Abstract
The density of NK cells in tumors correlates positively with prognosis in many types of cancers. The average number of infiltrating NK cells is, however, quite modest (approximately 30 NK cells/sq.mm), even in tumors deemed to have a “high” density of infiltrating NK cells. It is unclear how such low numbers of tumor-infiltrating NK cells can influence outcome. Here, we used ovalbumin-expressing tumor cell lines and TCR transgenic, OVA-specific cytotoxic T lymphocytes (OT-I-CTLs) to determine whether the simultaneous attack by anti-tumor CTLs and IL-2-activated NK (A-NK) cells synergistically increases the overall tumor cell kill and whether upregulation of tumor MHC class-I by NK cell-derived interferon-gamma (IFNγ) improves tumor-recognition and kill by anti-tumor CTLs.
At equal E:T ratios, A-NK cells killed OVA-expressing tumor cells better than OT-I-CTLs. The cytotoxicity against OVA-expressing tumor cells increased by combining OT-I-CTLs and A-NK cells, but the increase was additive rather than synergistic. A-NK cells adenovirally-transduced to produce IL-12 (A-NKIL-12) produced high amounts of IFNγ. The addition of a low number of A-NKIL-12 cells to OT-I-CTLs resulted in a synergistic, albeit modest, increase in overall cytotoxicity. Pre-treatment of tumor cells with NK cell-conditioned medium increased tumor MHC expression and sensitivity to CTL-mediated killing. Pre-treatment of CTLs with NK cell-conditioned medium had no effect on CTL cytotoxicity. In vivo, MHC class-I expression by OVA-expressing B16 melanoma lung metastases increased significantly within 24–48 hours after adoptive transfer of A-NKIL-12 cells. OT-I-CTLs and A-NKIL-12 cells localized selectively and equally well into OVA-expressing B16 lung metastases and treatment of mice bearing 7-days-oldOVA-B16 lung metastases with both A-NKIL-12 cells and OT-I-CTLs lead to a significant prolongation of survival.
Thus, an important function of tumor-infiltrating NK cells may be to increase tumor cell expression of MHC class-I through secretion of IFNγ, to prepare them for recognition by tumor-specific CTLs.
Keywords: Cytotoxic T lymphocytes (CTL), Natural killer cells (NK cells), Cancer Immunotherapy, Adoptive cell transfer, Cytotoxicity, Cell traffic
1.0 Introduction
It was the ability of natural killer (NK) cells to lyse tumor cells within minutes after first contact that led to their discovery in 1975 [1, 2]. Since then, the importance of NK cell-mediated cytotoxicity in the setting of cancer and infection, has been extensively investigated [3–8]. The relatively low density of infiltrating NK cells found in most solid tumors has made it difficult to accept NK cell cytotoxicity as an effector function of major importance for immune-mediated control of established malignancies. In contrast, many studies have clearly demonstrated that NK cells play an important role in the defense against hematogeneous metastasis via their ability to eliminate circulating tumor cells as they navigate through the capillaries of the lungs [9–15] and possibly other organs as well [16]. Lately, an increasing number of studies have shown that NK cells play a major role in immune-regulation, bridging the gap between innate and adaptive immune responses [17–20]. This includes cross-talk, mediated by cytokines as well as membrane-bound receptors, with dendritic cells (DCs) in the periphery, leading to DC activation and maturation [21–27]. NK/DC cross-talk can also occur in lymph nodes, where NK cells play an important role in the Th1/Th2 biasing of the mature immune response [20].
Interestingly, the density of NK cells in tumor tissue has been found to correlate positively with prognosis. This was first shown in colorectal cancer by Coca et al. [28] and has later been found to also be in case in many other types of cancers as well, including gastric and hepatocellular carcinoma, adenocarcinoma of the lungs, renal cancer, and squamous cell cancers of the esophagus, lungs, and vulva [29–36]. It is, however, hard to envision that the NK cells’ cytotoxic activity alone is able to influence outcome, since even in tumor tissues found to have the “high” density of infiltrating NK cells associated with better prognosis, these high densities appear to be very modest, i.e., usually well below 100 cells per sq.mm when counted in 2–10 micron thick tissue sections. These low densities are not supportive of NK cell mediated lysis of tumor cells as the sole mechanism behind the positive correlation between NK density and prognosis. It is more likely that NK cells provide the initial source of antigens via their tumor cell killing and then establish cross-talk with nearby DCs, to support their maturation and production of IL-12 [25, 37], as these activities are critical in the development of a Th1-biased anti-tumor T cell response [38, 39].
Here, we present evidence that NK cells may help anti-tumor CTLs at the tumor sites by killing MHC-negative tumor cells and, more importantly, by preparing the tumor tissue for recognition by CTLs via IFNγ-induced upregulation of tumor cell MHC class-I expression.
2.0 Materials and Methods
2.1 Animals
Female C57BL/6 and B6.129S7-Rag1tm1Mom Tg(TcraTcrb)1100Mjb (OT-I) mice, 8–12 weeks of age, were obtained from Taconic Biosciences, Inc. Congenic B6.Pl–Thy-1aCy (Thy1.1) male and B6.SJL-Ptprca Pep3b/BoyJ (CD45.1) female mice, 8–12 weeks of age, were obtained from Jackson (Bar Harbor, ME, USA). The use of animals for the experiments described below was approved by the Institutional Animal Care and Use Committee, University of Pittsburgh.
2.2 Tumor Cell Lines
The subline F10.P1 of the B16 melanoma (C57BL/6 origin) was established in our laboratory from a B16-F10 lung metastasis. The chicken OVAlbumin-transduced M05 variant of the B16 melanoma cell line (expressing the SIINFEKL peptide in H-2Kb) was a kind gift from Dr. Louis Falo, University of Pittsburgh [40]. Lewis lung carcinoma (3LL) and Panc02 adenocarcinoma cells were purchased from The American Type Culture Collection (ATCC). The MC38 colon carcinoma was a gift from Dr. M. Shurin, University of Pittsburgh. MC38 and Panc02 tumor cells were transfected to produce OVA-expressing variants, MC38OVA and Panc02 OVA, respectively. All cell-lines were maintained in RPMI-1640 medium (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% heat inactivated fetal calf serum, 2 mM glutamine, 20 mM Hepes buffer, 0.8 g/l streptomycin and 1.6×105 U/l penicillin (from hereon referred to as complete medium, CM). Adherent cells were detached by exposure to 0.02% EDTA for 2–3 min and washed three times in RPMI-1640. Cell viability, judged by trypan blue dye exclusion test, was always >95%. Murine pulmonary metastases were established by tail vein injection of 0.2–0.4×106 cells in 0.3 ml of RPMI-1640 into C57BL/6 mice, pretreated on day −1 with 40 μl anti-asialoGM1 antiserum (Wako Pure Chemicals, Wako, TX, USA).
2.3 Preparation of A-NK cells
Spleens were removed aseptically from C57BL/6 and CD45.1 congenic B6.SJL-Ptprca Pep3b/BoyJ mice and a single-cell suspension was prepared in RPMI-1640. Erythrocytes were lysed by incubation with ammonium chloride–potassium buffer at room temperature for 3 min and the spleen cells were subsequently washed twice in RPMI-1640. CD3 and B220 positive cells were magnetically removed following incubation of the cell culture with rat anti-CD3 and rat-antiB220 antibody and subsequently with anti-rat coated magnetic beads (Dynal Biotech, Lake Success, NY, USA). The CD3/B220-depleted cells were resuspended in fresh CM containing 6,000 IU/ml rhIL-2 (kindly provided by Novartis Pharma AG, Basel, Switzerland) to a final concentration of 1×105 cells/ml and cultured in tissue culture flasks (Falcon, B&D, Franklin Lakes, NJ, USA) at 37°C in an atmosphere of 5% CO2. Fresh CM containing 6,000 IU/ml IL-2 was added every 2–3 days as needed. After 5–7 days of culture, non-adherent cells and adherent cells were harvested after a brief treatment with 0.02% EDTA and washed twice in RPMI-1640 before use. Routinely, on day 5 of culture, the A-NK cells were >95% CD45.1+ (or CD45.2+), >95% Thy1.2+, >95% asGM1+, >90% NK1.1+, >90% NKp46+ <2% CD8+, <2% CD4+.
2.4 Preparation of anti-tumor CTLs
To produce Thy1.1 and Thy1.2 double-positive anti-tumor CTLs specific for B16-M05 cells, splenocytes from F1[B6.Pl–Thy-1aCy x B6.129S7-Rag1tm1Mom Tg(TcraTcrb)] mice were prepared and, after red blood cell lysis, transferred to T150 plastic flasks at 2×105 cells/ml of CM supplemented with 100 IU/ml of rhIL-2 and 8 μg/ml of PHA-P (phytohemagglutinin-P; DIFCO, Detroit, MI, USA). After 24–48 hours aggregated splenocytes were isolated by light centrifugation and transferred to new culture flasks with fresh CM supplemented with 100 IU/ml of rhIL-2/ml. Fresh CM with IL-2 was added every 2–3 days as needed. After 5–7 days of culture, non-adherent cells and adherent cells were harvested after a brief treatment with 0.02% EDTA and washed twice in RPMI-1640 before use.
2.5 NK cell-conditioned medium (NKCM)
On day 5–6 of culture with IL-2, A-NK cells were harvested, adjusted to 1 million cells/ml in CM with 6,000 IU of IL-2 and cultured for 24 hours. After 24 h of culture, the supernatant was harvested and centrifuged at 3,000 rpm for 15 minutes to remove cells and debris. The supernatant was then passed through a 0.4 micron filter, aliquoted and stored at −80 C.
2.6 Cytotoxicity assays
Standard 51Cr-release assays were performed in 96-well, round-bottomed microtitre plates. Approximately 5 × 106 target cells were resuspended in 100 μl sodium chromate (Na51CrO4: DuPont, Germany) for 45 min at 37C. After labelling cells were washed three times in cold RPMI-1640. Effector cells were mixed with 1 × 104 target cells at various effector: target ratios. Plates were incubated at 37C, 5% CO2 atmosphere for 4 h. After centrifugation for 5 min at 600 g, 100 μl of supernatant from each well was removed and counted in a gamma counter. Spontaneous release was estimated by culturing target cells in medium alone. Maximum release was estimated by adding 100 μl of 10% Triton X-100 to target cells. All experiments were performed in triplicate, and the percentage of cytotoxicity was calculated as: % Specific lysis = (cpm of test − cpm spontaneous release)/(cpm maximum release − cpm spontaneous release)× 100.
