Abstract
This study determined if high-frequency biphasic stimulation can induce nerve conduction block that persists after the stimulation is terminated, i.e. post-stimulation block. The frog sciatic nerve-muscle preparation was used in the study. Muscle contraction force induced by low frequency (0.5 Hz) nerve stimulation was recorded to indicate the occurrence and recovery of nerve block induced by the high-frequency (5 or 10 kHz) biphasic stimulation. Nerve block was observed during high-frequency stimulation and after termination of the stimulation. The recovery from post-stimulation block occurred in two distinct phases. During the first phase, the complete block induced during high-frequency stimulation was maintained. The average maximal duration for the first phase was 107±50 seconds. During the second phase, the block gradually or abruptly reversed. The duration of both first and second phases was dependent on stimulation intensity and duration but not frequency. Stimulation of higher intensity (1.4–2 times block threshold) and longer duration (5 minutes) produced the longest period (249±58 seconds) for a complete recovery. Post-stimulation block can be induced by high-frequency biphasic stimulation, which is important for future investigations of the blocking mechanisms and for optimizing the stimulation parameters or protocols in clinical applications.
Keywords: Nerve, Block, High-frequency, Stimulation, Frog
1 Introduction
Nerve conduction block by high-frequency (kHz) biphasic stimulation has been known for more than 70 years [16,17]. This nerve block method has attracted greater attention in recent years due to its potential clinical applications to treat obesity [18], restore urinary function after spinal cord injury [8,21], or suppress chronic pain of peripheral origin [6,19]. However, the mechanisms underlying this high-frequency nerve block are not fully understood [24].
Previous animal studies [2–4,10–12,21,22] revealed that the nerve block can recover quickly within a second after termination of high-frequency biphasic stimulation. However, recent animal studies [13,23] have also shown that high-frequency biphasic stimulation can cause a complete suppression or a change in shape of the compound action potential during a post-stimulation period ranging from seconds to minutes. Since both animal and computer simulation studies [13,24] have shown that axonal conduction velocity is reduced following high-frequency biphasic stimulation, a suppression or change in the shape of compound action potentials could simply be due to slowing action potential conduction differently in different axons and not necessarily due to a conduction block. Therefore, previous studies [13,23] by recording compound action potentials did not provide direct evidence that axonal conduction can be blocked by high frequency biphasic stimulation during the post-stimulation period. The goal of this study using the frog sciatic nerve-muscle preparation was to answer the following questions: 1. Are neurally evoked muscle contractions blocked by high-frequency biphasic axonal stimulation during the post-stimulation period and 2. How is the post-stimulation block influenced by different stimulation parameters (intensity, duration, and frequency)? Muscle contraction force was recorded instead of recording the compound action potential in order to directly determine whether a nerve block occurred.
It is important to determine whether a post-stimulation block can be induced by high-frequency biphasic stimulation because the blocking mechanisms during post-stimulation period are also likely to be present during the stimulation. However, the large stimulation artifacts occurring during the stimulation that interfere with electrical recordings will be absent during the post-stimulation period, thereby providing a significant advantage for electrophysiological experiments to record the neural activity during the post-stimulation period and investigate the underlying mechanisms of nerve block. In addition, understanding the post-stimulation block induced by high-frequency biphasic stimulation could optimize the stimulation protocols and promote further clinical applications of this therapy [6,8,18,21].
2 Materials and Methods
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
The experiments were performed on sciatic nerve-muscle preparations from 16 adult Xenopus laevis frogs. After the frog was euthanized by double pithing, the right sciatic nerve was exposed and cut at the location close to the spinal cord. Then, two stimulation electrodes (Stim.A and Stim.B in Fig. 1) were placed around the exposed sciatic nerve. Stim.A was a bipolar hook electrode to deliver electrical pulses (0.2 ms pulse width) at a low frequency (0.5 Hz) and induce muscle twitches. Stim.B was a tripolar cuff electrode (NCE113, MicroProbes Inc., Gaithersburg, MD, USA) to deliver the high-frequency (5 kHz or 10 kHz) biphasic stimulation used to block axonal conduction and the 0.5 Hz muscle twitch. The low frequency (0.5 Hz) stimulation was provided by a Grass S88 stimulator (Grass Technologies, RI, USA) via a stimulation isolator (SIU5, Grass Technologies, RI, USA). The high-frequency (5 or 10 kHz) biphasic signal was generated by a computer running a Labview program (National Instruments, TX, USA) and delivered via a stimulation isolator (A395, World Precision Instruments, FL, USA). The nerve-muscle preparation was immersed in Ringer’s solution at room temperature (20–26 ºC). Multiple pins were inserted through the quadriceps and hamstrings muscles to fix the frog leg above the knee to the bottom of the recording chamber leaving the shank and ankle free to move (Fig. 1). A thread tied on the foot was attached to a force transducer (FT03, Grass Technologies, RI, USA) to measure the muscle contraction force that was amplified (TBM4M, World Precision Instruments, FL, USA) with a gain of 1000, recorded on a chart recorder (TA4000, Gould, USA), and saved in a computer with a 12-bit precision via an analog-to-digital converter (PCI-6024E, National Instruments, TX, USA) with a sampling rate of 1000 Hz. The thread connecting the foot to the transducer had a baseline tension about 5 gram-force.
