Abstract
Specific anti-venom used to treat scorpion envenomation is usually obtained from horses after hyperimmunization with crude scorpion venom. However, immunized animals often become ill because of the toxic effects of the immunogens used. This study was conducted to evaluate the toxic and immunogenic activities of crude and detoxified Tityus serrulatus (Ts) venom in sheep during the production of anti-scorpionic anti-venom. Sheep were categorized into three groups: G1, control, immunized with buffer only; G2, immunized with crude Ts venom; and G3, immunized with glutaraldehyde-detoxified Ts venom. All animals were subjected to clinical exams and supplementary tests. G2 sheep showed mild clinical changes, but the other groups tolerated the immunization program well. Specific antibodies generated in animals immunized with either Ts crude venom or glutaraldehyde-detoxified Ts venom recognized the crude Ts venom in both assays. To evaluate the lethality neutralization potential of the produced sera, individual serum samples were pre-incubated with Ts crude venom, then subcutaneously injected into mice. Efficient immune protection of 56.3% and 43.8% against Ts crude venom was observed in G2 and G3, respectively. Overall, the results of this study support the use of sheep and glutaraldehyde-detoxified Ts venom for alternative production of specific anti-venom.
Keywords: Tityus serrulatus, anti-venom, glutaraldehyde detoxification, scorpion, sheep
Introduction
Scorpion envenomation, which has increased in the past few years, causes alarming health problems, mainly in tropical and sub-tropical countries [21]. Envenomation by Tityus serrulatus (Ts) is of particular concern in South America because its venom is highly toxic. This species is involved in the most severe cases of toxicity, which often results in cardiac failure and pulmonary oedema [6].
Scorpion venoms are complex mixtures of molecules, and it is estimated that more than 100,000 different components are present in the venom of scorpion from around the world. However, not all of these venoms have been described in detail to date [27]. The molecules identified in scorpion venoms to date include histamine, serotonine, peptides, nucleotides, amino acids, salts, hyaluronidases, proteases, phospholipases, enzyme inhibitors and neurotoxins. Among these, neurotoxins are primarily responsible for causing the symptoms that occur in response to scorpion envenomation [2].
The recommended treatment for scorpion envenomation in severe cases is immunotherapy with specific anti-venom [11]. Hyperimmune serum is obtained from large producer animals, typically horses, after several immunizations with the venomof-interest, followed by bleeding of the animals. However, the immunized animals often become ill and have lower life expectancies as a result of the toxic effects of immunogens [5].
In Brazil, horses are the producer animals of choice for anti-venom production because of their large size and good response to antigenic stimulus, which favours the production of large amounts of sera [9]. However, there are some advantages to substituting these animals with sheep such as ease in handling, low acquisition and maintenance costs, good tolerance to adjuvants [33], and potential use of hyperimmune sheep sera in patients allergic to equine proteins [19].
Development of alternative immunogens that are effective at eliciting neutralizing antibodies, but are non-toxic to the producer animals, is fundamental to improving the quality of life of the immunized animals. Currently, many methods can be employed in venom detoxification. One such method is polymerization using glutaraldehyde [12,26], which is a commonly found reagent that is inexpensive, easy to prepare, and efficient at reducing the toxicity of venoms without impairing their immunogenicity.
Glutaraldehyde is a molecule with two aldehyde groups in its extremities. These groups are extremely efficient at crosslinking proteins and binding to available amine groups to form Schiff's base [1]. This approach has already been successfully tested [12,20], and glutaraldehyde cross-linking protocol for venom detoxification is easy to perform.
To the present study was conducted to determine the toxicity of both crude and glutaraldehyde-detoxified Ts venom in sheep subjected to one cycle of immunization for anti-venom production. We determined the biochemical and immunological profiles in response to both crude and detoxified venoms and evaluated and compared the resulting neutralizing antibodies to determine the possibility of using sheep as producer animals for scorpion anti-venom production.
Materials and Methods
Animals and venoms
All procedures, treatments and animal care were approved by the Ethics Committee on Animal Experimentation at the Universidade Federal de Minas Gerais-UFMG (protocol No. 202/2012). Twelve healthy crossbred sheep 7 months of age weighting approximately 30 kg that were clinically healthy, vaccinated against chlostridiosis and wormed were used. The sheep were purchased from a rural property located in the city of Baldim, Minas Gerais, Brazil. Animals were housed in collective stalls in the Veterinary School of UFMG (Belo Horizonte, Brazil), where they received a diet consisting of granulated commercial feed (300 g/animal/day), mineral salt for growing sheep, hay, and water ad libitum. Before the beginning of the immunization cycle, the animals spent 60 days adapting to the environment. The animals were divided into three groups of four animals, and each group was housed in a separate stall.
Mice used for the in vitro toxicity assays were acquired from the Biotherium Center (CEBIO) of the Federal University of Minas Gerais (UFMG), and maintained at the biotherium of the Veterinary College of UFMG. The animals were placed in plastic boxes and divided into groups of four animals each. All animals received water and food under controlled environmental conditions. The guidelines for ethical conduct in the care and use of nonhuman animals in research from the American Psychological Association were followed.
Ts scorpions were collected from Belo Horizonte, Minas Gerais, Brazil and maintained in the Immunochemistry Laboratory of the Institute of Biological Sciences-UFMG. The venom was obtained by electrostimulation of the telson at 20 V and was stored in its liquid form in the dark at −20℃.
Ts venom was detoxified by coupling with glutaraldehyde according to the method described by Machado-de-Ávila et al. [20], with minor modifications. Briefly, 10 mg of crude venom was diluted in 1 mL of phosphate buffered saline (PBS). Next, 1 mL of 1% glutaraldehyde solution was slowly added to the venom, after which the solution was constantly agitated for 1 h at room temperature. The glutaraldehyde-conjugated venom was then stored at −20℃ until use.
Toxicity assays
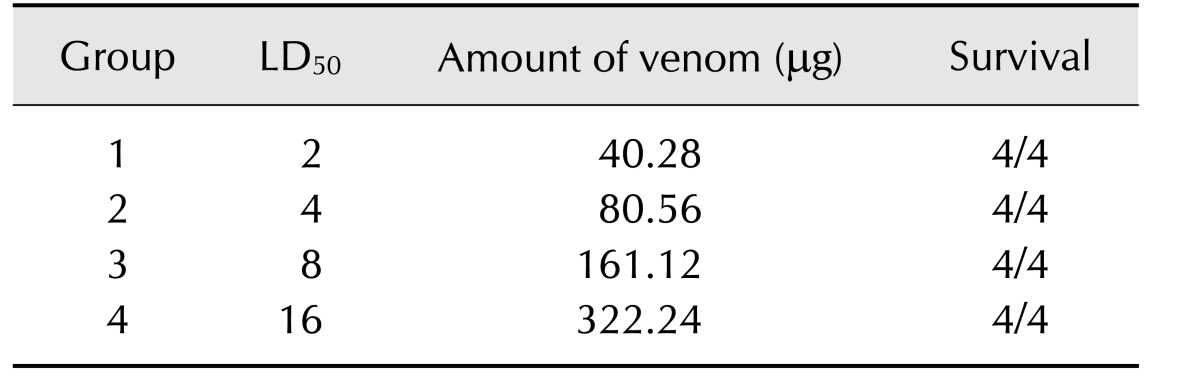
The lethal dose 50 (LD50) of Ts venom was determined by subcutaneously injecting crescent doses diluted in 0.2 mL of PBS-0.1% bovine serum albumin (BSA) into female Swiss mice weighing 18 to 22 g. Dead animals were counted 24 h after injection. To verify the effectiveness of glutaraldehyde conjugation as a venom detoxification procedure, quantities of glutaraldehyde-conjugated venom equivalent to 2, 4, 8, and 16-fold of the LD50 of the non-conjugated crude Ts venom were injected into mice following the same protocol described above.
