Abstract
Clinical and preclinical studies on attention deficit hyperactivity disorder (ADHD) show that juvenile males that are exposed to methylphenidate (MPH) show reduced risk for substance use later in life. In contrast, little is known about whether females have the same enduring treatment response to stimulants and how gonadal hormones influence their behavior later in life. Females received either a sham or 6-hydroxydopamine (6-OHDA) microinjection in the prefrontal cortex (PFC) at postnatal day (P)10. Subjects were then treated with Vehicle or MPH (2 mg/kg, p.o.) between P20–35 and tested during late adolescence/young adulthood (P60); half of these subjects underwent ovariectomy at P55 to determine hormonal influences. Females with 6-OHDA were depleted of PFC dopamine by 61% and demonstrated increased impulsive choice (delayed discounting) and preferences for cocaine-associated environments relative to control females. Both MPH and ovariectomy reduced impulsive choice and cocaine preferences in 6-OHDA females, but had no enduring effect in Sham females. Ovariectomy itself did not significantly affect impulsivity. Juvenile MPH interacted strongly with 6-OHDA to increase D4, D5, Alpha-1A, Alpha-2A, and 5-HT-1A mRNA receptor expression in the PFC. MPH alone effected D1 mRNA, while 6-OHDA increased BDNF; all markers were decreased by ovariectomy. Together, these data suggest that 6-OHDA changes in dopamine are not only relevant for ADHD-like behaviors, but their long-term modulation by treatment and the influence of cyclical differences in menstrual cycle.
Keywords: BDNF, Discounting, Dopamine, Estrogen, Impulsive choice, Norepinephrine
1. Introduction
The childhood disorder of attention deficit hyperactivity disorder (ADHD) has an average onset of seven years of age (Merikangas et al., 2010). Symptoms of ADHD include increased impulsivity, elevated novelty seeking, and insensitivity to changes in reward contingencies (Sonuga-Barke, 2003). These behaviors are risk factors for substance use disorder (SUD), which is elevated in individuals with ADHD (Kollins, 2003). The risk for SUD is two-fold higher in females with ADHD compared with males with ADHD or typical females (Biederman et al., 2006). Pre-pubertal treatment with stimulants, including methylphenidate (MPH), does not increase and may actually reduce the risk of developing SUD in some ADHD cases (Mannuzza et al., 2008; Wilens et al., 2003). Preclinical studies parallel this clinical observation and show that exposure to MPH in male juvenile rats (P20-P35) reduces drug seeking (Andersen et al., 2002b; Warren et al., 2011).
Females are rarely examined in research on ADHD in humans (Nussbaum, 2012) or in animal models, including the spontaneously hypertensive rat (SHR (Somkuwar et al., 2013)), dopamine transporter knockouts (Gainetdinov et al., 1999), and rats with dopamine depletions using 6-hydroxydopamine (6-OHDA; (Davids et al., 2002; Freund et al., 2014). 6-hydroxydopamine depletions in immature, but not mature (Breese et al., 2005), animals increase ADHD-like behaviors of hyperactivity and impair working memory (Davids et al., 2002), but see (Boyce and Finlay, 2005). Localized prefrontal (PFC) 6-OHDA depletions during development (Freund et al., 2014) may better recapitulate observations of young adults with ADHD who have decreased DOPA decarboxylase in this region (Ernst et al., 1998). Early postnatal 6-OHDA depletions in juvenile female rats increased novelty seeking and impulsivity and D1 receptors on glutamatergic cells in the PFC (Freund et al., 2014), which has been associated with elevated cocaine self-administration.
The ADHD-like risk behaviors of impulsivity and increased sensitivity to cocaine-associated environments found following PFC 6-OHDA depletions (Freund et al., 2014) can aid in determining whether early MPH treatment increases (e.g., as we found in typically developing females (Brenhouse et al., 2009)) or decreases risk behaviors in older females. Ovarian hormones may also influence the expression of lasting treatment effects on behavior and on underlying mechanisms. Similar to females with ADHD (Van Voorhees et al., 2012), estrogen facilitates drug-seeking behavior in adult rats possibly by increasing dopamine levels in the brain (Becker, 1990; Russo et al., 2003).
The biochemical underpinnings of 6-OHDA, MPH, and the effects of ovariectomy (OVX) within a developmental context have received even less attention than typical females. This study focused on dopamine, noradrenergic, and 5-HT1A receptors given their role in ADHD-like behaviors and treatment approaches. Impulsive choice is modulated by dopaminergic (Fernando et al., 2012), serotonergic (Pattij and Vanderschuren, 2008; Winstanley et al., 2006), and noradrenergic (Chamberlain et al., 2007) signaling in the PFC. MPH targets both dopaminergic and noradrenergic systems (Berridge et al., 2006) within the PFC to reduce impulsivity. Prior studies in non-lesioned male rats exposed to MPH as juveniles show an enduring decrease in D3 mRNA at P60 with no significant change in the other dopamine receptors (Andersen et al., 2008). An increase in brain-derived growth factor (BDNF) has also been observed in juvenile MPH-exposed males (Andersen and Sonntag, 2014; Simchon Tenenbaum et al., 2015). In contrast, BDNF decreased in adolescent SHR rats treated with MPH (Fumagalli et al., 2010); whether these differences are attributable to the model or age of exposure is not known. Regardless, little is known about females. Together, the current study may help fill the gap between clinical and preclinical knowledge about whether early medication exposure affects behavior and biochemistry under differing levels of gonadal hormones and PFC dopamine levels.
2. Experimental procedures
2.1. Subjects
Lactating female Sprague-Dawley rats and their litters obtained from Charles River (Worcester, MA) were housed on a 12:12 h light: dark cycle, with lights on at 06.00 h and food and water provided ad libitum. Litters with an n=10 pups and a 5/5 male/female ratio arrived on postnatal day 6 (P6) and were weaned and group-housed (4/cage) on P21. Only females were used (N=103), with one subject/litter in any individual condition. A total of 14 different litters were used, with extra females and males distributed to different studies. The timeline of the study is shown in Figure 1A. All animals were treated in accordance with the policies established by NIH and the McLean Hospital Institutional Animal Care and Use Committee.
