Abstract
BACKGROUND
Mucopolysaccharidoses (MPS) are a group of inborn errors of metabolism that are progressive and usually result in irreversible in skeletal, visceral and/or brain damage, highlighting a need for early diagnosis.
METHODS
This pilot study analyzed 2,862 dried blood spots (DBS) from newborns and 14 DBS from newborn patients with MPS (MPS I, n = 7; MPS II, n = 2; MPS III, n = 5). Disaccharides were produced from polymer GAGs by digestion with chondroitinase B, heparitinase, and keratanase II. Heparan sulfate (0S, NS), dermatan sulfate (DS) and mono- and di-sulfated KS were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS). Median absolute deviation (MAD) was used to determine cutoffs to distinguish patients from controls. Cutoffs were defined as median + 7× MAD from general newborns.
RESULTS
The cutoffs were as follows: HS-0S> 90ng/mL; HS-NS> 23 ng/mL, DS> 88 ng/mL; mono-sulfated KS> 445 ng/mL; di-sulfated KS> 89 ng/mL and ratio di-KS in total KS> 32%. All MPS I and II samples were above the cutoffs for HS-0S, HS-NS and DS, and all MPS III samples were above cutoffs for HS-0S and HS-NS. The rate of false positives for MPS I and II was 0.03% based on a combination of HS-0S, HS-NS, and DS, and for MPS III was 0.9% based upon a combination of HS-0S and HS-NS.
CONCLUSIONS
Combination of levels of two or more different GAGs improves separation of MPS patients from unaffected controls, indicating that GAG measurements are potentially valuable biomarkers for newborn screening for MPS.
Keywords: newborn screening, mucopolysaccharidoses, tandem mass spectrometry, glycosaminoglycans, disaccharides
Introduction
Mucopolysaccharidoses (MPS) are inherited progressive and heterogeneous disorders caused by a deficiency of specific lysosomal enzymes responsible for the degradation of glycosaminoglycans (GAGs): chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), and hyaluronan. The MPS are classified according to the deficient enzyme as well as the undegraded GAGs and comprise 11 distinct subtypes, with an estimated combined incidence of 1 in 25,000 live births (Neufeld and Muenzer 2001).
Accumulation of undegraded GAGs leads to progressive tissue damage in multiple organs causing disease-specific manifestations (e.g. coarse facial features, skeletal dysplasia, hepatosplenomegaly, corneal clouding, joint rigidity or laxity, cardiac and respiratory complications, and neurological impairment).
Although clinical manifestations usually do not appear at birth, accumulation of GAGs can be detected in the human fetus (MPS I, II, III, IVA) and placenta (MPS II, VI), (Beck et al., 1992; Martin et al., 1983; Baldo et al., 2011) indicating that the disease process starts and can be detected prior to appearance of clinical signs and symptoms. Several MPS-subtypes have specific enzyme replacement therapy available, and there are many indications that MPS patients treated at an early age do better than those treated later in life (Auclair et al., 2003; McGill et al., 2010; Gabrielli et al., 2010; Schulze-Frenking et al., 2011; Baldo et al., 2013; Poe et al., 2014; Muenzer J, 2014; Tomatsu et al., 2016). Newborn screening programs for MPSs using dried blood spots (DBS) were proposed in 2001 and high-throughput technologies such as tandem mass spectrometry (MS/MS) are now under investigation (Meikle et al., 2006; Gelb et al., 2006; Wolfe et al., 2011; de Ruijter et al., 2012; Spacil et al., 2013; Scott et al., 2013; Lin et al., 2013; Tomatsu et al., 2013; Tomatsu et al., 2014; Shimada et al., 2014a; Liao et al., 2014; Gucciardi et al., 2014; Ruijter et al., 2014; Gelb et al., 2015; Hopkins et al., 2015). Methods currently under development include assays for activity of deficient enzymes (Gelb et al., 2015; Hopkins et al., 2015), measurement of accumulated GAGs (Oguma et al., 2007; Tomatsu et al., 2013; Lawrence et al., 2012, 2014).
In the present study, we analyzed 2,862 de-identified DBS from unselected newborns and 14 DBS from newborns known to have MPS I, II, or III.
