Abstract
Proteomics has identified potential pathways involved in platelet storage lesions, which correlate with untoward effects in the recipient, including febrile non-haemolytic reactions. We hypothesize that an additional pathway involves protein mediators that accumulate in the platelet supernatants during routine storage in a donor gender-specific fashion.
Apheresis platelet concentrates were collected from 5 healthy males and 5 females and routinely stored. The 14 most abundant plasma proteins were removed and the supernatant proteins from days 1 and 5 were analyzed via 1D-SDS-PAGE/nanoLC-MS/MS, before label-free quantitative proteomics analyses. Findings from a subset of 18 proteins were validated via LC-SRM analyses against stable isotope labeled standards.
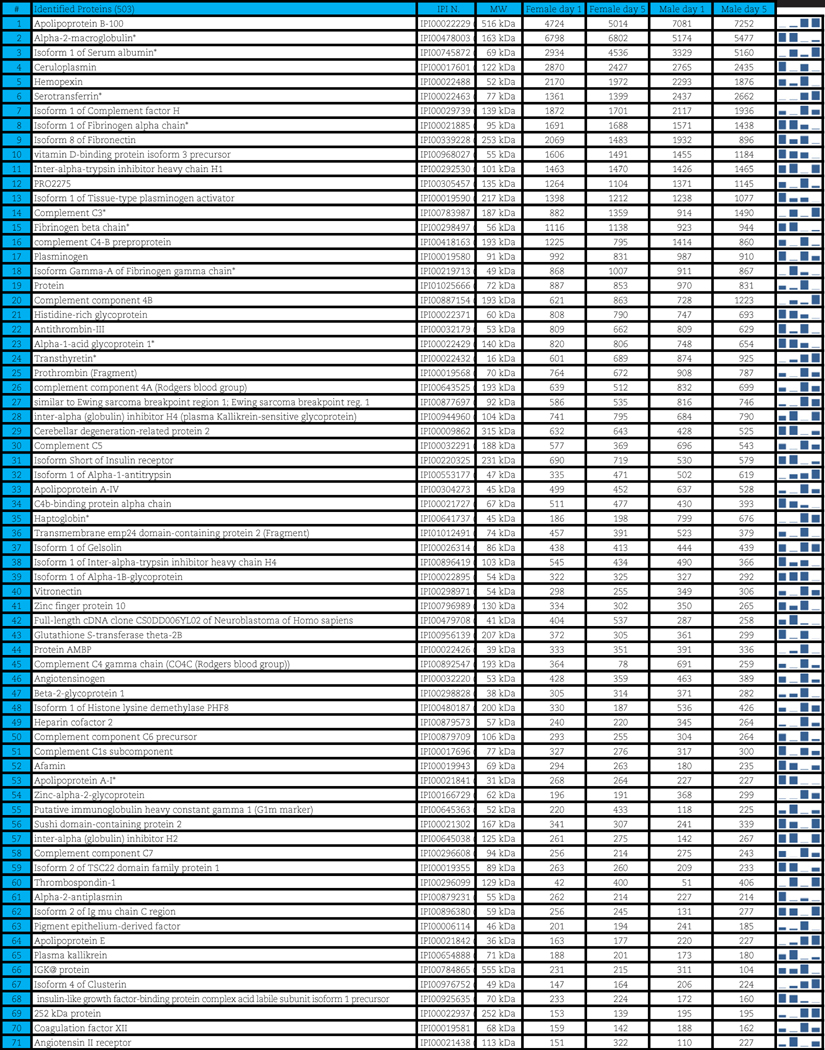
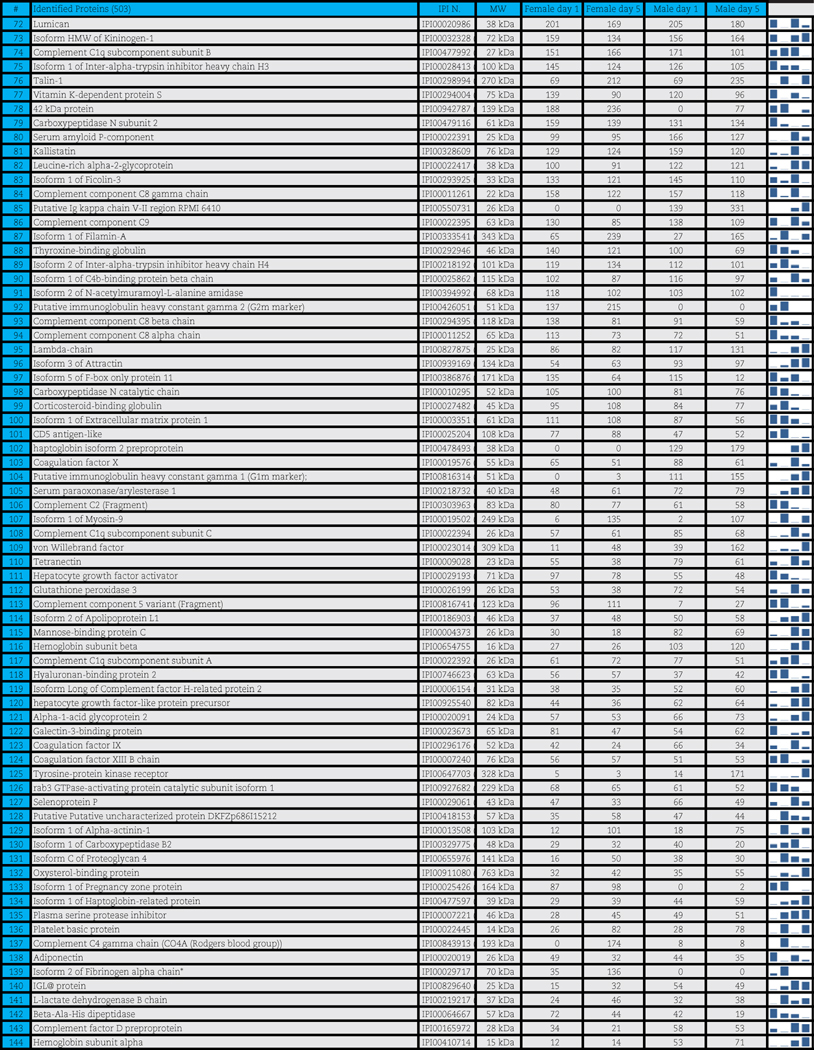
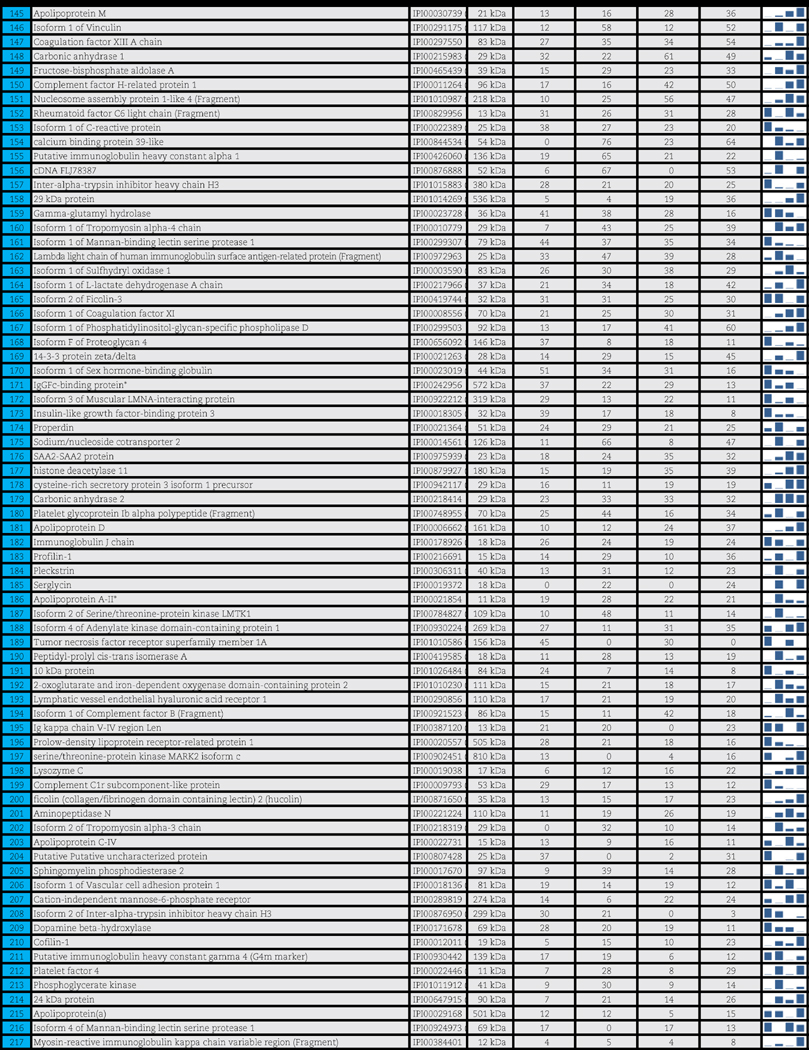
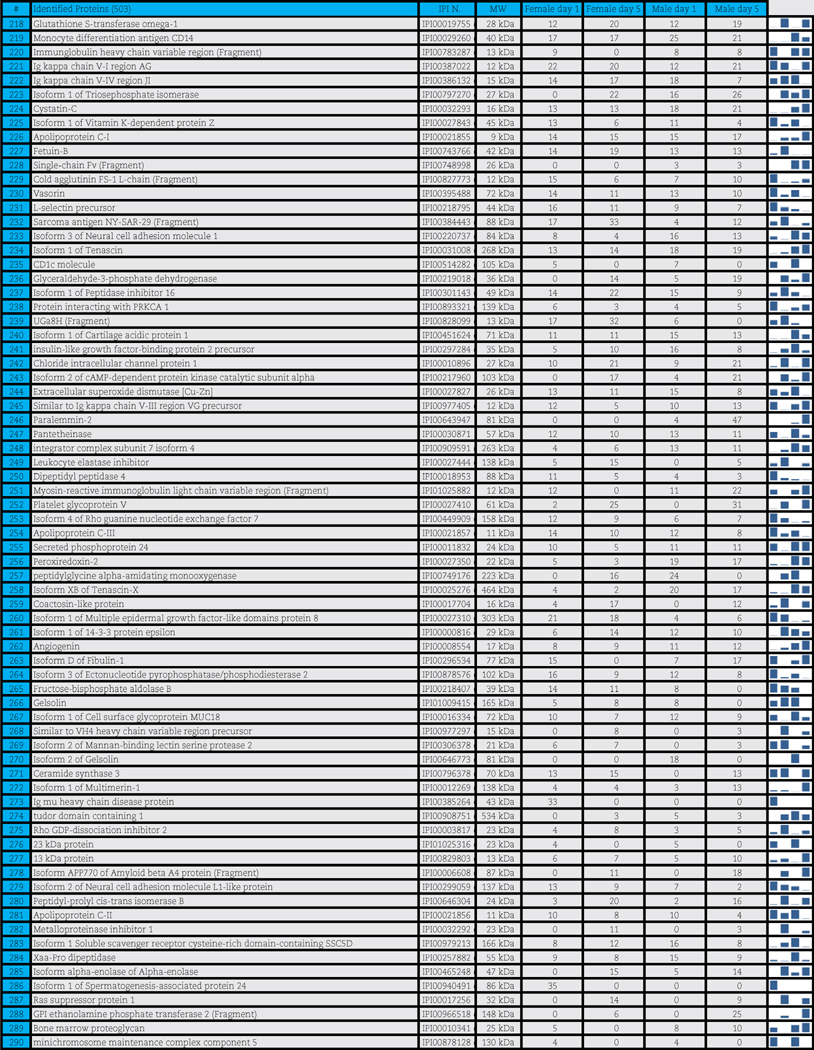
A total of 503 distinct proteins were detected in the platelet supernatants from the 4 sample groups: female or male donor platelets, either at storage day 1 or 5. Proteomics suggested a storage and gender-dependent impairment of blood coagulation mediators, pro-inflammatory complement components and cytokines, energy and redox metabolic enzymes. The supernatants from female donors demonstrated increased deregulation of structural proteins, extracellular matrix proteins and focal adhesion proteins, possibly indicating storage-dependent platelet activation.