2.7 Live-cell microscopy
OT-I-CTLs were incubated with Cell Tracker Orange (Invitrogen, cat.no. C2927) at 1 microM in RPMI1640 for 30 minutes. After wash, the CTLs were resuspended in complete medium with 100 IU IL-2/ml and added (3:1) to 8-well chamber slides (Ibidi, cat.no. 80826) with GFP+ B16-M05 tumor cells, which had been pre-exposed to A-NK cell-conditioned medium for 18–24 hours or with non-treated (control) GFP+ B16-M05 tumor cells. All NKCM and control medium was removed from the chamber wells before addition of the CTLs. Using an Olympus (IX81 Inverted) live cell microscope with a stage incubation chamber (Tokai Hit, INUH-IX3D-F1) kept at 37 C, 5% C02 and 100% humidity), the cell cultures were observed for 10–24 hours. DIC and green and red fluorescence images were acquired from multiple fields every 3.16 minutes. DIC and red-fluorescence images, as well as red and green fluorescence images were merged (ImageJ), time-stamped (hr:min:sec) and saved in video format.
2.8 Adenoviral vectors
The recombinant adenoviral vectors used in this study were obtained from Dr. Andrea Gambotto, (Preclinical Vector Core Facility, University of Pittsburgh). The cDNA encoding murine IL-12 or IL-18 was inserted into the E1 region and high titer recombinant adenoviruses were generated as described previously [41, 42]. For viral transduction, A-NK cells were collected (0.02% EDTA), washed with CM and resuspended in RPMI without serum at a concentration of 10×106 cells/ml (or 40×106 cells/ml for in vivo studies). Virus was added at an MOI of 25 and the cell suspensions were incubated at 37C for 90–120 min. During the incubation period the tubes were gently shaken every 15 min. The cell suspensions were then placed into culture flasks containing CM and 6,000 IU/ml IL-2 for A-NK and incubated for 24 hours at 37C. Before each assay and the in vivo tumor experiments, cells were harvested and washed three times in a large volume of CM to remove unincorporated virus.
2.9 Cytokine measurements
Adv-IL-12 and Adv-IL-18 transduced A-NK cells were cultured in 6,000 IU/ml rhIL-2 for 48 hours. Supernatant was collected and cytokine production was determined by the Luminex Core Laboratory within the Cancer Proteomics Facility (CPF), University of Pittsburgh Cancer Institute, using a Mouse Cytokine Magnetic 20-Plex Panel (LMC0006M, NOVEX, Life Technologies).
2.10 Adoptive transfer of A-NK cells and OT-I-CTLs
At 24 hours after transduction with Adv-IL-12 or Adv-mock vectors, A-NK cells were harvested and washed extensively. Groups of mice (n=4–6) with well-established, 7 days old B16-M05 lung metastases received one i.v. injection of either four million A-NK cells, three million OT-I-CTLs, or a mixture of four million A-NK cells plus three million OT-I-CTLs in 0.4 ml. In some groups, the transferred cells were supported by a single ip injection of 60,000 IU of IL-2 in complex with polyethylene glycol (PEG-IL-2 [43], a kind gift from the Chiron Corporation, Emeryville, CA).
2.11 Estimation of A-NK cell and OT-I-CTL infiltration of lung metastases
The intratumoral densities of adoptively transferred A-NK cells (Thy1.2+/CD45.1+) and OT-I-CTLs (Thy1.1+/Thy1.2+/CD45.2+) in the Thy1.2+/CD45.2+ recipients were determined as described previously [44]. Briefly, eight-micron thick sections of fresh frozen lung tissue were stained with PE-anti-Thy1.1 and FITC-anti-CD45.1 antibodies (Pharmingen), both at 1:200 dilution, to identify OT-1-CTLs and A-NK cells, respectively. For background control, sections were stained with PE- and FITC-conjugated IgG2b Ab controls. Fluorescence and DIC images of the tissue sections were obtained using a Nikon fluorescent microscope and analyzed for cell densities in lung tumors and the surrounding normal lung tissue using MetaMorph image analysis software.
2.12 Measurement of tumor MHC-I expression
B16-M05 lung tumors were established in Thy1.2+ C57BL/6 mice as described. Seven days later, three groups of mice (n=5) received i.v. injections of five million A-NKIL-12 cells, five million A-NKmock cells and saline, respectively. Immediately after the i.v. injection, all mice received a single i.p. injection of 60,000 IU of Peg-IL-2. Fourty hours later, lungs were removed and fresh frozen. Eight-micron thick sections were cut from ten randomly chosen areas of each set of lungs and stained with PE-anti-H-2Kb (553570, BD Pharmingen™). One or two tumors per section were identified by bright-field microscopy by blinded observers. The chosen tumors were outlined and DIC and fluorescence images were acquired using the same microscope and camera settings for all images (Nikon Eclipse 800 Microscope, QImaging Retiga 4000R digital camera). The average PE-fluorescence per pixel above background threshold level within the tumor outline was measured using ImageJ. The background threshold level was defined as the threshold which excluded 95% of all PE-fluorescence in tumors stained with PE-conjugated IgG 2a,κ isotype control antibody (553457, Pharmingen).,
2.13 Survival experiments
Mice were code numbered and monitored for survivability by two observers with no knowledge of the code. When reaching a premorbid condition, as defined by our IACUC, mice were euthanized and the lungs and other organs were removed and fresh frozen. In one pre-experiment, lungs were dried and weighed. The average dry weight of the lungs from Adv-mock and Adv-IL-12 transduced ANK groups were 0.189g±0.01g versus 0.163g±0.03g, respectively, ensuring that the mice, upon sacrifice, had reached roughly the same stage of tumor development.
2.14 Statistical analysis
Two-tailed, unpaired Student’s t-tests were performed with a 95% confidence interval. For survivability curves, data were plotted according to the Kaplan–Meier method with statistical significance determined using the log-rank test.
3.0 RESULTS
3.1 Cytotoxicity of CTLs, A-NK cells and mixtures of CTLs and A-NK cells
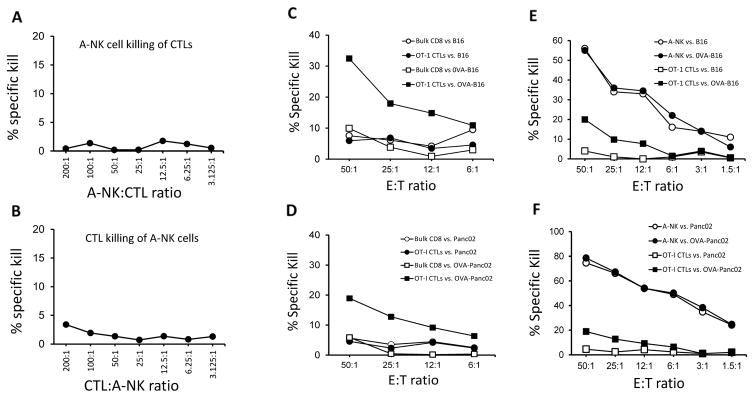
It has been suggested that NK cells, which accumulate early at sites of viral infection, rapidly become eliminated by virus-specific T cells that begin to arrive at the site a few days later [45]. Thus, to determine whether activated CTLs also kill highly activated A-NK cells, we performed standard 51-Cr-release assays over a large range of E:T ratios (from below 1:1 to 200:1) with CTLs and A-NK cells as either effectors or targets. We found no indication that any of these effector cell types lyse each other (Figure 1A–B).
Figure 1. Cytotoxicity against wt. and OVA-expressing tumor cells by A-NK cells and OVA-specific and non-specific CTLs.
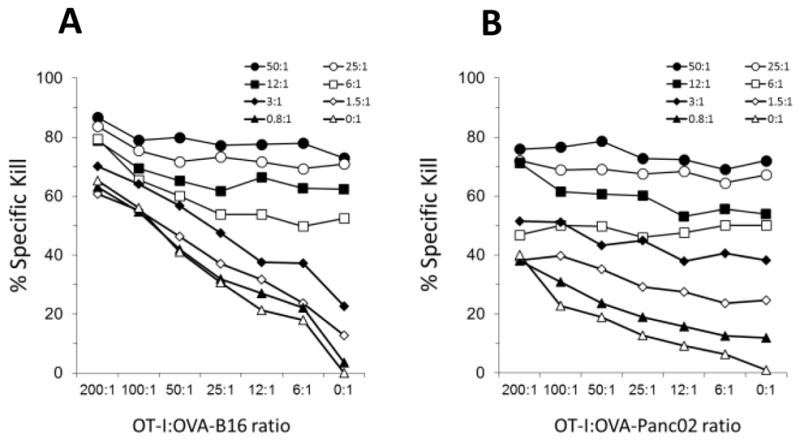
A–B. To determine if highly activated A-NK cells and CTLs kill each other, CTLs and A-NK cells were used as either effector cells or target cells in standard four hour 51-Cr-release assays. A: Percent Specific lysis of CTL by A-NK cells. B: Percent Specific lysis of A-NK cells by CTLs. C–F: Cytotoxicity of A-NK cells OVA-specific and non-specific CTLs was determined in standard four hour 51-Cr-release assays. C: Percent Specific lysis of wt. B16 and OVA-expressing B16-M05 tumor cells by OVA-specific (OT-I) CTLs and bulk-activated, non-specific CD8 cells. D: Percent Specific lysis of wt. and OVA-Panc02 tumor cells by OVA-specific (OT-I) CTLs and bulk-activated, non-specific CD8 cells. E: Percent Specific lysis of B16 and OVA-expressing B16-M05 tumor cells by OVA-specific (OT-I) CTLs and A-NK cells. F: Percent Specific lysis of wt. and OVA-Panc02 tumor cells by OVA-specific (OT-I) CTLs and NK cells. Representative of two (Panc02) and multiple (B16) experiments.