2. Material and methods
2.1 Materials
Chondroitinase B, heparitinase, keratanase II, chondrosine (internal standard-IS), and the unsaturated disaccharides: heparan ΔDi-0S [2-acetamido-2-deoxy-4-O-(4- deoxy-α- L-threo-hex- 4-enopyranosyluronic acid)-D-glucose] (HS-0S), heparan ΔDi-NS [2-deoxy-2-sulfamino 4-O-(4-deoxy-α-L-threo-hex-4-enopyranosyluronic acid)-D-glucose] (HS-NS), chondro Δ Di-4S [2-acetamido-2-deoxy 3-O-(β-D-gluco-4-enepyranosyluronic acid)-4-O-D-sulfo-galactose] (Di-4S), mono-sulfated KS [Galβ1-4GlcNAc(6S)], and di-sulfated KS [Gal(6S) Galβ1-4GlcNAc(6S) were all provided by Seikagaku Co (Tokyo, Japan). Stock solutions of HS-0S (100 μg/mL), HS-NS (100 μg/mL), Di-4S (250 μg/mL), mono- and di-sulfated KS (1,000 μg/mL), and IS (5 μg/mL) were prepared in ddH20 (Millipore Milli-Q Reference A+ System). Standard curves were prepared using dilutions of these stocks as follows: HS-0S, HS-NS, Di-4S, and mono- and di-sulfated KS (78.13, 156.25, 312.50, 625, 1,250, 2,500, 5,000, and 10,000 ng/mL). Standards were digested with chondroitinase B, heparitinase, and keratanase II. Acetonitrile optima® (A996-4), ammonium hydroxide ammoniaque optima® (A470-500), ammonium acetate optima ® LC/MS (A114-50), bovine serum albumin (BP1600-100), Corning Costar® Assay plate 96 well ref 3797 (07-200-105), Thermo Scientific Hypercarb™ Porous Graphitic Carbon LC Columns (35005-052130), Thermo Scientific™ Uniguard™ Direct-connection guard cartridge holder (85200), Hypercarb 5 μm 10 × 2.1mm drop-in guards (35005-012101), Thermal adhesive sealing film (08-408-240), and Tris Base (BP152-500) were purchased from Thermo Fisher Scientific (Ottawa, Ontario). Acroprep™ advance 96 filter plates 10 K Omega (PN 8034) were purchased from Pall Co (Ann Arbor, MI).
2.2 Samples
DBS samples of 2,862 newborns were collected during routine newborn screening by Shimane University, Osaka City University, and Nagasaki University after informed consent was obtained. The samples were collected between 3 and 7 days after birth. Fourteen de-identified newborn DBS samples with MPS (MPS I: 7; MPS II: 2; MPS IIIA:3, MPS IIIB: 1, MPS IIIC: 1) were provided by the University of Amsterdam (The Netherlands) and St. Mary’s Hospital (UK). All samples were shipped to Nemours/AIDHC and stored at −20 °C until the GAG assay was conducted. This study was approved by IRBs at local institutes and Nemours/AIDHC.
2.3 Sample preparation
Two Disks (3.3mm) were cut from DBS samples using a DBS puncher (PerkinElmer®; Waltham, MA) and placed into a 96 well omega 10K filter plate with 100 μL of 0.1% BSA on a 96 well receiver plate. Samples were incubated for 15 min and then centrifuged for 15 min at 2500 g. The filter plate was transferred to a new receiver plate and a cocktail mixture of: 90 μL of 50 mM Tris HCL (pH 7.0), 10 μL of 5 μg/mL IS, 10 μL of 0.5 mU chondroitinase B (in 1% BSA), 1 mU heparitinase (in 1% BSA), 10 μL of 1 mU keratanase II (in 1% BSA) was added to each DBS. Samples were incubated at 37 °C water bath overnight. The next day, the samples were centrifuged for 15 min at 2,500 g. The filter plate was discarded and receiver plates stored at −20 °C until injection of samples into the LC-MS/MS.
2.4 LC-MS/MS
The apparatus consisted of a 1290 Infinity LC system with a 6460 triple quad mass spectrometer (Agilent Technologies, Palo Alto, CA). Disaccharides were separated on a Hypercarb column (2.0 mm i.d. 50 mm length; 5 μm particles; Thermo Scientific, USA), thermostated at 60 °C. The method was modified from that developed by Oguma et al. (2007). The mobile phase was a gradient elution of 5 mM ammonium acetate, pH 11.0 (solution A) to 5 mM ammonium acetate in 90% acetonitrile (solution B). The flow rate was 0.7 mL/min, and the gradient was as follows: 0 min 100% solution A, 1 min 70% solution A, 2 min 70% solution A, 2.20 min 0% solution A, 2.60 min 0% solution A, 2.61 min 100% solution A, 5 min 100% solution A. The mass spectrometer was operated with electrospray ionization in the negative ion mode (Agilent Jet Stream technology) with drying gas temperature 350 °C, drying gas flow 11 L/min, nebulizer pressure 58 psi, sheath gas temperature 400 °C, sheath gas flow 11 L/min, capillary voltage 4,000 V, nozzle voltage 2,000 V. Specific precursor and product ions, m/z, were used to quantify each disaccharide respectively (IS, 354.3, 193.1; DS, 378.3, 175.1; mono-sulfated KS, 462, 97; di-sulfated KS 542, 462; HS-NS 416, 138; HS-0S 378.3, 175.1) (Oguma et al., 2007; Zhang et al., 2005; Saad et al., 2005; Wei et al., 2011). DS was measured as Di-0S after digestion of Di-4S by a 4S-sulfatase present in the preparation of chondroitinase B. The injection volume was 5 μL with a running time of 5 min per sample. Ratio di-sulfated KS in total KS was calculated as di-sulfated KS divided by (mono-sulfated KS + di-sulfated KS) × 100%. The lower limit of quantitation (LLOQ) was defined as the lowest level of the signal with an accuracy of better than 20% and the lower limit of detection (LOD) as a signal to noise ratio of <10 according to the Food and Drug Administration (FDA-2001).