Immunization protocols
The adopted immunization protocol was based on the first cycle of the immunization program used by Fundação Ezequiel Dias (FUNED), a government institution responsible for the production of scorpion anti-venom in the state of Minas Gerais, Brazil. The protocol of one cycle of immunization consisted of six doses. After the first cycle, the obtained immune sera had their neutralization properties evaluated and, if necessary, reimmunization cycles were performed until the desired neutralization potential was reached. The first dose of the immunization cycle was emulsified in Freund's complete adjuvant at a 1 : 1 ratio (1.5 mL of adjuvant plus 0.5 mg of Ts venom in 1.5 mL of PBS), and the next two doses were given in Freund's incomplete adjuvant after 21-day intervals. The last three doses were injected without any adjuvant (booster) after intervals of 3 days.
For the first three doses, animals in the control group (G1) received only PBS emulsified in Freund's adjuvant. The other groups received either 0.5 mg of crude Ts venom (G2) or 0.5 mg of detoxified Ts venom (G3) in a 1 : 1 ratio that was emulsified in complete or incomplete Freund's adjuvant. The following three booster injections had one-third of the initial dose diluted only in PBS. All doses had a volume of 3 mL. Doses were subcutaneously injected into the dorsal-lumbar region at two separate sites. The injection sites were disinfected with ethanol prior to each injection. Bleedings (50 mL from each animal) were performed 11 days after the last booster injection (67 days after the immunization started).
Clinical examination and electrocardiography
All animals received full clinical exams before the immunizations (time 0) and 20 min, 24 h and 48 h after the immunizations. Examinations evaluated behavior, cardiac frequency, respiratory frequency, arterial pulse, rectal temperature, ruminal movements frequency, mucosa (ocular and oral), capillary perfusion time and lymph node palpation (retropharyngeal, mandibular, parotidal, cervical, sub-iliac, and popliteal), in addition to analysis of the injection site and dehydration status.
Computer ECG tracings of 15 min long (Teb ECGPC, Brazil) were performed before T0 and after each immunogen administration. Heart rate, heart rhythm, and measurement of waves and intervals were evaluated. ECG was recorded in standard bipolar limb leads (I, II, III) and augmented unipolar leads (aVR, aVL and aVF) at a speed (50 mm/sec) and a sensitivity (1 cm = 1 mV). The electrodes were placed in forelimbs and hindlimbs [36]. Animals were at rest and placed in lateral recumbency.
Haematology and biochemical profile
Blood was withdrawn before immunizations (day 0), as well as immediately and 48 h after each dose of venom was administered on days 0, 22, 43, 50, 53, and 56, resulting in 19 blood collections/animal. The animals were bled by puncturing the jugular vein and the blood was collected into vacuum flasks containing 10% ethylenediaminetetraacetic acid with either sodium fluorite as an anti-coagulant or without any anticoagulant to obtain plasma (for glucose dosage) and serum, respectively. The vacuum flasks were then centrifuged at 800 × g for 10 min. Finally, individual samples were stored at −20℃ until use.
Complete blood counts were performed using an electronic counter (CELM CC 530 Vet; Companhia Equipadora de Laboratórios Modernos, Brazil) and blood smears on glass slides were stained with Panoptic for differential counting of leucocytes and platelets [34]. Packed cell volume (PCV) was determined by microhematocrit and total protein concentration was determined using a refractometer (Ningbo Utech International, China).
Plasma glucose and serum aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), urea, creatinine, and amylase levels were determined by kinetic colorimetric methods using commercial kits (Bioclin, Brazil) in a semiautomatic analyser (TP Analyzer Basic, Thermoplate, Brazil).
Enzyme-linked immunosorbent assay (ELISA)
Microplates of 96 wells (Becton, Dickinson and Company, USA) were pre-coated overnight at 4℃ with 100 mL of a 10 µg/mL Ts crude venom solution in carbonate buffer pH 9.6. The assay was performed as described by Chávez-Olórtegui et al. [4]. Briefly, after washing with saline solution containing 0.05% Tween, wells were blocked with 3% non-fat milk in PBS for 1 h at 37℃. Wells were washed once and different dilutions (1 : 100 to 1 : 50,000) of each sheep sera were added in PBS-0.1% non-fat milk and then incubated for 1 h at 37℃. After washing again, the secondary antibody (anti-sheep IgG conjugated to peroxidase, diluted 1 : 4,000) was added and the plate was incubated for 1 h at 37℃. The plate was then rinsed and 100 µL of a solution composed of 0.33 mg/mL of o-phenilenediamine in citrate buffer, pH 5.2, with 0.04% of H2O2, was added to the wells. The plate was then incubated for 20 min, after which 20 µL of a 1 : 20 solution of diluted H2SO4 was added to terminate the reaction. Finally, the absorbance values at 490 nm were determined using a 680 Microplate Reader (Bio-Rad Laboratories, USA). All experiments were performed in duplicate.
Western blotting procedure
The reactivity of individual serum samples was visualized by Western blotting. Briefly, Ts venom (20 µg) was subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) using an 18% acrylamide gel. The gel was then transferred to a nitrocellulose membrane [38] that was subsequently blocked for 1 h with PBS-Tween 0.35%. After three 5 min washes with PBS-Tween 0.05%, the membrane was incubated for 1 h and 30 min with a 1 : 1,000 dilution of sera from the immunized sheep. The membrane was washed three more times with PBS-Tween 0.05%, then incubated for one additional hour with a 1 : 2,000 dilution of anti-sheep immunoglobulin G (IgG) antibody conjugated to peroxidase. After washing, the reaction was detected using 3,3′-diaminobenzidine/chloronaphthol substrate according to the manufacturer's instructions. An anti-Ts venom sheep serum from our laboratory stock was used as the positive control.