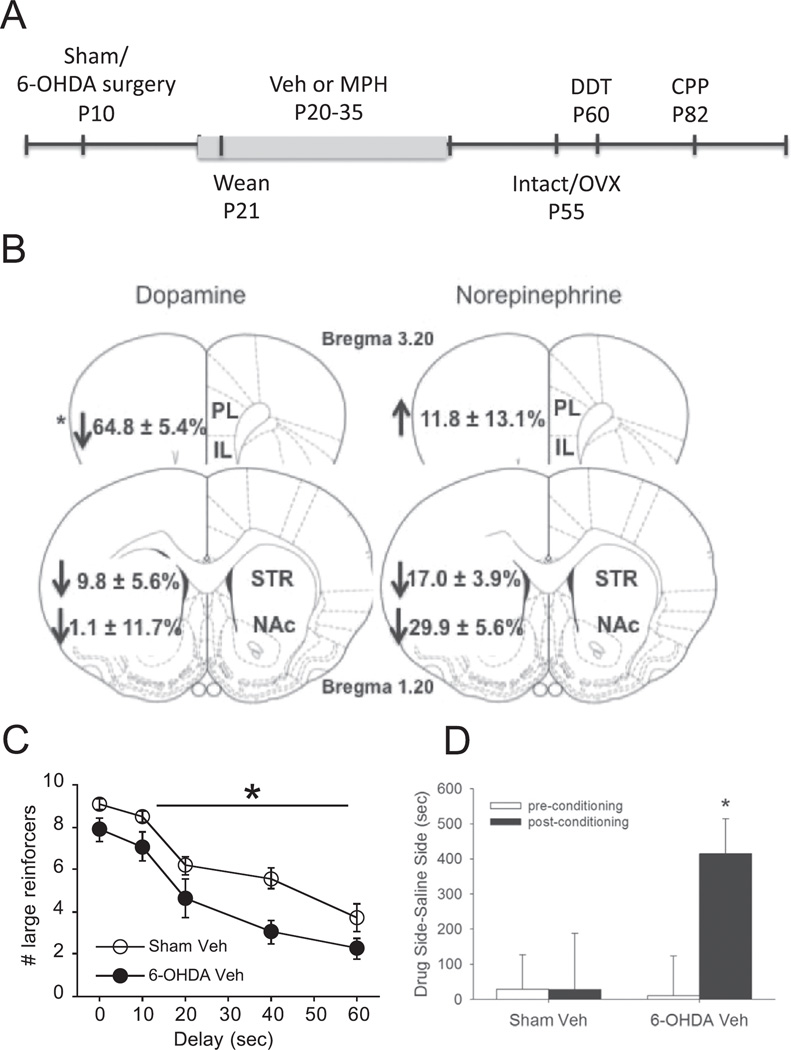
Figure 1.
(A) Timeline illustrating experimental design. (B) Percent change in DA and NE levels of 6-OHDA females relative to Shams in the prelimbic (PL) and infralimbic (IL) PFC (combined), nucleus accumbens (NAc), and striatum (STR). C left) Enduring effects of 6-OHDA plPFC lesions in females on impulsive choice; * and the line indicate the delay periods when significant differences were observed between groups; and right) place conditioning on 6-OHDA and Sham females. Means ± SE presented. *P<0.05.
2.2. Surgeries
2.2.1. 6-OHDA lesions
On P10, female rats were pretreated with desipramine (20 mg/ml in a 25 µl injection) to protect noradrenergic terminals. Subjects were anesthetized by hypothermia and bilaterally injected with 0.5 µg in 0.5 µl of either 6-hydroxydopamine (6-OHDA) or 0.9% saline into the plPFC (AP: +2.8, ML: ±0.5, DV: 2.6; following our previous methods (Freund et al., 2014)).
2.2.2. Ovariectomy (OVX)
On P55, all females that were involved in behavioral studies were injected with a ketamine/xylazine mixture (80/12 mg/kg) and underwent either sham or OVX surgery (Andersen et al., 2002a). Briefly, subjects were anesthetized with ketamine/xylazine anesthesia, and under aseptic conditions, an incision was made either at the midline. The ovaries were removed and oviduct tied off. The main incision was sutured with continuous absorbable sutures.
2.2.3. Drugs
d,l-Methylphenidate HCl (MPH) and cocaine HCl were obtained from Sigma (St Louis, MO). Each drug was dissolved in 0.9% saline (Vehicle, Veh) in a volume of 1 ml/kg.
Experiment 1. Enduring effects of MPH and ovarian hormones on delay discounting and place conditioning
Females ingested MPH (2 mg/kg) or Veh via Frootloop (General Mills) twice daily between P20–35 (Brenhouse et al., 2009). This MPH dose was based on previous preclinical studies and represents a clinically relevant dose for humans (Andersen et al., 2002b; Brenhouse et al., 2009).
2.2.4. Delay discounting
At P57, females (n=8–9/group) were food-deprived to 90% of free-feeding weight and began training at P60 to measure changes in impulsive choice in an operant chamber with a Bussey-Saksida Touch Screen (Lafayette Instruments, Lafayette, IN). Subjects underwent four phases of training to assure that each phase was learned sufficiently before the next phase following our previous protocols (Lukkes et al., 2015). Phase 1 trained subjects to initiate each session with a nose poke in the food hopper, which produced a square on one side of the touch screen. Poking the square produced one food pellet (45 mg, Dustless Precision Pellets, Bio-Serv, French-town, NJ) and subjects needed to reach a criterion of 60 rewards in 90 trials to move to Phase 2. Phase 2, initiated by a magazine nose poke, now produced two symbols on the screen that were counterbalanced across, but not within, subjects. One symbol was associated with the delivery of an immediate reward and the other with a delayed reward that was used throughout the rest of the experiment. Criterion was responding 60 of 90 trials to the delay square for two consecutive days to index learning. Phase 3 trained subjects to discriminate between larger (4 pellets) vs. smaller (1 pellet) rewards. Criterion was set at 45 responses out of 50 for the square that was the assigned delay square for two consecutive days. Phase 4 presented a block of delays comprised of 12 trials of 0,10, 20, 40 and 60 seconds in succession. The presentation of one square at the beginning of a block signaled a change in delay condition, and was followed by two forced choice trials. After a nose poke and the delivery of the reinforcer, the magazine light extinguished for 100 seconds and the next trial began with the presentation of the symbols. Data from the last 10 trials at each delay were averaged across three consecutive days or until the within-subject factor of “day” was not significant. Most subjects stabilized within 3–4 days on Phase 4.
2.2.5. Place conditioning
Following delay discounting, the same subjects in the four groups (n=8–9/group; Veh/intact; Veh/OVX; MPH/intact; MPH/OVX) were tested at P82 for unbiased place conditioning to cocaine (10 mg/kg) according to the methods of (Andersen et al., 2002b). Ten mg/kg is a threshold dose of cocaine that we have repeatedly used for determining enduring MPH effects (Andersen et al., 2002b; Brenhouse et al., 2009; Carlezon et al., 2003). The place conditioning chambers had compartments that differed in color, floor texture, and lighting and were separated by a middle compartment (Med Associates, St. Albans, VT). Screening occurred for 30 min on Day 1 and subjects that showed a bias (4 1080 seconds spent on one side) were eliminated from the experiment. During two 60 min conditioning sessions on Days 2 and 3, rats received a 1 ml/kg i.p. injection of Veh in the morning (09.00 h) and were confined to one side and four hours later, received 10 mg/kg i.p. cocaine and confined to the other side in the afternoon to avoid any possible carryover effects of cocaine. On Day 4, rats freely explored the entire apparatus for 30 min in a drug-free state. Place conditioning scores were determined as the difference of the time spent on the drug side - time spent on the saline side.
2.2.6. Dopamine and Norepinephrine enzyme-linked immunoassay (ELISA)
Ninety minutes following the onset of day 4 of CPP, subjects were rapidly decaped, brains collected, and the plPFC microdissected and then stored at −80 °C until assayed for DA and NE levels. Tissue was homogenized in 0.01 N HCl in the presence of EDTA and sodium metabisulfite. Measurement of plPFC, ilPFC, accumbens, and striatal DA and NE levels were performed using a dopamine and norepiphrine enzyme linked immunoassay kit (2-CAT [N-D] Research ELISA; Rocky Mountain Diagnostics, CO). Samples were run in duplicate and 100 µl of the extracted homogenate was used for each sample. All samples were processed according to the manufacturer’s directions.
Experiment 2. Quantitative, real time-polymerase chain reaction (qRT-PCR)
A separate set of Sham/6-OHDA females exposed to Veh or MPH were used and staged into high or low estrous states based on vaginal cytology and sacrificed. Monoamine receptor and BDNF mRNA levels were measured using qRT-PCR as previously described (Andersen et al., 2008).
