Abstract
Biodiversity experiments have generated robust empirical results supporting the hypothesis that ecosystems function better when they contain more species. Given that ecosystems provide services that are valued by humans, this inevitably suggests that the loss of species from natural ecosystems could diminish their value. This raises two important questions. First, will experimental results translate into the real world, where species are being lost at an alarming rate? And second, what are the benefits and pitfalls of such valuation exercises? We argue that the empirical results obtained in experiments are entirely consistent with well-established theories of species coexistence. We then examine the current body of work through the lens of niche theory and highlight where closer links with theory could open up opportunities for future research. We argue that niche theory predicts that diversity–functioning relationships are likely to be stronger (and require more species) in the field than in simplified experimental settings. However, we caution that while many of the biological processes that promote coexistence can also generate diversity–function relationships, there is no simple mapping between the two. This implies that valuation exercises need to proceed with care.
Keywords: biodiversity, ecosystem functioning, niche, coexistence, value, ecosystem services
1. Introduction
The ongoing loss of biodiversity [1] has prompted ecologists to investigate the role of species in ecosystems in much the same way as molecular biologists investigate how individual genes affect the functioning of organisms. Molecular biologists often create ‘knock-out mutants’—individuals that lack a gene of interest—and compare their structure and function with the respective wild-type [2]. Losing a gene can have major, sometimes fatal, consequences for the functioning of the organism; but genomes have also evolved redundancy, in which there is total or partial compensation for the ‘lost’ gene and phenotypic effects are minimized [3].
Over the past 20 years, ecologists have begun to investigate the role of species in ecosystems in a similar way. Biodiversity experiments measure the functioning of experimental ecological communities from which chosen species have been deliberately left out. Ecosystem functioning is used as a collective term for various biogeochemical processes, including stocks and flows of both energy and matter. These include processes familiar to ecosystems ecologists such as biomass production and the cycling of elements (nutrients) and water. The ecosystems used in these experiments have mostly been communities of grasses and herbaceous plants (but more recently forests, marine and freshwater ecosystems) owing to the relative ease of manipulating diversity and in measuring the response of different aspects of functioning.
Currently, we do not know enough about complex natural systems to understand how they function in detail (for example, the global carbon cycle is still not fully understood [4]). It is also unclear whether, and to what degree, evolution may act on ecosystem processes directly. Theory predicts that ecosystem-level selection, if it exists, should be relatively weak [5] and currently, we are not aware of any evidence that ecosystems have been selected to maximize any particular process or function (like productivity). However, we do know that natural ecosystems inadvertently contribute to the regulation of the biosphere in many ways that are beneficial to humans via the so-called ecosystem services [6,7]. Examples include the provision of clean air and water, and regulation of the climate. Somewhat contentiously, ecosystems have therefore been assigned monetary value [8].
However, ecosystems depend on the organisms within them. Over the past quarter century, biodiversity experiments have revealed that communities with fewer species generally function less efficiently [5,7,9–11]. This literature is now large and has been well synthesized, including several detailed meta-analyses [12,13]. Net primary productivity (or a surrogate variable) is one of the most widely examined response variables because it is easy to measure and is an energy bottleneck for organisms at higher trophic levels. Although the diversity dependence of functions varies among systems, and depends on which species are lost, the overwhelming majority of studies show that loss of species will decrease ecosystem functioning [12,13]. The shape of the relationship between diversity and functioning is often decelerating, although this is not always the case [14], particularly in long-term studies [15]. Hence the on-going loss of biodiversity is predicted to have increasingly negative impacts on ecosystem functioning.
While relatively few species may be required to support a particular function—at least in the short term—different functions generally require different species. This has led to the concept of ecosystem multi-functionality [16–21]. In general, studies have found that the more functions they consider, the greater the number of species that play a functionally important role. Similarly, if functions are measured over a wider range of environmental conditions or over a longer period of time then a greater number of species are shown to play a role [17,22]. The relationship between diversity and stability has been studied since at least the 1950s, when the focus tended to be on the stability of community composition and structure rather than the associated ecosystem functions [23,24]. More recently, the field of ecosystem functioning has focused on measures of long-term temporal stability (e.g. the coefficient of variation of biomass production over time) and measures of resistance to, and recovery from (resilience), large perturbations such as extreme climatic events. Biodiversity experiments often show that year-to-year stability of biomass production increases with diversity [25–27]. Diversity can also increase the resistance of ecosystem productivity to climatic extremes [28].
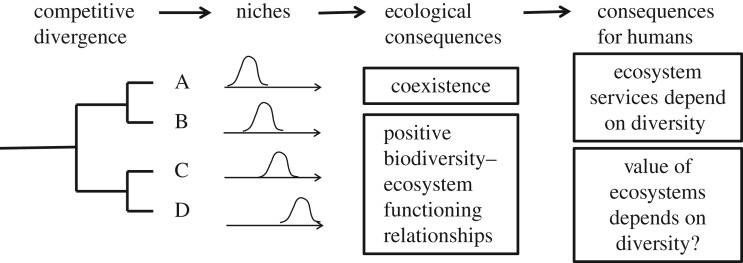
If ecosystems have value because they provide services to humans and ecosystems function better when they contain more species, then the loss of species could diminish the value of ecosystems. This raises two important questions. First, can we ever hope to know the precise nature of the relationships between species, ecosystem functioning, services and value in the real world (figure 1)? Second, is it sensible or desirable to value species in such a narrow way? To answer the first question, we suggest that niche theory provides a strong theoretical framework for understanding relationships between species and ecosystem functioning, which can (among other things) help us to decide how widely we can apply lessons learned in experiments to the real world. To answer the second question, we briefly review the merits and perils of valuation systems.
Figure 1.
Competitive divergence creates niches and the presence of niches has two major ecological consequences. First, niches create stabilizing forces that lead to coexistence. Second, the presence of niches makes it highly likely that ecosystem functioning will be enhanced when more species are present, owing to better coverage of the niche space. Thus, the services delivered by ecosystems are likely to be diversity-dependent, as is the value that we place on ecosystems. However, the precise mapping between species, ecosystem functioning, services and value is likely to be complex.