Fig. 1.
Experiment setup showing that the exposed sciatic nerve is stimulated by a bipolar hook electrode (Stim.A) and blocked by a tripolar cuff electrode (Stim.B). Stim.C is used to confirm the nerve block. The frog legs are immersed in a Ringer’s solution bath and fixed by multiple pins. A thread is attached to the foot and the force transducer to record the muscle contraction force. Stim.A and Stim.C: frequency 0.5 Hz, pulse width 0.2 ms; Stim.B: 5 kHz or 10 kHz.
Initially, the intensity of the low frequency (0.5 Hz) stimulation (Stim.A in Fig. 1) was gradually increased to induce a muscle twitch force slightly greater than 10 gram-force in order to avoid stimulation current spreading at a high stimulation intensity. Then, the high-frequency (5 or 10 kHz) biphasic stimulation (Stim.B in Fig. 1) was applied for 10 s at different intensities (1–10 mA) starting at 1 mA and with 1 mA increments to determine the minimal intensity (i.e., the block threshold) required to completely block the muscle contractions induced by the high-frequency stimulation at the end of the 10 s stimulation. Once the block threshold (T) was determined, the high-frequency biphasic stimulation of different durations (10 s, 1 min, 2 min, 3 min, 4 min, and 5min) was applied at 1T or at an intensity ranging from 1.4–2T in an increasing order of the stimulation durations to block the 0.5 Hz muscle twitch and determine if a post-stimulation block was induced. A resting period of 2 minutes without stimulation was inserted after full recovery from the nerve block induced by each individual stimulation test. The experimental trial of serial stimulation tests was performed only once on each animal in order to eliminate the potential influence of repeated experimental trials. At the end of the experiment, a low frequency stimulation (0.5 Hz, 0.2 ms) was applied to the nerve via a bipolar hook electrode at a site (Stim.C in Fig. 1) distal to the blocking electrode (Stim.B) during high-frequency block to confirm that the nerve conduction block only occurs locally at the blocking electrode (Stim.B).
Measurements from different animals under the same experimental conditions were averaged and reported as mean ± standard error in each figure. Statistical significance (p<0.05) was detected by ANOVA followed by Bonferroni multiple comparison.
3 Results
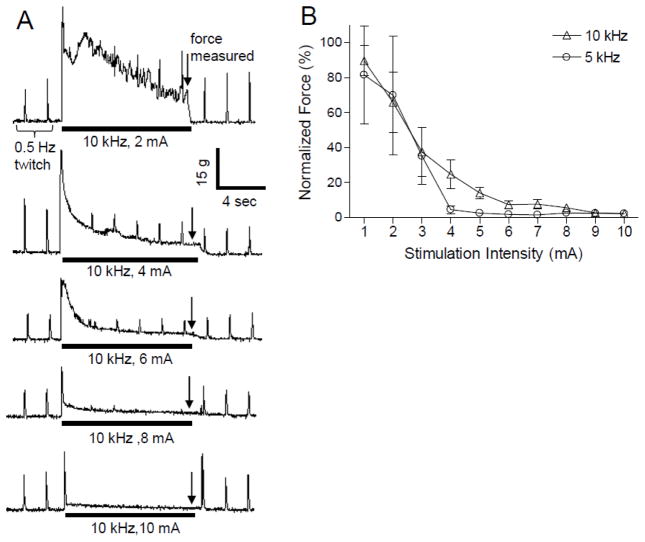
Fig. 2A shows the typical responses induced by 10 kHz biphasic stimulation (Stim.B) of different intensities while the 0.5 Hz stimulation (Stim.A) was applied continuously. Prior to the start of the 10 kHz stimulation the 0.5 Hz stimulation induced muscle twitches of relatively constant amplitude (Fig. 2A). The muscle contraction responses induced by 5 or 10 kHz stimulation at different intensities consisted of an initial large amplitude twitch followed by a tetanic contraction that gradually declined in amplitude over time (Fig. 2A). The high-frequency stimulation at low intensity (1–2 mA) induced a strong muscle contraction that gradually declined during stimulation and became only an initial muscle twitch at the beginning of the stimulation as the intensity increased to 8–10 mA (see Fig. 2A). The muscle contraction forces measured at the end of high-frequency stimulation (marked by the downward arrows in Fig. 2A) were normalized to the maximal measurement at 1 or 2 mA intensity for either 5 kHz or 10 kHz stimulation and the normalized results are shown in Fig. 2 B. A complete block of all nerve fibers could occur at the intensities above 4–7 mA (Fig. 2B). However, the 10 kHz required a slightly higher minimum stimulation intensity (6–7 mA) than 5 kHz (about 4 mA) to completely block the muscle contraction responses induced by the high-frequency stimulation itself (Fig. 2B). Note that when the large amplitude muscle contractions induced by the high-frequency stimulation (at Stim. B) were completely blocked, the muscle twitches induced by the 0.5 Hz stimulation (at Stim. A) were also blocked (Fig. 2A).