2.2.7. qRT-PCR
For qRT-PCR analyses, subjects were dichotomized into high and low hormonal states based on vaginal cytology at the time of sacrifice. Based on (Emanuele et al., 2003) low levels of estrogen are found during meta- and di-estrous phases of the cycle and high estrogen levels are found during pro- and estrous phases of the cycle. Briefly, the plPFC was the main target of dissection for this analysis, and HPLC analysis confirmed that the ilPFC demonstrated significant depletion effects as well (the implications are discussed below). The total mRNA from the plPFC (n=6/group) was prepared, processed to cDNA (50 ng/ µl mRNA total mRNA equivalent), and analyzed with qRT-PCR using the IQ SYBR Green SuperMix (BioRad, Hercules, CA). Primer sequences were based on published methods and are given in Table 1 for GAPDH, BDNFtotal, D1 to D5 (Andersen et al., 2008), Alpha-1A, Alpha-2A (Sun et al., 2012), and 5-HT1A ((Perez-Garcia et al., 2006). Gene expressions were normalized to the housekeeping gene product glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCt method (Livak and Schmittgen, 2001), and plotted as a fold change relative to Sham intact Veh females.
Table 1.
Primers used to characterize monoamine receptors, BDNF, and GAPDH.
| D1-F: | 5′-AGATGACCCCCAAAGCAG-3′ | R:5’-ACGTCCTGCTCAACCTTG-3′ |
| D2-F: | 5′-CAGACCATGCCCAATGGC-3′ | R:5’-CACACCGAGAACAATGGC-3′ |
| D3-F: | 5′-AAGCGCTACTACAGCATCTGC-3′ | R:5’-GGATAACCTGCCGTTGCTGAG-3′ |
| D4-F: | 5′-CCTGATGTGTTGGGACGCCTTTC-3′ | R:5’-TGGTGTAGATGATGGGGTTGAGGG-3′ |
| D5-F: | 5′-AAAGACTGGCTTCCCTTGTGTC-3′ | R:5’-CTGATGTTTACCGTCTGCACTG-3′ |
| Alpha1A-F: | 5′-GCGAATCCAGTGTCTTCGCAG-3′ | R:5’-ACCATGTCTCTGTGCTGTCCC-3′ |
| Alpha2A-F: | 5′- CTGTTCACCGTGTTTGGCAAC-3′ | R:5’-AAAGGGAATGACCAGCGTGG-3′ |
| 5-HT1A-F: | 5′- CCAAAGAGCACCTTCCTCTG-3′ | R:5’-CTTGCGCTTTGCTTCAGC-3′ |
| BDNF total-F: | 5′- ACTCTGGAGAGCGTGAATGG- 3′ | R: 5’- TACTGTCACACACGCTCAGC- 3′ |
| GAPDH-F: | 5′-AACTCCCATTCTTCCACCTTTG-3′ | R: 5’-CCCTGTTGCTGTAGCCATATTC-3′ |
2.3. Statistical analysis
Between-subject ANOVAs (SPSS v. 22) were used to analyze the delay discounting data from Phase 4 with depletion (2: Sham/6-OHDA), treatment (2: Veh/MPH), and hormones (2: intact/OVX) were used as between-subjects measures and delay (5) and day (3) as within-subject variables. The criterion for sensitivity to delay was when delay was significant or significantly interacted with the other between-subjects variables (Mar and Robbins, 2007). All subjects displayed such a sensitivity to delay and no effect of day was present in any group. An alternative way of capturing impulsivity is to determine the average of the number of large reinforcers received across three days of testing. While similar to “K” the indifference point (Mazur, 2007), the DDT50 is the delay period when 50% of the large reinforcers are received (Lukkes et al., 2015). Place conditioning data was analyzed similarly: depletion (2: Sham/ 6-OHDA), treatment (2: Veh/MPH), and hormones (2: intact/OVX) were used as between-subjects measures and pre- and post-conditioning as a within-subject variable. Post-hoc analyses were corrected with Bonferroni and qRT-PCR. Significance was set at P<0.05. Correlational analyses with a two-tailed Pearson’s r test were conducted to determine the relationship between discounting and place conditioning scores.
3. Results
Experiment 1. Enduring effects of MPH and ovarian hormones on delay discounting and place conditioning
3.1. Depletion confirmation
Dopamine and noradrenergic levels were characterized by ELISA and revealed that dopamine in Veh subjects was: 6-OHDA: 0.12 ± 0.02 vs Sham: 0.21 ± 0.07 ng/mg wet plPFC tissue weight, similar to other reports (Boyce and Finlay 2005); depletion was also evident in the ilPFC: 6-OHDA: 0.09 ± 0.02 vs VEH: 0.38 ± 0.14 ng/mg wet tissue weight. Norepinephrine levels remained stable following 6-OHDA lesions in the plPFC (Figure 1B). The specificity of 6-OHDA to the plPFC is evidenced by the absence of effects in downstream regions of the striatum and nucleus accumbens (Figure 1B).
3.2. Delay discounting and place conditioning in the model
To establish whether the behavioral effects of neonatal PFC 6-OHDA depletions endure into adulthood (Freund et al. [2014] tested juveniles), comparisons were made between Sham Veh and 6-OHDA Veh rats for delay discounting and place conditioning behavior. Learning to discriminate larger versus smaller reinforcers in Phase 3 of the discounting task was faster in 6–4 OHDA Veh females (3.9 ± 0.9 days to reach criterion) compared to Sham Veh females (8.2 ± 0.9 days; P<0.005), suggesting that 6-OHDA did not produce learning or motivational deficits. In testing Phase 4, developmental 6-OHDA depletions increased impulsive choice compared to Sham control subjects (Figure 1C). Main effects of depletion (F1,11 =8.28, P<0.05) and [delay] (F4,44=12.82 P<0.001) indicated that 6-OHDA increased impulsive choice; [day] was not significant. Only 6-OHDA subjects demonstrated a significant place preference for cocaine-associated environments and not Sham subjects (main effect of depletion: F1,12=4.79, P<0.05) that was driven by the post-conditioning phase (Bonferroni post-hoc comparison, P<0.05; Figure 1D).
3.3. Effects of MPH and OVX on delay discounting
The interactive effects of juvenile MPH exposure and hormones on delay discounting were tested with a five-way mixed ANOVA of 2 (hormones) × 2 (treatment) × 2 (depletion) × [delay] × [day], which was not significant. A depletion × treatment interaction: (F1,47=4.94, P<0.05) and main effects of hormones (F1,47=5.34, P<0.05), depletion (F1,47=6.1, P<0.05), and [delay] (F4,188=38.8, P<0.001) were observed. Both 6-OHDA and OVX increased discounting overall, although treatment decreased discounting in the 6-OHDA animals and not the Sham controls. A main effect of [delay] indicated that subjects decreased responding to the large reinforcer as the duration of the delay grew. To better understand whether the enduring effects of juvenile MPH treatment and hormones on impulsive choice depended on the animal model, subsequent analyses were divided into 6-OHDA (Figure 2A and C) and Sham (Figure 2B and D) groups.
