Abstract
Background
Multidrug-resistant tuberculosis (MDR-TB) is an important global health problem. Furthermore, the time to identify a positive sputum culture is an important risk factor for the spread of tuberculosis, and several factors can predict a prolonged time to culture conversion. Moreover, the relationship between poor nutritional status and infectious disease is clearly established. Therefore, the present study aimed to investigate the association between body mass index (BMI) and sputum culture conversion within 3 months among patients with MDR-TB.
Materials and Methods
We retrospectively evaluated 218 patients with MDR-TB who were treated at a large tuberculosis referral hospital in South Korea between January 2005 and December 2010. The outcome of interest was defined as sputum culture conversion within 3 months, and we analyzed the association between BMI and this outcome.
Results
Among the 218 patients, 53 patients (24.3%) had a low BMI (<18.5 kg/m2). In the multivariate Cox proportional-hazards regression analysis, failure to achieve sputum culture conversion within 3 months was independently associated with having a low BMI (hazard ratio [HR]: 1.741, 95% confidence interval [CI]: 1.006–3.013; P = 0.047) and a positive sputum smear at the initiation of therapy (HR: 8.440, 95% CI: 1.146–62.138, P = 0.036).
Conclusion
Low BMI (<18.5 kg/m2) was an independent risk factor for failure to achieve sputum culture conversion within 3 months among patients with MDR-TB.
Keywords: Tuberculosis, Multidrug-resistant, Nutritional status, Risk factors, Sputum
Introduction
There were an estimated 9.7 million cases of tuberculosis (TB) in 2014 [1], and multi-drug resistant tuberculosis (MDR-TB) is a major global health problem, despite global control efforts. Furthermore, the increasing incidence of MDR-TB can create challenges regarding TB control in many countries. In this context, MDR-TB is defined as resistance to at least rifampicin and isoniazid, which are the two most effective first-line anti-TB drugs. In 2014, there were an estimated 300,000 MDR-TB cases (range: 220,000-370,000), and approximately 54% of these cases occurred in India, China, and the Russian Federation [2]. Moreover, in 2014, 53% of the MDR-TB cases were among new cases and 47% of the MDR-TB cases were previously treated TB cases [2].
TB is the important infectious disease in South Korea, and nation-wide reporting identified 787 patients with MDR-TB in 2015, which represented an 8.1% decrease from the previous year [3]. However, among the Organization for Economic Co-operation and Development countries, South Korea still maintains the highest ranking in terms of the incidence, prevalence, and mortality of MDR-TB [1]. Although most patients with drug-susceptible TB can be cured using a 6-month course of treatment, most MDR-TB cases are treated for ≥20 months using the daily administration of drugs that are more toxic, more expensive, and less effective, compared to the drugs that are used to treat drug-susceptible TB [4,5]. Furthermore, treatment is only successful in approximately one-half of patients with MDR-TB, which is related to the high rates of loss to follow-up (28%) and death (15%) [5].
Positive sputum culture results from patients with pulmonary TB are the most important factor in controlling the spread of MDR-TB, as these patients expel droplets that can carry infectious bacilli through coughing or sneezing [6]. There are also several factors that can predict a prolonged time to culture conversion: cavitary lesions on radiographic images, uncontrolled diabetes mellitus, old age, and the total number of administered anti-TB drugs [7]. Furthermore, the relationship between poor nutritional status and infectious disease is clearly established [8], some reports have demonstrated that malnutrition could be a risk factor for TB mortality [9], and other reports have suggested that malnutrition is related to treatment failure [10]. Therefore, the present study aimed to evaluate the association between body mass index (BMI; as a measure of nutritional status) and sputum culture conversion within 3 months among a population of South Korean patients with MDR-TB.
Materials and Methods
1. Study population and ethical considerations
This retrospective study’s design was approved by the ethics committee of the National Masan Tuberculosis Hospital (Korean National Institute of Health). We identified all patients who were treated for MDR-TB between January 2005 and December 2010 at a tertiary TB referral hospital in South Korea (the National Masan Hospital). Suspected cases of MDR-TB are generally identified by various units at the National Masan Tuberculosis Hospital or other healthcare facilities, and are referred to the MDR-TB clinic at the National Masan Tuberculosis Hospital. The patients’ medical records were reviewed to collect data regarding their demographic characteristics, TB treatment history, chest radiographs, comorbidities, drug susceptibility testing (DST) results, and clinical outcomes. Patients were excluded from the present study if their records did not contain information regarding their height and weight. All patients had been followed-up every month until they completed their treatment regimen.
