Summary
Claudins are discovered to be key players in renal epithelial physiology. They are involved in developmental, physiological and pathophysiological differentiation. In the glomerular podocytes, claudin-1 is an important determinant of cell junction fate. In the proximal tubule, claudin-2 plays important roles in paracellular salt reabsorption. In the thick ascending limb, claudin-14, -16 and -19 regulate the paracellular reabsorption of calcium and magnesium. Recessive mutations in claudin-16 or -19 cause an inherited calcium and magnesium losing disease. Synonymous variants in claudin-14 have been associated with hypercalciuric nephrolithiasis by genome wide association studies (GWAS). More importantly, claudin-14 gene expression can be regulated by extracellular calcium levels via the calcium sensing receptor. In the distal tubules, claudin-4 and -8 form paracellular chloride pathway to facilitate electrogenic sodium reabsorption. Aldosterone, WNK4, Cap1 and KLHL3 are powerful regulators of claudin and the paracellular chloride permeability. The lessons learned on claudins from the kidney will have a broader impact on tight junction biology in other epithelia and endothelia.
Keywords: tight junction, claudin, kidney, ion channel, glomerulus, epithelium, polarity, calcium, magnesium, kidney stone, hypertension
Introduction
The tight junction (TJ) is composed of a series of direct membrane contacts of adjacent cells in polarized epithelia (16). The known integral membrane proteins of the tight junction include occludin (21), the Junctional Adhesion Molecules (JAMs) (13), and the claudins (19, 22). Freeze-fracture electron microscopy has revealed the tight junction as a branching and anastomosing reticulum of “fibrils” or “strands” on the P fracture face (31). These fibrils have been demonstrated to be partly composed of integral membrane proteins directly involved in cell-cell interactions.
Claudins are the key integral membrane proteins of TJs and are 21–28 kDa proteins that consist of four transmembrane (TM) domains, two extracellular loops (ECL1 and 2), amino- and carboxyl-terminal cytoplasmic domains, and a short cytoplasmic turn (38). Claudins cis associate within the plasma membrane of the cell into dimers, or higher oligomeric states. These associations are followed by trans interactions between claudins in adjacent cells, and additional cis interactions to assemble claudin oligomers into TJ strands. The cis interaction can involve a single type of claudin (homomeric interaction) or different types of claudins (heteromeric interaction); further the trans interaction can operate in a homotypic or heterotypic mode (23). Studies have shown that claudin-4, -5, -8, -11 and -14 selectively decrease the permeability of cations (4, 11, 85, 91, 96), specifically to Na+, K+, H+ and ammonium, while claudin-2 and -15 increase cation permeability (20, 86). These and other studies have led to the model of claudins forming the paracellular channel, a novel class of channels of 4–7 Å in diameter and oriented perpendicular to the membrane plane to join two extracellular compartments (81, 87).
Bowman’s Capsule
Glomerulus is the filtering unit of the kidney. The whole glomerulus is bounded by a bowl-like enclosure called Bowman’s capsule that is formed by a layer of squamous epithelia called parietal epithelial cells (PECs) (61). The inner core of the glomerulus a highly intricate and specialized microvascular bed that is formed by glomerular endothelial cells (GECs) (64). Intimately wrapped as a monolayer around glomerular capillaries are stellate-shaped cells called visceral epithelial cells, or podocytes.
Parietal Epithelial Cells (PECs)
PECs resemble squamous epithelial cells, with a small cell body size ranging in thickness from 0.1 to 0.3 µm, increasing to 2.0–3.5 µm at the nucleus (73). Adjacent PECs adhere to the underlying Bowman's basement membrane and express the TJ proteins in normal rat, mouse, and human glomerulus (90) (Figure 1). Among the claudin subtypes, claudin-1 is expressed in the TJs of PECs and is therefore regarded as a marker of PECs (47, 66) (Figure 2). Claudin-1 staining was positive from the S-phase onward in both PECs and podocytes but persisted in mature PECs only (60). ZO-1 staining was first detected in both cell types in the S-phase, with increased staining intensity noted during the capillary loop phase. Staining for ZO-1 persisted in the normal adult PEC and podocyte (70). Claudin-2, a cation-permeable claudin isoform predominantly expressed in the proximal tubule, has also been reported to be expressed in the parietal epithelium (66).
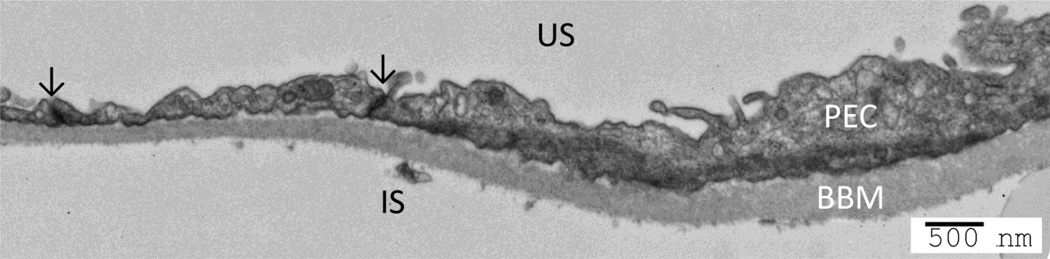
Figure 1.
Structure of tight junction in parietal epithelial cells. The parietal epithelial cells (PEC) are flat cells and the tight junctions (arrow) are found at points of close apposition between the lateral membranes. US: urinary space; IS: interstitial space; BBM: Bowman’s basement membrane. Bar: 500nm.