2.5 Statistical analysis
Sensitivity, specificity, false positive, false negative and median absolute deviation (MAD) were performed using R software (R Core team, 2014). As most of the disaccharides were below the LLOQ (59% for ΔDiHS-0S, 94% for ΔDiHS-NS, 98% for mono-sulfated KS, and 99.8% for di-sulfated KS) in the control samples, raw calculated values were used for statistical analysis GAG levels in the blood can be elevated for reasons other than MPS, resulting in a non-normal distribution and consequently the standard methodology using mean ± standard deviation to define cutoff values is not appropriate for this study. The median absolute deviation (MAD) is a robust method of central tendency, which is not sensitive to outliers (Leys et al., 2013). Cutoffs were defined as median + 7× MAD of newborn control samples. False positive samples were defined as controls that were above the cutoff values, and false negatives were defined as MPS newborn samples that were below the cutoffs. Coefficient of variation (CV) was determined at three different concentrations for the HS-0S standard (std8: 1,000 ng/mL, std5: 125 ng/mL and std3: 31.25 ng/mL). Three separate preparations of each dilution were measured 5 times, and CV calculated as the standard deviation divided by mean × 100. It is estimated that each 3.3 mm dried blood spot disc corresponds to 3.6 μL of blood (Blanchard et al., 2008), and that concentrations of all the GAGs analyzed in this study were expressed as ng/mL of blood.
3. Results
3.1 Coefficient of variation (CV), lower limit of quantitation (LLOQ), and lower limit of detection (LOD)
Imprecision was calculated by replicate analysis of three different concentrations (std 8, std 5 and std 3, respectively) of HS-0S, HS-NS and DS standard (Sup. 1). LLOQs and LOD were defined for HS-0S, HS-NS and DS (Sup. 1).
3.2 Median absolute deviation (MAD)
In order to calculate median and MAD for levels of these disaccharides in control samples, it was necessary to use calculated concentrations of these samples even though the accuracy of measurement will be low. MAD values for the control newborn samples were 10 ng/mL for HS-0S, 2 ng/mL for HS-NS, 10 ng/mL for DS, 47 ng/mL for mono-sulfated KS, 10 ng/mL for di-sulfated KS, and 2 % for ratio di-sulfated KS in total KS. To assure no false negatives, cutoffs were determined as values higher than median + 7× MAD (all patients were above cutoff for all GAGs). 7 × MAD is equivalent to more than 4 standard deviations for normally distributed data) (table 1).
Table 1.
Values for HS-0S, HS-NS, DS, mono-sulfated KS, di-sulfated KS, and ratio di-KS/total KS% for 14 newborn MPS (I, II, III) patients
| DS (ng/mL) |
HS-NS (ng/mL) |
HS-0S (ng/mL) |
Mono-KS (ng/mL) |
Di-KS (ng/mL) |
ratio Di-KS (%) |
|
|---|---|---|---|---|---|---|
| I-1 | 209# | 61# | 337# | 97 | 24 | 35 |
| I-2 | 160# | 31# | 195# | 171 | 38 | 29 |
| I-3 | 276# | 68# | 374# | 213 | 63 | 41 |
| I-4 | 170# | 76# | 431# | 258 | 65 | 34 |
| I-5 | 99# | 40# | 336# | 220 | 87 | 42 |
| I-6 | 150# | 50# | 259# | 242 | 21 | 22 |
| I-7 | 226# | 35# | 216# | 54 | 27 | 58 |
| II-1 | 91# | 42# | 240# | 196 | 82 | 40 |
| II-2 | 453# | 34# | 266# | 65 | 12 | 16 |
| III-1a | 31 | 57# | 397# | 115 | 24 | 31 |
