Abstract
Rationale
The purpose of this study was to begin researching the effects of very low nicotine content cigarettes in smokers especially vulnerable to dependence to assess their potential as a less dependence-producing alternative to current commercial cigarettes.
Methods
Participants were 26 adult, daily cigarette smokers from one of three populations: economically disadvantaged women of reproductive age (n = 9), opioid-dependent individuals (n = 11), individuals with affective disorders (n = 6). Participants completed fourteen 2–4 hrs experimental sessions in a within-subjects research design. Sessions were conducted following brief smoking abstinence. Four research cigarettes varying in nicotine content (0.4, 2.4, 5.2, 15.8 mg/g) were studied under double-blind conditions, assessing smoking topography, subjective effects, and relative reinforcing effects of varying doses in concurrent choice tests. Results were collapsed across vulnerable populations and analyzed using repeated measures ANOVA.
Results
No significant differences between doses were discernible in smoking topography. All doses were equi-effective at reducing nicotine withdrawal. Ratings of satisfaction from smoking were lower at the 0.4 compared to 15.8 mg/g dose. Participants preferred the 15.8 mg/g dose over the 0.4 and 2.4 but not the 5.2 mg/g doses in concurrent choice testing; no differences between the two lowest doses were noted.
Conclusions
All cigarettes effectively reduced nicotine withdrawal with no differences in smoking topography, suggesting minimal compensatory smoking. Dependence potential was lowest at the 0.4 mg/g dose. These initial results are promising regarding the feasibility of lowering nicotine content in cigarettes to very low levels in vulnerable populations without untoward effects.
Keywords: cigarette smoking, very low nicotine content cigarettes, nicotine, vulnerable populations, acute exposure, abuse liability, concurrent choice testing, smoking topography, nicotine dependence, nicotine withdrawal, tobacco
Introduction
Cigarette smoking continues to have a staggering adverse impact on public health, contributing to an estimated 480,000 premature deaths annually in the U.S. and 5.4 million globally (Centers for Disease Control and Prevention, 2016; U.S. Department of Health and Human Services, 2014). In the U.S., the 2009 Tobacco Control Act granted regulatory authority over the manufacturing and marketing of cigarettes and other tobacco products to the Food and Drug Administration (FDA) (Family Smoking Prevention and Tobacco Control Act, 2009). That legislation included authority to reduce, although not eliminate, nicotine content of cigarettes if doing so was deemed important to protecting public health.
There is broad scientific consensus that the reinforcing effects of nicotine delivered in combusted cigarette smoke underpin the development and persistence of chronic smoking, dependence, and, for many, premature death. Hence, a question of potential critical importance to the regulation of cigarettes by the FDA, or other regulatory agencies outside of the U.S., is whether nicotine content can be set below a threshold level necessary to produce and maintain dependence. Doing so would allow current smokers greater opportunity to make choices about continuing to smoke unencumbered by the adverse effects of nicotine withdrawal and related unpleasant effects of smoking abstinence and lower the likelihood of developing dependence among those newly introduced to cigarette smoking.
This idea of setting nicotine content levels in cigarettes below a dependence threshold was first introduced in a prescient editorial by Benowitz and Henningfield (1994). While this idea was well received by health experts, various legal developments and obstacles that have been outlined previously (Donny et al., 2015) precluded a concerted scientific examination of its feasibility and efficacy before passage of the Tobacco Control Act. Passage of that legislation has been followed by a considerable increase in research on this topic. Indeed, results from a series of experimental studies indicate that reducing nicotine content to very low levels reduces smoking rates, exposure to nicotine and other harmful constituents in tobacco smoke, and nicotine dependence levels, while increasing attempts to quit or abstain from smoking (Benowitz et al., 2007; Benowitz et al., 2009; Donny et al., 2007; Donny et al., 2015; Hatsukami et al., 2010).
It is important to note a fundamental distinction between this effort to reduce the harmful effects of cigarette smoking and earlier efforts to do so through the development of “light” cigarettes. This current effort involves reducing the nicotine content of the cigarette to low levels while in the earlier effort the content remained unchanged and the goal was to reduce nicotine/tar yield by engineering the cigarette to increase ambient air ventilation which was intended to dilute the smoke and hence reduce exposure levels (Kozlowski et al., 2001). Unfortunately, smokers often make various adjustments to how they smoke in order to compensate for the reduced delivery of nicotine, including smoking more cigarettes or taking larger puffs, blocking ventilation holes in the filter, and inhaling smoke deeper into their lungs that result in lower than estimated reductions in nicotine exposure and introduce additional adverse health outcomes (U.S. Department of Health and Human Services, 2001). By contrast, there has been relatively little evidence of sustained compensatory smoking with very low nicotine cigarettes (Donny et al., 2015; Hatsukami et al., 2015; MacQueen et al., 2012).
Understandably, initial studies on very low nicotine content cigarettes were conducted with relatively healthy and socially stable smokers. However, the promising results from those studies raise the important question of how smokers with co-morbid health, socioeconomic and other problems (i.e., more vulnerable populations) will respond to reductions in nicotine content. Cigarette smoking is overrepresented in these vulnerable populations and they often experience a disparate risk for dependence and burden of other smoking-related adverse health impacts (Higgins et al., 2016; Higgins & Chilcoat, 2009; Hiscock et al., 2012). The acute behavioral, subjective and cognitive effects of very low nicotine content cigarettes in smokers with schizophrenia, a population that is highly vulnerable to persistent tobacco use and dependence, have been reported (AhnAllen et al., 2015; Tidey et al., 2013; 2016). However, to our knowledge, the present study represents the first effort to experimentally examine response to reductions in the nicotine content of cigarettes in other highly vulnerable populations. We studied economically disadvantaged women of reproductive age, a group with higher than average smoking prevalence and substantial risk for adverse health impacts should they become pregnant (Higgins et al., 2009; Higgins & Chilcoat, 2009), as well as smokers with opioid dependence and affective disorders, groups that also have greater than average smoking prevalence rates and risk for dependence and other adverse impacts (Hser et al., 2001; Lasser et al., 2000; Lawrence et al., 2009).