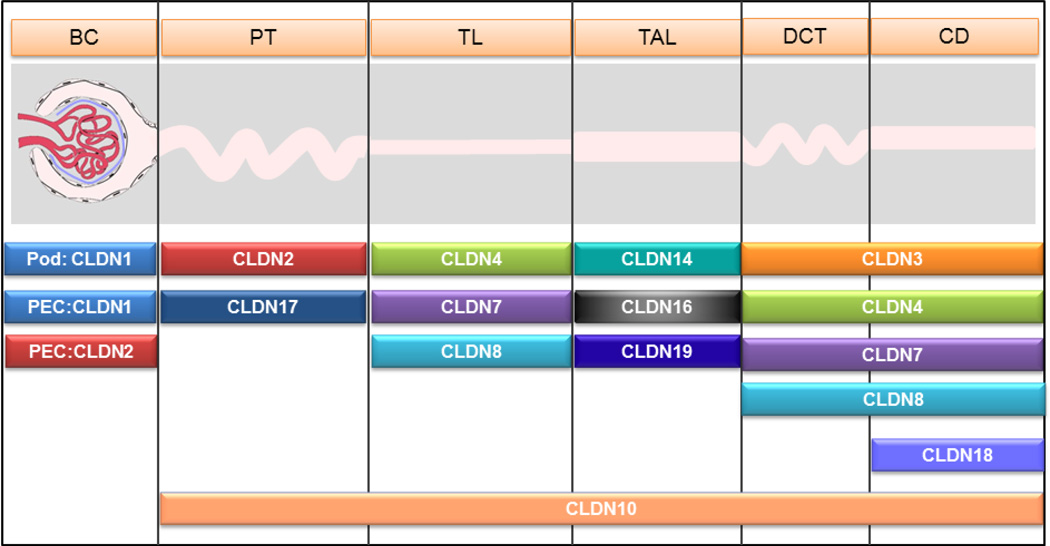
Figure 2.
Expression profile of claudin genes along the nephron of the kidney. Note that different nephron segments express a unique combination of claudin genes, which may underlie the specific transport functions of these segments. BC: Bowman’s capsule; PT: proximal tubule; TL: thin limb; TAL: thick ascending limb; DCT: distal convoluted tubule; CD: collecting duct; Pod: podocyte; PEC: parietal epithelial cell. Note: the claudin-1 gene is only expressed in podocytes under nephrotic condition.
PECs are thought to limit filtered proteins ‘escaping’ into the peri-glomerular space. The inter-cellular TJs between PECs, together with the underlying Bowman’s Basement membrane, serve as a second barrier to urinary filtrate (14). TJs are disrupted in experimental anti-glomerular basement membrane (GBM) disease, accompanied by reduced levels of TJ proteins claudin-1, ZO-1, and occludin. In an attempt to determine the biological consequences of these changes, Ohse and colleagues performed an in-vivo permeability assay using different-sized labeled tracers (60). After experimental induction of glomerulonephritis, the permeability to tracers similar in size to albumin was increased between adjacent PECs. The macromolecule tracers can be observed leaking into the space between the parietal epithelium and the underlying basement membrane of Bowman’s capsule, as well as the extraglomerular space (60). The roles of PECs in podocyte regeneration, crescent formation and focal segmental glomerulosclerosis are under intense investigation (2, 6, 77, 78, 89).
Podocytes
The podocyte is a highly specialized, terminally differentiated epithelial cell with unique morphological and functional features in the Bowman's capsule of the kidneys that wrap around capillaries of the glomerulus (72). Podocytes are important for the maintenance of the glomerular filter in the kidney and its malfunction is central to many glomerular diseases. In immature glomeruli, the presumptive podocytes are connected by apically localized TJs, which form between podocytes near their apical surfaces during the comma and S-shape stages of glomerular formation (65). As podocytes enter the subsequent capillary loop stage, they begin to establish their characteristic complex cell architecture including transformation from a columnar epithelium toward a highly arborized cellular morphology and developing elaborate foot processes that interdigitate with processes from neighboring podocytes (62). At this stage, their cell junctions relocate more basally and are transformed from TJs into slit diaphragm (SD) (Figure 3). The mechanism of apical junctional complexes migrating basolaterally between these cellular projections and converting into the SD complex between foot processes is not fully understood. The first hypothesis proposes that immature podocytes disassemble their temporary TJs and reconnect via filopodia-like protrusions that eventually transform into foot processes (51). Alternatively, they might remain attached at their lateral faces throughout their maturation and remodel their TJs into SDs while the progressive spreading of podocytes causes cytoplasmic projections that mature into foot processes (51).
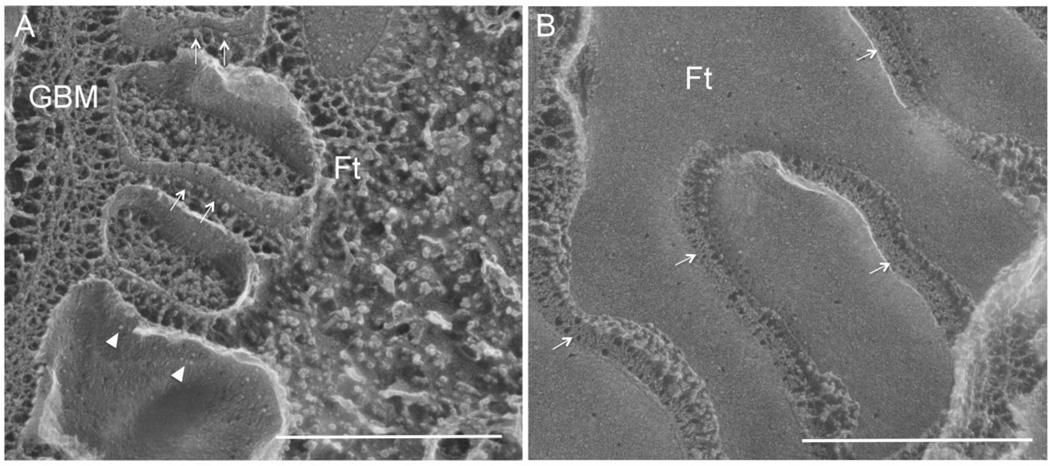
Figure 3.