| III-2a | 11 | 45# | 212# | 62 | 37 | 32 |
| III-3a | 56 | 30# | 145# | 71 | 37 | 26 |
| III-4c | 15 | 35# | 187# | 116 | 22 | 17 |
| III-5b | 58 | 72# | 464# | 66 | 26 | 49 |
Samples above the cutoffs
MPS IIIA
MPS IIIB
MPS IIIC
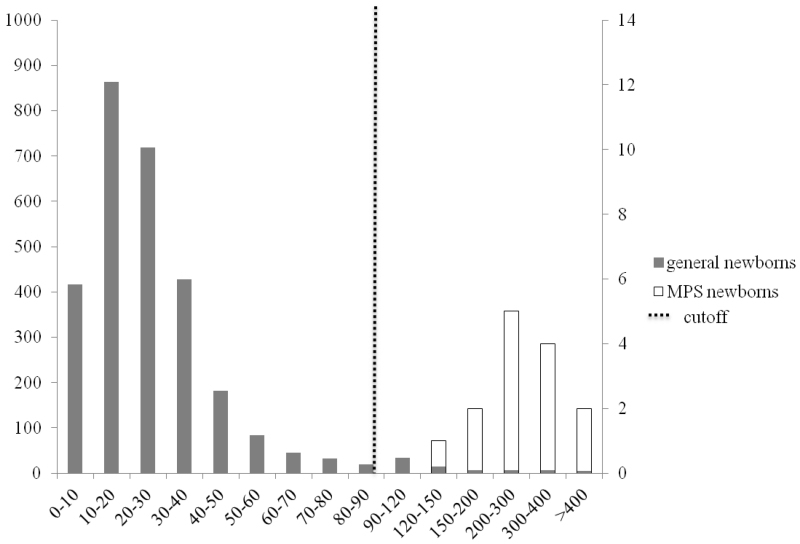
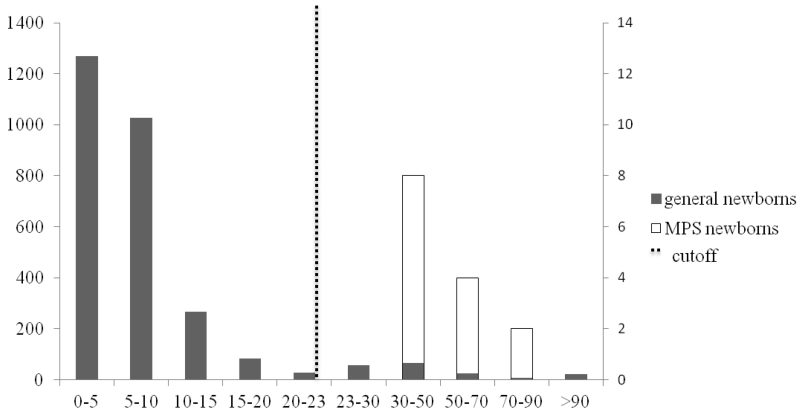
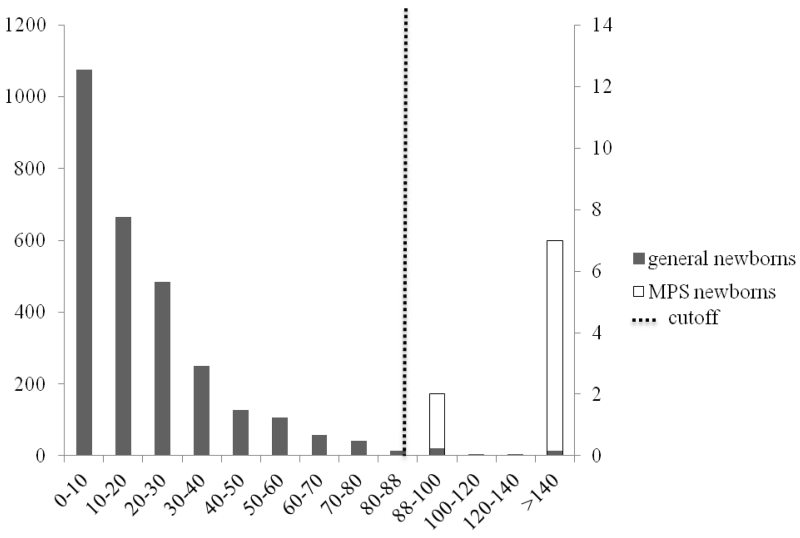
Cutoffs were >90 ng/mL for HS-0S, and > 23 ng/mL for HS-NS, >88 ng/mL for DS, >445 ng/mL for mono-sulfated KS, > 89 ng/mL for di-sulfated KS and >32% for di-sulfated KS in total KS. Values for MPS newborn samples ranged from 145 to 463 ng/mL for HS-0S, 30 to 76 ng/mL for HS-NS, 54 to 257 ng/mL for mono-sulfated-KS, 12 to 87 ng/mL for di-sulfated KS, and 16 to 58% for ratio di-sulfated KS in total KS. DS values for newborn samples with MPS I and II were between 91 and 453 ng/mL. All 9 MPS I and II newborn patients had a significant elevation of DS while none of 5 MPS III patients showed elevation of DS, consistent with the catabolic pathway of DS.
3.3 Sensitivity and specificity
Sensitivity was 100% for HS-0S, HS-NS, and DS (0% false negative rate). MPS I, II and III do not affect KS levels, so the significance of cutoffs for KS cannot be determined. Specificity was 97% for HS-0S, 94% for HS-NS, 98% for DS. Thus, the positive predictive value (PPV) for HS-0S was 16%, 7% for HS-NS and 18% for DS. The negative predictive value (NPV) was 97% for HS-0S, 94% for HS-NS, and 98% for DS (Sup. 2).
HS-0S (p = 2.25576E-07), HS-NS (p = 4.15E-07), DS (p = 0.002), and ratio di-KS in total KS (p = 0.0001) were significantly higher in DBS from this cohort of MPS patients, compared to control DBS.
2.5% of the controls samples were above the cutoffs (median + 7×MAD) for HS-0S (fig. 1), 6.2% for HS-NS (fig. 2), 1.5% for DS (fig. 3), 0.3% mono-sulfated KS, 1.6% for di-sulfated KS, and 2.3% for ratio di-sulfated KS in total KS. However, after combination of elevated levels for HS-0S, HS-NS, and DS, the false positive rates decreased to 0.03% for MPS I and II and 0.9% with combination of HS-0S and HS-NS for MPS III (table 2). There were no false negative samples in the patient population.
Figure 1.
HS-0S in general newborns and MPS newborns
Primary left y-axis represents solid bars for the number of general newborns (n=2862) among the different intervals; secondary right y-axis represents open bars for the number of MPS newborns (n=14) among the different intervals; dashed line represents the cutoff (median + 7× MAD for control newborns).
Figure 2.
HS-NS in general newborns and MPS newborns
Primary left y-axis represents solid bars for the number of general newborns (n=2862) among the different intervals; secondary right y-axis represents open bars for the number of MPS newborns (n=14) among the different intervals; dashed line represents the cutoff (median + 7× MAD for control newborns).
Figure 3.
DS in general newborns and MPS newborns
Primary left y-axis represents solid bars for the number of general newborns (n=2862) among the different intervals; secondary right y-axis represents open bars for the number of MPS newborns (n=14) among the different intervals; dashed line represents the cutoff (median + 7× MAD for control newborns).