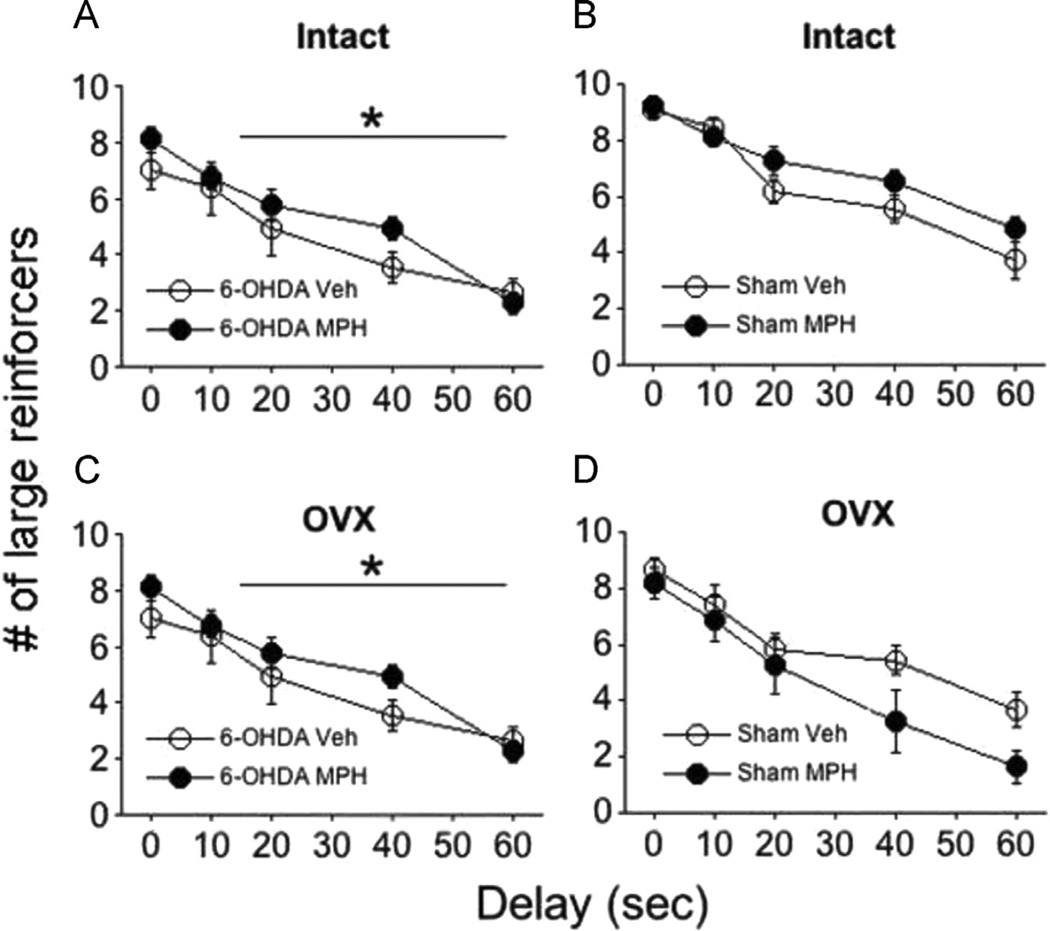
Figure 2.
Enduring effects of juvenile MPH on the number of large reinforcers during delay discounting in (A, C) intact or ovariectomized (OVX) 6-OHDA or (B, D) Sham females. The *line indicates an overall significant main effect of treatment between groups; Means ± SE presented. *P<0.05.
Within 6-OHDA subjects, a significant main effect of treatment was observed (F1,28=6.93, P<0.05). MPH increased responding for the large reinforcer in both intact and OVX 6-OHDA rats overall (Figure 2A and C). A significant main effect of [delay]: (F4,112=24.7, P<0.001) indicated that subjects selected fewer large reinforcers as the delay interval increased. Unlike 6-OHDA females, neither juvenile MPH exposure (treatment: P=0.51) nor OVX (P=0.08) effected discounting in Sham females (Figure 2B, D). However, a main effect of [delay]: (F4,72=15.87, P<0.001) was observed in Sham females similar to 6-OHDA females.
3.4. Effects of MPH and OVX on place conditioning
A four-way mixed ANOVA of 2 (hormones) × 2 (treatment) × 2 (depletion) × [conditioning] was significant (F1,49=4.58, P<0.05). The interaction between treatment × depletion × [conditioning] was also significant (F1,49=4.53, P<0.05). Conditioning overall exerted a significant main effect (F1,49=13.41, P<0.001), and pre-conditioning did not interact with any of these factors as this was a non-biased paradigm (all P’s>0.4). To better understand how 6-OHDA influenced place conditioning, analyses were divided into 6-OHDA and Sham groups.
In the 6-OHDA depletion group, a three-way significant interaction (hormones × treatment × [conditioning]: F1,29 = 6.18, P<0.05) was observed, demonstrating that MPH reduced place preferences in intact 6-OHDA subjects (Figure 3A,B). OVX reduced place preferences in both Veh and MPH treated 6-OHDA subjects to similar levels as those found in intact, MPH treated 6-OHDA females. In contrast, Sham subjects treated with MPH demonstrated significant place preferences ([conditioning]: F1,20 = 5.84, P<0.05) with no other significant interactions or main effects (Figure 3B).
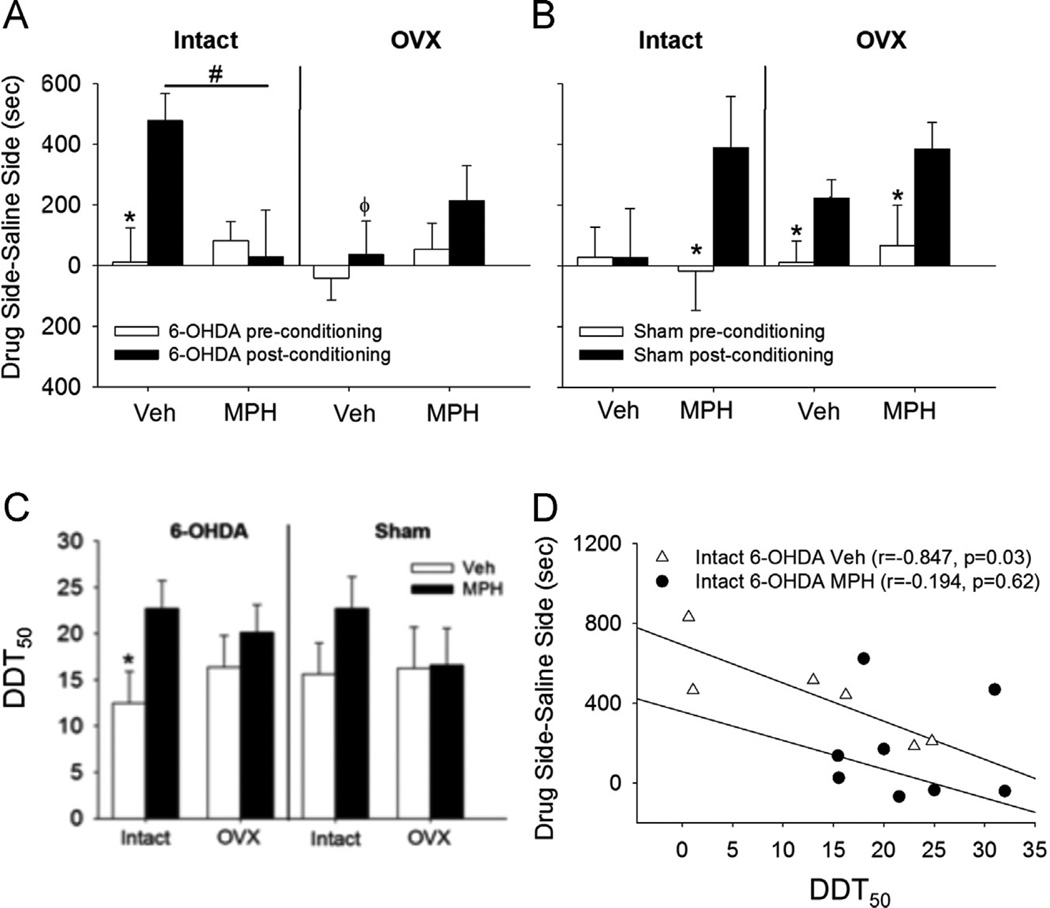
Figure 3.
