Dear Editor,
Essential thrombocythemia (ET) is a myeloproliferative neoplasm (MPN) that primarily involves the megakaryocytic lineage, and is characterized by increased numbers of large, mature megakaryocytes in bone marrow as well as sustained thrombocytosis. Mutations in JAK2 or calreticulin (CALR) are present in about 50% and 25% of patients with ET, respectively, and these mutations are thought to drive MPN [1]. CALR and JAK2 mutations are mutually exclusive in MPNs [1]. Compared with patients with JAK2-mutated ET, patients with CALR-mutated ET have lower Hb levels and lower numbers of granulocytes, but higher numbers of platelets [2,3,4,5,6]. The CALR-mutated patients also have a lower incidence of thrombosis during their clinical course. Genetic background such as race may influence the risk of thrombosis, and recent study reported that Japanese ET patients with JAK2 mutation had a higher cumulative incidence of thrombosis than those with CALR mutations, although the differences were not significant [6]. Therefore, we analyzed the impact of JAK2 and CALR mutations on clinical features and thrombotic events in Japanese patients with ET.
One hundred forty-nine patients diagnosed as having ET at the Department of Gastroenterology and Hematology, University of Miyazaki or other participating institutions in Japan between February 2007 and December 2012, according to the 2008 or 2001 WHO diagnostic criteria [7,8] were included in this study. The coding sequences of JAK2 (exon 14), MPL (exon 10), and CALR (exons 9) in them were examined by Sanger sequencing. JAK2 mutation was examined at diagnosis, and the status of MPL and CALR mutation was evaluated for this study by using frozen DNA samples. Their clinical and hematological features based on their genetic mutations were retrospectively analyzed. Univariate analyses comparing variables between ET patients with JAK2 mutation and those with CALR mutation were done with the t test for continuous variables, and Pearson's χ2 test with Yates' continuous correction for 2×2 tables. The number of leukocytes was compared with the Wilcoxon rank-sum test because data were heavily skewed. This study was approved by the Research Ethics Committee of the University of Miyazaki.
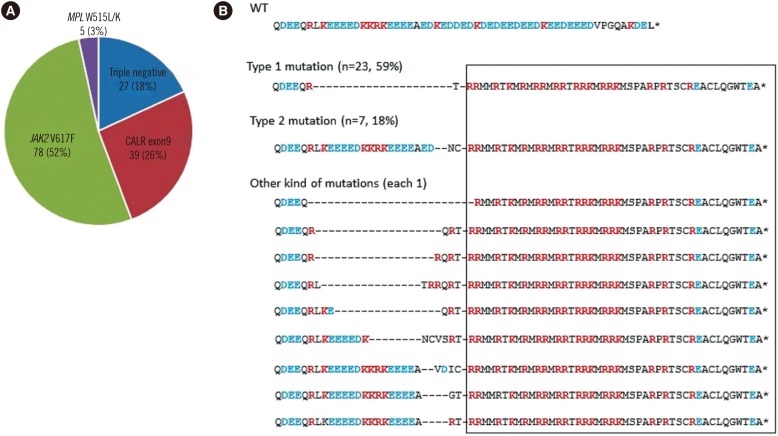
The median age of the 149 patients with ET was 59 yr (range, 8-94), and 71 subjects (48%) were male. The JAK2 V617F mutation was detected in 78 (52%) patients, CALR exon 9 mutations in 39 (26%), and MPL W515L/K mutations in 5 (3%). The remaining 27 cases (18%) exhibited none of these three mutations and were considered triple-negative MPNs (Fig. 1A). Our result confirmed previous findings regarding mutational status in patients with ET; about a half of the patients with ET harbored a JAK2 V617F mutation, and about one-quarter of the patients with ET had a CALR mutation. The proportion of patients with triple-negative ET was 18% in our study, which was similar to the range of 10-19% reported in previous studies [2,3,4,6].
Fig. 1. Mutational status in 149 patients with ET. (A) Frequency of JAK2, CALR, and MPL mutations in ET. Eighteen percent of patients were negative for all three kinds of mutations. (B) Analysis of the CALR C-terminal amino acid (AA) sequence. In addition to the common Type 1 and Type 2 mutations, nine types of CALR exon 9 mutations were observed in one case each. All mutation types resulted in +1 bp frameshifts and led to a novel C-terminal peptide sequence, in which the KDEL motif was absent and positively charged AAs (red text) were substituted for negatively charged AAs (blue text).
Abbreviations: ET, essential thrombocythemia; WT, wild type.
Various CALR mutations were found in exon 9. Type 1 mutations, a 52-bp deletion in exon 9, were the most common and were observed in 23 cases (59%) (Fig. 1B). Type 2 mutations, a 5-bp insertion in exon 9, were identified in seven cases (18%), while nine other types of CALR exon 9 mutations occurred in one case each. All CALR mutations resulted in +1 bp frameshifts and led to a novel C-terminal peptide sequence. The newly formed C-terminus of CALR lacked the KDEL motif and contained positively charged amino acids (AAs) such as lysine and arginine instead of negatively charged AAs such as aspartic acid and glutamic acid.
