Abstract
Abstract: Objective
Osteoimmunology investigates interactions between skeleton and immune system. In the light of recent discoveries in this field, a new reading register of osteoporosis is actually emerging, in which bone and immune cells are strictly interconnected. Osteoporosis could therefore be considered a chronic immune mediated disease which shares with other age related disorders a common inflammatory background. Here, we highlight these recent discoveries and the new landscape that is emerging.
Method
Extensive literature search in PubMed central.
Results
While the inflammatory nature of osteoporosis has been clearly recognized, other interesting aspects of osteoimmunology are currently emerging. In addition, mounting evidence indicates that the immunoskeletal interface is involved in the regulation of important body functions beyond bone remodeling. Bone cells take part with cells of the immune system in various immunological functions, configuring a real expanded immune system, and are therefore variously involved not only as target but also as main actors in various pathological conditions affecting primarily the immune system, such as autoimmunity and immune deficiencies, as well as in aging, menopause and other diseases sharing an inflammatory background.
Conclusion
The review highlights the complexity of interwoven pathways and shared mechanisms of the crosstalk between the immune and bone systems. More interestingly, the interdisciplinary field of osteoimmunology is now expanding beyond bone and immune cells, defining new homeostatic networks in which other organs and systems are functionally interconnected. Therefore, the correct skeletal integrity maintenance may be also relevant to other functions outside its involvement in bone mineral homeostasis, hemopoiesis and immunity.
Keywords: Osteoimmunology, Osteoporosis, Inflammation, Lymphocytes, Bone, Immune system
Introduction
Recently, extensive reciprocal interactions between the immune and skeletal systems have been demonstrated, resulting in the establishment of a new interdisciplinary research field, named “osteoimmunology”, which is focused on the understanding of the crosstalk between the immune and bone systems [1-3].
In particular, osteoimmunology investigates how the immune system impacts on bone turnover in physiological and pathological conditions through the immunoskeletal interface [1]. Researchers recently hav-Egained better understanding of the dialogue between immune and bone cells and a new reading register of bone remodeling is emerging, in which the various phases of bone formation and resorption, that coexist in a dynamic equilibrium, are under strict immunological control [2]. Bone and immune system are functionally integrated through complex homeostatic networks and, in all respects, osteoporosis could be considered a chronic immune mediated inflammatory disease which shares clinical and biological features with many other inflammatory conditions [3], as well as of other immune mediated diseases. In this context immune and bone systems appear to be integrated and sharing signaling pathways and regulatory mechanisms whose understanding provides a framework for obtaining new insights for the discovery of novel treatments for diseases related to both systems.
Thus osteoimmunology appears definitely as an interdisciplinary research and clinical field which also allowed new pathogenetic and clinical interpretations of well-known and common diseases, such as osteoporosis [4]. Its fields of interest are constantly expanding, thus enriching with an increasing number of translational implications, even in clinical practice and in various branches of medicine. Here, the most important concepts in osteoimmunology are addressed in the context of physiopatological states bridging these two organ systems, including osteoporosis, ageing, menopause, inflammatory arthritis, cancer, dysmetabolism and neurological disorders. Purpose of this review is to outline a new panorama of osteoimmunology that is not limited to immune mediated bone turnover but also consider, in the light of the latest findings in this field, interesting connections with other systems and regulatory functions over bone remodeling.
Cellular and molecular mechanisms of bone remodeling
The Basic Multicellular Unit
Bone is a dynamic tissue formed by a protein and mineral salt matrix in which are embedded the bone cells, osteocytes (OCy), osteoblasts (OB) and osteoclasts (OC). In addition, many other types of cells take part in bone composition, including cartilage, stromal, hematopoietic and mesenchimal stem cells, all linked by a dense network of signals. Antagonistic signaling between skeletal stem cell-derived subsets is a key mechanism in skeletal subset lineage commitment [5]. Bone tissue undergoes continuous adaptation during lifetime to preserve the structure of the skeleton and to control the mineral homeostasis. Bone turnover requires two coordinated processes: bone formation, driven by OB and bone resorption, mediated by OC [6].
OCy, through a complex network of tiny channels, transmit mechanical and microtraumatic signals leading to the activation of repair processes. They exert a fundamental role in the control of OB and OC functions. Moreover, OCy synthesize the bone matrix proteins which, along with the mineral component, determine the quality of the bone [1].
OB are the precursors of Ocy, i.e, the structural cells in the bone, and interact with OC to drive their differentiation and function. The mesenchymal stem cell (MSC) from which the OB originate, can also give rise to chondrocytes, marrow stromal cells, and adipocytes. There are multiple subpopulations of perisinusoidal mesenchymal stem/progenitor cells (MSPCs), that have specific relationships with the different kinds of niche, i.e. the surrounding microenvironment in which the self-renewal and multilineage stem cells proliferate and differentiate [7-9]. The stem cells that maintain and repair the postnatal skeleton is an osteochondroreticular (OCR) stem cell that generate OB, chondrocytes, and reticular marrow stromal cells, but not adipocytes. They are characterized by the expression of the bone morphogenetic protein (BMP) antagonist gremlin 1 (Grem 1). The perisinusoidal MSC population also contains Nes-GFP, leptin receptor (Lepr)-cre and CD146 expressing cells with osteogenic and adipogenic potential [10].
The osteoblast precursor cells (OBP) after increasing the osteopontin receptor (CD44) and the receptor for stromal cell-derived factor 1 - SDF1 (CXCR4) expression, migrate and become mature OB, attracted by vascular endothelial cells expressing SDF1 along chemotactic gradients into regions of bone formation [11].
OC are multinucleated myeloid cells, specialized to remove mineralized bone matrix through the production of lysosomal enzymes, such as tartrate-resistant acid phosphatase (TRAP) and catepsin k, against which a selective inhibitor (odanacatib) has been recently synthesized to be employed in osteoporotic patients [12]. They derive from a bone marrow precursor which gives rise also to professional antigen presenting cells (APC), i.e. dendritic cells and macrophages. OC may be therefore considered specialized immune cells.
OB, OCy and OC continuously communicate with each other to optimize the quality of the bone. For example, OB provide essential signals for the differentiation of the myeloid lineage precursors of OC by producing macrophage colony-stimulating factor (M-CSF), receptor activator of nuclear factor-kB (NF-kB) ligand (RANKL) and other co-stimulatory factors [13].
The Receptor Network
The binding of RANK receptor on OC and their precursors by its ligand RANKL, expressed by OB and stromal cells, is the main activation signal for bone resorption. The OB derived M-CSF links to its receptor c-fms on the surface of osteoclast cell precursors (OCP), enabling the RANK/RANKL signal. Osteoprotegerin (OPG) inhibits osteoclastogenesis by acting as a decoy receptor of RANKL, thus preventing bone resorption [14].
RANK receptor on OC, through the adapter protein tumor-necrosis-factor-receptor-associated factor 6 (TR-AF6), bound to its cytoplasmic tail, activates NF-kB and other transcription factors, such as MAPKs, c-fos, activator protein 1 (AP1), up to nuclear factor of activated T cells (NFATc1), the hub of various signaling pathways. Simultaneously, the activation of RANK induces the phosphorylation of Ig-like receptor associated adaptor proteins, such as the immunoreceptor tyrosine-based activation motif (ITAM) and Fc-receptor common gamma (FcRγ) subunit. In the nucleus NFATc1, together with other transcription factors, such as AP1, PU.1, microphthalmia-associated transcription factor (MITF) and cyclic AMP responsive-element-binding protein (CREB), induces OC specific genes, including those codifying for calcitonin receptor, cathepsin k and TRAP, leading to OC differentiation and proliferation [15,16].
Many other receptor pathways interact with RANK, some costimulators and amplificators, others inhibitors and modulators, and many of these are shared by immune cells. An inhibitor receptor system for RANK signal is ephrin (Eph) B2/B4. EphB2 receptor on OC, stimulated by EphB4 ligand on OB, inhibits the OC differentiation blocking c-fos and the NFATc1 transcriptional cascade. A peculiar property of this membrane receptor complex is its capacity to control bone turnover through bidirectional signals: the cell expressing the receptor and the one that expresses the ligand influence each other at the same time. Therefore, EphB4 activation on OB, through the induction of osteogenetic regulatory genes, contemporaneously favours the coupling of bone formation and resorption [17]. The canonical Wingless (Wnt)/β catenin pathway, involved mainly in the response to mechanical load, promotes differentiation, proliferation and mineralization activity of OB and also inhibits their apoptosis. It encompasses a family of proteins that bind to complex transmembrane receptors, formed by the association of Frizzled (Fz) proteins and low density lipoprotein related receptors (LRP-5, LRP-6) which stabilize the β-catenin substrate that concentrates in the cytosol and migrates into the OBP nucleus to regulate the transcription of target genes to induce OB differentiation and bone formation.
The entire osteoblastogenesis is affected by the canonical Wnt pathway. Wnt signaling inhibits MSC commitment towards adipogenic and chondrogenic lineages, stimulating the differentiation towards osteoblastogenesis. Wnt-β-catenin signaling also indirectly inhibits osteoclastogenesis and bone resorption by increasing OPG secretion in OB and OCy [18,19].
The BMP pathway acts as a Wnt associative stimulator signal in skeletogenesis by expanding primitive mesenchymal cells and thus laying the foundation for subsequent endochondral ossification [10]. At the same time, various natural inhibitors, produced both by OB and OCy, exert a negative feed-back control on Wnt system, such as the Wnt/β-catenin signaling pathway inhibitor Dickkopf Homolog-1 (DKK-1), the secreted frizzled related protein (sFRP) and the sclerostin, synthesized by the SOST gene, which binds to LRP5/6 receptors [19]. The inflammatory cytokine tumor necrosis factor-α (TNF-α) induces OB apoptosis and reduces osteoblastogenesis by stimulating DKK-1 and Sost expression. Cortison augments DKK-1 and sFRP expression thus suppressing the Wnt/β-catenin signal on OB inducing osteoporosis. An anti-sclerostin MoAb has recently been produced as a new osteoanabolic drug useful in the therapy of osteoporosis [20]. Other potential osteoanabolic drugs acting on the Wnt pathway are also DKK inhibitor molecules, now in the experimental stage [19].
Crosstalk between the immune and bone system: the immunoskeletal interface
Shared Signaling Pathways
Both clinical observations and basic research demonstrated that mediators driving inflammatory processes are also closely involved in bone remodeling. Inflammation and bone turnover share the same mediators, such as cytokines and transcription factors. Various molecules mediating communication between bone cells have been identified and several immunological mediators are involved in this crosstalk.
T lymphocytes resident in the bone marrow are the key immune cells that regulate bone remodeling and responsiveness of bone cells to parathyroid hormone (PTH), in physiological and pathological conditions. During inflammatory diseases or in conditions characterized by low-grade systemic inflammation, such as menopause and aging, OC bone resorption is driven by inflammatory cytokines produced by activated T lymphocytes. However, bone marrow T cells also support bone homeostasis by inducing bone formation via direct interactions with bone cells. Two mechanisms are involved: the binding of T cell costimulatory molecules to their counter receptors on bone cells and their precursors, and the release of cytokines and Wnt ligands that activate Wnt signaling in osteoblastic cell lineage. The final effect of T lymphocytes on bone depends on their activation state and their specific phenotype. The prevailing bone marrow T cells are activated central memory CD8+ lymphocytes, secreting relatively high levels of effector cytokines, mainy TNF-α. These cells are abundant in postmenopausal women with osteoporotic fractures. T helper (Th17) cells are capable of stimulating bone resorption and play a pivotal role in the bone loss of inflammatory conditions such as psoriasis, rheumatoid arthritis, periodontal disease, and inflammatory bowel disease. T regulatory (Treg) cells inhibit osteoclastogenesis and support bone formation. Th17 cells induce osteoclastogenesis by secreting interleukin (IL)-17, RANKL, TNF-α, IL-1, and IL-6, along with low levels of IFN-γ. Moreover, IL-17 stimulates the release of RANKL by OB and OCy and upregulate RANK expression on OC. Treg exert anti-osteo-clastogenic activity by producing suppressor cytokines, including IL-4, IL-10, and TGF-β [21].