Freeze fracture electron micrographs showing the replica of fractured podocytes from both the perpendicular view (A) and the parallel view (B) relative to the glomerular basement membrane. In A, arrow indicates the slit diaphragm as protein particles anchored on the E-face of the fractured membrane and arrowhead indicates the slit diaphragm protein particles on the P-face of the fractured membrane. In B, arrow indicates the slit diaphragm as zipper-like structure. GBM: glomerular basement membrane; Ft: foot process. Bar: 500nm. Adapted from reference (28).
SDs are usually considered to represent a modified TJ because they derive from the tight junctional complexes during glomerular development (18). The morphology of SDs is apparently different from that of TJs. The intercellular space bridged by the SD is 30 to 40 nm wide, which is to our knowledge the widest intercellular contact known to date (32). TJs are the closest known contacts between adjacent cells which obliterate the intervening intercellular space (40). In spite of the structural difference between them, the SDs and TJs do share a set of similarities. Like the TJs which restrict paracellular ion permeability, SDs serves as the exit port for primary urinary filtrate and is now well recognized as essential in the selective retention of high-molecular-weight plasma components such as albumin (72). In addition, both junctions participate in the regulation of apicobasal polarity and cell growth, differentiation, and dedifferentiation (3, 5, 54, 56, 62, 74).
Under nephrotic conditions, SDs frequently dislocate or disappear, and the TJs reappear in lieu of SDs between the retracted podocyte foot processes. Farquhar and colleagues first recognized the presence of TJs in the residual slits with both thin-section EM and freeze-fracture EM (10, 15). Using several techniques including fractionation, immunofluorescence, and immunoelectron microscopy, TJ proteins such as JAM-A, occludin, coxsackievirus and adenovirus receptor (CAR) and ZO-1 have been found in the SD of the mature podocyte (18, 70). The TJs scaffold protein ZO-1 is essential for the normal interdigitation of foot processes and the formation of SD. Lack of ZO-1 triggers early-onset proteinuria with podocyte effacement with the progressive development of glomerulosclerosis (45). Several claudins have been detected in the glomerulus of adult mouse kidneys. With transcriptional profiling, Doné and colleagues have found that claudin-3 was the only gene significantly upregulated in nephrin knockout mouse podocytes, which was normally absent in glomeruli (12). Claudin-1, which is primarily expressed at TJs of the glomerular parietal epithelium in healthy mouse kidneys, has been found profoundly upregulated in podocytes from animals with diabetic nephropathy (35) (Figure 2). Using a podocyte specific transgenic approach, Gong and colleagues have shown that induction of claudin-1 in mature podocytes caused SD to TJ transition, accompanied by profound proteinuria (28). Gong’s data attest to a new concept that SD and TJ are interchangeable during development and nephrotic diseases.
Proximal tubule
The proximal tubule is a leaky nephron segment, with transepithelial resistance of < 10 Ω•cm2 (9). The predominant claudins expressed in the proximal tubule are claudin-2, claudin-10 and claudin-17 (47, 52) (Figure 2). Claudin-2 functions in vitro as a cation selective paracellular channel (95). Genetic ablation of claudin-2 in mouse kidneys caused defects specific to the proximal tubule, indicating decreases in proximal tubular reabsorption of salt and calcium (57). While the claudin-2 knockout animals showed normal renal metabolism of salt when fed with normal salt diet, high salt infusion to these animals uncovered a mechanism compatible with salt reabsorption defects in the proximal tubules (57). Ex vivo perfusion of the proximal tubules from the claudin-2 knockout mice showed a marked decrease in paracellular ion conductance and selectivity (PNa/PCl) (57) (Figure 4A). Pei and colleagues tried to provide a physiologic explanation of claudin-2’s role in maintaining renal energy efficiency by rationalizing a compensatory mechanism through the transcellular Na-K-2Cl transport activity in the thick ascending limb of Henle (63). They hypothesized that reduction of paracellular salt reabsorption in the proximal tubule may lead to increased transcellular salt reabsorption in the downstream tubules at the expense of higher oxygen consumption. Such additional energy consumption will trigger renal tubular injury under ischemic conditions (63).
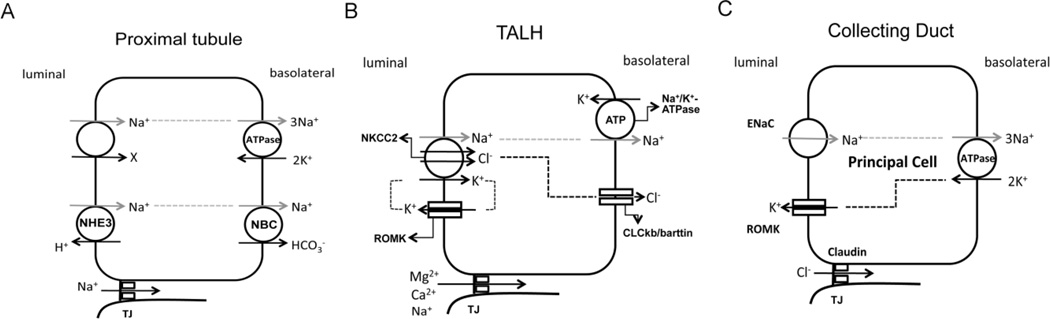
Figure 4.
