Abstract
Study Design
In vivo patient biomechanical study.
Objective
To investigate the dimensions of lumbar intervertebral foramen (LIVF) of patients with degenerative disc disease (DDD) during a flexion-extension motion of the body.
Summary of Background Data
LIVF narrowing may result in nerve root compression. The area changes of degenerated and adjacent non-degenerated LIVFs in DDD patients under physiologic loading conditions are unknown.
Methods
Nine symptomatic low back pain patients with radiological evidence of L4-S1 DDD were recruited. Each subject was MRI scanned for construction of 3D lumbar vertebral models, and fluoroscopically imaged when the body extended from 45° flexion to full extension for reconstruction of LIVF dimensions. The data of the adjacent segment L3/4 and diseased segment L4/5 and L5/S1 were compared with a normal control group at 45° flexion, upright, and full extension of the body.
Results
The mean LIVF areas of DDD segments were significantly smaller than those of the normal subjects in all positions (p<0.05). In upright position, the LIVF areas of the DDD patients were 32.8% and 33.6% smaller than the normal subjects for L4/5 and L5/S1, respectively. For the adjacent L3/4, the LIVF area of the DDD patients was 32.3% smaller than that of the normal controls (p<0.05). The total change of L3/4 LIVF area in DDD patients from flexion to extension was significantly smaller than that of the normal subjects, but the changes in L4/5 and L5/S1 LIVF areas were similar between the two groups (p>0.05).
Conclusion
Similar reductions of the LIVF dimensions were observed at the adjacent and the involved levels of the DDD patients, implying that biomechanical changes might have already occurred at the adjacent segment despite the lack of radiographic evidence of degeneration. Subsequent research should focus on the effects of surgical fusion on the biomechanical features of the adjacent segment.
Level of Evidence
N/A
Keywords: Lumbar intervertebral foramen, foramen area, degenerative disc disease, stenosis, flexion, extension, in vivo, adjacent segment degeneration, low back pain
Introduction
Lumbar degenerative disc disease (DDD) results in loss of disc height which is correlated with lumbar intervertebral foramen (LIVF) stenosis 1. Hasegawa et al. in a cadaver study described the critical dimension of 4mm or less of posterior disc space height and 15 mm of foraminal height that lead to compression of the nerve root in the foramen. The geometric changes could cause compression of the nerve root in the lower lumbar spine 2, resulting in lower back pain and lower limb radiculopathy in adults 3.
Numerous studies have suggested altered biomechanics at levels adjacent to a fusion are responsible for the development of adjacent segment degeneration 4-7. However it remains unclear whether these changes began developing with degeneration of the index level prior to any surgical intervention. Wang et al have described differences in the disc deformation of healthy appearing cephalic adjacent above L4 to S1 discs with degeneration when compared to normal subjects 8. There is limited literature detailing the in vivo dynamic biomechanical effects of disc degeneration on the LIVF areas of involved levels and non-degenerated adjacent segment levels 9. Previous in vitro cadaveric studies have described a decreased range of motion and decreased intervertebral foraminal area from flexion to extension 9,10. Comparatively, in vivo studies have highlighted posture-dependent LIVF geometry changes, demonstrating larger LIVF dimensions in non-weightbearing positions and decreased dimensions in weightbearing hyperlordotic positions 11. The in vivo changes of the LIVF dimensions in DDD patients under physiologic loading conditions are still not well understood. A quantitative understanding of the geometric characters of the LIVF is critical for improvement of the diagnosis and treatment of LIVF stenosis 12.
The purpose of this study was to investigate the in vivo LIVF dimensional changes in patients with DDD during a flexion-extension movement of the body. DDD patients were studied using a combined dual fluoroscopic imaging system and MRI based 3D modeling technique. The data was compared with a previously investigated normal subject group. We hypothesize that DDD affects not only the LIVF of the involved motion segments, but also the adjacent, non-degenerated motion segment.