2. Niche theory
Niche theory is the centre of gravity of community ecology. Niche theory states that guilds of competing species will diverge, leading to reduced niche overlap [29], otherwise all but one of them will be driven extinct (the competitive exclusion principle). This means that we expect, a priori, that species will not only look different in ways that enable us to easily identify them, but that they will be ecologically different in ways that are functionally important. Indeed, Darwin [30,31] recognized this consequence of his principle of divergence [32]. The ubiquity of ecological niches provides a general explanation for the positive relationship between diversity and functioning: through competitive divergence each species only covers some part of the total niche space available in a community [33].
In traditional theory, the niche space is defined by a number of niche axes, but in practice these are difficult to measure, especially for plants. One scheme [34] distinguishes niche axes that relate to: (i) resources (that decrease when the abundance of focal organisms increases); (ii) natural enemies (that increase as the abundance of focal organisms increases) and (iii) abiotic ‘stress’ factors (that neither increase nor decrease as the abundance of focal organisms changes). Thus, species might differ in how they use resources, in their resistance to different natural enemies and in their response to abiotic conditions. Of course many species are highly plastic, especially plants. When competitors are removed, the remaining species might expand their niche space (fundamental versus realized niches)—which probably explains the nonlinear response to diversity loss. However, we would still expect a loss of function, through a loss of efficiency. Species generally outcompete others because they are ‘better’ at using the resource in question. Hence, the impacts of species loss are reduced by plasticity, but they are not removed entirely.
A recent re-framing of niche theory provides a more explicit way in which the field of biodiversity research could potentially be linked with coexistence theory. Chesson suggested that we could understand the coexistence of species by identifying and distinguishing between equalizing and stabilizing forces [35]. Species differences affect both of these forces [36]. On the one hand, differences between species can lead to differences in fitness, which can lead to competitive exclusion. But, differences between species inevitably arise as they evolve into different niches, providing a stabilizing force. Coexistence occurs when the differences between species lead to stabilizing forces that are strong enough to overcome fitness differences [37]. The larger the fitness differences, the larger the stabilizing forces needed to offset them. Small differences between species can lead to small fitness differences requiring only weak stabilizing forces to ensure coexistence.
There are potential parallels between the Chesson scheme for coexistence and the additive partitioning method that is commonly applied to biodiversity experiments [38]. The additive partition defines a net biodiversity effect and splits it into selection and complementarity effects. The selection effect quantifies the covariance between monoculture yields and relative performance in mixture. Positive selection effects indicate that high-yielding species dominate mixtures and negative selection effects indicate the reverse. The complementarity effect (or overyielding) is positive when increases in the relative yields of some species are not exactly compensated by decreases in others and is commonly interpreted as evidence for complementarity, for example, with regard to resource use, natural enemy impacts or other diversity-dependent factors. Unfortunately, however, there is no one-to-one mapping between the two schemes [39–41]. Selection and complementarity effects are influenced by both fitness differences and niche differences.
While a formal one-to-one mapping between coexistence theory and the field of biodiversity and ecosystem functioning research may not be possible, the two areas of research are highly interrelated and should draw more strength from each other. In the following section, we highlight how niche theory in its broadest sense can help to inform current results from biodiversity experiments and illuminate how the lessons learned using experimental platforms are likely to apply in the real world.
3. Understanding biodiversity experiments through niche theory
In this section, we focus on three key results from biodiversity experiments: biomass overyielding, ecosystem multi-functionality and temporal stability. In each case, we demonstrate how niche theory can help us to better understand these results. We also highlight where confusion remains and indicate potential directions for future research.
Community overyielding occurs when increases in the relative yields of species that are doing better than expected in mixture (based on monoculture yields and planted proportions) are not perfectly compensated by concomitant declines in the relative yields of their competitors [38]. In the context of niche theory, Vandermeer [42] and Loreau [5,43] have shown that this is directly analogous to the reduction of interspecific relative to intraspecific competition that is necessary for coexistence of species within the Lotka–Volterra framework. There are a number of ways in which this can come about. The interpretation of the earliest biodiversity experiments often focused on differential use of resources: resource-use complementarity. Different functional groups and species of plants have been shown to be complementary in their use of a range of resources, including the form of nitrogen, water and light [44–46]. Hence, resource-use complementarity seen in biodiversity experiments is generally consistent with resource-based theories of coexistence [33,47]. However, we have previously shown that complementarity effects can arise even when stabilizing forces are insufficient for long-term coexistence [39]. The development of better methods for assessing the evidence for resource-use complementarity and long-term coexistence in biodiversity experiments is an important direction for future research [48].
Overyielding can also result from partitioning enemy-free niche space [5]. For example, diversity can lead to overyielding when pests and diseases have reduced incidence and severity when species are grown in mixture [49]. The impacts of pests and diseases in natural communities of plants are still poorly known and there have been mixed results from biodiversity experiments [50–52]. But a higher incidence of pests and diseases has been recorded in monocultures of some species in forestry [53] and in agriculture [54,55] and is one reason to favour the intercropping of different species. We expect species in diverse communities to be less affected by specialist pests and diseases because vulnerable hosts are interspersed with species that are invulnerable (or at least less vulnerable [56]). This might mean that specialist pests are less able to effectively locate their hosts in mixtures (‘associational resistance’) [57] or that pest and diseases achieve lower population densities in mixtures (‘resource concentration effects’), perhaps owing to reduced transmission rates. The higher observed incidence of specialist pests and diseases in monocultures and low-diversity ecosystems is well established in community ecology, for example, through the so-called Janzen–Connell effects [58,59] and negative soil feedbacks [60]. Quantifying the role of natural enemies in diversity-function experiments relative to resource partitioning and abiotic factors would be an exciting future direction.
The link between niche theory and ecosystem multi-functionality is inevitably more complex than the link to a single ecosystem function such as productivity. One complexity is that different ecosystem functions are rarely independent. Instead, it is likely that in many cases pairs of functions are positively or negatively correlated [18,19]. For example, across monocultures drawn from a plant community we might expect biomass to be negatively correlated with end-of-season soil nutrient levels (if plants are equally efficient at converting nutrients into biomass). Surprisingly, these correlations between functions are currently poorly explored (but see [20]). Niche theory predicts that negative correlations between functions are expected to be widespread because of ecological trade-offs (for example, between growth and defence [2]).