Routine storage of platelet concentrates induces changes in the supernatant proteome, which may have effects on the transfused patient, some of which are related to donor gender.
Biological significance
The rationale behind this study is that protein components in platelet releasates have been increasingly observed to play a key role in adverse events and impaired homeostasis in transfused recipients.
In this view, proteomics has recently emerged as a functional tool to address the issue of protein composition of platelet releasates from buffy coat-derived platelet concentrates in the blood bank. Despite early encouraging studies on buffy coat-derived platelet concentrates, platelet releasates from apheresis platelets have not been hitherto addressed by means of extensive proteomics technologies. Indeed, apheresis platelets are resuspended in donors' plasma, which hampers detection of less abundant proteins, owing to the overwhelming abundance of albumin (and a handful of other proteins), and the dynamic range of protein concentrations of plasma proteins.
In order to cope with these issues, we hereby performed an immuno-affinity column-based depletion of the 14 most abundant plasma proteins. Samples were thus assayed via GeLC-MS, a workflow that allowed us to cover an unprecedented portion of the platelet supernatant proteome, in comparison to previous transfusion medicine-oriented studies in the literature.
Finally, we hereby address the issue of biological variability, by considering the donor gender as a key factor influencing the composition of apheresis platelet supernatants. As a result, we could conclude that platelet supernatants from male and female donors are not only different in the first place, but they also store differently. This conclusion has been so far only suggested by classic transfusion medicine studies, but has been hitherto unsupported by actual biochemistry/proteomics investigations.
In our opinion, the main strengths of this study are related to the analytical workflow (immunodepletion and GeLC-MS) and proteome coverage, the translational validity of the results (from a transfusion medicine standpoint) and the biological conclusion about the intrinsic (and storage-dependent) gender-related differences of platelet supernatants.
Keywords: Donor, Transfusion medicine, Proteomics, Human, Label-free quantitation, Mass spectrometry
1. Introduction
Platelets (PLTs) are key mediators of hemostasis, and PLT concentrates (PCs) are an important component of supportive care for thrombocytopenic patients. Despite decades of improvements, preparation of PLT concentrates still represents one of the major challenges to the blood bank, in light of standard platelets storage limitations [1–3]. Indeed, routine storage results in decreased PLT morphology scores (loss of disk shape) and responses to agonist (shape change), increased hypotonic shock response, volume and density heterogeneity, PLT activation marker expression (e.g. CD62P, PAC-1 binding/ expression of the fibrinogen binding site on the αIIβ3 complex), release of PLT α-granules, cytosolic proteins and inflammatory mediators, increased pro-coagulant activity, and altered supernatant pH and glycoprotein expression [1–4].
In most countries, PCs are stored for 5–7 days, depending on the national guidelines. This short lifespan results in a significant amount of the PLT inventory becoming outdated and discarded. Lifespan limitations also impose constraints regarding the narrow time window to complete donor testing, shipment of PCs, and leaves even less time for the recipient hospitals to transfuse these units.
Proteomics is an ideal tool to investigate in vitro changes of stored PLTs [5]. Owing to their anucleate nature, platelets have limited protein synthesis capacity [6] and a rather stable proteome (∼5,000 proteins) that, in comparison to other cell types, is less affected by biological variability issues [7–10]. Translational application of proteomics to PLT-related transfusion medicine issues has focused on many aspects of PLT product preparation, including the effects of preparation (cell processing and pathogen inactivation) on in vivo viability [11–18].
PLT supernatants are recognized mediators of hypercoagulability after injury [19], as they become increasingly enriched with PLT releasates [20], lipid [21] and oncogenic, angiogenic and invasion-promoting mediators [22,23]. In this view, it has become increasingly accepted that protein components in PLT releasates might play a non-secondary biological role in mediating untoward responses in the recipients [24]. Despite recent breakthroughs, the intrinsic limitations of the gel-based approaches [5,25] (limited coverage of the proteome, impaired detection of very high molecular weight and hydrophobic proteins) leave room for further improvements in this research endeavor, with evident benefit for the transfusion community.
In the present study we hypothesize that a significant number of proteins accumulate during routine PLT storage which are directly affected by donor gender and may in turn affect the recipient. The differences in male and female donors were addressed at the two extreme storage time points, as to assess whether the PLT proteomes of freshlydonated apheresis PLTs differed as a function of storage time, either in a storage or gender-dependent fashion.
2. Materials and methods
2.1. Sample collection
After obtaining informed consent under a protocol approved by the Combined Multiple Institution Review Board, 10 healthy donors (5 females –mean age: 54.0 ± 5.7 and 5 males –mean age 47.7 ± 1.2) donated one unit of apheresis platelets using a Cobe Trima apparatus with appropriate leukoreduction (<5 × 106/unit per Bonfils Blood Center standard operating procedures and consistent with AABB guidelines, as determined by direct leukocyte counting by flow cytometry). Duration of the apheresis procedure was 92.0 ± 2 and 97.3 ± 1.5 min for female and male donors, respectively. All of the donors gave double, with one donating a triple, apheresis units. So the platelet numbers are all a total of 6 × 1011 with one being 9 × 1011 by definition and cutoffs. All the females were post-menopausal; none on hormone replacement. Samples were taken serially on days 1 and 5 via sterile couplers and centrifuged first at 5,000 g for 7 min to remove the majority of platelets and centrifuged again at 12,500 g for 6 min to remove all platelets and acellular debris. The supernatants were aliquoted and stored at –80 °C. All samples were prepared by the identical protocol. Therefore, artifacts caused by preparation should affect all samples similarly.
2.2. QConCAT design and expression
In agreement with Pratt et al. [26], QConCAT constructs were designed to quantify plasma and PLTs specific proteins. The selection of target peptides was based on the result of previous non-targeted experiments of plasma and publicly accessible databases, including PeptideAtlas (www.peptideatlas.org) SRM Atlas (www.srmatlas.org) and Global Proteome Database (http://gpmdb.thegpm.org/). The QConCAT DNA construct was synthesized de novo and cloned into pET21a by Genscript (Piscataway, NJ ). E. coli strain BL21(l)DE3 was transformed with the vector and cultured in minimal medium supplemented either with unlabeled or 13C6 arginine and 13C6 lysine at 0.1 mg/ml (Sigma Aldrich). The cells were grown to mid log phase (A600 0.6–0.8), at which point expression was induced by adding 1 mM isopropyl-D-1-thiogalactopyranoside. The cells were lysed with the BugBuster Protein Extraction Reagent (EMD Millipore). Inclusion bodies were suspended in 20 mM phosphate buffer, 6 M guanidinium chloride, 0.5 M NaCl, 20 m M imidazole, pH 7.4. QConCAT proteins were purified by affinity chromatography using a nickel-based resin. The purified QConCAT was desalted by three rounds of dialysis against 100 volumes of 10 mM ammonium bicarbonate, pH 8.5.
2.3. Depletion of the most 14 abundant plasma proteins in platelet superantants and protein digestion
Multiple Affinity Removal System™ columns (4.6 × 100 mm) designed to deplete 14 abundant plasma proteins (albumin, IgG, antitrypsin, IgA, transferrin, haptoglobin, fibrinogen, alpha2-macroglobulin, alpha1-acid glycoprotein, IgM, apolipoprotein AI, apolipoprotein AII, complement C3, and transthyretin) were purchased from Agilent (Palo Alto, CA). Depletion was performed at room temperature on an AKTAmicro (GE Healthcare Life Sciences) system. Platelet supernatant samples were diluted fourfold using the load/ wash buffer supplied by the manufacture and remaining particulates in the diluted supernatants were removed by centrifugation through a 0.22-μm spin filter 1 min at 16,000 ×g. After equilibration with the load/wash buffer, the Multiple Affinity Removal System™ column was loaded with 160 μL of the diluted plasma at a low flow rate (0.125 mL min – 1) for 4 min. Flow-through fractions, representing depleted platelet supernatants were collected and saved. The bound proteins were released with elution buffer at 1.0 mL/min for 10 min. The column was then washed with the load/wash buffer for 11 min at a flow rate of 1 mL/min. Each depletion cycle took 38 min of total run time. Each flow-through portion was individually concentrated using 5000 Da molecular weight cutoff spin concentrators (Agilent Technologies, Palo Alto, CA), followed by buffer exchange with 50 mM NH4HCO3 and protein concentrations were determined by a Bradford protein assay.