Immune activation may directly induce osteoclastogenesis and bone resorption through RANKL. For example it is interesting to note how even the cells of the immune sytem, mainly activated T and B lymphocytes and dendritic cells, express RANKL. Moreover, RAN-KL, the principal osteoclastogenic mediator, stimulates bone resorption through the NFATc1, which is also a crucial factor in the immune system regulation [13].
RANKL, initially regarded as activator of APC by T lymphocytes, also plays an important role in the generation of Treg, which suppress the development of CD8+ lymphocytes into cytotoxic cells.
The expression of RANKL on T lymphocytes is also central for the differentiation of medullary epithelial cells which are responsible for self-reactive T lymphocyte negative selection in the thymus. Thus, depending on the context in which it acts, RANKL can stimulate or suppress immune reactions [3]. Other examples of shared receptor signals are the immunoglobulin (Ig)-like receptors which amplify the NFATc1 signal. The Toll like receptors (TLR), stimulated by pathogen associated molecular patterns (PAMP), utilize TRAF6 in their cascade signaling [22]. TLR are able to activate both the synthesis and release of proinflammatory and osteoclastogenic cytokines from immune cells, leading to bone resorption stimulation. Their involvement in the bone remodeling process provides a further key in the comprehension of the osteoporosis of infectious diseases.
The osteoclast associated receptor (OSCAR), which belongs to the Ig-like receptor family, mediates interactions between OB and OC [23] and is also involved in the regulation of both the adaptive and innate immunity. It associates with the adaptor molecule FcRγ subunit, which harbors an immunoreceptor tyrosine-based activation motif (ITAM) critical for calcium signaling activation in the immune system [24].
Also, the ITAM-harboring adaptor DAP12 plays a role in OC differentiation and function [35]. Therefore, receptors such as DAP12 and Ig-like receptors associated with FcRγ, initially characterized in myeloid cells and in natural killer lymphocytes, are also involved in RANK induced osteoclastogenesis [25].
TNF induces the expression of OSCAR and other receptors important for OC differentiation on the surface of monocytoid peripheral blood cells [26]. Other membrane receptors of the monocyte lineage downregulate OC differentiation. For example, CD80/CD86 blocks OC generation by binding to CTLA4, an inhibitory molecule of the monocyte induced T lymphocyte costimulation, which is highly expressed on Treg surface [27].
Cathepsin K is expressed in OC and plays a central role in the degradation of bone matrix components, such as type I collagen. In addition to osteoclastic bone resorption, cathepsin k is also implicated in dendritic cell activation through TLR 9. Moreover, cathepsin k supports the secretion of IL-6 and IL-23, inflammatory cytokines involved in the production of Th17 cells, which in turn promote osteoclastic bone resorption [28].
Eph receptors and their associated ligands, expressed by cells found within the bone marrow microenvironment, including OB and OC, are implicated in the regulation of physiological and pathological bone remodeling [29], but also are central in many other different cellular processes including, in addition to immune regulation, angiogenesis, neuronal development and neoplastic metastatization.
Osteoclast semaphorin 4D sustains bone resorption by inhibiting osteoblastogenesis. Since Sema4D also regulates a variety of immune functions, such as antigen presentation, B lymphocyte activation and chemotaxis of monocytes, it could be regarded as an osteoimmunological mediator [30].
The matrix glycoprotein osteopontin (OPN), produced by different types of cells, including immune cells, OC, endothelial and epithelial cells, increases bone resorption by inducing the expression of the osteoclastic immune receptor CD44, essential for cell migration, and by directly enhancing OC attachment to bone extracellular matrix (ECM), required for OCP activation. As a consequence of bone resorption, more OPN is further released from the ECM into the bone microenvironment and into the blood, thus amplifying local and systemic osteoclastogenesis [31].
RANKL and CD40L expressed on T cells, APC, stromal cells and OB, activate the cognate receptors RANK and CD40 in OCP and OB, respectively. CD40/CD40L signaling promotes macrophage activation and differentiation, antibody isotype switching, and the development of B cell memory. CD40 activation in B cells promotes their OPG production thereby decreasing bone resorption. CD40L also increases the commitment of MSC to the osteoblastic lineage. Through CD80/86 signaling in OCP, T cells suppress OC differentiation [21].
Parathyroid Hormone, Lymphocytes and Bone
PTH is an endocrine regulator of calcium and phosphorus metabolism. Primary hyperparathyroidism induces accelerated bone loss, increased bone turnover and osteopenia. Secondary hyperparathyroidism is involved in the pathogenesis of senile osteoporosis. Boh these conditions are mimicked by continuous PTH infusion (cPTH), whereas daily or intermittent PTH injection (iPTH), therapeutically employed in several osteoporotic conditions, stimulates bone formation [32].
PTH binds its receptors (PPR) on stromal cells, OB, and OCy but also on T cells and macrophages. The catabolic effect of cPTH is mostly mediated by enhanced production of RANKL and decreased production of OPG by OB and stromal cells. The PTH anabolic effect is mediated by Wnt signaling activation: PTH increases β-catenin levels in OB, promotes LRP6 signaling and decreases the production of sclerostin [21]. cPTH stimulates bone cells and immune cells to release growth factors and cytokines, including IL-6 and TNF-α, which induce Th17 cell differentiation and the production of IL-17, that plays a pivotal role in the PTH induced bone loss [21]. TNF-α in turn stimulates OC formation and activity via multiple mechanisms, including increased RANKL production. Moreover, TNF-α upregulates the expression of CD40 in OB and stromal cells, increasing their sensitivity to cPTH and suppressing OPG. Bone marrow T cells provide cell surface signals and secrete cytokines that direct the differentiation of mesenchimal progenitors towards OB characterized by a high sensitivity to PTH. Therapy with teriparatide, a form of iPTH treatment, increases the bone marrow levels of Wnt10β. Bone marrow CD8+ T cells potentiate the anabolic activity of PTH by providing Wnt10β [32].
Cytokine and Cell Network
Inflammation results in disturbances in the immunoskeletal interface, i.e. the convergence of cells and cytokines that regulate both the immunity that the bone, causing osteoporosis [33,34]. The inflammatory cytokines TNF-α, IL-1, IL-6 and IL-17 are crucial in acute and chronic inflammation and strong inducers of bone resorption. Cytokines involved in bone remodeling and their main effects are summarized in Table 1. An excessive or abnormal immune activation can induce osteoporosis, as for example in autoimmune diseases, infections and also in senile and postmenopausal osteoporosis. All these conditions go along with an increased inflammatory background and the presence of RANKL producing activated T cells.
Table 1.
Main cytokines involved in bone remodeling.
| Cytokine | Proresorptive effects | Antiresorptive effects |
|---|---|---|
| IL-1 | . TRAF6 expression stimulation, NF-kB induction and MAPK activation . OC maturation induction . PGE2 and RANKL expression induction on OB . enhancement of RANKL induced osteoclastogenesis . amplification of RANKL secretion by stromal cells induced by TNF-α |
|
| IL-4 | . induction of TNF-α expression on macrophages through IL-6 action . IL-1 increase |
. inhibition of RANKL and TNF-α induced osteoclastogenesis . decrease of COX-2 activity and PG production . IL-1 signaling inhibition . c-Fos and NFATc1 expression inhibition via STAT6 . RANKL expression inhibition and OPG expression stimulation |
| IL-6 | . inhibition of SHP2/MEK2/ERK and SHP2/PI3K/Akt2 mediated OB differentiation . RANKL production by synovial and bone marrow stromal cells . synergy with IL-1, TNF-α and PGE . generalized inflammatory effects |
. OPG increase |
| IL-7 | . T and B cell activation . promotion of RANKL and osteoclastogenic cytokine production by T cells . synergy with IL-1, TNF-α and IFN-γ |
. osteoclastogenesis decrease directly acting on OC |
| IL-8 | . RANKL increase | . NO increase |
| IL-10 | . induction of TNF-α expression (in synergy with IL-4) | . NO increase . increase of OPG and decrease of RANKL expression . inhibition of NFATC1, cFos and cJun downstream RANK signaling . anti-inflammatory Th1 cell differentiation . inflammatory and osteoclastogenic cytokine inhibition |
| IL-11 | . increase of RANKL/OPG ratio | . antagonism with IL-6 |
| IL-12 | . IL-1 and Th1 derived cytokine induction | . decrease of T cell induced osteoclastogenesis . direct inhibitory effect on osteoclasts . synergy with IFN-γ and IL-18 |
| IL-13 | . OB inhibition . mineralization blocking |
. OC inhibition |
| IL-15 | . OC differentiation . synergy with TNF-α |
|
| IL-17 | . induction of RANKL expression on OB, synovial cells and fibroblasts . synthesis induction of bone matrix degrading enzymes (MMP) . induction of TNF-α and IL6 production by inflammatory cells . synergy with TNF-α, IL-1 and PGE |
|
| IL-18 | . induction of OPG production from stromal cells . induction of GM-CSF and IFN-γ production from T cells . RANKL signaling inhibition |
|
| Cytokine | Proresorptive effects | Antiresorptive effects |
| IL-23 | . OC activation in inflammatory pathologies . Th17 increase . IL-17 and RANKL induction |
. OC inhibition in physiological condition . fusion inhibition of OCP . inhibition of OC survival . decrease OC maturation via T cells . synergy with IL-18 in inhibiting osteoclastogenesis |
| IL-31 | . stimulation of proinflammatory cytokine, chemokine and matrix metalloproteinase secretion (TNF-α, IL-1β, IL-8, MMP) | |
| IL-32 | . proinflammatory cytokine production stimulation . OCP differentiation induction |
. OC maturation blocking |
| IL-33 | . TNF-α expression induction . bone resorption induction (in synergy with IL-6) |
. synergy with IL-4 . OCP and OC inhibition |
| TNF-α | . M-CSF and RANKL expression induction and inflammation . OC transcription factor (TRAF2, NF-kB) activation through TNF-R1 . OBP maturation inhibition, OB activity inhibition and OB apoptosis induction . induction of negative regulators of the Wnt pathway expression . inhibition of genes involved in bone formation (APH, 1,25-(OH)2 D3 receptor, PTH receptor) |
|
| TGF-β | . promotion of RANKL induced osteoclastogenesis | . inhibition of T cell activation and bone resorption . proinflammatory cytokine production down-modulation . induction of OC apoptosis and OPG production by OB . OBP migration to sites of bone resorption and OBP differentiation |
| INF-γ | . MHCII antigen expression induction on APC . antigen presentation enhancement and T cell activation . osteoclastogenic cytokine production enhancement . synergy with IL-12 |
. TRAF6 proteosomal degradation through specific OC receptors . cathepsin K decrease . decrease of 1,25-(OH)2 D3, PTH and IL-1 mediated osteoclastogenesis . antagonism with TNF-α |
| SOFAT | . direct promotion of osteoclastogenesis in a RANKL-independent manner . induction of IL-6 production by OB |
|
| M-CSF | . RANK expression upregulation on OCP . OCP pool expansion . OC formation (in synergy with RANKL) . monocyte/macrophage lineage differentiation |
|
| G-CSF | . OB apoptosis . MSC apoptosis |
. RANKL signaling inhibition . granulocyte differentiation |
| HMGB1 | . RAGE-dependent RANKL-induced OC differentiation |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; M-CSF, monocyte/macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; RANK, receptor activator of nuclear factor-κB; RANKL, receptor activator of nuclear factor-κB ligand; Th, T-helper; TNF, tumor necrosis factor; TGF, transforming growth factor; OC, osteoclast; OCP, osteoclast precursor; OB, osteoblast; OBP, osteoblast precursor; SOFAT, secreted osteoclastogenic factor of activated T cells; NFATc, nuclear factor of activated T cells; OPG, osteoprotegerin; NO, nitric oxide; TRAF, TNF receptor associated factor; STAT, signal transducer and activator of transcription; PTH, parathyroid hormone; PG, prostaglandin; MHCII, major histocompatibility complex-class II; APC, antigen presenting cells; 1,25-(OH)2 D3, dihydroxyvitamin D3; APH, alkaline phosphatase; MMP, matrix metalloproteinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TNF-R, TNF-receptor; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinases; PI3K, phosphatidylinositol-3-kinase; Wnt, Wingless pathway; HMGB1, high mobility group box 1.