Competition is predicted to generate differences in traits among species associated with ecological specialization and hence with stabilization. But, constraints imposed by life-history trade-offs ensure that not all trait combinations are possible and differences are unlikely to have neutral consequences for fitness [61]. Differences in traits therefore lead to both fitness differences and niche differences. Many of these traits also affect ecosystem functioning—hence they are often termed functional traits [62]. Species can either be assigned to discrete functional groups, or differences in trait values can be used to calculate a continuous measure of functional difference (FD, [63]) by analogy with phylogenetic difference. Trait-based approaches have identified how the average value of plant traits varies among communities but have arguably been less successful in explaining variation in functioning within a given ecosystem [64]. This might be because of a lack of one-to-one mapping between traits and ecological functions. One study with plants found that average fitness differences were often correlated with single functional traits, for example, late phenology and deep rooting. But niche differences could only be described by combinations of traits, perhaps reflecting the multi-dimensional nature of ecological niches [65]. Gaining a better understanding of how traits define the niche space and how this in turn contributes to ecosystem functioning is an important goal for future research.
The potential mechanisms that generate stability in diverse communities are complex in their fine detail, but the key ingredient is differential responses of species to environmental variation. Put simply, asynchronous fluctuations in the performance of different species over time mean that a bad year for some species will inevitably be a better year for other competing species. These differential responses of individual species stabilize fluctuations in the biomass of the whole community. This idea is encapsulated in the two leading theories on the stability of ecosystem functioning [66]: the insurance effect [67] and compensatory dynamics [68]. Once again, we can intuitively understand the effects of diversity on stability in terms of niche theory. There are various theories of ‘fluctuation-dependent coexistence’ in which species partition environmental variation [69] to achieve different temporal niches. Once again, the biological processes that promote coexistence are also the potential mechanisms by which diversity can impact ecosystem functioning—or in this case, its temporal stability. Identifying the trade-offs that lie behind the seemingly idiosyncratic responses of species to environmental variation is an important future direction (for an example, see [70]).
4. Using niche theory to predict effects in the field
Although biodiversity experiments generally convey a consistent story, a question mark inevitably hangs over the relevance of any work undertaken under controlled conditions for understanding effects in the real world [71]. Real-world scenarios of species loss might differ from those represented in biodiversity experiments in three key ways: (i) species might not be lost at random, (ii) rare species might be lost more easily than common species and (iii) environmental heterogeneity in the real world is much higher than in experiments. While it is relatively easy to list the ways in which the real word differs from experimental plots, we argue that it is more useful to ask whether changing these assumptions will lead to a fundamentally different relationship between diversity and functioning in the real world. If the results obtained in experiments had no theoretical underpinning, then this would not be possible, but again we suggest that niche theory can help to supply answers. Further, we examine the common criticism that direct loss of species from ecosystems is likely to be a lesser threat than the indirect loss of species that occurs through other drivers of global change, such as habitat loss or eutrophication [71]. Throughout this section, we highlight the limits of current understanding and the challenges for future work.
The first biodiversity experiments generated communities of different species richness using randomly assembled subsets of some larger community. However, extinction drivers in the real world are unlikely to affect species equally. One of the specific criticisms levied at the experimental work therefore concerns the order of species loss. For example, there is evidence from animal communities that the species most likely to be lost: (i) are large-bodied, (ii) occur at low relative abundance and (iii) are found at the top of food chains [72]. In plants, species with large individuals are again often targeted (e.g. selective logging of large tree species with desirable wood qualities). But in other cases it can be smaller, rarer species that are preferentially lost [73]. Clearly, the impact of species loss on ecosystem functioning will be affected by species identity (see below). But niche theory predicts that the loss of species is expected to reduce ecosystem functioning, regardless of their specific traits, hence changing the order of species loss is unlikely to lead us to conclude that species loss has no impact on ecosystems. Indeed, there are now several biodiversity experiments that have mimicked a targeted sequence of species loss based on vulnerability to extinction. As expected, in some cases the impacts of this targeted species loss on ecosystem functioning are reduced compared with random species loss [74], while in others the effects are more intense [75] or even mixed [76].
Many species in real-world ecosystems are rare. How does this change our expectations? Again, following niche theory this is simply a question of degree. In the short term, we would naively expect the loss of an abundant species to have a greater impact on functioning than the loss of a rare one [74]. But over the longer term, the complexity of food webs allows for impacts that may be disproportionate to the abundances of the species that are lost. Indeed, this is the basis of the keystone species concept. For plants, effects on functioning have been predicted to be proportional to their relative abundance (the mass-ratio hypothesis; [77]) but it is easy to think of cases where this is unlikely to be the case. For example, some plants fix atmospheric nitrogen and can therefore have impacts (e.g. on nitrogen cycling) disproportionate to their biomass. Furthermore, abundances can change. A rare species may well be rare because the prevailing ecological conditions are less than ideal, but during unusual years (such as those with extreme climatic events), the species may greatly increase in abundance. This may bring stability to the community and prevent a dramatic decline in ecosystem functions. Indeed, there is a growing realization that the functioning of many of our ecosystems—both terrestrial and marine—may have been more severely impacted by the loss of rare species, such as large predators, than was previously realized. In several biodiversity experiments, rare species have had a disproportionately large effect on ecosystem functioning [78] or on multi-functionality [79]. More generally, the ubiquity of species-level overyielding (and underyielding) observed across hundreds of biodiversity experiments indicates that species rarely influence ecosystem functioning in proportion to their relative abundance. Yet unfortunately this assumption remains common, particularly in observational studies [80].
By definition, experiments aim to manipulate one (or a small number) of variables while holding other factors constant. Biodiversity experiments therefore tend to include lower levels of environmental heterogeneity than is likely to be seen in real-world ecosystems. Do the higher levels of environmental heterogeneity seen in natural ecosystems lead us to expect a weaker relationship between biodiversity and ecosystem functioning? No, quite the reverse. Theory predicts that higher levels of heterogeneity make it harder for one species to cover the niche space, and therefore we expect that more species are required to support ecosystem functioning when environmental heterogeneity is high. Experiments that have included heterogeneity support this point already—when a greater range of environmental conditions are included in an experiment, more species are found to play a role in supporting functioning [17,81]. Indeed, a spatial version of the insurance hypothesis has been developed based on the similar (although not identical) effects of spatial and temporal heterogeneity on the relationship between diversity and function [82].