In agreement with Dzieciatkowska et al. [27], a portion of the sample (30 μg) was diluted into SDS-PAGE sample buffer, heated at 70 °C for 10 min and loaded in a single lane on a 1.5-mm-thick 4–12% Bis-Tris gel (Invitrogen). After separation, the gel was stained with SimplyBlue SafeStain (Invitrogen). Each lane of the gel was divided into 24 equal-sized bands and proteins in the gel were digested as follows. Bands were destained in 200 μl of 25 mM ammonium bicarbonate in 50 % v/v ACN for 15 min, and then 200 μl of 100% ACN was applied for 15 min at room temperature. Dithiothreitol (DTT) was added to a final concentration of 10 mM and incubated at 65 °C for 30 min to reduce the disulfide bonds. Protein cysteines were alkylated with 55 mM iodoacetamide for 30 min at room temperature in the dark. The iodoacetamide was then removed, and washes were performed with 200 μl of distilled water followed by addition of 100 μl of ACN. Then ACN was removed, and 50 μl of the 0.01 μg/μl trypsin solution was added to each plug. The gel plugs were allowed to rehydrate at 4 °C for 30 min and then placed at 37 °C and allowed to digest overnight. The tryptic mixtures were acidified with formic acid up to a final concentration of 1%. Peptides were extracted three times from the gel plugs using 50% ACN, 1% FA, concentrated under vacuum (SpeedVac, Savant ThermoFisher) to approximately ∼20 μL, and subjected to LC-MS/MS analysis. If necessary, they were stored at –20 °C.
2.3.1. Targeted quantitation assay via single reaction monitoring
For all targeted quantification analyses, a subset of three (out of five of the label-free approach) male and female units of platelet supernatants after days 1 and 5 of storage were analyzed independently with the isotopic QConCAT standard. For trypsin proteolysis, known amounts of the recombinant isotopically labeled QConCAT protein were mixed with the albumin and IgG depleted platelets supernatant. The samples were reduced, alkylated, and digested with sequencing grade modified trypsin (Promega) according to the FASP protocol using a 10 kDa molecular weight cutoff filter. In brief, samples were mixed in the filter unit within 8 M urea in 0.1 M Tris-HCl, pH 8.5 and centrifuged at 14000 g for 15 min. The proteins were reduced by addition of 100 μL of 10 mM DTT in 8 M urea in 0.1 M Tris-HCl, pH 8.5, incubation for 30 min at RT and the device was centrifuged. Subsequently, 100 μl of 55 mM iodoacetamide in 8 M urea in 0.1 M Tris-HCl, pH 8.5 were added to the samples, incubation for 30 min at RT in dark followed by centrifugation. Afterward, three washing steps with 100 μL of 8 M urea in 0.1 M Tris-HCl, pH 8.5 solution were performed, followed by three washing steps with 100 μL of 50 mM ABC buffer. Proteins were digested with trypsin overnight at 37 °C. Peptides were recovered from the filter using 30% ACN. The volume of the eluted sample was reduced to ∼2 μL in a vacuum centrifuge and reconstituted to 50 μL with 0.1% formic acid. Following digestion, the resultant peptide mixture was analyzed in duplicate by LC-SRM. Further details about the peptides are provided in the Supplementary File 1.
2.4. Liquid chromatography-tandem mass spectrometry
Samples were analyzed on an LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) coupled to an Eksigent nanoLC-2D system through a nanoelectrospray LC - MS interface. A volume of 8 μL of sample was injected into a 10 μL loop using the autosampler. To desalt the sample, material was flushed out of the loop and loaded onto a trapping column (ZORBAX 300SB-C18, dimensions 5×0.3 mm 5 μm) and washed with 0.1% FA at a flow rate of 5 μL/min for 5 min. The analytical column was then switched on-line at 600 nl/min over an in house-made 100 μm i.d. × 150 mm fused silica capillary packed with 4 μm 80 Å Synergi Hydro C18 resin (Phenomex; Torrance, CA). After 10 min of sample loading, the flow rate was adjusted to 350 nL/min, and each sample was run on a 90-min linear gradient of 5–40% ACN with 0.1% formic acid to separate the peptides. LC mobile phase solvents and sample dilutions used 0.1% formic acid in water (Buffer A) and 0.1% formic acid in acetonitrile (Buffer B) (Chromasolv LC–MS grade; Sigma-Aldrich, St. Louis, MO). Data acquisition was performed using the instrument supplied Xcalibur™ (version 2.1) software. The mass spectrometer was operated in the positive ion mode. Each survey scan of m/z 400–2,000 was followed by collision-assisted dissociation (CAD) MS/MS of twenty most intense precursor ions. Singly charged ions were excluded from CID selection. Normalized collision energies were employed using helium as the collision gas.
2.5. Selected reaction monitoring analysis
A targeted Selected Reaction Monitoring (SRM) approach was performed using the QTRAP® 5500 interfaced with a capillary HPLC system (Agilent 1200, Palo Alto, Calif). 10ul of each sample was injected and directly loaded onto an Agilent C18 column (Zorbax SB-C18, 5 μm 150×0.5 mm) with 5%ACN, 0.1% FA at 30 μl/min for 3 min. Chromatography was performed with Solvent A (Milli-Q water with 0.1% formic acid) and Solvent B (acetonitrile with 0.1% formic acid). A gradient of 5– 28% ACN was run for 61 min to differentially elute QConCAT peptides. The mass spectrometer was run in positive ion mode with the following settings: a source temperature of 200 °C, spray voltage of 5300 V, curtain gas of 20 psi, and a source gas of 35 psi (nitrogen gas). Multiple MRM transitions were monitored using unit resolution in both Q1 and Q3 quadrupoles to maximize specificity. SRM assay optimization was performed with the aid of Skyline v1.2 software [28]. Collision energies (CE) and declustering potential (DP) were optimized for each transition. The energy was ramped around the predicted value in 5 steps on both sides with 1 V increments. Method building and acquisition were performed using the instrument supplied Analyst® Software (Version 1.5.2). Each sample was run in duplicate.
2.6. Data analysis
MS/MS spectra were extracted from raw data files and converted into mgf files using a PAVA script (UCSF, MSF, San Francisco, CA). These mgf files were then independently searched against Human IPI database using an in-house Mascot™ server (Version 2.2.06, Matrix Science). Mass tolerances were +/-15 ppm for MS peaks, and +/-0.6 Da for MS/MS fragment ions. Trypsin specificity was used allowing for 1 missed cleavage. Met oxidation, protein N-terminal acetylation, and peptide N-terminal pyroglutamic acid formation were allowed for variable modifications while carbamidomethylation of Cys was set as a fixed modification. Alternative searches were performed indicating semi-trypsin digestion, while maintaining the other search criteria unaltered. Scaffold (version 4.3.2, Proteome Software, Portland, OR, USA) was used to validate MS/MS based peptide and protein identifications. All mascot DAT files, for each subject (10 bands each) were loaded together as one “biological sample” within Scaffold. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least two identified unique peptides in the first set of experiments.
All data files generated on the triple quadrupole mass spectrometer during LC-SRM analyses were imported to Skyline v2.2 software for data processing. Quantification was based on the ratio of corresponding light and heavy peak areas. The peak integration was done automatically by the software, using Savitzky-Golay smoothing, and all the data were manually inspected to confirm correct peak detection.
2.6.1. Heat maps and clustering
Quantitative results from Scaffold or Skyline were exported into .xls files and loaded into GENE-E (v. 3.0.200 - Broad Institute, Inc.) to plot heat maps and perform hierarchical clustering analyses (one minus Pearson correlation). Raw results were Z-score normalized and heat maps were plotted as to regroup proteins showing (1.5 fold change) increases or decreases in a gender or storage day-dependent fashion. Gene ontology annotations for biological functions and cell compartments were performed either with Scaffold or David v. 6.7 (David Bioinformatics services).
