Abstract
Humans and other mammals as well as honeybees learn a unilateral association between an olfactory stimulus presented to one side and a reward. In all of them, the learned association can be behaviourally retrieved via contralateral stimulation, suggesting inter-hemispheric communication. However, the underlying neuronal circuits are largely unknown and neural correlates of across-brain-side plasticity have yet not been demonstrated. We report neural plasticity that reflects lateral integration after side-specific odour reward conditioning. Mushroom body output neurons that did not respond initially to contralateral olfactory stimulation developed a unique and stable representation of the rewarded compound stimulus (side and odour) predicting its value during memory retention. The encoding of the reward-associated compound stimulus is delayed by about 40 ms compared with unrewarded neural activity, indicating an increased computation time for the read-out after lateral integration.
Keywords: olfaction, neurophysiology, insects, plasticity, lateral integration, mushroom body
1. Introduction
Bilaterally symmetric organization is conserved across most phyla in the animal kingdom. Bilateral protostomes and deuterostomes share mirror symmetric representations of sensory information. In the insect olfactory system, the two antennae differently receive an odour stimulus depending on the spatial stimulus pattern and its temporal evolution. This difference can be exploited by the nervous system to extract behaviourally relevant spatial information about the olfactory scene (e.g. a pollinator may orient itself inside the flower in an optimal position to transfer the pollen [1]). Olfactory input from each antenna is first processed ipsilaterally in the respective antennal lobe, and then projected almost exclusively to the mushroom body (MB) and the lateral horn on the same side of the brain [2]. The MB integrates different sensory modalities, and is crucial for learning and memory formation (for review, see [3–5]). Crosstalk between the two sides of the bee brain is confined predominantly to the mushroom body output neurons (MBONs) and their target neurons [6].
Unilateral olfactory conditioning leads to a stable memory that can be initially retrieved only via the antenna that was stimulated during training [7]. Several hours later the association could be behaviourally recalled via the contralateral antenna [8], at least to a certain extent, which suggests across-brain-side interactions. This inter-hemispheric transmission of olfactory information has also been demonstrated in behavioural experiments with rats [9,10] and humans [11], and may reflect lateral integration, which is necessary to solve complex forms of learning and memory formation. In honeybees, for example, non-elemental learning tasks are solved when both antennae and MBs are involved [12–17]. However, if only one antenna is stimulated [16] or only one MB is functioning, bees are no longer able to solve side-spanning learning tasks, do not resolve contradictory information during differential conditioning and cannot learn negative and positive patterning tasks [18].
We studied the encoding of odour valence in the MB in relation to the animal's behaviour in a side-specific learning task. To this end, we recorded from a subset of MBONs (electronic supplementary material, figure S1) in one brain side of behaving honeybees, and compared their activity before, during and after applying a unilateral training protocol to the antenna contralateral to the recording electrode position. MBONs are known to change their response properties during learning and memory processing. Extracellular long-term recordings of a single identified MBON, the PE1, indicate a reduction of its response to the reward-associated odour [19] and supported, therefore, earlier findings of Mauelshagen [20], who recorded intracellularly from the same neuron. MBONs from the A1, A2, A4, A5 and A7 clusters that exit the MB at the ventral region of the alpha-lobe are specifically activated by the rewarded odour as an effect of olfactory learning, and thus encode stimulus valence [21]. Inhibitory MB feedback neurons which exit the MB at the lateral side (A3d and A3v neurons [6]) increase their responses to the learned odour [22–24]. Filla & Menzel [25] showed that learning of odours in a specific context led to changes of response rates of these neurons that varied according to both the odour (whether rewarded or not rewarded) and the context (whether combined with the rewarded or the not rewarded odour). Here we used a well-established method to extracellularly record and extract single unit activity of target neurons in insects that was previously used to record from MBONs in the honeybee [21,26,27]. We demonstrate lateral integration in these MBONs after classical conditioning merging information about odour identity, side-specific occurrence and reward as indicated by memory retention tests.
2. Material and methods
(a). Animals
Honeybee foragers (Apis mellifera) were anaesthetized on ice and harnessed in metal tubes such that only the mandibles, proboscis and antennae could freely move [28]. Heads were fixed with wax onto the metal tube, and the scapi of the antennae were fixed with low-melting-point wax on the head capsule. Antennae were spatially separated using a piece of transparent plastic (3 × 3 cm; 0.2 mm thick) in which the silhouette of each bee's head was cut. Gaps between the cuticle and the plastic were filled with wax. A small window (1.5 × 1.5 mm) was cut unilaterally between the left compound eye and the glued-on plastic wall. Head glands and trachea sacks were removed, and the electrode was positioned at the ventral part of the alpha-lobe at a depth between 100 and 250 µm (cf. electronic supplementary material). Following insertion, the hole in the head capsule was filled with silicon (KWIK-SIL, Sarasota, FL, USA) in order to prevent the brain from drying and to anchor the electrode within the brain and the head capsule, avoiding electrode drift. To avoid a confusion of potential effects of a hemispheric specialization [29] and lateralization [30] with the outcome of our learning protocol, we always recorded from the left brain side.
(b). Odour stimulation
The two antennae were stimulated separately (figure 1a) using a custom-built olfactometer [21]. A constant air stream (1.5 m s−1 speed) was split into two Teflon tubes (diameter 6 mm). Five syringes (5 ml) were inserted into each tube. Filter papers (2 cm2) were soaked with 10 µl of odour solution or paraffin oil alone (control) and placed in the syringes. During the 3 s of odour stimulation, only half of the syringes’ air volume was injected into the air stream. An exhaust hood was placed behind the bee to remove all odour molecules. The odour delivery as well as the data acquisition software (Cheetah, Neuralynx, Bozeman, MT, USA) was synchronized using a Visual Basic script (VBA v. 6.0, Microsoft, USA).
Figure 1.