Materials and Methods
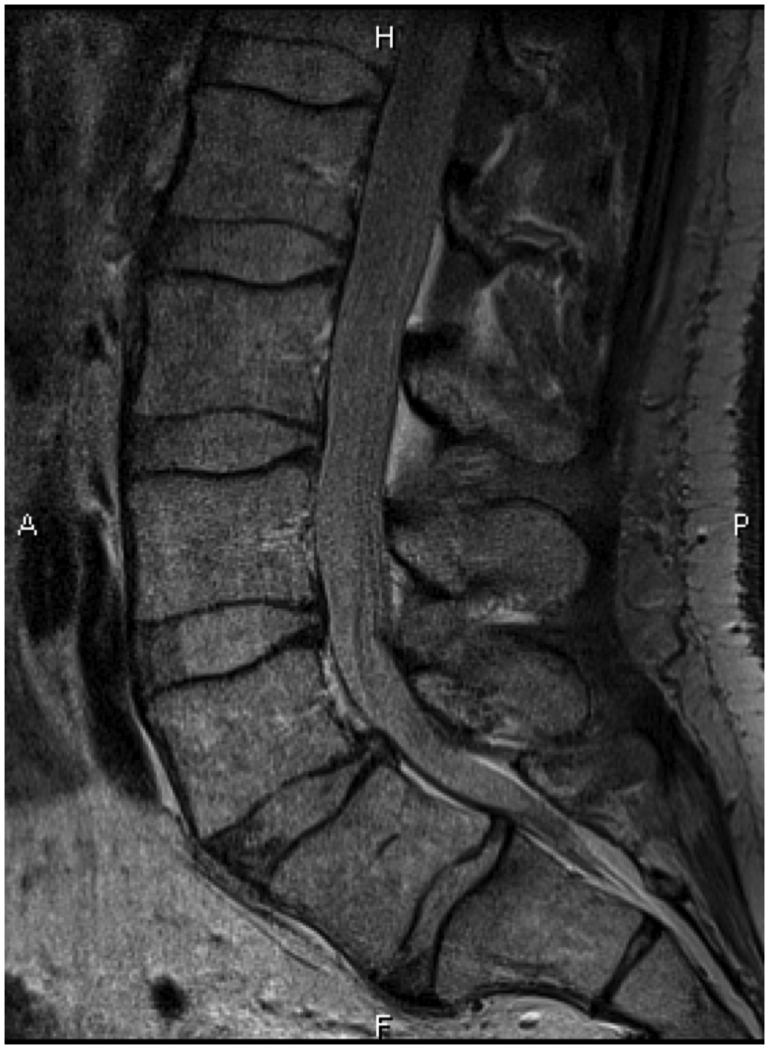
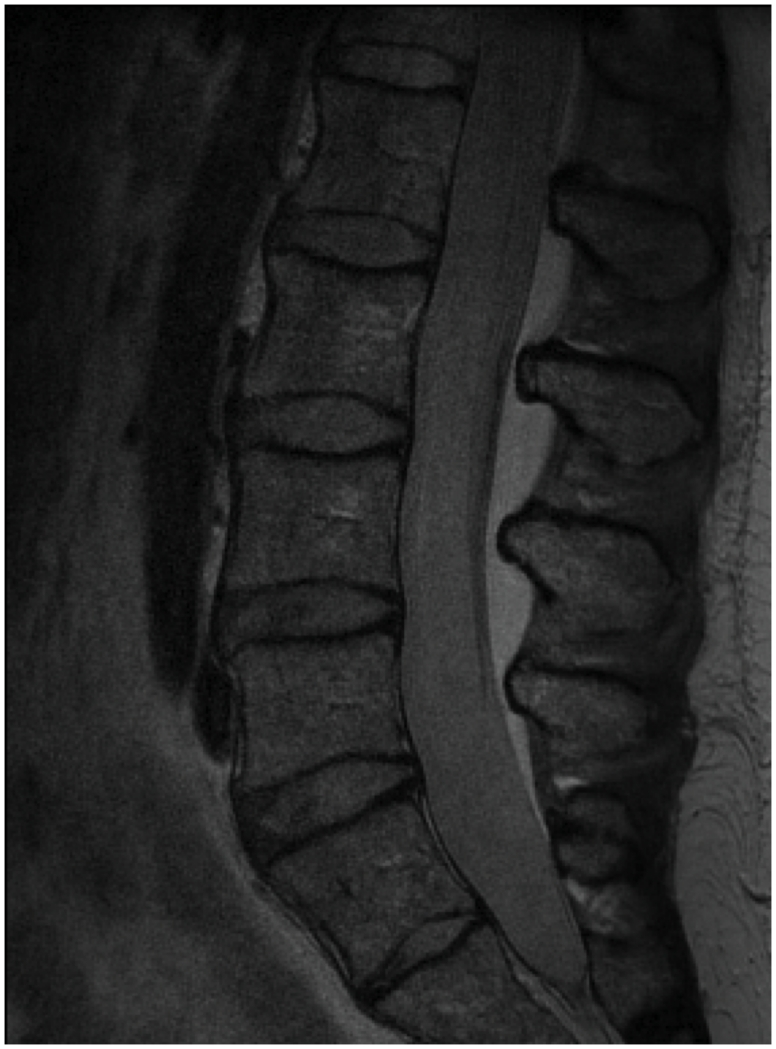
Nine symptomatic patients (three females, six males, aged 50 to 60 years, mean BMI 23.8kg/m2) with evidence of lumbar degenerative disc disease (DDD) visible on a T2-weighted MRI were recruited for this prospective study. All DDD patients in this study reported having both back and radicular leg symptoms corresponding to their L4/5 or L5/S1 dermatomes. We used the Pfirrmann classification13 to determine the grade of lumbar intervertebral disc degeneration from T2-weighted MRI for each patient from L2 to S1 (Figure 1A and 1B). Grades of I and II indicate mostly normal disc physiology while grades of IV and V indicate abnormal, severely degenerated discs. The L3/4 disc had a grade of 1.6 ± 0.9, L4/5 of 4.1 ± 0.6, and L5/S1 of 4.4 ± 0.5.