Methods
Participants Recruitment and Inclusion/Exclusion Criteria
Participants were recruited through ads placed on bulletin boards throughout the surrounding community, buses, as well as in local newspapers. Inclusion criteria that applied across subpopulations included being 18 years of age or older, reporting smoking five or more cigarettes per day, and providing an expired breath carbon monoxide (CO) level of more than 8 ppm (CoVita, Haddonfield, NJ). Participants also had to provide a negative urine toxicology screen for illicit drug use (i.e., amphetamine, methamphetamine, cocaine, barbiturates, benzodiazepines, buprenorphine, opiates, methadone, oxycodone, phencyclidine) except for marijuana (THC) (Rapid CHECK 9 panel Multi-Drug Test Card, and Single Panel Dipstrip for buprenorphine, Craig Medical, Vista, CA). Opioid-dependent participants were not expected to test negative for their prescribed medication (buprenorphine or methadone). All participants had to provide a breath alcohol level (BAL) at < .01 (Alco-Sensor IV, Intoximeter, Inc, St Louis, MO) at the intake assessment session for inclusion into the study. Exclusion criteria that applied across subpopulations included intention to quit smoking within the next 30 days, use of other tobacco products on more than 9 of the previous 30 days, currently pregnant or trying to become pregnant, currently breastfeeding, and exclusive use of “roll your own” cigarettes, and current suicidal ideation or recent suicide attempt (past 10 years for women of reproductive age and opioid dependent; past year for those with affective disorders). Specific inclusion criteria for women of reproductive age were females only, ages 18–44 with less than an Associate’s degree. Inclusion criteria specific to opioid-dependent smokers were males and females ages 18–70 years currently receiving methadone or buprenorphine maintenance treatment for opioid dependence; they also had to be stable on their maintenance dose which was defined as < 30% urine toxicology samples positive for illicit drug use in past month as confirmed by their provider. Inclusion criteria specific to smokers with affective disorders were males and females ages 18–70 years with current or past year major depressive disorder, dysthymic disorder, generalized anxiety disorder, post-traumatic stress disorder, obsessive compulsive disorder, phobia or panic disorder with or without agoraphobia as determined by the MINI structured clinical interview (Sheehan et al., 1998). The local institutional review board at each participating research site (University of Vermont, Brown University, Johns Hopkins University) approved this study and all participants provided written informed consent.
Research Cigarettes
Study products were Spectrum investigational research cigarettes manufactured by 22nd Century Group (Clarence, NY) and obtained from the National Institute on Drug Abuse following submission of an application for an Investigational Tobacco Product with the Center for Tobacco Products, U.S. Food and Drug Administration. These products have been described previously (Donny et al., 2015; Perkins et al., 2016). Four nicotine dose conditions were investigated using research cigarettes defined according to the nicotine content, averaged across menthol and non-menthol products (assignment of a menthol or non-menthol product was based on a participant’s reported usual brand): 15.8, 5.2, 2.4, and 0.4 mg of nicotine per gram of tobacco (mg/g). These cigarettes also differed in the content or yield of minor alkaloids and nitrosamines and in the application of casings, including sugars (which were higher in the cigarettes with 15.8 mg/g than in the reduced-nicotine cigarettes in order to balance the ratio of nicotine to sugar) (Donny et al., 2015). All sessions were conducted under double-blind conditions with the varying dose cigarettes being represented by letter codes. The dose and letter code combinations were determined randomly.
Procedure
Participants completed fourteen 2–4 hrs experimental sessions (≥ 48 hrs between sessions) in a within-subjects design. Experimental sessions were conducted in ventilated observation rooms (at least 4.3×5.9× 7.3 ft) equipped with Acer Aspire ES1-111 series laptops with 11.6″ monitors that were used for completing questionnaires and for indicating cigarette preference in concurrent choices sessions (described below). Rooms were also equipped with Dell Optiplex 740 series computers that ran on Windows XP Professional and with 15″ CRT monitors that were used for controlling smoke exposure (described below).
Experimental sessions were conducted following brief smoking abstinence (≤ 50% baseline breath CO level). Participants were instructed that they should try to abstain from smoking for at least 6–8 hrs in order to meet the breath CO criterion. Experimental sessions could be scheduled in mornings, afternoons, or evenings, but were conducted at approximately the same time of day across sessions within individual participants. Upon arrival at the laboratory, participants completed a brief battery of physiological measures, including breath CO, BAL, urine toxicology screen for drugs of abuse, urine pregnancy test, weight, heart rate, and blood pressure. Experimental sessions were rescheduled for those who failed to meet the ≤ 50% baseline breath CO criterion or had a BAL > .03%. Those with a positive drug screen were administered a field sobriety test: if passed the session was conducted and if failed the session was rescheduled. A positive pregnancy test resulted in discontinuation from the study.