However, in addition to osteoclastogenic cytokines, there are also cytokines which counteract bone resorption and exert osteoblastogenic properties, resulting in a complex bone remodeling cytokine network [56]. Each cytokine has also pleiotropic functions and it is therefore not surprising that same cytokines can exert different and often contrasting effects depending on the specific context in which they act, the maturation stage of target cells and/or the influence of other cytokines. For example, the pleiotropic cytokine IFN-γ exerts anti-osteoclastogenic effects in physiological bone remodeling, by binding to specific OC receptors and inducing TRAF6 proteosomal degradation with consequent inhibition of the transduction signal mediated by RANKL. However, in postmenopausal osteoporosis, inflammation or infections, the final effect of IFN-γ is skewed towards bone resorption through T lymphocyte activation and RANKL expression. In fact, IFN-γ is a powerful stimulator of class II major histocompatibility complex (MHCII) antigen expression on APC, with consequent increased stimulation of T cells through their antigen specific receptor (TCR), inducing further immune activation and production of osteoclastogenic proinflammatory cytokines [35-40].
A cascade of cytokines drives OCP homing, differentiation and activation [41]. Circulating bone marrow produced OCP function as a tank of progenitor cells for several effector cells, in relation to the different cytokines implicated. Furthermore, OCP are also capable of producing proinflammatory cytokines amplifying inflammatory processes. The OCP secreted chemokines preferentially recruit CD8 T lymphocytes. The activation of CD8 T cells by OC induces IL-2, IL-6, IL-10 and IFN-γ production. Individually, these cytokines can activate or suppress OC resorption. OCP can also enhance the expression of the suppressor of cytokine signaling (SOCS) [42].
Treg, whose main marker is the transcription factor FoxP3, balance IL-17 induced bone resorption closely interacting with OC and expressing CTLA-4, which in turn inhibits OC activity [43]. Endothelial cells, activated by IL-1 and TNF-α, attract circulating OCP at sites of inflammation where they migrate through high endothelial venules driven by the expression of cell adhesion molecules (CAM), such as ICAM-1 and CD44. These CD14+ monocytoid cells, under the stimulation of RANKL, become activated bone resorbing OC [44].
Resident tissue macrophages of bone, termed osteal macrophages, are predominantly located adjacent to OB. Osteal macrophages play diverse roles in skeletal homeostasis, their specific functions depending on the macrophage subset considered. They are key mediators of fracture repair. A central function of macrophages is their phagocytic ability. In particular, efferocytosis (phagocytosis of apoptotic cells) is a critical process in both clearing dead cells and replacement of progenitor cells to maintain bone homeostasis [45].
Finally, not only the immune system regulates bone remodeling, but also the bone is able to influence the immune system, actively interacting with immune cells. The same bone cells would then be able to influence or even also perform many immune functions, such as cytokine production and antigen presentation [46-48]. In this sense, the bone would be regarded as a sort of expanded immune system.
Cytokines secreted by bone cells drive naive T cell differentiation into several lineages, leading to expansion of mature T cell populations that further regulate bone homeostasis. OC selectively recruit and activate CD8+ T cells expressing CD25 and FoxP3 (OC-induced regulatory CD8 T cells). In turn, these CD8+ Treg cells suppress bone resorption, decrease inflammatory/osteoclastogenic cytokine production, and stimulate bone formation, creating a regulatory loop: OC and RANKL induce Treg, and then Treg blunt osteoclastic bone resorption [21].
CXCL12 expressing osteolineage cells influence B cell progenitors and OCy affect thymic function. Osteocalcin, expressed on mature bone cells, regulates the production of thymic-seeding T lymphoid progenitors. Mesenchimal skeletal progenitor cells exhibit immunomodulatory profiles. They secrete a variety of proinflammatory and immunosuppressive factors. A subset of mesenchimal cells expressing Osterix, a marker of bone precursors, regulate the maturation of early B lymphoid precursors by promoting pro-B to pre-B cell transition through insulin-like growth factor 1 (IGF-1) production [49]. Skeletal stem cells are also able to recruit and activate neutrophils via the release of IL-6 and IL-8, IFN-β, GM-CSF and MIF [50]. They inhibit B cell proliferation, differentiation, and antibody production, and can also directly inhibit T cell function, rendering them anergic or shifting their phenotype to that of functional regulatory cells. MSC induce macrophages to switch from classically activated proinflammatory (M1) to alternatively activated anti-inflam-matory (M2) phenotype, and inhibit mast cell degranulation attenuating allergic reactions [51]. MSC express active TLR, through which they sense bone microenvironment, recognizing exogenous (bacterial products) and endogenous (heat shock proteins, RNA) danger signals. The common TLR signaling feature is the activation of the NF-κ B transcription factors implicated in controlling the expression of inflammatory cytokines and cell maturation molecules [50].
Osteoporosis and inflammation: the lesson of rheumatoid arthritis
Osteoporosis is a systemic disease of the skeleton, whose main features are loss of bone mass, bone mineral density (BMD) decrease and disruption of bone microarchitecture, so the skeleton becomes fragile, exposing patients to increased risk of fractures [2,4]. Aside from senile and postmenopausal osteoporosis, the first recognized types of primary osteoporosis, many other causes of secondary osteoporosis have been subsequently recognized, for example vitamin D and calcium deficits, lack of sun exposure, immobility, drugs such as cortisone, malabsorption syndromes, endocrine and dismetabolic diseases such as diabetes, disthyroidisms, hypercortisolism, and so on [52]. Only later, clinical and experimental findings evidenced a close connection between osteoporosis and immune mediated inflammatory conditions, for example, rheumatoid arthritis (RA) [53] and acquired immune deficiency syndrome (AIDS) [54], and a common inflammatory background has been finally discovered as pathogenetic factor even in conditions of major osteoporotic risk, such as old age [55,56] and estrogen deficiency [57-59]. More recently, other unpredictable pathological conditions, such as obesity, are coming out as potential osteoporotic risk factors. Even in these cases, the main pathogenetic mechanism leading to bone tissue alteration seems to be inflammation [60]. From this point of view osteoporosis could be therefore regarded as an immune mediated disease in which immune activation, through the induction of cytokine production and inflammation, leads to a remodeling of OC and OB activity and dysregulation of bone turnover with consequent increased bone resorption and osteoporosis.
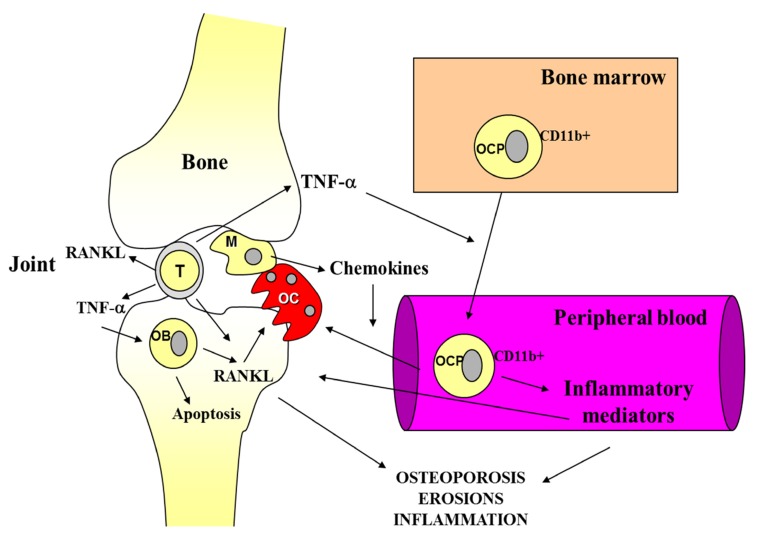
Paradigmatic examples of the link between inflammation and osteoporosis are inflammatory arthritis, mainly RA (Fig. 1). RA is an autoimmune disease that is characterized by inflammation of the synovial joint, leading to severe structural damage and bone destruction. An increased bone resorption is the main pathogenetic mechanism in both disease progression leading to juxta-articular bone erosions and irreversible joint damage and systemic osteoporosis. Bisphosphonates, drugs used for some time in the therapy of osteoporosis, are potent inhibitors of OC activity both in the primitive and secondary osteoporosis, such as that associated with autoimmune diseases [61,62]. A decreased BMD in the spine and hip and higher prevalence of osteoporosis have been described in RA patients [63]. In early untreated RA, BMD is related to longer symptom duration, the presence of rheumatoid factor (RF) and cyclic citrulinated peptide antibodies (anti-CCP), disease activity score, and the presence and progression of joint damage [1, 19]. Bone is therefore a target of inflammation in RA. Monoclonal antibodies (MoAb) against various proinflammatory cytokines and their receptors, such as TNF-α, are useful in preventing and/or reversing bone erosions as well as systemic osteoporosis [64].
Fig. (1).
Shared immune mediators in rheumatoid arthritis and osteoporosis.
Autoimmune reactions induce RANKL expression and subsequent osteoclastogenesis. That the RANK/ RANKL/OPG pathway is central to the osteoporosis pathogenesis is confirmed by the elevated antiresorptive capacity of denosumab, an anti-RANKL MoAb, utilized in osteoporosis therapy [65]. Activated immune cells at sites of inflammation produce a wide spectrum of proinflammatory and osteoclastogenic cytokines, resulting in bone erosions, osteitis, and peri-inflammatory and systemic bone loss. Local peri-inflammatory bone loss and osteitis occur early and precede and predict erosive bone destruction in RA [66]. Moreover, peri-inflammatory bone formation is impaired, resulting in non-healing of erosions, and this allows a local vicious circle of inflammation between synovitis, osteitis, and local bone loss. OC have a central role in RA pathogenesis. RANKL is highly expressed in the RA synovium, and inflammation-mediated bone damage is largely attributable to its abnormally high expression.
In addition to activated T lymphocytes and macrophages, a pivotal role in inducing bone erosions is also played by RANKL-expressing B cells, as highlighted by the observation of the therapeutic effect of anti-CD20 antibody in RA [67,68].
CD4+T-cells, especially Th17 cells, play a prominent role, particularly in the initiation of systemic immune response in RA. The interaction between immune and mesenchymal cells in joints, including synovial fibroblasts, which are characterized by hyperproliferative and hyperactive properties in response to an inflammatory environment, is of paramount importance in rheumatoid inflammation [69,70]. In Fig. (2) the role of Th17 lymphocytes in RA is illustrated.
Fig. (2).
The role of Th17 lymphocytes in rheumatoid arthritis.
Synovial cells in RA hyperproduce both inflammatory cytokines and matrix degrading enzymes. Thus, in the affected joints, hyperplasia of the synovial membrane is characterized by both hyperproliferation of synovial fibroblasts and massive infiltration of inflammatory immune cells, including CD4+ T cells and innate immune cells.