3. Results
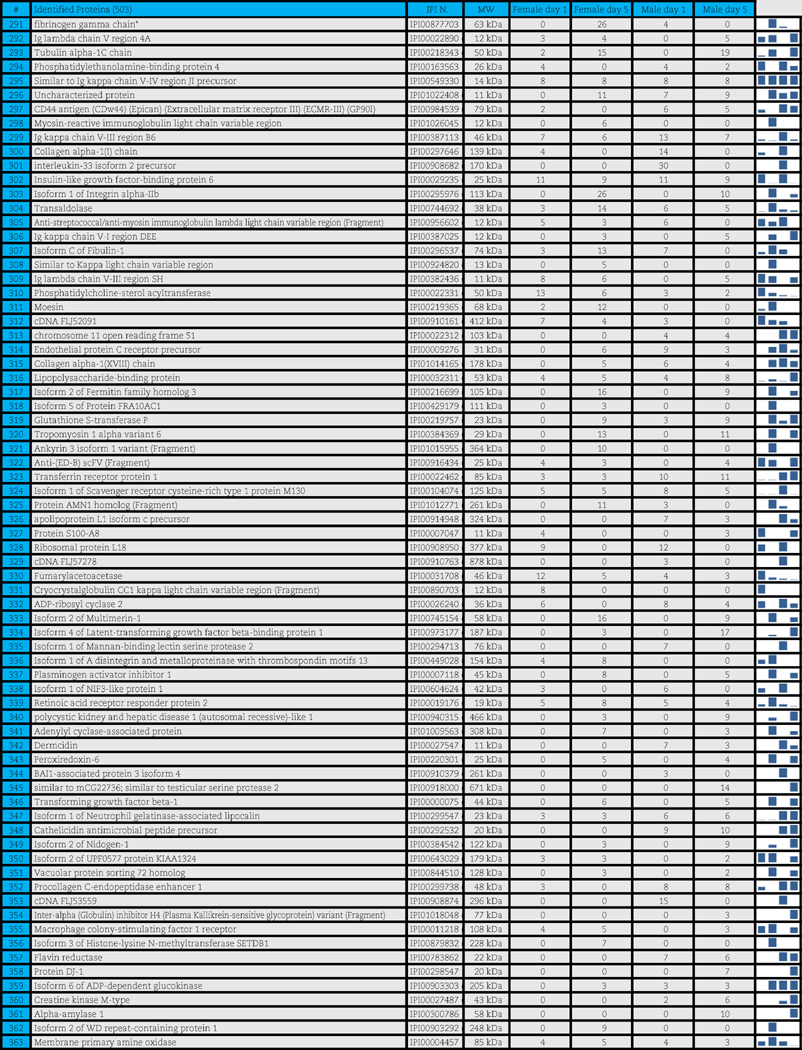
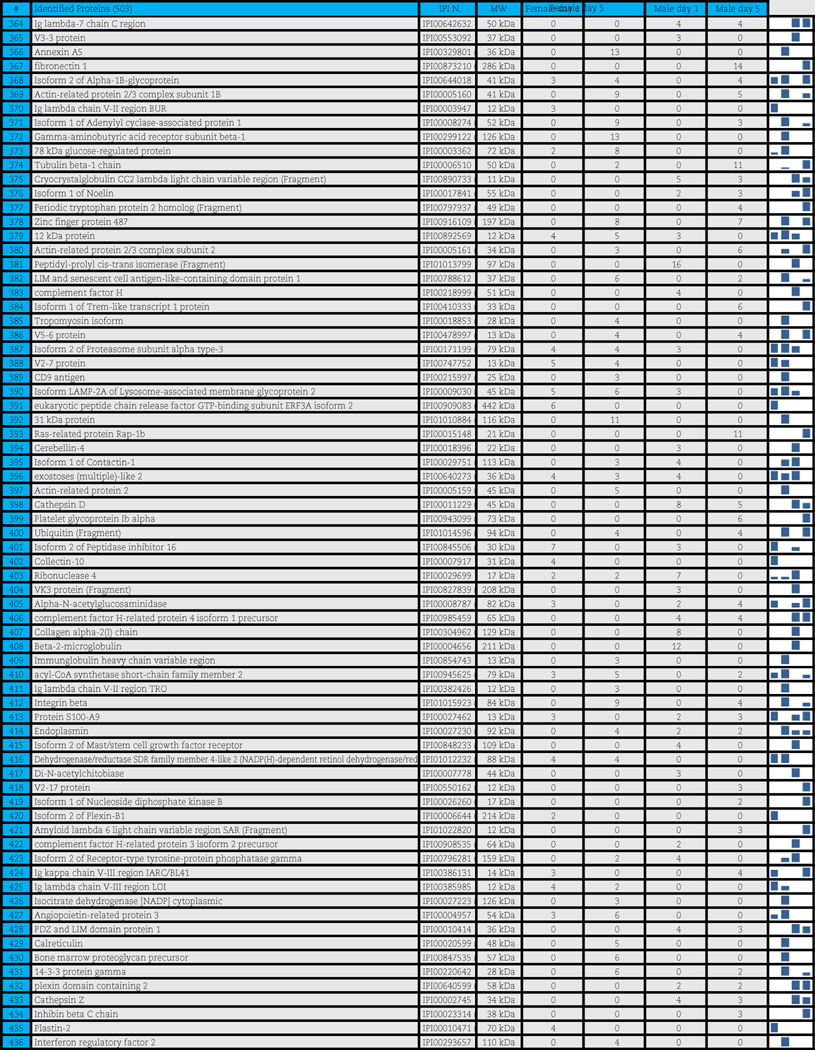
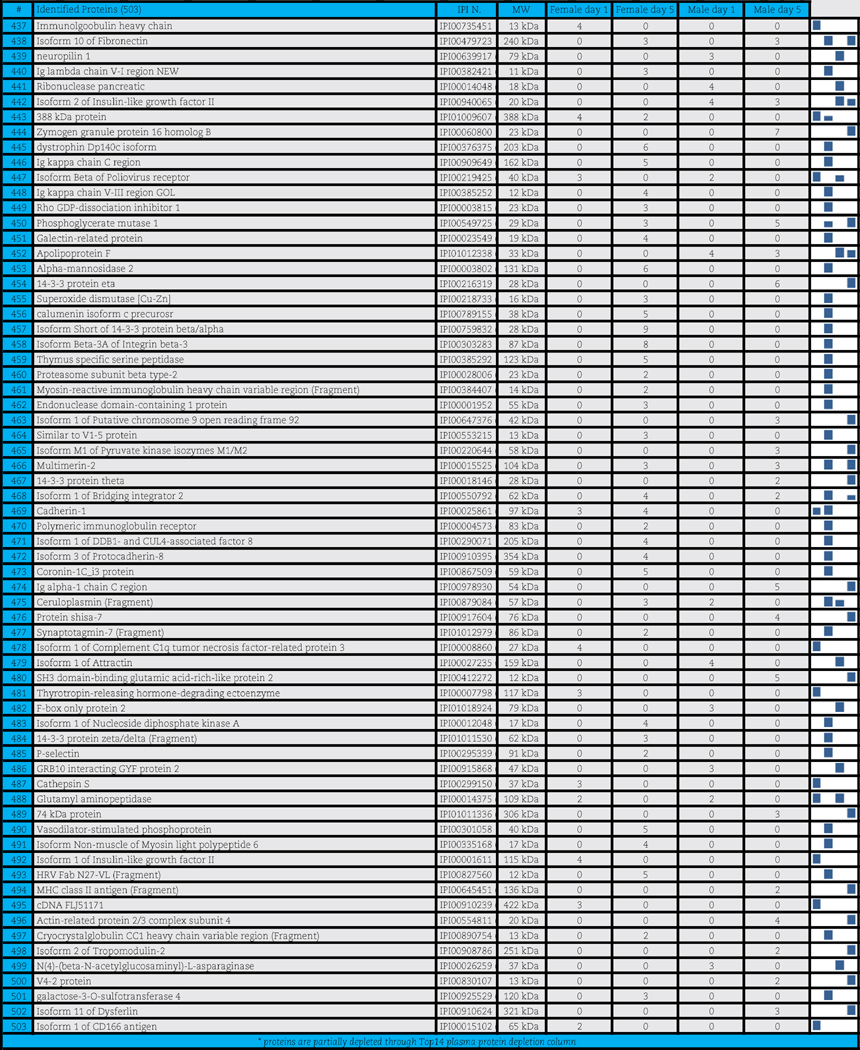
A total of 503 distinct proteins were detected in the platelet supernatants from the 4 sample groups: female or male at storage day 1 or 5. Proteins are enlisted in Table 1, together with the IPI identification numbers, the theoretical molecular weight and the mean quantitative emPAI values as calculated for each of the four groups. These quantitative results were thus exported for hierarchical clustering analysis (1-Pearson correlation), as graphed in Supplementary Fig. 1.
In order to ease data analysis and interpretation, we thus exported sub-clusters either including proteins:
constitutively higher in the supernatants of PLTs from female (Fig. 1.A) or male (Fig. 1.B) donors, not affected by storage duration;
decreasing or increasing (Supplementary Table 2) from storage day 1 to day 5 both in PLTs from female and male donors (Fig. 2.A and B, respectively);
decreasing in a storage-dependent fashion in female (Fig. 3.A) or male (Fig. 3.B) PLT supernatants only;
increasing in a storage-dependent fashion in female (Fig. 4.A) or male (Fig. 4.B) PLT supernatants only;
showing storage-dependent reverse trends in a gender-specific fashion (Fig. 5).
Fig. 1.
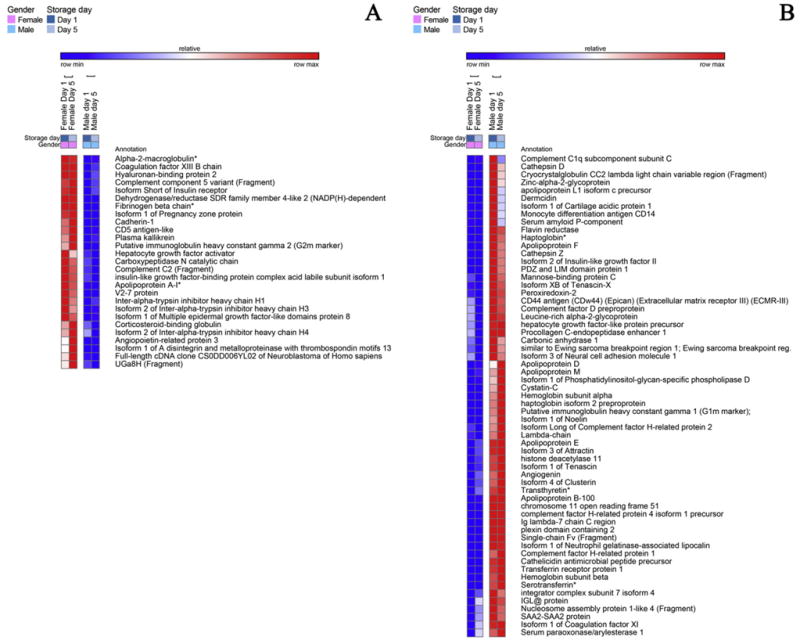
A detail of heat maps showing quantitative dynamic proteomics changes in apheresis platelet supernatants from male and female donors at storage day 1 and 5. Proteins constitutively higher (storage-independent gender-dependent) in female (A) and male (B) donors are mapped.
Fig. 2.
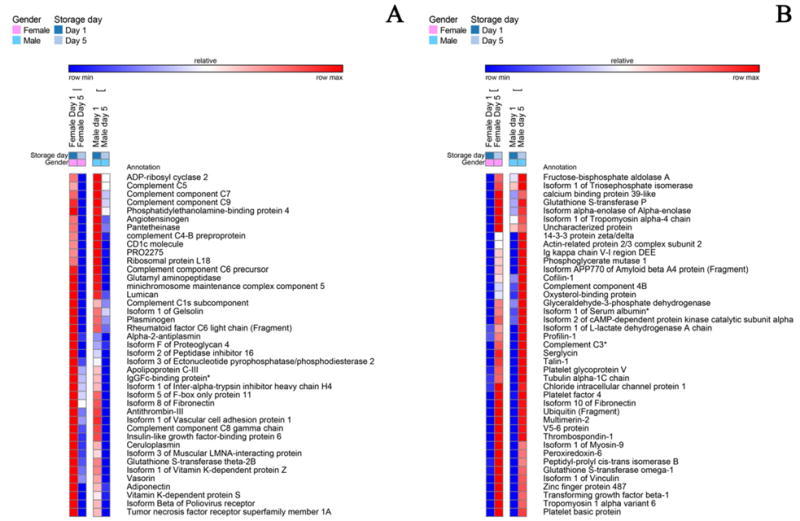
A detail of heat maps showing quantitative dynamic proteomics changes in apheresis platelet supernatants from male and female donors at storage day 1 and 5. Proteins decreasing (A) or increasing (B) in a storage-dependent gender-independent) fashion in female and male donors are mapped.