At the beginning of each experimental session, participants were instructed to take two puffs from their usual brand cigarette under staff observation to equate time since last cigarette across study participants. Experimental sessions began 30 min following completion of the two puffs. During that 30-min wait period, participants completed the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986) and the Questionnaire of Smoking Urges-Brief scale (QSU-Brief; Cox et al., 2001). No eating or drinking other than water was permitted during sessions.
The 14 sessions were organized into three phases: Phase 1 (Sessions 1–5) involved assessment of smoking topography and subjective effects; Phase 2 (Sessions 6–11) involved assessment of preference between all dose-pairs of the four research cigarettes using free-operant concurrent choice procedures; Phase 3 (Sessions 12–14) involved assessment of preference for each of the three lower doses at a fixed relatively low response requirement (Fixed-Ratio 10) vs. the highest dose, which was available on a progressive ratio schedule. Due to technical problems results from Phase 3 are not included in the present report.
Phase 1 (Sessions 1–5)
Participants smoked usual brand cigarettes in Session 1; in Sessions 2–5, participants were exposed to the different dose research cigarettes, one dose per session with order of exposure randomized across sessions and participants.
Participants smoked two cigarettes per session using a CReSS (Clinical Research Support System) Desktop smoking topography device (Borgwaldt KC, Richmond, VA). Individuals smoked the cigarettes through a plastic cigarette holder that was attached to an air-filled tube, which leads to a pressure transducer. The device measures and records a number of smoking topography parameters, including (1) total number of puffs, (2) inter-puff interval, (3) puff volume, (4) puff duration and (5) maximum flow rate. The first cigarette was smoked ad libitum to assess potential differences in smoking topography upon initial exposure to each of the research cigarettes. Following completion of smoking participants were encouraged to make detailed notes about the cigarette (identified by letter code) for use in Phases 2 and 3 when they would have the opportunity to choose between smoking the different research cigarettes.
Approximately 2 min after extinguishing the first cigarette, participants were instructed to light a second cigarette of the same dose and smoked it in a controlled manner as described below. This was designed to introduce participants to the controlled puffing procedures that would be used in Phases 2 and 3. Participants lit the cigarette without inhaling, inserted the cigarette into the cigarette holder filter, and then proceeded to begin puffing until a 60 mL volume of smoke had been inhaled which was displayed visually on the computer screen by a counter that incremented as puff volume increased; a second counter immediately next to the running counted showed the goal volume of 60 mL. Participants were instructed to hold the inhaled puff in their lungs for 5 s that was also displayed on a running counter. There was a 25 s inter-puff interval, also displayed as a running counter on the computer screen, after which participants were to initiate a second puff following the same regimen. Participants followed this regimen until the cigarette was smoked down to just above the filter.
Upon completion of smoking the second cigarette, participants completed the Cigarette Purchase Task (CPT; Jacobs & Bickel, 1999; MacKillop et al., 2008) and the modified Cigarette Evaluation Questionnaire (mCEQ; Cappelleri et al., 2007; Westman et al., 1992). Breath CO as well as withdrawal and craving using the MNWS (Hughes & Hatsukami, 1986) and the QSU-brief (Cox et al., 2001) were measured every 15 min in the hour following completion of smoking the second cigarette.
Phase 2 (Sessions 6–11)
Upon arrival at the laboratory, participants completed the same battery of physiological measures as described above, took two puffs from their usual brand cigarette and completed the MNWS and QSU-brief questionnaires during the 30-min wait period between puffs and start of the experimental session.
Upon initiation of the 3-hrs experimental session, two different packs of research cigarettes were made available to participants, each with a different letter code. These letter codes corresponded to the same letter codes used with individual participants in the exposure sessions in Phase 1. Participants were encouraged to consult any notes they had made about each of the research cigarettes during the exposure sessions. Each of the possible 6 dose pairs was evaluated once per participant in random order and under double-blind conditions. Participants were instructed to smoke as much or little of either of the research cigarettes they preferred and they were also free to forgo smoking either of the available cigarettes. The computer screen displayed two 1.25 inch squares with each having one of the two letter codes of the cigarettes available for that session embedded within each square. When participants wished to smoke, they used the computer mouse to direct the cursor to the desired square and associated letter code and clicked the mouse ten times (Fixed-Ratio 10). After completion of the response requirement, the screen changed colors displaying a printed instruction indicating that during the next three minutes the participant could take two puffs from the selected cigarette adhering to the same controlled puffing protocol outlined above. A counter was displayed that counted down the 3-min interval during which 2 puffs on the selected cigarette were available. Once two puffs were taken from an earned cigarette, participants extinguished the cigarette and deposited the butt in a designated container for that cigarette code. They were to use a new cigarette for each subsequent two puffs earned. This controlled-puffing procedure is used so that differences in nicotine exposure that should be associated with smoking the varying dose cigarettes is not altered by between- or within-participant changes in smoking topography or confounding of dose and rod length at the time of smoking the different dose cigarettes. Upon completion of the session, participants completed the MNWS and the QSU-Brief.
