent, such that NET inhibitors produced greater substitution for smaller compared to larger cocaine training doses in squirrel monkeys (Spealman 1995). The present cocaine training dose (0.32 mg/kg) was towards the larger cocaine training doses used in the squirrel monkey study. Given that (±)-MDMA is approximately equipotent at NET and SERT (Rothman et al. 2001), the present results and the existing literature provide further evidence for a noradrenergic component of the MDMA discriminative stimulus.
Implications
Overall, these structure activity relationship studies suggest 3 main conclusions regarding the behavioral consequences of structural modifications involving an N-methyl, β-ketone, or 3,4-methylenedioxy addition alone or in combination on the subjective-like discriminative stimulus effects of amphetamine. First, an N-methyl addition to both (±)-amphetamine and β-ketoamphetamine (±-cathinone) slightly increased the potency to produce cocaine-like stimulus effects, although only (±)-methcathinone was significantly more potent than cathinone as denoted by non-overlapping confidence limits. Furthermore, the N-methyl addition shortened the duration of cocaine-like effects to a greater extent for cathinone (Smith et al. 2016) compared to amphetamine across equally efficacious doses. Second, a β-ketone addition did not alter the potency of either amphetamine or methamphetamine to produce cocaine-like stimulus effects, but tended to shorten the duration of cocaine-like effects. Finally, a 3,4-methylenedioxy addition attenuated the cocaine-like discriminative stimulus effects of amphetamine and cathinone to a greater extent than methamphetamine and methcathinone (Smith et al. 2016). Overall, these results suggest that the behavioral pharmacology of substituted cathinone analogs cannot be solely predicted based on their amphetamine counterparts and that the substituent on the N-terminus might be important for determining the expression of subjective-like discriminative stimulus effects.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award number R01DA031718 and R01DA012970, and institutional professional development funds. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We acknowledge Crystal Reyns for technical assistance, Kevin Costa for coding the original behavioral program version, and David E. Nichols for the generous (±)-MDMA HCl donation.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS:
Experiments were conducted in accordance with the eighth edition of Guide for the Care and Use of Laboratory Animals (2011) and under Institutional Animal Care and Use Committee-approved research and enrichment protocols.
AUTHORSHIP CONTRIBUTIONS
Participated in research design: Banks
Conducted experiments: Smith
Contributed new reagents: Blough
Recorded data analysis: Banks
Wrote or contributed to the writing of the manuscript: Smith, Blough, and Banks
CONFLICT OF INTEREST:
Mr. Smith declares no conflicts. Dr. Blough declares NIH has funded his research. Dr. Banks declares NIH has funded his research. During the past 3 years, he has received compensation as a collaborator with Purdue pharmaceutical for projects related to opioid pharmacology and drug development. Dr. Banks declares that the present study was not related to this professional relationship and should not be perceived as constituting a conflict of interest.
References
- Banks ML. Effects of the nicotinic acetylcholine receptor antagonist mecamylamine on the discriminative stimulus effects of cocaine in male rhesus monkeys. Exp Clin Psychopharmacol. 2014;22:266–273. doi: 10.1037/a0035274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Bauer CT, Blough BE, Rothman RB, Partilla JS, Baumann MH, Negus SS. Abuse-related effects of dual dopamine/serotonin releasers with varying potency to release norepinephrine in male rats and rhesus monkeys. Exp Clin Psychopharmacol. 2014;22:274–284. doi: 10.1037/a0036595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Role of phenmetrazine as an active metabolite of phendimetrazine: Evidence from studies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend. 2013;130:158–166. doi: 10.1016/j.drugalcdep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Blough BE, Poklis JL, Negus SS. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: Effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int J Neuropsychopharmacol. 2015;18:pyv009. doi: 10.1093/ijnp/pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Smith DA, Kisor DF, Poklis JL. Relationship between discriminative stimulus effects and plasma methamphetamine and amphetamine levels of intramuscular methamphetamine in male rhesus monkeys. Pharmacol Biochem Behav. 2016;141:58–65. doi: 10.1016/j.pbb.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, Cozzi NV, Partilla JS, Baumann MH, Negus SS. Quantitative structure–activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. Br J Pharmacol. 2015;172:2433–2444. doi: 10.1111/bph.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council National Rearrch. Guide for the care and use of laboratory animals. 8. National Academies Press; Washington DC: 2011. [Google Scholar]
- Cozzi NV, Brandt SD, Daley PF, Partilla JS, Rothman RB, Tulzer A, Sitte HH, Baumann MH. Pharmacological examination of trifluoromethyl ring-substituted methcathinone analogs. Eur J Pharmacol. 2013;699:180–187. doi: 10.1016/j.ejphar.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. Cathinone: An Investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- DEA; Office of Diversion Control DoJ, editor. National Forensic Laboratory Information System Special Report: Synthetic cannabinoids and synthetic cathinones reported in NFLIS, 2010–2013. Drug Enforcement Administration; Springfield, VA: 2014. [Google Scholar]
