Abstract
The specification of distinct neural cell types in central nervous system development crucially depends on positional cues conferred to neural stem cells in the neuroectoderm. Here, we investigate the regulation and function of the epidermal growth factor receptor (EGFR) signalling pathway in early development of the Drosophila brain. We find that localized EGFR signalling in the brain neuroectoderm relies on a neuromere-specific deployment of activating (Spitz, Vein) and inhibiting (Argos) ligands. Activated EGFR controls the spatially restricted expression of all dorsoventral (DV) patterning genes in a gene- and neuromere-specific manner. Further, we reveal a novel role of DV genes—ventral nervous system defective (vnd), intermediate neuroblast defective (ind), Nkx6—in regulating the expression of vein and argos, which feed back on EGFR, indicating that EGFR signalling stands not strictly atop the DV patterning genes. Within this network of genetic interactions, Vnd acts as a positive EGFR feedback regulator. Further, we show that EGFR signalling becomes dependent on single-minded-expressing midline cells in the posterior brain (tritocerebrum), but remains midline-independent in the anterior brain (deuto- and protocerebrum). Finally, we demonstrate that activated EGFR controls the proper formation of brain neuroblasts by regulating the number, survival and proneural gene expression of neuroectodermal progenitor cells. These data demonstrate that EGFR signalling is crucially important for patterning and early neurogenesis of the brain.
Keywords: dorsoventral patterning genes, epidermal growth factor receptor, rhomboid, vein, argos, brain neuroblasts
1. Introduction
The central nervous system in insects and mammals arises from multipotent neural stem cells that give rise to a vast array of distinct cell types. The underlying molecular genetic mechanisms have been extensively studied in the developing embryonic truncal nervous system (reviewed in [1,2]). In Drosophila, the ventral nerve cord (VNC) is generated by segmental arrays of neural stem cells, called neuroblasts, which delaminate from the truncal neuroectoderm (NE). Each neuroblast acquires a unique identity that is reflected by the typical developmental time point and position, and the combination of developmental control genes it expresses [3], which finally determines the number and types of progeny it generates [4,5]. The identity of each neuroblast is initially determined by the combinatorial code of positional cues in the NE, provided by the products of early patterning genes (reviewed in [1]).
A group of genes essentially involved in patterning of the VNC is the evolutionary conserved dorsoventral (DV) genes. Their activity subdivides the NE along the DV axis into longitudinal columns: ventral nervous system defective (vnd) is expressed in the ventral, intermediate neuroblasts defective (ind) in the intermediate and muscle segment homeobox (msh; Drop [Dr], FlyBase) in the dorsal neuroectodermal column, where they control the formation of neuroblasts (except msh) and specify aspects of their fate [6–16]. The expression domains of DV genes and, accordingly, the DV boundaries of the NE are regulated by the graded activity of the nuclear factor Dorsal and Bone morphogenetic protein [17–19]. Another signalling pathway that controls the regionalized expression of DV genes is the epidermal growth factor receptor (EGFR) pathway [17,20,21], which is highly conserved from fly to human (reviewed in [22,23]). The EGFR pathway is required to induce ind expression and to maintain vnd expression [17,24,25], and thus to control the formation of intermediate and the identity of ventral neuroblasts [20,24]. Localized EGFR activation depends on the neuregulin-like ligand Vein (Vn) and the TGF-α homologue Spitz (Spi) [26–28]. Spi is processed and secreted by the combined activity of the transmembrane protease Rhomboid (Rho) and the chaperone Star (S), and serves as the cardinal activating ligand [28–31]. Expression of rho is tightly controlled and represents the key to the dynamic activation of EGFR, whereas the inactive Spi precursor and S are rather broadly expressed in the NE [32–35]. The spatio-temporal pattern of EGFR activity also depends on the inhibiting ligand Argos (Aos), which is induced in response to high levels of EGFR activity [36], and antagonizes EGFR activation by sequestering Spi [27,37–39]. Although EGFR signalling is initially induced by ligands (Spi, Vn) expressed and secreted from the ventral NE [20,26,27,40], by gastrulation EGFR signalling becomes dependent on Spi, which is secreted from the ventral midline [20,35,41,42].
Similar to the situation in the trunk, it has been shown that EGFR signalling is required in midline cells of the embryonic head, which follow a particular mode of neurogenesis to give rise to the larval visual system, stomatogastric nervous system and most medial parts of the brain [43–46]. However, the regulation of EGFR signalling and its role in neuroectodermal patterning and specification of cell fate along the DV axis are not well understood in the early embryonic brain. The NE of the embryonic brain gives rise to an array of about 100 neuroblasts in each hemisphere, which can be subdivided (from anterior to posterior) into the presumptive proto- (PC), deuto- (DC) and tritocerebrum (TC) [47,48]. Based on a distinct combination of regulatory genes expressed, each brain neuroblast acquires a unique identity [49], which suggests that the expression of patterning genes in the overlying NE has to be precisely controlled during neuroblast formation. In previous reports, we showed that the regionalized expression of DV genes exhibits neuromere-specific differences in the NE and neuroblasts of the embryonic brain [50,51]. We then uncovered a genetic network in which evolutionarily conserved factors encoded by DV genes (vnd, ind, msh, Nkx6) and AP patterning genes (empty spiracles, engrailed) closely interact to properly pattern the NE and specify neuroblast identity along the DV axis of the brain [52,53].
In this study, we shed light on the regulation and function of EGFR signalling in early embryonic brain development. We show that a neuromere-specific deployment of activating (Spi, Vn) and inhibiting (Aos) ligands controls the localized activation of EGFR in the brain NE, which, in turn, is necessary for the spatially restricted expression of all DV genes. We also show that DV genes (vnd, Nkx6, ind) regulate the expression of vn and aos, which indicates that within the genetic network EGFR stands not strictly atop the DV genes. Moreover, Single-minded (Sim), a master regulator of CNS midline cells [54], is needed for EGFR signalling specifically in TC, but not in the anterior brain (DC, PC). Finally, we show that activated EGFR promotes the formation of brain neuroblasts, as it controls the number, survival and proneural gene expression of neuroectodermal progenitor cells. Thus, EGFR signalling plays a central role in DV patterning and early neurogenesis of the fly brain.
2. Results
2.1. The pattern of EGFR activity compared with DV gene expression in the early brain
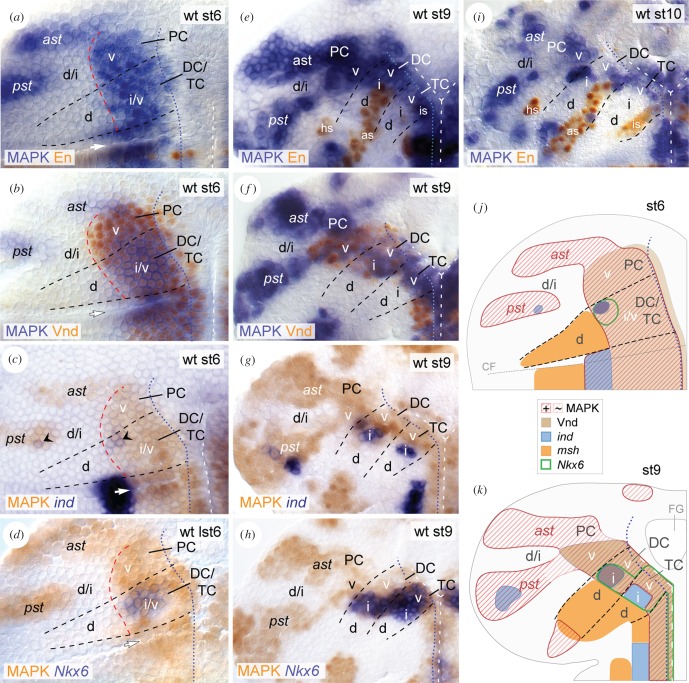
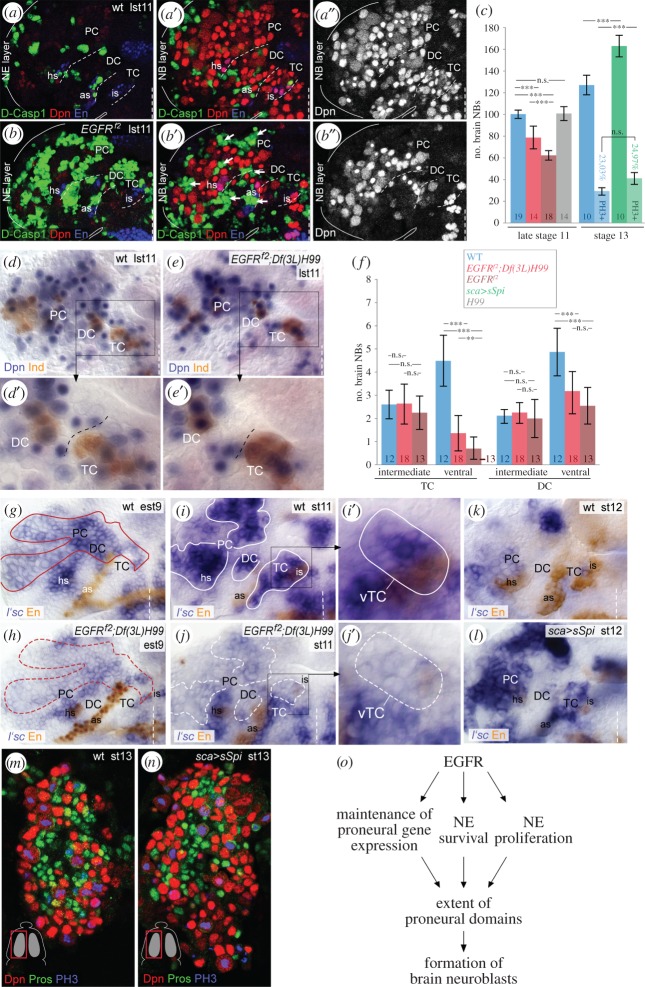
To test whether EGFR controls DV patterning in the early brain, we first compared the activation pattern of EGFR with the known expression patterns of the DV genes vnd, ind, Nkx6 and msh [52,53]. We visualized EGFR activity in the brain NE by using an antibody against double-phosphorylated (activated) MAPK [27]. Additionally, the segmental marker Engrailed (En) was used to delineate the borders between brain neuromeres (according to [48,50]). MAPK is initiated at stage 5 in a broad longitudinal stripe (data not shown), which by stage 6 precisely overlaps with expression of vnd, and includes expression of ind and Nkx6 (figure 1a–d,j). Thus, EGFR signalling in the presumptive TC and DC is confined to the ventral and intermediate NE (and ventrally adjacent mesectoderm). In the presumptive PC, MAPK overlaps with Vnd in the ventral NE, but is additionally detected in two large stripes in the intermediate/dorsal NE (the ‘anterior stripe’ and ‘posterior stripe’; according to [45]). By stage 9, MAPK is kept at strong levels in PC, DC and ventral TC, but is largely vanished from the intermediate TC (figure 1e–h,k). Interestingly, ind expression, which depends on EGFR in the developing VNC [17], is not initiated in the intermediate TC before MAPK has vanished (figure 1g), unlike in PC and DC (figure 1c). From early stage 10 onwards, MAPK becomes confined to smaller cell clusters in the brain NE (figure 1i). During stages 5–11, MAPK remains complementarily expressed to msh (figure 1j,k; electronic supplementary material, figure S1a,b). EGFR is therefore not active in the dorsal NE of TC and DC. During stages 8–11, MAPK is also transiently detected in subsets of neuroblasts that mostly develop from the MAPK-positive brain NE (electronic supplementary material, figure S2a,b).
Figure 1.
MAPK pattern in the brain NE. (a–i) Flat preparations displaying the wild-type (wt) head ectoderm of the left hemisphere at stage 6 (st6) (a–d), stage 9 (st9) (e–h), and stage 10 (st10) (i); anterior is up. MAPK pattern is combined with En (a,e,i), Vnd (b,f), ind (c,g) and Nkx6 (d,h). (c,g) ind expression in DC/PC (black arrowheads in (c)), but not in TC (g) initiates within MAPK-positive NE. (j,k) Schematic representation of gene expression patterns in (a–i) (also including msh expression; see also electronic supplementary material, figure S1). ast, anterior and pst, posterior protocerebral MAPK stripe. v, ventral; i, intermediate; d, dorsal. Dashed lines in black indicate borders between trito- (TC), deuto- (DC), protocerebrum (PC). At stage 6 (which is slightly prior to the expression of the segmental marker En in the brain NE), tentative boundaries between presumptive brain neuromeres were estimated with regard to the distance from the cephalic furrow (CF; white arrow in (a–d)) in AP axis and the AP extent of DV gene expression domains (i.e. ind in (c) and Nkx6 in (d); see also [52,53]). Dashed lines in white indicate the ventral midline. Dashed lines in red (in (a–d)) mark the border between intermediate/dorsal NE in TC/DC, and ventral/intermediate NE in PC. Dotted lines in blue indicate border between NE and mesectoderm. FG, foregut; hs, en head spot; as, en antennal stripe; is, en intercalary stripe. See the main text for further details.
