Abstract
Paclitaxel was isolated from the bark of the Pacific yew, Taxus brevifolia, and used as an anticancer agent. Paclitaxel prevents cancer cell division by inhibiting spindle fiber function, inducing cell death. A recent study demonstrated that paclitaxel binds to myeloid differentiation protein-2 of Toll-like receptor 4 and prevents the signal transduction of lipopolysaccharide (LPS). Paclitaxel converts immune cells hypo-responsive to LPS. In this study, we investigated whether paclitaxel can inhibit the phenotype and function of immune cells. To accomplish this, we used spleen cells, a major type of immune cell, LPS, a representative inflammatory agent and a mitogen for B lymphocytes. LPS profoundly increased the activation and cytokine production of spleen cells. However, paclitaxel significantly inhibited LPS-induced hyper-activation of spleen cells. Furthermore, we found that paclitaxel induced cell death of LPS-treated spleen cells. These results suggest that paclitaxel can inhibit the hyper-immune response of LPS in spleen cells via a variety of mechanisms. These findings suggest that paclitaxel can be used as a modulating agent for diseases induced by hyper-activation of B lymphocytes. Taken together, these results demonstrate that paclitaxel inhibits the function of spleen cells activated by LPS, and further induces cell death.
Keywords: cell death, hyper-activation, lipopolysaccharide, paclitaxel, spleen cells
Introduction
Paclitaxel is an anti-cancer drug known as taxol [20,23] that stabilizes microtubules during cell division, thus arresting the process. As a result, paclitaxel has been used as an anticancer agent for treatment of breast, ovary, and lung cancer. However, since paclitaxel cannot select between cancer and normal cells, it has side effects including myelosuppression [5,15].
To date, few studies have investigated the effects of paclitaxel on immune cells. It has been reported that paclitaxel modulates the activity of various immune cells [8]. Specifically, paclitaxel binds to myeloid differentiation protein-2 (MD-2), which is an accessory protein of Toll-like receptor 4 (TLR4), the main receptor of lipopolysaccharide (LPS) [11]. As a result, paclitaxel can block TLR4-mediated nuclear factor (NF)-κB signaling of LPS on cells [25]. LPS is the primary causative agent of inflammation by Gram negative bacteria and sepsis [21]. However, the immunomodulatory activity of paclitaxel remains unclear. Paclitaxel induces the maturation of dendritic cells (DCs) associated with up-regulation of major histocompatibility complex class II molecules [9] and increases DC viability [14], while it makes splenic lymphocytes hyporesponsive to LPS [16].
In this study, we investigated whether paclitaxel modulates the activation and function of spleen cells activated by LPS as an inflammatory agent. The functional changes in LPS-treated spleen cells in response to paclitaxel focused on alteration of spleen cell proliferation, cell morphology, presence of apoptosis, and production of cytokines.
Materials and Methods
Animals and reagents
BALB/c or C57BL/6 mice were purchased from ORIENT BIO (Korea) and maintained in our animal facility. Mice used in this study were 7- to 12-weeks-old. All animal experiments conformed to the institutional guidelines of Jeju National University for laboratory animal use and care. LPS purified from Escherichia coli and paclitaxel were purchased from Sigma (USA) and dissolved in phosphate buffered saline. The concentration of dimethyl sulfoxide in culture medium was 0.1% (v/v).
Preparation of spleen cells
Spleen cells were prepared from the spleens of mice [10]. Briefly, spleens were smashed and treated with ammonium chloride potassium lysis buffer to remove red blood cells. The cells were then passed through a 70 µm cell strainer to obtain individual cells, which were cultured in a complete culture medium for lymphocytes, RPMI 1640 medium containing 10% fetal bovine serum, 2 mM L-glutamine, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 100 IU/mL penicillin/streptomycin, and 50 µM 2-mercaptoethanol.