In vivo neutralization assays
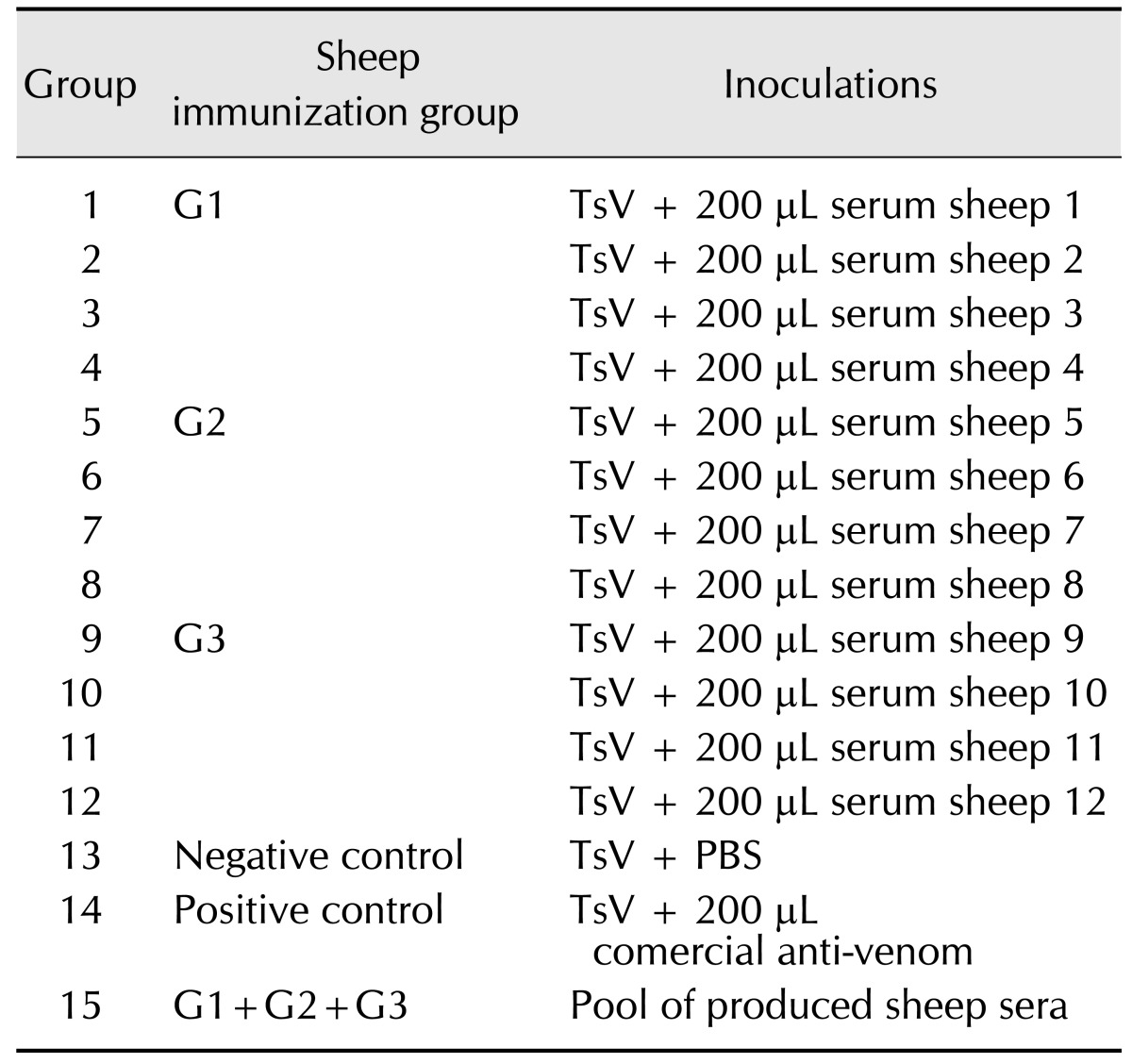
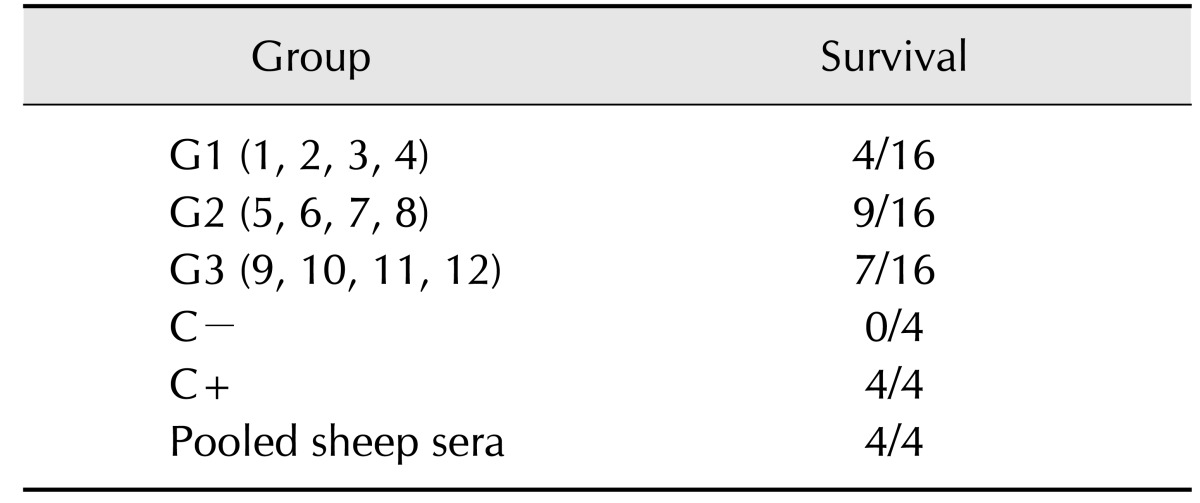
Swiss mice were challenged with a dose equivalent to twice the LD50 of Ts venom diluted in PBS-0.1% BSA. The venom was incubated for 1 h under constant agitation at 37℃ with 200 µL of sera from either the immunized animals or the commercial anti-venom from FUNED in a final volume of 250 µL. Two control groups were used, one that received only venom in PBS-0.1% BSA and another that received only a mixture of sheep sera (Table 1). Altogether, 15 groups were formed, with four animals randomly distributed to each group. Groups 1 to 4 received Ts venom pre-incubated with serum from animals 1 to 4, respectively, which were members of the control group. Groups 5 to 8 received Ts venom pre-incubated with serum from animals 5 to 8 respectively, which were members of group G2, immunized with crude Ts venom. Groups 9 to 12 received Ts venom pre-incubated with serum from animals 9 to 12 respectively, which are members of group G3, immunized with glutaraldehyde Ts venom. Group 13 received Ts venom pre-incubated with only PBS, representing a negative control. Group 14 received Ts venom pre-incubated with commercial anti-scorpionic anti-venom from FUNED, representing a positive control for neutralization. Group 15 did not receive any venom, but did receive a pool of all sheep sera to verify whether there was a toxic reaction or anaphylaxis in treated mice. The group distributions in this experiment are summarized in Table 1.
Table 1. Serum neutralization in mice challenged with Tityus serrulatus venom (TsV).
Groups 1 to 14 received amounts corresponding to 2 LD50 of TsV that had been preincubated for 1 h at 37℃ with individual sheep serum (groups 1 to 12), PBS (group 13, negative control) or commercial anti-venom (group 14, positive control). Group 15 received only 200 µL of pooled sheep sera to verify any adverse events.
Animals were observed for 4 h after injection to visualize the onset of typical symptoms of scorpion envenoming. In addition, the number of dead animals was counted 24 h after experimental envenoming. The primary clinical signs observed were behavioural changes, sweating, salivation, bleeding, respiratory distress, itching, hyper-responsiveness to external stimuli, tearing, and agitation.
Statistical analyses
The experimental design was completely randomized in subdivided plots (split-plot design) [31]. The plots correspond to groups (G1, control; G2, crude Ts venom; and G3, glutaraldehyde-detoxified Ts venom), the sub-plots to the immunization step (first to sixth dose), and sub-subplots to bleedings (t = 0, 24, and 48 h) within each immunization step.
For variables that were not normally distributed, the data were log transformed, and the means were compared by ANOVA followed by Tukey's test. The number of eosinophils was determined by differential leukocyte counts using ANOVA followed by the Kruskal-Wallis non-parametric test when comparing groups and Friedman analysis when comparing the collection times. The significance level was set at p < 0.05.
Results
LD50
The LD50 of the sample of Ts crude venom used throughout this work was determined to be 20.14 µg/20 g of mice. The venom pool used for LD50 determination was divided in two, one part that was used to immunize animals from G2 and another that was submitted to detoxification using the glutaraldehyde cross-link protocol and used to immunize G3.
Detoxication process
To verify the efficacy of the detoxification process, increasing amounts of glutaraldehyde-detoxified Ts venom were injected to mice. All animals subjected to this challenge survived and did not show any clinical signs of poisoning (Table 2).
Table 2. Toxicity test of Ts venom treated with glutaraldehyde.
Groups of four mice were injected with quantities of detoxified venom equivalent to 2, 4, 8 and 16 LD50 of Ts crude venom. Animals were observed for 4 h to verify toxicity symptoms, and deaths were counted after 24 h.