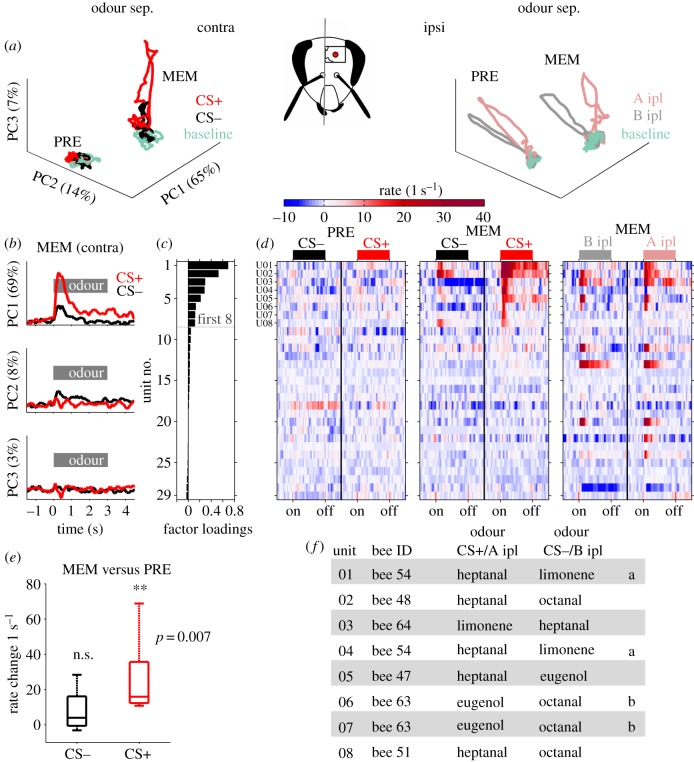
Classical conditioning of an odour-side compound stimulus. (a) The antennae were spatially separated using a plastic wall. The electrode was inserted into the ventral part of the alpha-lobe (red dot). The contralateral or the ipsilateral antenna relative to the electrode position was stimulated. The activity of muscle M17 involved in the PER was monitored by inserting an electrode through the cuticle between the right ocellus and the compound eye. The lower scheme illustrates the four odour-side compound stimuli. Odours A and B were presented either contralaterally (red, black) or ipsilaterally (pink, grey). (b) Experimental protocol. Prior to conditioning (PRE) all four odour-side compound stimuli were presented 10 times each in pseudorandom order. During the acquisition phase (ACQ) odour A presented to the contralateral antenna (CS+) was paired with a sugar reward (US), while odour B presented to the contralateral antenna (CS−) was presented without reward. During the memory retention phase (MEM) 3 h after conditioning, the stimulation protocol of the PRE phase was repeated. (c) Bees (n = 17) learned to discriminate the rewarded odour-side compound stimulus (CS+, red line) from the unrewarded compound stimulus (CS−, black line) significantly (Wilcoxon's rank sum test, p < 0.005). (d) During 10 memory retention trials (MEM) the animals significantly separated the CS+ (A contra) from all other odour-side compound stimuli including the same odour but presented with inverted spatial information (A ipl) (Wilcoxon's rank sum test, individual p-values given in the inset).
(c). Conditioning of an odour-side compound stimulus
Two out of four different odours (eugenol, heptanal, octanal, limonene; Sigma-Aldrich Chemie GmbH) were chosen randomly for each experiment. Odours were diluted in paraffin oil (Sigma-Aldrich Chemie GmbH) to a 0.01 concentration. During a pre-conditioning phase (PRE) the two odours (A and B) were presented in pseudo-randomized order 10 times to the contralateral as well as to the ipsilateral antennae relative to the recording position (figure 1b). We used an inter-trial interval (ITI) of 1 min. Twenty minutes after the PRE test we performed differential conditioning (acquisition = ACQ) always to the contralateral antenna (figure 1a). Odour A contralateral was paired with a reward (CS+) while odour B contralateral was presented without reward (CS−). The ITI was again 1 min. Odour stimuli lasted 3 s. A 30% sucrose reward stimulus (US) followed the CS+ odour-side compound. It was applied to both antennae and the proboscis ensuring the activation of the unilateral (antenna) as well as the bilateral (proboscis) US component [31]. The US started 2 s after odour onset and lasted for about 3 s. Three hours later, a memory retention test (MEM) was performed by repeating the initial test phase.
(d). Data acquisition
The electrode consisted of three closely spaced wires (polyurethane-coated copper wire, 14 µm in diameter; Electrisola, Escholzmatt, Switzerland) which are very flexible. Extracellular neural signals were measured differentially from all three electrode pair combinations using the Patch Panel ERP-27 (Neuralynx, Bozeman, MT, USA) with a sampling rate of 20 kHz. A silver wire with a diameter of 25 µm (Nilaco, Tokyo, Japan) inserted into the right compound eye served as a ground electrode. We used a 16-channel analogue recording system (Neuralynx, Bozeman, MT, USA) for data acquisition. An example recording is shown in the electronic supplementary material.
(e). Single unit activity
To obtain single unit activity, we applied a semi-automatic spike sorting (template-matching) provided with the Spike2 software (Cambridge Electronic Design, Cambridge, UK). All details can be found in the electronic supplementary material, S3. Following our criteria we could extract 1–3 units per bee. In total, we extracted 29 units out of 17 bees.
(f). Data analysis
Data analyses were carried out with Matlab (MathWorks GmbH, Ismaning, Germany) and the FIND open source toolbox (http://find.bccn.uni-freiburg.de/; [32]). The firing rate during a single trial was estimated using a kernel convolution [32,33]. We used an asymmetric kernel with the kernel shape ‘ALP’ (alpha function) and time-resolution τ. The trial-averaged firing rates were used to construct stimulus-dependent neuronal population vectors in the following way. For a given stimulus configuration a (odour identity, stimulation phase and stimulation side) and an ensemble of n neurons, we constructed the n-dimensional rate vector va at each point in time during a 6000 ms time window (1500 ms before odour onset, 3000 ms during odour stimulation, 1500 ms following odour offset). All vectors together were used for principal component analysis (PCA) in figure 3a. Before we applied a separate PCA on the population vectors representing the CS+ and CS− induced activity during memory retention (figure 3b), we averaged the baseline activity of 1000 ms before stimulus onset in every single unit and subtracted it from the unit's firing rate (baseline correction). The factor loadings of each single unit after PCA were used to rank the units with respect to their contribution to the first principal component (PC1, figure 3c). The same procedure was used to extract eight units (highest factor loadings) which responded to ipsilateral stimulation during MEM. We calculated stimulus response latencies for a given stimulus from individual neurons. To this end, we convolved the pooled spike trains across all 10 trials with a kernel of exponential shape. We then estimated the latency as the relative time after stimulus onset when the spike rate function crossed a threshold of 4 × s.d. of the spontaneous spike rate before stimulus onset. To calculate the Euclidean distances (EDs) (L2-Norm), we performed a pairwise subtraction of the respective population vector couples 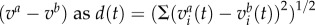 . Maximal firing rates were always extracted in the phasic response window (500 ms following odour onset).