Cell morphology
To observe the effects of paclitaxel, the morphological changes of spleen cells were investigated. Briefly, cells were cultured at a concentration of 2 × 106 cells/mL in 96- or 6-well culture plates in the absence or presence of 1.0 µg/mL LPS and 5.0 µg/mL paclitaxel for 3 days. The cell morphology was then observed and photographed using an inverted optical microscope (Olympus, Japan) with a digital camera.
Assessment of cellular viability
The viability of LPS and paclitaxel-treated spleen cells was measured by a cellular viability assay [1,12]. Briefly, spleen cells were seeded at a concentration of 2 × 106 cells/mL in 96-well plates and treated with 5-fold serial dilutions of LPS (0, 0.008, 0.04, 0.2, 1.0, 5.0 µg/mL) and paclitaxel (5.0 µg/mL). After 3 days of culture, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma) was treated at a concentration of 0.5 mg/mL for 4 hr. The insoluble violet crystal generated by viable cells was dissolved by 100 µL/well 10% sodium dodecyl sulfate solution for 2 h, after which the optical density of samples was measured at 570 nm using a microplate reader (Multiskan FC; Thermo Fisher Scientific, USA).
Measurement of cytokine production
A range of concentrations of LPS (0, 0.04, 0.2, 1.0 µg/mL) and paclitaxel (5 µg/mL) were added to 2 × 106 cells/mL of spleen cells in 96-well culture plates. After 3 days, the culture supernatants were collected and used to quantify tumor necrosis factor-alpha (TNF-α) and interleukin-10 (IL-10), which are critical inflammatory and anti-inflammatory cytokines, respectively. For these assays, enzyme-linked immunosorbent assay (ELISA) using CytoSet antibody pairs (Thermo Fisher Scientific) was performed based on the manufacturer's instructions.
Flow cytometry analysis
Flow cytometry analysis was performed as previously described [7,13]. Briefly, spleen cells were cultivated in 6-well culture plates and treated with 1 µg/mL LPS and 5 µg/mL paclitaxel for 3 days. To check the membrane potential of the mitochondria, the cells were stained with 10 µg/mL rhodamine 123 (Sigma) for 30 min at room temperature. To measure apoptosis, the cells were stained with 2 µg/mL propidium iodide (PI). The cluster of differentiation (CD)25 and CD69 on the surface of the LPS- and paclitaxel-treated cells were stained with purified anti-mouse CD25 antibody, followed by phycoerythrin (PE)-labeled anti-rat IgM antibody, and PE-labeled anti-mouse CD69 antibody (all from BD Biosciences, USA). The stained cells were analyzed using FACSCalibur flow cytometer and the CellQuest software (both from BD Biosciences).
Statistical analysis
The means ± standard deviations (SD) were determined and then compared by ANOVA, followed by Tukey-Kramer multiple comparisons test. A p value of < 0.05 was considered significant. *, **, *** indicate p < 0.05, 0.01, 0.001 compared to the control.
Results
Paclitaxel inhibits the activity of LPS-treated spleen cells
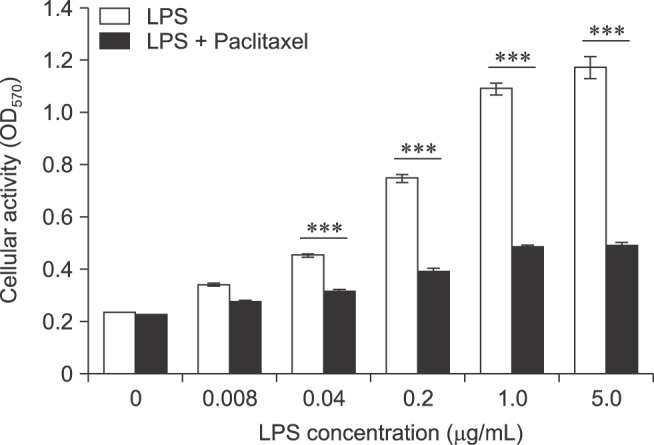
To measure the cellular activity and proliferation rate, MTT assay was performed (Fig. 1). LPS alone increased the activity and proliferation of spleen cells in a concentration-dependent manner (0–5 µg/mL). However, paclitaxel significantly decreased the activity and proliferation of LPS-treated spleen cells (0.04–5 µg/mL). These results suggest that paclitaxel might prevent the effects of LPS on spleen cells.