Fig. 3.
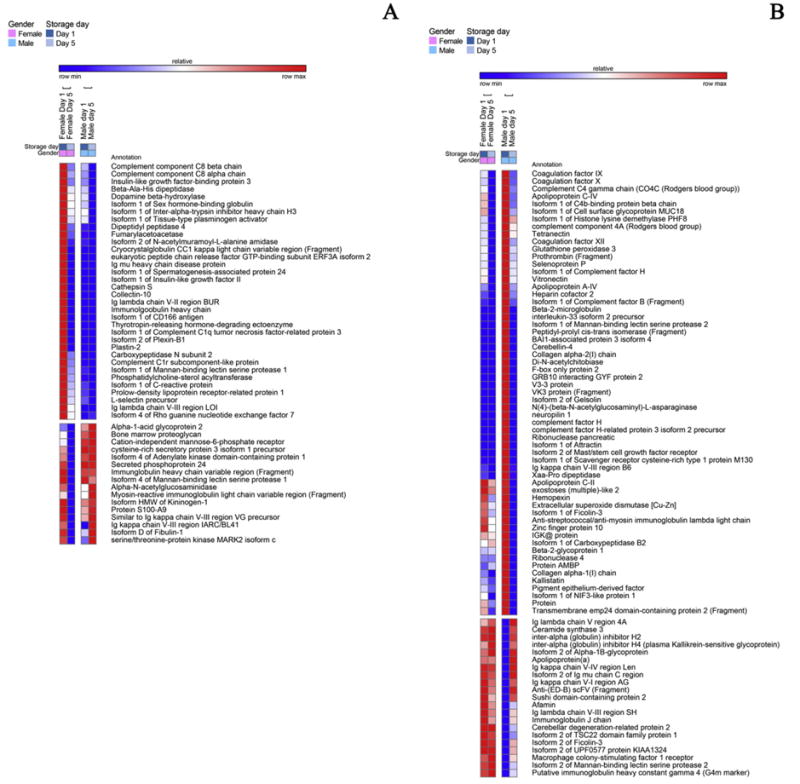
A detail of heat maps showing quantitative dynamic proteomics changes in apheresis platelet supernatants from male and female donors at storage day 1 and 5. Proteins decreasing either in female (A) or male donors (B) in a storage and gender-dependent fashion are mapped.
Fig. 4.
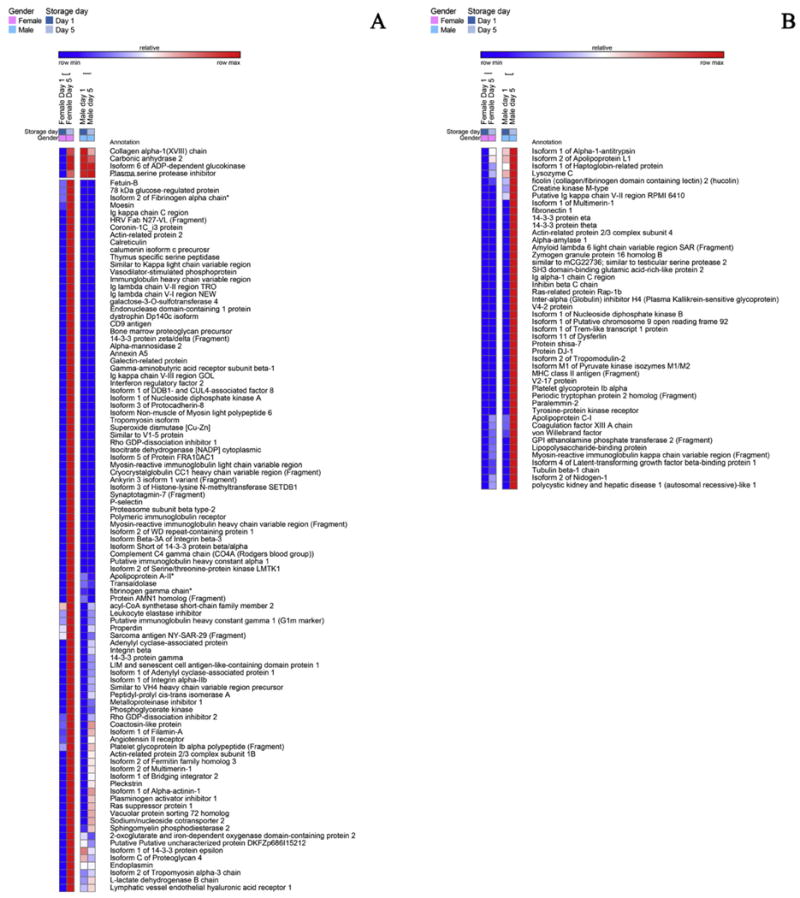
A detail of heat maps showing quantitative dynamic proteomics changes in apheresis platelet supernatants from male and female donors at storage day 1 and 5. Proteins increasing either in female (A) or male donors (B) in a storage and gender-dependent fashion are mapped.
Fig. 5.
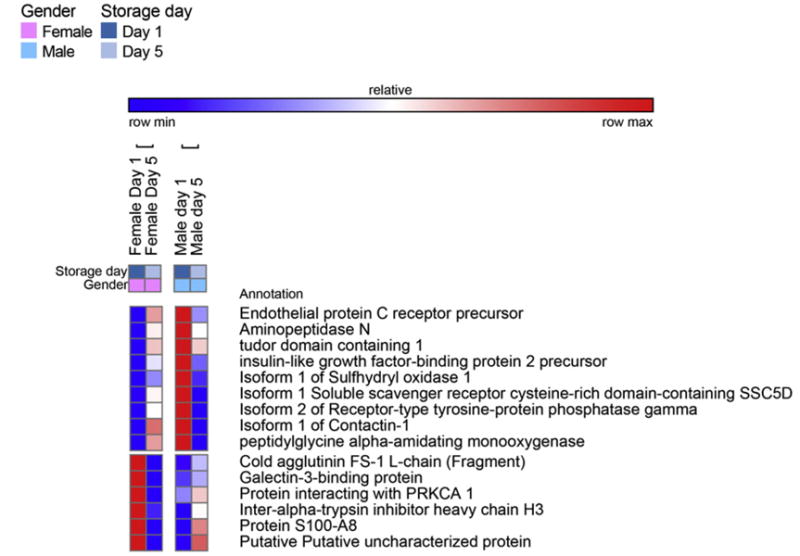
A detail of heat maps showing quantitative dynamic proteomics changes in apheresis platelet supernatants from male and female donors at storage day 1 and 5. Proteins showing opposite gender-dependent trends in a storage-dependent fashion are mapped.
Bioinformatic elaboration for functional GO term enrichment via DAVID is reported for each of these groups in Supplementary Table 1 (except group (v), owing to the limited number of entries). The observed proteins were primarily grouped into secreted extracellular (GO:0005576) proteins of the platelet granules (GO:0031091) and granule lumen(GO:0031093), other than membrane-bound proteins (GO:0031988) gene ontology groups (Supplementary Table 1).
It is worth noting that parallel increases and decreases in the levels of different proteins involved in the same biological pathways were observed, both when addressing the storage and the gender effect on the proteomes of apheresis PLT supernatants (Supplementary Table 1). Indeed, we identified either female or male, storage day 1 or day 5 specific proteins involved in endopeptidase inhibitor activity (GO:0004866) or blood coagulation (GO:0007596). This might be due also to the different half-lives of proteins originally present in plasma supernatants of apheresis PLTs, or proteins shed through PLT releasates or from trace amounts of contaminating cells (such as WBCs) over storage progression. Additionally, it should be noted that although protein extraction has been performed using a widely accepted procedure [20,29,30], PLT centrifugation steps in the absence of prostacyclin might have promoted release of PLT proteins in the supernatant, thereby affecting the supernatant proteome profiles to some extent, both in PLTs from male and female donors.
3.1. LC-SRM targeted quantitative validation against QConCAT standards
Despite the exploratory nature of the study, we validated results for a subset of 18 proteins using LC-SRM targeted assays against stable isotope labeled peptides. Proteins were selected on the basis of relative trends in label-free analyses (Fig. 6.A), including five proteins increasing in apheresis PLT supernatants during storage duration (CXCL7, PLF4, GPV, THBS1, SRGN – UniProt gene names), six proteins constitutively higher in samples from female (FGB, A2M, APOA1, CRP, F13B) or male donors (HBA1, HBB, CA1, CLU, vWF, TTR) or a protein decreasing during storage duration (Fig. 6.B). An example of the skyline workflow is provided in Supplementary Fig. 2. Despite the limited number of biological and technical replicates of the untargeted label-free approach, targeted quantitation confirmed trends for most of the selected proteins. A discrepancy was observed for Vitamin K-dependent protein S (PROS1), possibly resulting from technical caveats associated with the less accurate label-free semi-quantification approach used in the untargeted experiments. Indeed, PROS1 decreased in a storage-dependent fashion in both label free (Fig. 6.A) and targeted assays (Fig. 6.B). However, PROS1 appeared to be 14% higher in day 1 female donor supernatants in comparison to day 1 male in the label free assays, while in targeted analyses it was measured as 20% higher in the day 1 male group. Analogous observations were made for C-reactive protein (CRP) and vWF, with constitutively higher levels in female and male donor supernatants, respectively, in targeted analyses, while in label free analyses they decreased or increased in a storage-dependent fashion, respectively.
Fig. 6.