2. Sputum cultures and DST
The diagnosis of MDR-TB was established using sputum cultures and DST results. Sputum specimens were processed using sodium hydroxide and N-acetyl-L-cysteine to screen for acid-fast bacilli, based on the Ziehl-Neelsen method. All cultures were performed using the MB/BacT liquid culture system (bioMérieux, Durham, NC, USA) and Ogawa agar slants (egg-based medium). DST was performed using Lowenstein-Jensen agar slants and the following drugs: isoniazid (0.2 mg/mL), rifampicin (40 mg/mL), ethambutol (2.0 mg/mL), streptomycin (10 mg/mL), kanamycin (40 mg/mL), ofloxacin (2.0 mg/mL), ethionamide (40 mg/mL), cycloserine (30 mg/mL), and para-aminosalicylic acid (1 mg/mL). Drug resistance for all drugs was defined as growth of >1% (vs. the control) in Lowenstein-Jensen media [11]. Sputum culture conversion was defined as two consecutive negative sputum cultures at least 30 days apart. We defined time to conversion as the time from the initiation of therapy to the first of the two negative sputum cultures.
3. Treatment
The Korean guidelines for TB treatment were used to treat patients based on their DST results and treatment history. Treatment for each patient was only started or modified after discussion between a group of TB specialists. The typical treatment regimen consisted of at least four targeted oral drugs and one injectable drug. Where possible, the four oral drugs were administered for the full term of treatment and the injectable drug was administered for 8 months. The injectable drug was administered 6 times per week for the first 2 months, and then 3 times per week thereafter. Renal function, hepatic function, smears, and cultures were assessed monthly until the end of treatment. Some patients were followed-up after their discharge from the hospital, and the typical duration of treatment was 12–36 months, based on the culture results, changes in the chest radiographs, and history of TB treatment.
4. Definition
Pre-treatment BMI was measured for each patient, and was calculated as body mass divided by height squared (kg/m2). The BMI value attempts to quantify an individual’s tissue mass, and individuals are categorized as underwent, normal weight, overweight, or obese, based on their BMI value. The commonly accepted BMI ranges are <18.5 kg/m2 for underweight and 18.5–25 kg/m2 for normal weight. The patients in the present study were categorized as having a low BMI (<18.5 kg/m2) or assigned to the control group (BMI of ≥18.5 kg/m2). The outcome of interest was defined as sputum culture conversion within 3 months, and we analyzed the association between BMI and this outcome.
5. Data analysis
Descriptive analyses were performed using Pearson’s chi-square test (for categorical variables) and the t-test (for continuous variables). The characteristics of the low BMI and control groups were compared, and differences with a P-value of <0.05 were considered statistically significant. Cox proportional-hazards regression analysis was used to investigate the association between BMI or other factors and sputum culture conversion within 3 months. All analyses were performed using SPSS software (version 18.0, IBM Corp., Armonk, NY), and missing data were not replaced or imputed.
Results
Between January 2005 and December 2010, 218 patients were treated for MDR-TB at our hospital. A total of 164 patients (75.2%) were men, and the overall mean patient age was 41.7 ± 14.2 years (men: 41.2 ± 13.2 years, women: 43.4 ± 16.7 years). Ninety-two patients (42.2%) were resistant to one or more injectable drugs, and 48 patients (22.0%) were resistant to one or more fluoroquinolone. Lung cavitation was confirmed on the chest radiographs for 120 patients (55.0%), and 136 patients (62.4%) had been treated for TB. Positive sputum cultures at the start of treatment were observed for 84.4% of the patients. The mean time to culture conversion was 3 months, and approximately 70% of the patients achieved sputum culture conversion within 3 months The mean overall BMI was 20.0 ± 3.3 kg/m2, and 53 patients (24.3%) had a BMI of <18.5 kg/m2 (Table 1). The patients received different empirical initial treatments, and the most commonly used drugs were prothionamide, cycloserine, para-aminosalicylic acid, levofloxacin, pyrazinamide, moxifloxacin, ofloxacin, and ethambutol; streptomycin or kanamycin were commonly used as injectable drugs (Table 2). We did not detect any significant differences in the drugs that were used for the low BMI and control groups.