To determine if the co-presence of tumor-specific CTLs and A-NK cells would, either positively or negatively, influence the overall tumor cell lysis, we first compared the killing of OVA-expressing and control tumor cells by OVA-specific CTLs produced from OT-I animals (OT-1 CTLs). Killing was also tested with polyclonal bulk-activated CD8+ T cells produced by incubation of splenocytes from normal C57BL/6 animals with PHA and IL-2 for 5 days (referred to as T-LAK cells - [46]). As expected, only OT-I-CTLs were able to kill OVA-expressing tumor cells, whereas none of the activated T cells (OT-I and T-LAK) were able to kill any tumor cells not expressing ovalbumin (Figure 1C–F). In contrast, A-NK cells efficiently killed all tumor cells, irrespective of ovalbumin expression (Figure 1E–F). On a per cell basis, the A-NK cells killed considerably more tumor cells within 4 hours than the CTLs.
When tumor-specific CTLs and A-NK cells were mixed, the percent specific lysis was in many cases higher than the lysis achieved at the same ratio by any of the two effector cells types alone (Figure 2A–B). Negative interference between the two effector cell types was occasionally seen. An example is shown in Figure 2B, where the 51% specific lysis of OVA-Panc02 tumor cells by A-NK cells at E:T = 6:1 decreases rather than increases as the number of OT-I-CTL effector cells increases (the % specific lysis by the 200:6:1 mixture of OT-1 CTLs, A-NK cells and OVA-Panc02 tumor cells was only 47%). In accordance, isobolograms (not shown) revealed that the combined cytotoxicity of the tumor-specific, MHC-restricted CTLs and the MHC non-restricted A-NK cells in these short-term assays was not synergistic, but, for the most, additive.
Figure 2. Additive killing of B16-M05 and OVA-Panc02 tumor cells by OVA-specific (OT-I) CTLs and non-specific A-NK cells.
Cytotoxicity of mixtures of OVA-specific (OT-I) CTLs and A-NK cells was determined in standard four hour 51-Cr-release assays. CTLs and A-NK cells were mixed with tumor cells at ratios from 0:1 to 200:1 and 0:1 to 50:1, respectively. A: Percent specific lysis of B16-M05 tumor cells (representative of multiple experiments). B: Percent specific lysis of OVA-Panc02 tumor cells (representative of two experiments). Legend identifies A-NK cell:tumor cell ratios.
3.2 Cytokine-production by IL-12 and IL-18 stimulated A-NK cells
The ability of A-NK cells to kill MHC-negative tumor cells may make up for this void in the cytotoxic T cells’ anti-tumor capabilities. However, the A-NK cells’ ability to secrete high amounts of IFNγ and TNFα, both of which upregulate MHC-expression in both healthy, infected and malignant cells, may be the NK cells’ most important function during CTL-based immune responses. While IL-2 activation induces proliferation of A-NK cells and significantly increases their cytotoxicity, their ability to produce high amounts of IFNγ requires co-stimulation by either IL-12 (or IL-18 ) [47–55] We therefore incubated A-NK cells with mrIL-12 and mrIL-18 (data not shown) or adenovirally transduced A-NK cells with the genes for murine IL-12 and IL-18 and determined the ability of the transduced A-NK cells to produce IFNγ and other cytokines 48 hours later by multiplexed ELISA (Table 1).
Table 1.
Cytokine-production by A-NKmock, A-NKIL-12 and A-NKIL-18 cells.
| Cytokine (picogram/ml/10^6 cells/day) | |||||||
|---|---|---|---|---|---|---|---|
| MIP-1a | GM-CSF | RANTES | IFN-γ | IL-6 | IL-10 | TNF-α | |
| A-NKmock | 8,289 | 1,495 | 106 | 1,209 | 5 | 143 | 221 |
| A-NKIL-12 | 11,423* | 4,685* | 147 | 17,298* | 5 | 12,292** | 694* |
| A-NKIL-18 | >20,000*** | 9,306* | 738* | 14,062* | 133*** | 236 | 618* |
Significantly different from A-NKmock, p<0.01
Significantly different from A-NKIL-18, p<0.01
Significantly different from A-NKIL-12, p<0.01
As expected, both IL-12 and IL-18 substantially increased the A-NK cells’ secretion of IFNγ (by at least 10 fold). Both cytokines moderately increased secretion of TNFα. MIP-1α and GM-CSF production also increased, especially after IL-18 gene-transduction. While IL-12, but not IL-18 gene-transduction increased the NK cells’ secretion of IL-10 by nearly 100 fold, only IL-18 gene-transduction increased the A-NK cells’ secretion of IL-6 and RANTES. Non-transduced A-NK cells produced low amounts of IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-7, IL-9, IL-13, IL-15, IL-17, G-CSF, IP-10, MCP-1 and KC and the production of these cytokines did not change after IL-12 or IL-18 gene-transduction.
3.3 Regulation of tumor cells’ MHC-class-I expression by A-NK cell-produced cytokines
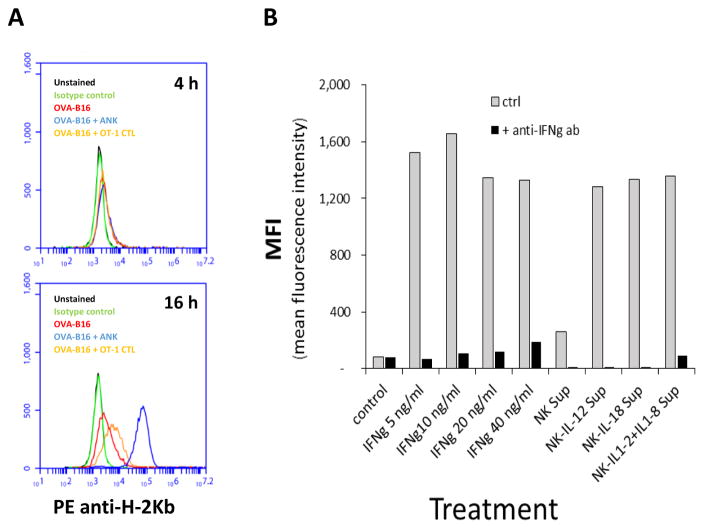
To determine if A-NK cells, via their secretion of IFNγ, are capable of upregulating expression of MHC class-I by tumor cells during a standard four hour 51-Cr release assays, B16-M05 cells that were still viable after four hours of incubation with A-NK cells and OT-I-CTLs were isolated and stained with anti-H-2Kb antibody. No change in MHC expression was observed. Similarly, when tumor cells were incubated with supernatants from A-NK cells or OT-I-CTL cultures, no changes were seen in MHC-expression within the initial 4–8 hours. However, after 12–16 hours of incubation, the tumor cells’ MHC-expression increased substantially (Figure 3A). Similar data was obtained using Panc02 and MC38 tumor cells (data not shown). When tumor cells were incubated with murine IFNγ, a substantial increase in MHC class-I expression was also seen after 12–16 hours (Figure 3B). When culture medium from IL-12 and IL-18 gene-transduced A-NK cells was used, MHC class-I expression reached 5-fold higher levels than those of tumor cells incubated in medium from non-transduced A-NK cells (Figure 3B). The MHC inducing capacity of all the A-NK cell conditioned media was efficiently abrogated by addition of blocking anti-IFNγ antibodies (Figure 3B).
Figure 3. Tumor cell expression of H-2Kb following incubation with IFNγ, CTLs, A-NK cells, or medium from CTL and A-NK cell cultures.
A: Tumor cell expression of H-2Kb after four and sixteen hours of co-culture with OVA-specific (OT-I) CTLs and A-NK cells. Expression of MHC class-I by B16-M05 tumor cells, which had been incubated with OVA-specific (OT-I) CTLs or A-NK cells for 4–16 hours in the presence (black columns) or absence (grey columns) of blocking antibodies to IFNγ was determined by flow cytometry. MHC class-1 was visualized by staining with PE-conjugated H-2Kb antibody. Black: non-stained cells; Green: isotype control; Red: B16-M05 cells; Blue: B16-M05 cells + A-NK cells (E:T=1:1); Orange: B16-M05 cells + CTLs (OT-I). Representative of two experiments. B: H-2Kb expression by B16-M05 tumor cells was measured by flow cytometry after 16 hours of incubation with IFNγ (5–40ng/ml) or conditioned medium from mock, IL-12, IL-18, and IL-12+IL-18 gene-transduced A-NK cells (collected at 72 hours after the transduction). All incubations were performed in the presence (black columns) or absence (grey columns) of blocking antibodies to IFNγ Values represent Mean Fluorescence Intensity (MFI) Representative of two (IFNγ) and three (NK-supernatants) experiments.
3.4 A-NK cell-produced IFNγ increases tumor cells’ sensitivity to CTL-mediated killing in vitro
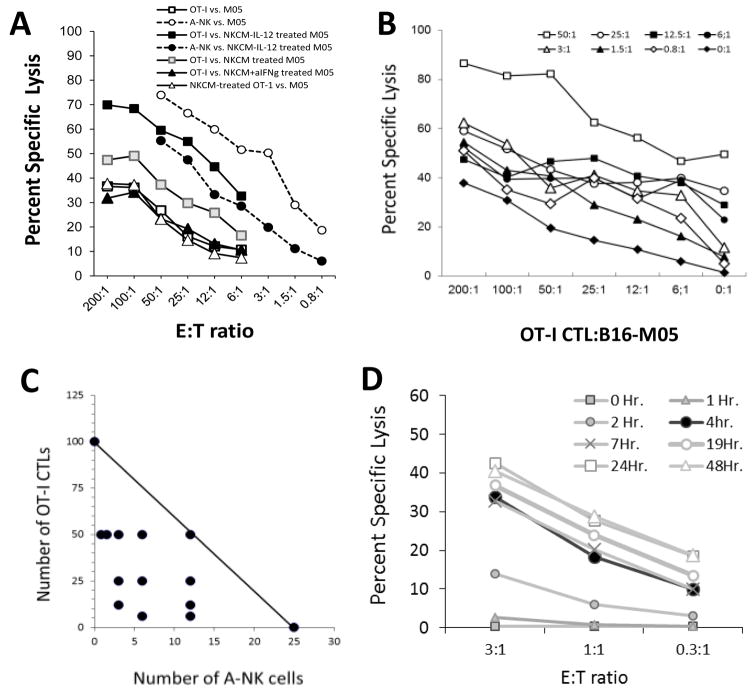
Pre-incubation of tumor cells in medium from A-NK cell cultures increased their sensitivity to lysis by tumor-specific CTLs (Figure 4A). The increase in CTL-mediated lysis was even more pronounced against tumor cells pre-incubated in medium from IL-12 (and IL-18 – data not shown) gene-transduced A-NK cells (Figure 4A). In contrast, lysis of tumor cells pretreated with A-NKIL-12-conditioned medium by A-NK cells decreased compared to control. These effects were totally abrogated by addition of anti-IFNγ antibodies to the NKCM (Figure 4A). Pretreatment of the CTLs with NKCM (or IFNγ, data not shown) did not change the CTLs’ ability to kill B16-M05 tumor cells (Figure 4A).