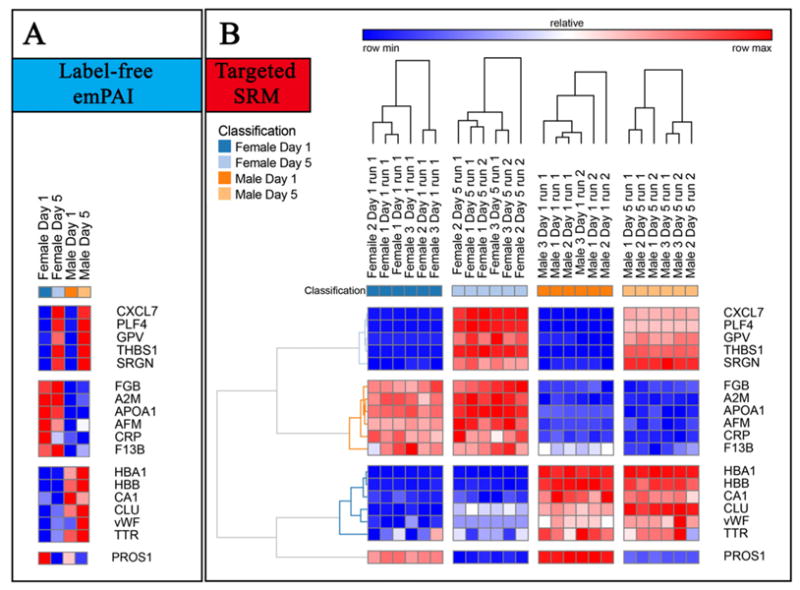
In A, a detail of a subset of proteins from apheresis platelet supernatants showing significant quantitative variations with respect to storage duration (top and bottom clusters), gender (second and third cluster from the top), as determined through label-free mass spectrometry-based approaches. In B, heat maps represent quantitative fluctuations of the very same proteins, as determined through targeted approaches based upon LC-SRM against labeled QConCAT standards.
3.2. Gender-specificity of PLT supernatants
Apheresis PLT supernatants were characterized by genderspecific proteins (Fig. 1). For example, the isoform 1 of pregnancy zone protein, a carrier and modulator of placental protein-14 in T-cell growth and cytokine production that is associated with late pregnancy [31], was identified only in female donor supernatants (Fig. 1.A). Female apheresis PLT supernatants were characterized by the highest storage-dependent increase in protein concentrations. A subset of female-specific PLT alterations were classified as involved in cell adhesion (GO:0007155 - Supplementary Table 1 - insulinlike growth factor-binding protein complex acid labile subunit isoform 1, angiopoietin-related protein 3, cadherin-1, Isoform 1 of A disintegrin and metalloproteinase with thrombospondin motifs 13, hyaluronan-binding protein 2 – Fig. 1.A). On the other hand, apheresis PLT supernatants from male donors were enriched in antimicrobial proteins (e.g. cathelicidin, cathepsin D and Z, dermicidin) and inflammation/immune responses (attractin, immunoglobulins and complement components), iron transporters (haptoglobin, transthyretin - TTR and serotransferin, despite the latter two proteins being previously depleted by affinity chromatography), apolipoproteins/serum amyloid proteins (apolipoproteins APOB100, APOD, APOE, APOF, APOL1, APOM; serum amyloid A2, serum amyloid P component) (Fig. 1.B). Since 100% depletion of high abundance proteins is practically impossible to achieve, relative quantitation from label free proteomics investigations should be viewed with caution. Validation was hereby provided for TTR via LC-SRM, suggesting that, since depletion protocols were the same for both male and female PLT supernatants, male donors might have constitutively higher TTR levels, that are only partially depleted, or forms of the protein missing the depletion epitope altogether. Male-donor PLT supernatants were also characterized by higher levels of extracellular matrix (ECM) proteins, including cartilage acidic protein 1, tenascin (TNC), factor X, and α-hemoglobin and β-hemoglobin subunits (as confirmed by targeted LC-SRM assays).
3.3. Coagulation and inflammation
Storage led to decreased levels of pro-coagulation factors (GO:0009611), such as angiotensinogen, fibronectin, gelsolin, plasminogen, but also of anti-coagulation factors (e.g. alpha 2-antiplasmin, antithrombin-III, Inter-alpha trypsin inhibitor heavy chain H4, vitamin K-dependent protein S – PROS1, validated through LC-SRM – Fig. 2.A). At the same time, male-donor PLT supernatants were characterized by higher levels of coagulation factors IX, X, XI, XII, prothrombin, heparin cofactor 2, gelsolin isoform 2, kallistatin, though they tended to decrease in a storage-dependent fashion (Fig. 3.B). On the other hand, PLT supernatants from female donors were characterized by constitutively and storage duration independent levels of alpha-2 macroglobulin (confirmed by LC-SRM – Fig. 6.B), coagulation factor XIIIB, fibronegen beta chain (validated against stable isotope labeled standards – Fig. 6.B), plasma kallikrenin, and serine-protease inhibitors (SERPINs, alpha trypsin inhibitor heavy chain H1, H3 isoform 2, H4, isoform 2 – Fig. 1.A), or storage-dependent increases in fibrinogen alpha and gamma chains and plasminogen activator inhibitor (also increasing in male donor PLT supernatants, though to a lesser extent – Fig. 4.A).
3.4. Complement system and inflammation
There was a storage-dependent and gender-independent decrease in the levels of complement components, classical pathway (GO:0006958-C1s, C5, C6, C7, C8, C9 – Fig. 2.A). On the other hand, PLT supernatants from male donors were constitutively enriched with components of the alternative complement pathway (Complement factor D, H-related protein 1, 2 and 4 isoform 1). The alternative complement pathway is responsive to microbial infection activation. Therefore, these results should be interpreted together with the observed constitutive (Fig. 1.B) or storage-dependent (e.g. lysozyme, lipopolysaccharide binding protein – Fig. 4.B) up-regulation of antimicrobial proteins and peptides in the supernatants of apheresis PLTs from male donor. However, the late effector complement component C3, which is at the crossroads between classical and alternative complement system pathways, was found to increase in a gender-independent storage-dependent fashion (Fig. 3.B), despite being one of the targets of depletion via affinity columns. Analogously, complement component 4B (another late player in the complement system activation cascade) increased in a storage-dependent fashion, especially in the male group (Fig. 3.B), while properdin, a positive regulator of the alternate pathway, was increased in the female group (Fig. 3.A), suggesting a progressive activation of this pathway in the supernatant of stored PLTs. (Fig. 3.B). Moreover, in male donors storage-dependent increases were observed in the supernatant levels of the isoform 4 of latent transforming growth-factor beta (Fig. 4.B). In line with this, vasorin, a potential TGFB-signaling inhibitor, was found to decrease in a storage-dependent fashion (Fig. 2.A). Storage-dependent increases in cytokine components were hereby observed as well (platelet factor 4, platelet basic protein – Fig. 2.B).
3.5. Apoptosis
Decreases in gelsolin supernatants levels were observed and were independent of donor gender (Fig. 1.A). Consistently, other proteins potentially involved in apoptosis (GO:0043065 – Supplementary Table 1) were less abundant in day 5 supernatants, including, tumor necrosis factor alpha receptor family member 1A, other than the previously mentioned complement components C6 and C9 (Fig. 2.A).
3.6. Cell adhesion
Storage promoted a broad deregulation of extra-cellular matrix (ECM- GO:0007596) and structural proteins (GO:0003779). Storage and gender-independent decreases were observed in the levels of lumican, isoform F of proteoglycan 4, isoform 1 of vascular cell adhesion protein 1, isoform 3 of muscular LMNA-interacting protein (Fig. 2.A). Gender-specific storage-dependent decreases were observed as well, such as in the case of plastin-2 (female donors – Fig. 3.A) or vitronectin (male donors – Fig. 3.B). However, storage-dependent up-regulation of other components were observed as well, including cofilin 1, 14-3-3 protein zeta/delta, actin-related protein 2/3 complex subunit 2, tropomyosin alpha-4 and 6, tubulin alpha chain, profilin-1, talin-1, platelet glycoprotein V (validated – Fig. 6.B), multimerin 2, thrombospondin 1 (both in label-free and targeted MS assays – Fig. 6.B), vinculin (Fig. 2.B). Gender-specific storage dependent increases were observed as well, both in female donors (collagen alpha-1 (XVIII), coronin 1C, actin-related protein 2, moesin, non-muscle isoform of myosin light polypeptide 6, protocadherin-8, tropomyosin isoform, ankyrin 3, synaptotagmin-7, p-selectin, metalloproteinase inhibitor 1, fermitin family homolog 3, pleckstrin, alpha-actinin 1 – Fig. 4.A) and male donors (isoform 1 of multimerin, 14-3-3 protein eta and theta, fibronectin, actin-related protein 2/3 complex subunit 4, nidogeni-1, tubulin beta chain – Fig. 4.B). Increased storage-dependent levels of “cell free” platelet glycoprotein Ib and IIb alpha in female and male donors, respectively (Fig. 4.A and B), might be more or less directly related to the previously reported structural remodeling via integrin αIIbβ3 signaling pathways [14].
3.7. Metabolism and oxidative stress
Storage resulted in the progressive supernatant accumulation of proteins involved in cell energy and redox-metabolism (GO:0006096, IPR010987), both in a gender-independent (Fig. 2.B) or dependent (Fig. 4.A and B) fashion. The release of these enzymes might stem either from cell lysis, resulting in subsequent release in the supernatant of intracellular products, or from vesiculation mechanisms.
The list of proteins in this group includes glycolytic enzymes (fructose biphosphate aldolase, triosephosphate isomerase, glyceraldehyde 3-phosphate dehydrogenase, phosphoglycerate mutase 1, lactate dehydrogenase A) and anti-oxidant enzymes (glutathione S-transferase omega-1 and P, peroxiredoxin 6) in all donors (Fig. 2.B). Female-specific storage-dependent increases in metabolic enzymes include nucleoside diphosphate kinase A, phosphoglyerate kinase, transaldolase, sphingomyelin phosphodiesterase 2 and L-lactate dehydrogenase B (Fig. 4.A). On the other hand, male donors showed late storage increases in creatine kinase M, nucleoside diphosphate kinase B and pyruvate kinase M1/M2(Fig. 4.B).
Storage corresponded to a decrease in the levels of ceruloplasmin (Fig. 2.A), a copper protein involved in iron metabolism, and an increase of antioxidant enzymes other than peroxiredoxins and GSH-transferases, including superoxide dismutase [Cu-Zn] in female donors (Fig. 4.A) and protein DJ-1 in the male group (Fig. 4.B).
4. Discussion
PLT concentrates can be obtained either from pooling of whole blood-derived buffy coats from multiple donors, or apheresis (also referred to as platepheresis) from a single donor. Differential storage lesions in the cell fraction of both blood-derived therapeutics have been extensively documented in the literature [11–14]. However, the proteomics lesions accumulating in the supernatants of stored PLTs have been so far limited to buffy coat concentrates [29,30], while proteome changes in apheresis-derived PLT supernatants have only focused on platelet factor 4 and β-thromboglobulin [20].