Table 1. Patient characteristics according to body mass index.
| All patients No. (%) (n = 218) | BMI of ≥18.5 kg/m2No. (%) (n = 165) | BMI of <18.5 kg/m2No. (%) (n = 53) | P value | |
|---|---|---|---|---|
| BMI (kg/m2) | 20.0 ± 3.3 | 21.4 ± 2.5 | 15.8 ± 1.5 | |
| Mean age (years) | 41.7 ± 14.2 | 41.4 ± 14.6 | 42.8 ± 12.8 | 0.543 |
| Male sex | 164 (75.2) | 119 (72.1) | 45 (51.1) | 0.069 |
| Previously treated for tuberculosisa | 136 (62.4) | 103 (62.4) | 33(62.3) | 1.000 |
| With cavitation (n = 198)b | 120 (55) | 88 (53.3) | 32 (60.4) | 0.238 |
| Smear positive at the start of treatment | 184 (84.4) | 137 (83) | 47 (88.7) | 0.390 |
| Side effectsc | 82 (37.6) | 62 (37.6) | 20 (37.7) | 1.000 |
| Fluoroquinolone resistanced | 48 (22) | 38 (23) | 10 (18.9) | 0.574 |
| Injectable drug resistancee | 92 (42.2) | 73 (44.2) | 19 (35.8) | 0.338 |
Data are reported as mean ± standard deviation or number (%). Differences were evaluated using the independent samples t-test or Pearson’s chi-square test, as appropriate.
aPatients who denied receiving any anti-tuberculosis treatment or having a >30–day history of anti-tuberculosis treatment.
bChest radiography or computed tomography was performed for all 198 patients.
cSide effects: drug eruption, nausea, vomiting, abdominal discomfort, peripheral neuropathy, nephrotoxicity, hepatic toxicity, ototoxicity, and hypothyroidism.
dIncluded levofloxacin, moxifloxacin, or ofloxacin
eIncluded kanamycin, streptomycin, or amikacin.
BMI, body mass index.
Table 2. Anti-tuberculosis drugs used for 218 patients with multidrug-resistant tuberculosis.
| BMI of ≥18.5 kg/m2No. (%) (n = 165) | BMI of <18.5 kg/m2No. (%) (n = 53) | P value | |
|---|---|---|---|
| Ethambutol | 19 (11.5) | 9 (17) | 0.346 |
| Pyrazinamide | 72 (43.6) | 28 (52.8) | 0.269 |
| Streptomycin | 81 (49.1) | 31 (58.5) | 0.27 |
| Kanamycin | 85 (51.5) | 22 (41.5) | 0.212 |
| Amikacin | 2 (1.2) | 2 (3.8) | 0.249 |
| Prothionamide | 161 (97.6) | 53 (100) | 0.574 |
| Para-aminosalicylic acid | 132 (80) | 44 (83) | 0.693 |
| Cycloserine | 161 (97.6) | 49 (92.5) | 0.1 |
| Levofloxacin | 86 (52.1) | 26 (49.1) | 0.753 |
| Ofloxacin | 37 (22.4) | 9 (17) | 0.445 |
| Moxifloxacin | 62 (37.6) | 21 (39.6) | 0.871 |
BMI, body mass index
The univariate Cox proportional-hazard regression analyses revealed that failure to achieve sputum culture conversion within 3 months was significantly associated with a positive sputum smear at the initiation of treatment (hazard ratio [HR]: 11.276, 95% confidence interval [CI]: 1.564–81.298; P = 0.016) and a BMI of <18.5 kg/m2 (HR: 1.974, 95% CI: 1.191–3.272; P = 0.008). Age, sex, cavitation, side effects during treatment, resistance to fluoroquinolone, and resistance to injectable drugs were not significantly associated with failure to achieve sputum culture conversion. The multivariate Cox proportional-hazard regression analysis confirmed that failure to achieve sputum culture conversion was independently associated with a BMI of <18.5 kg/m2 (HR: 1.741, 95% CI: 1.006–3.013; p = 0.047) and a positive sputum smear at the initiation of treatment (HR: 8.440, 95% CI: 1.146–62.138; P = 0.036) (Table 3, Fig. 1). A total of 200 patients (91.7%) achieved sputum culture conversion during their treatment. Among the 53 patients with a BMI of <18.5 kg/m2, 47 patients (88.7%) achieved sputum culture conversion (Table 4).