Autoimmune diseases, including arthritis, often result from an imbalance between Treg cells and Th17 cells. The Th17 cells derived from Foxp3+ T cells in RA comprise a novel Th17 cell subset with a distinct pattern of gene expression and arthritogenic properties. The T cell-synovial fibroblast interaction amplifies the local immune dysregulation. The fate of plastic Foxp3+ T cells may be a key determinant of the Treg/Th17 balance that is critically involved in the self-tolerance and autoimmunity [71].
Mesenchymal cells are a determinant of the development of RA that links the systemic immune response and the local disorder in the joints. Mesenchymal cells contribute to the Th17 mediated chronic inflammation by promoting the migration of Th17 cells to the inflamed joint and concomitant increase in IL-17 production. Th17-related cytokines enhance osteoclastogenesis, mainly via synovial fibroblasts. Thus, the interaction of immune and mesenchymal cells plays a key role in both the chronic inflammation and bone destruction in RA. In particular, pathogenetic autoreactive immune cells migrate into joints and activate the mesenchymal cells resident in joint, such as synovial fibroblasts. Moreover, since soluble inhibitors of the Wnt pathway, such as DKK-1, produced by synovial fibroblasts, are upregulated by TNF-α, antibodies against DKK1 could be able to both promote bone formation and prevent bone erosions in RA [72,73].
Menopausal and senile osteoporosis
Osteoporosis has long been regarded as simply the consequence of the menopausal estrogen decline. Os-
The progression of articular erosions is clearly driven by proinflammatory and osteoclastogenic cytokines produced by immune cells in the inflamed joint. Both activated lymphocytes and macrophages stimulate osteoclast differentiation by producing proinflammatory mediators. Activated lymphocytes of the synovial pannus overexpress RANKL and TNF-α that stimulate bone marrow CD11b+ OCP to proliferate and enter the bloodstream where they themselves produce inflammatory factors amplifying inflammation. Activated macrophages in the inflamed joints produce various chemokines which drive the localization of periarticular bone OCP. The stimulation of osteoclastogenesis induced by the high concentrations of RANKL and TNF-α results in bone resorption. In addition, TNF-α decreases the formation of bone tissue by enhancing OB apoptosis.
Th17 cells in the synovial pannus are IL-17 producing helper lymphocytes. These cells do not produce antiosteoclastogenic cytokines, such as IFN-γ or IL4, while expressing high levels of RANKL and secrete IL-17, that in turn stimulates OB, synoviocytes and fibroblasts to express RANKL, and induces macrophages to produce proinflammatory cytokines, such as TNF-α and IL-6 in the synovium amplifying local inflammation. IL-17 also induces the synthesis of enzymes capable of degrading the bone matrix such as matrix metalloproteinases (MMP). These effects are balanced by Treg that inhibit bone destruction by suppressing OC formation through both cell-cell contact and the secretion of inhibitory cytokines such as TGF-β, IL-4 and IL-10.
teoimmunology, suggesting that immune cells take part in the bone changes typical of menopause, has led to a shift in the concept of osteoporosis, that is currently considered an inflammatory condition [74,75]. Post-menopausal osteoporosis is a clear example of the mutual influences between immune system, bone and endocrine system. Like many other hormones, in addition to specific target organs (breast and reproductive system), estrogens have their receptors also on immune cells and bone, as well as on bone marrow precursors. Fig. (3) shows the effects of estrogen deficiency on cells and molecules involved in bone metabolism. Menopausal estrogen decline leads to proliferation and activation of T cells by increasing MHCII expression on monocytes and APC function and by inhibiting T cell apoptosis. These effects leads to the expansion of activated T lymphocytes resulting in hyperproduction of inflammatory cytokines and chronic OC stimulation which is responsible for bone loss and increased fracture risk [76,77].
Fig. (3).
The effects of estrogen deficiency on cells and molecules involved in bone metabolism.
Maintenance of inflammatory reactions leading to bone resorption and skeletal fragility is also present during senescence and inflammaging (Fig. 4), that is the condition of chronic inflammation characterizing aging, as the result of the immune system’s ability to cope with stressors. Inflammaging is now considered the background of a broad range of age-related diseases with an inflammatory pathogenesis [78]. Many of the biological mechanisms implicated in the aging process, such as cell senescence, proinflammatory immune profile, apoptosis and metabolism imbalance, are also implicated in bone remodeling. Also in the absence of overt inflammatory diseases, the heightened catabolic signals induced by inflammation enhance apoptosis of OB and muscle cells, causing both osteoporosis and sarcopenia. During aging, the lifelong exposure to oxidative stress and chronic antigenic load leads to the loss of the regulatory process which counteracts T cell activation induced bone resorption [79]. In aged people, lipid oxidation mediated by ROS increased production and Wnt signaling suppression, contribute to bone formation decline. Oxidized polyunsaturated fatty acids induce the association of PPAR with β-catenin and promote its degradation. Oxidized lipids also potentiate oxidative stress, enhance OB apoptosis and inhibit BMP-2 induced OB differentiation. Antioxidant agents seem to have some action on bone remodeling: resveratrol decreases NF-k activation induced by RANKL and OC differentiation and also promotes osteogenesis in MSC via the Sirt1/FoxO3 axis stimulation [80]. During senescence, besides the impaired Treg function, the number of effector memory cells is increased [81]. These are senescent cells with proinflammatory properties, secreting several inflammatory cytokines able to influence bone remodeling. Interestingly, this immunological pattern (accumulation of activated cells and memory/effector lymphocytes secreting proinflammatory cytokines) characterizing immunosenescence, is also peculiar of other immunological conditions associated with osteoporosis, such as RA, AIDS, chronic viral infections, etc. [54].
Fig. (4).
Senile osteoporosis.
Correlations with other systems and functions
The already complex cross-talk between bone and immune system is further expanding as they emerge new mediators involved. Interestingly, the scenario of osteoimmunolgy is increasingly including interconnections with other organs and systems, therefore influencing several homeostatic functions in addition to bone turnover and immunity, such as hemopoiesis, metabolic and neuro-endocrine functions.
Hemopoiesis and Tumour Growth
The crosstalk between skeletal and bone marrow is crucial to hematopoietic stem cell (HSC) function and throughout the hemopoiesis [3]. HSC occupy specific locations in the bone marrow microenvironment, commonly referred to as niches, comprising multiple cell types able to regulate HSC proliferation and differentiation. Both HSC and the cells of the niches are heterogeneous [7]. Hematopoietic and skeletal stem cell homeostasis are closely related: MSC progeny express numerous cytokines necessary for HSC maintenance and hematopoiesis, including Kit ligand and stromal-derived factor, but also stem and progenitor cells of the hematopoietic system may reciprocally regulate skeletal progenitors by expressing myriad factors associated with skeletogenesis, whose cognate receptors are highly expressed by skeletal stem cells. Interestingly, these latter exhibit receptors to circulating hormones, such as leptin and thyroid-stymulating hormone, suggesting that skeletal stem cells and their progeny may link systemic exocrine regulation to both skeletal and hematopoietic system [5]. Primitive mesenchymal progenitors are more critical for HSC function, whereas lineage-restricted mesenchymal cells control more committed hematopoietic progenitors [49]. Bone microenvironment is therefore a composite of specialized niches providing distinctive functional units to regulate hematopoiesis.
Menopause leads to relevant immune and skeletal changes due to the estrogen decline. The estrogen deficiency induces a marked increase of inflammatory cytokines and mediators of inflammation, in particular IFN-γ, M-CSF, TNF-α, IL-1, IL-6 and IL-7, in addition to prostaglandins (PGE) and reactive oxygen species (ROS), which play a driving role in the development of osteoporosis. Of particular interest is the expansion in the peripheral blood from postmenopausal women of two particular lymphocyte subsets, CD3+ CD56+ T lymphocytes, major producers of TNF-α, and B220+ IgM-B lymphocyte precursors, that under certain stimuli can differentiate into OCP and are strong producers of inflammatory cytokines. The enhanced TNF-α production by activated T cells has a central role in bone loss due to estrogen decline in menopausal women. The same IFN-γ, which in some situations could be protective against osteoporosis, during estrogen deficiency has a prevailing osteoclastogenic stimulating action. The estrogen decline also results in the decrease of OPG and TGF-β that normally contrast the effects of inflammatory mediators on bone.
Immunosenescence and osteoporosis share the same immune profile. The age-related increase of inflammatory cytokines results from a chronic activation of macrophages and memory/effector T cells, in addition to an impaired Treg function. A peculiar finding of immunosenescence is the increased number of senescent memory cells expressing RANKL and secreting osteoclastogenic cytokines, mainly TNF-α, IL-1, IL-6 and IL-17. These cytokines are able to facilitate OCP expansion which amplify the systemic inflammation by producing further proinflammatory factors able to recruit other inflammatory cells and perpetuating the flogistic vicious cycle. Both the increased transcriptional activity of NF-kB due to genotoxic, inflammatory, and oxidative stresses in aging, and the chronic p53 activation induced by the age-related progressive loss of telomere length, result in impaired OB proliferation and osteoporosis development.
Cells of the immune system and bone cells derive from bone marrow precursors and develop in the same bone marrow microenvironment. Hematopoietic and immune cells share same signaling pathways with cells of the bone and their precursors, which surprisingly do not take part in the regulation of immunity and hemopoiesis [2].
OB, together with MSC, are crucial components of the niche of growth of HSC. OB overexpression of the WNT antagonist Dkk1 reduces WNT signaling in HSC and disrupts HSC self-renewal potential [85]. The WNT antagonist secreted frizzled-related protein 1 (Sfrp1) is involved in osteoblastic mediated HSC regulation; through its regulation of OB, PTH controls the hematopoietic niche function, involving crosstalk with WNT signaling [86]. The upregulation of Notch ligand protein jagged-1 (Jag1) expression in OB and the increased Notch signaling are involved in increasing HSC numbers; angiopoietin-1 receptor activation on HSC by OB produced angiopoietin-1 promotes strict adhesion of HSC to the niche, inducing quiescence of these cells. Osteopontin, an OB-secreted protein, participates in HSC location and is a negative regulator of their proliferation. Also, OCy derived from OB that become embedded within the bone matrix, are involved in the complex regulation of hemopoiesis through the osteoimmune interface: mainly by the production of sclerostin, they appear to have an inhibitory effect on HSC support [7,41].
Even OC, as well as activated endothelial cells, are likely involved in the mobilization of HSC by cytokine induced CAM expression. Being the only cells capable of bone resorption, in addition to allowing the renewal of the skeleton, they also open the space in the bone marrow for hematopoietic cells. OC contribute to HSC release via enzyme secretion, which enhances mobilization. Moreover, osteoclastic bone resorption releases calcium, which attracts and retains calcium sensing receptor expressing HSC at the endosteal region. Bone resorption also produces TGF-β which can act on HSC.
Macrophages play diverse roles in the bone and marrow. At the sites of bone remodeling, they are juxtaposed with endosteal OB and participate in bone mineralization. Moreover, CD169+ macrophages promote HSC retention in the bone marrow. A subset of HSC is located in close proximity to megakaryocytes in the bone marrow, where OB undergo rapid expansion in response to the secretion of megakaryocyte-derived mesenchymal growth factors, such as platelet derived growth factor (PDGF)-β, to promote HSC proliferation [7].