Fig. 2.
Intensity-dependent block of muscle twitch by high-frequency biphasic stimulation. A. Muscle twitch forces generated by 0.5 Hz stimulation (Stim.A) in the same animal are gradually blocked by the high-frequency 10 kHz biphasic stimulation (Stim.B) as the stimulation intensity increases. The thick black line under the force trace indicates the duration (10 seconds) of 10 kHz biphasic stimulation. The 0.5 Hz stimulation was applied continuously throughout each experimental trial. B. The force amplitudes measured at the end of the high-frequency stimulation (indicted by downward arrows in A) were normalized to the maximal measurement at 1 or 2 mA intensity for either 5 or 10 kHz stimulation. Stim.A: 0.5 Hz, 0.2 ms, 0.7–7 V. N = 7 frogs for 5 kHz data; N = 9 frogs for 10 kHz data.
Nerve conduction block induced by high frequency biphasic stimulation was confirmed at the end of each experiment by applying 0.5 Hz stimulation at a site (Stim.C) distal to the blocking electrode (Stim.B in Fig. 1) during continuous stimulation at the Stim.A site. Since the 0.5 Hz stimulation at Stim.C could still induce a muscle twitch after the high frequency stimulation at Stim.B completely blocked the nerve conduction (see Fig. 2 and Fig. 3), the blockade was not caused by muscle fatigue or block of neuromuscular transmission. Therefore, it could only be due to a local nerve conduction block at the site of blocking electrode (Stim.B). After termination of the 10 s high-frequency stimulation, the nerve conduction recovered quickly and the muscle twitch induced by 0.5 Hz stimulation reappeared within a second (Fig. 2A), i.e. post-stimulation block was not induced.
Fig. 3.
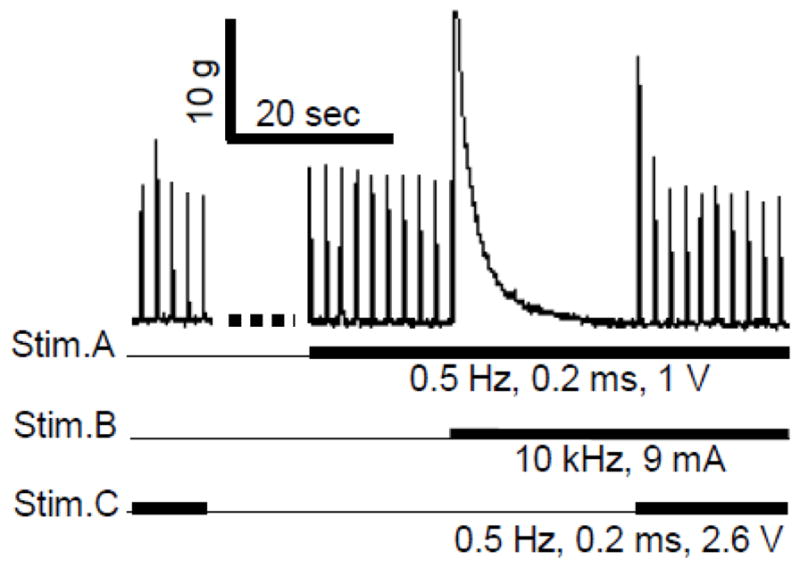
Nerve conduction block occurred locally at the site of Stim.B. The thick black lines under the trace indicate the duration of different stimulations. After the high frequency Stim.B completely blocked muscle contractions, an additional stimulation (0.5 Hz) applied at the site of Stim.C distal to the site of Stim.B could still induced muscle twitches, indicating the block was not due to muscle fatigue.