Figure 4. A-NK cell-produced IFNγ increased sensitivity of B16-M05 to killing by OVA-specific (OT-I) CTLs, but lowers their sensitivity to A-NK cell-mediated lysis.
B16-M05 cells were incubated in conditioned medium from A-NK cells and IL-12 gene-transduced A-NK cells for 16 hours, washed and then tested in four hour 51-Cr-release assays against A-NK cells and OT-I-CTLs. A: Percent specific lysis by OT-I-CTLs against B16-M05 cells pre-incubated in: control medium (white squares); NKCM from A-NK cells (grey squares); NKCM from NKIL-12 cells (black squares); NKCM from NKIL-12 cells + 0.1 microg/ml blocking anti-IFNγ Ab (black triangles). Percent specific lysis by OT-I-CTLs pre-incubated in NKCM from NKIL-12 cells against non-treated B16-M05 (white triangles). Percent specific lysis by A-NK cells against B16-M05 cells pre-incubated in: control medium (white circles); NKCM from A-NKIL-12 cells (black circles). Lysis of NKCM pretreated B16-M05 cells by OT-I-CTLs was significantly higher at all E:T ratios compared to non-treated B16-M05 (p<0.01). Lysis of NKCM-IL-12 pretreated B16-M05 cells by OT-I-CTLs was significantly higher at all E:T ratios compared to non-treated and NKCM-treated B16-M05 (p<0.01). Lysis of NKCM-IL-12+ anti-IFNγ pretreated B16-M05 cells by OT-I-CTLs was significantly lower at all E:T ratios compared to lysis of NKCM-IL-12 pretreated B16-M05 (p<0.005) and was not significantly better than OT-I-CTL-mediated lysis of non-treated B16-M05. At the E:T ratios tested, lysis of B16-M05 cells by NKCM pre-treated OT-I-CTLs was equal to or lower than lysis of B16-M05 cells by non-treated OT-I-CTLs. B: Cytolysis of B16-M05 tumor cells with heterogeneous expression of MHC class-1: B16-M05 cells were pre-incubated in control medium and in conditioned medium from A-NKIL-12 cells. After incubation, the tumor cells were mixed 1:1 and used as target cells for mixtures of CTLs and A-NK effector cells at E:T ratios from 0:1 to 200:1 and 0:1 to 50:1, respectively, as indicated by the legends. C: Isobolograms were constructed to determine if the concerted cytotoxicity by the CTLs and A-NK cells against the B16-M05 tumor cells was synergistic. The isobole at 30% lysis indicates the CTL:A-NK cells ratios between 100 CTLs:zero A-NK cells and Zero CTL:25 A-NK cells that should produce a 30% lysis of the tumor cells, if their combined cytolytic capacity is additive. Many A-NK cell:CTL mixtures with a total number of effector cells below the 30% isobole were able to kill 30% or more of the tumor cells (black circles), indicating that the combined cytolysis by these effector cell mixtures, was synergistically enhanced. D: Lysis of B16-M05 tumor cells by A-NK cells at 1h (grey squares), 2 h (grey triangles), 4 h (black circles), 7 h (grey x), 19 h (white circle), 24 h (white square), and 48 h (white triangle). Representative of two experiments.
To mimic the in vivo conditions, some B16-M05 cells were pre-incubated for 16 hours with supernatant from IL-12 gene-transduced A-NK cells and then mixed 1:1 with non-treated B16-M05 cells. OT-I-CTLs and A-NK cells were mixed in different ratios (as in Figure 2) and added to the tumor cells. After four hours, the specific lysis was calculated (Figure 4B). Interestingly, isobolograms now demonstrated synergy between the two cell types (Figure 4C).
By extending the cytotoxic assays to 24 hours, we observed that killing of tumor cells by A-NK cells reached its maximum after 4–8 hours (Figure 4D). Most of the tumor cells that were still alive at this time, were never killed by the A-NK cells. This rapid decline in A-NK cells cytotoxicity coincided with the observed increase in tumor cell expression of MHC induced at approximately 8 hours by IFNγ released by the A-NK cells (Figure 3A). In contrast, tumor cell killing by tumor specific CTLs commenced at a much slower pace, but steadily continued until most or all tumor cells were killed, which could take several days, especially at the lower E:T ratios.
3.5 Rapid lysis of NK cell-conditioned tumor cells by tumor-specific CTLs
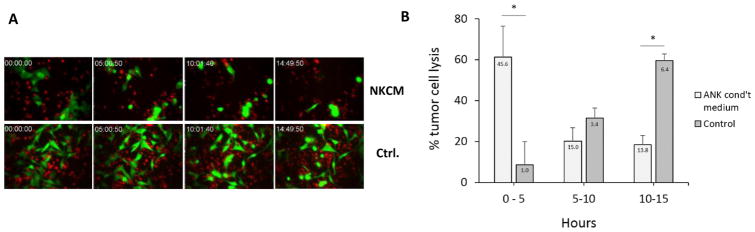
To determine the influence of pre-treatment with NKCM on the kinetics of tumor cell lysis by tumor-specific CTLs, CellTracker Orange-labelled OT-I-CTLs were mixed 3: 1 with NKCM-treated and non-treated GFP+ B16/M05 tumor cells and observed by live-cell microscopy for 15 hours (Figure 5 and Movie 1). While the OT-I-CTLs managed to kill about 75% of all NKCM pre-treated tumor cells within 15 hours of co-incubation, they killed only about 10% of the control, non-treated tumor cells within the same period (p<0.005). Of all NKCM-treated tumor cells that were killed within the 15 hour period, more than 60% were killed during the first 5 hours of co-culture. In contrast, only 8% of all the non-treated tumor cells killed by the OT-I-CTLs were killed within the initial 5 hours. Killing of non-treated tumor cells slowly increased, however, with 32% being killed between 5 and 10 hours and 60% being killed between 10 and 15 hours.
Figure 5. Rapid lysis of NK cell-conditioned tumor cells by tumor-specific CTLs.
To determine the influence of pre-treatment with NKCM on the kinetics of tumor cell lysis by tumor-specific CTLs, B16-M05 tumor cells were incubated with 50% NKCM for 18–25 hours. NKCM pretreated and non-treated, GFP+ B16-M05 tumor cells were mixed with CellTracker Orange labeled OT-I-CTLs (E:T = 3:1) and observed by live cell microscopy for 15 hours (DIC, red and green fluorescence images were acquired every 3 minutes). A: Images of co-cultures of OT-I-CTLs and NKCM pretreated (upper row: NKCM) and non-treated (lower row: Control) B16-M05 tumor cells acquired after approximately 0 h, 5 h, 10 h and 15 h of incubation. Left panels show merged DIC and red fluorescence images. Right panels show merged red and green fluorescence images. B: The time of lysis of individual tumor cells within 0–5, 5–10 and 10–15 hours of culture was determined in 12 randomly chosen areas of each co-culture (NKCM and Control). Data are expressed as the percentage of NKCM-treated (light columns) and control (dark columns) tumor cells killed within a 5 hour period relative to all NKCM-treated and control tumor cells, respectively, killed between 0 h and 15 h. *: p<0.05. Numbers in columns indicate the percentage of tumor cells killed within a 5 hour period relative to all tumor cells that could have been killed between 0 h and 15 h. Error bars indicate SE. Representative of three individual experiments.
3.6 Accumulation of A-NK cells and CTLs at tumor sites
The findings described above support the notion that tumor-infiltrating, IFNγ-producing A-NK cells may prepare the tumor for MHC-dependent, TCR-mediated recognition and cytolysis by tumor-specific T cells. To determine if A-NK cells and CTLs influence each other with respect to accumulation at tumor sites, we adoptively transferred A-NK cells, CTLs and a mixture of both into mice with established lung metastases. The densities of A-NK cells and CTLs in the lung tumors at 48 hours after injection did not differ whether only one effector cell type or both types were injected into the animals. The average density per sq.mm tumor tissue of A-NK cells was 599 ± 38; of OT-I cells: 872 ± 122; and 513 ± 73 and 819 ± 49 of A-NK cells and OT-I cells, respectively, when injected together. Thus, tumor-infiltrating A-NK cells and CTLs do not seem to impact each other’s ability to reach and/or extravasate at the tumor sites, where they are found in very close proximity of each other. This has been observed in the OVA-MC38 tumor model (Figure 5A–D) as well as other models based on B16, 3LL, and Panc02 (data not shown).
3.7 Increase in tumor MHC expression induced by adoptively transferred, IFNγ-producing A-NK cells
To determine if infiltration of tumors by adoptively transferred, IFNγ-secreting A-NK cells leads to upregulation of the tumors’ MHC expression, we injected A-NK and A-NKIL-12 cells into lung tumor-bearing animals. Forty hours later, we removed and fresh-froze the lungs. Cryosections of the lung tissue were stained with PE-conjugated anti-H-2Kb antibody and the fluorescence intensity of tumors tissue was determined by image. While tumors from non-treated and A-NK cell treated animals expressed only moderate amounts of MHC, a substantial and significantly higher MHC expression was found in tumors from animals treated with IL-12 gene-transduced A-NK cells (Figure 6).