Table 2.
Number of samples above the cutoffs (median + 7× MAD)
| control samples (n=2862) | MPS patients (n=14) | |
|---|---|---|
| > cutoff HS-0S and HS-NS | 26 | 14 |
| > cutoff HS-0S and DS | 6 | 9* |
| > cutoff HS-NS and DS | 8 | 9* |
| > cutoff HS-0S, HS-NS and DS | 1 | 9* |
14 MPS patients (I-7; II-2; III-5)
MPS III patients not included
4. Discussion
A major advantage of developing measurement of GAGs as a newborn screen for MPS is that levels of one or more GAGs should be elevated in any MPS, making a single initial screen more sensitive and cost-effective for these rare disorders. A potential limitation is that GAGs can be elevated due to other conditions unrelated to MPS giving false positives [e.g.: Mucolipidosis (Tomatsu et al., 2005); diabetes (Komosinska-Vassev et al., 2005); arthritis (Mankin et al., 1971); cancer (Hennessey et al., 1981)], as seen in the general NBS samples in this study. However, MPS I and II cause an elevation in three GAGs and MPS III causes an elevation in two GAGs, reducing the number of false positives. For this study we were able to obtain newborn DBS from 14 MPS I, II, or III patients to compare GAG levels in these samples with the ones observed in 2,862 DBS from a random control sample using a cutoff of median + 7× MAD of the control sample. We were able to distinguish all of the patient samples from 99.1% of the controls (26 out of 2,862 samples) by measuring levels of HS-0S in combination with HS-NS and could distinguish the MPS I and II patients from 99.97% (1 out of 2,862 samples) when levels of DS, HS-NS and HS-0S were combined. Considering that the combined incidence of MPS is approximately 1:20,000, it is unlikely that positive samples in the control sample set are due to MPS. The control samples were all de-identified so we were not able to determine the cause of elevated GAGs in these samples. None of the newborn MPS samples had levels of mono-sulfated KS or di-sulfated KS above the cutoffs, but eight patient samples (57%) showed elevated levels of ratio di-sulfated KS in total KS. The ratio of di-sulfated KS in total KS was significantly higher in MPS newborn patients compared to general newborns (p = 0.0001) (table 1). We have previously shown a secondary elevation of total KS and increase in ratio of di-sulfated KS in blood from older patients with other MPS types, where 40% for MPS II patients and 54% of MPS IVA had significantly higher levels of ratio di-sulfated KS in total KS (Shimada et al., 2015; Tomatsu et al., 2005; Tomatsu et al., 2014). We did not see a secondary elevation of KS in the MPS II newborn samples, possibly due to low accumulation during early stages of bone development. KS levels are age-dependent with expected accumulation during growth and development of the skeleton. MPS IV samples would be expected to show elevated KS due to impaired GALNS metabolism, but MPS IVA newborn samples were not available to test this hypothesis.
The standard deviation for the control samples is abnormally high due to a pronounced skew of the data towards higher levels, most likely due to elevated GAG levels in some newborns due to other unidentified conditions. For a population with a normal distribution, the number of false positives at mean + 2SD would be 5%, and mean and median values would be the same. Using the MAD, we were able to limit the effects of the high outliers.
To ensure that the screen includes the majority of the patients, we based cutoffs on values from known patient samples. Availability of newborn patient samples is limited, preventing detailed statistical analysis of patient data. For MPS I and II, by measuring three GAGs (HS-0S, HS-NS, and DS) we were able to distinguish all 9 patients from all but one of the 2,862 controls. We did not determine whether this “control” had MPS I or II and for the purposes of this study we considered this to be a false positive. This false positive rate of 0.03% is much lower than those for the single newborn screens for the LSDs of Pompe, Fabry, Gaucher, and MPS I that give false positive rates of 0.17, 0.4, 023 and 0.8 percent respectively (Hopkins et al., 2015).
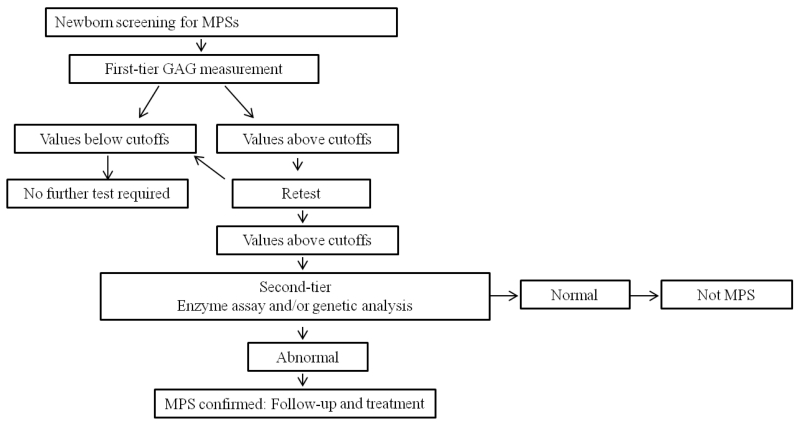
When MPS I, II and III were included the false positive rate increased to 0.9%. Chace et al (2010) and Turgeon et al (2010) suggest that a first-tier newborn screen could be performed with less selective methodologies if they give few false negatives and limited false positives that could then be followed by a more selective second-tier screen (fig. 4) (Chace et al., 2010; Turgeon et al., 2010). A better understanding of the cause of high levels of GAGs in the “control” population might help eliminate some false positive, but a first screen that eliminates 99% of the unaffected samples would be particularly valuable in reducing the costs of more expensive enzyme or genetic tests to define the specific metabolic defect.