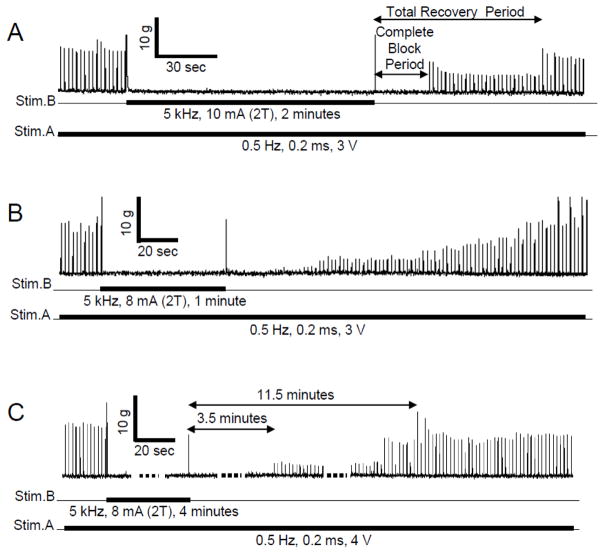
However, significant post-stimulation block of nerve conduction was observed when the high-frequency biphasic stimulation lasted more than 4–5 minutes (Fig. 4 and Fig. 5). In order to show different types of block recovery during an entire experimental trial, short periods (1–2 minutes) of high-frequency biphasic stimulation producing post-stimulation block are presented in Fig. 4 A and B. After termination of the high-frequency stimulation, the recovery from post-stimulation block consisted of two distinct phases. During the first phase, a complete block was maintained and no muscle twitch could be induced by 0.5 Hz stimulation. This phase is termed the complete block period (Fig. 4A). During the second phase, the muscle twitch induced by 0.5 Hz stimulation partially recovered (Fig. 4). The total recovery period is defined as the post-stimulation duration required for the muscle twitch response to recover to 95% of pre-stimulation level (Fig. 4A). It is worth noting that the recovery occurred abruptly in 13 out of 16 animals (Fig. 4A). A gradual recovery was only observed in 3 animals (Fig. 4B). A longer stimulation duration (4 minutes) can produce a much longer complete/partial post-stimulation block (Fig. 4C).
Fig. 4.
Recovery of the muscle twitch response after a long duration (1–4 minutes) of high-frequency biphasic stimulation at 2 times threshold intensity (2T) for completely blocking the nerve conduction. The thick black lines under the trace indicate the duration of different stimulations. The 0.5 Hz muscle twitch responses induced by Stim.A were completely blocked by the 5 kHz stimulation at Stim.B. A. The response recovered partially and abruptly. B. In another experiment, the response recovered gradually. C. A very long (4 minutes) stimulation can induce a complete post-stimulation block lasting 3.5 minutes followed by a partial post-stimulation block lasting for many minutes.
Fig. 5.
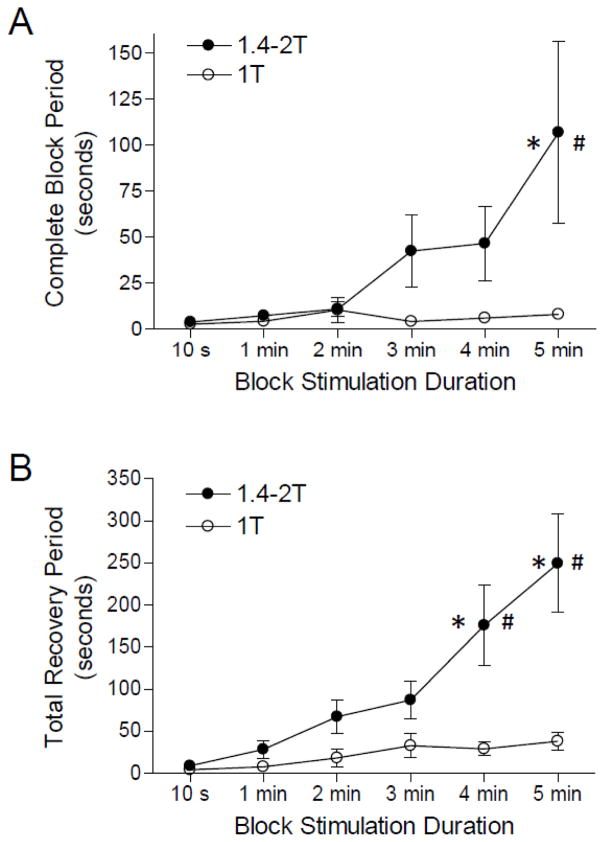
The duration of post-stimulation block is dependent on the intensity and duration of the high-frequency biphasic stimulation. A. Complete block period. B. Total recovery period. T – the intensity threshold for high-frequency stimulation to completely block the nerve. Stim.A: 0.5 Hz, 0.2 ms, 0.7–7 V. Stim.B: 5 kHz or 10 kHz, T = 4-7 mA. The results obtained by 5 kHz or 10 kHz stimulation are plotted together, because 5 kHz and 10 kHz stimulation produced similar results. * indicates a significantly different from the 10 second duration (one-way ANOVA). # indicates a significant difference between low and high intensity stimulations (two-way ANOVA). N = 16 frogs.