Blood profile alterations
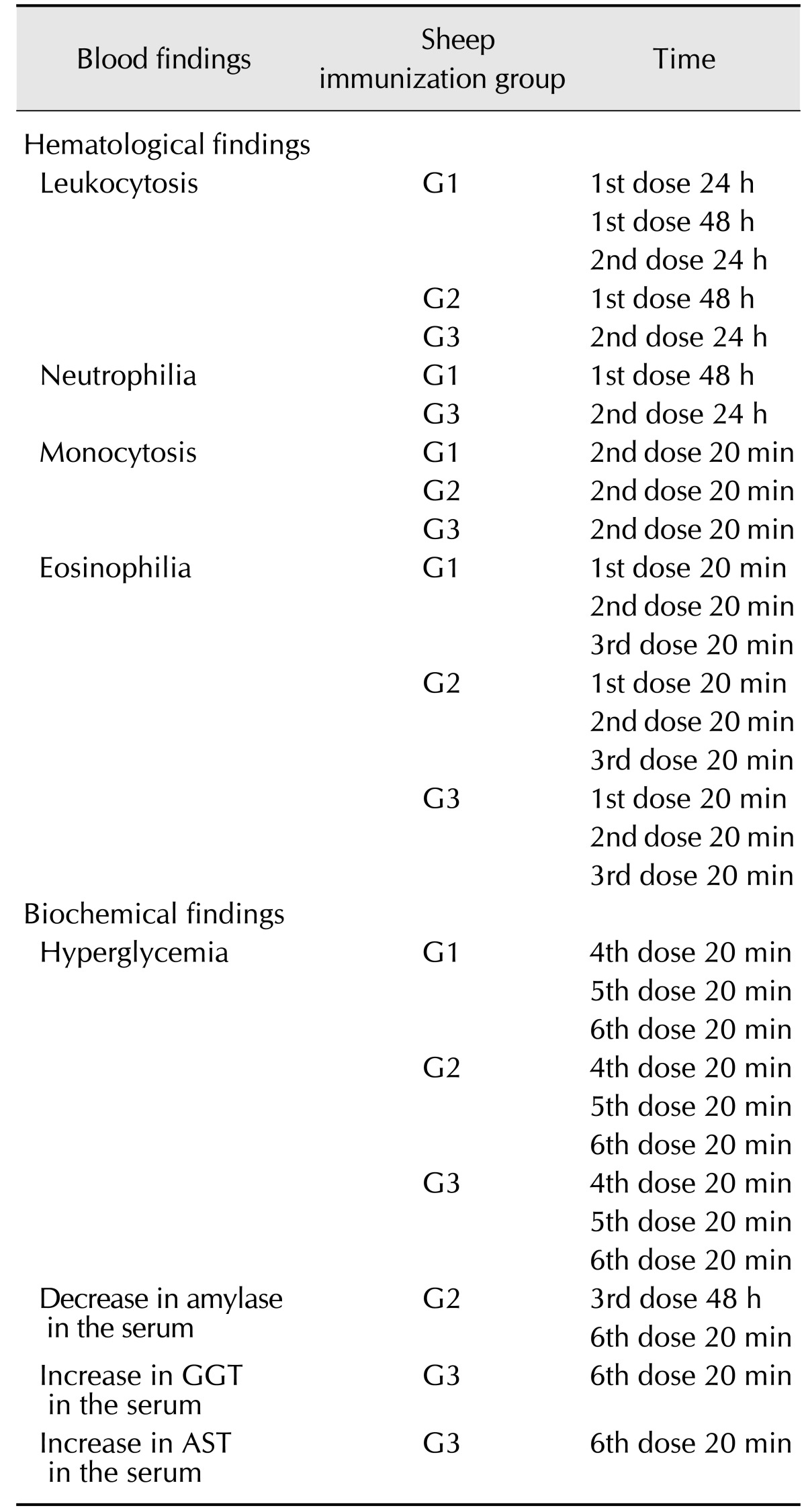
After confirming the venom detoxification, the immunization of three groups of four sheep proceeded according to the protocol described. G1 was injected only with PBS, G2 was immunized with crude Ts venom and G3 received the glutaraldehyde-detoxified Ts venom as an immunogen. In addition to clinical examination performed immediately, 24 h and 48 h after each dose, the haematological and biochemical profiles of sheep were evaluated during the immunization process. The main hematological and biochemical alterations are summarized in Table 3.
Table 3. Main alterations found in sheep during the immunization protocol.
GGT, gamma-glutamyltransferase; AST, aspartate aminotransferase.
The total erythrocyte and platelet counts, PCV, and haemoglobin concentrations (data not shown) did not show any significant variations among groups, and all results were within the respective reference values. The normal values of total leukocytes were established at time 0 for the investigated animals and were within the range of 7,000 to 16,000 cells/µL. Total white cell, neutrophil and eosinophil counts showed the same pattern for all three groups when Freund's adjuvant was used. Mild leukocytosis, neutrophilia, and eosinophilia were observed in all groups. During the booster injections, when no adjuvant was used, the values for these parameters decreased and stabilized. When the second dose was administered, there was a significant (p < 0.05) increase in the monocyte counts in all groups to values above the reference (0.5–0.73 × 103/µL). Moreover, from the third immunization onward there was a significant decrease in lymphocyte counts, although the values remained within physiological limits (1.56–8.88 × 103/µL).
The plasmatic concentration of total proteins (6.4–7.9 g/dL) also remained within physiological limits during the entire immunization protocol. G2 (immunized with crude venom) showed the highest values; however, the difference was not significant.
Serum glucose levels differed depending on the injected dose and whether adjuvant was included. In samples taken after the first three doses, the serum glucose levels were within the normal range (70–106 mg/dL) in all groups, but hyperglycemia (up to 147 mg/dL) was observed after the booster doses were administered.
The serum concentration of urea did not show a consistent pattern throughout the immunization process. Rather, urea concentrations oscillated, ranging from below to above the established (50–65 mg/dL) normal levels. Significant variations in creatinine levels, which ranged from 0.84 to 2.29 mg/dL, were not observed.
The serum activities of amylase in all animals from the present study ranged from 10.6 to 39.4 U/L. A decrease in the serum amylase values in the G2 group occurred on the third and sixth doses, with values below the reference limits for the species (12.1–37.1 U/L). However, this difference was not significant relative to other groups. The serum activities of GGT from all sheep ranged from 24.3 to 103.9 U/L. An increase in GGT levels occurred in G3 after administration of the last dose, with values above the physiological limit (24–92 U/L). However, significant differences compared to other groups were not observed. GGT increase was accompanied by an increase in AST levels as well, although they remained within normal levels (70–280 U/L).
Clinical alterations
Clinical evaluation of animals immunized with crude venom (G2) revealed mild changes in clinical signs, such as increased heart (> 70 to 90 bpm) and respiratory rates (> 12 to 20/min), mainly at the 5th immunization when animals showed tracheal fine crackles. The group receiving detoxified venom (G3) showed better clinical status at the end of the immunization cycle. Concerning the measurement of rectal temperature, groups that received venom behaved in the same way, regardless of whether it was crude or detoxified (G2 and G3). Moreover, there was an elevation of mean temperature values at some points of the immunization protocol (40.3℃ and 40.5℃). Inspection and palpation revealed an increase of superficial cervical lymph nodes in all groups after the first immunogen inoculation. There was no painful sensitivity to palpation or hard consistency of the lymph nodes observed, nor was there any adherence to other organs.