Enduring effects of juvenile MPH on preferences for cocaine-associated environments in A) intact or OVX 6-OHDA or B) intact or OVX Sham females. Means ± SE presented. *P<0.05 comparison between pre- and post-conditioning; #P<0.05 comparison of post-conditioning effects of Vehvs MPH in the intact condition; and ϕP<0.05 comparison between intact and OVX inVeh6-OHDA for post-conditioning effects. C) The enduring effects of juvenile MPH on the indifference point for selecting large reinforcers in delay discounting in 6-OHDA and Sham females. *P<0.05 comparison between Vehicle and MPH of the intact 6-OHDA group; D) A negative correlation is found between the DDT50 of discounting and place preference in intact 6-OHDAVeh females (r= −0.847, P=0.03; open triangles). This correlation is absent when 6-OHDA females are exposed to MPH as juveniles (r= −0.194, P=0.62; closed circles).
3.5. Inter-relationship between delay discounting and place conditioning
We investigated the enduring effects of juvenile MPH exposure by examining the delay discounting point where the subject selected 50% of the large reinforcers (DDT50). This DDT50 was based on the non-linear fit of the relationship between delay and the number of large reinforcers received for each subject (Lukkes et al., 2015). A hormones × treatment × depletion ANOVA revealed a main effect of MPH treatment (F1,47=4.62, P<0.05) on the DDT50, independent of hormones or depletion (Figure 3C), suggesting that MPH decreased impulsivity (an elevated DDT50).
Correlations between the amount of impulsive choice (represented by DDT50) and place conditioning scores indicated a significant negative correlation in the intact 6-OHDAVehrats (r=−0.847, P<0.05; Figure 3D); these data suggest greater impulsivity (lower DDT50) was associated with greater cocaine preferences in the 6-OHDA model. MPH disrupted this relationship in intact 6-OHDA rats. Correlations in the Sham females were not significant (both P’s>0.25).
Experiment 2. Quantitative, real time-polymerase chain reaction (qRT-PCR)
An overall hormones × treatment × depletion interaction was not observed for any of the receptors studied (P’s 0.3–0.8). However, hormones × depletion interactions were observed for all receptors studied (all P’s ≤ 0.03, even following Bonferroni correction for multiple comparisons). With minor exception, 6-OHDA reduced mRNA levels for all receptors. Within the 6-OHDA group, treatment with MPH increased mRNA levels for D4, D5, Adra-1A, Adra-2A, 5-HT-1A, and BDNF. Treatment alone had no significant effect unless dopamine levels were altered either by 6-OHDA or by hormones. Higher levels of hormones increased mRNA levels in 6-OHDA females, but decreased mRNA levels in Sham females. Fold-change in mRNA expression relative to Sham intact rats and P values for all statistical analyses are presented in Table 2.
Table 2.
Fold changes in plPFC mRNA expression in Sham and 6-OHDA female rats treated with VEH or MPH.
| Low E2 (Meta- and Di-Estrous) |
High E2 (Pro- and Estrous) |
P values |
|||||
|---|---|---|---|---|---|---|---|
| VEH | MPH | VEH | MPH | OH × Tmt | Deplete × Tmt | Deplete × OH | |
| 6-OHDA | |||||||
| D1 | 0.26±0.05 | 0.31±0.06 | 0.40±0.03 | 1.43±0.22 | 0.05 | 0.154 | 0.006 |
| D2 | 0.18±0.04 | 0.24±0.05 | 0.3±0.04 | 0.66±0.15 | 0.01 | 0.110 | 0.001 |
| D3 | 0.19±0.05 | 0.14±0.03 | 0.41±0.14 | 0.65±0.16 | 0.03 | 0.100 | 0.001 |
| D4 | 0.10±0.03 | 0.15±0.04 | 0.41±0.12 | 0.78±0.14 | 0.03 | 0.008 | 0.001 |
| D5 | 0.15±0.04 | 0.23±0.06 | 0.39±0.08 | 1.02±0.24 | 0.02 | 0.040 | 0.001 |
| BDNF total | 0.53±0.09 | 0.45±0.06 | 0.62±0.05 | 0.91±0.16 | 0.002 | 0.800 | 0.02 |
| Alpha-1A | 0.04±0.01 | 0.15±0.04 | 0.22±0.06 | 0.70±0.24 | 0.004 | 0.001 | 0.001 |
| Alpha-2A | 0.09±0.02 | 0.14±0.05 | 0.29±0.07 | 0.70±0.10 | 0.006 | 0.003 | 0.001 |
| 5-HT-1A | 0.08±0.03 | 0.14±0.03 | 0.30±0.02 | 0.52±0.16 | 0.13 | 0.040 | 0.002 |
| Sham | Tmt | Deplete | OH | ||||
| D1 | 1.0±0.21 | 0.73±0.25 | 0.37±0.12 | 0.59±0.17 | 0.06 | 0.88 | 0.29 |
| D2 | 1.0±0.16 | 0.54±0.19 | 0.11±0.04 | 0.36±0.13 | 0.1 | 0.1 | 0.18 |
| D3 | 1.0±0.18 | 0.51±0.16 | 0.25±0.10 | 0.31±0.11 | 0.5 | 0.08 | 0.57 |
| D4 | 1.0±0.12 | 0.48±0.16 | 0.27±0.17 | 0.23±0.10 | 0.7 | 0.9 | 0.9 |
| D5 | 1.0±0.17 | 0.60±0.20 | 0.24±0.10 | 0.39±0.15 | 0.3 | 0.3 | 0.91 |
| BDNF total | 1.0±0.18 | 0.56±0.17 | 0.28±0.06 | 0.83 ± 0.21 | 0.4 | 0.7 | 0.82 |
| Alpha-1A | 1.0±0.19 | 0.31±0.10 | 0.13±0.05 | 0.16 ± 0.05 | 0.8 | 0.18 | 0.42 |
| Alpha-2A | 1.0±0.26 | 0.36±0.08 | 0.19±0.05 | 0.18 ± 0.07 | 0.57 | 0.14 | 0.49 |
| 5-HT-1A | 1.0±0.29 | 0.46±0.16 | 0.34±0.10 | 0.28 ± 0.09 | 0.46 | 0.02 | 0.58 |
OH=ovarian hormones; MPH=methylphenidate; Tmt=treatment; VEH=vehicle.