In conclusion, critics of biodiversity experiments often focus on the limitations of experiments, as outlined above. It is therefore tempting to conclude that biodiversity experiments cannot illuminate how species loss will affect ecosystem functioning in the real world. However, critics rarely explain why such extrapolations are unlikely to be valid. Niche theory suggests that not only should the basic results of biodiversity experiments translate into real-world situations, but also that the relationship between biodiversity and ecosystem functioning will be stronger, not weaker, when assessed over larger areas and longer time scales.
5. Effects of biodiversity loss versus other global change drivers
Biodiversity experiments investigate the effect of species loss on ecosystems by removing species while holding other variables constant. But is this realistic? In the real world, species can be lost in a variety of ways. For example, hunting or over-harvesting may often deplete or remove targeted species while having relatively little effect on other aspects of the ecosystem (although this is not always the case). However, species may also be lost as a secondary consequence of global change drivers that have other direct effects on ecosystem functioning, for example, climate change, the introduction of exotic species or habitat alterations by humans. One of the best examples of a human-induced change that has both direct and indirect effects on productivity is eutrophication by nitrogen (and other soil nutrients). Nitrogen addition often causes loss of plant diversity [83–85] while having a direct fertilizing effect on productivity [86]. We use the example of eutrophication to explore these more complex scenarios that involve teasing apart both the direct effects of the change on functioning and the indirect effects acting via species loss.
Once again it is helpful to begin with general concepts from niche theory. The loss of species is predicted to impact ecosystem functions because it leads to vacant or under-used niches, for example, by leaving unconsumed resources or unused enemy-free niche space. This is the scenario represented by most biodiversity experiments (where species are deliberately omitted from communities while keeping all other conditions constant), and occurs in the real world when extinctions are caused by over-exploitation. By contrast, when species are lost from ecosystems because changing conditions mean that their niche no longer exists then the same argument for impacts on ecosystem functioning would not seem to apply (because if a niche is absent then it cannot be empty or under-used). But fundamental changes to an ecosystem can create new niches, so new species may be required to fill them. These species may or may not be available, depending on dispersal opportunities and on whether the changed conditions create truly novel conditions, to which no species are currently adapted. If this is the case, then functions in an altered ecosystem could still be diversity-dependent.
Studies have revealed that nitrogen enrichment can impact diversity in a variety of ways. In grasslands, fertilization usually leads to an increase in standing biomass and to more intense shading of the understory [87]. This enhanced shading has been experimentally shown to reduce diversity: artificially countering the effects of increased shading by supplementing light in the understory prevents the loss of diversity [88]. However, the story is sometimes more complex. For example, some forms of nitrogen fertilization lead to enhanced species loss through acidification [84,89]. In this case, it is still unclear whether the additional species loss occurs because some species simply cannot tolerate the lower pH (species lose their fundamental niche) or whether the more acidic conditions further alter fitness differences in a way that leads to additional exclusion (species lose their realized niche).
If nitrogen additions fundamentally alter the niche structure, then we might expect that the secondary loss of species following fertilization has no further consequences for ecosystem functioning. However, Isbell et al. [90] demonstrated that, in a US prairie, increased nitrogen deposition greatly increased productivity when first applied, but that this effect diminished rapidly over time. The rate of decrease was strongly linked to the rate of species loss, indicating that the changing species composition and diversity were partly responsible for the decline in productivity. The loss of the dominant plant species in this case had a much greater effect than would be expected for the loss of an average species chosen at random, as assessed by comparison with an adjacent biodiversity experiment.
Eutrophication can also affect other ecosystem metrics, such as stability. Hautier et al. [91] used a global network of fertilization experiments to investigate how the effects of diversity on stability measured in grassland biodiversity experiments compared with those seen under fertilization. Diversity–stability patterns in unfertilized communities were similar to those from biodiversity experiments, while fertilization appeared to have direct effects on stability but little or no effect via the loss of species. However, this may have been a type II error (failure to detect a real effect). An expanded follow-up analysis revealed that a broad range of factors that cause indirect losses of diversity (nitrogen, fire, elevated CO2, herbivory and water) did indeed reduce stability via changes in diversity [92].
Finally, the new niche space that results from the action of global change drivers such as nitrogen enrichment may also produce a community that delivers a poorer quality of service, as subjectively judged by humans. A green algal soup might be productive, but it is unlikely to attract tourists to visit a watercourse near you.
In conclusion, it is certainly true that the threats to ecosystems are often wider and more profound than simply the direct loss of native species. In particular, abiotic changes like eutrophication have reconfigured ecosystems: directly changing productivity, which indirectly causes the loss of species. Nevertheless, niche theory tells us that we should still be concerned about the loss of species, however it occurs. Overall, we therefore conclude that it still seems reasonable to support the current consensus that emerges from experimental studies: reduction of diversity has negative impacts on ecosystem functioning both in the short term and with respect to long-term stability [7,9,10], and these impacts of biodiversity loss on ecosystem functioning are of a similar order of magnitude to other major drivers of global change [93,94].
6. The nature of value
Only 25 years ago, we had little idea of what species did in ecosystems. The intervening period has seen dramatic advances in our understanding of the underlying natural science. More recently, it has also seen an overlap of the natural science of ecology with its socio-economic aspects. In particular, there are now many efforts to value the importance of the services that ecosystems provide to humans. These efforts are highly controversial [95–100]. Put simply, some people object to these valuations on principle, while others believe that the efforts will be too complex to properly quantify and protect the true value of nature to humanity. On the other hand, others argue that failure to assign monetary value to ecosystem services may result in ecosystems having no value at all [101–103].
Supporters of valuation exercises focus on the failure of more conventional conservations efforts to halt the overall global decline in biodiversity (including recent assessments of the efforts of the Convention on Biological Diversity). Our focus here is on the importance of biodiversity for ecosystem functioning (and by extension ecosystem services). Given the link between diversity and function, it seems only a matter of time before the attempts to value the importance of biodiversity (as a whole) to ecosystem services extends into efforts to place a monetary value on the importance of individual species to ecosystem services. We close by re-examining our overview of the research on the value of biodiversity to ecosystem functioning and asking what it implies for these efforts—how well could we do this and how wise would it be?
The fact that biodiversity has a measurable impact on ecosystem functioning and, by extension, on ecosystem services has often been viewed as scientific support for a utilitarian worldview, whereby biodiversity maps onto effects on ecosystem functioning through niche differences, which in turn map onto ecosystem services, human well-being and, ultimately, monetary value. While monetary valuation appears to provide a transparent and explicit expression of relative preferences in principle, it is not clear how well this simplistic view works in practice, as it assumes a linear chain of cause and effect that is not currently supported by scientific evidence.