Table 3. Cox proportional hazards regression analysis of risk factors for sputum culture conversion within 3 months among 218 patients with multidrug-resistant tuberculosis.
| Risk factors | Total | Conversionan (%) | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | Pb value | HR (95% CI) | Pb value | |||
| BMI of <18.5 kg/m2 | 53 | 30 (56.6) | 1.974 (1.191–3.272) | 0.008 | 1.741 (1.006–3.013) | 0.047 |
| Age of ≥40 years | 110 | 74 (67.3) | 1.286 (0.795–2.081) | 0.305 | 1.304 (0.767–2.218) | 0.327 |
| Male sex | 164 | 110 (67.1) | 1.548 (0.843–2.842) | 0.158 | 1.724 (0.878–3.385) | 0.113 |
| Cavitation | 120 | 88 (66.7) | 1.269 (0.737–2.183) | 0.5 | 0.85 (0.481–1.503) | 0.577 |
| Smear positivec | 184 | 118 (64.1) | 11.276 (1.564–81.298) | 0.016 | 8.44 (1.146–62.138) | 0.036 |
| Side effects | 82 | 57 (69.5) | 0.899 (0.547–1.478) | 0.675 | 1.115 (0.642–1.935) | 0.7 |
| FQ resistance | 48 | 30 (62.5) | 1.523 (0.885–2.621) | 0.129 | 1.64 (0.890–3.021) | 0.113 |
| ID resistance | 92 | 60 (65.2) | 1.190 (0.736–1.924) | 0.477 | 1.029 (0.612–1.731) | 0.914 |
aWithin 3 months after the initiation of therapy.
bBoldface indicates a significant difference compared to the baseline characteristics.
cThe sputum smear was positive at the initiation of therapy.
HR, hazard ratio; CI, confidence interval; BMI, body mass index; FQ, fluoroquinolone; ID, injectable drug.
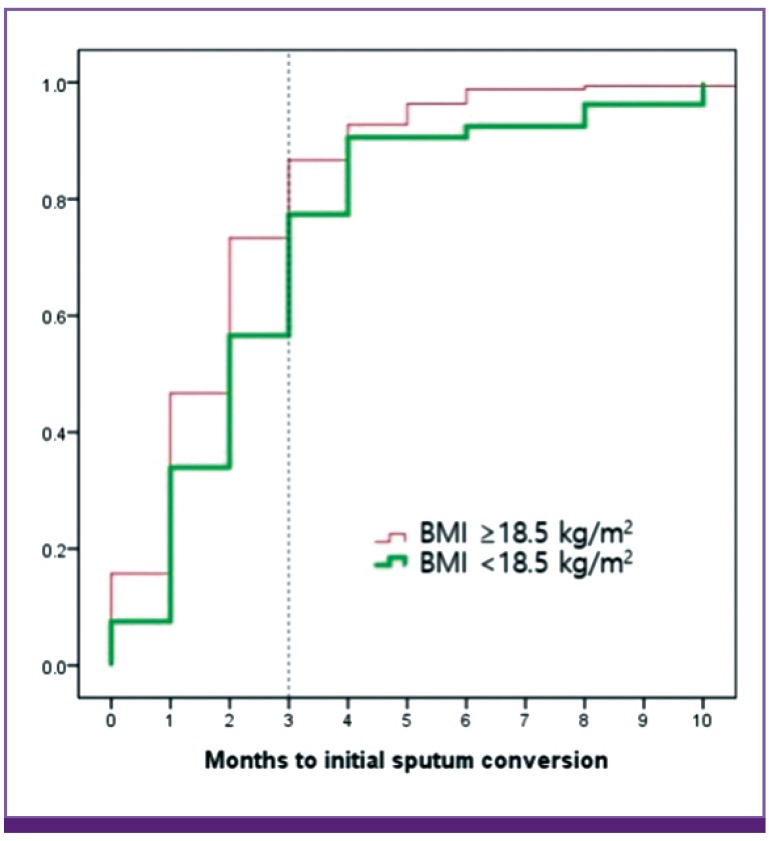
Figure 1.
Time to initial sputum culture conversion according to body mass index. The time to conversion among patients with a body mass index of <18.5 kg/m2 was significantly longer, compared to the group with a normal body mass index.