The bone marrow microenvironment can also act as a niche for the onset and progression of neoplastic diseases, including both hematologic malignancies and metastases of solid tumors, mainly breast and prostatic cancer. In multiple myeloma, an osteolytic hematological malignancy characterized by an important skeletal involvement, neoplastic plasma cells produce a large amount of mediators that induce osteoclastic bone resorption and block the activity of OB. In addition to osteolytic factors, myeloma cells produce DKK1, which inhibits OBP differentiation by binding to the LRP5/6 coreceptors expressed on their surface [82]. On the other hand, the same bone cells produce growth factors and angiogenetic cytokines able to support the development and progression of myeloma, perpetuating a vicious circle of mutual reinforcement [83,84].
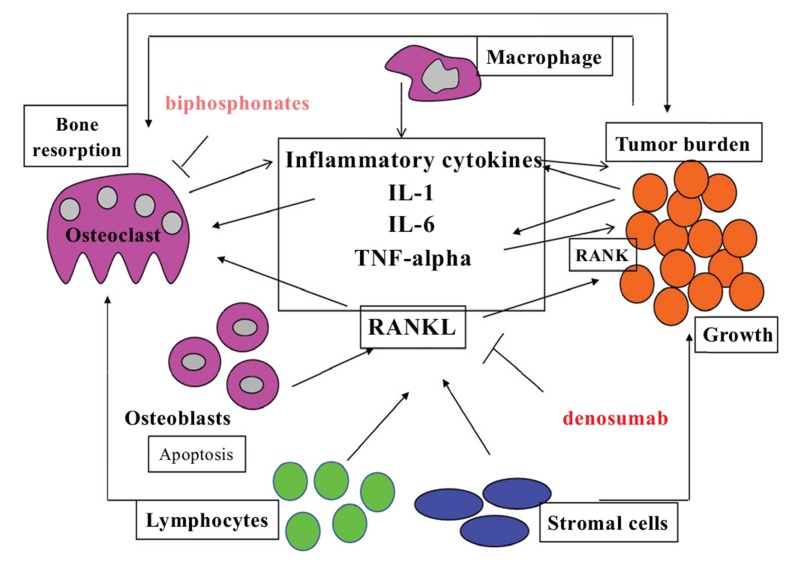
A promising new field of interest in this regard is the osteo-immune-oncology. There is in fact a close relationship between immune regulation of bone turnover and tumor growth. Often, cancer cells express RANK and in proneoplastic inflammation various cell types express RANKL that acts as chemotactic factor favoring the skeletal metastasis. Tumor cells in the bone cause activation of osteoclasts that mediate bone resorption and additional growth factor release from the bone matrix, fueling a vicious circle of impaired bone turnover and tumor proliferation (Fig. 5). In skeletal metastases, the production of OPG by OB is also decreased, thus favoring further osteolysis. Not always the tumor associated inflammation is the expression of an immune system that successfully opposes the neoplastic growth, as previously believed, but sometimes some patterns of immune activation can be facilitators of the development of tumors. This kind of proneoplastic inflammation is the same that supports a proresorptive immune phenotype. Then the block of RANKL, in addition to inhibiting bone resorption, also decreases tumor growth, enhances apoptosis of malignant cells and diminishes proneoplastic inflammation.
Fig. (5).
The vicious cycle of bone destruction and cancer growth.
Osteal macrophages, as well as macrophages in other tissues, have a role in tumor progression, supporting cancers which preferentially metastasize to the skeleton, in particular breast and prostate cancer. Macrophages, tumor cells and skeletal cells orchestrate complex immune reactions. Osteal macrophages expressing CD68, a phagocytic capacity marker of cells infiltrating metastatic lesions, could facilitate tumor establishment and growth. Tumor-derived PTHrP drives myeloid cell recruitment via OB produced CCL2, which is high in the bone microenvironment and whose levels are associated with poor prognoses in primary breast tumors [45].
Fat Tissue and Metabolic Syndrome
As for bone, adipose tissue is a kind of immune tissue and produces immunoregulatory factors. Adipocytes and OB derive from the same MSC, as well as visceral adipose tissue macrophages and OC derive from the same HSC. Shared transcription factors regulate the shift between the different cell lines and the subsequent differentiation cascade. By action of peroxisome proliferative activated receptor gamma (PPARγ), the principal regulator of adipogenesis, the MSC differentiates into adipocytes rather than into OB. In contrast, the expression of runt-related transcription factor 2 (Runx2), associated with OB differentiation, and Osterix, an OB zinc finger–containing transcription factor, shift the equilibrium towards the osteoblastogenesis [85,86]. In addition, through the induction of cfos, the PPARγ promotes osteoclastogenesis. Mechanical loading promotes OB differentiation and inhibits adipogenesis by down-regulating PPARγ or by stimulating a durable β-catenin signal [87]. Not surprisingly, PPAR agonists such as thiazolidinediones used to increase insulin sensitivity in type 2 diabetes, also increase the risk of osteoporotic fractures.
Adipokines are broadly divided into pro- and anti-inflammatory sub-groups. Leptin is a pro-inflammatory adipokine that exerts its actions via central nervous system regulation of feeding behavior, where it promotes satiety and prevents weight gain. Leptin can also directly signal through its receptor expressed on immunocytes, where it induces TNF-α and IL-6 production by monocytes, chemokines by macrophages, and Th1 cytokines from polarized CD4+ T cells. Leptin also supports proliferation of activated T cells. Adiponectin is an anti-inflammatory adipokine whose plasma levels are strongly correlated with insulin sensitivity and glucose tolerance. It can directly interfere with inflammatory cytokine production in macrophages and can induce expression of the anti-inflammatory cytokine IL-10. TNF-α and IL-6 inhibit adiponectin production in adipocytes [88]. Adipose tissue produced pro-inflammatory cytokines and adipokines further modulate the activity of OC and OB. Fat tissue, mainly visceral fat tissue, may increase bone resorption through the production of inflammatory cytokines such as IL- 6 and TNF-α, which stimulate OC activity through the regulation of the RANKL/RANK/OPG pathway [84]. Leptin and adiponectin act on the bone through different signaling pathways with contrasting effects [90] (Table 2). Leptin signaling regulates osteogenesis by skeletal stem cells. It promotes adipogenesis and inhibits osteogenesis in response to diet and adiposity by activating Jak2/Stat3 signaling. Therefore, leptin receptors on skeletal MSC function as a sensor of systemic energy homeostasis [8,9].
Table 2.
Leptin and adiponectin modulation of bone turnover.
| Adipokine | Mechanism of action |
|---|---|
| Leptin | • Stimulation of osteoblastic bone formation and OPG production through specific receptors (direct mechanism) • Stimulation of adrenergic receptors on OB inducing apoptosis (indirect neuro-mediated mechanism) • Induction of inflammatory osteoclastogenic cytokine production by cells of the immune system (indirect immune-mediated mechanism) • Enhancement of hepatic secretion of IGFBP-2, that increases both insulin sensitivity and OC differentiation • Decrease of renal expression of the 25-hydroxyvitamin D3 1α-hydroxylase gene and FGF-23 increased production by OB, resulting in reduced phosphate resorption and 1,25(OH)2D3 synthesis |
| Adiponectin | • Stimulation of OB proliferation, maturation and function • Enhancement of RANKL secretion by OB • Inhibition of OPG secretion by OB • Inhibition of RANKL induced osteoclastogenesis by interacting with its adaptor molecule APPL1 in OC |
Abbreviations: Osteoprotegerin (OPG); Osteoblasts (OB); IGF binding protein 2 (IGFBP-2); Osteoclasts (OC); 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3); Phosphaturic factor fibroblast growth factor 23 (FGF-23); Adaptor protein containing pleckstrin homology domain, phosphotyrosine domain, and leucine zipper motif (APPL1)
Various cell populations within the fat tissue can exacerbate the development of the chronic, low-grade inflammation associated with obesity and metabolic dysfunction. Adipocytes consist of subsets with distinct developmental origins and metabolic functions. White adipocytes store lipid as triglycerides within unilocular droplets, and are responsive to various stimuli, such as insulin. They are classified as subcutaneous or visceral, mainly abdominal, adipocytes. Brown adipocytes store lipid in multilocular droplets that are quickly catabolized for fuel when the tissue is stimulated. Beige adipocytes, dispersed within white adipose tissue, can dissipate heat, similarly to classical brown adipocytes, when exposed to cold temperatures or after prolonged highfat diet feeding. Various immunocytes infiltrate fat tissue and communicate closely with adipocytes. Visceral fat tissue infiltrating macrophages in the setting of diet-induced obesity, have a pro-inflammatory phenotype which initiates and/or exacerbates the chronic inflammation that contributes to adipocyte dysfunction in obesity. Conversely, in lean humans adipose tissue macrophages may promote tissue remodeling and temper inflammation by secreting anti-inflammatory cytokines [88].
Notwithstanding epidemiological evidence indicates that an increase in body mass index (BMI) is related to increased bone mass, probably due to the effects of the mechanical load of the body weight on the skeleton, not always obesity, and mainly the increase in visceral fat mass, has a positive effect on bone. Obesity is associated with increased leptin and decreased adiponectin serum levels [89]. Moreover, in the visceral adipose tissue there are activated macrophages producing cytokines. In obese subjects, especially with central obesity, in which the visceral fat is increased, there is a significant increase of several markers of inflammation such as C reactive protein (CRP), IL-1, IL-6 and TNF-α, that can alter the quality of the bone, making it more fragile. Therefore, these new clinical and experimental evidences definitively connect obesity and other related pathological conditions, such as metabolic syndrome, nonalcoholic fatty liver disease (NAFLD) and diabetes, to impaired bone health and fragility fractures [90-93]. In conclusion, it is currently emerging that adipose tissue, liver, bone and immune system modulate each other through a complex network of interconnected signals.
Both adipocytes and OB express OPG and RANKL and their modulation is influenced by adipokines, sex hormones, redox balance, PPARγ and liver X receptors (LXR). Osteocalcin, an OB secreted bone matrix noncollagen protein, takes part in calcium metabolism and in bone deposition, as well as in energy homeostasis and glucose metabolism. Fetuin-A, produced by the liver, is involved in the regulation of bone metabolism and insulin action, vascular calcification, neurodegenerative diseases and cancer cell proliferative signaling. OB lineage cells express receptors for adiponectin, leptin, angiotensin II, insulin and insulin-like growth factor-1, able to influence RANK/RANKL/OPG signaling pathway. Interestingly, these hormones are implicated in the pathogenesis of type-II diabetes, obesity and hypertension, all of which are risk factors for metabolic syndrome [31].
The development and progression of diabetes-associated osteoporosis are mediated by the interaction between advanced glycation end products (AGE) and receptor of AGE (RAGE). AGE are the end products of glucose, as well as other sugars, proteins, lipids, and nucleic acids via a non-enzymatic glycosylation reaction, able to bind to multiple membrane receptor proteins, including RAGE. AGE/RAGE interaction is involved in the pathophysiological processes of many inflammatory and dysmetabolic diseases. In particular, AGE are involved in the development and progression of osteoporosis by inhibiting proliferation and inducing OB apoptosis. The binding of AGE to organic bone matrix may also increase the fragility of bones. Autophagy is a metabolic process by which eukaryotic cells degrade and recover damaged macromolecules and organelles into autophagosomes Autophagy is upregulated in stressful conditions. However, excessive autophagy is harmful to cells and leads to damage or massive death of cells. AGE-induced autophagy is closely associated with OB proliferation and function. Autophagy deficiency increases oxidative stress levels in OB, decreases bone mineralization and induces RAN-KL secretion [94].
Neuroendocrine System and Major Depression
It is well eatablished that several neuroendocrine pathways modulate both immune responses and bone remodeling. In turn, immune factors not only influence neuroendocrine pathways but also induce bone remodeling alterations.