The complete block period and the total recovery period were dependent on the duration and intensity of high-frequency biphasic stimulation (Fig. 5). However, they were not dependent on the stimulation frequency since 5 kHz and 10 kHz stimulation produced similar post-stimulation block effects. Therefore, the results obtained from both 5 kHz and 10 kHz stimulations were plotted together in Fig. 5. At the threshold intensity (T) for producing complete block of nerve conduction, a longer stimulation duration did not significantly increase the complete block period (Fig. 5A) or the total recovery period (Fig. 5B). However, at a higher stimulation intensity (1.4-2T) these periods were significantly increased in duration by 4 or 5 minute high-frequency stimulation (Fig. 5). The complete block period was significantly (p<0.05) increased from 4±1 seconds to 107±50 seconds when stimulation duration increased from 10 seconds to 5 minutes (Fig. 5A). The total recovery period was significantly increased from 9±2 seconds to 176±48 (p<0.01) or 249±58 (p<0.001) seconds when stimulation duration increased from 10 seconds to 4 or 5 minutes, respectively (Fig. 5B).
4 Discussion
This study using a frog sciatic nerve-muscle preparation shows that high-frequency biphasic stimulation can induce nerve conduction block not only during the stimulation (Figs. 2–3) but also during the post-stimulation period (Fig. 4). The post-stimulation block is dependent on stimulation intensity and duration (Fig. 5) but not frequency. With high stimulation intensity and long stimulation duration, partial post-stimulation block can last for several minutes (Fig. 5). These results are important for further studies to better understand the underlying mechanisms of nerve block induced by high-frequency biphasic stimulation.
Muscle contraction force instead of compound action potential was used in this study to demonstrate nerve conduction block. This method is based on the following considerations. During the post-stimulation period, the action potential induced by 0.5 Hz stimulation (Stim.A in Fig. 1) may conduct through the blocking electrode site (Stim.B in Fig. 1) with a different delay in axons of different size [13,24]. Although the difference in conduction delay might be less than 1 ms, it could significantly suppress or change the shape of the compound action potential recorded at the location distal to the blocking electrode site. However, this small delay in action potential conduction should not change the muscle twitch force evoked by 0.5 Hz stimulation because the muscle contraction has a much longer time course than the delay of action potential conduction. While recording of compound action potentials might be a more sensitive measurement to indicate a post-stimulation effect on conduction velocity, the change in amplitude or shape of the compound action potential might not indicate that a nerve block actually occurred. Therefore, in this study the muscle contraction force instead of the compound action potential was used to indicate the conduction block.
The results obtained in this study by recording muscle contraction agree well with previous studies that used recordings of compound action potentials [10,13,23]. In unmyelinated Aplysia nerves, the compound action potential completely recovered within 5 s after terminating high-frequency biphasic stimulation with durations ranging from 30 s to 2 minutes [10]. The recovery time was longer for a longer stimulation duration but it was not dependent on stimulation frequency [10]. The short recovery period (<5 s) might be caused by the low stimulation intensity (close to the block threshold) used in this study [10]. In rat vagus nerve, high-frequency (5 kHz) biphasic stimulation of 1 minute duration suppressed the compound action potential during the post-stimulation period for 6–20 minutes [23]. Higher stimulation intensities induced a larger suppression of the compound action potential and longer recovery periods [23]. In frog sciatic nerve, high-frequency (10 kHz) biphasic stimulation of 5–60 s duration caused a change in shape of compound action potential during the post-stimulation period lasting up to 60 s [13]. This change was also dependent on stimulation duration and intensity [13]. The results from both previous studies and our current study indicate that the post-stimulation effect recovers more slowly for stimulation of longer duration or higher intensity. However, previous studies [10,13,23] only investigated the post-stimulation effect on compound action potential, while our current study provided direct evidence that post-stimulation block of neurally evoked muscle contractions did occur.