2.2. EGFR is required for neuromere-specific expression of ind, vnd and Nkx6
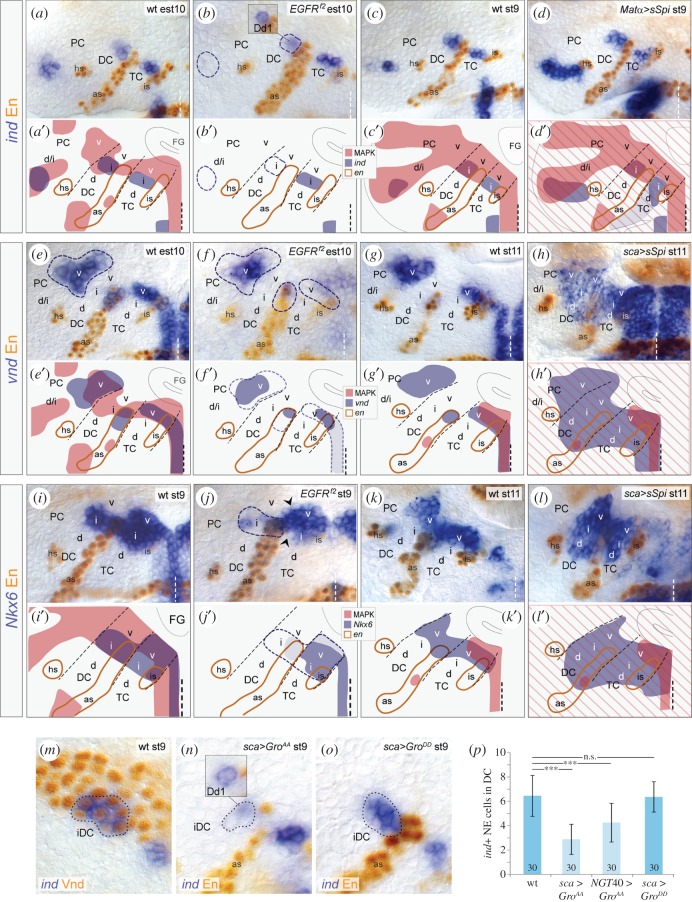
Because EGFR activity overlaps with the expression domains of vnd, Nkx6 and ind in the brain NE, we tested whether EGFR controls the expression of these genes. Analysing EGFRf2 mutants, we found that ind expression is delayed and strongly reduced in DC and PC (87% and 100% of brain hemispheres, respectively; n = 35; figure 2a,b), or entirely missing in DC (13% of hemispheres; n = 35). ind levels in TC of EGFRf2 mutants seemed to be unaffected, being consistent with ind expression complementary to MAPK in wild-type embryos. Ectopic activation of EGFR by overexpression of the secreted (i.e. active) EGFR ligand Spitz (sSpi) using the maternal Matα-Gal4 line (which drives expression ubiquitously; in the following termed Matα > sSpi) [55] led to ectopic activation of ind in intermediate/dorsal PC and dorsal TC (100% and 25% of hemispheres, respectively; n = 32), while ind was not affected in DC (figure 2c,d). Thus, EGFR signal is necessary for ind expression in DC and PC, and sufficient to activate ind expression in TC and PC.
Figure 2.
EGFR controls expression of ind, vnd and Nkx6. (a–l) Double stainings against ind (a–d), vnd (e–h), or Nkx6 mRNA (i–l) and Engrailed protein in different genetic backgrounds. Panels (a′–l′) show schematics of (a–l). (a,a′,b,b′) ind expression in the NE of DC/PC, but not TC, is strongly reduced in EGFRf2 mutants at early stage 10 (est10) (hatched areas in (b) indicate NE with a loss of ind expression), compared with wild-type (a). Note, ind expression is not affected in deutocerebral neuroblast Dd1 (inset in (b,b′)). (c,c′,d,d′) At stage 9, ind expression is dorsally expanded upon Matα > sSpi in TC/PC, but not in DC. (e,e′,f,f′) vnd expression is reduced in all neuromeres of EGFRf2 mutants at early stage 10 (areas are encircled with hatched line in (f,f′). (g,g′,h,h′) At stage 11, vnd expression is ectopically detected in the entire DC and intermediate/dorsal NE of TC upon sca > sSpi. (i,j′) At stage 9, Nkx6 expression is reduced in the EGFRf2-mutant intermediate DC (area encircled with a dashed line in (h)) and in a few cells in the intermediate/ventral TC (arrowheads in (h)). (k,k′,l,l′) Nkx6 expression is expanded into the intermediate and dorsal NE of TC/DC upon sca > sSpi at stage 11. (m) ind and Vnd are coexpressed in intermediate DC (encircled) at stage 9. (n) In sca > GroAA background, ind expression is reduced in the deutocerebral neuroectoderm, while unaffected in Dd1 (inset). (o) ind is not reduced upon sca > GroDD. (p) At stage 9, ind-expressing neuroectodermal cells in DC are significantly reduced after sca > GroAA (2,8 ± 1,2 cells) and NGT40 > GroAA (4,3 ± 1,6 cells), but not after sca > GroDD (6,4 ± 1,3 cells), compared with wild-type (wt) (6,5 ± 1,7 cells) (n = 30 each); error bars indicate s.d.; ***p < 0.0001; n.s., not significant; unpaired Student's t-test. For orientation and other abbreviations see figure 1.
Expression of vnd expression was activated normally in EGFRf2 mutants, but strongly reduced at early stage 10 in the NE of TC, DC (100% and 83% of hemispheres, respectively; n = 25) and, to a lower extent, PC (83% of hemispheres; n = 25; figure 2e,f). To ectopically activate EGFR, we misexpressed sSpi using the scabrous (sca)-Gal4 line [56] which drives expression within the NE stronger than the maternal driver used in this study. Because sca-Gal4 induces misexpression not before stage 7, thus later than the maternal driver, we usually took this driver to investigate effects at later stages (stages 11–13). In these sca > sSpi embryos, vnd was ectopically expressed in TC and DC, but not in PC (88% and 79% of hemispheres, respectively; n = 48; figure 2g,h). We conclude that EGFR is necessary for the maintenance and sufficient for induction of vnd expression especially in TC and DC. As with vnd, we observed similar effects of EGFR on the expression of Nkx6 in TC and DC (figure 2i–l). However, we did not observe effects of EGFR on the expression of scarecrow, another Nkx gene with close homology to vnd [57] (data not shown).
In TC and DC, we furthermore observed a slight derepression of msh in EGFRf2 mutants, and conversely, a reduction of msh expression in Matα > sSpi embryos (electronic supplementary material, figure S3a–d). To test whether EGFR signal directly represses msh, we analysed patterns of msh and MAPK (which normally exclude each other) in vnd6 mutants; in these mutants, msh was derepressed in the intermediate/ventral NE, and MAPK remained unaffected, both factors now being largely coexpressed in this NE (electronic supplementary material, figure S3e,f). These results suggest that activated EGFR regulates msh indirectly, through the activity of other DV genes vnd, ind and Nkx6 (as shown above).
2.3. EGFR induces ind expression in DC by phosphorylation of the co-repressor Groucho
vnd and ind are exceptionally coexpressed in the intermediate DC [51] (figure 2m), where EGFR is also activated. EGFR can regulate gene expression by MAPK-dependent phosphorylation and thus inactivation of the co-repressor Groucho (Gro) [58–60]. As Vnd is a Gro-dependent repressor [61], we hypothesized that EGFR signal phosphorylates Gro and thus inactivates Vnd/Gro-mediated repression of ind. To test this, we analysed ind expression after ectopically expressing unphosphorylatable (i.e. constitutively active; GroAA) and pseudo-phosphorylated (inactive; GroDD) Gro-constructs [58] in the brain NE using the sca-Gal4 and maternal NGT40-Gal4 [62] driver lines, which both drive Gal4 expression in the NE (although NGT40-Gal4 induces misexpression earlier and at weaker levels than sca-Gal4; data not shown). ind was significantly reduced in DC of sca > GroAA and NGT40 > GroAA embryos (figure 2n,p; data not shown), with effects being stronger with the later-initiating but stronger driver sca-Gal4, but unaltered in DC of sca > GroDD control embryos (figure 2o,p). Altogether, our results strongly suggest, that EGFR activity induces ind expression in DC specifically by phosphorylation of Gro, thus inactivating Vnd/Gro.
2.4. rho, vn and S are differentially expressed in the brain neuroectoderm and flanking mesectoderm
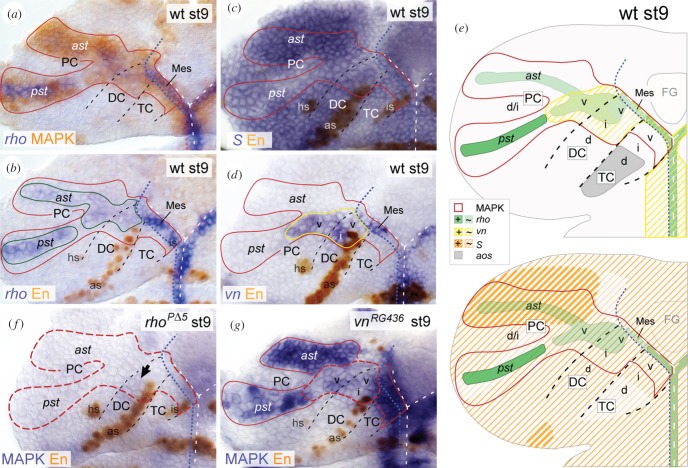
The complex spatio-temporal pattern of EGFR activation in the brain NE led us to determine the sources of activating Spi and Vn ligands. Spi is expressed as an inactive precursor and requires Rho and S to be secreted [28–31]. spi itself and S are rather broadly expressed, thus playing a minor role in controlling the spatio-temporal pattern of Spi secretion [32–35]. By contrast, rho expression is tightly controlled and represents the key regulatory step controlling Spi secretion in the developing VNC [35,40]. As we cannot exclude that the spatio-temporal Spi secretion is also regulated by spi and S expression in the early brain, we analysed rho, spi and S expression to determine Spi ligand sources in the brain NE. At stage 5, rho expression in the NE corresponded with the MAPK pattern, but became largely restricted to the sim-expressing mesectoderm by gastrulation (at stage 6), although rudimentary rho expression was still detectable in the NE of DC and PC (electronic supplementary material, figure S4a,b). By stage 9, when EGFR signalling and DV regionalization of the brain NE are most pronounced (figure 1) [52], rho expression was weakly detected in DC and ventral PC, and the anterior MAPK stripe of PC; stronger rho expression was observed in the posterior MAPK stripe and mesectoderm (ventral to TC and DC) (figure 3a,b). Thus, rho expression closely correlates with EGFR activity, even though rho expression levels vary within the brain NE and flanking mesectoderm. S and spi were broadly expressed in the brain NE (figure 3c; and data not shown). We noted that S expression was particularly strong in the anterior MAPK stripe of PC, where rho expression was weak, despite strong EGFR signalling (figure 3a–c). In contrast with Spi, Vn is expressed as a secreted protein which requires no further processing to be activated [26]. vn expression was codetected with MAPK in DC, ventral PC and mesectoderm (ventral to TC and DC) at stage 9 (figure 3d; electronic supplementary material, figure S4c). Because vn expression was never observed in both MAPK stripes of PC, this suggests that Vn only partially acts as a positive EGFR feedback regulator in the brain, in contrast with the VNC [42].
Figure 3.
rho, vn, S and aos are differentially expressed and required in distinct regions of the brain. Stage 9 (st9); red solid line demarcates the wild-typic MAPK domain, according to (a). (a,b) rho is expressed within MAPK domain in DC/PC (solid green line), and in the mesectoderm (‘midline cells’) ventral to TC/DC which are characterized by expression of Single-minded (shown in figure 5n,q). (c) S is broadly expressed in the brain NE, while expression levels vary. (d) vn is expressed in ventral PC and ventral/intermediate DC (solid yellow line), and mesectodermal cells. (e) Scheme summarizes expression data in (a–d), and the EGFR-independent expression of aos in dorsal TC (see also figure 4g and the main text for further explanations). (f) MAPK is strongly reduced in rhoPΔ5 mutant brain NE (red hatched line), when compared with wild-type (a); black arrow indicates faint MAPK in the mutant DC. (g) In vnRG436 mutants, MAPK is reduced particularly in the ventral PC, and ventral/intermediate DC (red hatched line). For orientation, other abbreviations and symbols see figure 1.