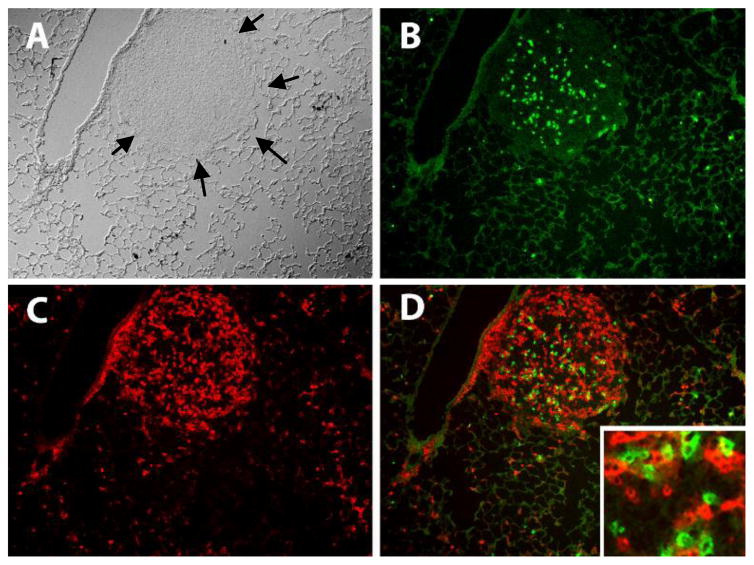
Figure 6. A-NK cells and tumor-specific CTLs accumulate preferentially in tumor tissue.
To determine if A-NK cells interfere with each other’s ability to localize at tumor sites, five million PHA and IL-2 stimulated, Thy1.1+ OT-I-CTLs and 2 million CD45.1 A-NK cells were mixed and injected into Thy1.2+, CD45.2+ mice with well-established, day 10 OVA-MC38 metastases. 10,000 IU Peg-IL-2 was given i.p. immediately after the effector cell injection and again 24 hours later. At ~48 hours after the cell injection, lungs were removed, snap frozen, cryo-sectioned and stained with PE-anti-Thy1.1 antibody and FITC-anti-CD45.1 antibody to in the lungs. Black arrows marks the border between tumor and normal lung tissue. B: FITC-positive (green) CD45.1+ A-NK cells. C: PE-positive (red) Thy1.1+ OT-I-CTLs. D: Overlay of B and C. Insert shows that the OT-I cells and A-NK cells are found in close proximity in the tumor tissue.
3.8 Increased anti-tumor effect by combined A-NK cell and CTL treatment
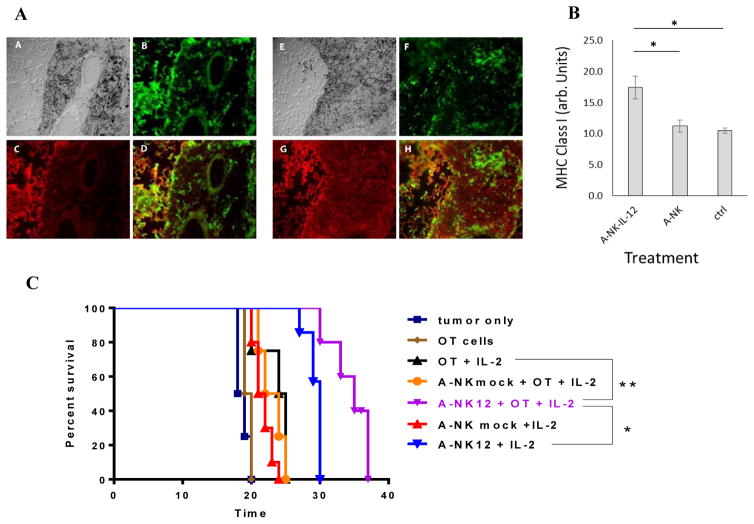
To evaluate the ability of NK cells to induce or increase MHC class-I expression in tumors, lungs with 10-days-old B16-M05 metastases were removed from animals adoptively transferred with A-NKmock and A-NKIL-12 cells 2 days earlier, and stained with anti-H-2Kb antibody. In contrast to transfer of A-NKmock cells, treatment with A-NKIL-12 cells significantly increased MHC class-I expression in the B16-M05 lung lesions (Figure 7A+B) compared to untreated animals. To determine if the anti-tumor effect of A-NK cells and tumor-specific CTLs improves if both effectors are present in the tumors at the same time, mice with 10 days old lung tumors received treatment with either A-NK cells, A-NKIL-12 cells, or CTLs, or with combinations of these effector cells. Treatment with tumor-specific CTLs (i.e., activated OT-1 cells) alone had no effect, but if just one injection of PegIL-2 was given together with the CTLs, they prolonged survival of the host by 5 days (Figure 7C). Treatment with a mixture of A-NK cells and OT-1-derived CTLs plus one injection of IL-2 did not further increase treatment effect. However, if the CTLs were co-injected with IL-12 gene-transduced A-NK cells plus one injection of Peg-IL-2, survival of the hosts was prolonged by 16.5 days. Survival after treatment with IL-12 gene-transduced A-NK cells plus one injection of PegIL-2 was clearly prolonged (by 11.5 days), but it was 5 days shorter (p=0.007) than treatment with the CTLs + A-NKIL-12 + PegIL-2. Thus, addition of IL-12 gene-transduced A-NK cells to the treatment with CTLs, significantly increased their anti-tumor effect compared to treatment with CTLs only plus PegIL-2 (+10.5 days, p=0.004).
Figure 7. Anti-tumor effect of combined treatment with IL-12 gene-transduced A-NK cells and tumor specific CTLs.
Tumor-homing by A-NK and IL-12-gene-transduced A-NK cells (A-NKIL-12) and changes in tumor MHC class-I expression was measured in mice with 0VA-B16 lung tumors. Tissue-sections were stained with FITC-anti-Thy1.1 to reveal the transferred A-NK cells and with PE-anti-H-2Kb to reveal MHC expression in the tissues. The expression of MHC was measured by image analysis of tissue-sections. A: A+E: DIC images of B16-M05 tumors from mice treated with A-NK cells (A) and A-NKIL-12 cells (E), respectively. B+F: FITC-Thy1.1 positive A-NK (B) and A-NKIL-12 (F) cells, respectively. C+G: PE-H-2Kb weakly and strongly positive tumors from mice treated with A-NK (C) and A-NKIL-12 (G) cells. D+H: Overlay of B and C (D) and F and G (H). B: Expression of H-2Kb (arbitrary units) in tumors from animals receiving IL-12 gene-transduced A-NK cells (A-NKIL-12), mock-transduced A-NK cells (A-NK), and no cells (ctrl). *: p<0.01. C: Mice bearing 7 days old B16-OVA lung tumors were injected i.v. with A-NK cells (4 × 106), OT-I cells (3 × 106), or both (n=4–6 animals per group). All mice (except those receiving neither A-NK, nor OT-I cells (“tumor only”) or only OT-I cells (“OT cells”) received one i.p. injection of 30,000 IU Peg-IL-2 immediately after the cell injection. Kaplan-Meyer survival curves were constructed. A-NK12 + OT + IL-2 vs. tumor only: +16.5 days (p<0.005); vs. A-NK12+IL-2: + 5 days (p<0.01); vs. A-NKmock + OT-I + IL-2: +12 days (p<0.005); vs. OT-I + IL-2: +11days (p=0.005); vs. OT-1: +15.5 days (p=0.005). *: p<0.01; **: p<0.005.
4.0 Discussion
Although the intratumoral density of NK cells has been found to correlate positively with outcome in many tumor types, it is unclear why this is the case. Previously, we estimated the densities of intratumoral NK cells associated with favorable prognoses based on the available literature [56]. In several studies, approximately 30 NK cells per mm2 of tumor tissue was considered as “high density”. This, however, translates into effector-to-target (E:T) ratios around 1:35. While it is hard to envision that the cytotoxicity of such low numbers of NK cells are directly responsible for better outcomes, it certainly is possible that a relatively low number of NK cells are able to support tumor-specific cytotoxic T lymphocytes at the tumor sites, e.g., via production of cytokines that may increase the sensitivity of the tumor cells to CTL-mediated lysis. Our data show that the overall in vitro cytotoxicity increases additively and even synergistically when A-NK cells and A-NKIL-12 cells, respectively, are allowed to attack tumor cells simultaneously with anti-tumor CTLs. However, although the observed increases in cytotoxicity were significant, the overall kill remained relatively modest, especially at lower (clinically relevant) E:T ratios. In contrast, preincubation of tumor cells with supernatant from A-NK and in particular A-NKIL-12 cell cultures substantially improved the killing efficiency OT-I-CTLs, even at low E:T ratios. This effect required longer than 4 hours of preincubation of the tumor cells with the NK cell-conditioned media to become apparent and coincided with a robust increase in tumor cell expression of H-2Kb (capable of presenting the OVA-derived peptide SIINFEKL [57]). While IFNγ and TNFα, as well as other cytokines, are able to upregulate MHC class-I expression in normal and malignant cells, addition of blocking IFNγ antibody to the NK cell-derived medium totally eliminated its H-2Kb-enhancing effect. Since NK cells transduced with the gene for IL-12 produce 10-fold higher amounts of IFNγ, but only 2–3 fold higher amounts of TNFα than mock-transduced NK cells, it is not surprising that IFNγ appears to be the main cytokine responsible for this effect.
While the NKCM clearly induces changes in tumor cells leading to more efficient killing by CTLs, it is possible that know and/or unknown cytokines contained in the NKCM induce changes in the CTLs as well, which may augment their anti-tumor functions. We found, however, no difference in cytotoxicity between OT-I-CTLs pre-incubated in NKCM (or with IFNγ at 0.1–1 microg/ml) and control CTLs (data not shown).