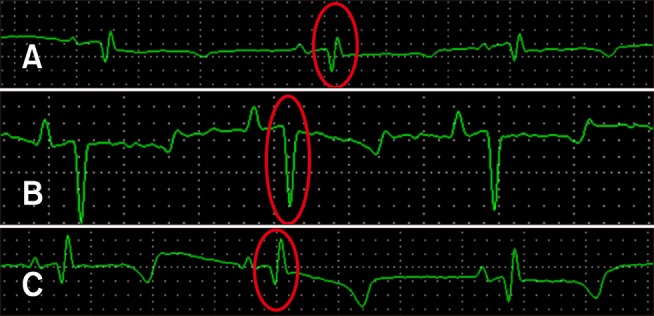
All electrocardiography (ECG) examinations revealed sinus rhythm. Neither arrhythmias nor conduction disturbances were detected. QRS morphology varied among the animals, even at T0, with QR, QS, and qR types detected (Fig. 1).
Fig. 1. Electrocardiogram of sheep inoculated with crude Ts venom. II derivation, 50 mm/sec, with 10 mm/mV sensibility. (A) QR complex (red circle). (B) QS complex (red circle). (C) qR complex (red circle).
Immunological evaluation
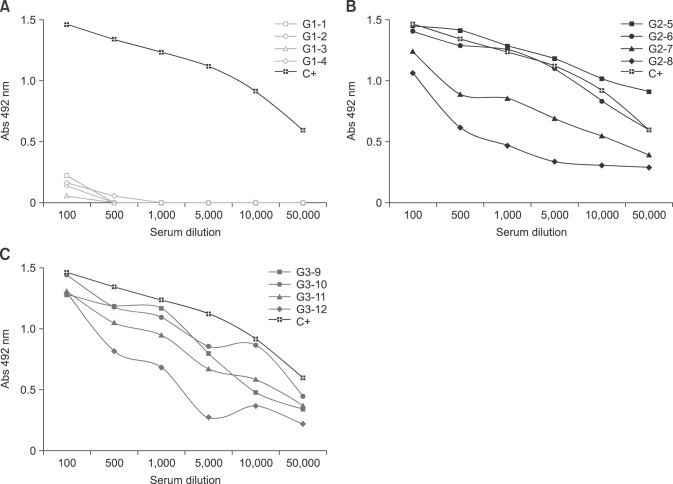
Antibodies produced by sheep immunization with either PBS (G1), crude Ts venom (G2) or glutaraldehyde detoxified Ts venom (G3) were characterized by ELISA (Figs. 2 and 3) and Western Blotting (Fig. 4). The results showed that the immunogenicity of Ts venom was preserved after glutaraldehyde detoxification. ELISA revealed that the antibody reactivity between the pool of sera from groups that received crude (G2) or detoxified (G3) Ts venom was similar (Fig. 2). Individual variations in antibody response between members of each group were also observed. In group G2, animals 5 and 6 produced antibodies with greater ELISA reactivity than animals 7 and 8 (panel B in Fig. 3). The same was also observed in group G3, in which animal 12 was found to have weaker serum reactivity upon ELISA (panel C in Fig. 3). Animals from the control group (PBS) did not produce significant antibody response against Ts venom (panel A in Fig. 3).
Fig. 2. Enzyme-linked immunosorbent assay (ELISA) characterization of the anti-Ts venom sheep antibodies, per group. Reactivity of sheep sera immunized with either PBS (G1, white bars), crude Ts venom (G2, black bars) or glutaraldehyde detoxified Ts venom (G3, grey bars). Serum from each animal was pooled by group and used at different dilutions (1/100; 1/500; 1/1,000; 1/5,000; 1/10,000 and 1/50,000). Plates were coated with 100 µL of Ts crude venom (10 µg/mL). Three independent experiments were conducted and plotted values are the means ± SEM of triplicates (error bars).
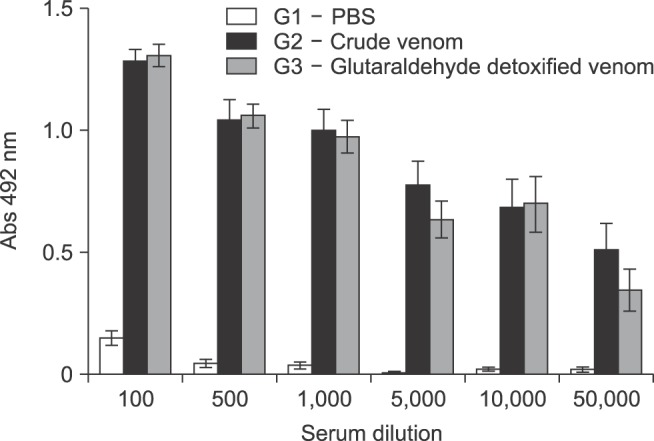
Fig. 3. ELISA characterization of the anti-Ts venom sheep antibodies, per animal. Reactivity of individual sheep serum. In G1, animals 1, 2, 3 and 4 were immunized with PBS (A). In G2, animals 5, 6, 7 and 8 were immunized with crude Ts venom (B). In G3, animals 9, 10, 11 and 12 were immunized with glutaraldehyde detoxified Ts venom (C). Serum from each animal was used at different dilutions (1/100; 1/500; 1/1,000; 1/5,000; 1/10,000 and 1/50,000). Plates were coated with 100 µL of Ts crude venom (10 µg/mL). Values are means of duplicates from two independent experiments. Anti-Ts sheep serum that was previously produced by the injection of eight doses of 500 µg of Ts venom and emulsified in Freund's adjuvant was used as a positive control (C+).
Fig. 4. Western blotting characterization of individual sheep sera. A total of 20 µg Ts venom were subjected to 18% sodium dodecyl sulphate polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. The membrane was then analysed against each individual serum at 1/1,000 dilution, and revealed with 3,3′-diaminobenzidine/chloronaphthol. P, molecular weight marker; Lanes 1–4, serum from animals immunized with PBS (G1); Lanes 5–8, serum from animals immunized with crude Ts venom (G2); Lanes 9–12, serum from animals immunized with glutaraldehyde-detoxified Ts venom (G3); Lane C+, anti-Ts sheep serum which was previously produced by the injection of eight doses of 500 µg of Ts venom emulsified in Freund's adjuvant.
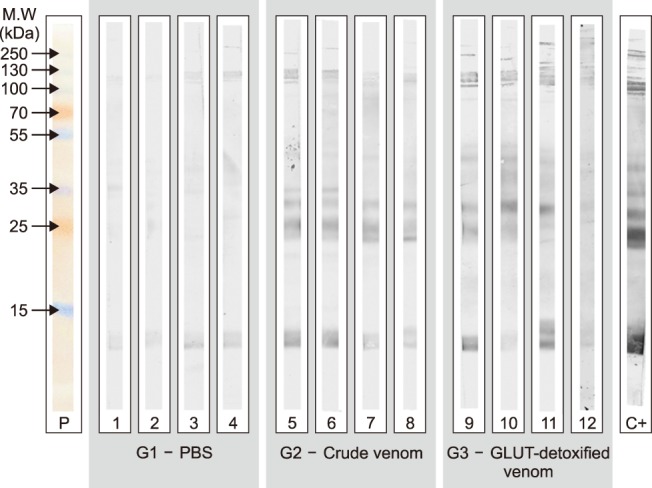
The produced sheep sera were also characterized by Western blotting (Fig. 4). Ts crude venom was run on 18% SDS-PAGE, blotted onto nitrocellulose membranes and probed against each individual serum. The obtained results were similar to those obtained by ELISA. Sera from G1 (PBS) did not present expressive reactions. Sera from G2 and G3 reacted with most of the components from Ts venom, but with different intensities.
Antibodies venom-neutralizing ability was determined by pre-incubating crude Ts venom with individual serum samples and then injecting it into mice. Group descriptions are summarized in Table 1. Using a dose equivalent to twice the LD50 of Ts venom, distinct protection was achieved in different groups. Mortality rates were 75.0% (12/16), 42.8% (7/16), and 56.3% (9/16) for G1, G2, and G3, respectively.