Statistical Methods
The analysis of Phase 1 results focused on examining differences between the different nicotine dose research cigarettes on smoking topography, CPT and CES questionnaire data, using repeated measures analysis of variance, with nicotine dose (0.4, 2.4, 5.2, 15.8mg/g) as the within-subjects factor for analysis of smoking topography measures, and the CPT and mCEQ questionnaires. The CO boost, and MNWS and QSU-Brief results were examined using a similar approach; however, time (pre- and post-cigarette within each session) was added as additional within-subject factors. All analyses also included a fixed effect for session. Time-by-dose interactions were included to test if CO boost, MNWS and QSU-brief ratings before and after smoking a cigarette differed by dose; however, none were statistically significant and these interaction effects were dropped from the models. Significant time and/or dose effects were followed by post-hoc tests to fully examine the nature of the differences. Differences in preference between all possible pairing of the four research cigarettes when presented in the two-choice concurrent arrangement (Phase 2) were similarly examined using a repeated measures analysis of variance, with each pairwise combination of doses as the within-subjects factor, controlling for the session number. Statistically significant dose-pair effects were followed by post-hoc tests to examine which specific dose elicited the greatest discrepancy in cigarette choices.
Data were complete for all but the MNWS, QSU, and smoking topography measures. Missing data for those measures amounted to 2% or less. All analyses were completed using maximum likelihood estimation procedures, which allow for the use of data from participants for the time period for which data are available without imputation of missing values. Significance for all tests was set at p < .05.
Results
Participant Characteristics
Twenty-six daily cigarette smokers were recruited from among educationally disadvantaged women of reproductive age (n = 9), adults with opioid dependence (n = 11), and adults with affective disorders (n = 6) (Table 1). They were mostly Caucasian females who were approximately 37 years of age with a high school or less education and unmarried. The vast majority smoked higher machine estimated nicotine yield cigarettes, had been smoking for over 20 years, and reported moderate levels of nicotine dependence.
Table 1.
Demographic and smoking characteristics.
| Participants (n=26) | |
|---|---|
| Age (M ± SD) | 36.69 ± 10.78 |
| Gender (% Female) | 76.92 |
| Race (%) | |
| White | 80.77 |
| American Indian/Alaskan Native | 7.69 |
| Asian | 0 |
| Black/African-American | 15.38 |
| Native Hawaiian/Pacific Islander | 0 |
| Education (%) | |
| Some High School | 23.08 |
| High School Grad./Equivalent | 42.31 |
| Some College/2-Yr Degree | 30.77 |
| College Graduate/4-Yr Degree | 3.85 |
| Marital Status (%) | |
| Married | 7.69 |
| Never Married | 65.38 |
| Divorced/Separated | 19.23 |
| Widowed | 7.69 |
| Cigarettes per Day (M ± SD) | 17.50 ± 10.15 |
| Primary Menthol Smoker (%) | 42.31 |
| Cigarette Type (%) | |
| Full Flavor | 80.00 |
| Light | 20.00 |
| Age Started Smoking Regularly (M ± SD) | 15.27 ± 2.07 |
| Fagerström Test for Nicotine Dependence (M ± SD) | 5.15 ± 2.68 |
Note. Data on cigarette type was not available for 1 participant.
Phase 1
Smoking topography and CO boost
Smoking topography and CO boost were of interest related to the potential for the low nicotine content cigarettes engendering compensatory smoking. There was no significant effect of nicotine dose across the six measures of smoking topography examined (puff volume, puff duration, inter-puff interval, maximal flow rate, puff number) (Table 2).
Table 2.
Smoking topography outcomes1.
| Smoking Topography Indices | Investigational Cigarettes | |||
|---|---|---|---|---|
| 0.4 mg/g | 2.4 mg/g | 5.2 mg/g | 15.8 mg/g | |
| Total Puff Volume | 411.88 ± 184.23 | 444.35 ± 200.09 | 370.72 ± 212.15 | 463.82 ± 211.94 |
| Mean Puff Volume | 38.62 ± 12.50 | 41.50 ± 12.60 | 35.58 ± 16.10 | 38.86 ± 15.12 |
| Puff Duration | 1.38 ± 0.36 | 1.39 ± 0.38 | 1.23 ± 0.45 | 1.35 v 0.40 |
| Inter-puff Interval | 21.29 ± 6.12 | 20.95 ± 8.24 | 27.77 ± 24.19 | 19.88 ± 9.34 |
| Max Flow Rate | 31.71 ± 9.00 | 34.33 ± 10.75 | 31.76 ± 11.87 | 34.14 ± 13.87 |
| Puff Number | 11.67 ± 3.12 | 11.25 ± 3.24 | 12.00 ± 4.98 | 15.00 ± 8.32 |
Data points represent means ± standard deviations for total and mean puff volume, mean puff duration, mean inter-puff interval, mean flow rate, and mean puff number observed during ad-libitum smoking of research cigarettes with varying nicotine content levels (0.4, 2.4, 5.2, 15.8 mg/g tobacco).
There was a significant main effect of time (F(3,309)=9.66, p<0.0001), but not nicotine dose nor interaction of dose and time on breath CO boost (not shown). CO increased comparably following smoking each of the doses and remained elevated through the 60-min assessment.