. Maximal firing rates were always extracted in the phasic response window (500 ms following odour onset).
Figure 3.
The MBON ensemble establishes a representation of the rewarded odour-side compound stimulus. (a) PCA of the population vectors across all units and stimuli for CS+ (red) and CS− (black; left) and the same odours but presented ipsilaterally (A ipl = pink, B ipl = grey; right) before (PRE) and after (MEM) conditioning. Trajectories of the first three principal components (PC1–3). Baseline activity before stimulation is drawn in green. The 3 s during odour stimulation are marked in the respective stimulus colour. Trajectories reflecting contralateral stimulation (left) illustrate that there was no stimulation induced activity in the PRE phase. A clear odour-induced population response for the CS+ was established during memory retention (MEM) 3 h after conditioning. Stimulation to the ipsilateral antenna (right) evoked clear odour-induced trajectories prior to (PRE) and after (MEM) conditioning. (b) A separate PCA was performed limited to the population vectors reflecting contralaterally induced activity during the MEM phase. Each panel illustrates the temporal response profile of one PC and indicates the explained variance. PC1 shows a clear odour-induced activation that resembles the average population rate response. (c) The single units were ordered with respect to their contributions to the variation of PC1 (factor loading). (d) Trial-averaged firing rate profiles of all units (ordered as in c). (e) The rate changes between the PRE and the MEM phase due to contralateral stimulation were calculated for the first 8 units (factor loading > 0.05). The distribution of rate changes is shown in a boxplot for CS+ (red) and CS− (black); the central mark indicates the median, edges indicate the 25th and 75th percentiles, whiskers extend to the most extreme data points. Only the CS+ showed a significant response rate increase (signed-rank test, p < 0.05). (f) Bee affiliation of the units and odour pairs used for differential conditioning. Identical letters indicate units recorded from the same animal.
(g). Monitoring behaviour
Simultaneously with the MBONs we recorded a myogram of the muscle M17, which reflects the proboscis extension response (PER) of the bee [34]. During the acquisition phase a behavioural response was detected if the activity of the M17 muscle started immediately after the odour onset and before the reward (US) was presented.
(h). Statistics
The differences in behavioural as well as neural performances between the different odour-side compound stimuli during ACQ and MEM were tested using the Wilcoxon rank sum test. Differences were considered to be significant if p < 0.05. The neural rate change between the ACQ and MEM phase for the CS+ as well as for the CS− were tested using the signed-rank test. The response rate latency distributions between odour A presented to the ipsilateral side (A ipl) and odour A presented to the contralateral side (CS+) during MEM were tested using the Wilcoxon rank sum test.
3. Results
(a). Unilateral odour discrimination
The behavioural learning in the acquisition phase (figure 1c) illustrates that unilateral conditioning rapidly leads to significant behavioural discrimination between the rewarded stimulus (CS+) and the unrewarded stimulus (CS−). During the memory test the bees discriminated the CS+ (A contralateral) and the CS− (B contralateral), as well as both odours presented to the ipsilateral antenna (A ipl, B ipl; figure 1d).
(b). Contralateral stimulation evokes neuronal CS+ response in conditioned but not in naive animals
None of the units recorded responded to contralateral odour stimulation before unilateral conditioning (figure 2a,b, PRE). This result can be explained by the bilateral symmetric organization and a morphological separation of the olfactory pathway between both brain sides up to the level of MB output [6]. However, 3 h after differential conditioning at the contralateral antenna the CS+ (odour A contralateral) evoked a clear average response (figure 2b, MEM) but not CS− stimulation (odour B contralateral), indicating that a learning-induced representation of the odour-side compound stimulus was established.
Figure 2.
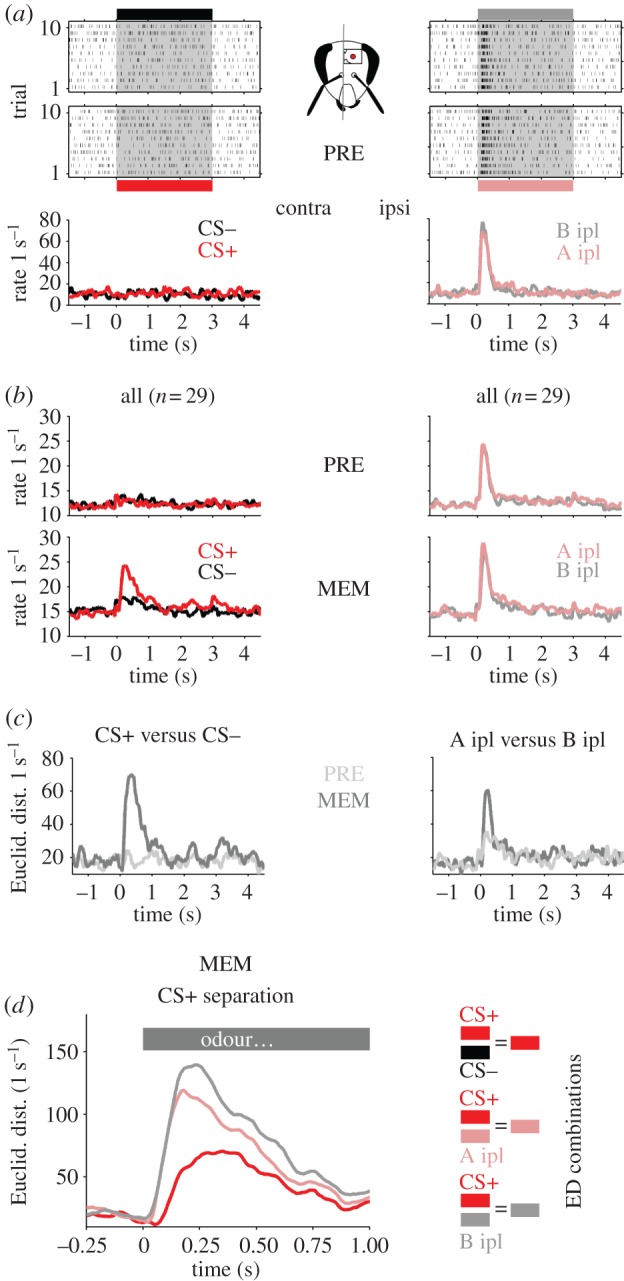
No neuronal responses to contralateral odour stimulation prior to conditioning but during memory retention. Activity in response to contralateral (ipsilateral) stimulation is shown on the left (right), respectively. (a) Initial test before conditioning (PRE). Spike raster plots and trial-averaged firing rates of a representative unit in response to odour A presented contralaterally (CS+, red), odour A presented ipsilaterally (A ipl, pink), odour B presented contralaterally (CS−, black) and odour B presented ipsilaterally (B ipl, grey). Odour stimulation is indicated by the grey shading. During the pretest this unit did not respond to contralateral stimulation. The same odours presented ipsilaterally evoked reliable responses to both odours. (b) Mean rates of all recorded units prior to conditioning (PRE) and during memory retention (MEM). (c,d) Reliable separation of odour identity and stimulation side during memory retention tests. (c) Euclidean distances (ED) between the population vectors of CS+ and CS− (left) and A ipl and B ipl (right). Before conditioning (PRE, light grey) the ED between CS+ and CS− was close to zero. The same odours presented to the ipsilateral side (A ipl and B ipl) resulted in a large ED. Three hours after conditioning (MEM, dark grey) the population response to the CS+ was now clearly separated from the population response to the CS−. (d) ED between the population vectors in response to CS+ versus CS−, CS+ versus A ipl and CS+ versus B ipl revealed a clear separation of the CS+ from all other stimuli.