First, each of these mappings is much more complex than traditionally believed [104]. A species' ecological niche is a multi-dimensional concept that involves a large number of biological traits [65]. Only some of these traits (sometimes called ‘effect traits’) have a measurable effect on ecosystem processes, and different traits are often involved in different ecosystem processes (as reviewed above). On the other hand, the so-called ‘response traits’ affect the ability of the species to respond to a wide array of environmental changes and thereby determine its contribution to ecosystem stability, and in many cases these response traits will be different from effects traits. As a result, biodiversity affects ecosystem functioning and stability through a complex network of interacting effects of species traits, which precludes a simple one-to-one mapping between any particular type or component of biodiversity and ecosystem functioning. As reviewed above, ecosystem functioning is also a multi-dimensional concept, which again precludes any simple one-to-one mapping between ecosystem functioning and ecosystem services. Indeed, many different ecosystem processes may be involved in a single ecosystem service, just as a single ecosystem process may be involved in many different ecosystem services [105].
Second, value itself is a multi-dimensional concept that includes such different dimensions as monetary value, use value, non-use value, cultural value and intrinsic value. Some provisioning services map onto monetary value in a relatively straightforward way when they concern the production of goods that are exchanged in a market, but most ecosystem services do not. And the intrinsic value of biodiversity is, by definition, foreign to any utilitarian framework and hence to any form of monetary evaluation. Values ultimately derive from human fundamental needs, and these needs include many non-utilitarian dimensions, including respecting and loving the natural world around us [106].
In conclusion, although recent research has vastly improved our knowledge of the ways in which ecosystems function and how biodiversity affects human societies, it reveals a complex, incomplete and limited view that should at least invite caution when assigning a monetary value to biodiversity. We concur with a recent call [107] for an inclusive approach to valuation that embraces a diversity of voices and would only emphasize that what ultimately matters is halting the catastrophic current decline in biodiversity. However you choose to value species, we are currently losing them far too quickly.
Acknowledgements
We thank two reviewers and Nat Seddon for helpful comments on the manuscript.
Authors' contributions
All authors discussed and agreed the initial content. L.A.T., A.H. and M.L. then drafted the manuscript with input from F.I. and D.W.P. All authors helped to revise the manuscript in line with the reviewers' comments and approved the final content.
Competing interests
We declare we have no competing interests.
Funding
No funding has been received for this article.
References
- 1.Newbold T, et al. 2016. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353, 288–291. ( 10.1126/science.aaf2201) [DOI] [PubMed] [Google Scholar]
- 2.Zuest T, Joseph B, Shimizu KK, Kliebenstein DJ, Turnbull LA. 2011. Using knockout mutants to reveal the growth costs of defensive traits. Proc. R. Soc. B 278, 2598–2603. ( 10.1098/rspb.2010.2475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowak MA, Boerlijst MC, Cooke J, Smith JM. 1997. Evolution of genetic redundancy. Nature 388, 167–171. ( 10.1038/40618) [DOI] [PubMed] [Google Scholar]
- 4.Smith MJ, Purves DW, Vanderwel MC, Lyutsarev V, Emmott S. 2013. The climate dependence of the terrestrial carbon cycle, including parameter and structural uncertainties. Biogeosciences 10, 583–606. ( 10.5194/bg-10-583-2013) [DOI] [Google Scholar]
- 5.Loreau M. 2010. From populations to ecosystems: theoretical foundations for a new ecological synthesis, vol. 46, pp. 1–18. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Daily GC, Matson PA. 2008. Ecosystem services: from theory to implementation. Proc. Natl Acad. Sci. USA 105, 9455–9456. ( 10.1073/pnas.0804960105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 8.Costanza R, et al. 1997. The value of the world's ecosystem services and natural capital. Nature 387, 253–260. ( 10.1038/387253a0) [DOI] [Google Scholar]
- 9.Naeem S, Duffy JE, Zavaleta E. 2012. The functions of biological diversity in an age of extinction. Science 336, 1401–1406. ( 10.1126/science.1215855) [DOI] [PubMed] [Google Scholar]
- 10.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. In Annual review of ecology, evolution, and systematics, vol. 45 (ed Futuyma DJ.), pp. 471–493. Palo Alto, CA: Annual Reviews. [Google Scholar]
- 11.Naeem S, Bunker DE, Hector A, Loreau M, Perrings C. 2009. Biodiversity, ecosystem functioning, and human wellbeing: an ecological and economic perspective. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Balvanera P, Pfisterer AB, Buchmann N, He J-S, Nakashizuka T, Raffaelli D, Schmid B. 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156. ( 10.1111/j.1461-0248.2006.00963.x) [DOI] [PubMed] [Google Scholar]
- 13.Cardinale BJ, Matulich KL, Hooper DU, Byrnes JE, Duffy E, Gamfeldt L, Balvanera P, O'Connor MI, Gonzalez A. 2011. The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 572–592. ( 10.3732/ajb.1000364) [DOI] [PubMed] [Google Scholar]
- 14.Mora C, Danovaro R, Loreau M. 2014. Alternative hypotheses to explain why biodiversity–ecosystem functioning relationships are concave-up in some natural ecosystems but concave-down in manipulative experiments. Sci. Rep. 4, 9 ( 10.1038/srep05427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reich PB, Tilman D, Isbell F, Mueller K, Hobbie SE, Flynn DFB, Eisenhauer N. 2012. Impacts of biodiversity loss escalate through time as redundancy fades. Science 336, 589–592. ( 10.1126/science.1217909) [DOI] [PubMed] [Google Scholar]