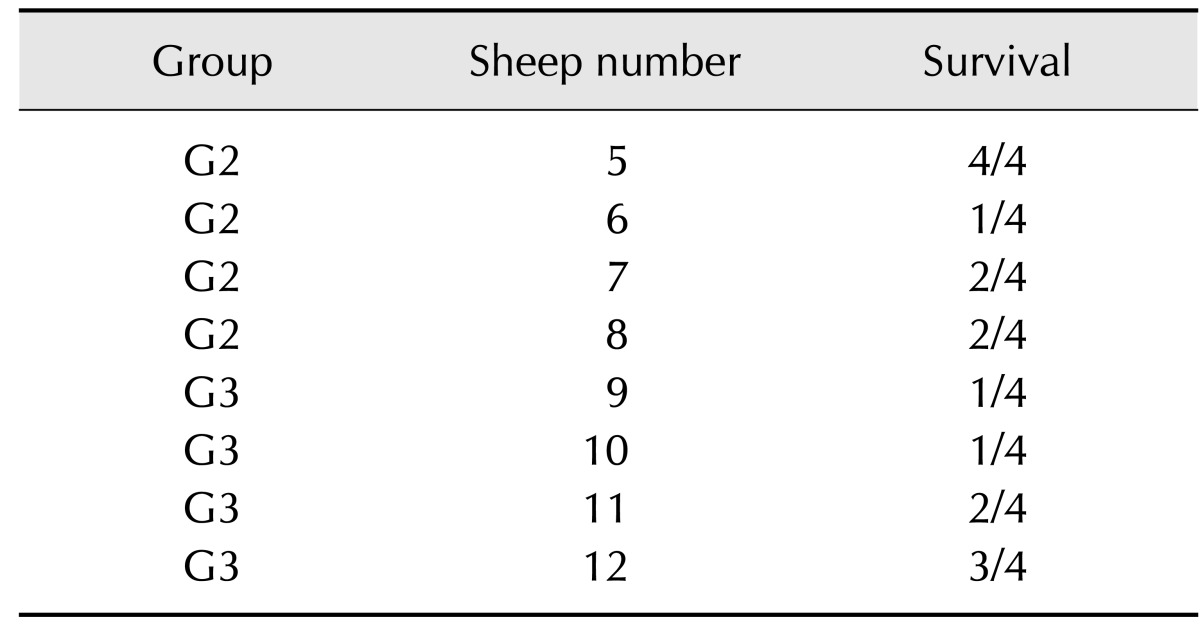
When individually analysed, serum from sheep number 5 from group G2 promoted 100% protection, whereas that from sheep number 6, also from this group, had only a 25% survival rate (Table 4). These results were in contrast to the reactivity observed by Western blotting, in which both sera presented a similar reactivity despite having diverse neutralization properties. Conversely, serum from sheep number 8 had the lowest reactivity in its group upon Western Blotting, but protected 50% of the injected mice.
Table 4. Survival rates for in vitro neutralization assay.
Measurement of survival rates after challenge with amounts corresponding to 2 LD50 of Ts crude venom pre-incubated with 200 µL of serum from each individual animal immunized with either PBS (G1, animals 1, 2, 3, and 4), crude Ts venom (G2, animals 5, 6, 7, and 8) or glutaraldehyde-detoxified Ts venom (G3, animals 9, 10, 11, and 12). As a negative control (C−), venom was preincubated with 200 µL of PBS, while as a positive control (C+), venom was preincubated with commercial horse anti-Ts. To detect possible adverse effects, one group was injected with only 200 µL of a pool of all sheep sera and no venom.
Analysis of survival rates of mice that received serum from sheep immunized with glutaraldehyde-detoxified venom (G3) showed that animal 12 was the best producer (75% survival) (Table 5). However, this animal showed the weakest reactivity upon Western Blotting and ELISA, indicating a poor correlation between in vitro antibody reactivity and the actual protection afforded by them.
Table 5. Survival following in vivo neutralization by individual serum.
Measurement of survival rates after challenge with amounts corresponding to 2 LD50 of Ts crude venom pre-incubated with 200 µL of serum from each individual animal. Sheep number 5, 6, 7 and 8 were immunized with Ts crude venom (G2) and sheep number 9, 10, 11 and 2 were immunized with glutaraldehyde-detoxified Ts venom (G3).
Discussion
The efficacy of the detoxification process was confirmed after injection of mice with glutaraldehyde-detoxified Ts venom. All challenged animals survived with no clinical signs of poisoning.
Our findings revealed no significant variations in the erythrograms among groups. These results contrast with those of earlier studies in which scorpion venom was found to be capable of triggering polycythaemia in dogs [29,30] and rabbits [7], as well as reducing platelet counts in rats [25]. Overall, these results indicate that sheep may be more resistant to scorpion venom toxicity than these other species, reinforcing their potential for use as anti-venom producing animals.
Lymphocyte counts presented a significant decrease from the third immunization. Lymphopenia is known to be an important aspect of sheep stress, which could explain this reduction [28]. Mild leukocytosis, neutrophilia, and eosinophilia were observed after the use of the Freund's adjuvant, indicating adjuvant-stimulated inflammatory response. Freund's adjuvant is commonly used for commercial anti-venom production in Brazil. When the second dose was injected, an increase in monocyte counts was observed in all groups. Monocytosis can indicate chronic inflammation or stress in small ruminants [28]. Many animals, regardless of treatment (G1, G2 or G3), presented abscesses at the inoculation point, indicating that this response is also typical of Freund's adjuvant use. Our results show that some alterations appear to be caused directly by the adjuvant. Accordingly, the use of alternative adjuvants that present less collateral effects must be pursued to develop a less aggressive protocol for anti-venom producer animals.
The plasmatic concentration of total proteins was higher in G2 (immunized with crude Ts venom), even though the difference was not statistically significant. Other studies analyzing envenoming by scorpions also showed higher plasmatic concentrations of total proteins [7,13,15,25]. A decrease in serum amylase values in G2 occurred following the third and sixth doses, but this decrease was not significant when compared to other groups. Other studies have reported serum amylase increases in scorpion-envenomed patients [7,23,25], suggesting that the decrease observed in our study may be specific to the ovine species.
Serum levels of glucose were within the normal range after the first three doses in all groups; however, hyperglycemia was observed after the booster doses were administered. Because there was no significant variation between groups, hyperglycaemia may be attributed to stress caused by daily manipulations during this period, as the secretion of cortisol induces gluconeogenesis [17]. Hyperglycaemia has been reported in cases of scorpion envenomation owing to venom-induced pancreatitis [6]. However, in our study, sheep treated with venom did not show increased serum amylase activities compared to other groups.
Moreover, urea serum concentration did not show a consistent pattern throughout the immunization process. When combined with creatinine levels, this variation is not clinically relevant, suggesting that venom does not alter renal function. These observations differ from the results obtained using envenomed rats [8,29] and humans [3], revealing once again that sheep are resistant to Ts venom toxicity.
Hepatic function was evaluated by measuring serum activities of GGT and AST, which were augmented in G3 after the 6th dose. Since no other signs or symptoms of hepatic lesions were observed, the increase in these enzymes may be due to the glutaraldehyde biotransformation process. The enzymatic systems of hepatic detoxification such as cytochrome P-450 are fast acting and have wide specificity, allowing the organism to be in contact with several xenobiotics without suffering intoxication [35].