(c). Mushroom body output neurons develop unique representation of the side-specific odour reward association
Next, we quantified the stimulus separation before (PRE) and after (MEM) contralateral conditioning in the recorded MBON population by calculating the pairwise ED between the population response vectors of two different stimuli. Ipsilateral stimulation with odours A and B evoked a distinct population response in both the PRE and the MEM phase (A ipl versus B ipl; figure 2c, right). By contrast, odour A and odour B presented to the contralateral side remained at baseline throughout stimulus presentation in the PRE-phase (CS+ versus CS−; figure 2c, left). However, during MEM contralateral stimulation resulted in a pronounced ED between both odours (CS+ versus CS−, MEM; figure 2c, left). Moreover, the population of MBONs had established a neuronal representation of the reward-associated odour-side compound that was distinct from all three other compound stimuli (figure 2d). The latter was supported by PCA across all experimental phases (figure 3a). Visualizing the stimulus-dependent evolution of the first three principal components (PC1–3) revealed a clear stimulus-specific response before conditioning (PRE) if odours A and B were presented on the ipsilateral antenna (A ipl, B ipl; figure 3a, right). During stimulation with the same odours but inverted side information the neural ensemble remained at baseline level (CS+, CS−; figure 3a, left).
During MEM two distinct changes in the population response were established. First, the baseline activity had changed (discussed in [21]) separating the experimental phases in PC space (figure 3a, left and right). This change dominates PC1, which explained 65% of variation. It reflected a change in baseline firing rates of some single units (some increased others decreased; data not shown). Second, contralateral stimulation with odour A (CS+) evoked a clear transient population response (figure 3a, left, MEM).
(d). Stimulus valence is encoded by a subset of initially silent mushroom body output neurons
Next, we identified individual units that are responsible for the increased CS+ activity during MEM. To remove the influence of the observed change in baseline activity, we subtracted the baseline firing rate from the time-resolved firing rate estimates in every single unit (cf. Material and methods), and performed PCA in the MEM phase on the CS+ (A contra) and the CS− (B contra) population response vectors. PC1 explained 69% of variation in the population activity and resembles the stimulation-induced response profile. PC2 explained 8% of the variation, mainly contrasting the CS+ and CS−. PC3 explained 3% of the variation (figure 3b). As PC1 resembled the dominant CS+ response profile during MEM, we ranked the single units with respect to their factor loading of PC1 (figure 3c). Units with high factor loadings did not show any significant CS+ response before conditioning (figure 3d, PRE). However, they became recruited as a consequence of the training procedure, showing pronounced responses evoked by the rewarded odour-side compound stimulus during MEM (figure 3d). Although there is partial overlap, ipsilateral stimulation during MEM activated a different set of MBONs (figure 3d). The maximal rate difference between the PRE and MEM phase for the units showing factor loadings (greater than 0.05) was significant for the side-specific CS+ but not for the CS− (figure 3e). These eight units were recorded in six different bees, which were trained with different rewarded and unrewarded odour-side combinations (figure 3f).
(e). Side-specific response development across experimental phases
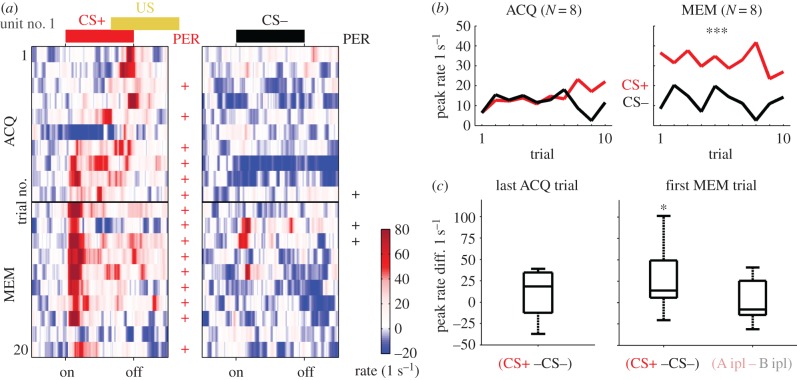
The strong CS+ response was carried by units that were silent in the PRE phase but recruited to encode the CS+ during MEM. The trial-resolved firing rate of such an example unit together with the bee's response behaviour is shown in figure 4a. To resemble the neuronal learning curves, we calculated the trial-resolved average peak rates during stimulation of the extracted eight units during ACQ and MEM (figure 4b). In contrast with the behavioural learning curves (figure 1c), the neural learning did not allow a differentiation between CS+ and CS− during ACQ (figure 4b). However, during MEM the units showed a significantly increased rate response for the CS+ (figure 4b). Calculating the difference between the CS+ and CS− induced peak rates in the last acquisition and the first memory retention trial for each unit revealed a significant differentiation at the first memory retention but not at the last acquisition trial (figure 4c).