MNWS and QSU-brief ratings
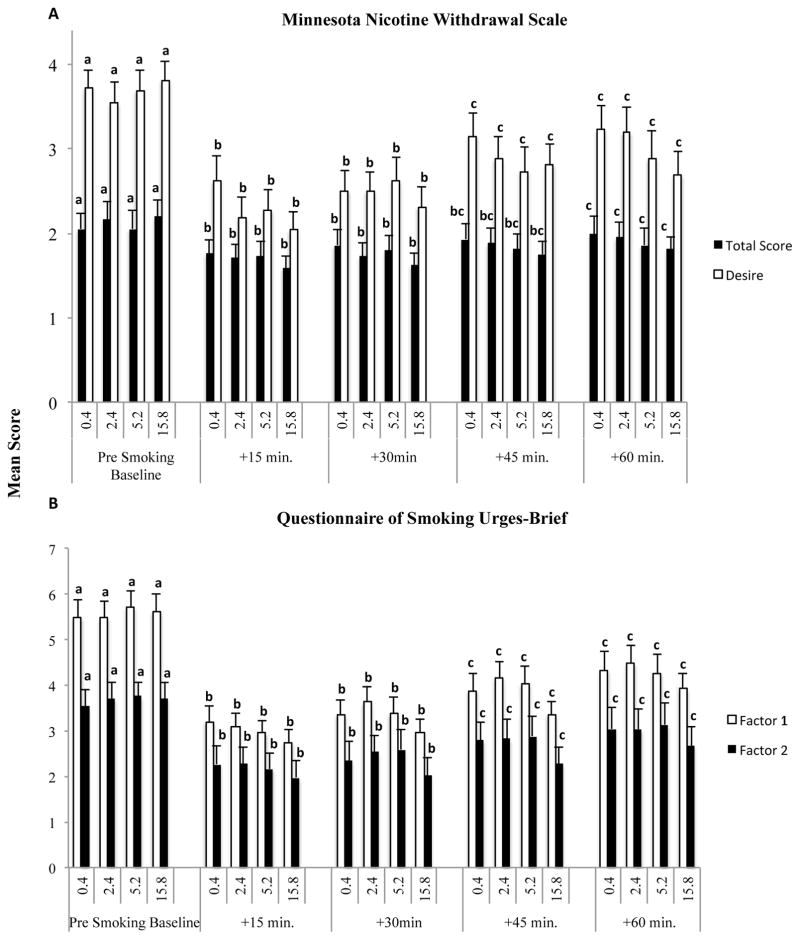
Nicotine withdrawal and craving were of interest to assess whether nicotine content affected relief from these states. There were significant main effects of time on the MNWS total score (F(4,100)=12.19, p<0.0001) and desire (F(4,100)=30.64, p<0.0001) ratings, but no main effect of dose nor interaction of dose and time (Figure 1, upper panel).
Figure 1.
Panel A: Mean Minnesota Nicotine Withdrawal Scale (MNWS) total scores (solid bars) and desire-to-smoke item (open bars) scores before and every 15 minutes for one hour after smoking research cigarettes with varying nicotine content levels (0.4, 2.4, 5.2, and 15.8 mg/g tobacco). Panel B: Mean Questionnaire of Smoking Urges-Brief (QSU-b) Factor 1 (solid bars) and Factor 2 (open bars) scores across the same nicotine doses and time-course of assessments as in Panel A. Error bars represent ± one standard error of the mean. Note that there was a main effect of time for MNWS Total Score and Desire subscales (ps < .01), as well as both QSU Factors 1 and 2 (ps<.01) no main effects of dose nor interactions of dose and time. Note also that data points that share a letter code do not differ significantly (p < .05) from one another.
Similar results were observed on the QSU-brief, with significant main effects of time on Factors 1 (F(4,99)=32.70, p<0.0001) and 2 (F(4,100)=29.46, p<0.0001) but not dose nor interaction of dose and time on either subscale (Figure 1, lower panel). Ratings on both instruments and subscales were elevated at baseline associated with recent abstinence and then decreased comparably following smoking each of the research cigarettes remaining below baselines levels throughout the 60-min assessment.
mCEQ ratings
The mCEQ was of interest to assess any differences across the varying nicotine content cigarettes related to Smoking Satisfaction, Psychological Reward, Aversion, Enjoyment of Respiratory Tract Sensations, and Craving Reduction subscales (Table 3). There was a significant main effect of nicotine dose (F(3,72)=4.03, p = 0.01) on Smoking Satisfaction, with ratings generally increasing as a function of increasing nicotine content. In post-hoc testing, ratings after smoking the 0.4 mg/g cigarette were significantly lower than those following the 15.8 mg/g dose (t(72)=3.46, p=0.001). There were no significant effects of nicotine dose on any of the other mCEQ subscales (Table 2).
Table 3.
Modified Cigarette Evaluation Questionnaire subscales across investigational cigarettes.
| Modified Cigarette Evaluation Questionnaire Subscales | Investigational Cigarettes | |||
|---|---|---|---|---|
| 0.4 mg/g | 2.4 mg/g | 5.2 mg/g | 15.8 mg/g | |
| Smoking Satisfaction* | 3.28 ± 0.41a | 3.68 ± 0.39ab | 3.77 ± 0.38ab | 4.17 ± 0.35b |
| Psychological Reward | 2.68 ± 0.37 | 2.62 ± 0.34 | 2.74 ± 0.34 | 3.02 ± 0.35 |
| Aversion | 1.60 ± 0.13 | 1.88 ± 0.25 | 1.62 ± 0.12 | 2.04 ± 0.22 |
| Enjoyment of Respiratory Tract Sensations | 2.85 ± 0.35 | 3.08 ± 0.36 | 3.00 ± 0.33 | 3.42 ± 0.34 |
| Craving Reduction | 3.88 ± 0.41 | 3.85 ± 0.41 | 3.46 ± 0.36 | 4.19 ± 0.38 |
Note. An asterisk indicates a statistically significant effect of dose on the subscale scores. Scores without superscript letters in common are statistically different from one another.