Figure 1.
(A) A lumbar T-2 weighted mid-sagittal MR image of a patient with degenerative disc disease at L4/5 and L5/S1; (B) MR image of a normal subject with healthy discs.
Institutional review board approval was obtained for this study. Informed consent was obtained from each subject prior to their participation in this experiment. Exclusion criteria for this study included patients with spondylolisthesis, pars interarticularis defects, congenital stenosis with shortened pedicles, history of vertebral fractures or tumors, or systemic musculoskeletal disease.
Subjects were scanned individually in a supine, relaxed position using a 3.0-T scanner (MAGNETOM Trio; Siemens, Erlangen, Germany) with a spine surface coil and a T2-weighted fat-suppressed 3D spoiled gradient-recalled echo sequence. One millimeter thick parallel slices were captured digitally with no gap at a resolution of 512 × 512 pixels. Using these digital MR images, a solid modeling software program (Rhinoceros; Robert McNeel & Associates, Seattle, WA, USA) was used to reconstruct a 3D model of lumbar vertebral segments from L2 to S1 9. The posterior vertebral body height (PVBH) was measured for each vertebra.
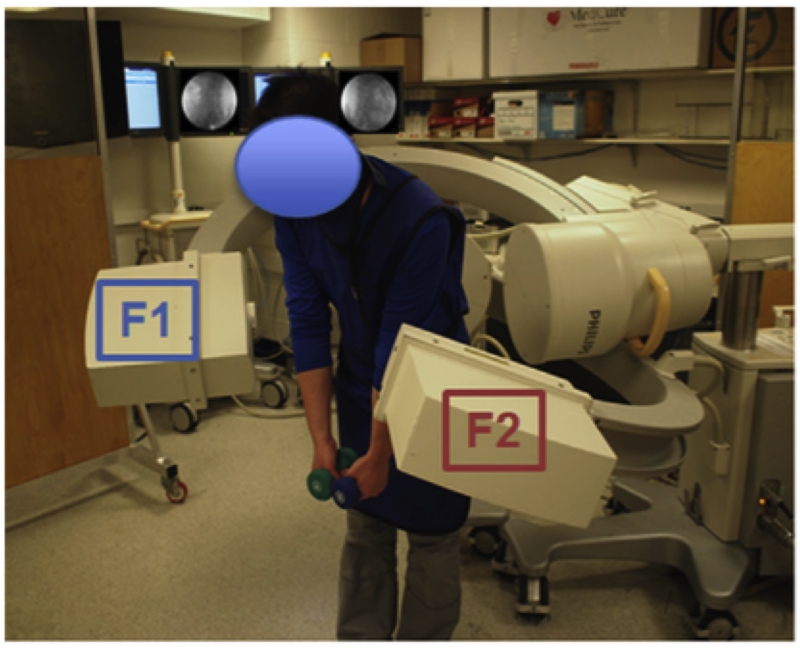
Each patient’s lumbar spine was then imaged using the dual fluoroscopic imaging system (DFIS) (BC Pulsera, Philips, Bothell, WA, USA) as the subject performed lumbar extension from a flexion position of 45° to a maximal extension position, with each hand holding an 8-pound dumbbell 14 (Figure 2A). Pelvis motion was limited throughout the lifting motion by a custom-built frame. The fluoroscopes captured the dynamic positions of the vertebrae at 30 frames per second with an 8 millisecond pulse width. The motion took approximately 2 seconds resulting in the collection of roughly 60 fluoroscopic image frames per scope. All subjects donned lead vests, skirts, and thyroid shields to minimize radiation exposure during fluoroscopic imaging.
Figure 2.
(A) Experimental set-up of the dual fluoroscopic imaging system; (B) Virtual dual fluoroscopic system used to reproduce lumbar vertebral motion. Illustration reproduced with permission of Elsevier (License 3796600837782).