According to Uemitsu et al. [39], the LD50 for subcutaneous injection of glutaraldehyde in rats is 2,390 mg/kg, while it is 1,430 mg/kg for glutaraldehyde administered subcutaneously to male mice. The amount of glutaraldehyde injected in each dose administered to sheep was 0.016 mg/kg, which is 100,000 times smaller than the established LD50 for this substance. As reported by Machado de Ávila et al. [20], dialysis of the glutaraldehyde-detoxified venom preparation could remove free glutaraldehyde molecules, minimizing possible toxic effects caused by this component. However, in order to be considered for adoption by anti-venom production centres, a venom detoxification protocol should be as simple, fast and cheap as possible. Since the increase in hepatic enzymes did not reflect any detectable physical damage to the animals, the use of small amounts of glutaraldehyde was shown to be safe for sheep, indicating that there is no need for any additional removal or inactivation steps.
Although differences in haematological and biochemical tests among groups were not observed, physical examination of animals immunized with crude venom (G2) revealed mild changes in clinical signs, such as increased heart and respiratory rates. Tracheal fine crackles were observed at the fifth immunization. The observed clinical changes are consistent with the effects of scorpion envenomation [14], and the group receiving the detoxified venom (G3) showed better clinical status at the end of the immunization cycle. Typical signs of scorpion envenoming such as piloerection, diarrhea, pain at the inoculation point, abdominal pain, dyspnea, pulmonary edema, and sialorrhea were not observed in the clinical exams of the immunized animals [6,21,30]. These results indicate that, even if the overall resistance to Ts venom toxicity is higher in sheep than in other animals, the use of glutaraldehyde-detoxified venom helps to further diminish its toxic effects in anti-venom producer animals.
The rectal temperature showed elevated values at some points of the immunization protocol (40.3℃ and 40.5℃). This increase could have been due to systemic inflammatory response caused by the release of cytokines such as IL-1 and IL-6 in response to the scorpion venom [24]. The ruminal motility was also similarly altered in both venom-immunized groups (G2 and G3), being reduced at some points (0.5 movements/min). These findings indicate that the immunization with venom caused some kind of discomfort [16].
No change in lymph nodes was found after inspection and palpation in any groups after the first immunogen inoculation. Freund's adjuvant can produce acute and chronic inflammatory reactions, sterile abscesses and/or ulcerative necrosis at the site of inoculation [34]. In this study, abscesses at the injection site were detected in some animals, including some of the control group, excluding the specific action of Ts venom. Following the first inoculation, the volume increased in the region, and possible abscessation was observed. However, these alterations were not observed in booster doses, indicating that they may be caused by the adjuvant.
ECG examinations revealed that QRS morphology varied among animals, which is in accordance with previous studies of sheep that showed the same variation in QRS morphology [18,32,37]. Antibodies produced by sheep immunization with either PBS, crude Ts venom or glutaraldehyde detoxified Ts venom were characterized by ELISA and Western Blotting. The results showed that, after glutaraldehyde detoxification, the immunogenicity of the venom was preserved. In general, the antibody reactivity between groups that received crude (G2) or detoxified (G3) venom were similar upon ELISA analysis. Sera from G2 and G3 reacted with most components from Ts venom, but at different intensities. Despite the immunological characterization of the produced sheep sera, the results obtained from the in vivo neutralization assay were fundamental to the evaluation of antibody efficacy for neutralizing the toxic effects of Ts venom.
The serum from sheep immunized with glutaraldehyde-detoxified venom that presented the best neutralizing potential showed a 75% survival rate. However, this animal presented the weakest reactivity upon Western Blotting and ELISA, indicating a poor correlation between in vitro reactivity of the antibodies and the actual protection afforded by them.
This lack of correlation between antibody titres detected by ELISA and the actual neutralization properties of anti-venoms has been observed elsewhere [22]. As a result of this characteristic, commercial anti-venom production primarily relies on in vivo assays of neutralization of venom lethality in mice. This detachment between the observed antibody reactivity and their neutralizing potential can occur because the immunogen used for anti-venom production, the crude venom, is a complex mixture of molecules with diverse biological roles that do not contribute equally to venom toxicity. Accordingly, one particular venom component can be highly immunogenic, eliciting high titers of antibodies that can be detected by ELISA and Western Blotting assays, but may not participate in establishment of the deleterious effects of venom. Conversely, highly toxic venom components can be inefficient at eliciting specific antibodies, contributing poorly to the polyclonal response, and therefore may not be neutralized by an anti-crude venom serum. Therefore, immunoassays should be carefully used in anti-venom characterization as a sign of antibody production, but not a guarantee of venom neutralization.
Reducing the use of experimental animals and their suffering is the principle of the 3R proposal (replacement, reduction and refinement) [10]. In the present study, the proposed immunization protocol seemed to cause less damage to animals, which were healthier than expected at the end of experimentation, indicating that refinement was achieved. This improved fitness may result in a higher life expectancy of producer animals and, consequently, in reduction of the number of animals used to this end. Unfortunately, the full replacement of animals for anti-venom production is not yet possible; accordingly, their welfare must be an important concern for anti-venom production-centers. The protocol proposed here can be seen as an initial step towards this goal.
In conclusion, sheep immunized with glutaraldehyde-detoxified Ts venom showed less adverse health effects than those induced by the crude venom. In addition, the glutaraldehyde detoxification protocol used here preserved venom immunogenicity and was able to induce neutralizing antibodies. This proposed protocol of using detoxified venom as the immunogen and sheep as the producer animal is a good alternative for anti-venom production because animal health is preserved to a greater degree and improved final product quality can be achieved. It is important to emphasize that only one cycle of immunization was performed in this study to ensure that the proposed protocol was safe to sheep. For commercial anti-venom production, several immunization cycles are used; therefore, further data obtained using prolonged protocols are needed to validate this protocol as an alternative to the one currently used for commercial anti-venom preparation. Nevertheless, the initial information presented herein indicates a promising path to be followed.
Acknowledgments
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil-CAPES (Toxinologia No. 23038000825/2011-63), Fundação de Amparo a Pesquisa do Estado de Minas Gerais, Brazil (FAPEMIG), Pró-Reitoria de Pesquisa da UFMG, funded by the INCTTOX Program of Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq).