CPT
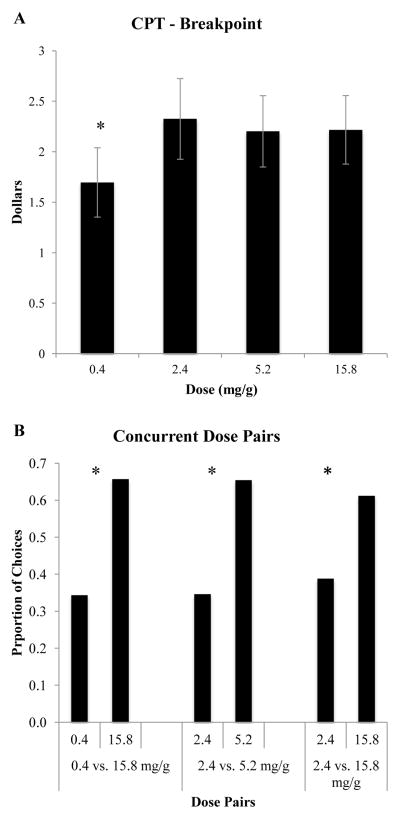
Sensitivity to price was of interest with regard to assessing the potential of the varying nicotine doses to function as economic substitutes for each other and their relative dependence potential. Breakpoint, the price where consumption falls to zero, varied significantly as a function of nicotine dose (F(3,69)=4.02, p = 0.01) (Figure 2, Panel A). In post-hoc testing, breakpoint was reached at a lower price with the 0.4mg/g dose than with the 2.4 mg/g (t69)=2.53, p=0.014), 5.2 mg/g (t(69)=2.69, p=0.009), and 15.8 mg/g (t(69)=3.14, p=0.003) doses, respectively. There were no significant effects of nicotine dose across the other CPT indices (Intensity, Omax, Pmax, elasticity of demand) (not shown). There was a non-significant trend for Pmax, the price at which consumption becomes elastic, to differ by nicotine dose, with the 0.4 mg/g dose trending towards greater elasticity than the other doses (F(3,69)=2.61, p = 0.06).
Figure 2.
Panel A: Mean Breakpoint from the Cigarette Purchase Task across experimental cigarettes differing in nicotine content (mg/g). Panel B: Mean proportion of choices allocated to different nicotine dose cigarettes during separate 3-hour two-dose concurrent choice sessions. All possible two-dose comparisons across the four nicotine dose cigarettes (0.4, 2.4, 5.2, and 15.8 mg/g tobacco) were examined; only those that differed significantly are shown. The two-dose comparisons are shown on the x-axis with mean proportion of choices allocated to each shown on the y-axis. Asterisks in Panels A and B indicate significant dose differences at p < .05.
Phase 2
Preference testing
A direct test of preference between dose pairs in a concurrent choice arrangement was of interest for assessing differences between doses in relative reinforcing effects (i.e., dependence potential). There was a significant effect of nicotine dose on choice (F(5,134)=2.29, p = 0.049). Post-hoc testing revealed three significant dose differences (Figure 2, Panel B). Participants choose the 15.8 mg/g dose significantly more than the 0.4 (t(134)=2.40, p < 0.05) and the 2.4 mg/g (t(134)=2.00, p < .05) doses. Participants also chose the 5.2 mg/g dose significantly more than the 2.4 dose (t(134)=2. 48, p = .01). There were no other significant differences in the preference tests.
Conclusions
The present study was conducted to begin examining potential impacts of a national policy of lowering the nicotine content of cigarettes to a sub-dependence threshold in three vulnerable populations of smokers. Results to date in the general population of adult smokers have been encouraging regarding the potential feasibility and efficacy of such a policy (Benowitz et al., 2007; Benowitz et al., 2009; Donny et al., 2007; Donny et al., 2015; Hatsukami et al., 2010; Hatsukami et al., 2013) as have results obtained among smokers with schizophrenia (AhnAllen et al., 2015; Tidey et al., 2013; 2016). As discussed below, the results of this initial study are similarly promising regarding the feasibility of reducing the nicotine content of cigarettes to very low levels without substantial adverse effects in these vulnerable populations of smokers.
First, we saw no evidence consistent with compensatory smoking during acute exposure to reduced nicotine content cigarettes. That is, there were no significant differences in smoking topography between the varying nicotine dose research cigarettes. As discussed above, compensatory smoking wherein smokers take more or larger puffs or otherwise alter how they smoke cigarettes in order to sustain a desired level of nicotine is a substantive concern because of the serious adverse health consequences that such changes can produce. These results suggest that pronounced compensatory smoking may not be a problem with very low nicotine content cigarettes in more vulnerable populations of smokers consistent with results on chronic exposure to comparable doses of reduced nicotine content cigarettes in healthier populations of smokers (Donny et al., 2015). The current results are partially consistent with Tidey et al. (2016) who found that smokers with schizophrenia and equally heavy smokers without psychiatric illness took longer puffs and had shorter inter-puff intervals when smoking very low nicotine content cigarettes than when they smoked their usual brand. However, because participants took fewer puffs when smoking very low nicotine content cigarettes, the net effect of these changes was a reduction in cigarette and session volume and no change in CO boost. Important to acknowledge, however, is the evidence from two prior acute-exposure studies examining smoking topography across commercially available low nicotine content cigarettes in relatively healthy smokers where total puff volume was significantly greater when smoking very low compared to moderately reduced nicotine content cigarettes (MacQueen et al, 2012; Strasser et al., 2007). One of those two studies also reported greater mean puff duration and shorter inter-puff intervals with the very low compared to the higher nicotine-dose cigarettes, although that effect was not evident across repeated exposures to the very low nicotine content cigarettes during the experimental session (MacQueen et al., 2012). Three between-study methodological differences may have contributed to the different results observed in the present compared to these two prior acute-exposure studies. First, both prior studies used commercial brand (Quest) cigarettes where the present study used research cigarettes. Second, neither of the prior studies included a nicotine dose comparable to full-flavor commercial brands as was done in the present study. It is compensation to maintain nicotine exposure at levels obtained from cigarettes with high nicotine content levels comparable to those found in commonly available commercial cigarettes that is the main concern. Worth noting is that Strasser et al. included participants own brand cigarettes as a comparison condition in their study and the increases in total puff volumes seen with the lowest nicotine content cigarettes fell below levels seen with usual brand. Third, the prior studies included approximately one-half male participants whereas the present study used slightly less than one-quarter males. Smoking in males appears to be more directly controlled by the reinforcing effects of nicotine than in females (Perkins, 2009). The present and prior acute- and chronic-exposure studies considered together suggest a pattern of relatively minimal compensatory smoking associated with use of very-low nicotine content cigarettes, but that it may occur in some individuals. While these results on the risk of compensatory smoking are encouraging, ongoing vigilance and additional research on this important topic are warranted.