Signals from the sympathetic nervous system are important regulatory components of the HSC niche. Sympathetic nerves produce catecholamines, which are delivered to the bone microenvironment by the blood circulation or by secretion from the nerve endings. HSC express catecholaminergic receptors, suggesting that they are able to directly respond to signals from the sympathetic nervous system. Adrenergic signaling reduces CXCL12 expression in the BM. affecting maintenance of HSC and the differential lineage commitment [7,95].
An association between major depression and osteoporosis has been recognized [96-99]. The prevalence of low BMD is higher in people with depression than the general population. Interestingly, patients on antidepressant therapy with selective serotonin reuptake inhibitors develop decreased BMD and increased risk of fracture compared to those treated with tricyclic antidepressants such as amitriptyline or patients with untreated depression, who also have a lower BMD compared with healthy controls [100, 101]. Neuroendocrine abnormalities of the hypothalamo-pituitary-adrenal (HPA) and sympathoadrenal axes are a common finding in depressed patients leading to increased catecholamine turnover and hypersecretion of corticotropin-releasing hormone (CRH). The hyperproduction of these mediators heavely impacts on bone health. Leptin is expressed in the hypothalamus and pituitary gland, where it modulates corticotropin-releasing hormone and ACTH secretion, probably acting in an autocrine-paracrine manner. Leptin dampens the HPA axis response to many kinds of stresses. It inhibits steroid-hormone secretion from the adrenal cortex but enhances catecolamine release from the adrenal medulla, activating the sympathoadrenal axis [102].
The neuromodulator serotonin or 5-hydroxy-tryptamine (5HT), which is an important vasoactive mediator of allergic reactions, is also likely involved in interactions between the central nervous and immune systems. In addition, it has recently been emerged that proinflammatory cytokines can modulate the central nervous system 5HT signaling, resulting in the conceptualization that 5HT participates in an integrated behavioral response to pathogens and inflammatory events. On the other hand, 5HT is also implicated in the regulation of bone remodeling. 5HT receptor expression on OCy and their precursors is involved in bone metabolism and its mechanoregulation. Moreover, serotonin blocks the proliferation of OCP through the suppression of intracellular transcription factor CREB, which regulates many genes involved in circadian rhythms in different tissues (period 1,2,3 and clock genes) [103].
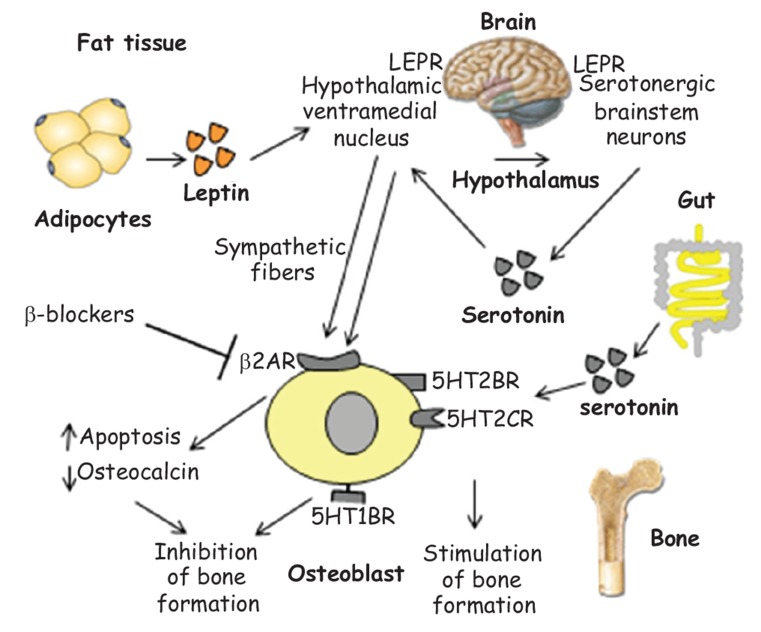
There are two anatomically and functionally distinct pools of serotonin: the first one, synthesized through the activity of the enzyme tryptophane-hydroxylase type 2 in the central nervous system, where it functions as neurotransmitter; the second one is synthesized in the periphery through the activity of the tryptophane-hydroxylase 1, regulated by the low-density lipoprotein receptor (LDLR)-related protein-5 (LRP5) [104]. The circulating serotonin does not exceed the blood brain barrier and is for 95% produced by intestinal enterochromaffin cells in the intestine, where it stimulates peristalsis in response to the meal. The peripherally produced serotonin acts as a hormone inhibiting bone formation, whereas serotonin produced in the brain functions as a neurotransmitter, enhancing bone formation and limiting bone resorption. The neurological mechanism of action of serotonin on bone implicates also the interaction with the adipokine leptin, in an integrated homeostatic network with fat tissue and metabolism [105] (Fig. 6).
Fig. (6).
Integrated roles of leptin and serotonin in bone remodeling.
A portion of 5HT is also produced by the mammary gland, where it acts as an autocrine-paracrine regulator of the epithelial homeostasis exerting antiproliferative and proapoptotic effects. In the course of pregnancy, lactation and menopause, the local serotonin production increases, contributing to the increased bone resorption typical of these phases of the woman's life. Small molecules able to specifically inhibit the intestinal tryptophane-hydroxylase and do not cross the blood brain barrier, have recently been proposed for the treatment of osteoporosis [106,107].
In Alzheimer’s disease (AD), a neurodegenerative disorder characterized by cortical and cerebrovascular amyloid β peptide (Aβ) deposits, neurofibrillary tangles, chronic inflammation, and neuronal loss, increased bone fracture rates and reduced BMD are commonly observed, suggesting common denominators between both disorders. Amyloid precursor protein (APP) is transmembrane protein involved in AD pathogenesis: APP gene mutations characterize early-onset AD, and many risk factors or genes associated with late-onset AD appear to affect APP trafficking and Aβ production. RAGE, acting as an APP/Aβ binding partner, is therefore implicated in the pathogenesis of both AD and osteoporosis. The role of RAGE in OC maturation and activation is also mediated by its interaction with proinflammatory associated Mac-1/β2 integrin, the S100 family, and the high mobility group box 1(HMGB1). In particular, HMGB1 is a proin-flammatory cytokine released from activated macro-phages, that promotes RANKL-induced OC differentiation in a RAGE-dependent manner [108].
Shared signaling pathways among the complex immunological machineries involved in bone remodeling activate vicious cycles underlying both bone destruction and cancer growth. Bone derived cytokines provide a chemotactic stimulus for tumor cell migration and homing. The release of growth factors in the skeletal microenvironment as a result of osteolysis induced by OC mediated bone resorption enhances metastases and cancer cell proliferation. In addition to the hyperproduction of proinflammatory cytokines, RANK /RANKL signal alterations are central in the pathogenesis of neoplastic osteolysis. The upregulation of RANKL, particularly in myeloma and breast cancer, promotes the growth of neoplastic cells which express RANK and protects them from DNA damage induced programmed cell death. In this context, bone cells may represent potential therapeutic targets in the treatment of both secondary neoplastic osteoporosis and the underlying neoplasia. For example, bisphosphonate treatment of individuals with multiple myeloma reduces osteolytic events and tumor burden as well. The block of RANKL by the monoclonal antibody denosumab, in addition to inhibiting bone resorption, is also useful in reducing tumor growth, in increasing apoptosis of malignant cells and in decreasing the inflammation that supports the neoplasia.
Adipocytes synthesize leptin, which increases in obesity proportionally to BMI. Its predominant effect on bone is through the central nervous system: by stimulating specific receptors (LEPR) on both serotonergic brainstem neurons and the hypothalamic ventromedial nucleus, which interact each other, it increases central sympathetic activity. Inhibitory signals are transmitted through sympathetic fibers from the hypothalamic ventromedial nucleus to β2-adrenergic receptors (β2-AR) expressed on OB, suppressing their differentiation and osteocalcin production. This explains the finding of a reduced risk fracture in patients taking β blockers. Serotonin is synthesized in serotonergic neurons of the central nervous system, exerting various functions in the brain; it is also synthesized in the gut, mediating different peripheral functions. It acts on bone cells using three different receptors: through 5HT1B receptors, it negatively regulates bone mass, while it enhances bone formation through 5HT2B and 5HT2C receptors.
Conclusion and perspectives
Immune system and skeletal system interact with each other both in physiological and pathological conditions. Considerable progress has been made in clarifying the crosstalk between bone and immune system that occurs in a complex and dynamic bone microenvironment. A central role in this crosstalk is played by proinflammatory and osteoclastogenic cytokines which drive bone resorption. Osteoimmunology therefore represents a new approach to studying osteoporosis that in the past was not considered an inflammatory condition. Moreover, the discoveries of the existence of the immunoskeletal interface has also highlighted interesting repercussions for a wide range of pathological conditions beyond osteoporosis, including infections, autoimmune diseases, and neoplasia, in which same pathogenetic pathways are shared. Osteoporosis could be therefore considered as a systemic model of integrated signaling pathways and cytokines working in a cooperative fashion.