Fig. 1. Paclitaxel decreases the activity and proliferation of lipopolysaccharide (LPS)-treated spleen cells. The cells were seeded at a concentration of 2 × 106 cells/mL in 96-well culture plates, then treated with 5-fold serial dilutions of LPS and 5.0 µg/mL paclitaxel for 3 days. MTT assay was performed as described in the Materials and Methods. The optical density (O.D.) of samples was measured at 570 nm using a microplate reader and expressed as mean ± standard deviation (SD).
Paclitaxel decreases the population of large cells in LPS-activated spleen cells
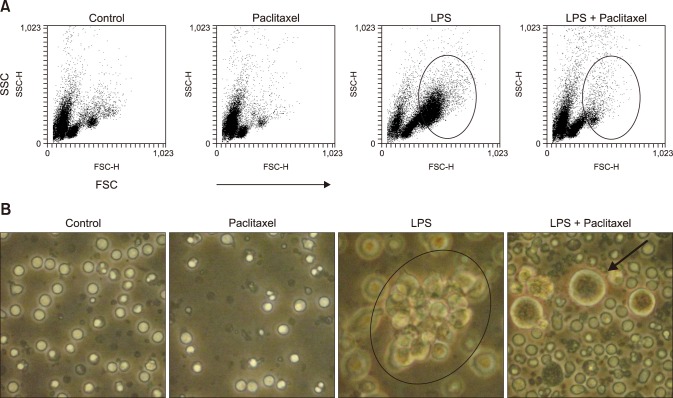
We used flow cytometry analysis and a microscope to investigate the effect of LPS and paclitaxel on the cell size and morphology of spleen cells (Fig. 2). The cell size was measured by forward scattered light (FSC) of flow cytometry and the cell morphology was observed using an inverted optical microscope. Flow cytometry analysis (panel A in Fig. 2) revealed that paclitaxel alone induced minor changes in cell size compared to the control, whereas LPS markedly increased the population of large cells (with high FSC, in circle). Interestingly, paclitaxel decreased the population of large cells in LPS-treated spleen cells. Microscopic analysis (panel B in Fig. 2) revealed growing clusters (a black circle) and large single cells in LPS-treated spleen cells. However, spleen cells treated with paclitaxel and LPS contained almost no clusters and very few large single cells (a black arrow).
Fig. 2. Paclitaxel alters the cell size and number of LPS-activated spleen cells. Cells were seeded at a concentration of 2 × 106 cells/mL in 6-well culture plates, then treated with a control (medium alone), paclitaxel (5.0 µg/mL), LPS (1.0 µg/mL), or LPS + paclitaxel. After 3 days of treatment, (A) the cells were analyzed using flow cytometry. The circles in the dot plots indicate the population of activated and big cells (forward scattered light [FSC]high cells). (B) The cell morphology was observed by an inverted optical microscope with a digital camera. The circle in the picture indicates a cluster of cells proliferating and the arrow indicates swollen cells.