Nicotine withdrawal and craving symptoms were significantly and comparably reduced by each of the research cigarette doses tested. This observation systematically replicates and extends to these vulnerable populations of smokers a consistent pattern of significant withdrawal and craving relief from smoking very low nicotine content cigarettes observed in a general population of smokers (Donny et al., 2015; Hatsukami et al., 2010; Hatsukami et al., 2013) and smokers with schizophrenia (Tidey et al., 2013). While more remains to be understood regarding the relative contributions of conditioned versus direct agonist effects to this remarkable level of relief from very low nicotine content cigarettes (Brody et al., 2009), there is now an abundance of evidence that very low nicotine content cigarettes provide smokers with significant relief. Note that our Phase 1 practice of having participants smoke a second cigarette as part of introducing them to controlled puffing but before they completed the battery of craving/withdrawal and other questionnaires can be expected to have enhanced effects of all of the doses studied above what would have been observed with smoking only a single cigarette. Whether the different doses produce different effects at this point in the procedure remains to be determined. Also, the present study only assessed craving and withdrawal relief following brief smoking abstinence in these vulnerable populations. It is well established that nicotine withdrawal extends over several weeks and thus further research assessing relief during extended exposure will be necessary for a thorough characterization of the degree of relief these more vulnerable smokers obtain from very low nicotine content cigarettes. Those trials are currently underway. Lastly, not including a cigarette with zero nicotine content in the study prevents a careful parsing out of how much of the effects of the 0.4 mg/g dose cigarette are attributable to the relatively low levels of nicotine delivered versus conditioned effects.
Regarding dependence liability, the 0.4 mg/g dose appears to have the greatest likelihood of falling below the dependence threshold discussed by Benowitz and Henningfield (1994). The 0.4 mg/g dose differed significantly from the 15.8 mg/g dose on several important measures of dependence liability, a dose that was included in the present study to represent nicotine content levels commonly found in commercial cigarettes. Results that parallel those found in healthier samples from the general population of smokers (Donny et al., 2015). Compared to the 15.8 mg/g dose, the 0.4 mg dose was rated as less satisfying, had a lower breakpoint in the Cigarette Purchase Task, and was chosen less often in the concurrent choice test where we directly compared the relative reinforcing effects of the two doses under double blind conditions. The 2.4 mg/g dose was also chosen significantly less than the 15.8 mg/g dose in the concurrent choice test, but did not differ significantly from the 15.8 mg/g dose on other measures relevant to assessing dependence liability. The dependence liability of the 5.2 mg/g dose appeared to be largely comparable to the 15.8 mg/g dose consistent with the ability of this dose to maintain smoking over a 6-week period (Donny et al., 2015), although in preference testing it was not reliably chosen over the 0.4 mg/g dose while the 15.8 mg/g dose clear was preferred. An additional point about outcomes observed with the 0.4 mg/g dose in the CPT merits mention. While participants indicated that they would forego smoking the 0.4 mg/g dose at a lower price than was observed with the other doses (i.e., lower breakpoint on CPT), they were willing to spend slightly more than $1.50 per cigarette for the 0.4 mg/g dose. That is, the 0.4 mg/g dose did have reinforcing effects. That was also clear in the intensity of demand measure in the CPT where participants indicated that they would smoke the 0.4 mg/g dose cigarettes at a rate comparable to the highest dose if the cigarettes were free. This could be important. While the goal is to find a dose that is below the dependence threshold, public acceptance of a new national policy of reduced nicotine content in cigarettes would likely be greater if the cigarettes retained at least a relatively modest level of reinforcing effects sufficient to serve as an inferior but nevertheless acceptable substitute for the currently available commercial cigarettes that they would be designed to replace. The challenge, at least in the initial stages of such a policy change, might be to find a nicotine content level that is adequate to function as a reinforcer, but not such a strong reinforcer so as to be valued over other options such as non-combusted nicotine/tobacco products or total abstinence when consumers elect to make choices about discontinuing cigarette smoking. The profile of effects associated with the 0.4 mg/g dose in the present and prior studies suggest that it has considerable promise to satisfy that more nuanced goal.