Currently, the mechanisms underlying the nerve block induced by high-frequency biphasic stimulation are not fully understood [24]. We presume that the same blocking mechanisms must be present before and immediately after terminating the stimulation. On the other hand, computer simulation analysis using both unmyelinated and myelinated axonal models shows that during the high-frequency biphasic stimulation the potassium channels are constantly open [20,24], because the potassium channels have slow kinetics and can not follow the alternating depolarization and hyperpolarization induced by the high-frequency biphasic stimulation. The constantly opened potassium channels can cause a nerve conduction block during the stimulation. Previous model analysis [24] also showed that the opened potassium channels close within 1 ms after terminating the high-frequency stimulation and a post-stimulation block does not occur. Closing the potassium channels during the 1 ms post-stimulation period can cause different conduction delays in axons of different size and therefore induce a suppression or a change in shape of the compound action potential [24]. However, previous modeling studies did show that high-frequency biphasic stimulation can induce pulsed sodium and potassium currents during the stimulation [20,24], which could increase the concentrations of intracellular sodium and extracellular potassium ions to cause a post-stimulation block and a slow recovery after terminating the stimulation. Unfortunately, the classical axonal models used in previous studies [7,9,14,15,20,24] do not include the sodium-potassium pump or stimulation-induced changes in ionic concentrations and therefore failed to simulate the post-stimulation block. Additional modeling studies to include these factors are needed in light of the results from this study using the frog sciatic nerve-muscle preparation. Since the ion channel properties are different between amphibian (frog) and mammalian nerves, whether these potential mechanisms and/or the post-stimulation block phenomenon are same in mammalian myelinated nerve still need to be determined.
It is worth noting that the threshold (T) for complete block of nerve conduction is about 4–5 times higher than the stimulation intensity that induced a maximal muscle contraction (Fig. 2). Meanwhile, the intensity for inducing a post-stimulation block (1.4-2T) is also higher than the complete block threshold (T) (Fig. 5). Based on previous model analysis [20,24], it is understood that a minimal frequency (>4–5 kHz) and a minimal intensity (T) are required in order to maintain the potassium channel open constantly and cause a block of axonal conduction. However, why does the post-stimulation block require an intensity higher than the block threshold? One possible explanation is that the post-stimulation block is maintained by the high concentrations of extracellular potassium or intracellular sodium ions that are accumulated during the stimulation. Such high ion concentrations can only be accumulated by stimulation with higher intensities (1.4-2T) that induce larger ion currents during each stimulation pulse. The larger currents would in turn induce larger changes in ion concentrations that could persist for variable periods after the stimulation. This interpretation may also explain why post-stimulation block is not sensitive to stimulation frequency. With the same stimulation intensity and duration, a higher frequency (10 kHz) should produce a similar amount of total ionic currents as the lower frequency (5 kHz) because the total durations for positive and negative pulses are the same. The results from this study are critical for designing further experiments to confirm or refute these proposals about blocking mechanisms. A possible animal experiment could be conducted in the future by measuring the extracellular potassium concentration using a microelectrode to determine if the change of potassium concentration is correlated with the period of post-stimulation block.
The abrupt recovery of nerve conduction instead of a gradual recovery is an interesting observation (Fig. 4A), indicating that many axons must recover conduction at the same time. A previous study using rat vagus nerve has shown that the large (Aδ-fiber) and small (C-fiber) axons respond to high-frequency block very differently [23]. The large axons are more sensitive to the block than the small axons and also require a longer period to recover from the block. Therefore, it could be speculated that axons of the same size might recover conduction at the same time, resulting in the abrupt appearance of the muscle twitch (Fig. 4A). This abrupt recovery could also be explained by the possible increase in intracellular sodium and extracellular potassium concentrations as mentioned above, because the time for the sodium-potassium pump to restore the ion concentrations will be the same for axons of the same size. More electrophysiological studies to record single nerve fiber activity are warranted in order to further understand axonal recovery from post-stimulation block.
Since muscle contraction force is used to indicate nerve block, only the myelinated A fibers in frog sciatic nerve responsible for sending action potentials to the muscles could be investigated in this study, while the small sensory C fibers were not investigated. The duration of post-stimulation block was monitored by a low frequency (0.5 Hz) stimulation that induced a muscle contraction of about 10 g force, which probably only activated the relatively large A fibers in the sciatic nerve. The sciatic nerve was also transected centrally to eliminate input from motoneurons in the spinal cord. When high frequency axonal block is induced in vivo with intact connections to the spinal motoneurons, it is possible that tonic activity originating in the spinal cord might influence the duration of post-stimulation block. This could be tested in future studies by determining the effects of a range of low frequency stimulation of the nerve at a site central to the block electrode.
The block threshold is defined as the minimal stimulation intensity required to completely block the muscle contractions induced by the high frequency stimulation itself, not induced by the 0.5 Hz Stim.A (see Fig. 2). This is because conduction block can not occur unless the high frequency stimulation blocks the nerve firing induced by the stimulation. Note that at the block threshold the high frequency stimulation must have completely blocked all the motor axons to the muscle generating the recorded tension, because: (1) The block threshold is about 4–5 times higher than the stimulation intensity that induces a maximal muscle contraction (Fig. 2); (2) As the stimulation intensity increases, the high frequency stimulation always excites a nerve fiber first before it can be blocked. Therefore, Fig. 2 shows that as stimulation intensity increases from low to high, the high frequency stimulation first produces a full activation of the sciatic nerve and a maximal muscle contraction, then it gradually blocks the nerve producing a low level of muscle contraction, and finally it completely blocks all nerve fibers without producing any muscle contraction. Therefore, at the block threshold the muscle twitching induced by the 0.5 Hz Stim.A is also blocked completely. It is worth noting that the post-stimulation block is determined by blocking the conduction of nerve activity induced by the 0.5 Hz Stim.A (see Fig. 4), although the block threshold is determined by blocking the high-frequency stimulation-induced tonic muscle contractions (see Fig. 2).