Paclitaxel increases death of LPS-treated spleen cells
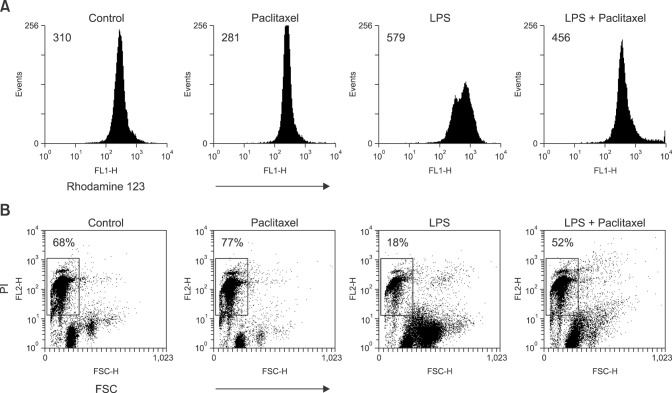
Rhodamine 123 and PI staining were used to investigate cell viability and death of the spleen cells treated with LPS and paclitaxel. Rhodamine123 staining revealed that LPS increased the geometric mean fluorescence intensity (MFI) of spleen cells compared to the control, whereas paclitaxel did not. Paclitaxel decreased the MFI of LPS-treated spleen cells (panel A in Fig. 3). Given that MFI in Rhodamine 123 staining indicates the stability of mitochondrial membrane potential, these results suggest that paclitaxel may affect the viability of LPS-treated spleen cells, indicating increased cell death. Indeed, PI staining demonstrated that paclitaxel consistently increased the death (FSClowPIhigh cells) of LPS-treated spleen cells (panel B in Fig. 3).
Fig. 3. Paclitaxel decreases the action potential of mitochondria and induces death of LPS-activated spleen cells. The cells were setup and treated as described in Fig. 2. (A) Cells were stained with Rhodamine 123 solution. (B) Cells were stained with propidium iodide (PI) solution. Cells in the area of FSClow and PIhigh indicate dead cells.
Paclitaxel decreases the expression of CD25 on LPS-treated spleen cells
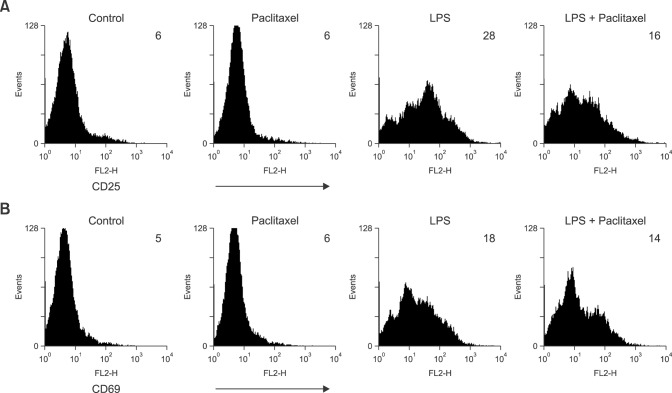
LPS is a representative activator of B lymphocytes. To investigate the effects of paclitaxel on the expression of activation markers on spleen cells, we measured the expression level of lymphocyte activation markers, CD25 and CD69 (Fig. 4). Flow cytometry analysis revealed that paclitaxel did not affect the expression of either marker on spleen cells compared to the controls, whereas LPS increased the expression of both markers. Paclitaxel decreased the expression of CD25 on LPS-treated spleen cells.
Fig. 4. Paclitaxel decreases the expression of cluster of differentiation (CD)25, an activation marker on LPS-activated spleen cells. The cells were cultured and treated as described in Fig. 2. The cells were stained for mouse CD25 or CD69 molecules, then analyzed by flow cytometry. The number of histograms indicates geometric mean fluorescence intensity.
Paclitaxel decreases the cytokine production of LPS-treated spleen cells
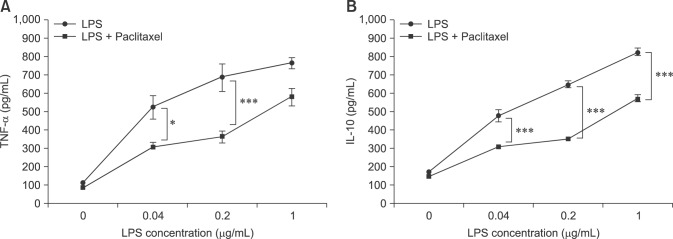
We measured cytokine production by ELISA to evaluate function. In this study, TNF-α and IL-10 were selected as representative inflammatory and anti-inflammatory cytokines, respectively. ELISA revealed that LPS enhanced the production of TNF-α and IL-10 in a concentration-dependent manner. Paclitaxel significantly inhibited LPS- induced production of both cytokines (Fig. 5).