In their pioneering study, Glenister and colleagues [29] exploited 2D gel electrophoresis (GE) maps of buffy coatderived PLT supernatant proteins to highlight changes to major plasma proteins. PLT-derived proteins were also identified, including tremlike transcript 1 and integrin-linked kinase, which may influence PLT–endothelium interactions. Moreover, the Authors detected increased storage-dependent accumulation in the supernatants of PLT derived cytokines including brain-derived neurotrophic factor (BDNF), CXCL7 (validated through LC-SRM – Fig. 6.B), epidermal growth factor, PLT-derived growth factor (PDGF), and CCL5 [29].
The biological relevance of PLT protein releasates has been previously documented to mediate PLT-triggered coagulation, inflammatory and acute phase responses [11]. However, the presence of plasma in the supernatant additive solution hampers the detection of low abundance proteins, and thus prevents any in-depth investigation of quantitative changes of supernatant proteins during storage in the blood bank. In a recent study, Egidi and colleagues have tackled this issue by performing initial selective enrichment of the buffy coat PLT supernatant low abundance proteome via combinatorial hexapeptide ligand libraries, followed by 2D-GE analyses [30].
In the present study, we performed a preliminary depletion of the most abundant 14 plasma proteins from apheresis PLT supernatants, coupled with 1D-SDS-PAGE/nanoLC-MS/MS analyses, a strategy that has been previously described in successful plasma proteomics investigations as a viable solution to cope with the widespread dynamic range of concentrations of proteins from this biological fluid [5,25]. This strategy favored an increased coverage of the PLT supernatant proteome in comparison to previous studies [11,20,29,30], enabling detection of 503 distinct proteins in PLT supernatants. Additionally, validation using a targeted MS-based approach confirmed quantitative trends determined through label-free assays for a subset of proteins showing significant fold-change variations in a gender or storage-dependent fashion.
4.1. Gender-specificity of PLT supernatants
Apheresis PCs are resuspended in donors' plasma. Concerns have recently arose about gender-related differences in PLT biology [32,33] and plasma proteomes [25]. The significance of gender-specific differences in the PLT proteome has previously been anticipated, as a likely contributing factor that could impact gender-influenced cardiovascular disease profiling, potentially resulting from differences in individual capacity of protein translation [6]. We previously documented gender-related plasma proteome differences [25] and were thus not surprised by differences in PC supernatant profiles from male and female donors.
Many gender-related specific proteins were identified in stored units both at day1 and at the end of the storage period, such as pregnancy zone protein, a protein associated with late-pregnancy sera from women donors [29] but also fund in non-pregnant women [34]. Functional classification of gender and storage-dependent proteomes included proteins involved in coagulation, complement and inflammation, apoptosis, PLT activation or energy and redox metabolism-associated proteins, as detailed below. Among the peculiar gender-specific proteins we observed, hemoglobin subunits alpha and beta were only detected in platelet supernatants from male donors. Although this result might be affected by hemolysis byproduct contamination, it is worth noting that mature PLTs harbor residual mRNAs to sustain limited de novo protein synthesis [6], and hemoglobin mRNAs have been identified in the mature PLT transcriptome [35].
4.2. Coagulation and inflammation
From a biological standpoint, it is worth recalling that the main purpose of issuing PCs (both the supernatant and cell fraction) is to restore hemostasis by improving coagulation time and efficiency. In this view, it is interesting to note that storage and gender affected proteins involved in coagulation cascades. PLT storage promoted a general decrease in hemostatic proteins, while male PLT supernatants were richer in coagulation factors from IX to XII, and female counterparts showed higher levels of alpha-2 macroglobulin (as confirmed both through label-free and targeted MS approaches) and SERPINs. Gender-specific levels of proteins related to hemostasis might suggest that PC from male and female donors might differently perform hemostatic functions upon transfusion, thus suggesting the necessity for further functional experiments.
4.3. Complement system and inflammation
Most of the components of the classical complement system decreased in all PCs. However, male donor PC supernatants were more enriched with alternative pathway complement components. It can be noted that the complement system plays a key role in immunity and inflammation, while previous studies had reported the storage dependent accumulation in the PLT releasates of pro-inflammatory mediators and cytokines, such as PLT factor 4 (PF4 – hereby confirmed through the LC-SRM approach – Fig. 6) and β-thromboglobulin (β-TG) [20], or BDNF, CCL5, PDGF and clusterin (a complement-mediated lysis inhibitor, apparently higher in abundance in male donor supernatants – Fig. 6.A and B), TLT-1 (having a role in PLT adhesion) and ILK (aggregation and adhesion to damaged endothelium) [29]. Cytokine expression can be modulated by tumor necrosis factor alpha and transforming growth factor beta (TGFB) [36], the latter appearing to increase in a storage-dependent gender-dependent fashion in PLT male supernatants.
4.4. Apoptosis
Previous studies on PLT storage had shown a storage-dependent alteration of intracellular gelsolin levels [12–14], a protein that was associated with an apoptosis-like event underlying PLT storage, together with increased beta-actin fragmentation and septin 2 deregulation. Storage-dependent decreases in gelsolin supernatants levels were detected, and were independent of donor gender. Gelsolin-depletion/ alteration had previously been reported in post shock mesenteric lymph in trauma models, whereby gelsolin is deemed to be a marker of apoptosis [37]. Gender-independent, storage-dependent decreases of gelsolin and other apoptosis markers in the supernatants were observed, and might be to some extent related to the previously reported storage-dependent increase in the intracellular level of these proteins [12–14].
4.5. Cell adhesion
Previous investigations on PLT storage have highlighted a progressive alteration of structural and cell adhesion proteins, utterly promoting morphological score impairment and altered functionality of long-stored PLTs [11–13]. Structural, signaling or focal adhesion proteins (e.g. beta-actin, coronin 1A, tropomosyin alpha-4 chain, 14-3-3 proteins, pleckstrin, α-actinin, moesin, vinculin, fermitin family homolog 3 (kindlin-3), and filamin A) had been found to be deregulated early upon storage in an integrin αIIbβ3 signaling pathway-mediated fashion [14]. In the present study we confirm a broad deregulation of ECM and structural proteins (e.g. lumican, isoform F of proteoglycan 4, isoform 1 of vascular cell adhesion protein 1, isoform 3 of muscular LMNA-interacting protein), while gender-specific storage-dependent decreases were observed in the levels of plastin-2 (female donors) and vitronectin (male donors). At the same time, storage promoted increases in the abundance of serglycin (SRGN – Fig. 6.A and B), a protein involved in MMP2 processing and thus, indirectly, in ECM remodeling [38]. Storage-dependent increases of other adhesion proteins might indirectly mirror storage-triggered gender-specific cascades, resulting in the progressive loss of PLT structural integrity and increased PLT activation (focal adhesion, ECM remodeling), with PLTs from female donors apparently being more sensitive to this phenomenon. Since structural proteins are strictly tied to PLT physiology and activation, gender and storage-dependent alterations of this class of proteins might mirror impairments of PLT function.
4.6. Metabolism and oxidative stress
PLT lysis or granule releasates might be responsible for the observed accumulation of metabolism and oxidative stressrelated proteins in the supernatants, especially in units from female donors. In the extracellular environment, these enzymes might either be inactive or preserve their activity, provided the absence of regulatory post-translational modifications and buffering of the pH drop associated with PLT storage [1–3]. Alternatively, extracellular enzymes might show as of yet undisclosed or underinvestigated catalytic functions, a typical phenomenon associated to the so-called “moonlighting proteins” [39]. Two examples of this phenomenon might be embodied by peroxiredoxins and glyceraldehyde 3-phosphate dehydrogenase (antioxidant and glycolytic enzymes, respectively), also playing a role in iron transport [40] and lipid metabolism [41], thereby mediating pro-oxidant and inflammatory responses [39]. Consistently, increased levels of oxidative-stress-related proteins were observed in day 5 PC supernatants, such as ceruloplasmin, peroxiredoxins and GSH-transferases, other than superoxide dismutase [Cu-Zn] (female donors) and DJ-1 (male donors).
5. Conclusion
The present study was designed to investigate storage and gender-specific proteomic alterations to the supernatants of PCs from male and female donors. Despite the exploratory nature of this study, addressing a limited cohort of donors without distinction of biological variables other than gender (e.g. smoking habitudes, age, etc.), we hereby observed a storage-dependent impairment of extracellular blood coagulation mediators, together with storage and gender-specific alterations in the levels of pro-inflammatory complement components and cytokines. In order to partially cope with intrinsic technical caveats deriving from label-free quantitative approaches and the limited number of technical and biological replicates, we performed a validation on a subset of proteins through targeted LC-SRM assays against QConCAT generated standard peptides. Both approaches highlighted the deregulation of structural proteins and increased supernatant levels of ECM and focal adhesion proteins are likely suggestive of an activated state of long-stored PLTs, especially from female donors. In addition, the observed increase in storage-dependent accumulation of energy and redox metabolic enzymes might result from cell lysis or granule release. The released proteins (especially enzymes) might still exert their normal catabolic activity, or rather perform alternative “moonlighting” biological functions [38].
Taken together, the observed results indicate gender specific traits in stored PLT supernatants, with the supernatants from female donors being characterized by a more sustained increase in the levels of several proteins, which might represent a further burden in PLT activation, ECM remodeling and focal adhesion components. Future functional studies will determine whether such a biological signature might correlate with untoward effects observed in recipients receiving transfusion of prestorage-leukoreduced stored PLT concentrates.
Finally, it should be noted that countries such as France and the United Kingdom do not allow the collection of platelets from women, due to the increased risk of TRALI. This risk is thought to be related to high anti-HLA or anti-neutrophil antibodies in plasma from parous women [42]. If confirmed, the presented results should help further inform policies regarding sex selection of PLT donors worldwide.
Supplementary Material
Table 1.
Platelet supernatants from female or male donors assayed as storage day 1 or 5.
Acknowledgments
Financial Support: This work was supported in part by grants from the National Institutes of Health, National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM049222, National Center for Research Resources (S10RR023015), and by NIH/NCATS Colorado CTSA (UL1 TR001082).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jprot.2014.08.016.
Conflict of interest: The authors disclose no conflict of interest.
Transparency Document: Transparency Document associated with this article can be found, in the online version.