Figure 4.
Neural response evolution during acquisition and memory retention trials. (a) Single trial response rates of one unit showing the highest factor loading (figure 3c) for all conditioning (ACQ) and memory retention (MEM) trials starting from top. CS+ stimulations are shown on the left, CS− stimulations on the right. Coloured bars on top indicate stimulus onset and duration. During ACQ the reward was presented (US, yellow). The black horizontal line indicates the 3 h pause between ACQ and MEM. A behavioural response (PER) of this bee is indicated by a plus symbol next to the respective trial. (b) Trial-resolved average peak rate (y-axis) across the extracted units. During ACQ there was no differentiation between CS+ (red) and CS− (black) (Wilcoxon's rank sum test, p = 0.43). During the MEM phase the neural responses differed significantly, separating the CS+ from the CS− (Wilcoxon's rank sum test, p = 0.0002). (c) Distribution across the differences between CS+ and CS− peak rate in the final ACQ trial and in the first MEM trial. Only the latter difference was significant, indicating stronger MBON responses to the CS+ than to the CS− (signed-rank test: first MEM trial, p < 0.05; last ACQ trial, p = 0.28). The same odours presented to the ipsilateral side did not show a significant difference (A ipl–B ipl; signed-rank test, p = 0.97).
(f). Increased computation time for the read-out of the compound stimulus value
Activation of MBONs evoked by ipsilateral odour stimulation represents unilateral processing within one hemisphere. However, activation of MBONs during contralateral stimulation, requires integration of information across hemispheres and represents lateral integration as found in tests during the MEM phase.
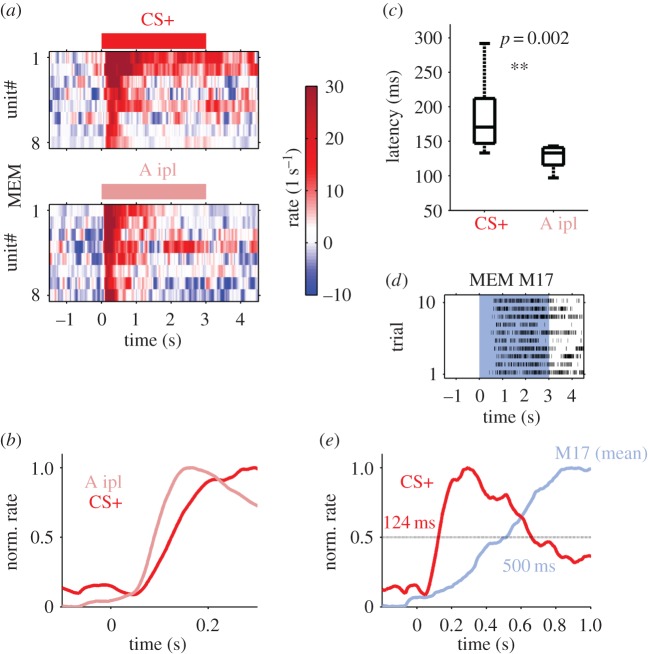
In order to test whether unilateral processing might be faster than processing after lateral integration, we analysed the neuronal response latencies for ipsi- and contralateral stimulation with odour A (A ipl versus CS+) during MEM. We compared the eight units extracted by their factor loadings (greater than 0.05) to compute for the CS+ during MEM (figures 3c, 5a top) with another set of eight units which showed the highest factor loadings for ipsilateral stimulation during MEM (figure 5a bottom; for details cf. electronic supplementary material, figure S2). The average firing rates of the different subsets of units revealed delayed responses for contralateral stimulation (figure 5b). Estimating the response latencies for each single unit in the trial-averaged firing rate profile (see Material and methods) revealed significantly longer latencies for the CS+ (lateral integration) than for the A ipl (unilateral processing) with an average delay of 37 ms (figure 5c). However, both ipsilateral computation as well as contralateral computation is fast enough to be integrated in the animals' decision process to extend or not extend the proboscis (figure 5d,e).
Figure 5.
Delayed response after lateral integration. (a) Trial-averaged firing rates of eight selected units (A contra, top panel; same selection as in figure 3) in comparison with eight units responding to the same odour but with inverted spatial information (A ipl, lower panel; for selection see methods) during memory retention (MEM). Stimulus identity and duration is indicated on top of each panel. (b) The average rate response revealed a temporal delay for units responding to contralateral stimulation with odour A (CS+) as compared with ipsilateral stimulation with A. Time zero indicates the stimulus onset. (c) Comparing the latency distributions across units revealed a significant difference (Wilcoxon's rank sum test, p < 0.001) with a median of 170 ms for CS+ and 133 ms for A ipl responses (37 ms delay between sides). (d) Trial-resolved activity of M17 muscle in a single animal in response to CS+ stimulation during the MEM phase. Each tick mark indicates a muscle potential. The 3 s of odour stimulation is marked by the blue shading. (e) M17 rate averaged across all animals (blue). The half-maximum of the M17 rate was reached 500 ms after stimulus onset (time 0). The mean firing rate of the MBON units in response to the CS+ is shown in red. MBON units reached the half-maximum response rate 124 ms after odour onset.
4. Discussion
(a). Lateral integration of side-specific odour information during memory retention
To form a side-specific odour memory, the information of both brain sides needs to be integrated to evoke a unique neural activity during memory recall. The existence of such stable side-specific memory was first postulated by Sandoz & Menzel [8] when they trained bees differently on both antenna such that the contingency of odours was reversed dependent on stimulation side. The result was a stable side-specific memory as expressed in the conditioned response behaviour.