In sum, rho, vn and S are differentially expressed during patterning of the brain (summarized in figure 3e). Primarily vn and little rho is expressed in DC and abutting ventral PC. By contrast, rho and S are expressed in the two protocerebral MAPK stripes, with weak rho and strong S expression levels in the anterior stripe, opposite to the posterior stripe. Only S, but not vn or rho, is expressed in the TC, although the adjacent mesectoderm reveals strong vn/rho expression levels. These data suggest that EGFR activity in the brain is regulated by a region-specific deployment of distinct ligands.
2.5. rho and vn are differentially required to activate EGFR in the brain
To test for requirements of Rho and Vn to activate EGFR, we analysed EGFR activity in the brain NE of rhoPΔ5 and vnRG436 mutant embryos. At stage 9, vnRG436 mutants exhibited a specific loss of MAPK only in DC and ventral PC (strong reduction in 40%, moderate reduction in 49%, normal in 11% of hemispheres; n = 35), whereas rhoPΔ5 mutants showed a near total loss of MAPK in the entire brain NE (100% of hemispheres; n = 22) (figure 3f,g). We conclude that Rho is required for EGFR activation in the entire brain NE, whereas Vn is required in addition to Rho for proper EGFR activation in DC and ventral PC. As MAPK was lost in DC/ventral PC in both, rho and vn mutants, we asked if vn expression depends on Rho. Indeed, vn expression was reduced in these brain regions in rhoPΔ5 mutants (electronic supplementary material, figure S4d), indicating that Rho-dependent factors normally induce vn expression, which in turn activates EGFR. However, we cannot rule out that low levels of Rho act in parallel to Vn to achieve proper EGFR activation in DC and ventral PC.
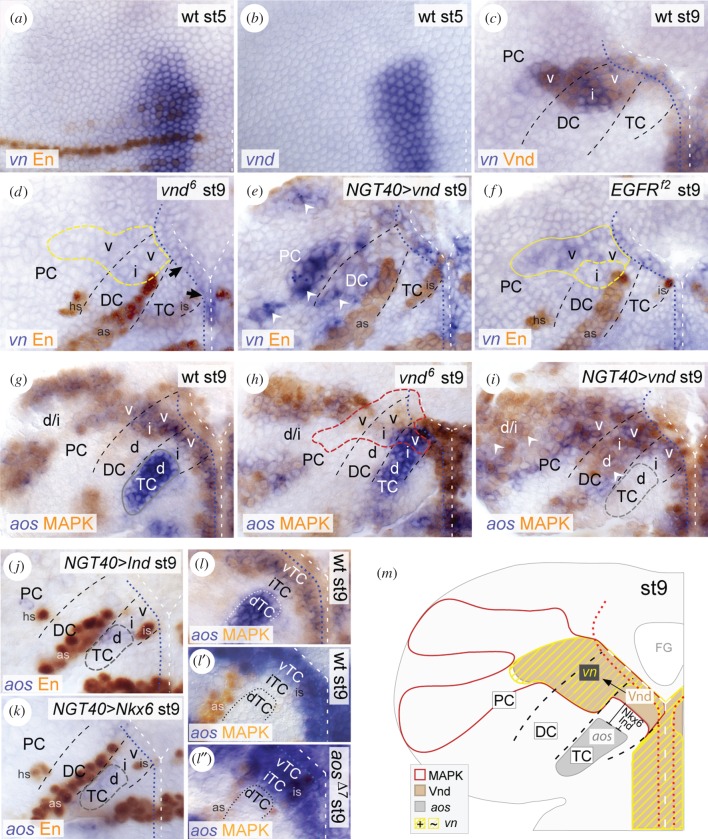
2.6. vn expression is controlled by Vnd in the brain
We found that expression of vn closely correlates with expression of vnd in the early brain NE (figure 4a–c). Therefore, we tested if Vnd controls vn expression. At stage 9, vnd6-mutant embryos showed strong reduction of vn expression in the brain NE (figure 4d). Correspondingly, we found ectopic vn expression in DC and PC upon vnd-overexpression (NGT40 > vnd) (figure 4e). These data suggest that Vnd is necessary, and to some extent sufficient, to activate vn expression. Next, we tested if vn expression is induced by activated EGFR, as it has been proposed in the VNC [42]. At stage 9, we observed a slight reduction of vn expression in the brain NE of EGFRf2 mutants (figure 4f), compared with the strong reduction in vnd6 mutants. Further, in EGFRf2 mutants, the reduction of vn expression was closely correlated with the reduction of vnd expression (as shown above); residual vn expression was always co-detected with residual vnd expression (electronic supplementary material, figure S4e). This suggests that in the brain, EGFR induces vn expression indirectly, via Vnd. Our data thus provide the first evidence that Vnd, a DV gene which has been considered as an EGFR target so far, regulates the expression of an EGFR ligand.
Figure 4.
Vnd controls EGFR activity by regulating expression of vn and aos. (a,b) Stage (st) 5, (c–l) stage 9. (a–c) In the presumptive brain NE, vn expression closely corresponds to vnd expression between stages (st) 5 to 9. (d–f) vn expression is lost in the brain NE in vnd6 mutants (yellow dashed outline in (d)); only a few mesectodermal cells ventral to TC remain vn-positive (black arrows in d). (e) Upon NGT40 > vnd, vn is ectopically induced in dorsal DC and PC (white arrowheads). (f) vn expression is reduced in the brain NE of EGFRf2 mutants (predominantly in intermediate DC; yellow dashed outline). (g–i) In wild-type, aos is strongly expressed in the MAPK-negative dorsal TC (and weaker within the MAPK domains; see below) (g). (h) In vnd6 mutants, aos is de-repressed in ventral/intermediate TC, and MAPK reduced in ventral TC and in the adjacent NE of DC and PC (red dashed outline). (i) Upon NGT40 > vnd, aos is specifically missing in dorsal TC (grey dashed outline), while MAPK is ectopically detected in dorsal DC and PC (white arrowheads). Note that in these different genetic backgrounds at stage 9 aos is always co-detected with MAPK in the brain NE, because aos is induced downstream of EGFR/MAPK [36], except in dorsal TC (see also electronic supplementary material, figure S4j,k). Accordingly, upon NGT40 > vnd ectopic aos expression within the enlarged MAPK domain (in (i)) is most probably due to ectopic EGFR activation. (j,k) aos is strongly reduced in dorsal TC (grey dashed outline) upon NGT40 > ind (J) or NGT40 > Nkx6 (k). (l) EGFR is inactive in intermediate TC, which adjoins aos expression in dorsal TC (dotted outline in (l–l″)). In aosΔ7 mutants, EGFR is ectopically activated in intermediate TC. (m) Schematic summary. Vnd activates vn expression in the ventral brain NE, and represses aos in ventral TC, whereas Ind/Nkx6 repress aos in intermediate TC. For orientation, other abbreviations and symbols see figure 1.
2.7. aos is expressed in dorsal TC independently of EGFR but under control of Vnd, Ind and Nkx6, and inhibits EGFR signalling
Argos (Aos) is a secreted EGFR regulator that antagonizes Spi activity [38]. We detected aos expression within the neuroectodermal MAPK domains in all brain neuromeres (figure 4g), which was expected because aos is known as a negative EGFR feedback regulator induced downstream of EGFR/MAPK [36]. Consistently, aosΔ7 mutants showed a massive overactivation of MAPK in the brain NE at stage 11, when endogenous EGFR activity is largely downregulated (electronic supplementary material, figure S4f,g). Interestingly, we recognized a prominent aos expression domain outside the MAPK pattern, in dorsal TC (figure 4g; electronic supplementary material, figure S4 h,i). Accordingly, this aos domain was not affected in EGFRf2 embryos, whereas the remaining aos expression was entirely missing in the brain (electronic supplementary material, figure S4j,k). We conclude that aos is regulated independently of EGFR in dorsal TC.
Expression of aos restricted to dorsal TC suggests a regulation by DV genes. Therefore, we tested whether vnd, ind or Nkx6, expressed in ventral/intermediate TC (see §2.1.), act to restrict aos to the dorsal TC. In vnd6 mutants, which are characterized by an additional loss of ind and Nkx6 in the TC [52] (electronic supplementary material, figure S4l,m), aos is derepressed in the ventral/intermediate TC (figure 4h). Conversely, aos is efficiently repressed in NGT40 > vnd embryos (lost in 87%, strongly reduced in 13% of hemispheres; n = 30) (figure 4i). We did not find substantial effects on aos expression in ind or Nkx6 mutants (data not shown), most probably because both factors act redundantly [52]. However, overexpression of either ind or Nkx6 with NGT40-Gal4 led to a strong reduction of aos expression in dorsal TC (figure 4j,k). We conclude that vnd, ind and Nkx6 act in concert to restrict aos to dorsal TC. This provides further evidence of DV patterning genes regulating the regionalized production of EGFR ligands.
Being controlled by the DV gene network, we asked whether Aos is involved in regulating EGFR activity along the DV axis in TC. At stage 9, EGFR is activated only in ventral TC. However, in aosΔ7 mutants, EGFR was additionally activated in intermediate TC (figure 4l–l″), indicating that Aos normally inhibits EGFR signalling in this NE. Thus, Aos is crucial for regulation of EGFR activity along the DV axis in TC.
2.8. Vnd is part of a positive feedback loop controlling EGFR activity in the brain
As Vnd controls the activity of both activating EGFR ligands, Vn and Spi (via aos, as shown above), we analysed the effect of Vnd on EGFR activity in the brain NE. At stage 9, vnd6 mutants exhibited a reduction of MAPK in TC, DC and adjacent ventral PC (figure 4h). In TC, this reduction is probably due to the ventrally expanded domain of aos expression which inhibits Spi-induced EGFR activation. In the vnd6-mutant DC/ventral PC, elevated Aos levels, secreted from the enlarged aos domain in TC, come together with the loss of Vn (figure 4d,h), which we propose to mutually account for the strong reduction of EGFR activation. To test whether Vnd is sufficient to induce EGFR, we analysed MAPK after vnd-overexpression. In NGT40 > vnd embryos, we found ectopic MAPK in PC and dorsal DC (figure 4i). We assume that EGFR is overactivated in PC primarily due to ectopic Vn (figure 4e), and in DC due to elevated levels of Vn and Spi, the latter owing to the loss of Aos in dorsal TC (figure 4e,i).
Taken together, these data demonstrate that Vnd plays a central role for the spatial activity of EGFR as it controls the ligand activity of Vn and Spi (figure 4m). As the maintenance of vnd expression in turn depends on activated EGFR (as shown in §2.2.), Vnd acts as a positive EGFR feedback regulator in the brain.
2.9. EGFR signalling crucially depends on Tll in PC, and on mesectodermal Sim in TC
As EGFR activity in PC emerges largely in the tailless (tll) expression domain (figure 5a–c), we analysed if Tll regulates EGFR activity. tlll49 mutants exhibited a loss of rho in the intermediate/dorsal PC while tll-overexpression (NGT40 > tll) induced ectopic rho expression predominantly in ventral/intermediate TC and DC, but not in PC where endogenous Tll is expressed (figure 5d–f). Consistent with these results, we detected in corresponding brain regions a loss of MAPK in tlll49 mutants and ectopic MAPK in NGT40 > tll embryos (figure 5g–i; electronic supplementary material, figure S4n). Further, we observed a loss of vn expression in ventral PC of tlll49 mutants (figure 5j). As Vnd, necessary for vn expression (as shown in §2.6.), is also lost in these mutants (figure 5k,l), we conclude that in ventral PC, Tll normally induces vn expression via Vnd. Taken together, in PC, Tll induces production of both ligands, Vn (in ventral PC) and Spi (in intermediate/dorsal PC), by regulating the expression of vnd and rho, respectively (figure 5m).
Figure 5.