As shown in Fig. 3 when low frequency electrical stimulation (0.5 Hz) was applied distal to the site of high frequency stimulation (10 kHz) which produced a complete block of axonal conduction and a completely relaxed muscle, the low frequency stimulation still produced a normal muscle contraction indicating that neural and muscle mechanisms distal to the axonal block were normal and that neither muscle fatigue nor suppression of neuromuscular transmission contributed to the high frequency block of contractions. Following longer duration blocking stimulation (1–5 min) the muscle remained unresponsive to nerve stimulation for many minutes after an initial transient contraction suggesting that similar mechanisms contribute to the axonal conduction block during and after stimulation.
Only 5 kHz and 10 kHz were investigated in this study. The rationale for choosing these two frequencies is: (1). 5 kHz is the minimal frequency known to block nerve conduction in previous studies [4,16,17]; (2). 5 kHz and 10 kHz were used in previous studies [13,23] to investigate the post-stimulation effects on compound action potentials; (3). Recent clinical applications have used 5 kHz or 10 kHz [18,19]. Therefore, we studied 5 kHz and 10 kHz so that our results could be easily compared with previous studies of post-stimulation effects and at the same time be clinically relevant. It is worth noting that 5–10 kHz stimulation may also block axonal conduction in the brain [5]. However, deep brain stimulation at this high frequency is ineffective in suppressing movement disorders [1], thus treatment of these disorders must depend on mechanisms other than axonal block.
Finally, this study shows that the post-stimulation block is reversible (Fig. 4), indicating that high-frequency biphasic stimulation probably induces a physiological change in the nerve rather than damage that should be irreversible. The reversibility and safety of high-frequency biphasic stimulation was also proven by its chronic application in human subjects to block the vagus nerve and treat obesity [16]. In addition, high-frequency nerve block has been recently applied clinically to treat post-amputation pain [19]. These results further emphasize the importance of the biphasic, charge-balanced, stimulation waveform for a safe clinical application. Understanding post-stimulation block will be very useful to optimize the stimulation parameters for clinical applications to treat chronic pain.
5 Conclusions
In summary, the novel results in this study are: (1) the first demonstration of post-stimulation block of motor axons using direct measurement of neurally evoked muscle contractions rather than by recording compound action potential; (2) the first examination of the influence of stimulation parameters (intensity, frequency and duration) on post-stimulation block. These results (Figs. 4–5) are critical for understanding the mechanism of action of high frequency induced axonal block and focus attention on slow processes such as restoration of intra-axonal ion concentrations by ion pumps instead of short-lasting mechanisms such as depolarization/hyperpolarization. Our results are also important for designing effective clinical stimulation protocols because continuous stimulation might not be necessary for many clinical applications once we learn more about the properties of post-stimulation block.
Acknowledgments
This study is supported by NIH/NIDDK under grants DK-068566
Biographies

Guangning Yang was a Visiting Researcher of Urology at the University of Pittsburgh. His research interests include electrical nerve stimulation and neural control of bladder function.

Zhiying Xiao was a Visiting Scholar of Urology at the University of Pittsburgh. Her research interests include electrical nerve stimulation and neurotransmitters involved in neuromodulation.

Jicheng Wang is a Research Associate of Urology at the University of Pittsburgh. His research interests include model analysis of nerve response to electrical stimulation and neural control of bladder function.

Bing Shen is a Research Associate of Urology at the University of Pittsburgh. Her research interests include electrical nerve stimulation and bladder neuromodulation in treatment of overactive bladder.

James R. Roppolo is an Assistant Professor of Pharmacology and Chemical Biology at the University of Pittsburgh. His research interests include electrical nerve stimulation, and neural control of the lower urinary tract.

William C. de Groat is a Distinguished Professor of Pharmacology and Chemical Biology at the University of Pittsburgh. His research interests include neurophysiology, neuropharmacology, and neuroanatomy of the lower urinary tract.
Changfeng Tai is an Associate Professor of Urology, Bioengineering, Pharmacology and Chemical Biology at the University of Pittsburgh. His research interests include electrical nerve stimulation and neuro-urology.