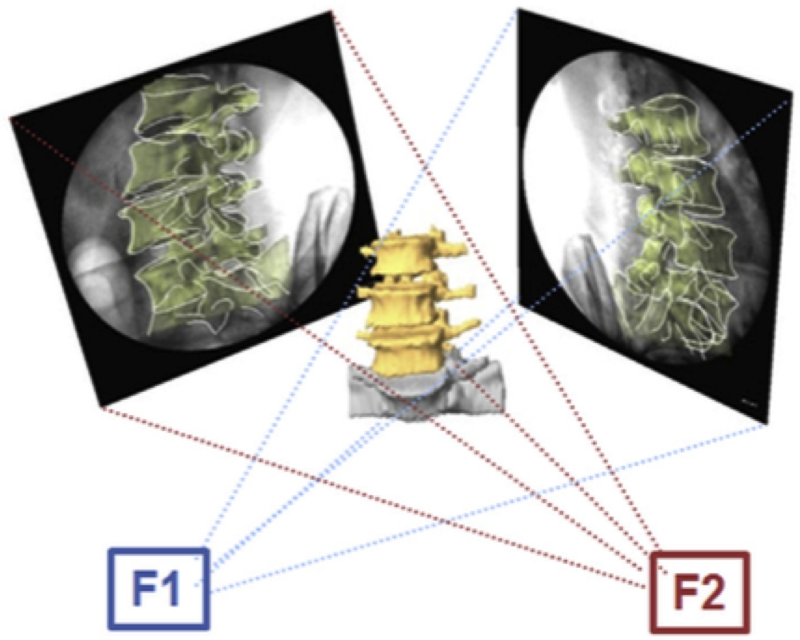
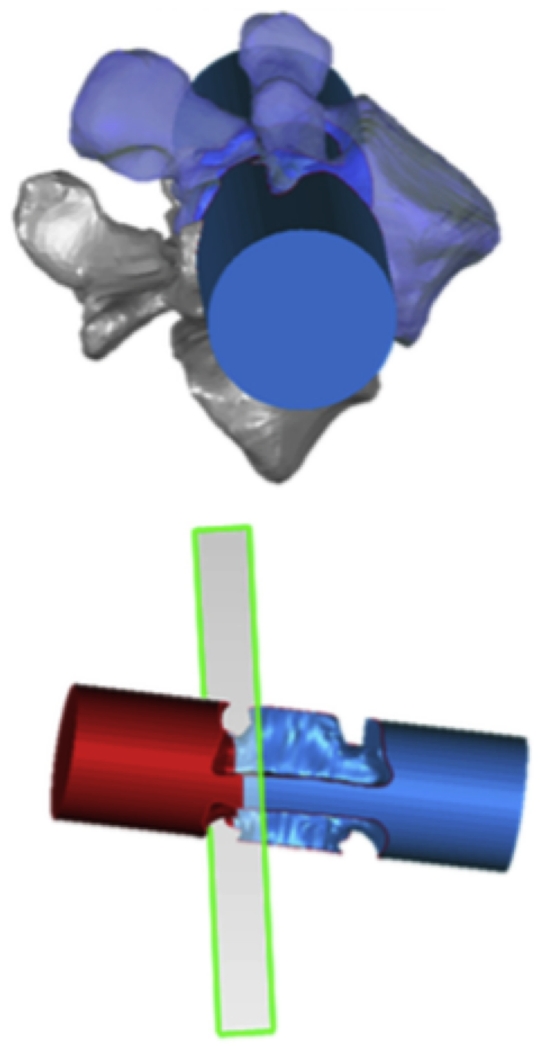
The in vivo positions of the vertebrae along the dynamic motion path of the weight-lifting activity were reproduced in the Rhinoceros software using the 3D vertebral models and the fluoroscopic images 15 (Figure 2B). This required the reproduction of the DFIS environment in the software and performing six degrees of freedom (6DOF) adjustments of the 3D vertebral models to match the fluoroscopic images. This technique has been previously validated and has been shown to have an error margin of 0.3 mm for dynamic vertebral position and 0.7° for vertebral orientation 15.