We found neural correlates of this lateral integration accompanied by a subset of MBONs that became recruited to encode the side-specific odour reward association during a post-acquisition memory-processing period (figure 3). Interestingly, approximately 50% of these units had also not responded to ipsilateral stimulation before conditioning. Thus, the activity of a small set of recruited units was sufficient to separate the reward-associated odour-side compound stimulus from all other tested stimuli including the same odour but presented to the ipsilateral antennae at the ensemble level (figure 2d). The fact that the same odour activated a different set of MBONs for ipsi- and contralateral stimulation (figure 3d; electronic supplementary material, figure S2) and only the CS+ combination evoked a conditioned response behaviour (figure 1d) shows that the activity measured during memory retention reflects lateral integration. The result is a stable neural representation of the valence of a specified stimulus constellation consisting of odour identity and stimulation side.
(b). Consolidation-dependent short- and mid-term memory at distinct locations
In honeybees, loci for learning-related olfactory plasticity have been determined with optophysiological and electrophysiological methods at different processing stages along the olfactory path, and in the AL [35–43], the MB calyces [44] and different MBONs [19,21,23–25,45]. In addition, paired presynaptic stimulation and postsynaptic recording suggested plasticity of the synapses between KCs and MBONs [46]. This suggests multiple consolidation-dependent serial and parallel kinds of learning-related memory traces distributed between different processing levels (reviewed in [4]).
Consolidation within the MB circuits may lead to a rearrangement of memory traces as it was described for Drosophila [47,48]. Our data indicate separate locations of plasticity for early short-term memory as behaviourally expressed during ACQ, and for consolidation-dependent short- to mid-term memory as tested 3 h after conditioning during MEM. Behavioural responses reflecting early short-term memory were already initiated during the first few acquisition trials (figure 1c). This behavioural effect was not seen in the neural learning curves of the MBONs recorded at the contralateral side, and is consistent with our earlier experiments where we stimulated both antennae and found acquisition-induced plasticity in MBONs only 3 h after but not during conditioning [21].
We therefore hypothesize that during acquisition short-term memory was established in parallel pathways possibly within another subset of MBONs of the ipsilateral or the contralateral brain side, which were not recorded in our experimental paradigm, nor in an earlier stage of unilateral processing, such as in the antennal lobe (cf. references above) or in the micro-glomerular structure of the calycal input region to the MB [49,50]. The latter might include plasticity of the GABAergic feedback synapses, as previously suggested [44,51]. The unilateral processing of the early memory phase is consistent with the model of Sandoz et al. [31], who predicted one association centre (network) for each of the two input sides. Based on the fact that a unilaterally established memory trace could be retrieved 3 h after training from the contralateral antennae [8], their model postulates a crosstalk between brain sides during later phases of memory formation. Our data support a substantial aspect of their model, namely the crosstalk and the formation of a side-spanning memory trace, which needs up to 3 h of consolidation. In contrast with Sandoz et al. [8], we did not observe a behavioural response to ipsilateral stimulation with the rewarded odour, which could have two reasons, both related to the experimental design. In particular, Sandoz and colleagues have used only four training trials, while here we used 10 conditioning trials. The number of conditioning trials might have an impact on the ability to learn and consolidate the side specificity and it also affects the animals' satiation level 3 h after conditioning as animals are fed in each conditioning trial. Thus, the motivation to generalize between stimulation sides in the present case might be lower. Another reason could be the extensive pre-conditioning phase in which the animals were exposed to 40 unilateral odour presentation (20× left, 20× right), which may have caused an acclimatization to unilateral stimulation which was not permitted by Sandoz et al. [8].
(c). Prolonged encoding of stimulus value to evaluate behavioural options
Comparing the latencies of the contralaterally recruited units with units that responded to the same odour A if presented to the ipsilateral side (A ipl) revealed a latency difference of approximately 37 ms. Thus, ipsilateral processing was faster than contralateral activation (figure 5) and could be based on the morphology, as the contralateral induced odour signal has to cross more synapses when crossing the midline of the brain. Because we always recorded the left brain side to ensure that our experiments are consistent across animals and do not confuse learning mechanisms and hemispheric specialization [29] and lateralization [30], the latency difference might be also caused by asymmetric odour computation as reported by Regosi et al. [29]. To confirm that it would be necessary to test naive animals and exclude influences of consolidation. However, in naive animals we found no odour-induced activity due to contralateral stimulation in MBON. We therefore assume that the delayed implementation of the contralateral information during MEM reflects a generally prolonged computation for the reward-associated stimulus because a temporal shift in the same range was found in MBONs to occur for the separation of the reward-associated odour from non-rewarded control odours after classical conditioning including both antennae [21]. Therefore, encoding the value of a behaviourally relevant (i.e. reward-associated) stimulus appears to be prolonged compared with the representation of non-reward-associated stimuli. We hypothesize that this temporal shift reflects the activation of stored memory for evaluating the current stimulus conditions as a prerequisite for eliciting the conditioned behaviour.
Supplementary Material
Acknowledgement
We thank Theodore Gray Smith for English proofreading.
Ethics
All experiments used honeybees maintained in a standard breeding programme at the Free University of Berlin, Germany.
Data accessibility
The dataset with all recorded single units is made publicly available on the database of the German Neuroinformatics Node (www.g-node.org).
Authors' contributions
M.F.S.-B. designed and performed all experiments, analysed the data and drafted the manuscript. M.P.N. discussed results and analysis, and helped draft the manuscript. R.M. discussed experimental design and results and helped drafting the manuscript. All authors commented on the manuscript and agreed to the final version.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Bundesministerium für Bildung und Forschung (BMBF) through grant no. 01GQ0413 to the Bernstein Center for Computational Neuroscience Berlin and grant no. 01GQ0941 to the Bernstein Focus Learning and Memory in Decision Making.