Further important research horizons are opened with the extension of the discoveries of osteoimmunology and the disclosure of the immunoskeletal interface, whose practical implications may provide novel therapies for diseases other than osteoporosis, by targeting specific transcription factors, cytokines and their receptors. The correct understanding and decoding of the complex language through which immune system and bone communicate, although still in its dawn, is the essential requirement for the identification of such potentially useful therapeutic targets for both osteoporosis and other correlated inflammatory conditions, which share same mediators and signaling pathways.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7(4):292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 2.Greenblatt M.B., Shim J.H. Osteoimmunology: a brief introduction. Immune Netw. 2013;13(4):111–115. doi: 10.4110/in.2013.13.4.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takayanagi H. Osteoimmunology in 2014: Two-faced immunology-from osteogenesis to bone resorption. Nat. Rev. Rheumatol. 2015;11(2):74–76. doi: 10.1038/nrrheum.2014.219. [DOI] [PubMed] [Google Scholar]
- 4.Geusens P., Lems W.F. Osteoimmunology and osteoporosis. Arthritis Res. Ther. 2011;13(5):242. doi: 10.1186/ar3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan C.K., Seo E.Y., Chen J.Y., Lo D., McArdle A., Sinha R., Tevlin R., Seita J., Vincent-Tompkins J., Wearda T., Lu W.J., Senarath-Yapa K., Chung M.T., Marecic O., Tran M., Yan K.S., Upton R., Walmsley G.G., Lee A.S., Sahoo D., Kuo C.J., Weissman I.L., Longaker M.T. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1-2):285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mensah K.A., Li J., Schwarz E.M. The emerging field of osteoimmunology. Immunol. Res. 2009;45(2-3):100–113. doi: 10.1007/s12026-009-8093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birbrair A., Frenette P.S. Niche heterogeneity in the bone marrow. Ann. N. Y. Acad. Sci. 2016;1370(1):82–96. doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yue R., Zhou B.O., Shimada I.S., Zhao Z., Morrison S.J. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18(6):782–796. doi: 10.1016/j.stem.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Worthley D.L., Churchill M., Compton J.T., Tailor Y., Rao M., Si Y., Levin D., Schwartz M.G., Uygur A., Hayakawa Y., Gross S., Renz B.W., Setlik W., Martinez A.N., Chen X., Nizami S., Lee H.G., Kang H.P., Caldwell J.M., Asfaha S., Westphalen C.B., Graham T., Jin G., Nagar K., Wang H., Kheirbek M.A., Kolhe A., Carpenter J., Glaire M., Nair A., Renders S., Manieri N., Muthupalani S., Fox J.G., Reichert M., Giraud A.S., Schwabe R.F., Pradere J.P., Walton K., Prakash A., Gumucio D., Rustgi A.K., Stappenbeck T.S., Friedman R.A., Gershon M.D., Sims P., Grikscheit T., Lee F.Y., Karsenty G., Mukherjee S., Wang T.C. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1-2):269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rauner M., Sipos W., Thiele S., Pietschmann P. Advances in osteoimmunology: pathophysiologic concepts and treatment opportunities. Int. Arch. Allergy Immunol. 2013;160(2):114–125. doi: 10.1159/000342426. [DOI] [PubMed] [Google Scholar]
- 12.Panwar P., Søe K., Guido R.V., Bueno R.V., Delaisse J.M., Brömme D. A novel approach to inhibit bone resorption: exosite inhibitors against cathepsin K. Br. J. Pharmacol. 2016;173(2):396–410. doi: 10.1111/bph.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teitelbaum S.L. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 14.Brown J.P., Roux C., Ho P.R., Bolognese M.A., Hall J., Bone H.G., Bonnick S., van den Bergh J.P., Ferreira I., Dakin P., Wagman R.B., Recknor C. Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos. Int. 2014;25(7):1953–1961. doi: 10.1007/s00198-014-2692-7. [DOI] [PubMed] [Google Scholar]
- 15.Gohda J., Akiyama T., Koga T., Takayanagi H., Tanaka S., Inoue J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005;24(4):790–799. doi: 10.1038/sj.emboj.7600564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S., Miller C.H., Giannopoulou E., Hu X., Ivashkiv L.B., Zhao B. RBP-J imposes a requirement for ITAM-mediated costimulation of osteoclastogenesis. J. Clin. Invest. 2014;124(11):5057–5073. doi: 10.1172/JCI71882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo K. Eph and ephrin interactions in bone. Adv. Exp. Med. Biol. 2010;658:95–103. doi: 10.1007/978-1-4419-1050-9_10. [DOI] [PubMed] [Google Scholar]
- 18.Lories R.J., Luyten F.P. Osteoimmunology: Wnt antagonists: for better or worse? Nat. Rev. Rheumatol. 2009;5(8):420–421. doi: 10.1038/nrrheum.2009.144. [DOI] [PubMed] [Google Scholar]
- 19.Deal C. Bone loss in rheumatoid arthritis: systemic, periarticular, and focal. Curr. Rheumatol. Rep. 2012;14(3):231–237. doi: 10.1007/s11926-012-0253-7. [DOI] [PubMed] [Google Scholar]
- 20.Klontzas M.E., Kenanidis E.I., MacFarlane R.J., Michail T., Potoupnis M.E., Heliotis M., Mantalaris A., Tsiridis E. Investigational drugs for fracture healing: preclinical & clinical data. Expert Opin. Investig. Drugs. 2016;25(5):585–596. doi: 10.1517/13543784.2016.1161757. [DOI] [PubMed] [Google Scholar]
- 21.Pacifici R. T cells, osteoblasts, and osteocytes: interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann. N. Y. Acad. Sci. 2016;1364:11–24. doi: 10.1111/nyas.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassem A., Henning P., Kindlund B., Lindholm C., Lerner U.H. TLR5, a novel mediator of innate immunity-induced osteoclastogenesis and bone loss. FASEB J. 2015;29(11):4449–4460. doi: 10.1096/fj.15-272559. [DOI] [PubMed] [Google Scholar]
- 23.Barrow A.D., Raynal N., Andersen T.L., Slatter D.A., Bihan D., Pugh N., Cella M., Kim T., Rho J., Negishi-Koga T., Delaisse J.M., Takayanagi H., Lorenzo J., Colonna M., Farndale R.W., Choi Y., Trowsdale J. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Invest. 2011;121(9):3505–3516. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koga T., Inui M., Inoue K., Kim S., Suematsu A., Kobayashi E., Iwata T., Ohnishi H., Matozaki T., Kodama T., Taniguchi T., Takayanagi H., Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428(6984):758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 25.Humphrey M.B., Nakamura M.C. A comprehensive review of immunoreceptor regulation of osteoclasts. Clin. Rev. Allergy Immunol. 2016;51(1):48–58. doi: 10.1007/s12016-015-8521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemeth K., Schoppet M., Al-Fakhri N., Helas S., Jessberger R., Hofbauer L.C., Goettsch C., Al-Fakhri N. The role of osteoclast-associated receptor in osteoimmunology. J. Immunol. 2011;186(1):13–18. doi: 10.4049/jimmunol.1002483. [DOI] [PubMed] [Google Scholar]
- 27.Schett G. Osteoimmunology in rheumatic diseases. Arthritis Res. Ther. 2009;11(1):210. doi: 10.1186/ar2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H. Sphingosine-1-phosphate receptor 2 regulates proinflammatory cytokine production and osteoclastogenesis. PLoS One. 2016;11(5):e0156303. doi: 10.1371/journal.pone.0156303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards C.M., Mundy G.R. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int. J. Med. Sci. 2008;5(5):263–272. doi: 10.7150/ijms.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y.H., Buhamrah A., Schneider A., Lin Y.L., Zhou H., Bugshan A., Basile J.R. Semaphorin 4D promotes skeletal metastasis in breast cancer. PLoS One. 2016;11(2):e0150151. doi: 10.1371/journal.pone.0150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musso G., Paschetta E., Gambino R., Cassader M., Molinaro F. Interactions among bone, liver, and adipose tissue predisposing to diabesity and fatty liver. Trends Mol. Med. 2013;19(9):522–535. doi: 10.1016/j.molmed.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Li J.Y., Walker L.D., Tyagi A.M., Adams J., Weitzmann M.N., Pacifici R. The sclerostin-independent bone anabolic activity of intermittent PTH treatment is mediated by T-cell-produced Wnt10b. J. Bone Miner. Res. 2014;29(1):43–54. doi: 10.1002/jbmr.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Amelio P., Sassi F. Osteoimmunology: from mice to humans. Bonekey Rep. 2016;18:5–802. doi: 10.1038/bonekey.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz P., Jørgensen N.R., Abrahamsen B. Status of drug development for the prevention and treatment of osteoporosis. Expert Opin. Drug Discov. 2014;9(3):245–253. doi: 10.1517/17460441.2014.884067. [DOI] [PubMed] [Google Scholar]
- 35.Kasagi S., Chen W. TGF-beta1 on osteoimmunology and the bone component cells. Cell Biosci. 2013;3(1):4. doi: 10.1186/2045-3701-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ginaldi L., De Martinis M., Ciccarelli F., Saitta S., Imbesi S., Mannucci C., Gangemi S. Increased levels of interleukin 31 (IL-31) in osteoporosis. BMC Immunol. 2015;16:60. doi: 10.1186/s12865-015-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa H., Mukai K., Kawano Y., Minegishi Y., Karasuyama H. Th2-inducing cytokines IL-4 and IL-33 synergistically elicit the expression of transmembrane TNF-α on macrophages through the autocrine action of IL-6. Biochem. Biophys. Res. Commun. 2012;420(1):114–118. doi: 10.1016/j.bbrc.2012.02.124. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Wu N.N., Mou Y.Q., Chen L., Deng Z.L. Inhibitory effects of recombinant IL-4 and recombinant IL-13 on UHMWPE-induced bone destruction in the murine air pouch model. J. Surg. Res. 2013;180(2):e73–e81. doi: 10.1016/j.jss.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Salem S., Gao C., Li A., Wang H., Nguyen-Yamamoto L., Goltzman D., Henderson J.E., Gros P. A novel role for interferon regulatory factor 1 (IRF1) in regulation of bone metabolism. J. Cell. Mol. Med. 2014;18(8):1588–1598. doi: 10.1111/jcmm.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raychaudhuri S.P., Raychaudhuri S.K. IL-23/IL-17 axis in spondyloarthritis-bench to bedside. Clin. Rheumatol. 2016;35(6):1437–1441. doi: 10.1007/s10067-016-3263-4. [DOI] [PubMed] [Google Scholar]
- 41.Jones D., Glimcher L.H., Aliprantis A.O. Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J. Clin. Invest. 2011;121(7):2534–2542. doi: 10.1172/JCI46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X., Alnaeeli M., Singh B., Teng Y.T. Involvement of SOCS3 in regulation of CD11c+ dendritic cell-derived osteoclastogenesis and severe alveolar bone loss. Infect. Immun. 2009;77(5):2000–2009. doi: 10.1128/IAI.01070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchwald Z.S., Kiesel J.R., DiPaolo R., Pagadala M.S., Aurora R. Osteoclast activated FoxP3+ CD8+ T-cells suppress bone resorption in vitro. PLoS One. 2012;7(6):e38199. doi: 10.1371/journal.pone.0038199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sućur A., Katavić V., Kelava T., Jajić Z., Kovačić N., Grčević D. Induction of osteoclast progenitors in inflammatory conditions: key to bone destruction in arthritis. Int. Orthop. 2014;38(9):1893–1903. doi: 10.1007/s00264-014-2386-y. [DOI] [PubMed] [Google Scholar]
- 45.Sinder B.P., Pettit A.R., McCauley L.K. Macrophages: their emerging roles in bone. J. Bone Miner. Res. 2015;30(12):2140–2149. doi: 10.1002/jbmr.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W., Zhang X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues. Mol. Med. Rep. 2015;11(5):3212–3218. doi: 10.3892/mmr.2015.3152. [review]. [DOI] [PubMed] [Google Scholar]
- 47.Charles J.F., Aliprantis A.O. Osteoclasts: more than ‘bone eaters’. Trends Mol. Med. 2014;20(8):449–459. doi: 10.1016/j.molmed.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dallas S.L., Prideaux M., Bonewald L.F. The osteocyte: an endocrine cell and more. Endocr. Rev. 2013;34(5):658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu V.W., Lymperi S., Oki T., Jones A., Swiatek P., Vasic R., Ferraro F., Scadden D.T. distinctive mesenchymal-parenchymal cell pairings govern b cell differentiation in the bone marrow. Stem Cell Rep. 2016;2016:S2213. doi: 10.1016/j.stemcr.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S.H., Das A., Chai J.C., Binas B., Choi M.R., Park K.S., Lee Y.S., Jung K.H., Chai Y.G. Transcriptome sequencing wide functional analysis of human mesenchymal stem cells in response to TLR4 ligand. Sci. Rep. 2016;6:30311. doi: 10.1038/srep30311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uccelli A., de Rosbo N.K. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann. N. Y. Acad. Sci. 2015;1351:114–126. doi: 10.1111/nyas.12815. [DOI] [PubMed] [Google Scholar]
- 52.Painter S.E., Kleerekoper M., Camacho P.M. Secondary osteoporosis: a review of the recent evidence. Endocr. Pract. 2006;12(4):436–445. doi: 10.4158/EP.12.4.436. [DOI] [PubMed] [Google Scholar]