4. Discussion
Early developmental reductions of PFC dopamine by 6-OHDA produced enduring increases in impulsivity and place preferences for cocaine-associated environments, and altered PFC mRNA receptor expression relative to Sham rats. Such behavioral increases are consistent with behavioral symptoms associated with ADHD, which include impaired discounting and increased risk for SUD that are related to hypofunction in PFC regions (Sonuga-Barke, 2003). Moreover, our study is the first to show that juvenile MPH treatment permanently reduced these behaviors in adult 6-OHDA females, in line with clinical findings of reduced substance use following treatment (Klassen et al., 2012). Reduced place preferences in MPH treated 6-OHDA rats are opposite to our previous findings in MPH treated typical adolescent rats (Brenhouse et al., 2009) or Sham rats in the current study, suggesting that treatment studies (at least in females) maybe more appropriate in subjects demonstrating an ADHD-like phenotype. The observation that MPH effectiveness depends on baseline dopamine levels is consistent with clinical observations (Volkow et al., 2005). Low PFC dopamine also led to an increase in the saliency of cocaine-associated environments. The more impulsive a female was, the more preference she had for cocaine-associated cues, but only in the 6-OHDA subjects. Juvenile MPH exposure disrupted the relationship between impulsive choice and cocaine preferences, consistent with clinical findings of reduced SUD in individual treated before puberty (Mannuzza et al., 2008). This increased sensitivity to the rewarding effects of cocaine in 6-OHDA rats did not manifest until later in life (this study) as our juvenile characterizations of 6-OHDA lesions produced no increase in cocaine place conditioning (Freund et al., 2014). SUD and its modulation by MPH does not manifest until adolescence, in concert with typical rising rates of drug use by teenagers (Wilens et al., 2003).
Notwithstanding, another major contribution of this study is the demonstration that the enduring effects of MPH interact with gonadal hormones to alter behavior and receptors. Ovarian hormones, especially estrogen, have pro-dopaminergic effects (Becker et al., 1984) that could reduce impulsivity much like MPH if the conditions were right. Hypotheses about how MPH reduces ADHD symptoms involve both modulation of autoreceptor activity to modulate dopamine overflow and a change in the signal-to-noise ratio of corticostriatal afferents (Volkow et al., 2005). Methylphenidate increases tonic dopamine levels that dampen noise while amplifying the phasic signal of corticostriatal afferents (Grace, 2000). When dopamine levels are low in 6-OHDA females, OVX further increased impulsivity (P=0.08) and attenuated place preferences for cocaine. Under these lower dopamine conditions, the enduring effects of MPH are even more effective (e.g., increasing the DDT50; reducing place preferences). Together, developmental 6-OHDA depletions in females increase ADHD-like behaviors into adulthood that are sensitive to early intervention with MPH and manipulation of ovarian hormones levels.
Previous studies show that developmental 6-OHDA manipulations often produce downstream effects that are generally the opposite of adult 6-OHDA depletions. For example, global 6-OHDA depletions in adulthood result in motor impairment (Breese et al., 1987). In contrast, global 6-OHDA dopamine depletions in juveniles increase other ADHD-like behaviors of hyperactivity and working memory deficits (Davids et al., 2002). More localized developmental 6-OHDA depletions in the PFC increase activity in males (but see (Boyce and Finlay, 2005)), but does not alter activity in females, and reduces drug nicotine self-administration later in life in females relative to controls (Rezvani et al., 2008). The current study shows that PFC 6-OHDA permanently increases impulsive choice that was initially observed in juvenile females (Freund et al., 2014). These results are similar to direct 6-OHDA lesions into the plPFC in adulthood, which reduce outcome evaluation in goal-directed responding (Balleine and Dickinson, 1998; Lex and Hauber, 2010). While direct depletion of the region of interest in adulthood is well-suited for testing the role of dopamine, develop-mentally, lesions of one region are likely to have subsequent effects on other brain regions (Pycock et al., 1980). The apparent lack of specificity of 6-OHDA that also reduced dopamine in the ilPFC may have to do with the spreading of developmental lesions (Teicher et al., 1998). Our previous study targeted and ‘hit’ the plPFC with 6-OHDA selectively (Freund et al., 2014). However, lesions with 6-OHDA are not always straight-forward. Rather, depletions that were performed under the identical situations can range from no depletion to a significant loss of dopamine. We have previously observed that an initial 6-OHDA lesion in the striatum progressively spread into a loss of dopamine in the nucleus accumbens with further maturation (Teicher et al., 1998). The same phenomenon may have occurred here with spreading from the plPFC to the ilPFC. Whether both plPFC and ilPFC are involved in these behaviors cannot be ruled out, although evidence shows plPFC involvement in delay discounting (Loos et al., 2009; Sonntag et al., 2014). However, all of the regional dissections for mRNA analysis were from plPFC. Other research models of ADHD-like behaviors have a delayed onset of behavioral impairment during adolescence (e.g., (Somkuwar et al., 2013)), whereas ADHD typically appears during childhood.
Neonatal 6-OHDA dopamine depletions affect more than just dopamine (Broaddus and Bennett, 1990), and changes have been found in glutamate (de Azeredo et al., 2010) and GABA receptors (Podkletnova et al., 2000). Our findings significantly expand this literature by further showing how PFC 6-OHDA depletions strongly interact with early treatment and later hormone levels to modulate receptor mRNA. Depletion with 6-OHDA significantly affected 5-HT-1A receptors, which also interacted with both treatment and hormone levels. The role of 5-HT-1A is consistent with previous findings of their role in impulsivity (Winstanley et al., 2003). The interaction of 6-OHDA with hormonal status increased D3 mRNA at a trend level in the high ovarian hormone state, whereas juvenile MPH exposure in typical, non-lesioned male rats reduced PFC D3 mRNA (Andersen and Sonntag, 2014). These findings indicate that dopamine levels are integral for varying levels of expression. 6-OHDA depletions also interacted with juvenile MPH to increase D4, D5, Alpha-1A, Alpha-2A, 5-HT-1A, and BDNF mRNA relative to Sham controls also exposed to MPH. These increases following MPH in 6-OHDA females under high hormone conditions implicate these receptors in decreased impulsivity and place conditioning in 6-OHDA females. MPH-induced increases in D4, D5, and Alpha1A in 6-OHDA subjects may work by enhancing GABAergic activity. The D4 polymorphism reduces the inhibitory function and has been associated with increased risk taking behavior (LaHoste et al., 1996). An increase in D4 following MPH could facilitate GABA functions in the PFC (Zhong and Yan, 2014), similar to D5, which is localized on PFC pyramidal neurons, but is also found on ~75% of parvalbumin-immunopositive GABA inter-neurons (Oda et al., 2010). Increased Alpha-2A may increase cortical excitability by disinhibition (Andrews and Lavin, 2006). Changes in Alpha-1A could reduce place preferences, although previous research in adult animals shows no effect of Alpha-1A formation of a place preference (reviewed in (Schmidt and Weinshenker, 2014)).
Juvenile versus adult MPH exposure produces opposite effects in typical animals in BDNF (Andersen, 2005), although 6-OHDA and hormonal state alters that relationship. Reduced PFC BDNF is associated with greater cocaine-seeking and reinstatement (reviewed in (Barker et al., 2015)), which is consistent with increased place preferences in our 6-OHDA females. Similarly, increased BDNF in the plPFC decreases drug-taking behavior in adult male rats (Berglind et al., 2007); juvenile MPH in 6-OHDA females increased BDNF and reduced cocaine place preferences. Overall, little work has examined the three way interaction of MPH (or cocaine) × BDNF × estrogen. Treatment with 17β-estradiol in intact adult females facilitates extinction of cocaine taking behavior (Larson and Carroll, 2007). Ours is the first study to show that elevated ovarian hormones may work through increased BDNF to reduce drug seeking. Indeed, the gene for BDNF contains an estrogen response element providing a mechanism where estrogen can easily increase BDNF (Harte-Hargrove et al., 2013). Here, both MPH treatment and 6-OHDA interacted with elevated ovarian hormones to increase BDNF levels that reduced impulsivity and cocaine preferences.