- 16.Hector A, Bagchi R. 2007. Biodiversity and ecosystem multifunctionality. Nature 448, 188–190. ( 10.1038/nature05947) [DOI] [PubMed] [Google Scholar]
- 17.Isbell F, et al. 2011. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202. ( 10.1038/nature10282) [DOI] [PubMed] [Google Scholar]
- 18.Lefcheck JS, et al. 2015. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 6, 6936 ( 10.1038/ncomms7936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrnes JEK, et al. 2014. Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions. Methods Ecol. Evol. 5, 111–124. ( 10.1111/2041-210X.12143) [DOI] [Google Scholar]
- 20.Gamfeldt L, Hillebrand H, Jonsson PR. 2008. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 89, 1223–1231. ( 10.1890/06-2091.1) [DOI] [PubMed] [Google Scholar]
- 21.Gamfeldt L, et al. 2013. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 4, 1340 ( 10.1038/ncomms2328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavaleta ES, Pasari J. R., Hulvey K. B., Tilman G. D. 2010. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc. Natl Acad. Sci. USA 107, 1443–1446. ( 10.1073/pnas.0906829107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pimm SL. 1984. The complexity and stability of ecosystems. Nature 307, 669–670. ( 10.1038/307669a0) [DOI] [Google Scholar]
- 24.Ives AR, Carpenter SR. 2007. Stability and diversity of ecosystems. Science 317, 58–62. ( 10.1126/science.1133258) [DOI] [PubMed] [Google Scholar]
- 25.Tilman D, Reich PB, Knops JMH. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441, 629–632. ( 10.1038/nature04742) [DOI] [PubMed] [Google Scholar]
- 26.Hector A, et al. 2010. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 91, 2213–2220. ( 10.1890/09-1162.1) [DOI] [PubMed] [Google Scholar]
- 27.Gross K, Cardinale BJ, Fox JW, Gonzalez A, Loreau M, Wayne Polley H, Reich PB, van Ruijven J. 2014. Species richness and the temporal stability of biomass production: a new analysis of recent biodiversity experiments. Am. Nat. 183, 1–12. ( 10.1086/673915) [DOI] [PubMed] [Google Scholar]
- 28.Isbell F, et al. 2015. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577. ( 10.1038/nature15374) [DOI] [PubMed] [Google Scholar]
- 29.MacArthur RH. 1970. Species-packing and competitive equilibrium for many species. Theor. Popul. Biol. 1, 1–11. ( 10.1016/0040-5809(70)90039-0) [DOI] [PubMed] [Google Scholar]
- 30.Darwin C. 1859. The origin of species by means of natural selection, ch. IV, p. 185. 1985 ed. London, UK: Penguin Classic. [Google Scholar]
- 31.Darwin C, Wallace AR. 1858. On the tendency of species to form varieties; and on the perpetuation of varieties and species by natural means of selection. J. Proc. Linnean Soc. Lond. Zool. 3, 45–62. ( 10.1111/j.1096-3642.1858.tb02500.x) [DOI] [Google Scholar]
- 32.Hector A, Hooper RE. 2002. ECOLOGY: Darwin and the first ecological experiment. Science 295, 639–640. ( 10.1126/science.1064815) [DOI] [PubMed] [Google Scholar]
- 33.Tilman D, Lehman CL, Thomson KT. 1997. Plant diversity and ecosystem productivity: theoretical considerations. Proc. Natl Acad. Sci. USA 94, 1857–1861. ( 10.1073/pnas.94.5.1857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chase JM, Leibold MA. 2003. Ecological niches: linking classical and contemporary approaches. Chicago, IL: University of Chicago Press. [Google Scholar]
- 35.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 36.Mayfield MM, Levine JM. 2010. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 13, 1085–1093. ( 10.1111/j.1461-0248.2010.01509.x) [DOI] [PubMed] [Google Scholar]
- 37.Adler PB, HilleRisLambers J, Levine JM. 2007. A niche for neutrality. Ecol. Lett. 10, 95–104. ( 10.1111/j.1461-0248.2006.00996.x) [DOI] [PubMed] [Google Scholar]
- 38.Loreau M, Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76. [Erratum: 413, 548.] ( 10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 39.Turnbull LA, Levine JM, Loreau M, Hector A. 2013. Coexistence, niches and biodiversity effects on ecosystem functioning. Ecol. Lett. 16, 116–127. ( 10.1111/ele.12056) [DOI] [PubMed] [Google Scholar]
- 40.Carroll IT, Cardinale BJ, Nisbet RM. 2011. Niche and fitness differences relate the maintenance of diversity to ecosystem function. Ecology 92, 1157–1165. ( 10.1890/10-0302.1) [DOI] [PubMed] [Google Scholar]
- 41.Loreau M, Sapijanskas J, Isbell FI, Hector A. 2012. Niche and fitness differences relate the maintenance of diversity to ecosystem function: comment. Ecology 93, 1482–1491. ( 10.1890/11-0792.1) [DOI] [PubMed] [Google Scholar]
- 42.Vandermeer J. 1981. The interference production principle: an ecological theory for agriculture. BioScience 31, 361–364. ( 10.2307/1308400) [DOI] [Google Scholar]
- 43.Loreau M. 2004. Does functional redundancy exist? Oikos 104, 606–611. ( 10.1111/j.0030-1299.2004.12685.x) [DOI] [Google Scholar]
- 44.McKane RB, et al. 2002. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415, 68–71. ( 10.1038/415068a) [DOI] [PubMed] [Google Scholar]
- 45.Jucker T, Bouriaud O, Avacaritei D, Dănilă I, Duduman G, Valladares F, Coomes DA. 2014. Competition for light and water play contrasting roles in driving diversity–productivity relationships in Iberian forests. J. Ecol. 102, 1202–1213. ( 10.1111/1365-2745.12276) [DOI] [Google Scholar]
- 46.Sapijanskas J, Paquette A, Potvin C, Kunert N, Loreau M. 2014. Tropical tree diversity enhances light capture through crown plasticity and spatial and temporal niche differences. Ecology 95, 2479–2492. ( 10.1890/13-1366.1) [DOI] [Google Scholar]
- 47.Loreau M. 1998. Biodiversity and ecosystem functioning: a mechanistic model. Proc. Natl Acad. Sci. USA 95, 5632–5636. ( 10.1073/pnas.95.10.5632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turnbull LA. 2014. Ecology's dark matter: the elusive and enigmatic niche. Basic Appl. Ecol. 15, 93–100. ( 10.1016/j.baae.2013.10.007) [DOI] [Google Scholar]