Scheme of coupled transcellular and paracellular transport pathways in the proximal tubule (A), the thick ascending limb (B) and the collecting duct (C). A, in the proximal tubule, Na+ is absorbed through the Na+/H+ exchanger (NHE3) and the Na+/glucose co-transporter localized in the luminal membrane and secreted into the basolateral side through the Na+/K+-ATPase and the Na+/HCO3− cotransporter (NBC). Additional Na+ can permeate through the tight junction (TJ) via the claudin-2 channels. B, in thick ascending limb (TALH), Na+, K+ and Cl− are absorbed through the luminal membrane Na+/K+/2Cl− cotransporter (NKCC2); Na+ is secreted into the basolateral side via the Na+/K+-ATPase; Cl− is secreted into the basolateral side via the chloride channel ClCkb/barttin; K+ is recycled into the luminal side through the renal outer medullary potassium channel (ROMK). Due to the continuous reabsorption of NaCl, a NaCl gradient develops from basolateral to luminal sides. The tight junction is permeable to Mg++ and Ca++ through the claudin-16 and -19 channels. C, in the collecting duct, Na+ is absorbed through the epithelial sodium channel (ENaC); Na+ is secreted into the basolateral side via the Na+/K+-ATPase; K+ is secreted into the luminal side via the renal outer medullary potassium channel (ROMK). Because of the unilateral Na+ absorption, a lumen-negative potential develops, which drives Cl− absorption through the tight junction via claudin-4 and -8 channels.
Whether the proximal tubule retains a paracellular water permeation pathway has long been controversial. Genetic knockout of Aqp1 in mice only caused a 50% reduction in renal water reabsorption rate, indicating an alternative water permeation pathway (71). In vitro in transfected MDCK cells, claudin-2 appeared to increase transepithelial water permeation dependent upon not only osmotic pressure gradient but also Na+ concentration gradient across the epithelial monolayer (67). Seemingly compatible with this hypothesis, in ex vivo perfused proximal tubules from claudin-2 knockout mice, the transepithelial reabsorption of volume was reduced compared to normal proximal tubules (57). However, the concept of tight junction making a water permeation pathway has been challenged by Spring and colleagues who, employing an advanced optical approach, have demonstrated that the water flow rate in the lateral intercellular spaces of MDCK cells are negligible beneath the tight junction (at least for bicellular tight junction) (50).
Claudin-10 and -17 are also expressed in the proximal tubule. The claudin-10 gene encodes two alternatively spliced isoforms of claudin-10 proteins – 10a and 10b, which differ in the first extracellular loop domain (88). Breiderhoff and colleagues show that in the proximal tubule claudin-10a is the expressed isoform and unaffected by the KSP-Cre knockout, whereas claudin-10b is expressed in thick ascending limb of Henle’s loop and almost completely abolished in the knockout (7). Claudin-10a functions as an anion channel while claudin-10b as a cation channel (88). The in vivo role of claudin-10 in the proximal tubule has not been addressed but its role in the thick ascending limb has become increasingly clear as a regulator of calcium and magnesium transport (vide infra). Studies of claudin-17 in several cell models indicate that it functions as an anion pore (52). Thus, claudin-10 or -17 could mediate the paracellular Cl− transport in the late proximal tubule where Cl− reabsorption is primarily paracellular and driven by its concentration gradient (48).
Thick ascending limb of Henle’s loop
The thick ascending limb of Henle’s loop is a particularly important nephron segment for reabsorbing calcium and magnesium via the paracellular pathway. The driving force for reabsorbing these divalent cations is the lumen positive electrical potential difference. This potential is generally considered to be a result of two additive mechanisms: (1) transcellular reabsorption of NaCl through the apical Na-K-2Cl co-transporter coupled with K+ secretion through the apical K channel, gives rise to a spontaneous positive charge to the apical membrane (8); (2) dilution of the luminal fluid through constant NaCl reabsorption creates a diffusion potential through the cation selective tight junction (33), which can add up to 30mV to the total luminal potential difference (34) (Figure 4B).
The major claudin species expressed in the thick ascending limb of Henle’s loop are claudin-10, -14, 16 and -19 (Figure 2). Simon and colleagues first discovered that mutations in claudin-16 caused a rare autosomal recessive disease – familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC; OMIM: 248250) (75). From the FHHNC patients carrying normal claudin-16 alleles, Konrad and colleagues identified a second locus harboring mutations in the claudin-19 gene (49). Hou and colleagues have generated the claudin-16 and -19 null mouse models and demonstrated that the loss of function in either claudin can cause FHHNC, compatible with its recessive transmission pattern (41, 44). More importantly, claudin-16 and -19 physically interact (42), and loss of one claudin from the tight junction can cause the other to be endocytosed from the plasma membrane (41). In both mammalian and insect cell membranes, recombinant claudin-16 and -19 proteins form a stable cis-dimer (27). Co-expression of claudin-16 and -19 into polarized epithelial cells can confer significant cation selectivity (PNa/PCl) to the tight junction (27, 42). In ex vivo perfused thick ascending limbs from the claudin-16 knockdown mice, the cation selectivity of tight junction is profoundly impaired (44). Will and colleagues using a global knockout approach have revealed a more selective decrease in relative divalent cation permeability including Ca++ and Mg++ in perfused thick ascending limbs (92).