Changes in D1 mRNA were evident in response to MPH, regardless of depletion state. High delay discounting is found in mice with elevated PFC D1/D5 receptors (Loos et al., 2009) or following viral-mediated overexpression of D1 on plPFC glutamatergic neurons (Sonntag et al., 2014). Prefrontal cortex 6-OHDA depletions reduced D1 mRNA and increased discounting relative to Shams. Our previous study in early 6-OHDA depleted juvenile females localized an increase in D1 to plPFC projection neurons to the accumbens (Freund et al., 2014). However, D1 mRNA from tissue cannot specifically localize these D1 receptors to projection neurons. The observation that MPH increased D1 and reduced discounting raises the possibility that these D1 receptors may be on non-projection neurons.
Finally, assessment of ovarian hormones by estrous cycle staging fully illustrates how different hormone states not only influence behavior as discussed above, but also receptor mRNA and the expression of treatment effect. Developmental reductions in PFC dopamine levels minimized the effect of MPH under low ovarian hormone conditions overall, consistent with place preferences following OVX in females (Russo et al., 2003). In the current study, we used 10 mg/kg dose of cocaine as a threshold dose and did not observe a significant place preference in our Sham-operated females. In non-lesioned females (with no PFC manipulation), others have found significant, but rather modest, place preferences to 10 mg/kg cocaine (Crawford et al., 2011) or less. For example, pre/post conditioning difference of ~250 sec was reported in one study (Bobzean et al., 2014) and a 37 s difference from a hypothesized difference of 0 at baseline reported in another study (Grotewold et al., 2014). However, this dose highlighted the increased sensitivity to cocaine-associations in the 6-OHDA females.
Depletion with 6-OHDA facilitated MPH-induced receptor mRNA expression changes when ovarian hormone levels were high. Previous studies found that estrogen increases dopamine release in the striatum of adult females by decreasing the affinity of the dopamine transporter (Becker, 1990) and dopamine receptor expression in vitro. Estrogen has neuroprotective effects also against dopamine cell loss (Baquet et al., 2005). An additional possible mechanism could involve estrogenic effects on GABA neurons. Increases in estrogen produce a decrease in GABA transmission (Almey et al., 2016), which could also explain the findings hormones × treatment interaction in the Sham females. In the intact females, estrogen would decrease GABA, and treatment reverses the effects via the receptors as discussed above. Estrogen manipulations have no apparent effect on PFC 5-HT-1A mRNA in the PFC (Landry and Di Paolo, 2003), although 6-OHDA may have unmasked a subtle influence. D1 and D5 dopamine receptors were also elevated in high ovarian hormone states, as shown previously (Frye and Vongher, 1999). Juvenile MPH in 6-OHDA females increased D1, D5, and Alpha-2A receptor mRNA expression during high ovarian hormone levels. While estrogen has received the most attention, progesterone reduces drug craving (Fox et al., 2013) and may also be involved. As the menstrual cycle likely influences acute treatment response, our data suggest the stage of cycle will also influence enduring treatment effects.
The results of this study demonstrate the importance of dopamine levels for the effectiveness of MPH on behavior in female rats. Early postnatal 6-OHDA lesions localized to the PFC resulted in low dopamine levels later in life. Depleted females demonstrated increased delay discounting and a greater sensitivity to cocaine-associated environments relative to Sham control females. These effects were reduced by juvenile MPH exposure, but not in Sham females. Relevant changes in dopamine, serotonin, and noradrenergic receptors also reflect both MPH and ovarian hormone levels. Future studies from our lab will determine the location of which cortical neuronal phenotype these receptor changes occur to better understand these ADHD-relevant findings in females.
Acknowledgments
The authors acknowledge the expert pipetting skills of Dr. Kai Sonntag.
Role of the funding source
Funding for this study was provided by NIDA Grant DA-015403 and MH-091114; Neither NIDA no NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
Authors Lukkes and Andersen designed the study, wrote the protocol, and managed the literature searches and analyses. Author Andersen undertook the statistical analysis, and author Lukkes wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
The authors have no conflict of interest to declare.
References
- Almey A, Milner TA, Brake WG. Estrogen receptor alpha and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum. Neurosci. Lett. 2016;622:118–123. doi: 10.1016/j.neulet.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S, Thompson A, Krenzel E, Teicher M. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002a;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol. Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Sonntag KC. Juvenile methylphenidate reduces prefrontal cortex plasticity via D3 receptor and BDNF in adulthood. Front. Synaptic Neurosci. 2014;6:1. doi: 10.3389/fnsyn.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Napierata L, Brenhouse HC, Sonntag KC. Juvenile methylphenidate modulates reward-related behaviors and cerebral blood flow by decreasing cortical D3 receptors. Eur. J Neurosci. 2008;27:2962–2972. doi: 10.1111/j.1460-9568.2008.06254.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat. Neurosci. 2002b;5:1314. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31:594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J. Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, De Vries TJ, Peters J. Brain-derived neurotrophic factor and addiction: pathological versus therapeutic effects on drug seeking. Brain Res. 2015;1628:68–81. doi: 10.1016/j.brainres.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME, Robinson TE. Striatal dopamine release stimulated by amphetamine or potassium: influence of ovarian hormones and the light-dark cycle. Brain Res. 1984;311:157–160. doi: 10.1016/0006-8993(84)91410-0. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur. J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Klein KL, Price JE, Faraone SV. Psychopathology in females with attention-deficit/hyperactivity disorder: a controlled, five-year prospective study. Biol. Psychiatry. 2006;60:1098–1105. doi: 10.1016/j.biopsych.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, Dennis TS, Perrotti LI. Acute estradiol treatment affects the expression of cocaine-induced conditioned place preference in ovariectomized female rats. Brain Res. Bull. 2014;103:49–53. doi: 10.1016/j.brainresbull.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce PJ, Finlay JM. Neonatal depletion of cortical dopamine: effects on dopamine turnover and motor behavior in juvenile and adult rats. Brain Res. Dev. Brain Res. 2005;156:167–175. doi: 10.1016/j.devbrainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Breese GR, Duncan GE, Napier TC, Bondy SC, Iorio LC, Mueller RA. 6-hydroxydopamine treatments enhance behavioral responses to intracerebral microinjection of D1- and D2-dopamine agonists into nucleus accumbens and striatum without changing dopamine antagonist binding. J. Pharmacol. Exp. Ther. 1987;240:167–176. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Criswell HE, Moy SS, Papadeas ST, Blake BL. The neonate-6-hydroxydopamine-lesioned rat: a model for clinical neuroscience and neurobiological principles. Brain Res. Brain Res. Rev. 2005;48:57–73. doi: 10.1016/j.brainresrev.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Napierata L, Kussmaul L, Leussis M, Andersen SL. Juvenile methylphenidate exposure and factors that influence incentive processing. Dev. Neurosci. 2009;31:95–106. doi: 10.1159/000207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP., Jr Postnatal development of striatal dopamine function. II. Effects of neonatal 6-hydroxydopamine treatments on D1 and D2 receptors, adenylate cyclase activity and presynaptic dopamine function. Brain Res. Dev. Brain Res. 1990;52:273–277. doi: 10.1016/0165-3806(90)90245-t. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol. Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Robbins TW, Sahakian BJ. The neurobiology of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61:1317–1319. doi: 10.1016/j.biopsych.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Baella SA, Farley CM, Herbert MS, Horn LR, Campbell RH, Zavala AR. Early methylphenidate exposure enhances cocaine self-administration but not cocaine-induced conditioned place preference in young adult rats. Psychopharmacology. 2011;213:43–52. doi: 10.1007/s00213-010-2011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology. 2002;160:92–98. doi: 10.1007/s00213-001-0962-5. [DOI] [PubMed] [Google Scholar]