- 49.Schnitzer SA, et al. 2011. Soil microbes drive the classic plant diversity–productivity pattern. Ecology 92, 296–303. ( 10.1890/10-0773.1) [DOI] [PubMed] [Google Scholar]
- 50.Koricheva J, Mulder CPH, Schmid B, Joshi J, Huss-Danell K. 2000. Numerical responses of different trophic groups of invertebrates to manipulations of plant diversity in grasslands. Oecologia 125, 271–282. ( 10.1007/s004420000450) [DOI] [PubMed] [Google Scholar]
- 51.Otway SJ, Hector A, Lawton JH. 2005. Resource dilution effects on specialist insect herbivores in a grassland biodiversity experiment. J. Anim. Ecol. 74, 234–240. ( 10.1111/j.1365-2656.2005.00913.x) [DOI] [Google Scholar]
- 52.Maron JL, Marler M, Klironomos JN, Cleveland CC. 2011. Soil fungal pathogens and the relationship between plant diversity and productivity. Ecol. Lett. 14, 36–41. ( 10.1111/j.1461-0248.2010.01547.x) [DOI] [PubMed] [Google Scholar]
- 53.Vehvilainen H, Koricheva J, Ruohomäki K, Johansson T, Valkonen S. 2006. Effects of tree stand species composition on insect herbivory of silver birch in boreal forests. Basic Appl. Ecol. 7, 1–11. ( 10.1016/j.baae.2005.05.003) [DOI] [Google Scholar]
- 54.Zhu Y, et al. 2000. Genetic diversity and disease control in rice. Nature 406, 718–722. ( 10.1038/35021046) [DOI] [PubMed] [Google Scholar]
- 55.Wilby A, Thomas MB. 2002. Natural enemy diversity and natural pest control: patterns of pest emergence with agricultural intensification. Ecol. Lett. 5, 353–360. ( 10.1046/j.1461-0248.2002.00331.x) [DOI] [Google Scholar]
- 56.Parker IM, Saunders M, Bontrager M, Weitz AP, Hendricks R, Magarey R, Suiter K, Gilbert GS. 2015. Phylogenetic structure and host abundance drive disease pressure in communities. Nature 520, 542–544. ( 10.1038/nature14372) [DOI] [PubMed] [Google Scholar]
- 57.Finch S, Collier RH. 2000. Host-plant selection by insects—a theory based on ‘appropriate/inappropriate landings’ by pest insects of cruciferous plants. AAPG Bull. 96, 91–102. ( 10.1046/j.1570-7458.2000.00684.x) [DOI] [Google Scholar]
- 58.Bagchi R, Gallery RE, Gripenberg S, Gurr SJ, Narayan L, Addis CE, Freckleton RP, Lewis OT. 2014. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature 506, 85–88. ( 10.1038/nature12911) [DOI] [PubMed] [Google Scholar]
- 59.Bell T, Freckleton RP, Lewis OT. 2006. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol. Lett. 9, 569–574. ( 10.1111/j.1461-0248.2006.00905.x) [DOI] [PubMed] [Google Scholar]
- 60.Petermann JS, Fergus AJF, Turnbull LA, Schmid B. 2008. Janzen-Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology 89, 2399–2406. ( 10.1890/07-2056.1) [DOI] [PubMed] [Google Scholar]
- 61.Purves DW, Turnbull LA. 2010. Different but equal: the implausible assumption at the heart of neutral theory. J. Anim. Ecol. 79, 1215–1225. ( 10.1111/j.1365-2656.2010.01738.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882–892. ( 10.1111/j.0030-1299.2007.15559.x) [DOI] [Google Scholar]
- 63.Petchey OL. 2002. Functional diversity (FD), species richness and community composition. Ecol. Lett. 5, 402–411. ( 10.1046/j.1461-0248.2002.00339.x) [DOI] [Google Scholar]
- 64.Paine CET, et al. 2015. Globally, functional traits are weak predictors of juvenile tree growth, and we do not know why. J. Ecol. 103, 978–989. ( 10.1111/1365-2745.12401) [DOI] [Google Scholar]
- 65.Kraft NJB, Godoy O, Levine JM. 2015. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl Acad. Sci. USA 112, 797–802. ( 10.1073/pnas.1413650112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loreau M, de Mazancourt C. 2013. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol. Lett. 16, 106–115. ( 10.1111/ele.12073) [DOI] [PubMed] [Google Scholar]
- 67.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tilman D, Lehman CL, Bristow CE. 1998. Diversity-stability relationships: statistical inevitability or ecological consequence? Am. Nat. 151, 277–282. ( 10.1086/286118) [DOI] [PubMed] [Google Scholar]
- 69.Chesson P. 2003. Quantifying and testing coexistence mechanisms arising from recruitment fluctuations. Theor. Popul. Biol. 64, 345–357. ( 10.1016/S0040-5809(03)00095-9) [DOI] [PubMed] [Google Scholar]
- 70.Angert AL, Huxman TE, Chesson P, Lawrence Venable D. 2009. Functional tradeoffs determine species coexistence via the storage effect. Proc. Natl Acad. Sci. USA 106, 11 641–11 645. ( 10.1073/pnas.0904512106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wardle DA. 2016. Do experiments exploring plant diversity–ecosystem functioning relationships inform how biodiversity loss impacts natural ecosystems? J. Veg. Sci. 27, 646–653. ( 10.1111/jvs.12399) [DOI] [Google Scholar]
- 72.Cardillo M, Mace GM, Jones KE, Bielby J, Bininda-Emonds ORP, Sechrest W, Orme CDL, Purvis A. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241. ( 10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 73.Leach MK, Givnish TJ. 1996. Ecological determinants of species loss in remnant prairies. Science 273, 1555–1558. ( 10.1126/science.273.5281.1555) [DOI] [Google Scholar]
- 74.Smith MD, Knapp AK. 2003. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 6, 509–517. ( 10.1046/j.1461-0248.2003.00454.x) [DOI] [Google Scholar]
- 75.Zavaleta ES, Hulvey KB. 2004. Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science 306, 1175–1177. ( 10.1126/science.1102643) [DOI] [PubMed] [Google Scholar]
- 76.Isbell FI, Losure DA, Yurkonis KA, Wilsey BJ. 2008. Diversity–productivity relationships in two ecologically realistic rarity–extinction scenarios. Oikos 117, 996–1005. ( 10.1111/j.0030-1299.2008.16692.x) [DOI] [Google Scholar]
- 77.Grime JP. 1998. Studies of the impacts of climate change on the structure and functioning of calcareous grasslands. TIGER IV 2c final report.