Figure 4.
Algorithm for two-tier newborn screening
We have previously shown that blood from patients with other forms of MPS (IV, VI, and VII) contain elevated levels of other GAGs, but we do not have access to newborn DBS from such patients at this time and cannot directly determine GAG levels expected in newborn DBS from all MPS types (Tomatsu et al., 2005; Rowan et al., 2013; Shimada et al., 2014b; Tomatsu et al., 2014; Shimada et al., 2015). Nevertheless, based on data from older patients, this newborn screen is likely to capture patients with a severe form of any MPS.
Prenatal lysosomal GAG storage has been demonstrated in MPS patients and animal models. Initial clinical signs and symptoms in newborn patients with MPSs include sacral dimple, gibbus, and abnormal shape of vertebrae in X-ray images (Ohashi et al., 2009). In human, fetuses aged 18–30 weeks gestation (MPS I, II, III, and IVA) have storage vacuoles in major organs (Martin et al., 1983; Beck et al., 1992). Newborn mice with MPS I, II, IVA, or VII, have storage vacuoles as well (Tomatsu et al., 2003; Vogler et al., 1990). Skeletal abnormalities represent the earliest clinical observations in MPS VII mice. Histological analysis of the growth plate, articular cartilage and cortical bone showed early pathology and progressive bone lesion (Tomatsu et al., 2015). It is noteworthy that all 14 MPS newborns have an elevation of GAGs consistent with the previous findings, (Shimada et al., 2014a; Tomatsu et al., 2013; Tomatsu et al., 2010; de Ruijter et al., 2012) demonstrating that accumulation of GAGs has already started before birth and that therapy should start at a newborn stage to prevent irreversible damage especially in bone and brain.
A limitation of this study is the low number of DBS from newborn MPS patients. Very few samples are available because MPS is not usually identified at birth and consequently DBS are not usually available. Thus while we were able to establish stringent cut-off values to distinguish all patient samples in this study from controls, we are not able to predict false negative rates for larger screening purposes. Another limitation is that the running time of the current LC-MS/MS (4 to 5 minutes per sample) is still a challenge for application to mass screening, compared to high-throughput MS/MS (10 to 12 seconds per sample) (Shimada et al., 2014). Although the main drawback of HT-MS/MS is that disaccharides with identical molecular weights cannot be distinguished, this may not be crucial for a method that is proposed as a first-tier screen to identify a high-risk group that may have an MPS. Further feasibility studies are required to compare LC-MS/MS and HT-MS/MS.
In conclusion, the combination of elevated HS-0S, HS-NS and DS seems to be a good biomarker for newborn screening of MPS I and II and HS-0S and HS-NS for MPS III. Elevated levels of GAGs will be valuable as a first-tier screen to identify a high-risk group that may have an MPS, and can be confirmed in a second-tier screen using a specific enzyme or genetic assays.
Supplementary Material
Take-home message.
Measurement of levels of multiple glycosaminoglycans in DBS from newborns improves discrimination of MPS patients from unaffected controls, enhancing the value of GAG analysis as a newborn screen for MPS.
Acknowledgements
This work was supported by grants from Japanese MPS Society, the Austrian MPS Society, The Bennett Foundation, and International Morquio Organization (Carol Ann Foundation). R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant numbers P20GM103464 and P30GM114736. S.T. and A.M were supported by National Institutes of Health grant 1R01HD065767. F.K. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, from Brazil (CNPq). The content of the article has not been influenced by the sponsors. We also appreciate individual patients with MPS, who participated in projects of “Newborn screening and biomarkers for Mucopolysaccharidoses.”
Footnotes
Conflict of interest:
Francyne Kubaski declares that she has no conflict of interest.
Robert W. Mason declares that he has no conflict of interest.
Akiko Nakatomi declares that he has no conflict of interest.
Haruo Shintaku declares that he has no conflict of interest.
Li Xie declares that she has no conflict of interest.
Naomi N. van Vlies declares that she has no conflict of interest.
Heather Church declares that she has no conflict of interest.
Roberto Giugliani declares that he has no conflict of interest.
Hironori Kobayashi declares that he has no conflict of interest.
Seiji Yamaguchi declares that he has no conflict of interest.
Yasuyuki Suzuki declares that he has no conflict of interest.
Tadao Orii declares that he has no conflict of interest.
Toshiyuki Fukao declares that he has no conflict of interest.
Adriana M. Montaño declares that she has no conflict of interest.
Shunji Tomatsu declares that he has no conflict of interest.
Compliance with Ethics Guidelines
Informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). The study was approved by Nemours IRB (protocol 281495). Informed consent was obtained from all patients for being included in the study.
Authorship contribution
Francyne Kubaski has contributed to the concept and planning of the project, collection of data, data analysis, and reporting of the work described.
Robert W. Mason has contributed to the concept and planning of the project, collection of data, analysis of data, and reporting of the work described.
Akiko Nakatomi has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Haruo Shintaku has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Li Xie has contributed to the data analysis and reporting of the work described.