We have previously shown that accumulation of adoptively transferred A-NK cells at tumor sites become significant within 8–16 hours and continues to increase within the following 2–3 days [58–64]. The A-NK cell accumulation translates into a significant delay in tumor growth [44] and increase in survival [41, 42]. Here we show that accumulation of adoptively transferred A-NKIL-12 cells at tumor sites induces a significant increase in tumor cell-expression of H-2Kb within 24–48 hours. The A-NK cells used in our model were stimulated with very high and probably non-physiologic amounts of IL-2. During viral infections and low dose IL-2 treatment, endogenous NK cells often become strongly activated, but whether they produce IFNγ to the same extent as the IL-12 gene-transduced A-NK cells used here, is an open question. However, the sustained presence of virally or low-dose IL-2 activated endogenous NK cells (compared to our A-NK-IL-12 cells, which circulate for less than 24 hours) may, within days, lead to changes in target cell MHC class-I expression similar to those observed here. We are currently investigating this question in models of virally infected tumors.
We also demonstrate that simultaneously injected A-NK cells and CTLs do not interfere negatively with each others ability to accumulate at tumor sites. In fact, within 24–48 hours after injection A-NK cells and CTLs are found in close proximity of each other in the tumor tissues. To determine if the increase in tumor expression of H-2Kb induced by the tumor-infiltrating A-NK cells is sufficient to increase the in vivo anti-tumor effect of simultaneously injected anti-tumor CTLs, we mixed A-NK cells and OT-I-CTLs and injected them into animals with well-established B16-M05 lung metastases. Indeed, injection of both OT-I-CTLs and A-NKIL-12 cells prolonged survival by 16 days compared to no treatment and by 11 days compared to treatment with OT-I-CTLs alone. Although treatment with A-NKIL-12 cells alone increased survival by 11 days compared to control (in accordance with previous findings [42]), this was significantly shorter than the 16 days prolongation of survival induced by the combined OT-I-CTL/A-NKIL-12 cell treatment. Thus, the loss in A-NK cell-mediated kill, an inevitable consequence of upregulating tumor cell MHC class-I, is fully justified by the much larger gain in CTL-mediated killing.
It is possible that the presence of NK cells in tumors above a certain threshold may correlate with prognosis without the tumor-infiltrating NK cells themselves having any functional influence on outcome. A certain density of NK cells in a patient’s tumor tissue may simply indicate that the NK cells are in general functional and able to contribute positively to the host’s anti-tumor immune responses as they develop outside of the tumor microenvironment; they may secrete critical Th1-stimulating cytokines during cross talk with dendritic cells in the secondary lymphoid tissues [20] or they inhibit metastases by the elimination of circulating tumor cells, which occurs preferentially in the lungs [9, 65, 66], but to some extent in other organs as well [56]. Nevertheless, our findings indicate that although tissue-infiltrating NK cells may kill some target cells and thereby help to liberate possible antigens and danger signals (PAMPS and DAMPS), their most important function in tumors and possibly in infected tissues as well, may be to prepare these tissue for recognition by infiltrating CTL, by secretion of IFNγ and other cytokines capable of forcing nearby cells to increase their surface expression of MHC molecules. Although CTLs are known to produce IFNγ, they do that especially after successful engagement of their TCR with MHC presenting their cognate antigen. Thus, in tumors expressing very little MHC, CTL-mediated killing and secretion of IFNγ may need to by “jump-started”. Our findings demonstrate that tumor-infiltrating NK cells have the capacity to serve as “jump starters” ofCTL-mediated tumor-lysis at the tumor site.
Supplementary Material
OT-I-CTLs labeled with CellTracker Orange were mixed (3:1) with GFP+ B16-M05 tumor cells, which had been pre-exposed to A-NK cell-conditioned medium for 24 hours or with non-treated GFP+ B16-M05 tumor cells. Using an Olympus live cell microscope with a stage incubation chamber, the cell cultures were observed for 15 hours. DIC and green and red fluorescence images were acquired from multiple fields every 3.16 minutes (282 frames). Top panel shows one representative field from a culture of OT-I-CTLs mixed with NKCM pre-treated B16-M05. Bottom panel shows a representative field from a culture of OT-I-CTLs mixed with untreated (Control) B16-M05 cells. The left part of each panel shows DIC and red-fluorescence overlays. The right part of each panel shows green and red fluorescence overlay. Time-stamp shows real time in hours:minutes:seconds.
Highlights.
The presence of both anti-tumor CTLs and IL-12 gene-transduced, IL-2 activated NK cells synergistically increases the overall kill of tumor cells in vitro.
Tumor cells pre-incubated with A-NK cell-conditioned medium (NKCM) express high levels of MHC and are more sensitive to CTL cytotoxicity (but less sensitive to A-NK cells) than non-treated tumor cells.
Both CTLs and A-NK cells localize selectively at tumor sites – and in close proximity to each other – following adoptive transfer of mixtures of CTLs and A-NK cells.
The simultaneous injection of anti-tumor CTLs and IL-12 gene-transduced A-NK cells is followed by a significant increase in tumor-expression of MHC and significantly prolongs survival of mice with well-stablished, 7-days-old pulmonary metastases compared to injection of each cell type alone.
IFNγ produced by A-NK cells and in particular IL-12 transduced A-NK cells increases tumor expression of MHC class-I and may in this way help to prepare the tumor tissue for recognition and kill by anti-tumor CTLs.
Acknowledgments
This study was supported by grants from the US-NIH (Grants No. R01CA104560 (PHB), R01 CA181450-01 (MTL) and RO1CA87672 (PHB)), the American Cancer Society (Grant No. RPG-00-221-01-CDD (PHB)) and the US Army Medical Research Breast Cancer IDEA Award (BC101672P1 (PHB)). None of these funding agencies were involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
This project used the UPCI Animal (AF), Cell and Tissue Imaging (CTIF), Cancer Proteomics (CPF), Cytometry (CF) and Biostatistics Facilities (BF) that are supported in part by award US-NIH P30CA047904.
We thank Dr. Andrea Gambotto, MD, Department of Surgery, University of Pittsburgh, for his expert help with production and application of the adenoviral vectors used in this study.
Abbreviations
- Ab
Antibody
- Adv-IL-
Adenoviral vector with gene for interleukin-
- A-NK
Activated natural killer cells
- A-NKIL-12
A-NK cells transduced with Adv-IL-12
- A-NKmock
A-NK cells transduced with empty Adv-
- CM
Complete Medium
- CPM
Counts per minute
- CTL
Cytotoxic T lymphocyte
- DAMP
Damage-associated molecular pattern
- DCs
dendritic cells
- E:T
Effector-to-target ratio
- GFP
Green fluorescent protein
- i.v.
intravenous
- IFNγ
Interferon-gamma
- IL-
Interleukin-
- IU
International unit
- M05
Ovalbumin-expressing B16 melanoma
- MHC
Major histocompatibility complex
- MHC
Major histocompatibility complex
- mr-/hr-
Murine recombinant-/human recombinant-
- NKCM
NK cell-conditioned medium
- OT-I-CTLs
CTLs derived from OT-I transgenic mice
- Ova
ovalbumin
- PAMP
Pathogen-associated molecular pattern
- PE
Phycoerythrin
- PEG-IL-2
IL-2 complexed with polyethylene glycol
- PHA
Phytohemagglutinin
- Sq.mm
Square millimeter
- TCR
T cell receptor
- Th1/Th2
T-helper-1/T-helper-2
- T-LAK cells
PHA and IL-2 stimulated bulk splenocytes
- TNFα
Tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. International journal of cancer. Journal international du cancer. 1975;16(2):216–229. doi: 10.1002/ijc.2910160204. http://www.ncbi.nlm.nih.gov/pubmed/50294. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. European journal of immunology. 1975;5(2):112–117. doi: 10.1002/eji.1830050208. http://www.ncbi.nlm.nih.gov/pubmed/1234049. [DOI] [PubMed] [Google Scholar]
- 3.Domogala A, Madrigal JA, Saudemont A. Natural Killer Cell Immunotherapy: From Bench to Bedside. Frontiers in immunology. 2015;6:264. doi: 10.3389/fimmu.2015.00264. http://www.ncbi.nlm.nih.gov/pubmed/26089820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gras Navarro A, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Frontiers in immunology. 2015;6:202. doi: 10.3389/fimmu.2015.00202. http://www.ncbi.nlm.nih.gov/pubmed/25972872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu LL, Pfefferle A, Yi Sheng VO, Bjorklund AT, Beziat V, Goodridge JP, Malmberg KJ. Harnessing adaptive natural killer cells in cancer immunotherapy. Molecular oncology. 2015;9(10):1904–1917. doi: 10.1016/j.molonc.2015.10.001. http://www.ncbi.nlm.nih.gov/pubmed/26604011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Sullivan TE, Sun JC. Generation of Natural Killer Cell Memory during Viral Infection. Journal of innate immunity. 2015;7(6):557–562. doi: 10.1159/000375494. http://www.ncbi.nlm.nih.gov/pubmed/25823611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidal SM, Khakoo SI, Biron CA. Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience. Current opinion in virology. 2011;1(6):497–512. doi: 10.1016/j.coviro.2011.10.017. http://www.ncbi.nlm.nih.gov/pubmed/22180766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biron CA. Natural killer cell regulation during viral infection. Biochemical Society transactions. 1997;25(2):687–690. doi: 10.1042/bst0250687. http://www.ncbi.nlm.nih.gov/pubmed/9191183. [DOI] [PubMed] [Google Scholar]