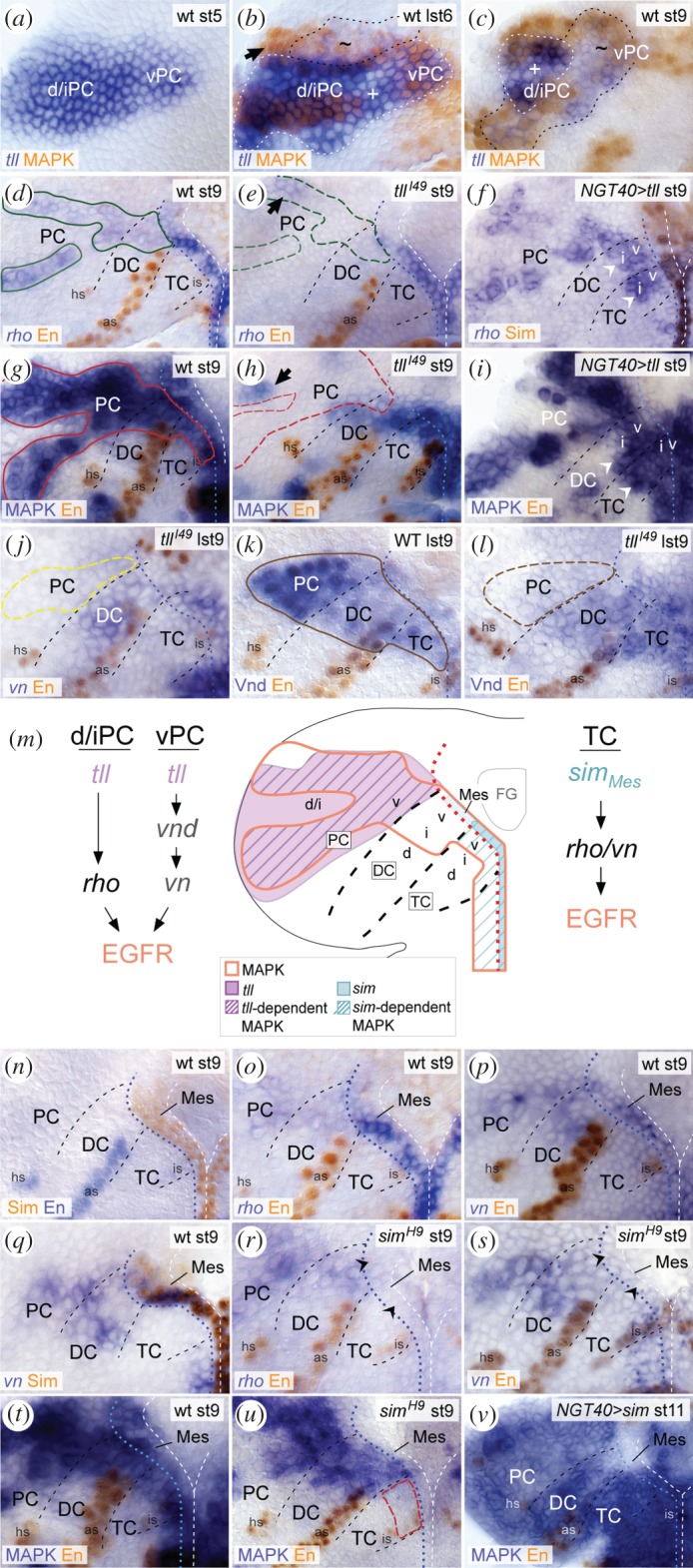
EGFR activity depends on Tll in PC, and on Sim in TC. (a–c) At stage 5 (st5) tll expression is activated exclusively in PC [63], prior to MAPK (a,b). (b) At late stage 6 (lst6), the protocerebral MAPK domain largely originates within the tll domain (encircled with black dashed line); indicated are subregions of strong (+) and weak (∼) tll expression levels. Few MAPK-positive cells (arrow) originate outside the tll-expressing NE. (c) Until stage 9, tll expression largely overlaps with MAPK in PC. (d–f) At stage 9, rho expression is missing in tlll49 mutant PC (green dashed outline in (e)), except in anteriodorsal NE (arrow), when compared with wild-type (solid green outline in (d)). (f) Upon NGT40 > tll, rho is ectopically expressed in ventral TC/DC (white arrowheads), and detected at stronger expression levels in ventral PC. Note that En expression is repressed in NGT40 > tll. Tentative segment boundaries were therefore estimated based on their wild-typic position with regard to the cephalic furrow, because the extent of the brain NE is largely unaffected in NGT40 > tll embryos. (g–i) At stage 9, compared to wild-type (g), MAPK is missing in tlll49 mutant PC (red hatched outline) in 80% of hemispheres (n = 20) (h) and ectopically activated in ventral/intermediate TC and DC upon NGT40 > tll (white arrowheads in (i)). We cannot exclude that lack of en expression in TC and DC partly evokes ectopic MAPK or rho expression (f) in these neuromeres. (j–l) At late stage 9, vn expression is lost in PC (yellow hatched outline; compared with wild-type in (p)). (l) Correspondingly, Vnd is lost in PC of tlll49 mutants (brown hatched outline), compared with wild-type (brown solid line in (k)). (m) Schematic summary of data in (a–l) and (n–v). Neuroectodermal Tll (violet) induces EGFR signalling in PC (hatched in violet), while mesectodermal Sim (turquoise) induces EGFR activity in ventral TC (hatched in turquoise). (n–s) At stage 9, coexpression of Sim (n,q), rho (o) and vn (p,q) in mesectodermal cells ventral to TC/DC. Expression of rho (o) and vn (p,q) is lost in simH9 mutant mesectoderm (black arrowheads in r,s). (b) MAPK is specifically lost in TC (red hatched line) and mesectoderm of simH9 mutants (u), and upon NGT40 > sim ubiquitously activated in the brain NE at stage 11 (v). For orientation, other abbreviations and symbols see figure 1.
Next, we analysed the role of single-minded (Sim), the main regulator of midline-dependent Spi secretion in VNC patterning [35,64,65], in controlling EGFR activity in the early brain. Onset of EGFR signalling occurs independently of Sim, because expression of rho initiates in the brain NE (at stage 5) before sim in the ventral mesectoderm (at stage 6) (electronic supplementary material, figure S4a,b). By stage 9, sim is expressed in the mesectoderm ventral to TC and DC (figure 5n), where it overlaps with rho and vn expression (figure 5o,p,q). Analysis of simH9 mutants revealed a loss of rho, vn and MAPK signal in this mesectoderm (figure 5r–u), accompanied by a loss of MAPK specifically in TC (figure 5t,u). This indicates that Sim is necessary for the production of Spi and Vn secreted from the mesectoderm to induce EGFR signalling in ventral TC, but is dispensable in DC and PC. Furthermore, sim overexpression (NGT40 > sim) was sufficient to induce ectopic MAPK in the brain NE by stage 11 (figure 5v), probably due to action of rho and vn, which both can be ectopically induced by Sim in the trunk [65]. Altogether these data suggest that mesectodermal Sim is normally important for ongoing EGFR signalling exclusively in TC.
2.10. EGFR controls formation of brain neuroblasts by regulating number, survival and proneural gene expression of neuroectodermal progenitor cells
Having established the regulation of EGFR signalling and its interaction with DV patterning genes in the procephalic NE, we asked if EGFR signal functions in the formation of brain neuroblasts. In stainings against Death caspase-1 (Dcp-1), a hallmark of cell death, we recognized extensive cell death in the brain NE (figure 6a,b; electronic supplementary material, figure S5a–d), which is accompanied by a significant loss of Deadpan (Dpn)-positive brain neuroblasts in all three neuromeres of EGFRf2-mutant brains at stage 11, when compared with wild-type (figure 6a′,a″,b′,b″,c). However, we did not detect apoptotic brain neuroblasts, although EGFR is transiently active in a subset of them (electronic supplementary material, figure S2a,b). To test if the loss of neuroblasts is caused by cell death of NE progenitor cells, we estimated the neuroblast number in EGFRf2;Df(3L)H99 double mutants which are cell death deficient [66]. In total, 20–25% of brain neuroblasts were lost in these double mutants, instead of 40% in EGFRf2 mutants (figure 6c–e′; electronic supplementary material, figure S5e,f), whereas neuroblast numbers were unaffected in Df(3L)H99 mutant control brains (electronic supplementary material, figure S5g,h). These data demonstrate that only a subfraction of 15–20% of brain neuroblasts is missing due to cell death of NE progenitor cells in EGFRf2 embryos.
Figure 6.
EGFR signalling is crucial for brain neuroblast formation. (a,a′,a″,b,b′,b″) Flat preparations of wild-type (a,a′,a″) and EGFRf2 mutants (b,b′,b″) at late stage 11 (lst11). The wild-typic and mutant NE (a,b) or neuroblast (NB) (a′,a″,b′,b″) layer depicted in these panels each represent a combined projection of an equal amount of confocal foci (in Z-axis). Dcp1-signal is broadly detected in the brain NE (a,b) of EGFRf2 mutants, but not in underlying neuroblasts (a′,a″,b′,b″). The brain neuroblast number is strongly reduced in the EGFRf2-mutant hemisphere, in which totally 56 neuroblasts were counted (in b′,b″), when compared with 104 neuroblasts in the wild-type hemisphere (in a′,a″). Note that at late stage 11 almost the entire NE undergoes cell death in EGFRf2 mutants. As the neuroectodermal layer dissolves, apoptotic neuroectodermal cells come to lie within the neuroblast layer (white arrows). (c) Quantification of the number of brain neuroblasts in different genotypes. Number of neuroblasts per hemisphere at stage 11: wt 100.3 ± 4.1; EGFRf2 62.3 ± 4.7; EGFRf2;Df(3L)H99 78.8 ± 10.8; H99 100.8 ± 6.7; at stage 13: wt 127.2 ± 9.2 (of those 29.0 ± 3.5 neuroblasts [23.0%] are PH3-labelled); sca > spi 163.4 ± 11.0 (of those 40.8 ± 5.5 neuroblasts [25.0%] are PH3-labelled); numbers within bars indicate n; error bars indicate s.d.; ***p < 0.0001, n.s., not significant; unpaired Student's t-test). (d,e) At late stage 11, the number of Dpn-positive brain neuroblast is significantly reduced in EGFRf2;Df(3L)H99 mutants. (d′,e′) Higher magnification of areas boxed in (d,e). The number of ventral (black asterisks) but not of (Ind-positive) intermediate neuroblasts (white asterisks) is reduced in TC/DC of those mutants. (f) Quantification of the number of intermediate/ventral neuroblasts in TC/DC (at stage 11) in different genotypes. Number of neuroblasts/hemisphere at stage 11: intermediate neuroblasts in TC: wt 2.6 ± 0.6; EGFRf2;H99 2.6 ± 0.9; EGFRf2 2.2 ± 0.7; intermediate neuroblasts in DC: wt 2.1 ± 0.3; EGFRf2;H99 2.3 ± 0.5; EGFRf2 2.0 ± 0.8; ventral neuroblasts in TC: wt 4.5 ± 1.1; EGFRf2;H99 1.4 ± 0.8; EGFRf2 0.7 ± 0.5; ventral neuroblasts in DC: wt 4.9 ± 1.0; EGFRf2;H99 3.2 ± 0.9; EGFRf2 2.6 ± 0.8); numbers within bars indicate n; error bars indicate s.d.; **p < 0.01, ***p < 0.0001, n.s., not significant; unpaired Student's t-test). (g,h) Wild-typic MAPK domain is outlined in red in (g), and for comparison in the EGFRf2;H99 mutant (h). l'sc expression is reduced in PC, DC and ventral TC particularly in the EGFRf2-mutant domain (indicated in (h)) at early stage 9 (est9). (i,i′,j,j′) Loss of L'sc expression at stage 11 (white hatched outlines in (i)), when compared with wild-type (white solid outline in (j)). (i′,j′) Higher magnification of NE in ventral TC discloses loss of l'sc expression, corresponding to the loss of ventral neuroblasts (see (e′)). (k,l) l'sc expression is widely downregulated after formation of brain neuroblast at stage 12 in wild-type (k), but maintained upon sca > sSpi (l). (m,n) Dorsal view on left hemisphere. Number of Dpn-positive/Pros-negative (Pros indicates ganglion mother cells) brain neuroblasts is increased by stage 13 in sca > sSpi embryos (n), when compared with wild-type (m); the mitotic index of neuroblasts (as judged by PH3-labelling) is unaltered (see (c)). (o) EGFR controls formation of brain neuroblast in multiple ways (see the main text). For orientation, other abbreviations and symbols see figure 1.
Because during the period of neuroblast formation EGFR is active in the NE of ventral/intermediate DC and ventral TC, we analysed the number of ventral and intermediate neuroblasts in both neuromeres of EGFRf2;Df(3L)H99 mutants. Ventral neuroblasts were reduced in TC and DC, whereas intermediate (Ind-positive) neuroblasts were all formed (figure 6d,d′,e, e′,f; electronic supplementary material, figure S5e′,f′i). Comparing the small number of neuroblasts missing in TC and DC with the total amount of brain neuroblasts missing in EGFRf2 mutants (figure 6c,f; electronic supplementary material, figure S5i), we conclude that neuroblast formation is primarily affected in PC.