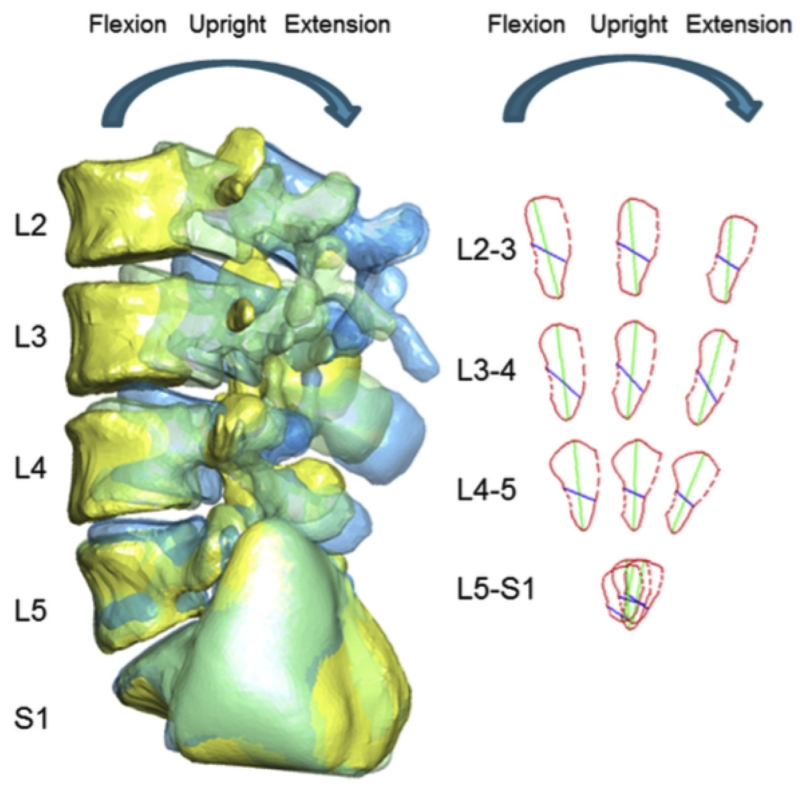
Figure 3A shows a typical 3D model of the reconstructed L3 to S1 lumbar segments. Subsequently, 3D models of the LIVF were obtained using the Boolean operator in the solid modeling software. The smallest cross sectional area of each LIVF was obtained from the pedicle cutting plane using another 3D modeling software (Geomagic, ThreeD Systems, Rock Hill, SC, USA) (Figures 3A and 3B)9. The LIVF cross section from which the area is derived was drawn according to the LIVF bony outline (solid red outline) and soft tissue (dashed red outline). Soft tissue was not accounted for in this study as soft tissue borders could not be visualized on dual fluoroscopy. For each intervertebral level, an average of the left and right LIVF dimensions was recorded.
Figure 3.
(A) 3D model of reconstructed L2 to S1 lumbar segments and measured positions of the lumbar spine and the foramen; (B) Construction of minimal LIVF area using 3D computation models. Illustration reproduced with permission of Elsevier (License 3796600837782).
We compared the LIVF areas of the L3-S1 vertebral motion segments of our DDD patients during the weightbearing flexion-extension activity against historical asymptomatic, normal controls without history of back pain, anatomic abnormality, or other spinal disorders (five males, five females, aged 40-60 years) 9. For the control group, the mean Pfirrmann grades were 1.2 ± 0.4, 1.7 ± 0.8 and 2.3 ± 1.3 for the L3/4, L4/5 and L5/S1 levels respectively. Three positions along the motion path were selected for analysis: 45° flexion, upright, and maximal extension. Range of motion (ROM) of each segment was obtained by measuring the angle (°) through which each vertebrae moved from 45° flexion to maximal extension (Table 1). Changes in LIVF areas when the body extended from flexion to full extension were also determined for the DDD segments and the adjacent segment. In addition, we measured the mean segmental angle of lumbar lordosis in the upright position (Table 1).
An ANOVA with Tukey’s post-hoc test for multiple comparisons was used to compare the LIVF dimensions between levels in the DDD patients. An unpaired Student’s t-test was used to compare the mean segmental lordosis, PVBHs, LIVF areas, and LIVF area changes of the DDD patients and the normal controls. Statistical significance was defined at p<0.05. Statistical analysis was performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Segmental morphological and ROM data
There was no statistical significant difference in segmental lordosis and posterior vertebral body heights between the DDD and normal patients (p>0.05) (Table 1). The ROM of L4/5 and L5/S1 in DDD patients were significantly lower than those of normal subjects (p<0.05), but no significant difference was found between DDD patients and normal subjects at the L3/4 adjacent segment.
LIVF areas
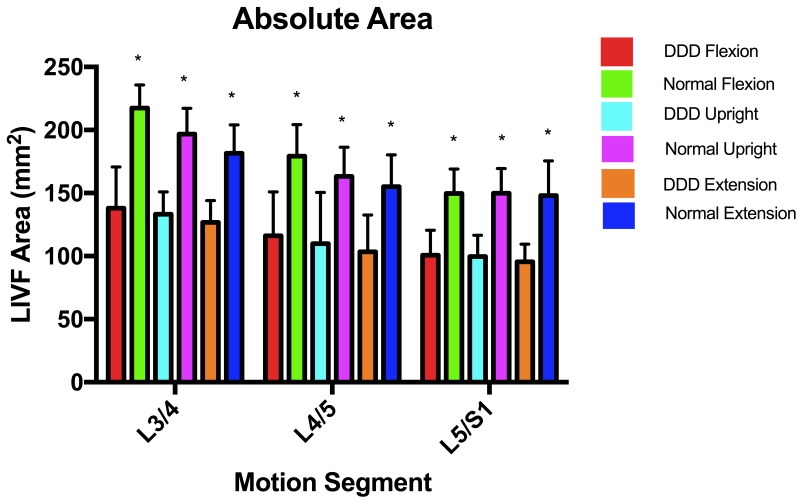
At each of the three body positions, the mean LIVF areas decreased monotonically from L3/4 to L5/S1, with L3/4 being significantly different from L5/S1 (Table 1, Figure 4A). LIVF area also decreased with extension of the body at each motion segment. At the upright position, the LIVF areas were 109.8 ± 40.8 mm± and 99.6 ± 17.0 mm±, respectively for L4/5 and L5/S1 of the DDD patients. For the adjacent L3/4 level, the LIVF area was 133.3 ± 17.6 mm±. Compared with the corresponding segments in normal subjects, the mean LIVF areas of DDD patients were significantly smaller at both the involved and the adjacent segment levels (p < 0.05) (Table 1, Figure 4A). At upright position, the average LIVF areas were reduced by 32.8% at L4/5 and 33.6% at L5/S1, and the adjacent level L3/4 was reduced by 32.3% compared to the corresponding segment of the normal subjects.