- de Azeredo LA, Marquardt AR, Frazzon AP, Barros HM. Cocaine reverses the changes in GABAA subunits and in glutamic acid decarboxylase isoenzymes mRNA expression induced by neonatal 6-hydroxydopamine. Behav. Pharmacol. 2010;21:343–352. doi: 10.1097/FBP.0b013e32833b33af. [DOI] [PubMed] [Google Scholar]
- Emanuele M, Wezeman F, Emanuele N. Alcohol’s effects on female reproductive function. Alcohol Res. Health 27 (online) 2003 [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J. Neurosci. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, Caprioli D, Moreno M, Hipolito L, Aspinall AT, Robbins TW, Dalley JW. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology. 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013;38:1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund N, MacGillivilray HT, Thompson BS, Lukkes JL, Stanis JJ, Brenhouse HC, Andersen SL. Sex-dependent changes in ADHD-like behaviors in juvenile rats following cortical dopamine depletion. Behav. Brain Res. 2014;270:357–363. doi: 10.1016/j.bbr.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. GABA(A), D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav. Brain Res. 1999;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Cattaneo A, Caffino L, Ibba M, Racagni G, Carboni E, Gennarelli M, Riva MA. Sub-chronic exposure to atomoxetine up-regulates BDNF expression and signalling in the brain of adolescent spontaneously hypertensive rats: comparison with methylphenidate. Pharmacol. Res. 2010;62:523–529. doi: 10.1016/j.phrs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Grace A. Psychostimulant actions on dopamine and limbic system function: relvance to pathophysiology and treatment of ADHD. In: Solanto M, Arnsten A, Castellanos F, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University; 2000. [Google Scholar]
- Grotewold SK, Wall VL, Goodell DJ, Hayter C, Bland ST. Effects of cocaine combined with a social cue on conditioned place preference and nucleus accumbens monoamines after isolation rearing in rats. Psychopharmacology. 2014;231:3041–3053. doi: 10.1007/s00213-014-3470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Maclusky NJ, Scharfman HE. Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen LJ, Bilkey TS, Katzman MA, Chokka P. Comorbid attention deficit/hyperactivity disorder and substance use disorder: treatment considerations. Curr. Drug. Abus. Rev. 2012;5:190–198. doi: 10.2174/1874473711205030190. [DOI] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict. Behav. 2003;28:1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, Kennedy JL. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol. Psychiatry. 1996;1:121–124. [PubMed] [Google Scholar]
- Landry M, Di Paolo T. Effect of chronic estradiol, tamoxifen or raloxifene treatment on serotonin 5-HT1A receptor. Brain Res. Mol. Brain Res. 2003;112:82–89. doi: 10.1016/s0169-328x(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen’s effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Lex B, Hauber W. The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning. Cereb. Cortex. 2010;20:873–883. doi: 10.1093/cercor/bhp151. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C(T))method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, Spijker S, van Gaalen MM. Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb. Cortex. 2009;20:1064–1070. doi: 10.1093/cercor/bhp167. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Thompson BS, Freund N, Andersen SL. The developmental inter-relationships between activity, novelty preferences, and delay discounting in male and female rats. Dev. Psychobiol. 2015;58:231–242. doi: 10.1002/dev.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, 3rd, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am. J. psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar AC, Robbins TW. Delay discounting and impulsive choice in the rat. Current protocols in neuroscience/editorial board, Jacqueline N. Crawley … [et al.] Chapter 8, Unit 8. 2007:22. doi: 10.1002/0471142301.ns0822s39. [DOI] [PubMed] [Google Scholar]
- Mazur JE. Rats’ choices between one and two delayed reinforcers. Learn. Behav. 2007;35:169–176. doi: 10.3758/bf03193052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics. 2010;125:75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum NL. ADHD and female specific concerns: a review of the literature and clinical implications. J. Atten. Disord. 2012;16:87–100. doi: 10.1177/1087054711416909. [DOI] [PubMed] [Google Scholar]
- Oda S, Funato H, Adachi-Akahane S, Ito M, Okada A, Igarashi H, Yokofujita J, Kuroda M. Dopamine D5 receptor immunoreactivity is differentially distributed in GABAergic interneurons and pyramidal cells in the rat medial prefrontal cortex. Brain Res. 2010;1329:89–102. doi: 10.1016/j.brainres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol. Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia G, Gonzalez-Espinosa C, Meneses A. An mRNA expression analysis of stimulation and blockade of 5-HT7 receptors during memory consolidation. Behav. Brain Res. 2006;169:83–92. doi: 10.1016/j.bbr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Podkletnova I, Makela R, Korpi ER, Luddens H, Helen P, Alho H. Neonatal 6-hydroxydopamine treatment affects GABA(A) receptor subunit expression in the frontal cortex but not the hippocampus of rats during postnatal development. Dev. Neurosci. 2000;22:296–302. doi: 10.1159/000017453. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Carter CJ, Kerwin RW. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J. Neurochem. 1980;34:91–99. doi: 10.1111/j.1471-4159.1980.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154:885–897. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Schmidt KT, Weinshenker D. Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Mol. Pharmacol. 2014;85:640–650. doi: 10.1124/mol.113.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchon Tenenbaum Y, Weizman A, Rehavi M. The impact of chronic early administration of psychostimulants on brain expression of BDNF and other neuroplasticity-relevant proteins. J. Mol. Neurosci.: MN. 2015;57:231–242. doi: 10.1007/s12031-015-0611-9. [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Jordan CJ, Kantak KM, Dwoskin LP. Adolescent atomoxetine treatment in a rodent model of ADHD: effects on cocaine self-administration and dopamine transporters in frontostriatal regions. Neuropsychopharmacology. 2013;38:2588–2597. doi: 10.1038/npp.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC, Brenhouse HC, Freund N, Thompson BS, Puhl M, Andersen SL. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: Comparison with adolescents. Psychopharmacology. 2014;231:1615–1626. doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci. Biobehav. Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Campbell A, Gelbard HA, Baldessarini RJ. Progressive accumbens degeneration after neonatal striatal 6- hydroxydopamine in rats. Neurosci. Lett. 1998;247:99–102. doi: 10.1016/s0304-3940(98)00281-x. [DOI] [PubMed] [Google Scholar]
- Van Voorhees EE, Mitchell JT, McClernon FJ, Beckham JC, Kollins SH. Sex, ADHD symptoms, and smoking outcomes: an integrative model. Med. Hypotheses. 2012;78:585–593. doi: 10.1016/j.mehy.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Warren BL, Iniguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, Bolanos-Guzman CA. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J. Neurosci. 2011;31:10347–10358. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin. Psychol. Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology. 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Zhong P, Yan Z. Distinct physiological effects of dopamine D4 receptors on prefrontal cortical pyramidal neurons and fast-spiking interneurons. Cereb. Cortex. 2014;26:180–191. doi: 10.1093/cercor/bhu190. [DOI] [PMC free article] [PubMed] [Google Scholar]