To ascertain if the failure in neuroblast formation in EGFRf2 mutants is due to a deregulation of proneural genes, we investigated expression of lethal of scute (l'sc), the key proneural factor for the development of brain neuroblasts [47,48]. In EGFRf2;Df(3 L)H99 mutants, l'sc expression is strongly reduced in regions where EGFR is normally active (figure 6g–j). Correspondingly, l'sc expression is significantly prolonged upon overexpression of activated Spi (sca > sSpi) (figure 6k,l). To test whether ectopic EGFR activation in those embryos is also sufficient to generate additional brain neuroblasts, we counted neuroblast numbers at stage 13 (until when sca-Gal4 is broadly active; data not shown). The number of Dpn-positive brain neuroblasts was significantly increased (figure 6m,n), but not the mitotic index of those neuroblasts: stainings against the M-phase marker Phospho-Histone3 (PH3) showed that 23% of neuroblasts were mitotic in wild-type and 25% in sca > sSpi (n = 10 hemispheres each). Thus, EGFR activation is sufficient to induce proneural gene expression, and the formation of ectopic brain neuroblasts, but does not enhance their proliferative activity.
As EGFR is activated in the brain NE from stage 5 onwards, we finally asked if early EGFR signalling already impacts the extent of the NE by controlling the early mitotic activity (see [67]), and thus the final number of NE progenitor cells from which brain neuroblasts develop. Therefore, we analysed PH3-labellings with focus on the ventral/central head NE where EGFR is largely activated, and observed that the amount of mitotic NE cells is reduced by approximately 20% in EGFRf2 mutants (n = 14 hemispheres) (electronic supplementary material, figure S5j–m). Nevertheless, this reduction in NE progenitor cell number alone does not account for the observed reduction in l'sc expression domains.
In sum, our data provide evidence that EGFR signalling affects formation of brain neuroblasts at multiple steps of development: first, by controlling mitosis in the NE anlagen to establish the proper number of neuroectodermal progenitor cells; second, by positively regulating expression of proneural gene l'sc in those cells; and third, by ensuring their survival (summarized in figure 6o).
3. Discussion
3.1. Localized EGFR signalling in the embryonic brain is controlled by neuromere-specific deployment of distinct ligands
In the VNC, EGFR is activated in two phases. In the early, midline-independent phase, EGFR is induced by Rho (via processing of Spi) and Vn, which are both expressed in the ventral/intermediate NE; rho expression becomes restricted to the midline during gastrulation, while vn expression restricts towards the ventral NE [20,26,40,41]. In the following midline-dependent phase, EGFR activity essentially depends on Sim, a master regulator of midline development which induces rho expression (and Spi secretion) in the ventral midline and is required for vn expression in the ventral NE [35,64,65,68,69]. As summarized in figure 7, in the brain we observe a similar early period of EGFR activity before Sim expression is initiated in ventral mesectodermal cells (corresponding to the ventral midline; see also [43]). EGFR is initially induced by Rho and Vn expressed in the ventral/intermediate NE of TC, DC and ventral PC. However, only in the posterior brain, the TC, do both factors become confined towards the ‘ventral midline’, followed by a midline-dependent phase of EGFR signalling (figure 7). These findings support that patterning in the TC closely resembles the situation in the trunk, but is more derived in DC and PC where EGFR activity remains midline-independent, even though weakly Sim-positive midline cells ventral to DC are likely to secrete limited amounts of Spi and Vn. Our data show that in DC/PC, EGFR activity relies on neuroectodermal sources of Vn and Spi (processed by Rho) that are controlled by the DV gene vnd (as discussed below) and the terminal gap gene tll. Surprisingly, Vn, which plays only a minor role in VNC patterning [20,26,42,70], proved to be a major activating EGFR ligand in DC and ventral PC. The reason is probably the exceptionally low level of rho expression in this area, leading to low Spi-levels that alone cannot sufficiently activate EGFR. By contrast, Rho-dependent Spi is the only ligand in the largest part of PC (i.e. intermediate/dorsal PC). Despite a close spatial correlation, however, rho and MAPK signal levels correlate poorly in PC: a strong MAPK signal is detected in the anterior stripe despite very low rho-expression levels, whereas MAPK is weaker in the posterior stripe despite stronger rho expression levels. We noted that S expression levels correlate with MAPK activity, being strong in the anterior MAPK stripe, and weaker in the posterior MAPK stripe. S is responsible for transport of the Spi precursor to the Golgi, where Rho-dependent secretion of Spi occurs [30,31]. Rho also cleaves and inactivates S, thus compromising the levels of secreted Spi [71]. Therefore it seems likely that S together with Rho modulate localized levels of secreted Spi, and hence the activity of EGFR within distinct regions of the brain NE.
Figure 7.
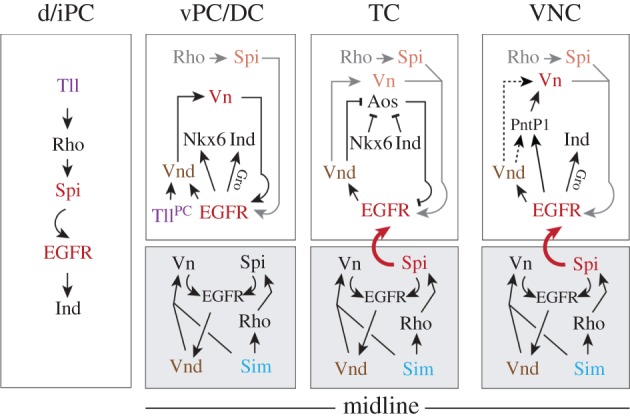
A model of genetic interactions between EGFR and other factors that pattern the brain in the DV axis, when compared with the VNC. Separated boxes show proposed genetic interactions in the NE (white boxes) of dorsal/intermediate (d/i) PC (left), ventral PC/DC (middle left), TC (middle right), VNC (right) and the ventrally adjacent mesectoderm (or midline; grey boxes), as deduced from the data in this study. In TC/DC, genetic factors/ligands active during the early period of EGFR signalling are indicated in grey/orange, respectively. Bent arrows indicate activity of secreted factors. Genetic control of EGFR is different in TC, DC and PC. For example, EGFR activity becomes midline-dependent only in TC (indicated by bent arrow in red), similar to the situation in the VNC. In the VNC, genetic interactions for PntP1 refer to [42]; it is unknown if EGFR signal induces vn expression via Vnd and PntP1 (indicated by stippled arrows). The potential role of PntP1 in the brain is unclear. See the main text for further details.
The nuclear Dorsal gradient is active in the early embryo to activate expression of rho, vnd and presumably vn in the truncal NE (each being expressed in a uniform longitudinal domain in the ventral NE) [9,72–74]. As the early expression domains of rho, vnd and vn cover in addition the ventral NE of the presumptive TC, DC and PC (figure 4a,b; electronic supplementary material, figure S4a), we assume that the early expression of these genes is likewise activated by the nuclear Dorsal gradient. In dorsal/intermediate PC, however, the induction of the slightly later emerging two stripes of rho expression might rather depend only on Tll (figure 7). The positive regulation of EGFR ligands by Tll in PC seems to be opposed to its function in the development of the larval visual system, where Tll has been proposed to block transcriptional programmes induced by EGFR (via Spi) [44].
Thus, during early brain development diverse ligands produced in distinct tissues (mesectoderm, NE) control localized activity of EGFR in a neuromere-specific manner (figure 7): in TC depending on midline-specific production of Vn and Spi (and Aos secreted from the dorsal TC, as discussed below), and in DC and PC primarily on Vn and Spi (via Rho), respectively, secreted from defined neuroectodermal domains. Other factors are known to control EGFR signalling, such as the feedback regulators Sprouty and Kekkon [75–78], MAPK phosphatase MKP-3 [79,80], or extracellular regulators of ligand travelling/activity such as Sulf1 and CG4096 [81]. Except Sulf1 and CG4096, which we did not detect in the embryonic brain NE (data not shown), the potential role of the other regulators in patterning of the embryonic brain has to be clarified in further investigations.
3.2. DV genes control activity of EGFR through regulation of vn and aos
In this study, we demonstrate a novel role for Vnd, Nkx6 and Ind in regulating the ligands of EGFR, which indicates that within the hierarchical gene network EGFR stands not strictly atop the DV genes (figure 7). The function of Vnd in this context is far-reaching, because Vnd induces vn expression in all brain neuromeres; this is of importance particularly in DC where Vn is the main EGFR ligand. In the VNC, vn expression is induced by the transcriptional activator Pointed P1 in response to EGFR signal [42] (figure 7). We cannot exclude that during posterior brain development Vnd induces vn expression by positively regulating Pointed P1.
Notably, Vnd also regulates the expression of the inhibitory ligand Aos in TC. Vnd suppresses aos expression in the ventral (and early intermediate) TC, and keeps Aos secretion limited to the dorsal TC (figure 7). Accordingly, Spi elicited from the ventral midline is able to activate EGFR only in ventral TC, where it maintains vnd expression. Vnd is also required for inducing expression of Nkx6 and Ind [52], which both keep aos suppressed in the intermediate TC after Vnd is downregulated.
Thus, via the deployment of EGFR ligands (Vn, Aos), Vnd acts positively on itself, and thus stabilizes at later stages the cross-repressive interactions between Msh (dorsal)/Nkx6 (intermediate) and Ind (intermediate)/Vnd (ventral), essential for establishing the boundaries of DV neuroectodermal and corresponding stem cell domains [52,53,82].
3.3. EGFR regulates expression of DV genes in the embryonic brain in a neuromere-specific manner
Subdivision of the NE into discrete gene expression domains is essential for the correct specification of neural stem cells. During DV patterning of the truncal NE, EGFR is necessary for regionalized expression of vnd and ind (summarized in [83]). In previous reports, we uncovered a network of genetic interactions underlying DV patterning in the brain (including vnd, ind, msh, Nkx6, Ems, En) [51–53]. Here, we expand on this knowledge and show that EGFR strongly participates in the control of DV gene expression in the early brain. EGFR signal is necessary for the maintenance of the expression of two Nkx genes, Nkx6 and vnd, similar to its role for vnd expression in the VNC [17]. It is likely that EGFR signal regulates the regionalized expression of other patterning genes. For example, ectopic En is detected in a few NE cells in EGFRf2-mutant DC (figure 2a,b), making it likely that EGFR is involved in the control of en in this neuromere. As we previously showed that En negatively regulates the expression of ind [53], possibly the reduction of ind in the EGFRf2-mutant DC is partly due to ectopic En.
EGFR signal is necessary for ind activation in the trunk [17], whereas its effect on ind expression in the brain strongly differs between neuromeres (figure 7). In TC, ind expression is activated independently of EGFR, which is unique in the entire embryo. However, in aos mutants in which EGFR is ectopically activated in the intermediate TC (figure 4l′′), we observed a significant reduction of ind expression (data not shown). Thus, we propose that in TC, EGFR controls ind expression indirectly via the maintenance of the ind-repressor Vnd which in wild-type fades early in the intermediate TC to allow for ind expression.
In DC, ind activation requires both, EGFR signal and phosphorylation of the co-repressor Gro. Gro has been shown to be directly phosphorylated (and thereby inactivated) by EGFR/MAPK activity (and other RTK/MAPK pathways) [59], thereby regulating ind expression in the trunk [60]. Given that Ind is co-expressed with its Gro-dependent repressor Vnd [61], our results strongly suggest that EGFR allows ind expression in DC by phosphorylation of Gro, thus inactivating Vnd/Gro repressor complexes. We noted that in the ventral NE of the trunk and TC, unlike in DC, Vnd manages to repress ind expression despite EGFR being active and Gro being phosphorylated. As Vnd has been shown to form multiple complexes in the embryo [84], a possible explanation is that Vnd associates with RTK-insensitive co-repressors in trunk segments, and exclusively with Gro in DC (thus being sensitive to Gro inactivation). In PC, EGFR is also necessary for ind activation, but not sensitive to Gro-phosphorylation (data not shown), indicating an EGFR-dependent regulatory mechanism different from VNC and other brain neuromeres.