Naomi N. van Vlies has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Heather Church has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Roberto Giugliani has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Hironori Kobayashi has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Seiji Yamaguchi has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Yasuyuki Suzuki has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Tadao Orii has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Toshiyuki Fukao has contributed to the concept and planning of the project, collection of samples and reporting of the work described.
Adriana M. Montaño has contributed to the planning, data analysis, and reporting of the work described.
Shunji Tomatsu is a Principal Investigator for this project and has contributed to the concept and planning of the project, analysis of data, and reporting of the work described.
References
- Auclair D, Hopwood JJ, Brooks DA, et al. Replacement therapy in Mucopolysaccharidosis type VI: advantages of early onset of therapy. MGM. 2003;78:163–74. doi: 10.1016/s1096-7192(03)00007-6. [DOI] [PubMed] [Google Scholar]
- Baldo G, Matte U, Artigalas O, et al. Placenta analysis of prenatally diagnosed patients reveals early GAG storage in mucopolysaccharidoses II and VI. MGM. 2011;103:197–8. doi: 10.1016/j.ymgme.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Baldo G, Mayer FQ, Martinelli BZ, et al. Enzyme replacement therapy started at birth improves outcome in difficult-to-treat organs in mucopolysaccharidosis I mice. MGM. 2013;109:33–40. doi: 10.1016/j.ymgme.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Beck M, Braun S, Coerdt W, et al. Fetal presentation of Morquio disease type A. Prenat Diagn. 1992;12:1019–9. doi: 10.1002/pd.1970121207. [DOI] [PubMed] [Google Scholar]
- Blanchard S, Sadilek M, Scott CR, et al. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin Chem. 2008;54:2067–70. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chace DH, Hannon WH. Impact of second-tier testing on the effectiveness of newborn screening. Clin Chem. 2010;56:1653–5. doi: 10.1373/clinchem.2010.153494. [DOI] [PubMed] [Google Scholar]
- de Ruijter J, Minke HR, Wagemans T, et al. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses type I, II and III. MGM. 2012;107:705–10. doi: 10.1016/j.ymgme.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Gabrielli O, Clarke LA, Bruni S, et al. Enzyme-replacement therapy in a 5-month-old boy with attenuated presymptomatic MPS I: 5-year follow-up. Pediatrics. 2010;125:183–7. doi: 10.1542/peds.2009-1728. [DOI] [PubMed] [Google Scholar]
- Gelb MH, Turecek F, Scott CR, et al. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. JIMD. 2006;29:397–404. doi: 10.1007/s10545-006-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb MH, Scott CR, Turecek F. Newborn screening for lysosomal storage diseases. Clin Chem. 2015;61:335–46. doi: 10.1373/clinchem.2014.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gucciardi A, Legini E, Di Gangi IM, et al. A column-switching HPLC-MS/MS method for mucopolysaccharidosis type I analysis in a multiplex assay for the simultaneous newborn screening of six lysosomal storage disorders. Biomed Chromatogr. 2014;28:1131–9. doi: 10.1002/bmc.3133. [DOI] [PubMed] [Google Scholar]
- Hennessey PT, Hurst RE, Hemstreet GP, et al. Urinary glycosaminoglycan excretion as a biochemical marker in patients with bladder carcinoma. Cancer Res. 1981;41:3868–73. [PubMed] [Google Scholar]
- Hopkins PV, Campbell C, Klug T, et al. Lysosomal storage disorder screening implementation: findings from the first six months of full population pilot testing in Missouri. J Pediatr. 2015;166:172–7. doi: 10.1016/j.jpeds.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Komosinska-Vassev K, Olczyk K, Kozma EM, et al. Alterations of glycosaminoglycan metabolism in the development of diabetic complications in relation to metabolic control. Clin. Chem. Lab. Med. 2005;43:924–9. doi: 10.1515/CCLM.2005.158. [DOI] [PubMed] [Google Scholar]
- Lawrence R, Brown JR, Al-Mafraji K, et al. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat. Chem. Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R, Brown JR, Lorey F, et al. Glycan-based biomarkers for mucopolysaccharidoses. Mol. Genet. Metab. 2014;111:73–83. doi: 10.1016/j.ymgme.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys C, Ley C, Klein O, et al. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J Exp Soc Psychol. 2013;49:764–766. [Google Scholar]
- Liao HC, Chiang CC, Niu DM, et al. Detecting multiple lysosomal storage diseases by tandem mass spectrometry- a national newborn screening program in Taiwan. Clin Chem Acta. 2014;431:80–6. doi: 10.1016/j.cca.2014.01.030. [DOI] [PubMed] [Google Scholar]
- Lin SP, Lin HY, Wang TJ, et al. A pilot newborn screening program for Mucopolysaccharidosis type I in Taiwan. Orphanet J Rate Dis. 2013;22:1–8. doi: 10.1186/1750-1172-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin HJ, Lippiello L, et al. The glycosaminoglycans of normal and arthritic cartilage. J. Clin. Invest. 1971;50:1712–19. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JJ, Ceuterick C. Prenatal pathology in mucopolysaccharidoses: a comparison with postnatal cases. Clin Neuropathol. 1983;2:122–7. [PubMed] [Google Scholar]