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Avrameas S, Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorvents. Immunochemistry. 1969;6:53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- 2.Becerril B, Marangoni S, Possani LD. Toxins and genes isolated from scorpions of the genus Tityus. Toxicon. 1997;35:821–835. doi: 10.1016/s0041-0101(96)00198-5. [DOI] [PubMed] [Google Scholar]
- 3.Bouaziz M, Bahloul M, Kallel H, Samet M, Ksibi H, Dammak H, Ahmed MNB, Chtara K, Chelly H, Hamida CB, Rekik N. Epidemiological, clinical characteristics and outcome of severe scorpion envenomation in South Tunisia: multivariate analysis of 951 cases. Toxicon. 2008;52:918–926. doi: 10.1016/j.toxicon.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Chávez-Olórtegui C, Kalapothakis E, Ferreira AMBM, Ferreira AP, Diniz CR. Neutralizing capacity of antibodies elicited by a non-toxic protein purified from the venom of the scorpion Tityus serrulatus. Toxicon. 1997;35:213–221. doi: 10.1016/s0041-0101(96)00133-x. [DOI] [PubMed] [Google Scholar]
- 5.Chippaux JP, Goyffon M. Venoms, antivenoms, and immunotherapy. Toxicon. 1998;36:823–846. doi: 10.1016/s0041-0101(97)00160-8. [DOI] [PubMed] [Google Scholar]
- 6.Cupo P, Hering SE. Cardiac troponin I release after severe scorpion envenoming by Tityus serrulatus. Toxicon. 2002;40:823–830. doi: 10.1016/s0041-0101(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 7.Cusinato DAC, Souza AM, Vasconcelos F, Guimarães LFL, Leite FP, Gregório ZMO, Giglio JR, Arantes EC. Assessment of biochemical and hematological parameters in rats injected with Tityus serrulatus scorpion venom. Toxicon. 2010;56:1477–1486. doi: 10.1016/j.toxicon.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.de Sousa Alves R, do Nascimento NRF, Barbosa PSF, Kerntopf MR, Lessa LMA, de Sousa CM, Martins RD, Sousa DF, de Queiroz MGR, Toyama MH, Fonteles MC, Martins AMC, Monteiro HSA. Renal effects and vascular reactivity induced by Tityus serrulatus venom. Toxicon. 2005;46:271–276. doi: 10.1016/j.toxicon.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Fan HW, França FOS. Serotherapy. In: Schvartsman S, editor. Poisonous Plants and Venomous Animals. São Paulo: Sarvier; 1992. pp. 176–181. [Google Scholar]
- 10.Flecknell P. Replacement, reduction and refinement. ALTEX. 2002;19:73–78. [PubMed] [Google Scholar]
- 11.Gazarian KG, Gazarian T, Hernández R, Possani LD. Immunology of scorpion toxins and perspectives for generation of anti-venom vaccines. Vaccine. 2005;23:3357–3368. doi: 10.1016/j.vaccine.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Guidolin R, Da Silva WD, Higashi HG, Caricati CP, Lima MLSR, Morais JF, Pinto JR, Marcelino JR. Hyperimmunization of horses producing anti-venom serum with botrypic and crotalic venoms. Mem Inst Butantan. 1989;51:85–90. [Google Scholar]
- 13.Guimarães PT, Pinto MCL, Labarrère CR, Verçosa D, Jr, Melo MM. Biochemical and anatomo-histopathogical alterations caused by Tityus fasciolatus scorpion venom in mice. Rev Bras Cien Vet. 2009;16:51–57. [Google Scholar]
- 14.Hering SE, Azevedo-Marques MM, Cupo P. Scorpionism. In: Schvartsman S, editor. Poisonous Plants and Venomous Animals. São Paulo: Sarvier; 1992. pp. 216–227. [Google Scholar]
- 15.Ismail M, Abd-Elsalam MA. Are the toxicological effects of scorpion envenomation related to tissue venom concentration? Toxicon. 1988;26:233–256. doi: 10.1016/0041-0101(88)90215-2. [DOI] [PubMed] [Google Scholar]
- 16.Jackson PGG, Cockcroft PD. Clinical Examination of Farm Animals. Ames: Blackwell Publishing Company; 2002. [Google Scholar]
- 17.Kaneko JJ, Harvey JW, Bruss ML. Clinical Biochemistry of Domestic Animals. San Diego: Academic Press; 2008. [Google Scholar]
- 18.Lago EP, Melo MM, Araújo RB, Nascimento EF, Silva EF, Melo MB. Electrocardiographical and echocardiographical profiles in experimental Mascagnia rigida Griseb. (Malpighiaceae) toxicosis in sheep. Arq Bras Med Vet Zootec. 2009;61:853–862. [Google Scholar]
- 19.Landon J, Smith DS. Merits of sheep antisera for antivenom manufacture. J Toxicol Toxin Rev. 2003;22:15–22. [Google Scholar]
- 20.Machado de Avila RA, Alvarenga LM, Tavares CAP, Molina F, Granier C, Chávez-Olórtegui C. Molecular characterization of protective antibodies raised in mice by Tityus serrulatus scorpion venom toxins conjugated to bovine serum albumin. Toxicon. 2004;44:233–241. doi: 10.1016/j.toxicon.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Marcussi S, Arantes EC, Soares AM. Scorpions: Biology, Poisoning and Action Mechanism of Their Toxins. São Paulo: FUNPEC Editora; 2011. p. 140. [Google Scholar]
- 22.Maria WS, Velarde DT, Alvarenga LM, Nguyen C, Villard S, Granier C, Chávez-Olórtegui C. Localization of epitopes in the toxins of Tityus serrulatus scorpions and neutralizing potential of therapeutic antivenoms. Toxicon. 2005;46:210–217. doi: 10.1016/j.toxicon.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Novaes G, Queiroz AC, Queiroz AP, Santos RR, Flores DG, Henkes G, Fernandes BJD. Histopathologic study in rats' pancrease after injection of venom from the scorpion Tityus serrulatus. Rev Cien Med Biol. 2002;1:1–6. [Google Scholar]
- 24.Pessini AC, Santos DR, Arantes EC, Souza GEP. Mediators involved in the febrile response induced by Tityus serrulatus scorpion venom in rats. Toxicon. 2006;48:556–566. doi: 10.1016/j.toxicon.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Pinto MCL, Melo MM, Costa MER, Labarrére CR. Hematological and biochemical profiles of rats submitted to experimental poisoning with Tityus serrulatus venom. Arq Bras Med Vet Zootec. 2010;62:350–356. [Google Scholar]
- 26.Possani LD. Structure of scorpion toxins. In: Tu AT, editor. Handbook of Natural Toxins: Insects Poisons, Allergens and Other Invertebrate Venoms. New York: Dekker; 1984. pp. 514–545. [Google Scholar]
- 27.Possani LD, Alagón AC, Fletcher PL, Jr, Erickson BW. Purification and properties of mammalian toxins from the venom of the Brazilian scorpion Tityus serrulatus Lutz and Mello. Arch Biochem Biophys. 1977;180:394–403. doi: 10.1016/0003-9861(77)90053-4. [DOI] [PubMed] [Google Scholar]
- 28.Pugh DG. Sheep and Goat Medicine. Philadelphia: Saunders; 2002. p. 468. [Google Scholar]
- 29.Ribeiro EL, Melo MM, Pinto MCL, Labarrère CR, Guimarães PTC, Paes PRO, Leme FOP. Canine blood profile after experimental envenomation by Tityus serrulatus. Arq Bras Med Vet Zootec. 2009;61:135–143. [Google Scholar]
- 30.Ribeiro EL, Melo MM, Silva EF, Melo MB, Labarrère CR, Merlo FA, Veado JCC. Cardiovascular and clinical evaluation of dogs after Tityus serrulatus venom experimentally inoculation. MedveP: Rev Cien Med Vet Pequeno Anim Anim Estim. 2010;28:21–28. [Google Scholar]
- 31.Sampaio IBM. Estatística Aplicada à Experimentação Animal. 3rd ed. Belo Horizonte: FEPMVZ-UFMG; 2007. p. 264. [Google Scholar]
- 32.Schultz RA, Pretorius PJ, Terblanche M. An electrocardiographic study of normal sheep using a modified technique. Onderstepoort J Vet Res. 1972;39:97–106. [PubMed] [Google Scholar]
- 33.Sjostrom L, al-Abdulla IH, Rawat S, Smith DC, Landon J. A comparison of ovine and equine antivenoms. Toxicon. 1994;32:427–433. doi: 10.1016/0041-0101(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 34.Stills HF., Jr Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J. 2005;46:280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 35.Thrall MA. Veterinary Hematology and Clinical Chemistry. Philadelphia: Lippincott Williams & Wilkins; 2003. p. 518. [Google Scholar]
- 36.Tilley LP. Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment. Philadelphia: Lea & Febiger; 1992. p. 470. [Google Scholar]
- 37.Torío R, Cano M, Montes A, Prieto F, Benedito JL. Comparison of two methods for electrocardiographic analysis in Gallega sheep. Small Rumin Res. 1997;24:239–246. [Google Scholar]
- 38.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uemitsu N, Kawasaki H, Furuhashi T, Miyoshi K, Ohtaka T, Nomura A, Hasegawa T, Shimizu Y, Nakazawa M. Acute and subacute toxicity studies and local irritation study of glutaraldehyde. Oyo Yakuri. 1976;12:11–32. [Google Scholar]