3.4. EGFR controls the formation of brain neuroblasts at different developmental steps
About 40% of brain neuroblasts are missing in EGFRf2-mutant embryos at embryonic stage 11. Our data suggest that EGFR function affects the formation of brain neuroblasts at multiple steps of development. First, activated EGFR positively controls the early mitotic activity within the neuroectodermal anlagen, in accordance with EGFR function in other developmental contexts (e.g. [85–88]). Second, as many neuroectodermal progenitor cells undergo premature cell death in EGFRf2 mutants, EGFR signalling is critical for their survival (see also [43,45]). EGFR-dependent survival has been reported also for midline glial cells in which Spi-activated EGFR suppresses the proapoptotic protein Hid [89,90]. Also in the large dorsal/intermediate PC, where apoptosis of EGFRf2-mutant neuroectodermal cells is substantial, Spi seems to be the only EGFR ligand, raising the possibility that a similar mechanism regulates neuroectodermal cell survival. We identified a small number of brain neuroblasts with activated EGFR, but never apoptotic neuroblasts in EGFRf2 mutants, suggesting that EGFR signal is rather dispensable for their survival. Third, in addition to the decrease of neuroectodermal progenitor cells, impairment of neuroblast formation in all brain neuromeres of EGFRf2 mutants is due to the loss of proneural gene expression; l'sc needs EGFR signal to be properly activated, in compliance with findings in the larval optic lobe [91]. In accordance with the distribution of activated EGFR in wild-type, in cell-death-deficient EGFRf2;Df(3L)H99 mutants, we found a large population of neuroblasts to be missing at all DV positions in PC, and specifically ventral neuroblasts in the posterior brain (TC/DC). By contrast, in the EGFR-mutant VNC intermediate neuroblasts do not develop, whereas ventral neuroblasts usually form but are often misspecified [20]. In VNC, EGFR promotes the formation of intermediate neuroblasts by activating ind expression [17,20]. At least in PC, it is likely that activity of Ind, in addition to L'sc, both of which are strongly reduced in EGFRf2 mutants, control the development of a small subset of protocerebral neuroblasts. Even though EGFR is active, in DC it does not impact the formation of intermediate neuroblasts; as these neuroblasts, opposed to the NE, express ind in EGFRf2 mutants, this further suggests that EGFR activity is dispensable for ind expression in these progenitors. In TC, onset of ind expression is delayed and regulated independently of EGFR (in NE and neuroblasts), explaining that intermediate neuroblasts are unaffected in the EGFRf2-mutant TC. However, in the mutant TC, we recognized an almost entire loss of ventral neuroblasts, which develop late. Moreover, L'sc was largely lacking there, and Vnd dissipated in the NE before these neuroblasts normally develop. Thus, it is likely that Vnd (see also [92]), together with L'sc, promote formation of these late-developing ventral neuroblasts. As Vnd is still expressed in the remaining ventral brain neuroblasts in EGFRf2 mutants (electronic supplementary material, figure S5f′), this suggests that vnd (similar to ind) expression is differently regulated in NE and neuroblasts (see also [24]), and further, that these ventral brain neuroblasts do not undergo a fate shift towards intermediate identity, as has been observed for approximately 50% of ventral neuroblasts in the EGFR-mutant VNC [20].
3.5. Phylogenetic considerations of EGFR-regulated patterning in the brain
The key components of the EGFR signalling pathways are evolutionarily highly conserved from fly to human. In vertebrates, 4 EGFR family members (ErbB1-4, with ErbB1 homologous to EGFR) and 11 EGF-like ligands are known (reviewed in [93,94]). In the forebrain, ErbB ligands secreted from a narrow region between the dorsal and ventral telencephalon (called ‘antihem’) have been proposed to assist in maintaining DV fates, which suggests a possible involvement of EGFR signalling in regional patterning of the cerebral cortex [95]. ErbB signalling might also be involved in patterning and differentiation of structures at the midbrain–hindbrain boundary (reviewed in [96]). However, ErbB signalling has not been connected with regulation of DV patterning genes (i.e. vnd/Nkx2, Nkx6, ind/Gsh). Instead, several other extrinsic signalling molecules are involved in their regulation, including the key player Shh, which is secreted from the floorplate (reviewed in [97–99]). This suggests that different upstream signalling pathways are used to control the expression of DV patterning genes in insect and vertebrate brains, even though the regionalized expression of these genes exhibits certain similarities in the embryonic brain of both animal phyla [52,100].
4. Material and methods
4.1. Drosophila genotypes
The following fly strains were used: Oregon R (wild-type); aosΔ7 [37], Df(3L)H99 [66], EGFRf2 [101], UAS-sim [102]; UAS-sSpi [21]; Matα-Gal4 [55], NGT40-Gal4 [62], simH9 [64], tlll49 [103] (all provided by Bloomington Drosophila Stock Center); rhoPΔ5 [37] (provided by Marta Llimargas Casanova); sca-Gal4 [57] (provided by Uwe Hinz); UAS-GroAA, UAS-GroDD [58] (provided by Ze'ev Paroush); UAS-tll [104] (provided by Mitsuhiko Kurusu); vnRG436 [105] (provided by Amanda Simcox); UAS-vnd [13], vnd6 [6] (provided by James Skeath).
4.2. Staging, flat preparation and mounting of embryos
Flat preparations of the head/truncal ectoderm of stained embryos and mounting were carried out as described previously [106].
4.3. Immunohistochemistry
Embryos were dechorionated, fixed and immunostained according to previously published protocols [48]. The following primary antibodies were used: mouse-anti-Dachshund 2–3 (1 : 250) [107], mouse-anti-Invected 4D9 (1 : 7) [108], mouse-anti-Prospero (1 : 10) (all provided by DSHB); rabbit-anti-Death caspase-1 (#9578) (1 : 50), rabbit-anti-p44/42-MAPK (1 : 500) (both provided by Cell Signalling Technology); guinea pig-anti-Deadpan (1 : 5000) [109] (provided by Jürgen Knoblich); rabbit-anti-Engrailed (1 : 800) (Santa Cruz Biotechnology); rabbit-anti-Ind (1 : 1000) [110] (provided by Tonia von Ohlen); mouse-anti-p44/42-MAPK (1 : 2000) (provided by Sigma Aldrich); rabbit-anti-PH3 (1 : 500) (provided by Merck Millipore); guinea pig-anti-Runt (1 : 300) [111] (provided by Ralf Pflanz); guinea pig-anti-Sim (1 : 1500) [112] (provided by Stephen Crews); rabbit-anti-Vnd (1 : 2000) [16] (provided by Marshall Nirenberg); sheep-anti-DIG alkaline-phosphatase conjugated (1 : 1000) (provided by Roche Diagnostics). The secondary antibodies were either biotinylated, conjugated with alkaline-phosphatase, or DyLight, Cyanine (all Jackson Immunoresearch) and Alexa (Life technologies) fluorescent dyes (all diluted 1 : 500). Tyramide signal amplification (TSA biotin system; PerkinElmer) was used in DAB stainings according to the manufacturer's protocol.
4.4. Whole mount in situ hybridization
Probes were synthesized using either linearized cDNA/EST-clones, cloned PCR products or PCR products containing a RNA polymerase adapter (both from genomic DNA) (electronic supplementary material, table S1) as a template. T3, T7 or SP6 Polymerase and DIG-RNA Labelling Mix (all Roche Diagnostics) were used for probe synthesis according to the manufacturers protocol. In situ hybridization was performed as described previously [51] and the probes processed with NBT/BCIP or VectorRed (Vector Labs) solution. Afterwards, the embryos were immunolabelled with a second primary antibody followed by incubation with biotinylated secondary antibodies, and processed with DAB.
4.5. Documentation
The non-fluorescent stainings were documented on a Zeiss Axioplan. Pictures were digitized with a CCD camera (Contron progress 3012). Fluorescent confocal images were acquired on a Leica TCS SP5 II. Pictures were processed with ImageJ, Adobe Photoshop CS4 and Adobe Illustrator CS4. Data (shown in figures 2p and 6c,f; electronic supplementary material, figure S5i,m) were analysed with a two-tailed unpaired Student's t-test.
Supplementary Material
Acknowledgements
We thank Dagmar Volland for excellent technical assistance and Gerd Technau for critical reading of the manuscript. We are indebted to James Skeath, Ralf Pflanz, Tonia von Ohlen, Joachim Urban, Jürgen Knoblich, Chris Doe, Stephen Crews, Marshall Nirenberg, Marta Llimargas Casanova, Uwe Hinz, Amanda Simcox, Mitsuhiko Kurusu, Ze'ev Paroush, Ethan Bier, the Bloomington Drosophila Stock Center, the Developmental Studies Hybridoma Bank and the Drosophila Genomic Resource Center for providing flies, antibodies and cDNA.
Authors' contributions
R.U. conceived the study. D.J. and R.U. designed experiments. D.J. performed the majority of experiments and analysed the majority of data. J.v.H. participated in the analysis of the wild-typic MAPK pattern and EGFR-dependent regulation of DV genes. D.J. prepared all figures. D.J. and R.U. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (UR163/2-2, UR163/3-3 to R.U.).
References
- 1.Skeath JB, Thor S. 2003. Genetic control of Drosophila nerve cord development. Curr. Opin. Neurobiol. 13, 8–15. (doi:10.1016/S0959-4388(03)00007-2) [DOI] [PubMed] [Google Scholar]
- 2.Dessaud E, McMahon AP, Briscoe J. 2008. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489–2503. (doi:10.1242/dev.009324) [DOI] [PubMed] [Google Scholar]
- 3.Doe CQ. 1992. Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development 116, 855–863. [DOI] [PubMed] [Google Scholar]
- 4.Bossing T, Udolph G, Doe CQ, Technau GM. 1996. The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev. Biol. 179, 41–64. (doi:10.1006/dbio.1996.0240) [DOI] [PubMed] [Google Scholar]
- 5.Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM. 1997. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev. Biol. 189, 186–204. (doi:10.1006/dbio.1997.8660) [DOI] [PubMed] [Google Scholar]
- 6.Jiménez F, Campos-Ortega JA. 1990. Defective neuroblast commitment in mutants of the achaete-scute complex and adjacent genes of D. melanogaster. Neuron 5, 81–89. (doi:10.1016/0896-6273(90)90036-F) [DOI] [PubMed] [Google Scholar]
- 7.Skeath JB, Panganiban GF, Carroll SB. 1994. The ventral nervous system defective gene controls proneural gene expression at two distinct steps during neuroblast formation in Drosophila. Development 120, 1517–1524. [DOI] [PubMed] [Google Scholar]
- 8.Jiménez F, Martin-Morris LE, Velasco L, Chu H, Sierra J, Rosen DR, White K. 1995. vnd, a gene required for early neurogenesis of Drosophila, encodes a homeodomain protein. EMBO J. 14, 3487–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellerick DM, Nirenberg M. 1995. Dorsal-ventral patterning genes restrict NK-2 homeobox gene expression to the ventral half of the central nervous system of Drosophila embryos. Dev. Biol. 171, 306–316. (doi:10.1006/dbio.1995.1283) [DOI] [PubMed] [Google Scholar]
- 10.D'Alessio M, Frasch M. 1996. msh may play a conserved role in dorsoventral patterning of the neuroectoderm and mesoderm. Mech. Dev. 58, 217–231. (doi:10.1016/S0925-4773(96)00583-7) [DOI] [PubMed] [Google Scholar]
- 11.Buescher M, Chia W. 1997. Mutations in lottchen cause cell fate transformations in both neuroblast and glioblast lineages in the Drosophila embryonic central nervous system. Development 124, 673–681. [DOI] [PubMed] [Google Scholar]
- 12.Isshiki T, Takeichi M, Nose A. 1997. The role of the msh homeobox gene during Drosophila neurogenesis: implication for the dorsoventral specification of the neuroectoderm. Development 124, 3099–3109. [DOI] [PubMed] [Google Scholar]