A recent genome wide association study has identified claudin-14 as a major risk gene for hypercalciuric nephrolithiasis (83). The common, synonymous variant (rs219780[C]) is predicted to have 1.64-time greater risk of developing the disease in homozygous carriers compared to noncarriers (83). Gong and colleagues have found that the claudin-14 protein interacts with claudin-16 and inhibits its permeability in vitro (26). Transgenic overexpression of claudin-14 in mouse kidneys generated renal defects featured by uncontrolled loss of calcium and magnesium (25). More intriguingly, claudin-14 gene expression can be regulated by extracellular calcium changes induced by either serum calcium alterations or calcimimetic drugs such as cinacalcet via the calcium sensing receptor (25, 26). MicroRNAs (miR-9 and miR-374) are the key second messengers for this process (24).
Claudin-10 appears to play an opposite role to claudin-16 and -19 in the thick ascending limb of Henle’s loop. Kidney specific deletion of claudin-10 in mice generated serum electrolyte imbalances of hypermagnesemia, allegedly due to hyperabsorption of magnesium in the thick ascending limb of Henle’s loop (7). The paracellular sodium permeability in perfused thick ascending limbs from claudin-10 knockout animals mysteriously decreased, accompanied by relative increases in paracellular calcium and magnesium permeabilities (7). According to Hebert and colleagues (36, 37), the paracellular Na+ reabsorption in the thick ascending limb of Henle’s loop accounts for around 50% of total transepithelial Na+ reabsorption. The reduction in paracellular Na+ reabsorption in claudin-10 knockout kidneys may have led to a compensatory increase in the transcellular component such as the NKCC2/ROMK activity, which is reflected by the pronounced increase in the lumen-positive furosemide-inhibitable spontaneous potential (Vte) (7). The increase in Vte may also contribute to hyperabsorption of divalent cations such as Ca++ and Mg++. Milatz and colleagues have recently proposed a new hypothesis to reconcile the renal phenotypes of claudin-10 knockout mice with claudin-16 or -19 knockout mice. Milatz et al found that the TJs in thick ascending limbs possessed a mosaic expression pattern separating claudin-10 from the claudin-16/-19 complex (55). Furthermore, TJs from the medullary thick ascending limb dominated by claudin-10 appear to favor Na+ over Mg++ whereas TJs from the cortical thick ascending limb dominated by claudin-16 favor Mg++ over Na+ (55). These results would suggest an axial heterogeneity in thick ascending limb paracellular permeability to various cations.
Aldosterone sensitive distal nephron
The aldosterone sensitive distal nephron comprises the distal convoluted tubule, the connecting tubule and the collecting duct. It is the last nephron segment made of tight epithelia with transepithelial resistance of > 100 Ω•cm2 (58, 59). While the tight junction function in the distal convoluted tubule is not well studied, the paracellular pathway in the connecting tubule and the collecting duct has become increasing clear as an important route for Cl− reabsorption, in addition to the well-established transcellular pathway made of the Cl−/HCO3− exchanger - pendrin. The paracellular Cl− pathway is essential to maintain electrical coupling with the electrogenic Na+ reabsorption that takes place via the epithelial sodium channel on the luminal membrane (69) (Figure 4C). The claudins making the paracellular Cl− pathway were found by Hou and colleagues to include claudin-4 and -8 (43), both of which were predominantly localized in the connecting tubule and the collecting duct (47) (Figure 2). Claudin-4 knockout in mouse kidneys caused significant increases in urinary NaCl excretion; the animals developed hypochloremia and low blood pressure, consistent with systemic loss of extracellular fluid volume (30). The claudin-8 knockout mice phenocopied the claudin-4 knockout mice in many ways including renal loss of salt and volume but developed more severe hypotension (29). The phenotypic similarity between claudin-4 and claudin-8 null animals could derive from the molecular interaction between these two molecules (43). Fujita and colleagues showed that deletion of claudin-4 in mouse kidneys impaired the TJ localization of claudin-8 (17). Gong and colleagues have demonstrated that loss of claudin-8 in mice can render claudin-4 delocalization from the tight junction of the collecting duct cell (29). Claudin-7 is also expressed in the aldosterone sensitive distal nephron. Genetic ablation of claudin-7 made experimental mice develop severe renal salt wasting phenotypes and hypovolemia, which eventually led to acute renal failure (82). Nevertheless, it is difficult to conclude that claudin-7 directly contributes to the paracellular Cl− pathway, because both overexpression and knockdown of its expression in LLC-PK1 cells paradoxically reduced Cl− permeability (1, 39).
Mineralocorticoids such as aldosterone can regulate the paracellualr pathway in the collecting duct. For example, aldosterone reduces paracellular Na+ permeability in the inner medullar collecting duct, suggesting the need to limit the paracellular backleak of Na+ in face of salt and volume losses (68). In the cortical collecting duct, on the other hand, aldosterone rapidly (< 1hr) increased the Cl− permeability of the paracellular pathway through claudin-4 hyperphosphorylation, in line with its primary role to couple with the electrogenic Na+ reabsorption (53). The electrical coupling itself can be regulated by a proteinase known as Cap1 (channel activating proteinase 1). Cap1 was first discovered as a stimulator of the epithelial sodium channel from a functional screening assay (84). Recently, Gong and colleagues have shown that Cap1 is also able to regulate the trans-interaction of claudin-4 and the paracellular Cl− permeability in the collecting duct cells (30). WNK4 and its pseudohypoaldosteronism type II (PHA-II) causing mutations have been found to augment the paracellular Cl− permeability in the collecting duct, presumably through hyperphosphorylation of claudin-4 or -7 (46, 93). These studies would suggest a role of the paracellular pathway in PHA-II pathogenic mechanisms. Consistent with such a concept, claudin-8 has been shown to be a direct substrate of the KLHL3 ubiquitinase, another causal gene of PHA-II (29). The dominant PHA-II mutation in KLHL3 impaired the claudin-8 binding, ubiquitination, and degradation (29).