- 9.Riccardi C, Puccetti P, Santoni A, Herberman RB. Rapid in vivo assay of mouse natural killer cell activity. Journal of the National Cancer Institute. 1979;63(4):1041–1045. http://www.ncbi.nlm.nih.gov/pubmed/480377. [PubMed] [Google Scholar]
- 10.Seth R, Tai LH, Falls T, de Souza CT, Bell JC, Carrier M, Atkins H, Boushey R, Auer RA. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Annals of surgery. 2013;258(1):158–168. doi: 10.1097/SLA.0b013e31826fcbdb. http://www.ncbi.nlm.nih.gov/pubmed/23108132. [DOI] [PubMed] [Google Scholar]
- 11.Kruschinski C, Hama Y, Skripuletz T, Vaske B, Knapp WH, Schmidt RE, Pabst R, von Horsten S, Hofmann M. In vivo monitoring of natural killer cell-dependent clearance of lung metastasis using dynamic positron emission tomography. In vivo. 2012;26(2):191–195. http://www.ncbi.nlm.nih.gov/pubmed/22351657. [PubMed] [Google Scholar]
- 12.Shakhar G, Bar-Ziv I, Ben-Eliyahu S. Diurnal changes in lung tumor clearance and their relation to NK cell cytotoxicity in the blood and spleen. International journal of cancer. Journal international du cancer. 2001;94(3):401–406. doi: 10.1002/ijc.1477. http://www.ncbi.nlm.nih.gov/pubmed/11745421. [DOI] [PubMed] [Google Scholar]
- 13.Toft P, Dagnaes-Hansen F, Tonnesen E, Basse PM. The effect of surgical stress and endotoxin-induced sepsis on the NK-cell activity, distribution and pulmonary clearance of YAC-1 and melanoma cells. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 1999;107(4):359–364. doi: 10.1111/j.1699-0463.1999.tb01565.x. http://www.ncbi.nlm.nih.gov/pubmed/10230687. [DOI] [PubMed] [Google Scholar]
- 14.Page GG, Ben-Eliyahu S, Taylor AN. The development of sexual dimorphism in natural killer cell activity and resistance to tumor metastasis in the Fischer 344 rat. Journal of neuroimmunology. 1995;63(1):69–77. doi: 10.1016/0165-5728(95)00132-8. http://www.ncbi.nlm.nih.gov/pubmed/8557827. [DOI] [PubMed] [Google Scholar]
- 15.Algarra I, Ohlen C, Perez M, Ljunggren HG, Klein G, Garrido F, Karre K. NK sensitivity and lung clearance of MHC-class-I-deficient cells within a heterogeneous fibrosarcoma. International journal of cancer. Journal international du cancer. 1989;44(4):675–680. doi: 10.1002/ijc.2910440420. http://www.ncbi.nlm.nih.gov/pubmed/2507453. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Goding SR, Hokland ME, Basse PH. Antitumor activity of NK cells. Immunologic research. 2006;36(1–3):13–25. doi: 10.1385/IR:36:1:13. http://www.ncbi.nlm.nih.gov/pubmed/17337762. [DOI] [PubMed] [Google Scholar]
- 17.Stein-Streilein J, Sonoda KH, Faunce D, Zhang-Hoover J. Regulation of adaptive immune responses by innate cells expressing NK markers and antigen-transporting macrophages. Journal of leukocyte biology. 2000;67(4):488–494. doi: 10.1002/jlb.67.4.488. http://www.ncbi.nlm.nih.gov/pubmed/10770280. [DOI] [PubMed] [Google Scholar]
- 18.Kos FJ, Engleman EG. Immune regulation: a critical link between NK cells and CTLs. Immunology today. 1996;17(4):174–176. doi: 10.1016/0167-5699(96)80616-5. http://www.ncbi.nlm.nih.gov/pubmed/8871349. [DOI] [PubMed] [Google Scholar]
- 19.Murphy WJ, Longo DL. NK Cells in the Regulation of Hematopoiesis. Methods. 1996;9(2):344–351. doi: 10.1006/meth.1996.0039. http://www.ncbi.nlm.nih.gov/pubmed/8812687. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nature immunology. 2004;5(12):1260–1265. doi: 10.1038/ni1138. http://www.ncbi.nlm.nih.gov/pubmed/15531883. [DOI] [PubMed] [Google Scholar]
- 21.Mahmood S, Upreti D, Sow I, Amari A, Nandagopal S, Kung SK. Bidirectional interactions of NK cells and dendritic cells in immunotherapy: current and future perspective. Immunotherapy. 2015;7(3):301–308. doi: 10.2217/imt.14.122. http://www.ncbi.nlm.nih.gov/pubmed/25804481. [DOI] [PubMed] [Google Scholar]
- 22.Pampena MB, Levy EM. Natural killer cells as helper cells in dendritic cell cancer vaccines. Frontiers in immunology. 2015;6:13. doi: 10.3389/fimmu.2015.00013. http://www.ncbi.nlm.nih.gov/pubmed/25674087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel T, Hentges F, Zimmer J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Frontiers in immunology. 2012;3:403. doi: 10.3389/fimmu.2012.00403. http://www.ncbi.nlm.nih.gov/pubmed/23316194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreira da Silva R, Graf C, Munz C. Cytoskeletal stabilization of inhibitory interactions in immunologic synapses of mature human dendritic cells with natural killer cells. Blood. 2011;118(25):6487–6498. doi: 10.1182/blood-2011-07-366328. http://www.ncbi.nlm.nih.gov/pubmed/21917751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzello V, Bonaccorsi I, Dongarra ML, Fink LN, Ferlazzo G. Role of natural killer and dendritic cell crosstalk in immunomodulation by commensal bacteria probiotics. Journal of biomedicine & biotechnology. 2011;2011:473097. doi: 10.1155/2011/473097. http://www.ncbi.nlm.nih.gov/pubmed/21660136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. Journal of innate immunity. 2011;3(3):258–263. doi: 10.1159/000323923. http://www.ncbi.nlm.nih.gov/pubmed/21411969. [DOI] [PubMed] [Google Scholar]
- 27.Walzer T, Dalod M, Vivier E, Zitvogel L. Natural killer cell-dendritic cell crosstalk in the initiation of immune responses. Expert opinion on biological therapy. 2005;5(Suppl 1):S49–59. doi: 10.1517/14712598.5.1.S49. http://www.ncbi.nlm.nih.gov/pubmed/16187940. [DOI] [PubMed] [Google Scholar]
- 28.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79(12):2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. http://www.ncbi.nlm.nih.gov/pubmed/9191519. [DOI] [PubMed] [Google Scholar]
- 29.Cozar JM, Canton J, Tallada M, Concha A, Cabrera T, Garrido F, Ruiz-Cabello Osuna F. Analysis of NK cells and chemokine receptors in tumor infiltrating CD4 T lymphocytes in human renal carcinomas. Cancer immunology, immunotherapy: CII. 2005;54(9):858–866. doi: 10.1007/s00262-004-0646-1. http://www.ncbi.nlm.nih.gov/pubmed/15887015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donskov F. Interleukin-2 based immunotherapy in patients with metastatic renal cell carcinoma. Danish medical bulletin. 2007;54(4):249–265. http://www.ncbi.nlm.nih.gov/pubmed/18208677. [PubMed] [Google Scholar]
- 31.Hsia JY, Chen JT, Chen CY, Hsu CP, Miaw J, Huang YS, Yang CY. Prognostic significance of intratumoral natural killer cells in primary resected esophageal squamous cell carcinoma. Chang Gung medical journal. 2005;28(5):335–340. http://www.ncbi.nlm.nih.gov/pubmed/16086548. [PubMed] [Google Scholar]
- 32.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577–583. http://www.ncbi.nlm.nih.gov/pubmed/10649250. [PubMed] [Google Scholar]
- 33.Sznurkowski JJ, Zawrocki A, Biernat W. Subtypes of cytotoxic lymphocytes and natural killer cells infiltrating cancer nests correlate with prognosis in patients with vulvar squamous cell carcinoma. Cancer immunology, immunotherapy: CII. 2014;63(3):297–303. doi: 10.1007/s00262-013-1511-x. http://www.ncbi.nlm.nih.gov/pubmed/24368339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. The Journal of thoracic and cardiOVAscular surgery. 2001;121(6):1058–1063. doi: 10.1067/mtc.2001.113026. http://www.ncbi.nlm.nih.gov/pubmed/11385371. [DOI] [PubMed] [Google Scholar]
- 35.Taketomi A, Shimada M, Shirabe K, Kajiyama K, Gion T, Sugimachi K. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer. 1998;83(1):58–63. doi: 10.1002/(sici)1097-0142(19980701)83:1<58::aid-cncr8>3.0.co;2-a. http://www.ncbi.nlm.nih.gov/pubmed/9655293. [DOI] [PubMed] [Google Scholar]
- 36.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung cancer. 2002;35(1):23–28. doi: 10.1016/s0169-5002(01)00292-6. http://www.ncbi.nlm.nih.gov/pubmed/11750709. [DOI] [PubMed] [Google Scholar]
- 37.Zitvogel L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. The Journal of experimental medicine. 2002;195(3):F9–14. doi: 10.1084/jem.20012040. http://www.ncbi.nlm.nih.gov/pubmed/11828015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker C, Wirtz S, Neurath MF. Stepwise regulation of TH1 responses in autoimmunity: IL-12-related cytokines and their receptors. Inflammatory bowel diseases. 2005;11(8):755–764. doi: 10.1097/01.mib.0000172808.03877.4d. http://www.ncbi.nlm.nih.gov/pubmed/16043992. [DOI] [PubMed] [Google Scholar]
- 39.Dredge K, Marriott JB, Todryk SM, Dalgleish AG. Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer immunology, immunotherapy: CII. 2002;51(10):521–531. doi: 10.1007/s00262-002-0309-z. http://www.ncbi.nlm.nih.gov/pubmed/12384803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. The Journal of experimental medicine. 1996;183(1):283–287. doi: 10.1084/jem.183.1.283. http://www.ncbi.nlm.nih.gov/pubmed/8551233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goding S, Yang Q, Mi Z, Robbins PD, Basse PH. Targeting of products of genes to tumor sites using adoptively transferred A-NK and T-LAK cells. Cancer gene therapy. 2007;14(5):441–450. doi: 10.1038/sj.cgt.7701019. http://www.ncbi.nlm.nih.gov/pubmed/17273184. [DOI] [PubMed] [Google Scholar]