- 13.Chu H, Parras C, White K, Jiménez F. 1998. Formation and specification of ventral neuroblasts is controlled by vnd in Drosophila neurogenesis. Genes Dev. 12, 3613–3624. (doi:10.1101/gad.12.22.3613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss JB, Von Ohlen T, Mellerick DM, Dressler G, Doe CQ, Scott MP. 1998. Dorsoventral patterning in the Drosophila central nervous system: the intermediate neuroblasts defective homeobox gene specifies intermediate column identity. Genes Dev. 12, 3591–3602. (doi:10.1101/gad.12.22.3591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald JA, Holbrook S, Isshiki T, Weiss J, Doe CQ, Mellerick DM. 1998. Dorsoventral patterning in the Drosophila central nervous system: the vnd homeobox gene specifies ventral column identity. Genes Dev. 12, 3603–3612. (doi:10.1101/gad.12.22.3603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao X, Koizumi K, Nosworthy N, Tan DP, Odenwald W, Nirenberg M. 2002. Regulatory DNA required for vnd/NK-2 homeobox gene expression pattern in neuroblasts. Proc. Natl Acad. Sci. USA 99, 113–117. (doi:10.1073/pnas.012584599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Ohlen T, Doe CQ. 2000. Convergence of dorsal, dpp, and egfr signalling pathways subdivides the Drosophila neuroectoderm into three dorsal-ventral columns. Dev. Biol. 224, 362–372. (doi:10.1006/dbio.2000.9789) [DOI] [PubMed] [Google Scholar]
- 18.Mizutani CM, Meyer N, Roelink H, Bier E. 2006. Threshold-dependent BMP-mediated repression: a model for a conserved mechanism that patterns the neuroectoderm. PLoS Biol. 4, e313 (doi:10.1371/journal.pbio.0040313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong J-W, Hendrix DA, Papatsenko D, Levine MS. 2008. How the Dorsal gradient works: insights from postgenome technologies. Proc. Natl Acad. Sci. USA 105, 20 072–20 076. (doi:10.1073/pnas.0806476105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skeath JB. 1998. The Drosophila EGF receptor controls the formation and specification of neuroblasts along the dorsal-ventral axis of the Drosophila embryo. Development 125, 3301–3312. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G, Wheeler SR, Skeath JB. 2007. Genetic control of dorsoventral patterning and neuroblast specification in the Drosophila central nervous system. Int. J. Dev. Biol. 51, 107–115. (doi:10.1387/ijdb.062188gz) [DOI] [PubMed] [Google Scholar]
- 22.Shilo B-Z. 2005. Regulating the dynamics of EGF receptor signalling in space and time. Development 132, 4017–4027. (doi:10.1242/dev.02006) [DOI] [PubMed] [Google Scholar]
- 23.Lemmon MA, Schlessinger J. 2010. Cell signalling by receptor tyrosine kinases. Cell 141, 1117–1134. (doi:10.1016/j.cell.2010.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao G, Skeath JB. 2002. The Sox-domain containing gene Dichaete/fish-hook acts in concert with vnd and ind to regulate cell fate in the Drosophila neuroectoderm. Development 129, 1165–1174. [DOI] [PubMed] [Google Scholar]
- 25.Ajuria L, et al. 2011. Capicua DNA-binding sites are general response elements for RTK signalling in Drosophila. Development 138, 915–924. (doi:10.1242/dev.057729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnepp B, Grumbling G, Donaldson T, Simcox A. 1996. Vein is a novel component in the Drosophila epidermal growth factor receptor pathway with similarity to the neuregulins. Genes Dev. 10, 2302–2313. (doi:10.1101/gad.10.18.2302) [DOI] [PubMed] [Google Scholar]
- 27.Gabay L, Seger R, Shilo BZ. 1997. In situ activation pattern of Drosophila EGF receptor pathway during development. Science 277, 1103–1106. (doi:10.1126/science.277.5329.1103) [DOI] [PubMed] [Google Scholar]
- 28.Urban S, Lee JR, Freeman M. 2002. A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J. 21, 4277–4286. (doi:10.1093/emboj/cdf434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweitzer R, Shaharabany M, Seger R, Shilo BZ. 1995. Secreted Spitz triggers the DER signalling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev. 9, 1518–1529. (doi:10.1101/gad.9.12.1518) [DOI] [PubMed] [Google Scholar]
- 30.Lee JR, Urban S, Garvey CF, Freeman M. 2001. Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell 107, 161–171. (doi:10.1016/S0092-8674(01)00526-8) [DOI] [PubMed] [Google Scholar]
- 31.Tsruya R, Schlesinger A, Reich A, Gabay L, Sapir A, Shilo B-Z. 2002. Intracellular trafficking by Star regulates cleavage of the Drosophila EGF receptor ligand Spitz. Genes Dev. 16, 222–234. (doi:10.1101/gad.214202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutledge BJ, Zhang K, Bier E, Jan YN, Perrimon N. 1992. The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal-ventral axis formation and neurogenesis. Genes Dev. 6, 1503–1517. (doi:10.1101/gad.6.8.1503) [DOI] [PubMed] [Google Scholar]
- 33.Heberlein U, Hariharan IK, Rubin GM. 1993. Star is required for neuronal differentiation in the Drosophila retina and displays dosage-sensitive interactions with Ras1. Dev. Biol. 160, 51–63. (doi:10.1006/dbio.1993.1285) [DOI] [PubMed] [Google Scholar]
- 34.Kolodkin AL, Pickup AT, Lin DM, Goodman CS, Banerjee U. 1994. Characterization of Star and its interactions with sevenless and EGF receptor during photoreceptor cell development in Drosophila. Development 120, 1731–1745. [DOI] [PubMed] [Google Scholar]
- 35.Golembo M, Raz E, Shilo BZ. 1996. The Drosophila embryonic midline is the site of Spitz processing, and induces activation of the EGF receptor in the ventral ectoderm. Development 122, 3363–3370. [DOI] [PubMed] [Google Scholar]
- 36.Golembo M, Schweitzer R, Freeman M, Shilo BZ. 1996. Argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development 122, 223–230. [DOI] [PubMed] [Google Scholar]
- 37.Freeman M, Klämbt C, Goodman CS, Rubin GM. 1992. The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye. Cell 69, 963–975. (doi:10.1016/0092-8674(92)90615-J) [DOI] [PubMed] [Google Scholar]
- 38.Schweitzer R, Howes R, Smith R, Shilo BZ, Freeman M. 1995. Inhibition of Drosophila EGF receptor activation by the secreted protein Argos. Nature 376, 699–702. (doi:10.1038/376699a0) [DOI] [PubMed] [Google Scholar]
- 39.Klein DE, Nappi VM, Reeves GT, Shvartsman SY, Lemmon MA. 2004. Argos inhibits epidermal growth factor receptor signalling by ligand sequestration. Nature 430, 1040–1044. (doi:10.1038/nature02840) [DOI] [PubMed] [Google Scholar]
- 40.Bier E, Jan LY, Jan YN. 1990. rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev. 4, 190–203. (doi:10.1101/gad.4.2.190) [DOI] [PubMed] [Google Scholar]
- 41.Udolph G, Urban J, Rüsing G, Lüer K, Technau GM. 1998. Differential effects of EGF receptor signalling on neuroblast lineages along the dorsoventral axis of the Drosophila CNS. Development 125, 3291–3299. [DOI] [PubMed] [Google Scholar]
- 42.Golembo M, Yarnitzky T, Volk T, Shilo BZ. 1999. Vein expression is induced by the EGF receptor pathway to provide a positive feedback loop in patterning the Drosophila embryonic ventral ectoderm. Genes Dev. 13, 158–162. (doi:10.1101/gad.13.2.158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumstrei K, Nassif C, Abboud G, Aryai A, Aryai A, Hartenstein V. 1998. EGFR signalling is required for the differentiation and maintenance of neural progenitors along the dorsal midline of the Drosophila embryonic head. Development 125, 3417–3426. [DOI] [PubMed] [Google Scholar]
- 44.Daniel A, Dumstrei K, Lengyel JA, Hartenstein V. 1999. The control of cell fate in the embryonic visual system by atonal, tailless and EGFR signalling. Development 126, 2945–2954. [DOI] [PubMed] [Google Scholar]
- 45.Dumstrei K, Wang F, Shy D, Tepass U, Hartenstein V. 2002. Interaction between EGFR signalling and DE-cadherin during nervous system morphogenesis. Development 129, 3983–3994. [DOI] [PubMed] [Google Scholar]
- 46.Chang T, Shy D, Hartenstein V. 2003. Antagonistic relationship between Dpp and EGFR signalling in Drosophila head patterning. Dev. Biol. 263, 103–113. (doi:10.1016/S0012-1606(03)00448-2) [DOI] [PubMed] [Google Scholar]
- 47.Younossi-Hartenstein A, Nassif C, Green P, Hartenstein V. 1996. Early neurogenesis of the Drosophila brain. J. Comp. Neurol. 370, 313–329. (doi:10.1002/(SICI)1096-9861(19960701)370:3<313::AID-CNE3>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 48.Urbach R, Schnabel R, Technau GM. 2003. The pattern of neuroblast formation, mitotic domains and proneural gene expression during early brain development in Drosophila. Development 130, 3589–3606. (doi:10.1242/dev.00528) [DOI] [PubMed] [Google Scholar]
- 49.Urbach R, Technau GM. 2003. Molecular markers for identified neuroblasts in the developing brain of Drosophila. Development 130, 3621–3637. (doi:10.1242/dev.00533) [DOI] [PubMed] [Google Scholar]
- 50.Urbach R, Technau GM. 2003. Segment polarity and DV patterning gene expression reveals segmental organization of the Drosophila brain. Development 130, 3607–3620. (doi:10.1242/dev.00532) [DOI] [PubMed] [Google Scholar]
- 51.Urbach R, Volland D, Seibert J, Technau GM. 2006. Segment-specific requirements for dorsoventral patterning genes during early brain development in Drosophila. Development 133, 4315–4330. (doi:10.1242/dev.02605) [DOI] [PubMed] [Google Scholar]
- 52.Seibert J, Volland D, Urbach R. 2009. Ems and Nkx6 are central regulators in dorsoventral patterning of the Drosophila brain. Development 136, 3937–3947. (doi:10.1242/dev.041921) [DOI] [PubMed] [Google Scholar]
- 53.Seibert J, Urbach R. 2010. Role of en and novel interactions between msh, ind, and vnd in dorsoventral patterning of the Drosophila brain and ventral nerve cord. Dev. Biol. 346, 332–345. (doi:10.1016/j.ydbio.2010.07.024) [DOI] [PubMed] [Google Scholar]
- 54.Nambu JR, Lewis JO, Wharton KA, Crews ST. 1991. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell 67, 1157–1167. (doi:10.1016/0092-8674(91)90292-7) [DOI] [PubMed] [Google Scholar]
- 55.Häcker U, Perrimon N. 1998. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12, 274–284. (doi:10.1101/gad.12.2.274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klaes A, Menne T, Stollewerk A, Scholz H, Klämbt C. 1994. The Ets transcription factors encoded by the Drosophila gene pointed direct glial cell differentiation in the embryonic CNS. Cell 78, 149–160. (doi:10.1016/0092-8674(94)90581-9) [DOI] [PubMed] [Google Scholar]
- 57.Zaffran S, Das G, Frasch M. 2000. The NK-2 homeobox gene scarecrow (scro) is expressed in pharynx, ventral nerve cord and brain of Drosophila embryos. Mech. Dev. 94, 237–241. (doi:10.1016/S0925-4773(00)00298-7) [DOI] [PubMed] [Google Scholar]
- 58.Hasson P, Egoz N, Winkler C, Volohonsky G, Jia S, Dinur T, Volk T, Courey AJ, Paroush ZE. 2005. EGFR signalling attenuates Groucho-dependent repression to antagonize Notch transcriptional output. Nat. Genet. 37, 101–105. (doi:10.1038/ng1486) [DOI] [PubMed] [Google Scholar]
- 59.Cinnamon E, Helman A, Ben-Haroush Schyr R, Orian A, Jiménez G, Paroush Z. 2008. Multiple RTK pathways downregulate Groucho-mediated repression in Drosophila embryogenesis. Development 135, 829–837. (doi:10.1242/dev.015206) [DOI] [PubMed] [Google Scholar]
- 60.Helman A, Cinnamon E, Mezuman S, Hayouka Z, Von Ohlen T, Orian A, Jiménez G, Paroush ZE. 2011. Phosphorylation of Groucho mediates RTK feedback inhibition and prolonged pathway target gene expression. Curr. Biol. 21, 1102–1110. (doi:10.1016/j.cub.2011.05.043) [DOI] [PubMed] [Google Scholar]
- 61.Yu Z, Syu L-J, Mellerick DM. 2005. Contextual interactions determine whether the Drosophila homeodomain protein, Vnd, acts as a repressor or activator. Nucleic Acids Res. 33, 1–12. (doi:10.1093/nar/gki140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tracey WD, Ning X, Klingler M, Kramer SG, Gergen JP. 2000. Quantitative analysis of gene function in the Drosophila embryo. Genetics 154, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudolph KM, Liaw GJ, Daniel A, Green P, Courey AJ, Hartenstein V, Lengyel JA. 1997. Complex regulatory region mediating tailless expression in early embryonic patterning and brain development. Development 124, 4297–4308. [DOI] [PubMed] [Google Scholar]