References
- 1.Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med. 2010;30:475–87. doi: 10.1016/j.cll.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Ohto H, Nollet KE. Overview on platelet preservation: better controls over storage lesion. Transfus Apher Sci. 2011;44:321–5. doi: 10.1016/j.transci.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Shrivastava M. The platelet storage lesion. Transfus Apher Sci. 2009;41:105–13. doi: 10.1016/j.transci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Goodrich RP, Li J, Pieters H, Crookes R, Roodt J, Heyns Adu P. Correlation of in vitro platelet quality measurements with in vivo platelet viability in human subjects. Vox Sang. 2006;90:279–85. doi: 10.1111/j.1423-0410.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 5.Liumbruno G, D'Alessandro A, Grazzini G, Zolla L. Blood-related proteomics. J Proteomics. 2010;73(3):483–507. doi: 10.1016/j.jprot.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Schubert P, Devine DV. De novo protein synthesis in mature platelets: a consideration for transfusion medicine. Vox Sang. 2010;99:112–22. doi: 10.1111/j.1423-0410.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 7.Burkhart JM, Gambaryan S, Watson SP, Jurk K, Walter U, Sickmann A, et al. What can proteomics tell us about platelets? Circ Res. 2014;114(7):1204–19. doi: 10.1161/CIRCRESAHA.114.301598. [DOI] [PubMed] [Google Scholar]
- 8.Senis Y, Garcia A. Platelet proteomics: state of the art and future perspective. Methods Mol Biol. 2012;788:367–99. doi: 10.1007/978-1-61779-307-3_24. [DOI] [PubMed] [Google Scholar]
- 9.Prudent M, D'Alessandro A, Cazenave JP, Devine DV, Gachet C, Greinacher A, et al. Proteome changes in platelets after pathogen inactivation—an interlaboratory consensus. Transfus Med Rev. 2014;28(2):72–83. doi: 10.1016/j.tmrv.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Zufferey A, Fontana P, Reny JL, Nolli S, Sanchez JC. Platelet proteomics. Mass Spectrom Rev. 2012;31:331–51. doi: 10.1002/mas.20345. [DOI] [PubMed] [Google Scholar]
- 11.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in atherosclerotic lesions. Blood. 2004;103:2096–104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 12.Thon JN, Schubert P, Duguay M, Serrano K, Lin S, Kast J, et al. Comprehensive proteomic analysis of protein changes during platelet storage requires complementary proteomic approaches. Transfusion. 2008;48:425–35. doi: 10.1111/j.1537-2995.2007.01546.x. [DOI] [PubMed] [Google Scholar]
- 13.Thiele T, Steil L, Gebhard S, Scharf C, Hammer E, Brigulla M, et al. Profiling of alterations in platelet proteins during storage of platelet concentrates. Transfusion. 2007;47:1221–33. doi: 10.1111/j.1537-2995.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- 14.Thiele T, Iuga C, Janetzky S, Schwertz H, Gesell Salazar M, Furll B, et al. Early storage lesions in apheresis platelets are induced by the activation of the integrin alphaIIbbeta(3) and focal adhesion signaling pathways. J Proteomics. 2012;76:297–315. doi: 10.1016/j.jprot.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 15.Prudent M, Crettaz D, Delobel J, Tissot JD, Lion N. Proteomic analysis of Intercept-treated platelets. J Proteomics. 2012;76:316–28. doi: 10.1016/j.jprot.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Marrocco C, D'Alessandro A, Girelli G, Zolla L. Proteomic analysis of platelets treated with gamma irradiation versus a commercial photochemical pathogen reduction technology. Transfusion. 2013;53:1808–20. doi: 10.1111/trf.12060. [DOI] [PubMed] [Google Scholar]
- 17.Schubert P, Culibrk B, Coupland D, Scammell K, Gyongyossy-Issa M, Devine DV. Riboflavin and ultraviolet light treatment potentiates vasodilator-stimulated phosphoprotein Ser-239 phosphorylation in platelet concentrates during storage. Transfusion. 2012;52:397–408. doi: 10.1111/j.1537-2995.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- 18.Prudent M, Sonego G, Abonnenc M, Tissot JD, Lion N. LC-MS/ MS analysis and comparison of oxidative damages on peptides induced by pathogen reduction technologies for platelets. J Am Soc Mass Spectrom. 2014;25(4):651–61. doi: 10.1007/s13361-013-0813-8. [DOI] [PubMed] [Google Scholar]
- 19.Harr JN, Moore EE, Chin TL, Gonzalez E, Wohlauer M, Banerjee A, et al. Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg. 2013;74(3):756–62. doi: 10.1097/TA.0b013e3182826d7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wurtz V, Hechler B, Ohlmann P, Isola H, Schaeffer-Reiss C, Cazenave JP, et al. Identification of platelet factor 4 and β-thromboglobulin by profiling and liquid chromatography tandem mass spectrometry of supernatant peptides in stored apheresis and buffy-coat platelet concentrates. Transfusion. 2007;47:1099–100. doi: 10.1111/j.1537-2995.2007.01260.x. [DOI] [PubMed] [Google Scholar]
- 21.Silliman CC, Bjornsen AJ, Wyman TH, Kehler M, Allard J, Bieber S, et al. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43(5):633–40. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanter J, Khan SY, Kelher M, Gore L, Silliman CC. Oncogenic and angiogenic growth factors accumulate during routine storage of apheresis platelet concentrates. Clin Cancer Res. 2008;14(12):3942–7. doi: 10.1158/1078-0432.CCR-07-4824. [DOI] [PubMed] [Google Scholar]
- 23.Dineen SP, Roland CL, Toombs JE, Kehler M, Silliman CC, Brekken RA, et al. The acellular fraction of stored platelets promotes tumor cell invasion. J Surg Res. 2009;153(1):132–7. doi: 10.1016/j.jss.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103(6):2096–104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 25.Silliman CC, Dzieciatkowska M, Moore EE, Kehler M, Banerjee A, Liang X, et al. Proteomic analyses of human plasma: Venus versus Mars. Transfusion. 2012;52(2):417–24. doi: 10.1111/j.1537-2995.2011.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratt JM, Simpson DM, Doherty MK, Rivers J, Gaskell SJ, Beynon RJ. Multiplexed absolute quantification for proteomics using concatenated signature peptides encoded by QconCAT genes. Nat Protoc. 2006;1(2):1029–43. doi: 10.1038/nprot.2006.129. [DOI] [PubMed] [Google Scholar]
- 27.Dzieciatkowska M, Hill R, Hansen KC. GeLC-MS/MS analysis of complex protein mixtures. Methods Mol Biol. 2014;1156:53–66. doi: 10.1007/978-1-4939-0685-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26(7):966–8. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glenister KM, Payne KA, Sparrow RL. Proteomic analysis of supernatant from pooled buffy-coat platelet concentrates throughout 7-day storage. Transfusion. 2008;48:99–107. doi: 10.1111/j.1537-2995.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- 30.Egidi MG, Rinalducci S, Marrocco C, Vaglio S, Zolla L. Proteomic analysis of plasma derived from platelet buffy coats during storage at room temperature. An application of ProteoMiner (TM) technology. Platelets. 2011;22:252–69. doi: 10.3109/09537104.2010.550348. [DOI] [PubMed] [Google Scholar]
- 31.Skornicka EL, Kiyatkina N, Weber MC, Tykocinski ML, Koo PH. Pregnancy zone protein is a carrier and modulator of placental protein-14 in T-cell growth and cytokine production. Cell Immunol. 2004;232(1–2):144–56. doi: 10.1016/j.cellimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Breet NJ, Sluman MA, van Berkel MA, van Wekum JW, Bouman HJ, Harmsze AM, et al. Effect of gender difference on platelet reactivity. Neth Heart J. 2011;19(11):451–7. doi: 10.1007/s12471-011-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biino G, Santimone I, Minelli C, Sorice R, Frongia B, Traglia M, et al. Age- and sex-related variations in platelet count in Italy: a proposal of reference ranges based on 40987 subjects' data. PLoS One. 2013;8(1):e54289. doi: 10.1371/journal.pone.0054289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen CM, Jensen PH, Bukh A, Sunde L, Lamm LU, Ingerslev J. Pregnancy zone protein: a re-evaluation of serum levels in healthy women and in women suffering from breast cancer or trophoblastic disease. Scand J Clin Lab Invest. 1990;50(5):479–85. doi: 10.1080/00365519009089162. [DOI] [PubMed] [Google Scholar]
- 35.Gnatenko DV, Dunn JJ, McCorkle SR, Weissmann D, Perrotta PL, Bahou WF. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood. 2003;101:2285–93. doi: 10.1182/blood-2002-09-2797. [DOI] [PubMed] [Google Scholar]
- 36.Silberstein FC, De Simone R, Levi G, Aloisi F. Cytokineregulated expression of platelet-derived growth factor gene and protein in cultured human astrocytes. J Neurochem. 1996;66(4):1409–17. doi: 10.1046/j.1471-4159.1996.66041409.x. [DOI] [PubMed] [Google Scholar]
- 37.Dzieciatkowska M, Wohlauer MV, Moore EE, Damle S, Peltz E, Campsen J, et al. Proteomic analysis of human mesenteric lymph. Shock. 2011;35(4):331–8. doi: 10.1097/SHK.0b013e318206f654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundequist A, Abrink M, Pejler G. Mast cell-dependent activation of pro matrix metalloprotease 2: A role for serglycin proteoglycan-dependent mast cell proteases. Biol Chem. 2006;387(10–11):1513–9. doi: 10.1515/BC.2006.189. [DOI] [PubMed] [Google Scholar]
- 39.Jeffery CJ. Moonlighting proteins: old proteins learning new tricks. Trends Genet. 2003;19(8):415–7. doi: 10.1016/S0168-9525(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 40.Polati R, Castagna A, Bossi AM, Alberio T, De Domenico I, Kaplan J, et al. Murine macrophages response to iron. J Proteomics. 2012;76:10–27. doi: 10.1016/j.jprot.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathion eperoxidase and phospholipase A2 activities. J Biol Chem. 2000;275:28421–7. doi: 10.1074/jbc.M005073200. [DOI] [PubMed] [Google Scholar]
- 42.Gajic O, Yilmaz M, Iscimen R, Kor DJ, Winters JL, Moore SB, et al. Transfusion from male-only versus female donors in critically ill recipients of high plasma volume components. Crit Care Med. 2007;35(7):1645–8. doi: 10.1097/01.CCM.0000269036.16398.0D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.