References
- 1.Schiestl F, Schlüter P. 2009. Floral isolation, specialized pollination, and pollinator behavior in orchids. Annu. Rev. Entomol. 54, 425–446. ( 10.1146/annurev.ento.54.110807.090603) [DOI] [PubMed] [Google Scholar]
- 2.Mobbs P. 1982. The brain of the honeybee Apis mellifera. I. The connections and spatial organization of the mushroom bodies. Phil. Trans. R. Soc. Lond. B 298, 309–354. ( 10.1098/rstb.1982.0086) [DOI] [Google Scholar]
- 3.Heisenberg M. 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275. ( 10.1038/nrn1074) [DOI] [PubMed] [Google Scholar]
- 4.Menzel R. 2012. The honeybee as a model for understanding the basis of cognition. Nat. Rev. Neurosci. 13, 758–768. ( 10.1038/nrn3357) [DOI] [PubMed] [Google Scholar]
- 5.Menzel R. 2014. The insect mushroom body, an experience-dependent recoding device. J. Physiol. Paris 108, 84–95. ( 10.1016/j.jphysparis.2014.07.004) [DOI] [PubMed] [Google Scholar]
- 6.Rybak J, Menzel R. 1993. Anatomy of the mushroom bodies in the honey bee brain: the neuronal connections of the alpha-lobe. J. Comp. Neurol. 334, 444–465. ( 10.1002/cne.903340309) [DOI] [PubMed] [Google Scholar]
- 7.Menzel R, Erber J, Masuhr TH. 1974. Learning and memory in the honeybee. In Experimental analysis of insect behaviour (ed. Barton Browne L.), pp. 195–217. Berlin, Germany: Springer. [Google Scholar]
- 8.Sandoz J, Menzel R. 2001. Side-specificity of olfactory learning in the honeybee: generalization between odors and sides. Learn. Mem. 8, 286–294. ( 10.1101/lm.41401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kucharski D, Hall W. 1987. New routes to early memories. Science 238, 786–788. ( 10.1126/science.3672125) [DOI] [PubMed] [Google Scholar]
- 10.Kucharski D, Hall W. 1988. Developmental change in the access to olfactory memories. Behav. Neurosci. 102, 340–348. ( 10.1037/0735-7044.102.3.340) [DOI] [PubMed] [Google Scholar]
- 11.Mainland J, Bremner E, Young N, Johnson B, Khan R, Bensafi M, Sobel N. 2002. One nostril knows what the other learns. Nature 419, 802 ( 10.1038/419802a) [DOI] [PubMed] [Google Scholar]
- 12.Chandra S, Smith B. 1998. An analysis of synthetic processing of odor mixtures in the honeybee (Apis mellifera). J. Exp. Biol. 201, 3113–3121. [DOI] [PubMed] [Google Scholar]
- 13.Deisig N, Lachnit H, Giurfa M, Hellstern F. 2001. Configural olfactory learning in honeybees: negative and positive patterning discrimination. Learn. Mem. 8, 70–78. ( 10.1101/lm.8.2.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deisig N, Lachnit H, Giurfa M. 2002. The effect of similarity between elemental stimuli and compounds in olfactory patterning discriminations. Learn. Mem. 9, 112–121. ( 10.1101/lm.41002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deisig N, Lachnit H, Sandoz JC, Lober K, Giurfa M. 2003. A modified version of the unique cue theory accounts for olfactory compound processing in honeybees. Learn. Mem. 10, 199–208. ( 10.1101/lm.55803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komischke B, Sandoz J-C, Lachnit H, Giurfa M. 2003. Non-elemental processing in olfactory discrimination tasks needs bilateral input in honeybees. Behav. Brain Res. 145, 135–143. ( 10.1016/s0166-4328(03)00105-0) [DOI] [PubMed] [Google Scholar]
- 17.Deisig N, Sandoz JC, Giurfa M, Lachnit H. 2007. The trial-spacing effect in olfactory patterning discriminations in honeybees. Behav. Brain Res. 176, 314–322. ( 10.1016/j.bbr.2006.10.019) [DOI] [PubMed] [Google Scholar]
- 18.Komischke B, Sandoz J, Malun D, Giurfa M. 2005. Partial unilateral lesions of the mushroom bodies affect olfactory learning in honeybees Apis mellifera L. Eur. J. Neurosci. 21, 477–485. ( 10.1111/j.1460-9568.2005.03879.x) [DOI] [PubMed] [Google Scholar]
- 19.Okada R, Rybak J, Manz G, Menzel R. 2007. Learning-related plasticity in PE1 and other mushroom body-extrinsic neurons in the honeybee brain. J. Neurosci. 27, 11 736–11 747. ( 10.1523/jneurosci.2216-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauelshagen J. 1993. Neural correlates of olfactory learning in an identified neuron in the honey bee brain. J. Neurophysiol. 69, 609–625. [DOI] [PubMed] [Google Scholar]
- 21.Strube-Bloss MF, Nawrot MP, Menzel R. 2011. Mushroom body output neurons encode odor-reward associations. J. Neurosci. 31, 3129–3140. ( 10.1523/jneurosci.2583-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grünewald B. 1999. Physiological properties and response modulations of mushroom body feedback neurons during olfactory learning in the honeybee, Apis mellifera. J. Comp. Physiol. A 185, 565–576. ( 10.1007/s003590050417) [DOI] [Google Scholar]
- 23.Haehnel M, Menzel R. 2010. Sensory representation and learning-related plasticity in mushroom body extrinsic feedback neurons of the protocerebral tract. Front. Syst. Neurosci. 4, 1–13. ( 10.3389/fnsys.2010.00161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haehnel M, Menzel R. 2012. Long-term memory and response generalization in mushroom body extrinsic neurons in the honeybee Apis mellifera. J. Exp. Biol. 215, 559–565. ( 10.1242/jeb.059626) [DOI] [PubMed] [Google Scholar]
- 25.Filla I, Menzel R. 2015. Mushroom body extrinsic neurons in the honeybee (Apis mellifera) brain integrate context and cue values upon attentional stimulus selection. J. Neurophysiol. 114, 2005–2014. ( 10.1152/jn.00776.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strube-Bloss MF, Herrera-Valdez MA, Smith BH. 2012. Ensemble response in mushroom body output neurons of the honey bee outpaces spatiotemporal odor processing two synapses earlier in the antennal lobe. PLoS ONE 7, 1–16. ( 10.1371/journal.pone.0050322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farkhooi F, Strube-Bloss M, Nawrot M. 2009. Serial correlation in neural spike trains: experimental evidence, stochastic modeling, and single neuron variability. Phys. Rev. E 79, 1–10. ( 10.1103/physreve.83.050905) [DOI] [PubMed] [Google Scholar]