As was mentioned above, the present study has several limitations, including use of too few participants for meaningful assessments of response within or comparisons between the three vulnerable populations of interest and no assessment of chronic exposure to these varying nicotine dose cigarettes. Additional studies of acute and chronic exposure to very low nicotine content cigarettes that are powered to separately characterize effects within these same three vulnerable populations are currently underway addressing each of those limitations. Assessing acute and chronic exposure in other vulnerable populations (e.g., pregnant smokers) will be important as well. Examining a wider range of doses at the lower end of the dose-range examined in the present study may be informative in terms of more definitively delineating the dependence threshold discussed by Benowitz and Henningfield (1994). These limitations notwithstanding, these initial results in more vulnerable populations of smokers are encouraging regarding the feasibility of a national policy to reduce the nicotine content of cigarettes to very low levels without substantial adverse effects. We saw no evidence of compensatory smoking, smokers obtained significant reductions in nicotine withdrawal and craving from the lowest content cigarette that was indistinguishable from higher doses, and the lowest dose had a significantly lower dependence liability profile than the highest dose that was included in the study to represent nicotine content levels common in commercial cigarettes.
Acknowledgments
We extend deep appreciation to the research assistants and other support staff at the University of Vermont, Brown University, and Johns Hopkins School of Medicine for their tireless efforts in implementing this study.
Funding: This project was supported by a Tobacco Centers of Regulatory Science (TCORS) award (P50DA036114) from the National Institute on Drug Abuse and Food and Drug Administration. Preparation of the reported was also supported in part by a Centers of Biomedical Research Excellence award (P20GM103644) from the National Institute on General Medical Sciences. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Food and Drug Administration.
Footnotes
Disclosures: The authors have nothing to declare related to this study and report.
References
- AhnAllen CG, Bidwell LC, Tidey JW. Cognitive effects of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine & Tobacco Research. 2015;17:510–4. doi: 10.1093/ntr/ntu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction: the implications for tobacco regulation. New England Journal of Medicine. 1994;331:123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P., III Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine & Tobacco Research. 2009;11:954–60. doi: 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P., III Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. International Journal of Neuropsychopharmacol. 2009;12:305–16. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addictive Behaviors. 2007;32:912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed 4/24/16];Global tobacco control. 2016 http://www.cdc.gov/tobacco/global/index.htm.
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al Abasi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK. Randomized trial of reduced nicotine standards for cigarettes. New England Journal of Medicine. 2015;373:1340–9. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–34. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- [Accessed 4/24/16];Family Smoking Prevention and Tobacco Control Act (H.R. 1256) 2009 https://www.govtrack.us/congress/bills/111/hr1256/text.
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence, and cessation. Addiction. 2010;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Hertsgaard LA, Vogel RI, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiology, Biomarkers & Prevention. 2013;22:1015–24. doi: 10.1158/1055-9965.EPI-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiology, Biomarkers & Prevention. 2015;24:472–476. doi: 10.1158/1055-9965.EPI-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Chilcoat H. Women and smoking: an interdisciplinary examination of socioeconomic influences. Drug & Alcohol Dependence, Suppl. 2009;1:S1–S5. doi: 10.1016/j.drugalcdep.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug & Alcohol Dependence. 2009;104(S1):S100–105. doi: 10.1016/j.drugalcdep.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Kurti AN, Redner R, White TJ, Keith DR, Gaalema DE, Sprague BL, Stanton CA, Roberts ME, Doogan NJ, Priest JS. Co-occurring risk factors for current cigarette smoking in a US nationally representative sample. Prevntive Medicine. 2016 Feb 21; doi: 10.1016/j.ypmed.2016.02.025. pii: S0091–7435(16)30004–4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock R, Bauld L, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Annals of the New York Academy of Science. 2012;1248:107–23. doi: 10.1111/j.1749-6632.2011.06202.x. [DOI] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. Archives of General Psychiatry. 2001;58:503–8. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Jacobs EA, Bickel WK. Modeling consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Experimental and Clinical Psychopharmacology. 1999;7:412–26. doi: 10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, O’Connor RJ, Sweeney CT. Smoking and Tobacco Control Monograph 13. Bethesda, MD, USA: US Department of Health and Human Services, Public Health Services, National Institutes of Health; 2001. Cigarette design; pp. 13–38. [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285. doi: 10.1186/1471-2458-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG, Ray LA, Eisenberg DT, Lisman SA, Lum JK, Wilson DS. Further validation of the cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Experimental and Clinical Psychopharmacology. 2008;16:57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- MacQueen DA, Heckman BW, Blank MD, Van Rensburg KJ, Evans DE, Drobes DJ. Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology (Berl) 2012;223:47–54. doi: 10.1007/s00213-012-2685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Nebraska Symposium on Motivation. 2009;55:143–69. doi: 10.1007/978-0-387-78748-0_9. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Karelitz JL, Michael VC, Donny EC. Threshold dose for discrimination of nicotine via cigarette smoking. Psychopharmacology (Berl) 2016;233:2309–17. doi: 10.1007/s00213-016-4281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Intervewi (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998 [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine & Tobacco Research. 2013;15:121–9. doi: 10.1093/ntr/nts098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Cassidy RN, Miller ME. Nicotine & Tobacco Research. 2016. Mar 19, Smoking topography characteristics of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. 2016. pii:ntw089. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Smoking and Tobacco Control Monograph 13: Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD, USA: US Department of Health and Human Services, Public Health Services, National Institutes of Health; 2001. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking---50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Westman EC, Levin ED, Rose JE. Smoking while wearing the nicotine patch: Is smoking satisfying or harmful? Clinical Research. 1992;40:871A. [Google Scholar]