- 53.Deal C. Bone loss in rheumatoid arthritis: systemic, periarticular, and focal. Curr. Rheumatol. Rep. 2012;14(3):231–237. doi: 10.1007/s11926-012-0253-7. [DOI] [PubMed] [Google Scholar]
- 54.Mallon P.W. Aging with HIV: osteoporosis and fractures. Curr. Opin. HIV AIDS. 2014;9(4):428–435. doi: 10.1097/COH.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 55.De Martinis M., Di Benedetto M.C., Mengoli L.P., Ginaldi L. Senile osteoporosis: is it an immune-mediated disease? Inflamm. Res. 2006;55(10):399–404. doi: 10.1007/s00011-006-6034-x. [DOI] [PubMed] [Google Scholar]
- 56.Khosla S. Pathogenesis of age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(10):1226–1235. doi: 10.1093/gerona/gls163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Amelio P. The immune system and postmenopausal osteoporosis. Immunol. Invest. 2013;42(7):544–554. doi: 10.3109/08820139.2013.822764. [DOI] [PubMed] [Google Scholar]
- 58.Weitzmann M.N., Pacifici R. T cells: unexpected players in the bone loss induced by estrogen deficiency and in basal bone homeostasis. Ann. N. Y. Acad. Sci. 2007;1116:360–375. doi: 10.1196/annals.1402.068. [DOI] [PubMed] [Google Scholar]
- 59.Rauner M., Sipos W., Thiele S., Pietschmann P. Advances in osteoimmunology: pathophysiologic concepts and treatment opportunities. Int. Arch. Allergy Immunol. 2013;160(2):114–125. doi: 10.1159/000342426. [DOI] [PubMed] [Google Scholar]
- 60.Zupan J., Jeras M., Marc J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med (Zagreb) 2013;23(1):43–63. doi: 10.11613/BM.2013.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwarz P., Jørgensen N.R., Abrahamsen B. Status of drug development for the prevention and treatment of osteoporosis. Expert Opin. Drug Discov. 2014;9(3):245–253. doi: 10.1517/17460441.2014.884067. [DOI] [PubMed] [Google Scholar]
- 62.Jones D., Glimcher L.H., Aliprantis A.O. Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J. Clin. Invest. 2011;121(7):2534–2542. doi: 10.1172/JCI46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panwar P., Søe K., Guido R.V., Bueno R.V., Delaisse J.M., Brömme D. A novel approach to inhibit bone resorption: exosite inhibitors against cathepsin K. Br. J. Pharmacol. 2016;173(2):396–410. doi: 10.1111/bph.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown J.P., Roux C., Ho P.R., Bolognese M.A., Hall J., Bone H.G., Bonnick S., van den Bergh J.P., Ferreira I., Dakin P., Wagman R.B., Recknor C. Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos. Int. 2014;25(7):1953–1961. doi: 10.1007/s00198-014-2692-7. [DOI] [PubMed] [Google Scholar]
- 65.Klontzas M.E., Kenanidis E.I., MacFarlane R.J., Michail T., Potoupnis M.E., Heliotis M., Mantalaris A., Tsiridis E. Investigational drugs for fracture healing: preclinical & clinical data. Expert Opin. Investig. Drugs. 2016;25(5):585–596. doi: 10.1517/13543784.2016.1161757. [DOI] [PubMed] [Google Scholar]
- 66.Geusens P., Lems W.F. Osteoimmunology and osteoporosis. Arthritis Res. Ther. 2011;13(5):242–257. doi: 10.1186/ar3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karmakar S., Kay J., Gravallese E.M. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum. Dis. Clin. North Am. 2010;36(2):385–404. doi: 10.1016/j.rdc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graves D.T., Oates T., Garlet G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011;3:5304. doi: 10.3402/jom.v3i0.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van den Berg W.B., Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009;5(10):549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 70.Hot A., Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann. Rheum. Dis. 2011;70(5):727–732. doi: 10.1136/ard.2010.143768. [DOI] [PubMed] [Google Scholar]
- 71.Komatsu N., Takayanagi H. Arthritogenic T cells in autoimmune arthritis. Int. J. Biochem. Cell Biol. 2015;58:92–96. doi: 10.1016/j.biocel.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Diarra D., Stolina M., Polzer K., Zwerina J., Ominsky M.S., Dwyer D., Korb A., Smolen J., Hoffmann M., Scheinecker C., van der Heide D., Landewe R., Lacey D., Richards W.G., Schett G. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007;13(2):156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 73.Hoes J.N., Bultink I.E., Lems W.F. Management of osteoporosis in rheumatoid arthritis patients. Expert Opin. Pharmacother. 2015;16(4):559–571. doi: 10.1517/14656566.2015.997709. [DOI] [PubMed] [Google Scholar]
- 74.Weitzmann MN. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica. 2013:125705. doi: 10.1155/2013/125705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weitzmann M.N. T-cells and B-cells in osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21(6):461–467. doi: 10.1097/MED.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mansoori M.N., Tyagi A.M., Shukla P., Srivastava K., Dev K., Chillara R., Maurya R., Singh D. Methoxyisoflavones formononetin and isoformononetin inhibit the differentiation of Th17 cells and B-cell lymphopoesis to promote osteogenesis in estrogen-deficient bone loss conditions. Menopause. 2016;23(5):565–576. doi: 10.1097/GME.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 77.Li H., Lu Y., Qian J., Zheng Y., Zhang M., Bi E., He J., Liu Z., Xu J., Gao J.Y., Yi Q. Human osteoclasts are inducible immunosuppressive cells in response to T cell-derived IFN-γ and CD40 ligand in vitro. J. Bone Miner. Res. 2014;29(12):2666–2675. doi: 10.1002/jbmr.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ginaldi L., Mengoli L.P., De Martinis M. Osteoporosis, Inflammation and Ageing. In: Fulop T., Franceschi C., Hirokawa K., Pawelec G., editors. Handbook on Immunosenescence: Basic Understanding and Clinical Applications. Springer-Verlag Press; 2009. pp. 1329–1352. [Google Scholar]
- 79.Föger-Samwald U., Vekszler G., Hörz-Schuch E., Salem S., Wipperich M., Ritschl P., Mousavi M., Pietschmann P. Molecular mechanisms of osteoporotic hip fractures in elderly women. Exp. Gerontol. 2016;73:49–58. doi: 10.1016/j.exger.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Tilstra J.S., Clauson C.L., Niedernhofer L.J., Robbins P.D. -κB in aging and disease. Aging Dis. 2011;2(6):449–465. [PMC free article] [PubMed] [Google Scholar]
- 81.Takayanagi H. SnapShot: Osteoimmunology. Cell Metab. 2015;21(3):502.e1. doi: 10.1016/j.cmet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Roodman G.D. Biology of osteoclast activation in cancer. J. Clin. Oncol. 2001;19(15):3562–3571. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- 83.Gkotzamanidou M., Dimopoulos M.A., Kastritis E., Christoulas D., Moulopoulos L.A., Terpos E. Sclerostin: a possible target for the management of cancer-induced bone disease. Expert Opin. Ther. Targets. 2012;16(8):761–769. doi: 10.1517/14728222.2012.697154. [DOI] [PubMed] [Google Scholar]
- 84.Sigl V., Penninger J.M. RANKL/RANK - from bone physiology to breast cancer. Cytokine Growth Factor Rev. 2014;25(2):205–214. doi: 10.1016/j.cytogfr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, Meguro H, Aburatani H, Nishimura R, Yoneda T. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J. Biol. Chem. 2008;24(283):29119–29125. doi: 10.1074/jbc.M801774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao Y., Zhou Z., de Crombrugghe B., Nakashima K., Guan H., Duan X., Jia S.F., Kleinerman E.S. Osterix, a transcription factor for osteoblast differentiation, mediates antitumor activity in murine osteosarcoma. Cancer Res. 2005;65(4):1124–1128. doi: 10.1158/0008-5472.CAN-04-2128. [DOI] [PubMed] [Google Scholar]
- 87.Monroe D.G., McGee-Lawrence M.E., Oursler M.J., Westendorf J.J. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492(1):1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DiSpirito J.R., Mathis D. Immunological contributions to adipose tissue homeostasis. Semin. Immunol. 2015;27(5):315–321. doi: 10.1016/j.smim.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cao J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madeira E., Mafort T.T., Madeira M., Guedes E.P., Moreira R.O., de Mendonça L.M., Lima I.C., de Pinho P.R., Lopes A.J., Farias M.L. Lean mass as a predictor of bone density and microarchitecture in adult obese individuals with metabolic syndrome. Bone. 2014;59:89–92. doi: 10.1016/j.bone.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Sun K., Liu J., Lu N., Sun H., Ning G. Association between metabolic syndrome and bone fractures: a meta-analysis of observational studies. BMC Endocr. Disord. 2014;14:13. doi: 10.1186/1472-6823-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kishida K., Funahashi T., Shimomura I. Adiponectin as a routine clinical biomarker. Best Pract. Res. Clin. Endocrinol. Metab. 2014;28(1):119–130. doi: 10.1016/j.beem.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 93.Cildir G., Akıncılar S.C., Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol. Med. 2013;19(8):487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Meng H.Z., Zhang W.L., Liu F., Yang M.W. Advanced glycation end products affect osteoblast proliferation and function by modulating autophagy via the receptor of advanced glycation end products/raf protein/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/ extracellular signal-regulated kinase (RAGE/Raf/MEK/ ERK) pathway. J. Biol. Chem. 2015;290(47):28189–28199. doi: 10.1074/jbc.M115.669499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khurana S., Melacarne A., Yadak R., Schouteden S., Notelaers T., Pistoni M., Maes C., Verfaillie C.M. SMAD signaling regulates CXCL12 expression in the bone marrow niche, affecting homing and mobilization of hematopoietic progenitors. Stem Cells. 2014;32(11):3012–3022. doi: 10.1002/stem.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fernandes B.S., Hodge J.M., Pasco J.A., Berk M., Williams L.J. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21–25. doi: 10.1007/s40266-015-0323-4. [DOI] [PubMed] [Google Scholar]
- 97.Mössner R., Lesch K.P. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav. Immun. 1998;12(4):249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 98.Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 2013;16(4-1):48–63. doi: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanaka K., Hirai T., Ishibashi Y., Izumo N., Togari A. Modulation of osteoblast differentiation and bone mass by 5-HT2A receptor signaling in mice. Eur. J. Pharmacol. 2015;762:150–157. doi: 10.1016/j.ejphar.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 100.Collet C., Schiltz C., Geoffroy V., Maroteaux L., Launay J.M., de Vernejoul M.C. The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J. 2008;22(2):418–427. doi: 10.1096/fj.07-9209com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yadav V.K., Oury F., Suda N., Liu Z.W., Gao X.B., Confavreux C., Klemenhagen K.C., Tanaka K.F., Gingrich J.A., Guo X.E., Tecott L.H., Mann J.J., Hen R., Horvath T.L., Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malendowicz L.K., Rucinski M., Belloni A.S., Ziolkowska A., Nussdorfer G.G. Leptin and the regulation of the hypothalamic-pituitary-adrenal axis. Int. Rev. Cytol. 2007;263:63–102. doi: 10.1016/S0074-7696(07)63002-2. [DOI] [PubMed] [Google Scholar]
- 103.Cui Y., Niziolek P.J., MacDonald B.T., Zylstra C.R., Alenina N., Robinson D.R., Zhong Z., Matthes S., Jacobsen C.M., Conlon R.A., Brommage R., Liu Q., Mseeh F., Powell D.R., Yang Q.M., Zambrowicz B., Gerrits H., Gossen J.A., He X., Bader M., Williams B.O., Warman M.L., Robling A.G. Lrp5 functions in bone to regulate bone mass. Nat. Med. 2011;17(6):684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ducy P., Karsenty G. The two faces of serotonin in bone biology. J. Cell Biol. 2010;191(1):7–13. doi: 10.1083/jcb.201006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yadav V.K., Oury F., Tanaka K.F., Thomas T., Wang Y., Cremers S., Hen R., Krust A., Chambon P., Karsenty G. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J. Exp. Med. 2011;208(1):41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Westbroek I., van der Plas A., de Rooij K.E., Klein-Nulend J., Nijweide P.J., Nijweide P.J. Expression of serotonin receptors in bone. J. Biol. Chem. 2001;276(31):28961–28968. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- 107.Meiser J., Weindl D., Hiller K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013;11(1):34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cui S., Xiong F., Hong Y., Jung J.U., Li X.S., Liu J.Z., Yan R., Mei L., Feng X., Xiong W.C. APPswe/Aβ regulation of osteoclast activation and RAGE expression in an age-dependent manner. J. Bone Miner. Res. 2011;26(5):1084–1098. doi: 10.1002/jbmr.299. [DOI] [PMC free article] [PubMed] [Google Scholar]