Perspective
Structural basis of claudin interaction
The crystal structure of claudin-15 monomer has provided critical information of how the extracellular loop domains are folded to expose the electrostatic interaction sites and create potential ionic permeation pores (79). A major unresolved question is how claudin monomers are polymerized to form a high-order structure of the tight junction strand. Several prevailing models all point to the antiparallel arrangement owing to claudin cis-interactions (27, 80). Nevertheless, no real experimental evidence could provide meaningful structural determination of such a high-order structure. Recent development in the cryo-EM technique has suggested an alternative approach to address this important question. Cryo-EM is in fact better suited to resolve high molecular weight complexes such as virions (76) or spliceosomes (94).
Single-channel conductance of paracellular channel
Due to the leaky currents through the cell-cell boundaries, the traditional patch clamp technique may become less suitable to isolate the tight junction specific conductance. The concept of “ion scanning” turns out to be ideal for recording the paracellular conductance (98). While the ion scanning techniques have several major limitations including low signal gain and contaminating transcellular currents, an important improvement has been proposed by Zhou and colleagues to utilize two coordinated patch clamps to neutralize the apical currents during the paracellular conductance scanning (97). Such an approach may eventually resolve the technical difficulty of tight junction recording.
Claudin expression at single cell level
Milatz’s seminal discovery of the mosaic expression pattern of claudin-10 and claudin-16 in the thick ascending limb (55) has triggered important new thinking of how tight junction may be regulated through combinatorial claudin expression. Such cellular heterogeneity of claudin expression may exist in other epithelia or endothelia and have important physiologic functions. Modern technique of single-cell RNA sequencing will allow addressing this knowledge deficiency of claudin expression mosaics. It will be also important to study how such gene expression mosaics is regulated on cell and organ levels and what role the mosaics may play in physiology and pathology.
Claudin and cell junction alteration
The role of glomerular claudins, particularly in podocytes, has become increasingly important. Gong and colleagues first demonstrated that transgenic introduction of claudin-1 to the mouse podocytes, a claudin normally absent from mature podocytes, can induce cell junction alteration, i.e. slit diaphragm to tight junction transition (28). This study also attests to the concept that single claudin molecule is sufficient to trigger the ultrastructural changes in the cell junction involving hundreds of proteins. The physiologic and pathologic significance of such ultrastructural transition will be a major new research direction.
Acknowledgments
This work is supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases - RO1DK084059 and Department of Defense - HDTRA1-11-16-BRCWMDBAA.
Reference
- 1.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. Journal of cell science. 2005;118:2683–2693. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 2.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. Journal of the American Society of Nephrology : JASN. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochimica et biophysica acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Human molecular genetics. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 5.Benzing T. Signaling at the slit diaphragm. Journal of the American Society of Nephrology : JASN. 2004;15:1382–1391. doi: 10.1097/01.asn.0000130167.30769.55. [DOI] [PubMed] [Google Scholar]
- 6.Berger K, Schulte K, Boor P, Kuppe C, van Kuppevelt TH, Floege J, Smeets B, Moeller MJ. The regenerative potential of parietal epithelial cells in adult mice. Journal of the American Society of Nephrology : JASN. 2014;25:693–705. doi: 10.1681/ASN.2013050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Muller D. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14241–14246. doi: 10.1073/pnas.1203834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burg MB, Green N. Function of the thick ascending limb of Henle's loop. The American journal of physiology. 1973;224:659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- 9.Burg MB, Orloff J. Electrical potential difference across proximal convoluted tubules. The American journal of physiology. 1970;219:1714–1716. doi: 10.1152/ajplegacy.1970.219.6.1714. [DOI] [PubMed] [Google Scholar]
- 10.Caulfield JP, Reid JJ, Farquhar MG. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Laboratory investigation a journal of technical methods and pathology. 1976;34:43–59. [PubMed] [Google Scholar]
- 11.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. American journal of physiology Cell physiology. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 12.Done S, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, Tryggvason K. Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney international. 2008;73:697–704. doi: 10.1038/sj.ki.5002707. [DOI] [PubMed] [Google Scholar]
- 13.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? Journal of cell science. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 14.Eng DG, Sunseri MW, Kaverina NV, Roeder SS, Pippin JW, Shankland SJ. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney international. 2015;88:999–1012. doi: 10.1038/ki.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farquhar MG, Palade GE. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. The Journal of experimental medicine. 1961;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farquhar MG, Palade GE. Junctional complexes in various epithelia. The Journal of cell biology. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita H, Hamazaki Y, Noda Y, Oshima M, Minato N. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PloS one. 2012;7:e52272. doi: 10.1371/journal.pone.0052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG. Slit diaphragms contain tight junction proteins. Journal of the American Society of Nephrology : JASN. 2009;20:1491–1503. doi: 10.1681/ASN.2008101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and-2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. The Journal of cell biology. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. The Journal of cell biology. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. The Journal of cell biology. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or-2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. The Journal of cell biology. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Y, Himmerkus N, Plain A, Bleich M, Hou J. Epigenetic regulation of microRNAs controlling CLDN14 expression as a mechanism for renal calcium handling. Journal of the American Society of Nephrology : JASN. 2015;26:663–676. doi: 10.1681/ASN.2014020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong Y, Hou J. Claudin-14 Underlies Ca++-Sensing Receptor-Mediated Ca++ Metabolism via NFAT-microRNA-Based Mechanisms. Journal of the American Society of Nephrology : JASN. 2014;25:745–760. doi: 10.1681/ASN.2013050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca(+)(+) transport in response to CaSR signalling via a novel microRNA pathway. Embo j. 2012;31:1999–2012. doi: 10.1038/emboj.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Y, Renigunta V, Zhou Y, Sunq A, Wang J, Yang J, Renigunta A, Baker LA, Hou J. Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Molecular biology of the cell. 2015 doi: 10.1091/mbc.E15-06-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong Y, Sunq A, Roth RA, Hou J. Inducible Expression of Claudin-1 in Glomerular Podocytes Generates Aberrant Tight Junctions and Proteinuria through Slit Diaphragm Destabilization. Journal of the American Society of Nephrology : JASN. 2016 doi: 10.1681/ASN.2015121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:4340–4345. doi: 10.1073/pnas.1421441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Y, Yu M, Yang J, Gonzales E, Perez R, Hou M, Tripathi P, Hering-Smith KS, Hamm LL, Hou J. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3766–E3774. doi: 10.1073/pnas.1406741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. The Journal of cell biology. 1970;45:272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nature reviews Nephrology. 2013;9:587–598. doi: 10.1038/nrneph.2013.169. [DOI] [PubMed] [Google Scholar]
- 33.Greger R. Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Archiv : European journal of physiology. 1981;390:30–37. doi: 10.1007/BF00582707. [DOI] [PubMed] [Google Scholar]
- 34.Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985;65:760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nature medicine. 2013;19:1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. The American journal of physiology. 1981;241:F412–F431. doi: 10.1152/ajprenal.1981.241.4.F412. [DOI] [PubMed] [Google Scholar]
- 37.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. The American journal of physiology. 1981;241:F432–F442. doi: 10.1152/ajprenal.1981.241.4.F432. [DOI] [PubMed] [Google Scholar]
- 38.Hou J. A connected tale of claudins from the renal duct to the sensory system. Tissue barriers. 2013;1:e24968. doi: 10.4161/tisb.24968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. The Journal of biological chemistry. 2006;281:36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- 40.Hou J, Rajagopal M, Yu AS. Claudins and the kidney. Annual review of physiology. 2013;75:479–501. doi: 10.1146/annurev-physiol-030212-183705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. The Journal of clinical investigation. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. The Journal of biological chemistry. 2007;282:17114–17122. doi: 10.1074/jbc.M700632200. [DOI] [PubMed] [Google Scholar]
- 45.Itoh M, Nakadate K, Horibata Y, Matsusaka T, Xu J, Hunziker W, Sugimoto H. The structural and functional organization of the podocyte filtration slits is regulated by Tjp1/ZO-1. PloS one. 2014;9:e106621. doi: 10.1371/journal.pone.0106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, Lifton RP. Paracellular Cl- permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14877–14882. doi: 10.1073/pnas.0406172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 48.Kokko JP, Burg MB, Orloff J. Characteristics of NaCl and water transport in the renal proximal tubule. The Journal of clinical investigation. 1971;50:69–76. doi: 10.1172/JCI106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. American journal of human genetics. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovbasnjuk O, Leader JP, Weinstein AM, Spring KR. Water does not flow across the tight junctions of MDCK cell epithelium. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6526–6530. doi: 10.1073/pnas.95.11.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreidberg JA. Podocyte differentiation and glomerulogenesis. Journal of the American Society of Nephrology : JASN. 2003;14:806–814. doi: 10.1097/01.asn.0000054887.42550.14. [DOI] [PubMed] [Google Scholar]
- 52.Krug SM, Gunzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cellular and molecular life sciences : CMLS. 2012;69:2765–2778. doi: 10.1007/s00018-012-0949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Moellic C, Boulkroun S, Gonzalez-Nunez D, Dublineau I, Cluzeaud F, Fay M, Blot-Chabaud M, Farman N. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. American journal of physiology Cell physiology. 2005;289:C1513–C1521. doi: 10.1152/ajpcell.00314.2005. [DOI] [PubMed] [Google Scholar]
- 54.Matter K, Balda MS. Signalling to and from tight junctions. Nature reviews Molecular cell biology. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 55.Milatz S, Himmerkus N, Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Muller D, Fromm M, Bleich M, Gunzel D. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg++ transport. PNAS. 2016 doi: 10.1073/pnas.1611684114. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mundel P, Shankland SJ. Podocyte biology and response to injury. Journal of the American Society of Nephrology : JASN. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 57.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8011–8016. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Neil RG, Boulpaep EL. Ionic conductive properties and electrophysiology of the rabbit cortical collecting tubule. The American journal of physiology. 1982;243:F81–F95. doi: 10.1152/ajprenal.1982.243.1.F81. [DOI] [PubMed] [Google Scholar]
- 59.O'Neil RG, Sansom SC. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. The Journal of membrane biology. 1984;82:281–295. doi: 10.1007/BF01871637. [DOI] [PubMed] [Google Scholar]