- 28.Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119. ( 10.1037/0735-7036.97.2.107) [DOI] [PubMed] [Google Scholar]
- 29.Rigosi E, Haase A, Rath L, Anfora G, Vallortigara G, Szyszka P. 2015. Asymmetric neural coding revealed by in vivo calcium imaging in the honey bee brain. Proc. R. Soc. B 282, 20142571 ( 10.1098/rspb.2014.2571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell AT, Niven JE. 2016. Strength of forelimb lateralization predicts motor errors in an insect. R. Soc. Biol. Lett. 12, 20160516 ( 10.1098/rsbl.2016.0516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandoz JC, Hammer M, Menzel R. 2002. Side-specificity of olfactory learning in the honeybee: US input side. Learn. Mem. 9, 337–348. ( 10.1101/lm.50502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meier R, Egert U, Aertsen A, Nawrot MP. 2008. FIND—a unified framework for neural data analysis. Neural Netw. 21, 1085–1093. ( 10.1016/j.neunet.2008.06.019) [DOI] [PubMed] [Google Scholar]
- 33.Nawrot M, Aertsen A, Rotter S. 1999. Single-trial estimation of neuronal firing rates: from single-neuron spike trains to population activity. J. Neurosci. Methods 94, 81–92. ( 10.1016/s0165-0270(99)00127-2) [DOI] [PubMed] [Google Scholar]
- 34.Rehder V. 1987. Quantification of the honeybee's proboscis reflex by electromyographic recordings. J. Insect Physiol. 33, 501–507. ( 10.1016/0022-1910(87)90115-6) [DOI] [Google Scholar]
- 35.Faber T, Joerges J, Menzel R. 1999. Associative learning modifies neural representations of odors in the insect brain. Nat. Neurosci. 2, 74–78. ( 10.1038/4576) [DOI] [PubMed] [Google Scholar]
- 36.Faber T, Menzel R. 2001. Visualizing mushroom body response to a conditioned odor in honeybees. Naturwissenschaften 88, 472–476. ( 10.1007/s001140100263) [DOI] [PubMed] [Google Scholar]
- 37.Sandoz JC, Galizia CG, Menzel R. 2003. Side-specific olfactory conditioning leads to more specific odor representation between sides but not within sides in the honeybee antennal lobes. Neuroscience 120, 1137–1148. ( 10.1016/s0306-4522(03)00384-1) [DOI] [PubMed] [Google Scholar]
- 38.Arenas A, Giurfa M, Farina WM, Sandoz JC. 2009. Early olfactory experience modifies neural activity in the antennal lobe of a social insect at the adult stage. Eur. J. Neurosci. 30, 1498–1508. ( 10.1111/j.1460-9568.2009.06940.x) [DOI] [PubMed] [Google Scholar]
- 39.Fernández PC, Locatelli FF, Person-Rennell N, Deleo G, Smith BH. 2009. Associative conditioning tunes transient dynamics of early olfactory processing. J. Neurosci. 29, 10 191–10 202. ( 10.1523/jneurosci.1874-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roussel E, Sandoz JC, Giurfa M. 2010. Searching for learning-dependent changes in the antennal lobe: simultaneous recording of neural activity and aversive olfactory learning in honeybees. Front. Behav. Neurosci. 4, 1–12. ( 10.3389/fnbeh.2010.00155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denker M, Finke R, Schaupp F, Grün S, Menzel R. 2010. Neural correlates of odor learning in the honeybee antennal lobe. Eur. J. Neurosci. 31, 119–133. ( 10.1111/j.1460-9568.2009.07046.x) [DOI] [PubMed] [Google Scholar]
- 42.Rath L, Galizia CG, Szyszka P. 2011. Multiple memory traces after associative learning in the honey bee antennal lobe. Eur. J. Neurosci. 34, 352–360. ( 10.1111/j.1460-9568.2011.07753.x) [DOI] [PubMed] [Google Scholar]
- 43.Chen JY, Marachlian E, Assisi C, Huerta R, Smith BH, Locatelli F, Bazhenov M. 2015. Learning modifies odor mixture processing to improve detection of relevant components. J. Neurosci. 35, 179–197. ( 10.1523/jneurosci.2345-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szyszka P, Galkin A, Menzel R. 2008. Associative and non-associative plasticity in Kenyon cells of the honeybee mushroom body. Front. Syst. Neurosci. 2, 1–10. ( 10.3389/neuro.06.003.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussaini SA, Menzel R. 2013. Mushroom body extrinsic neurons in the honeybee brain encode cues and contexts differently. J. Neurosci. 33, 7154–7164. ( 10.1523/jneurosci.1331-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menzel R, Manz G. 2005. Neural plasticity of mushroom body-extrinsic neurons in the honeybee brain. J. Exp. Biol. 208, 4317–4332. ( 10.1242/jeb.01908) [DOI] [PubMed] [Google Scholar]
- 47.Davis RL. 2011. Traces of Drosophila memory. Neuron 70, 8–19. ( 10.1016/j.neuron.2011.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Séjourné J, et al. 2011. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat. Neurosci. 14, 903–910. ( 10.1038/nn.2846) [DOI] [PubMed] [Google Scholar]
- 49.Groh C, Rössler W. 2011. Comparison of microglomerular structures in the mushroom body calyx of neopteran insects. Arthropod Struct. Dev. 40, 358–367. ( 10.1016/j.asd.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 50.Rybak J, Menzel R. 2010. Mushroom body of the honeybee. In Handbook of brain microcircuits (eds G Shepherd, S Grillner), pp. 433–438. New York, NY: Oxford University Press. [Google Scholar]
- 51.Haenicke J.2015. Modeling insect inspired mechanisms of neural and behavioral plasticity. Doctoral thesis, Freie Universität, Berlin, Germany. (urn:nbn:de:kobv:188-fudissthesis000000100699-0)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset with all recorded single units is made publicly available on the database of the German Neuroinformatics Node (www.g-node.org).