- McGill JJ, Inwood AC, Coman DJ, et al. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age- a sibling control study. Clin Genet. 2010;77:492–8. doi: 10.1111/j.1399-0004.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- Schulze-Frenking G, Jones SA, Roberts J, et al. Effects of enzyme replacement therapy on growth in patients with mucopolysaccharidosis type II. JIMD. 2011;34:203–8. doi: 10.1007/s10545-010-9215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle PJ, Grasby DJ, Dean CJ, et al. Newborn screening for lysosomal storage disorders. MGM. 2006;88:307–14. doi: 10.1016/j.ymgme.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Muenzer J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. MGM. 2014;111:63–72. doi: 10.1016/j.ymgme.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Neufeld E, Muenzer J. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th Ed. McGraw-Hill; New York: 2001. pp. 3421–52. [Google Scholar]
- Oguma T, Tomatsu S, Montaño AM, et al. Analytical method for determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal Biochem. 2007;368:79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Ohashi A, Montaño AM, Colón JE, et al. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr. 2009;98:768–9. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- Poe MD, Chagnon SL, Escolar ML. Early treatment is associated with improved cognition in Hurler syndrome. Ann Neurol. 2014;76:1–24. doi: 10.1002/ana.24246. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. URL http://www.R-project.org/ [Google Scholar]
- Rowan DJ, Tomatsu S, Grubb JH, et al. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIA, IVA and VII. JIMD. 2013;36:235–46. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter GJ, Goudriaan DA, Boer AM, et al. Newborn screening for hunter disease: a small-scale feasibility study. JIMD Re. 2014;14:23–7. doi: 10.1007/8904_2013_279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad OM, Ebel H, Uchimura K, et al. Compositional profiling of heparin/heparan sulfate using mass spectrometry: Assay for specificity of a novel extracellular human endosulfatase. Glycobiology. 2005;15:818–826. doi: 10.1093/glycob/cwi064. [DOI] [PubMed] [Google Scholar]
- Scott CR, Elliott S, Buroker N, et al. Identification of infants at risk for developing Fabry, Pompe, or Mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr. 2013;163:498–503. doi: 10.1016/j.jpeds.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Kelly J, LaMarr WA, et al. Novel heparan sulfate assay by using automated high-throughput mass spectrometry: application to monitoring and screening for mucopolysaccharidoses. MGM. 2014a;113:92–9. doi: 10.1016/j.ymgme.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Tomatsu S, Yasuda E, et al. Chondroitin 6-sulfate as a novel biomarker for mucopolysaccharidosis type I, IIIA, IVA and VII. JIMD Rep. 2014b;16:15–24. doi: 10.1007/8904_2014_311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Tomatsu S, Mason RW, et al. Di-sulfated KS as a novel biomarker for mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2015;21:1–13. doi: 10.1007/8904_2014_330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacil Z, Tatipaka H, Barcenas M, et al. High-Throughput assay of 9 lysosomal enzymes for newborn screening. Clin Chem. 2013;59:502–11. doi: 10.1373/clinchem.2012.189936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Orii KO, Vogler C, et al. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns−/−) produced by targeted disruption of the gene defective in Morquio A Disease. Hum Mol Genet. 2003;12:3349–58. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Okamura K, Maeda H, et al. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. JIMD. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oguma T, et al. Validation of keratan sulfate level in Mucopolysaccharidosis IVA by liquid tandem mass spectrometry method. JIMD. 2010;33:35–42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Fujii T, Fukushi M, et al. Newborn screening and diagnosis of mucopolysaccharidoses. MGM. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Shimada T, Mason RW, et al. Establishment of Glycosaminoglycan assays for Mucopolysaccharidoses. Metabolites. 2014;4:655–79. doi: 10.3390/metabo4030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Almeciga-Diaz CJ, Montano AM, et al. Therapies for the bone in mucopolysaccharidoses. MGM. 2015;114:94–109. doi: 10.1016/j.ymgme.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Azario I, Sawamoto K, et al. Neonatal cellular and gene therapies for mucopolysaccharidoses: the earlier the better? JIMD. 2016;39:189–202. doi: 10.1007/s10545-015-9900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon CT, Magera MJ, Cuthbert CD, et al. Determination of total homocysteine, methylmalonic acid, and 2-methylcitric acid in dried blood spots by tandem mass spectrometry. Clin Chem. 2010;56:1686–5. doi: 10.1373/clinchem.2010.148957. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM) Guidance for Industry. Bioanalytical method validation. 2001 Available on: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070107.pdf.
- Vogler C, Birkenmeier EH, Sly WS, et al. A murine model of mucopolysaccharidosis VII. Gross and microscopic findings in beta-glucuronidase-deficient mice. Am J Pathol. 1990;136:207–17. [PMC free article] [PubMed] [Google Scholar]
- Wei W, Ninñonuevo MR, Sharma A, et al. A comprehensive compositional analysis of heparin/heparan sulfate-derived disaccharides from human serum. Anal chem. 2011;83:3703–3708. doi: 10.1021/ac2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BJ, Blanchard S, Sadilek M, et al. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis II (Hunter syndrome) Anal Chem. 2011;83:1152–6. doi: 10.1021/ac102777s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Conrad AH, Tasheva ES, et al. Detection and quantification of sulfated disaccharides from keratin sulfate and chondroitin/dermatan sulfate during chick corneal development by ESI-MS/MS. Invest Ophthalmol Vis Sci. 2005;46:1604–14. doi: 10.1167/iovs.04-1453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.