BMI, body mass index
Table 4. The sputum culture conversion results.
| BMI of ≥18.5 kg/m2No. (%) | BMI of <18.5 kg/m2No. (%) | |
|---|---|---|
| Achieved SCC | 153 (92.7) | 47 (88.7) |
| Defaulted before achieving SCC | 2 (1.2) | 0 (0) |
| Failed SCC | 9 (5.5) | 4 (7.5) |
| Died before achieving SCC | 1 (0.6) | 2 (3.8) |
| Total | 165 | 53 |
BMI, body mass index; SCC, sputum culture conversion.
Discussion
It is important to control MDR-TB, and positive sputum culture results from patients with pulmonary TB can be used to help control the spread of MDR-TB. In this context, transmission occurs when a person inhales droplets that contain Mycobacterium tuberculosis, which subsequently enters the alveoli of the lungs. Thus, prolonged periods of infectiousness may increase the likelihood of spreading MDR-TB [6]. However, few studies have evaluated predictors of initial sputum conversion among patients with MDR-TB. In Latvia, the significant predictors of initial sputum culture conversion were previous treatment for MDR-TB using second-line drugs, a high initial sputum colony count (≥3+), bilateral cavitation, and a higher number of resistant drugs at the initial treatment [12]. In the present study, we found that a low BMI (<18.5 kg/m2) and a positive sputum smear at the initiation of treatment were independently associated with failed sputum culture conversion within 3 months. However, our overall sputum conversion rate (91.7%) was higher than the rates from previous studies [13,14], which indicates that the underlying management protocol functioned reasonably well. Nevertheless, patients with a positive smear culture at the initiation of treatment exhibited a high risk of failed sputum culture conversion (HR: 8.440, 95% CI: 1.146–62.138; P = 0.036), which may be related to these patients having a high initial high bacillary burden. For example, Holtz et al. have reported that a high initial sputum colony count (≥3+) was significantly associated with delayed sputum conversion (odd ratio: 10.8, 95% CI: 1.3–91.1) [12].
Malnutrition has well-established effects on immune function [15], and the accompanying decrease in immunity may increase susceptibility to many infectious diseases [16,17]. For example, Scrimshaw et al. have suggested that malnutrition reduced the concentrations of immunoglobulins, interleukin-2 receptors, and T-cell subsets (helper, suppressor-cytotoxic, and natural killer cells) among patients with TB [18]. The most useful measure of malnutrition in adults is BMI, and low BMI (<18.5 kg/m2) is associated with increased TB-related morbidity and mortality [19,20]. Furthermore, several study have demonstrated that low BMI is a risk factor of mortality among patients with TB who are co-infected with HIV [10], and underweight status (BMI of <18.5 kg/m2) was significantly associated with severe clinical symptoms and a higher proportional of positive sputum cultures. Moreover, in South Africa, underweight status was a significant predictor of more advanced TB disease (odds ratio: 14.2, 95% CI: 4.4–46.2) [1]. Our findings regarding the risk of being underweight are also similar to the findings of Kurbatova et al., who reported that underweight patients had a low sputum conversion rate (HR: 0.82, 95% CI: 0.72–0.93; P = 0.002), compared to patients with MDR-TB who had a BMI of ≥18.5 kg/m2 [20].
The present study has several limitations. First, we only evaluated patients from a single TB referral hospital, which may have introduced selection bias and limits the extrapolation of our findings to the entire population of South Korean patients with MDR-TB. Second, our study only evaluated a relatively small sample of MDR-TB cases, and larger studies are needed to validate our findings regarding the predictors of outcomes in cases of MDR-TB. Third, we were unable to perform long-term follow-up, which limits any conclusions regarding the long-term trends for this disease.
In the present study, underweight status and a positive initial sputum smear were associated with failing to achieve sputum culture conversion within 3 months among a single-center population of patients with MDR-TB. Our findings suggest that underweight status and a positive initial sputum smear might be risk factors for community transmission from patients with MDR-TB.
Acknowledgments
We acknowledge the outstanding contributions of the technicians and nursing staff at the National Masan Tuberculosis Hospital.
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.World Health Organization (WHO) Global tuberculosis report 2015. [Accessed 2 May 2016]. Available at: http://aidsdatahub.org/global-tuberculosis-report-2015-who-2015.
- 2.World Health Organization (WHO) Global tuberculosis report 2014. [Accessed 2 May 2016]. Available at: http://reliefweb.int/report/world/global-tuberculosis-report-2014.