- Evans SM, Johanson CE. Discriminative stimulus properties of (+/−)-3,4-methylenedioxymethamphetamine and (+/−)-3,4-methylenedioxyamphetamine in pigeons. Drug Alcohol Depend. 1986;18:159–64. doi: 10.1016/0376-8716(86)90048-7. [DOI] [PubMed] [Google Scholar]
- Garza RD, Johanson CE. The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav. 1983;19:145–8. doi: 10.1016/0091-3057(83)90323-4. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Forster MJ. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology. 2015;232:1197–1205. doi: 10.1007/s00213-014-3755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behavioural pharmacology. 2013;24:437–47. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of phenylisopropylamine derivatives. Drug Alcohol Depend. 1986;17:119–134. doi: 10.1016/0376-8716(86)90003-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R. MDA: a psychoactive agent with dual stimulus effects. Life Sci. 1984a;34:379–83. doi: 10.1016/0024-3205(84)90627-1. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R. MDA: an agent that produces stimulus effects similar to those of 3,4-DMA, LSD and cocaine. Eur J Pharmacol. 1984b;99:249–50. doi: 10.1016/0014-2999(84)90250-4. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R. Drug discrimination and in vivo structure-activity relationships. In: Glennon RA, Young R, editors. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. Wiley and Sons; Hoboken: 2011. pp. 163–181. [Google Scholar]
- Glennon RA, Young R, Hauck AE, McKenney JD. Structure-activity studies on amphetamine analogs using drug discrimination methodology. Pharmacol Biochem Behav. 1984;21:895–901. doi: 10.1016/s0091-3057(84)80071-4. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: A new and potent amphetamine-like agent. Pharmacol Biochem Behav. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- Huang D, Wilson MC. Comparative discriminative stimulus properties of dl-rmcathinone, d-amphetamine, and cocaine in rats. Pharmacol Biochem Behav. 1986;24:205–210. doi: 10.1016/0091-3057(86)90339-4. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Johanson CE, Schuster CR, Woolverton WL. The effects of (±)-methylenedioxymethamphetamine and (±)-methylenedioxyamphetamine in monkeys trained to discriminate (+)-amphetamine from saline. Drug Alcohol Depend. 1986;18:139–147. doi: 10.1016/0376-8716(86)90046-3. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Woolverton WL. A pharmacological analysis of the discriminative stimulus properties of d-amphetamine in rhesus monkeys. J Pharmacol Exp Ther. 1989;248:938–946. [PubMed] [Google Scholar]
- KankaanpÄÄ A, Meririnne E, Lillsunde P, SeppÄlÄ T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Khorana N, Pullagurla MR, Young R, Glennon RA. Comparison of the discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) and cocaine: asymmetric generalization. Drug Alcohol Depend. 2004;74:281–287. doi: 10.1016/j.drugalcdep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990;254:312–7. [PubMed] [Google Scholar]
- Kueh D, Baker LE. Reinforcement schedule effects in rats trained to discriminate 3,4-methylenedioxymethamphetamine (MDMA) or cocaine. Psychopharmacology. 2007;189:447–457. doi: 10.1007/s00213-006-0523-z. [DOI] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Hall E, Mello NK. Relationship between the discriminative stimulus effects and plasma concentrations of intramuscular cocaine in rhesus monkeys. Psychopharmacology. 1995;121:331–338. doi: 10.1007/BF02246072. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Henningfield JE. Human d-amphetamine drug discrimination: methamphetamine and hydromorphone. Journal of the experimental analysis of behavior. 1994;61:169–80. doi: 10.1901/jeab.1994.61-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JF, Nichols DE. Microdialysis studies on 3,4-methylenedioxyamphetamine and structurally related analogues. Eur J Pharmacol. 1991;200:53–8. doi: 10.1016/0014-2999(91)90664-c. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: Studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Schechter MD, Yamamoto BK. Effects of cathinone and amphetamine on the neurochemistry of dopamine in vivo. Neuropharmacology. 1990;29:1171–1176. doi: 10.1016/0028-3908(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Sakloth F, Kolanos R, Mosier PD, Bonano JS, Banks ML, Partilla JS, Baumann MH, Negus SS, Glennon RA. Steric parameters, molecular modeling and hydropathic interaction analysis of the pharmacology of para-substituted methcathinone analogues. Br J Pharmacol. 2015;172:2210–2218. doi: 10.1111/bph.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Stoops WW, Hays LR, Rush CR. Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther. 2009;328:1007–1018. doi: 10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Negus SS, Poklis JL, Blough BE, Banks ML. Cocaine-like discriminative stimulus effects of alpha-pyrrolidinovalerophenone, methcathinone and their 3,4-methylenedioxy or 4-methyl analogs in rhesus monkeys. Addict Biol. 2016 doi: 10.1111/adb.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- Suyama JA, Sakloth F, Kolanos R, Glennon RA, Lazenka MF, Negus SS, Banks ML. Abuse-related neurochemical effects of para-substituted methcathinone analogs in rats: Microdialysis studies of nucleus accumbens dopamine and serotonin. J Pharmacol Exp Ther. 2016;356:182–190. doi: 10.1124/jpet.115.229559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, English JA. Effects of some phenylethylamines in rhesus monkeys trained to discriminate (+)-amphetamine from saline. Drug Alcohol Depend. 1997;44:79–85. doi: 10.1016/s0376-8716(96)01322-1. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. Cocaine-stimulus generalization to two new designer drugs: Methcathinone and 4-methylaminorex. Pharmacol Biochem Behav. 1993;45:229–231. doi: 10.1016/0091-3057(93)90110-f. [DOI] [PubMed] [Google Scholar]