References
- 1.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 2.Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve. 2005;32:782–790. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- 3.Bhadra N, Kilgore KL. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation. 2014;17:242–254. doi: 10.1111/ner.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman BR, McNeal DR. Response of single alpha motoneurons to high-frequency pulse trains. Firing behavior and conduction block phenomenon. Appl Neurophysiol. 1986;49:121–138. doi: 10.1159/000100137. [DOI] [PubMed] [Google Scholar]
- 5.Couto J, Grill WM. Kilohertz frequency deep brain stimulation is ineffective at regularizing the firing of model thalamic neurons. Front Computational Neuroscience. 2016;10:22. doi: 10.3389/fncom.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuellar JM, Alataris K, Walker A, Yeomans DC, Antognini JF. Effect of high-frequency alternating current on spinal afferent nociceptive transmission. Neuromodulation. 2013;16:318–327. doi: 10.1111/ner.12015. [DOI] [PubMed] [Google Scholar]
- 7.Frankenhaeuser B, Huxley AF. The action potential in the myelinated nerve fibre of Xenopus Laevis as computed on the basis of voltage clamp data. J Physiol. 1964;171:302–315. doi: 10.1113/jphysiol.1964.sp007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaunt A, Prochazka A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehab Neural Repair. 2009;23:615–626. doi: 10.1177/1545968308328723. [DOI] [PubMed] [Google Scholar]
- 9.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph L, Butera R. Unmeylinated aplysia nerves exhibit a nonmonotonic blocking response to high-frequency stimulation. IEEE Trans Neural Syst Rehabil Eng. 2009;17:537–544. doi: 10.1109/TNSRE.2009.2029490. [DOI] [PubMed] [Google Scholar]
- 11.Joseph L, Butera R. High-frequency stimulation selectively blocks different types of fibers in frog sciatic nerve. IEEE Trans Neural Syst Rehabil Eng. 2011;19:550–557. doi: 10.1109/TNSRE.2011.2163082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilgore KL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput. 2004;42:394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Zhu L, Sheng S, Sun L, Zhou H, Tang H, Qiu T. Post stimulus effects of high frequency biphasic electrical current on a fibre’s conductibility in isolated frog nerves. J Neural Eng. 2013;10:036024. doi: 10.1088/1741-2560/10/3/036024. [DOI] [PubMed] [Google Scholar]
- 14.Rattay F. Analysis of models for extracellular fiber stimulation. IEEE Trans Biomed Eng. 1989;36:676–682. doi: 10.1109/10.32099. [DOI] [PubMed] [Google Scholar]
- 15.Rattay F, Aberham M. Modeling axon membranes for functional electrical stimulation. IEEE Trans Biomed Eng. 1993;40:1201–1209. doi: 10.1109/10.250575. [DOI] [PubMed] [Google Scholar]
- 16.Reboul J, Rosenblueth A. The action of alternating currents upon the electrical excitability of nerve. Am J Physiol. 1939;125:205–215. [Google Scholar]
- 17.Rosenblueth A, Reboul J. The blocking and deblocking effects of alternating currents on nerve. Am J Physiol. 1939;125:251–264. [Google Scholar]
- 18.Sarr MG, Billington CJ, Brancatisano R, Brancatisano A, Toouli J, Kow L, Nguyen NT, Blackstone R, Maher JW, Shikora S, Reeds DN, Eagon JC, Wolfe BM, O’Rourke RW, Fujioka K, Takata M, Swain JM, Morton JM, Ikramuddin S, Schweitzer M, Chand B, Rosenthal The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012;22:1771–1782. doi: 10.1007/s11695-012-0751-8. [DOI] [PubMed] [Google Scholar]
- 19.Soin A, Shah NS, Fang ZP. High-frequency electrical nerve block for postamputation pain: a pilot study. Neuromodulation. 2015;18:197–206. doi: 10.1111/ner.12266. [DOI] [PubMed] [Google Scholar]
- 20.Tai C, de Groat WC, Roppolo JR. Simulation analysis of conduction block in unmyelinated axons induced by high-frequency biphasic electrical currents. IEEE Trans Biomed Eng. 2005;52:1323–1332. doi: 10.1109/tbme.2005.847561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai C, Roppolo JR, de Groat WC. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J Urol. 2004;172:2069–2072. doi: 10.1097/01.ju.0000140709.71932.f0. [DOI] [PubMed] [Google Scholar]
- 22.Tanner JA. Reversible blocking of nerve conduction by alternating current excitation. Nature. 1962;195:712–713. doi: 10.1038/195712b0. [DOI] [PubMed] [Google Scholar]
- 23.Waataja J, Tweden K, Honda C. Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng. 2011;8:056013. doi: 10.1088/1741-2560/8/5/056013. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Roppolo JR, de Groat WC, Tai C. Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE Trans Biomed Eng. 2006;53:2445–2454. doi: 10.1109/TBME.2006.884640. [DOI] [PMC free article] [PubMed] [Google Scholar]