- 78.Connolly J, et al. 2013. An improved model to predict the effects of changing biodiversity levels on ecosystem function. J. Ecol. 101, 344–355. ( 10.1111/1365-2745.12052) [DOI] [Google Scholar]
- 79.Soliveres S, et al. 2016. Locally rare species influence grassland ecosystem multifunctionality. Phil. Trans. R. Soc. B 371, 20150269 ( 10.1098/rstb.2015.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winfree R, Fox JW, Williams NM, Reilly JR, Cariveau DP. 2015. Abundance of common species, not species richness, drives delivery of a real-world ecosystem service. Ecol. Lett. 18, 626–635. ( 10.1111/ele.12424) [DOI] [PubMed] [Google Scholar]
- 81.Griffin JN, Méndez V, Johnson AF, Jenkins SR, Foggo A. 2009. Functional diversity predicts overyielding effect of species combination on primary productivity. Oikos 118, 37–44. ( 10.1111/j.1600-0706.2008.16960.x) [DOI] [Google Scholar]
- 82.Loreau M, Mouquet N, Gonzalez A. 2003. Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl Acad. Sci. USA 100, 12 765–12 770. ( 10.1073/pnas.2235465100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hillebrand H, et al. 2007. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc. Natl Acad. Sci. USA 104, 10 904–10 909. ( 10.1073/pnas.0701918104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silvertown J, Poulton P, Johnston E, Edwards G, Heard M, Biss PM. 2006. The Park Grass Experiment 1856–2006: its contribution to ecology. J. Ecol. 94, 801–814. ( 10.1111/j.1365-2745.2006.01145.x) [DOI] [Google Scholar]
- 85.Harpole WS, Tilman D. 2007. Grassland species loss resulting from reduced niche dimension. Nature 446, 791–793. ( 10.1038/nature05684) [DOI] [PubMed] [Google Scholar]
- 86.Gruner DS, et al. 2008. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol. Lett. 11, 740–755. ( 10.1111/j.1461-0248.2008.01192.x) [DOI] [PubMed] [Google Scholar]
- 87.Grace JB, et al. 2016. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 529, 390–393. ( 10.1038/nature16524) [DOI] [PubMed] [Google Scholar]
- 88.Hautier Y, Niklaus PA, Hector A. 2009. Competition for light causes plant biodiversity loss after eutrophication. Science 324, 636–638. ( 10.1126/science.1169640) [DOI] [PubMed] [Google Scholar]
- 89.Stevens CJ, Thompson K, Grime JP, Long CJ, Gowing DJG. 2010. Contribution of acidification and eutrophication to declines in species richness of calcifuge grasslands along a gradient of atmospheric nitrogen deposition. Funct. Ecol. 24, 478–484. ( 10.1111/j.1365-2435.2009.01663.x) [DOI] [Google Scholar]
- 90.Isbell F, Reich PB, Tilman D, Hobbie SE, Polasky S, Binder S. 2013. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc. Natl Acad. Sci. USA 110, 11 911–11 916. ( 10.1073/pnas.1310880110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hautier Y, et al. 2014. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature 508, 521–525. ( 10.1038/nature13014) [DOI] [PubMed] [Google Scholar]
- 92.Hautier Y, Tilman D, Isbell F, Seabloom EW, Borer ET, Reich PB. 2015. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science 348, 336–340. ( 10.1126/science.aaa1788) [DOI] [PubMed] [Google Scholar]
- 93.Hooper DU, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108. ( 10.1038/nature11118) [DOI] [PubMed] [Google Scholar]
- 94.Tilman D, Reich PB, Isbell F. 2012. Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc. Natl Acad. Sci. USA 109, 10 394–10 397. ( 10.1073/pnas.1208240109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chee YE. 2004. An ecological perspective on the valuation of ecosystem services. Biol. Conserv. 120, 549–565. ( 10.1016/j.biocon.2004.03.028) [DOI] [Google Scholar]
- 96.McCauley DJ. 2006. Selling out on nature. Nature 443, 27–28. ( 10.1038/443027a) [DOI] [PubMed] [Google Scholar]
- 97.Ghazoul J. 2007. Recognising the complexities of ecosystem management and the ecosystem service concept. Gaia-Ecol. Perspect. Sci. Soc. 16, 215–221. [Google Scholar]
- 98.Redford KH, Adams WM. 2009. Payment for ecosystem services and the challenge of saving nature. Conserv. Biol. 23, 785–787. ( 10.1111/j.1523-1739.2009.01271.x) [DOI] [PubMed] [Google Scholar]
- 99.Vira B, Adams WM. 2009. Ecosystem services and conservation strategy: beware the silver bullet. Conserv. Lett. 2, 158–162. ( 10.1111/j.1755-263X.2009.00063.x) [DOI] [Google Scholar]
- 100.Norgaard RB. 2010. Ecosystem services: from eye-opening metaphor to complexity blinder. Ecol. Econ. 69, 1219–1227. ( 10.1016/j.ecolecon.2009.11.009) [DOI] [Google Scholar]
- 101.Daily GC. 1997. Nature's services. Washington, DC: Island Press. [Google Scholar]
- 102.Kareiva P, Watts S, McDonald R, Boucher T. 2007. Domesticated nature: shaping landscapes and ecosystems for human welfare. Science 316, 1866–1869. ( 10.1126/science.1140170) [DOI] [PubMed] [Google Scholar]
- 103.Ehrlich PR, Mooney HA. 1983. Extinction, substitution, and ecosystem services. Bioscience 33, 248–254. ( 10.2307/1309037) [DOI] [Google Scholar]
- 104.Naeem S, et al. 2016. Biodiversity as a multidimensional construct: a review, framework and case study of herbivory's impact on plant biodiversity. Proc. R. Soc. B 283, 20153005 ( 10.1098/rspb.2015.3005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bennett EM, Peterson GD, Gordon LJ. 2009. Understanding relationships among multiple ecosystem services. Ecol. Lett. 12, 1394–1404. ( 10.1111/j.1461-0248.2009.01387.x) [DOI] [PubMed] [Google Scholar]
- 106.Loreau M. 2014. Reconciling utilitarian and non-utilitarian approaches to biodiversity conservation. Ethics Sci. Environ. Policy 14, 27–32. ( 10.3354/esep00149) [DOI] [Google Scholar]
- 107.Tallis H, Lubchenco J. 2014. A call for inclusive conservation. Nature 515, 27–28. ( 10.1038/515027a) [DOI] [PubMed] [Google Scholar]