Fig. 5. Paclitaxel decreases the production of tumor necrosis factor-alpha (TNF-α) and IL-10 of LPS-activated spleen cells. The cells were treated with 5-fold serial dilutions of LPS and 5.0 µg/mL paclitaxel for 3 days in 96-well culture plates, after which the culture supernatants were used for TNF-α and IL-10 enzyme-linked immunosorbent assay. Data are presented as the means ± SD.
Discussion
The anticancer drug, paclitaxel, has been used to treat breast, ovary, and lung cancer. This drug inhibits microtubule depolymerization, resulting in cell cycle arrest and death of cancer cells. However, few studies have investigated the effects of paclitaxel on immune cells, although an action mechanism of paclitaxel on the immune system has been demonstrated [2,22]. Paclitaxel binds to MD-2 protein of TLR4 and sequentially prevents the signal transduction of LPS, an activator of B cells. Indeed, paclitaxel converts immune cells hypo-responsive to LPS through modification of the signal transduction [16]. In this study, we demonstrated that paclitaxel inhibits the function and further induces death of LPS-activated cells.
To investigate the effects of paclitaxel on immune cells, we measured the activation and proliferation of spleen cells treated with paclitaxel and LPS by MTT assay, flow cytometry, and microscopic analysis. Paclitaxel significantly decreased LPS-induced effects on the proliferation and activation of spleen cells. Moreover, microscopic analysis revealed that some LPS- and paclitaxel-treated cells were large and swollen.
We suspected that paclitaxel may induce the death of LPS-treated spleen cells; therefore, Rhodamine123 [24] and PI staining were performed. The results revealed that paclitaxel decreased the number of large cells induced by LPS treatment and increased the number of PI-highly stained small cells (dead cells). Therefore, these results confirm that paclitaxel induced death of LPS-induced spleen cells, including apoptosis. Additionally, we analyzed the expression of CD25 and CD69, activation markers of lymphocytes. CD25, which is the alpha chain of interleukin-2 (IL-2) receptor, converts lymphocytes sensitive to IL-2, and is thus related to the proliferation of lymphocytes [17]. CD69 is an early activation marker for lymphocytes [6]. Paclitaxel also decreased the expression of activation markers in LPS-treated spleen cells. We speculated that paclitaxel can inhibit activation of the spleen cells by LPS, resulting in a reduced number of activated cells.
We next investigated if paclitaxel also affects cytokine production of LPS-treated spleen cells. ELISA demonstrated that paclitaxel decreased the production of TNF-α and IL-10, which are representative inflammatory [3] and anti-inflammatory cytokines [19], respectively. Both cytokines are closely related to the immune responses generated by LPS [4]; thus, these findings suggest that paclitaxel modulates LPS-induced immune responses by inhibiting the production of related cytokines. Therefore, we speculate that paclitaxel not only induces hypo-response of immune cells to LPS via the inhibition of signal transduction, but also prevents LPS-induced immune response through inhibition of activation and proliferation, induction of apoptosis, and inhibition of cytokine production in immune cells.
Based on these results, paclitaxel can be applied to treat diseases caused by hyper-activation of B cell. Indeed, LPS causes a variety of diseases, including the generation of auto-reactive B cells [18]. We expect paclitaxel to be particularly useful for treatment of currently incurable autoimmune diseases. To accomplish this, the therapeutic effects of paclitaxel should be verified, especially its inhibitory effects on antigen-specific IgG production of B cells through in vivo experiments. Moreover, it is necessary to investigate which subsets of spleen cells can be affected since spleen cells are composed of multiple subsets and lineages of immune cells.
In conclusion, the results of this study provide evidence that paclitaxel inhibits the activation and proliferation of spleen cells by LPS, as well as induces the death of activated cells. These results will help broaden the use of paclitaxel in basic science and clinical areas beyond as an anti-cancer therapeutic agent.