- 64.Thomas JB, Crews ST, Goodman CS. 1988. Molecular genetics of the single-minded locus: a gene involved in the development of the Drosophila nervous system. Cell 52, 133–141. (doi:10.1016/0092-8674(88)90537-5) [DOI] [PubMed] [Google Scholar]
- 65.Chang J, Kim IO, Ahn JS, Kim SH. 2001. The CNS midline cells control the spitz class and Egfr signalling genes to establish the proper cell fate of the Drosophila ventral neuroectoderm. Int. J. Dev. Biol. 45, 715–724. [PubMed] [Google Scholar]
- 66.White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. 1994. Genetic control of programmed cell death in Drosophila. Science 264, 677–683. (doi:10.1126/science.8171319) [DOI] [PubMed] [Google Scholar]
- 67.Foe VE. 1989. Mitotic domains reveal early commitment of cells in Drosophila embryos. Development 107, 1–22. [PubMed] [Google Scholar]
- 68.Crews ST, Thomas JB, Goodman CS. 1988. The Drosophila single-minded gene encodes a nuclear protein with sequence similarity to the per gene product. Cell 52, 143–151. (doi:10.1016/0092-8674(88)90538-7) [DOI] [PubMed] [Google Scholar]
- 69.Nambu JR, Franks RG, Hu S, Crews ST. 1990. The single-minded gene of Drosophila is required for the expression of genes important for the development of CNS midline cells. Cell 63, 63–75. (doi:10.1016/0092-8674(90)90288-P) [DOI] [PubMed] [Google Scholar]
- 70.Schnepp B, Donaldson T, Grumbling G, Ostrowski S, Schweitzer R, Shilo BZ, Simcox A. 1998. EGF domain swap converts a Drosophila EGF receptor activator into an inhibitor. Genes Dev. 12, 908–913. (doi:10.1101/gad.12.7.908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsruya R, Wojtalla A, Carmon S, Yogev S, Reich A, Bibi E, Merdes G, Schejter E, Shilo B-Z. 2007. Rhomboid cleaves Star to regulate the levels of secreted Spitz. EMBO J. 26, 1211–1220. (doi:10.1038/sj.emboj.7601581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ip YT, Park RE, Kosman D, Bier E, Levine M. 1992. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev. 6, 1728–1739. (doi:10.1101/gad.6.9.1728) [DOI] [PubMed] [Google Scholar]
- 73.Stathopoulos A, Van Drenth M, Erives A, Markstein M, Levine M. 2002. Whole-genome analysis of dorsal-ventral patterning in the Drosophila embryo. Cell 111, 687–701. (doi:10.1016/S0092-8674(02)01087-5) [DOI] [PubMed] [Google Scholar]
- 74.Markstein M, Zinzen R, Markstein P, Yee K-P, Erives A, Stathopoulos A, Levine M. 2004. A regulatory code for neurogenic gene expression in the Drosophila embryo. Development 131, 2387–2394. (doi:10.1242/dev.01124) [DOI] [PubMed] [Google Scholar]
- 75.Musacchio M, Perrimon N. 1996. The Drosophila kekkon genes: novel members of both the leucine-rich repeat and immunoglobulin superfamilies expressed in the CNS. Dev. Biol. 178, 63–76. (doi: 10.1006/dbio.1996.0198) [DOI] [PubMed] [Google Scholar]
- 76.Ghiglione C, Carraway KL, Amundadottir LT, Boswell RE, Perrimon N, Duffy JB. 1999. The transmembrane molecule kekkon 1 acts in a feedback loop to negatively regulate the activity of the Drosophila EGF receptor during oogenesis. Cell 96, 847–856. (doi:10.1016/S0092-8674(00)80594-2) [DOI] [PubMed] [Google Scholar]
- 77.Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y. 1999. Sprouty: a common antagonist of FGF and EGF signalling pathways in Drosophila. Development 126, 2515–2525. [DOI] [PubMed] [Google Scholar]
- 78.Reich A, Sapir A, Shilo B. 1999. Sprouty is a general inhibitor of receptor tyrosine kinase signalling. Development 126, 4139–4147. [DOI] [PubMed] [Google Scholar]
- 79.Kim S-H, Kwon H-B, Kim Y-S, Ryu J-H, Kim K-S, Ahn Y, Lee W-J, Choi K-Y. 2002. Isolation and characterization of a Drosophila homologue of mitogen-activated protein kinase phosphatase-3 which has a high substrate specificity towards extracellular-signal-regulated kinase. Biochem. J. 361, 143–151. (doi:10.1042/bj3610143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim M, Cha G-H, Kim S, Lee JH, Park J, Koh H, Choi K-Y, Chung J. 2004. MKP-3 has essential roles as a negative regulator of the Ras/mitogen-activated protein kinase pathway during Drosophila development. Mol. Cell. Biol. 24, 573–583. (doi:10.1128/MCB.24.2.573-583.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butchar JP, et al. 2012. New negative feedback regulators of Egfr signalling in Drosophila. Genetics 191, 1213–1226. (doi:10.1534/genetics.112.141093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kammermeier L, Leemans R, Hirth F, Flister S, Wenger U, Walldorf U, Gehring WJ, Reichert H. 2001. Differential expression and function of the Drosophila Pax6 genes eyeless and twin of eyeless in embryonic central nervous system development. Mech. Dev. 103, 71–78. (doi:10.1016/S0925-4773(01)00328-8) [DOI] [PubMed] [Google Scholar]
- 83.Skeath JB. 1999. At the nexus between pattern formation and cell-type specification: the generation of individual neuroblast fates in the Drosophila embryonic central nervous system. Bioessays 21, 922–931. (doi:10.1002/(sici)1521-1878(199911)21:11<922::aid-bies4>3.0.co;2-t) [DOI] [PubMed] [Google Scholar]
- 84.Zhang H, Syu L-J, Modica V, Yu Z, Von Ohlen T, Mellerick DM. 2008. The Drosophila homeodomain transcription factor, Vnd, associates with a variety of co-factors, is extensively phosphorylated and forms multiple complexes in embryos. FEBS J. 275, 5062–5073. (doi:10.1111/j.1742-4658.2008.06639.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schweitzer R, Shilo BZ. 1997. A thousand and one roles for the Drosophila EGF receptor. Trends Genet. 13, 191–196. (doi:10.1016/S0168-9525(97)01091-3) [DOI] [PubMed] [Google Scholar]
- 86.Garoia F, Grifoni D, Trotta V, Guerra D, Pezzoli MC, Cavicchi S. 2005. The tumor suppressor gene fat modulates the EGFR-mediated proliferation control in the imaginal tissues of Drosophila melanogaster. Mech. Dev. 122, 175–187. (doi:10.1016/j.mod.2004.10.007) [DOI] [PubMed] [Google Scholar]
- 87.Marenda DR, Vrailas AD, Rodrigues AB, Cook S, Powers MA, Lorenzen JA, Perkins LA, Moses K. 2006. MAP kinase subcellular localization controls both pattern and proliferation in the developing Drosophila wing. Development 133, 43–51. (doi:10.1242/dev.02168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang H, Edgar BA. 2009. EGFR signalling regulates the proliferation of Drosophila adult midgut progenitors. Development 136, 483–493. (doi:10.1242/dev.026955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bergmann A, Tugentman M, Shilo BZ, Steller H. 2002. Regulation of cell number by MAPK-dependent control of apoptosis: a mechanism for trophic survival signalling. Dev. Cell 2, 159–170. (doi:10.1016/S1534-5807(02)00116-8) [DOI] [PubMed] [Google Scholar]
- 90.Hidalgo A. 2002. Interactive nervous system development: control of cell survival in Drosophila. Trends Neurosci. 25, 365–370. (doi:10.1016/S0166-2236(02)02186-0) [DOI] [PubMed] [Google Scholar]
- 91.Yasugi T, Sugie A, Umetsu D, Tabata T. 2010. Coordinated sequential action of EGFR and Notch signalling pathways regulates proneural wave progression in the Drosophila optic lobe. Development 137, 3193–3203. (doi:10.1242/dev.048058) [DOI] [PubMed] [Google Scholar]
- 92.Sprecher SG, Urbach R, Technau GM, Rijli FM, Reichert H, Hirth F. 2006. The columnar gene vnd is required for tritocerebral neuromere formation during embryonic brain development of Drosophila. Development 133, 4331–4339. (doi:10.1242/dev.02606) [DOI] [PubMed] [Google Scholar]
- 93.Bogdan S, Klämbt C. 2001. Epidermal growth factor receptor signalling. Curr. Biol. 11, R292–R295. (doi:10.1016/S0960-9822(01)00167-1) [DOI] [PubMed] [Google Scholar]
- 94.Iwakura Y, Nawa H. 2013. ErbB1-4-dependent EGF/neuregulin signals and their cross talk in the central nervous system: pathological implications in schizophrenia and Parkinson's disease. Front. Cell Neurosci. 7, 4 (doi:10.3389/fncel.2013.00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Assimacopoulos S, Grove EA, Ragsdale CW. 2003. Identification of a Pax6-dependent epidermal growth factor family signalling source at the lateral edge of the embryonic cerebral cortex. J. Neurosci. 23, 6399–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakamura H. 2013. Midbrain patterning: isthmus organizer, tectum regionalization and polarity formation. In PNS (eds Rubenstein J, Rakic P), pp. 45–60. New York, NY: Academic Press. [Google Scholar]
- 97.Rallu M, Corbin JG, Fishell G. 2002. Parsing the prosencephalon. Nat. Rev. Neurosci. 3, 943–951. (doi:10.1038/nrn989) [DOI] [PubMed] [Google Scholar]
- 98.Lupo G, Harris WA, Lewis KE. 2006. Mechanisms of ventral patterning in the vertebrate nervous system. Nat. Rev. Neurosci. 7, 103–114. (doi:10.1038/nrn1843) [DOI] [PubMed] [Google Scholar]
- 99.Tole S, Hebert J. 2013. Telencephalon patterning. In Patterning and cell type specification in the developing CNS and PNS (eds Rubenstein J, Rakic P), pp. 3–24. New York, NY: Academic press. [Google Scholar]
- 100.Urbach R, Technau GM. 2008. Dorsoventral patterning of the brain: a comparative approach. Adv. Exp. Med. Biol. 628, 42–56. (doi:10.1007/978-0-387-78261-4_3) [DOI] [PubMed] [Google Scholar]
- 101.Clifford R, Schüpbach T. 1994. Molecular analysis of the Drosophila EGF receptor homolog reveals that several genetically defined classes of alleles cluster in subdomains of the receptor protein. Genetics 137, 531–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao H, Hrdlicka LA, Nambu JR. 1996. Alternate functions of the single-minded and rhomboid genes in development of the Drosophila ventral neuroectoderm. Mech. Dev. 58, 65–74. (doi:10.1016/S0925-4773(96)00559-X) [DOI] [PubMed] [Google Scholar]
- 103.Pignoni F, Baldarelli RM, Steingrímsson E, Diaz RJ, Patapoutian A, Merriam JR, Lengyel JA. 1990. The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily. Cell 62, 151–163. (doi:10.1016/0092-8674(90)90249-E) [DOI] [PubMed] [Google Scholar]
- 104.Kurusu M, Maruyama Y, Adachi Y, Okabe M, Suzuki E, Furukubo-Tokunaga K. 2009. A conserved nuclear receptor, Tailless, is required for efficient proliferation and prolonged maintenance of mushroom body progenitors in the Drosophila brain. Dev. Biol. 326, 224–236. (doi:10.1016/j.ydbio.2008.11.013) [DOI] [PubMed] [Google Scholar]
- 105.Wurst G, Hersperger E, Shearn A. 1984. Genetic analysis of transdetermination in Drosophila. II. Transdetermination to wing of leg discs from a mutant which lacks wing discs. Dev. Biol. 106, 147–155. (doi:10.1016/0012-1606(84)90070-8) [DOI] [PubMed] [Google Scholar]
- 106.Jussen D, Urbach R. 2014. Non-fluorescent RNA in situ hybridization combined with antibody staining to visualize multiple gene expression patterns in the embryonic brain of Drosophila. Methods Mol. Biol. 1082, 19–35. (doi:10.1007/978-1-62703-655-9_2) [DOI] [PubMed] [Google Scholar]
- 107.Mardon G, Solomon NM, Rubin GM. 1994. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120, 3473–3486. [DOI] [PubMed] [Google Scholar]
- 108.Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS. 1989. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell 58, 955–968. (doi:10.1016/0092-8674(89)90947-1) [DOI] [PubMed] [Google Scholar]
- 109.Homem CCF, Reichardt I, Berger C, Lendl T, Knoblich JA. 2013. Long-term live cell imaging and automated 4D analysis of drosophila neuroblast lineages. PLoS ONE 8, e79588 (doi:10.1371/journal.pone.0079588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Von Ohlen TL, Moses C. 2009. Identification of Ind transcription activation and repression domains required for dorsoventral patterning of the CNS. Mech. Dev. 126, 552–562. (10.1016/j.mod.2009.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kosman D, Small S, Reinitz J. 1998. Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev. Genes Evol. 208, 290–294. (doi:10.1007/s004270050184) [DOI] [PubMed] [Google Scholar]
- 112.Wheeler SR, Stagg SB, Crews ST. 2008. Multiple Notch signalling events control Drosophila CNS midline neurogenesis, gliogenesis and neuronal identity. Development 135, 3071–3079. (doi:10.1242/dev.022343) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.