- 60.Ohse T, Chang AM, Pippin JW, Jarad G, Hudkins KL, Alpers CE, Miner JH, Shankland SJ. A new function for parietal epithelial cells: a second glomerular barrier. American journal of physiology Renal physiology. 2009;297:F1566–F1574. doi: 10.1152/ajprenal.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohse T, Pippin JW, Chang AM, Krofft RD, Miner JH, Vaughan MR, Shankland SJ. The enigmatic parietal epithelial cell is finally getting noticed: a review. Kidney international. 2009;76:1225–1238. doi: 10.1038/ki.2009.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiological reviews. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 63.Pei L, Solis G, Nguyen MT, Kamat N, Magenheimer L, Zhuo M, Li J, Curry J, McDonough AA, Fields TA, Welch WJ, Yu AS. Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. The Journal of clinical investigation. 2016;126:2509–2518. doi: 10.1172/JCI83942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pollak MR, Quaggin SE, Hoenig MP, Dworkin LD. The glomerulus: the sphere of influence. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:1461–1469. doi: 10.2215/CJN.09400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reeves W, Caulfield JP, Farquhar MG. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Laboratory investigation a journal of technical methods and pathology. 1978;39:90–100. [PubMed] [Google Scholar]
- 66.Reyes JL, Lamas M, Martin D, del Carmen Namorado M, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L. The renal segmental distribution of claudins changes with development. Kidney international. 2002;62:476–487. doi: 10.1046/j.1523-1755.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- 67.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. Journal of cell science. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 68.Sands JM, Nonoguchi H, Knepper MA. Hormone effects on NaCl permeability of rat inner medullary collecting duct. The American journal of physiology. 1988;255:F421–F428. doi: 10.1152/ajprenal.1988.255.3.F421. [DOI] [PubMed] [Google Scholar]
- 69.Sansom SC, Weinman EJ, O'Neil RG. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. The American journal of physiology. 1984;247:F291–F302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- 70.Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. The Journal of cell biology. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scott RP, Quaggin SE. Review series: The cell biology of renal filtration. The Journal of cell biology. 2015;209:199–210. doi: 10.1083/jcb.201410017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shankland SJ, Smeets B, Pippin JW, Moeller MJ. The emergence of the glomerular parietal epithelial cell. Nature reviews Nephrology. 2014;10:158–173. doi: 10.1038/nrneph.2014.1. [DOI] [PubMed] [Google Scholar]
- 74.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annual review of cell and developmental biology. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 75.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science (New York, NY) 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 76.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. The 3.8 A resolution cryo-EM structure of Zika virus. Science (New York, NY) 2016;352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Grone HJ, Floege J, Moeller MJ. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. Journal of the American Society of Nephrology : JASN. 2011;22:1262–1274. doi: 10.1681/ASN.2010090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. Journal of the American Society of Nephrology : JASN. 2009;20:2604–2615. doi: 10.1681/ASN.2009010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, Dohmae N, Tsukita S, Nureki O, Fujiyoshi Y. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science (New York, NY) 2014;344:304–307. doi: 10.1126/science.1248571. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki H, Tani K, Tamura A, Tsukita S, Fujiyoshi Y. Model for the architecture of claudin-based paracellular ion channels through tight junctions. Journal of molecular biology. 2015;427:291–297. doi: 10.1016/j.jmb.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 81.Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophys J. 2003;84:1660–1673. doi: 10.1016/S0006-3495(03)74975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tatum R, Zhang Y, Salleng K, Lu Z, Lin JJ, Lu Q, Jeansonne BG, Ding L, Chen YH. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. American journal of physiology Renal physiology. 2010;298:F24–F34. doi: 10.1152/ajprenal.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d'Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nature genetics. 2009;41:926–930. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 84.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 85.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. The Journal of clinical investigation. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. American journal of physiology Renal physiology. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 87.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. Journal of cell science. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 88.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. American journal of physiology Renal physiology. 2006;291:F1288–F1299. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 89.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB. Unraveling the role of podocyte turnover in glomerular aging and injury. Journal of the American Society of Nephrology : JASN. 2014;25:707–716. doi: 10.1681/ASN.2013050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Webber WA, Blackbourn J. The permeability of the parietal layer of Bowman's capsule. Laboratory investigation a journal of technical methods and pathology. 1971;25:367–373. [PubMed] [Google Scholar]
- 91.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Molecular and cellular biology. 2004;24:8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Gunzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Muller D. Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. American journal of physiology Renal physiology. 2010;298:F1152–F1161. doi: 10.1152/ajprenal.00499.2009. [DOI] [PubMed] [Google Scholar]
- 93.Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, Suda S, Hayama A, Sasaki S, Uchida S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4690–4694. doi: 10.1073/pnas.0306924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan C, Hang J, Wan R, Huang M, Wong CC, Shi Y. Structure of a yeast spliceosome at 3.6-angstrom resolution. Science (New York, NY) 2015;349:1182–1191. doi: 10.1126/science.aac7629. [DOI] [PubMed] [Google Scholar]
- 95.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. The Journal of biological chemistry. 2003;278:17350–17359. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 97.Zhou L, Zeng Y, Baker LA, Hou J. A proposed route to independent measurements of tight junction conductance at discrete cell junctions. Tissue barriers. 2015;3:e1105907. doi: 10.1080/21688370.2015.1105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Y, Chen CC, Weber AE, Zhou L, Baker LA, Hou J. Potentiometric-scanning ion conductance microscopy for measurement at tight junctions. Tissue barriers. 2013;1:e25585. doi: 10.4161/tisb.25585. [DOI] [PMC free article] [PubMed] [Google Scholar]