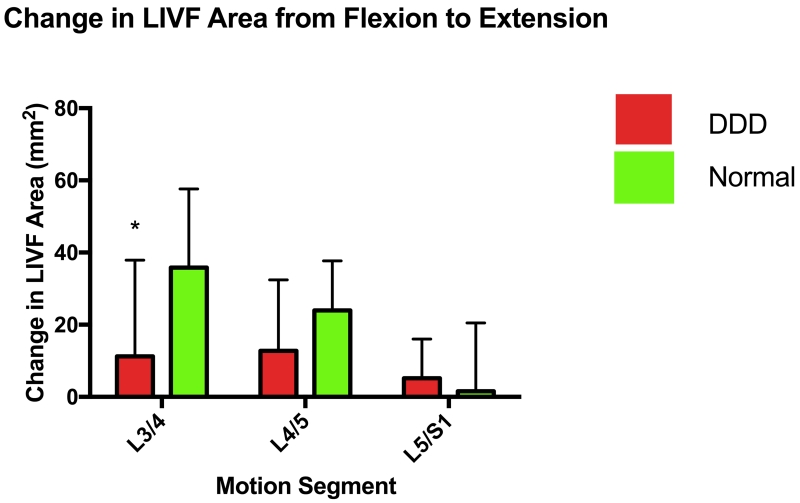
Figure 4.
(A) Absolute LIVF areas of DDD and Normal subjects; (B) Changes of LIVF areas of normal and DDD subjects between the flexion and extension positions.
Changes of foramen areas along the extension path
From 45° flexion to the maximal extension of the body, the change of LIVF area at the adjacent L3/4 motion segment was 11.3 ± 26.6 mm2 which was significantly smaller than that of the normal subjects (35.8 ± 21.8 mm2) (p<0.05) (Figure 4B). The changes of LIVF areas were 12.8 ± 19.6 mm2for the L4/5 and 5.2 ± 10.8 mm2 for the L5/S1 levels of the DDD patients. These were not significantly different compared with the normal subjects at both the L4/5 and L5/S1 levels (p>0.05) (Figure 4B).
Discussion
This study investigated the in vivo characteristics of the LIVF areas in symptomatic patients with degenerative disc disease at L4 to S1 during a flexion-extension movement and compared the data with that of a healthy, normal control group. The results revealed that the LIVF areas of the DDD patients are significantly smaller when compared with those of the normal subjects at both the degenerative levels and the non-degenerated adjacent level. From flexion to extension, the LIVF areas of the DDD and adjacent level displayed a similar decreasing trend. These data supported our hypothesis by showing that lumbar DDD at L4/5 and L5/S1 not only affects the LIVF of the diseased segments, but also affects the adjacent non-degenerated L3/4 segment.
Previous studies have investigated the LIVF geometry of normal subjects at different positions of the body using imaging techniques. For example, Zhong et al. found a decrease in the foramen dimensions of normal patients during extension of the body 9. However, no data has been reported on the effect of DDD on the geometric changes of the LIVF of living patients. Cadaveric studies by Hasegawa et al. and Iwata et al. 2,16 demonstrated decreased disc height and, in turn, decreased LIVF height, which is consistent with the findings regarding LIVF areas in the DDD cohort. In our study, the DDD patients had smaller LIVF areas at both the diseased L4/5 and L5/S1 levels and the non-degenerated adjacent L3/4 level through the flexion/extension movement when compared to normal subjects. The lack of a significant difference in ROM at the cranial adjacent segment (L3/4) between the DDD patients and normal subjects was consistent with a recent study by Lao et al 17. However, the higher mean range of motion in the cranial adjacent segment (L3/4) compared to the involved DDD segments was also consistent with other studies which have demonstrated increased mobility of segments adjacent to degenerated discs 8.