- 3.Park WS, Kang HY, Kim SJ, Cha JO, Son HJ, Park O. Notified tuberculosis status in Korea, 2015. Public Health Weekly Report of the KCDC. 2015;9:342–345. [Google Scholar]
- 4.Kwon YS, Kim YH, Suh GY, Chung MP, Kim H, Kwon OJ, Choi YS, Kim K, Kim J, Shim YM, Koh WJ. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008;47:496–502. doi: 10.1086/590005. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Companion hand book to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. [Accessed 4 May 2016]. Available at: http://www.who.int/tb/publications/pmdt_companionhandbook/en.
- 6.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care setting, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 7.Long R, Bochar K, Chomyc S, Talbot J, Barrie J, Kunimoto D, Tilley P. Relative versus absolute noncontagiousness of respiratory tuberculosis on treatment. Infect Control Hosp Epidemiol. 2003;24:831–838. doi: 10.1086/502145. [DOI] [PubMed] [Google Scholar]
- 8.Scrimshaw NS. Historical concepts of interactions, synergism, and antagonism between nutrition and infection. J Nutr. 2003;133:316S–21S. doi: 10.1093/jn/133.1.316S. [DOI] [PubMed] [Google Scholar]
- 9.Zachariah R, Spielmann MP, Harries AD, Salanioni FM. Moderate to severe malnutrition associated with early death in patients with tuberculosis is a risk factor. Trans R Soc Trop Med Hyg. 2002;96:291–294. doi: 10.1016/s0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 10.Podewils LJ, Holtz T, Riekstina V, Skripconoka V, Zarovska E, Kirvelaite G, Kreigere E, Leimane V. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect. 2011;139:113–120. doi: 10.1017/S0950268810000907. [DOI] [PubMed] [Google Scholar]
- 11.Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, Rist N, Smelev NA. Advances in techniques of testing mycobacterial drug sensitivity, and the used of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 12.Holtz TH, Sternberg M, Kammerer S, Laserson KF, Riekstina V, Zarovska E, Skripconoka V, Wells CD, Leimane V. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med. 2006;144:650–659. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hafkin J, Modongo C, Newcomb C, Lowenthal E, MacGregor RR, Steenhoff AP, Freidman H, Bisson GP. Impact of the human immunodeficiency virus on early multidrug-resistant tuberculosis treatment outcomes in Botswana. Int J Tuberc Lung Dis. 2013;17:348–353. doi: 10.5588/ijtld.12.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qazi F, Khan U, Khowaja S, Javaid M, Ahmed A, Salahuddin N, Hussain H, Becerra MC, Golub JE, Khan AJ. Predictors of delayed culture conversion in patients treated for multidrug-resistant tuberculosis in Pakistan. Int J Tuberc Lung Dis. 2011;15:1556–1559. doi: 10.5588/ijtld.10.0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82:2–8. doi: 10.1136/pgmj.2005.037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris T, Cook EF, Garrision R, Higgins M, Kannel W, Goldman L. Body mass index and mortality among nonsmoking older persons. The Framingham heart study. JAMA. 1988;259:1520–1524. [PubMed] [Google Scholar]
- 17.Lee YM, Kim SM, Park SJ, Lee SO, Choi SH, Kim YS, Woo JH, Kim SH. Factors associated with a strong response to the T-SPOT.TB in patients with extrapulmonary tuberculosis. Infect Chemother. 2014;46:248–252. doi: 10.3947/ic.2014.46.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–477S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 19.Mupere E, Malone L, Zalwango S, Chiunda A, Okwera A, Parraga I, Stein CM, Tisch DJ, Muqerwa R, Boom WH, Mayanja H, Whalen CC. Lean tissue mass wasting is associated with increased risk of mortality among women with pulmonary tuberculosis in urban Uganda. Ann Epidemiol. 2013;22:466–473. doi: 10.1016/j.annepidem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurbatova EV, Gammino VM, Bayona J, Becerra MC, Danilovitz M, Falzon D, Gelmanova I, Keshavjee S, Leimane V, Mitnick CD, Quelapio MI, Riekstina V, Taylor A, Viklepp P, Ziqnol M, Ceqielski JP. Predictors of sputum culture conversion among patients treated for multi-drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16:1335–1343. doi: 10.5588/ijtld.11.0811. [DOI] [PubMed] [Google Scholar]