- 42.Goding SR, Yang Q, Knudsen KB, Potter DM, Basse PH. Cytokine gene therapy using adenovirally transduced, tumor-seeking activated natural killer cells. Human gene therapy. 2007;18(8):701–711. doi: 10.1089/hum.2007.052. http://www.ncbi.nlm.nih.gov/pubmed/17678438. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman RJ, Aukerman SL, Katre NV, Winkelhake JL, Young JD. Schedule dependency of the antitumor activity and toxicity of polyethylene glycol-modified interleukin 2 in murine tumor models. Cancer research. 1989;49(23):6521–6528. http://www.ncbi.nlm.nih.gov/pubmed/2819708. [PubMed] [Google Scholar]
- 44.Yang Q, Hokland ME, Bryant JL, Zhang Y, Nannmark U, Watkins SC, Goldfarb RH, Herberman RB, Basse PH. Tumor-localization by adoptively transferred, interleukin-2-activated NK cells leads to destruction of well-established lung metastases. International journal of cancer. Journal international du cancer. 2003;105(4):512–519. doi: 10.1002/ijc.11119. http://www.ncbi.nlm.nih.gov/pubmed/12712443. [DOI] [PubMed] [Google Scholar]
- 45.Fogler WE, Volker K, Watanabe M, Wigginton JM, Roessler P, Brunda MJ, Ortaldo JR, Wiltrout RH. Recruitment of hepatic NK cells by IL-12 is dependent on IFN-gamma and VCAM-1 and is rapidly down-regulated by a mechanism involving T cells and expression of Fas. Journal of immunology. 1998;161(11):6014–6021. http://www.ncbi.nlm.nih.gov/pubmed/9834083. [PubMed] [Google Scholar]
- 46.Yamamoto RS, Coss J, Vayuvegula B, Gupta S, Beamer Y, Jacques S, Jeffes EW, 3rd, Carson WE, 3rd, Jakowatz J, Granger GA. Generation of stimulated, lymphokine activated T killer (T-LAK) cells from the peripheral blood of normal donors and adult patients with recurrent glioblastoma. Journal of immunological methods. 1991;137(2):225–235. doi: 10.1016/0022-1759(91)90028-e. http://www.ncbi.nlm.nih.gov/pubmed/2013699. [DOI] [PubMed] [Google Scholar]
- 47.Chace JH, Hooker NA, Mildenstein KL, Krieg AM, Cowdery JS. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clinical immunology and immunopathology. 1997;84(2):185–193. doi: 10.1006/clin.1997.4380. http://www.ncbi.nlm.nih.gov/pubmed/9245551. [DOI] [PubMed] [Google Scholar]
- 48.de Groen RA, Boltjes A, Hou J, Liu BS, McPhee F, Friborg J, Janssen HL, Boonstra A. IFN-lambda-mediated IL-12 production in macrophages induces IFN-gamma production in human NK cells. European journal of immunology. 2015;45(1):250–259. doi: 10.1002/eji.201444903. http://www.ncbi.nlm.nih.gov/pubmed/25316442. [DOI] [PubMed] [Google Scholar]
- 49.Duluc D, Tan F, Scotet M, Blanchard S, Fremaux I, Garo E, Horvat B, Eid P, Delneste Y, Jeannin P. PolyI:C plus IL-2 or IL-12 induce IFN-gamma production by human NK cells via autocrine IFN-beta. European journal of immunology. 2009;39(10):2877–2884. doi: 10.1002/eji.200838610. http://www.ncbi.nlm.nih.gov/pubmed/19728309. [DOI] [PubMed] [Google Scholar]
- 50.Galon J, Sudarshan C, Ito S, Finbloom D, O’Shea JJ. IL-12 induces IFN regulating factor-1 (IRF-1) gene expression in human NK and T cells. Journal of immunology. 1999;162(12):7256–7262. http://www.ncbi.nlm.nih.gov/pubmed/10358173. [PubMed] [Google Scholar]
- 51.Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, Parihar R, Karpa V, Papenfuss TL, LaPerle KM, Biller E, Lehman A, Chaudhury AR, Jarjoura D, Burry RW, Carson WE., 3rd IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-gamma production. Journal of immunology. 2011;186(6):3401–3409. doi: 10.4049/jimmunol.1000328. http://www.ncbi.nlm.nih.gov/pubmed/21321106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matikainen S, Paananen A, Miettinen M, Kurimoto M, Timonen T, Julkunen I, Sareneva T. IFN-alpha and IL-18 synergistically enhance IFN-gamma production in human NK cells: differential regulation of Stat4 activation and IFN-gamma gene expression by IFN-alpha and IL-12. European journal of immunology. 2001;31(7):2236–2245. http://www.ncbi.nlm.nih.gov/pubmed/11449378. [PubMed] [Google Scholar]
- 53.Mirjacic Martinovic K, Babovic N, Dzodic R, Jurisic V, Matkovic S, Konjevic G. Favorable in vitro effects of combined IL-12 and IL-18 treatment on NK cell cytotoxicity and CD25 receptor expression in metastatic melanoma patients. Journal of translational medicine. 2015;13:120. doi: 10.1186/s12967-015-0479-z. http://www.ncbi.nlm.nih.gov/pubmed/25889680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker W, Aste-Amezaga M, Kastelein RA, Trinchieri G, Hunter CA. IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-gamma. Journal of immunology. 1999;162(10):5894–5901. http://www.ncbi.nlm.nih.gov/pubmed/10229825. [PubMed] [Google Scholar]
- 55.Ye J, Ortaldo JR, Conlon K, Winkler-Pickett R, Young HA. Cellular and molecular mechanisms of IFN-gamma production induced by IL-2 and IL-12 in a human NK cell line. Journal of leukocyte biology. 1995;58(2):225–233. doi: 10.1002/jlb.58.2.225. http://www.ncbi.nlm.nih.gov/pubmed/7643015. [DOI] [PubMed] [Google Scholar]
- 56.Larsen SK, Gao Y, Basse PH. NK cells in the tumor microenvironment. Critical reviews in oncogenesis. 2014;19(1–2):91–105. doi: 10.1615/critrevoncog.2014011142. http://www.ncbi.nlm.nih.gov/pubmed/24941376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner SJ, Jameson SC, Carbone FR. Functional mapping of the orientation for TCR recognition of an H2-Kb-restricted OVAlbumin peptide suggests that the beta-chain subunit can dominate the determination of peptide side chain specificity. Journal of immunology. 1997;159(5):2312–2317. http://www.ncbi.nlm.nih.gov/pubmed/9278320. [PubMed] [Google Scholar]
- 58.Basse P, Herberman RB, Hokland M, Goldfarb RH. Tissue distribution of adoptively transferred adherent lymphokine-activated killer cells assessed by different cell labels. Cancer immunology, immunotherapy: CII. 1992;34(4):221–227. doi: 10.1007/BF01741789. http://www.ncbi.nlm.nih.gov/pubmed/1537054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basse P, Herberman RB, Nannmark U, Johansson BR, Hokland M, Wasserman K, Goldfarb RH. Accumulation of adoptively transferred adherent, lymphokine-activated killer cells in murine metastases. The Journal of experimental medicine. 1991;174(2):479–488. doi: 10.1084/jem.174.2.479. http://www.ncbi.nlm.nih.gov/pubmed/1856630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basse PH. Tissue distribution and tumor localization of effector cells in adoptive immunotherapy of cancer. APMIS. Supplementum. 1995;55:1–28. http://www.ncbi.nlm.nih.gov/pubmed/8534522. [PubMed] [Google Scholar]
- 61.Basse PH, Goldfarb RH, Herberman RB, Hokland ME. Accumulation of adoptively transferred A-NK cells in murine metastases: kinetics and role of interleukin-2. In vivo. 1994;8(1):17–24. http://www.ncbi.nlm.nih.gov/pubmed/8054506. [PubMed] [Google Scholar]
- 62.Basse PH, Nannmark U, Johansson BR, Herberman RB, Goldfarb RH. Establishment of cell-to-cell contact by adoptively transferred adherent lymphokine-activated killer cells with metastatic murine melanoma cells. Journal of the National Cancer Institute. 1991;83(13):944–950. doi: 10.1093/jnci/83.13.944. http://www.ncbi.nlm.nih.gov/pubmed/2067037. [DOI] [PubMed] [Google Scholar]
- 63.Kjaergaard J, Hokland M, Nannmark U, Hokland P, Basse P. Infiltration patterns of short- and long-term cultured A-NK and T-LAK cells following adoptive immunotherapy. Scandinavian journal of immunology. 1998;47(6):532–540. doi: 10.1046/j.1365-3083.1998.00339.x. http://www.ncbi.nlm.nih.gov/pubmed/9652820. [DOI] [PubMed] [Google Scholar]
- 64.Ribeiro U, Jr, Basse PH, Rosenstein M, Safatle-Ribeiro AV, Alhallak S, Goldfarb RH, Posner MC. Retention of adoptively transferred interleukin-2-activated natural killer cells in tumor tissue. Anticancer research. 1997;17(2A):1115–1123. http://www.ncbi.nlm.nih.gov/pubmed/9137458. [PubMed] [Google Scholar]
- 65.Barlozzari T, Reynolds CW, Herberman RB. In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in anti-asialo GM1-treated rats. Journal of immunology. 1983;131(2):1024–1027. http://www.ncbi.nlm.nih.gov/pubmed/6863925. [PubMed] [Google Scholar]
- 66.Basse P, Hokland P, Heron I, Hokland M. Fate of tumor cells injected into left ventricle of heart in BALB/c mice: role of natural killer cells. Journal of the National Cancer Institute. 1988;80(9):657–665. doi: 10.1093/jnci/80.9.657. http://www.ncbi.nlm.nih.gov/pubmed/3373554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OT-I-CTLs labeled with CellTracker Orange were mixed (3:1) with GFP+ B16-M05 tumor cells, which had been pre-exposed to A-NK cell-conditioned medium for 24 hours or with non-treated GFP+ B16-M05 tumor cells. Using an Olympus live cell microscope with a stage incubation chamber, the cell cultures were observed for 15 hours. DIC and green and red fluorescence images were acquired from multiple fields every 3.16 minutes (282 frames). Top panel shows one representative field from a culture of OT-I-CTLs mixed with NKCM pre-treated B16-M05. Bottom panel shows a representative field from a culture of OT-I-CTLs mixed with untreated (Control) B16-M05 cells. The left part of each panel shows DIC and red-fluorescence overlays. The right part of each panel shows green and red fluorescence overlay. Time-stamp shows real time in hours:minutes:seconds.