Numerous studies have also shown that DDD could cause reduction of LIVF area and thus nerve root compression1,17. Our data show that the LIVF areas at the diseased L4/5 and L5/S1 levels were reduced by over 30% compared to the normal control subjects. Interestingly, the LIVF area of the non-degenerated L3/4 adjacent level in DDD patients was also reduced similarly by over 30% when compared to normal controls despite having a segmental ROM which was not significantly different from the normal controls. This finding indicates that the LIVF area of the non-degenerated L3/4 adjacent level in DDD patients may have undergone similar geometric changes to its neighboring diseased levels. The mechanism of this phenomenon is unclear and warrants further investigation.
From the literature , the initial physiologic lordosis while standing was thought to affect the LIVF dimensions 18. A higher degree of initial lordosis could reduce the baseline LIVF area similarly to that occurring in extension of the body, thus confounding the comparison between the normal and DDD cohorts 9. However, no difference was found in segmental lumbar lordosis between the DDD and normal control groups in this study. Similarly, posterior vertebral body height (PVBH) has been postulated to affect LIVF area 2. However, we found no significant difference in the PVBH between the DDD patients and normal subjects. In addition, none of the DDD patients had evidence of congenital stenosis, shortened pedicles or spondylolisthesis which may have affected LIVF measurements. These findings suggest that there could be other factors, such as disc height that may, in part, contribute to the differences in LIVF dimensions between the DDD patients and normal controls. Further, the non-degenerated, cranially adjacent L3/4 level had no MRI evidence of disc degeneration nor nerve root compression-related pain among this group of patients. This could imply that radicular symptomology may only occur once the LIVF area reaches a certain threshold value that is related to the structural characteristics of the nerve roots 19. A future study should investigate the foraminal area changes of the adjacent segment with time using a longitudinal experimental design.
The results of the current study need to be interpreted in light of the potential limitations. First, this investigation, by nature of using MRI and fluoroscopy matching techniques, is unable to take into account other soft tissue structures that occupy the LIVF such as the posterior border of the intervertebral disc, ligamentum flavum, and posterior longitudinal ligament. Soft tissue contribution to foraminal stenosis has been studied in healthy cadaveric spines by Inufusa et al. and Fujiwara et al., and they also found that foramen area decreased during extension in normal subjects 10,19. Improved imaging technology that can capture in vivo, weightbearing motion of the spine, with both bony and soft tissue structures will allow better estimation of LIVF properties. Second, we acknowledge that our study has a small sample size. LIVF dimensions are determined by many biological variables such as the inter-subject variation which could be accounted for with a larger sample size 12. Third, we exclusively used the Pfirmann classification, which determines the quality of lumbar intervertebral discs from a non-loaded T2-weighted mid-sagittal MRI. We recognize that there are several alternative methods of determining intervertebral disc health, such as T2 relaxation times, which may indicate extant degenerative changes at levels that are considered non-degenerated under the Pfirrmann classification scheme 20,21. In addition, the use of axially-loaded MRI may also provide useful information regarding the quality of intervertebral discs under physiological loading conditions 22,23.
Lastly, the subjects were evaluated during an extension motion of the body. A recent review article indicated that this may not represent the loading the DDD patients would experience in daily life and the normal controls and the DDD patients may perform this activity differently 24. Therefore, future studies of DDD patients should be conducted under loading conditions experienced during typical daily activities such as walking. Despite these various limitations, this study was the first to provide quantitative data on LIVF areas of DDD segments and the non-degenerated adjacent level during in vivo weightbearing, flexion-extension motion of the body.
Conclusion
In conclusion, symptomatic patients with DDD have significantly smaller LIVF areas at flexion, standing, and extension when compared to normal controls. Decreased LIVF area was observed not only at the diseased level, but also at the non-degenerated cranial adjacent level which suggests that geometric changes may have occurred despite the lack of radiographic intervertebral disc degeneration of the adjacent segment. These results suggest a future study of the nerve root structures at the diseased segments and the adjacent level to help understand any influence degenerative disc disease can have on LIVF dimensional changes.
Supplementary Material
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). National Institute of Health (R21AR057989) and DePuy Synthes, Inc. funds were received in support of this work.
Relevant financial activities outside the submitted work: grants.