Footnotes
There is no conflict of interest.
References
- 1.Byon YY, Kim MH, Yoo ES, Hwang KK, Jee Y, Shin T, Joo HG. Radioprotective effects of fucoidan on bone marrow cells: improvement of the cell survival and immunoreactivity. J Vet Sci. 2008;9:359–365. doi: 10.4142/jvs.2008.9.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- 5.Guchelaar HJ, ten Napel CH, de Vries EG, Mulder NH. Clinical, toxicological and pharmaceutical aspects of the antineoplastic drug taxol: a review. Clin Oncol (R Coll Radiol) 1994;6:40–48. doi: 10.1016/s0936-6555(05)80367-x. [DOI] [PubMed] [Google Scholar]
- 6.Hamann J, Fiebig H, Strauss M. Expression cloning of the early activation antigen CD69, a type II integral membrane protein with a C-type lectin domain. J Immunol. 1993;150:4920–4927. [PubMed] [Google Scholar]
- 7.Jang JY, Moon SY, Joo HG. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells. Food Chem Toxicol. 2014;68:234–238. doi: 10.1016/j.fct.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci. 2009;38:283–290. doi: 10.1016/j.ejps.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Joo HG. Altered maturation of dendritic cells by taxol, an anticancer drug. J Vet Sci. 2003;4:229–234. [PubMed] [Google Scholar]
- 10.Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a β-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001;69:555–564. [PubMed] [Google Scholar]
- 11.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse Toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000;275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Kim MH, Byon YY, Park JW, Jee Y, Joo HG. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 2007;8:39–44. doi: 10.4142/jvs.2007.8.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008;115:138–143. doi: 10.1016/j.imlet.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Kim MH, Joo HG. The role of Bcl-xL and nuclear factor-κB in the effect of taxol on the viability of dendritic cells. J Vet Sci. 2009;10:99–103. doi: 10.4142/jvs.2009.10.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohler DR, Goldspiel BR. Paclitaxel (taxol) Pharmacotherapy. 1994;14:3–34. doi: 10.1002/j.1875-9114.1994.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Yea SS, Jeon YJ. Paclitaxel causes mouse splenic lymphocytes to a state hyporesponsive to lipopolysaccharide stimulation. Int J Immunopharmacol. 2000;22:615–621. doi: 10.1016/s0192-0561(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 17.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Möller G. Lipopolysaccharide as a tool to reveal autoreactive B cells. APMIS. 1988;96:93–100. doi: 10.1111/j.1699-0463.1988.tb05274.x. [DOI] [PubMed] [Google Scholar]
- 19.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nehate C, Jain S, Saneja A, Khare V, Alam N, Dubey RD, Gupta PN. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11:666–686. doi: 10.2174/1567201811666140609154949. [DOI] [PubMed] [Google Scholar]
- 21.Opal SM. The clinical relevance of endotoxin in human sepsis: a critical analysis. J Endotoxin Res. 2002;8:473–476. doi: 10.1179/096805102125001109. [DOI] [PubMed] [Google Scholar]
- 22.Resman N, Gradišar H, Vašl J, Keber MM, Pristovšek P, Jerala R. Taxanes inhibit human TLR4 signaling by binding to MD-2. FEBS Lett. 2008;582:3929–3934. doi: 10.1016/j.febslet.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 23.Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol. 1992;19:646–662. [PubMed] [Google Scholar]
- 24.Sugrue MM, Tatton WG. Mitochondrial membrane potential in aging cells. Biol Signals Recept. 2001;10:176–188. doi: 10.1159/000046886. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Li Y, Liu Y, Xiang X, Dong Z. Paclitaxel ameliorates lipopolysaccharide-induced kidney injury by binding myeloid differentiation protein-2 to block Toll-like receptor 4-mediated nuclear factor-κB activation and cytokine production. J Pharmacol Exp Ther. 2013;345:69–75. doi: 10.1124/jpet.112.202481. [DOI] [PMC free article] [PubMed] [Google Scholar]