We compared hematological and clinical features of patients with JAK2 mutations and CALR mutations (Table 1). Patients with CALR-mutated ET displayed a unique phenotype; compared with patients with JAK2-mutated ET, they were younger and predominantly male. They also exhibited lower Hb levels, but higher platelet counts. These characteristics are identical to the previous results [2,3,4,5,6], and indicate that patients with CALR-mutated ET display a phenotype favoring megakaryopoiesis as opposed to the skewed erythropoiesis found in patients with JAK2-mutated ET. Mutant CALR was reported to augment STAT5 activation in the presence of MPL, but not in the presence of the EPO receptor [9]. Increased STAT5 activation by mutant CALR in cells that express MPL might cause the preferential expansion of the megakaryocyte lineage. In contrast to previous reports, there was no difference in neutrophil counts between patients with JAK2-mutated and CALR-mutated ET in this study, but patients with CALR-mutated ET had lower leukocyte alkaline phosphatase scores compared with patients with JAK2-mutated ET.
Table 1. Clinical and hematological features of patients with essential thrombocythemia with respect to JAK2 and CALR mutation status.
| Variable | JAK2 V617F mutation (n = 78) | CALR mutation (n = 39) | P |
|---|---|---|---|
| Age median (yr, range) | 63.5 (21-85) | 52 (8-94) | < 0.05 |
| Sex (M/F) | 30/48 | 28/11 | < 0.01 |
| WBC ( × 109/L, median, range) | 10.6 (4.5-46.7) | 9.0 (3.4-22.4) | n.s. |
| Granulocytes ( × 109/L, mean ± SD) | 8.2 ± 5.5 | 6.9 ± 4.5 | n.s. |
| Hb ( g/L, mean ± SD) | 143 ± 21 | 136 ± 16 | < 0.05 |
| Plt ( × 109/L, mean ± SD) | 947 ± 525 | 1143 ± 599 | < 0.05 |
| Ferritin (μg/L, mean ± SD) | 85.0 ± 75.4 | 158.8 ± 87.2 | < 0.01 |
| Erythropoietin (IU/L, mean ± SD) | 11.8 ± 7.0 | 36.4 ± 9.9 | < 0.01 |
| Leukocyte alkaline phosphatase score (mean ± SD) | 270.6 ± 86.1 | 166.6 ± 52.9 | < 0.01 |
| Splenomegaly (presence/examined, %) | 23/52 (45%) | 11/24 (46%) | n.s. |
| Thrombosis during follow-up (presence/examined, %) | 21/77 (26%) | 3/39 (7.7%) | < 0.05 |
Distribution of variables was evaluated by using the Kolmogorov-Smirnov test, and all except for the number of WBC were distributed normally.
Abbreviations: WBC, white blood cell; Plt, platelet; n.s., no significance.
In the course of ET, thrombotic events occurred in 26% of patients with JAK2-mutated ET versus 7.7% of patients with CALR-mutated ET. Consistent with previous reports [2,3,4,5], patients with CALR-mutated ET in this study had a lower risk of thrombosis than patients with JAK2-mutated ET. The widely accepted risk factors for thrombosis in ET were age >60 yr old and a history of thrombosis. In addition, elevated white blood cell count (>11×109/L) and presence of JAK2 mutations were reported to be associated with thrombotic events, while elevated platelet count (>1,000×109/L) was associated with lower arterial thrombotic risk [10]. Clinical features observed in patients with CALR-mutated ET, including younger age, higher platelet count, and lack of JAK2 mutation might contribute to their lower risk of thrombosis.
Acknowledgments
This study was supported by a Grant-in-Aid for Clinical Research from Miyazaki University Hospital.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 2.Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123:1552–1555. doi: 10.1182/blood-2013-11-538983. [DOI] [PubMed] [Google Scholar]
- 3.Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123:1544–1551. doi: 10.1182/blood-2013-11-539098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CC, Gau JP, Chou HJ, You JY, Huang CE, Chen YY, et al. Frequencies, clinical characteristics, and outcome of somatic CALR mutations in JAK2-unmutated essential thrombocythemia. Ann Hematol. 2014;93:2029–2036. doi: 10.1007/s00277-014-2151-8. [DOI] [PubMed] [Google Scholar]
- 5.Park SH, Kim SY, Lee SM, Yi J, Kim IS, Kim HH, et al. Incidence, clinical features, and prognostic impact of CALR exon 9 mutations in essential thrombocythemia and primary myelofibrosis: an experience of a single tertiary hospital in Korea. Ann Lab Med. 2015;35:233–237. doi: 10.3343/alm.2015.35.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okabe M, Yamaguchi H, Usuki K, Kobayashi Y, Kawata E, Kuroda J, et al. Clinical features of Japanese polycythemia vera and essential thrombocythemia patients harboring CALR, JAK2V617F, JAK2Ex12del, and MPLW515L/K mutations. Leuk Res. 2016;40:68–76. doi: 10.1016/j.leukres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow SH, Campo E, editors. WHO classification of tumors of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008. [Google Scholar]
- 8.Jaffe ES, Harris NL. Pathology and genetics of tumors of haematopoietic and lymphoid tissues. 3rd ed. Lyon: IARC; 2001. [Google Scholar]
- 9.Chachoua I, Pecquet C, El-Khoury M, Nivarthi H, Albu RI, Marty C, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127:1325–1335. doi: 10.1182/blood-2015-11-681932. [DOI] [PubMed] [Google Scholar]
- 10.Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117:5857–5859. doi: 10.1182/blood-2011-02-339002. [DOI] [PubMed] [Google Scholar]