References
- 1.Cinotti G, De Santis P, Nofroni I, et al. Stenosis of lumbar intervertebral foramen: anatomic study on predisposing factors. Spine (Phila Pa 1976) 2002;27:223–9. doi: 10.1097/00007632-200202010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa T, An HS, Haughton VM, et al. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am. 1995;77:32–8. [PubMed] [Google Scholar]
- 3.Lee SY, Kim TH, Oh JK, et al. Lumbar Stenosis: A Recent Update by Review of Literature. Asian Spine J. 2015;9:818–28. doi: 10.4184/asj.2015.9.5.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strube P, Tohtz S, Hoff E, et al. Dynamic stabilization adjacent to single-level fusion: part I. Biomechanical effects on lumbar spinal motion. Eur Spine J. 2010;19:2171–80. doi: 10.1007/s00586-010-1549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata H, Schendel MJ, Transfeldt EE, et al. The effects of immobilization of long segments of the spine on the adjacent and distal facet force and lumbosacral motion. Spine (Phila Pa 1976) 1993;18:2471–9. doi: 10.1097/00007632-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Weinhoffer SL, Guyer RD, Herbert M, et al. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine (Phila Pa 1976) 1995;20:526–31. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Molz FJ, Partin JI, Kirkpatrick JS. The acute effects of posterior fusion instrumentation on kinematics and intradiscal pressure of the human lumbar spine. J Spinal Disord Tech. 2003;16:171–9. doi: 10.1097/00024720-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Xia Q, Passias P, et al. How does lumbar degenerative disc disease affect the disc deformation at the cephalic levels in vivo? Spine (Phila Pa 1976) 2011;36:E574–81. doi: 10.1097/BRS.0b013e3181f79e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong W, Driscoll SJ, Tsai TY, et al. In vivo dynamic changes of dimensions in the lumbar intervertebral foramen. Spine J. 2015;15:1653–9. doi: 10.1016/j.spinee.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara A, An HS, Lim TH, et al. Morphologic changes in the lumbar intervertebral foramen due to flexion-extension, lateral bending, and axial rotation: an in vitro anatomic and biomechanical study. Spine (Phila Pa 1976) 2001;26:876–82. doi: 10.1097/00007632-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Singh V, Montgomery SR, Aghdasi B, et al. Factors affecting dynamic foraminal stenosis in the lumbar spine. Spine J. 2013;13:1080–7. doi: 10.1016/j.spinee.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 12.Senoo I, Espinoza Orias AA, An HS, et al. In vivo 3-dimensional morphometric analysis of the lumbar foramen in healthy subjects. Spine (Phila Pa 1976) 2014;39:E929–35. doi: 10.1097/BRS.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 14.Wu M, Wang S, Driscoll SJ, et al. Dynamic motion characteristics of the lower lumbar spine: implication to lumbar pathology and surgical treatment. Eur Spine J. 2014;23:2350–8. doi: 10.1007/s00586-014-3316-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Passias P, Li G, et al. Measurement of vertebral kinematics using noninvasive image matching method-validation and application. Spine (Phila Pa 1976) 2008;33:E355–61. doi: 10.1097/BRS.0b013e3181715295. [DOI] [PubMed] [Google Scholar]
- 16.Iwata T, Miyamoto K, Hioki A, et al. In vivo measurement of lumbar foramen during axial loading using a compression device and computed tomography. J Spinal Disord Tech. 2013;26:E177–82. doi: 10.1097/BSD.0b013e318286f635. [DOI] [PubMed] [Google Scholar]
- 17.Stephens MM, Evans JH, O’Brien JP. Lumbar intervertebral foramens. An in vitro study of their shape in relation to intervertebral disc pathology. Spine (Phila Pa 1976) 1991;16:525–9. [PubMed] [Google Scholar]
- 18.Spivak JM, Kummer FJ, Chen D, et al. Intervertebral foramen size and volume changes in low grade, low dysplasia isthmic spondylolisthesis. Spine (Phila Pa 1976) 2010;35:1829–35. doi: 10.1097/BRS.0b013e3181ccc59d. [DOI] [PubMed] [Google Scholar]
- 19.Inufusa A, An HS, Lim TH, et al. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine (Phila Pa 1976) 1996;21:2412–20. doi: 10.1097/00007632-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Takashima H, Takebayashi T, Yoshimoto M, et al. Correlation between T2 relaxation time and intervertebral disk degeneration. Skeletal Radiol. 2012;41:163–7. doi: 10.1007/s00256-011-1144-0. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe A, Benneker LM, Boesch C, et al. Classification of intervertebral disk degeneration with axial T2 mapping. AJR Am J Roentgenol. 2007;189:936–42. doi: 10.2214/AJR.07.2142. [DOI] [PubMed] [Google Scholar]
- 22.Huang KY, Lin RM, Lee YL, et al. Factors affecting disability and physical function in degenerative lumbar spondylolisthesis of L4-5: evaluation with axially loaded MRI. Eur Spine J. 2009;18:1851–7. doi: 10.1007/s00586-009-1059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saifuddin A, Blease S, MacSweeney E. Axial loaded MRI of the lumbar spine. Clin Radiol. 2003;58:661–71. doi: 10.1016/s0009-9260(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 24.Malakoutian M, Volkheimer D, Street J, et al. Do in vivo kinematic studies provide insight into adjacent segment degeneration? A qualitative systematic literature review. Eur Spine J. 2015;24:1865–81. doi: 10.1007/s00586-015-3992-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.