Abstract
Trait-based ecology argues that an understanding of the traits of interactors can enhance the predictability of ecological outcomes. We examine here whether the multidimensional behavioural-trait diversity of communities influences community performance and stability in situ. We created experimental communities of web-building spiders, each with an identical species composition. Communities contained one individual of each of five different species. Prior to establishing these communities in the field, we examined three behavioural traits for each individual spider. These behavioural measures allowed us to estimate community-wide behavioural diversity, as inferred by the multidimensional behavioural volume occupied by the entire community. Communities that occupied a larger region of behavioural-trait space (i.e. where spiders differed more from each other behaviourally) gained more mass and were less likely to disband. Thus, there is a community-wide benefit to multidimensional behavioural diversity in this system that might translate to other multispecies assemblages.
Keywords: behavioural hypervolume, behavioural syndrome, behavioural niche, community, mixed species group, personality
1. Introduction
Individual variation in behavioural traits is ecologically notable because of its potential to change the outcome of a variety of processes, including population dynamics [1,2], range expansion/recession [3,4], trophic cascades [5,6], community assembly [7,8], extinction risk [9] (but see [10]) and species interaction networks [11]. In behavioural ecology, the majority of research on individual variation in behaviour has focused on single-species studies or two-species interactions [12–14]. For instance, individual differences in behaviour are known to shape foraging efficiency [15,16], diet breadth [17], susceptibility to predation [18,19] and propensity to be parasitized [20,21]. However, empirical information on how the impacts of such behavioural differences scale to influence species-rich ecological modules is still scant [22,23].
Here, we explore whether and how the multidimensional behavioural diversity exhibited by whole communities influences the success of each constituent species of the community, the success of the community as a whole and community persistence in the wild. Inspired by niche hypervolumes of general ecology [24,25] and recent advances in trait-based plant community ecology [26], we quantify behavioural-trait diversity as a multidimensional behavioural hypervolume. We define a behavioural hypervolume as the n-dimensional volume occupied by an individual, population or community in behavioural-trait space. To the extent to which behavioural differences map to niche differences, and there is some evidence that it should and does (e.g. [17,27–29]), we predict that communities that occupy a larger behavioural hypervolume will outperform behaviourally homogeneous communities. This prediction is analogous to classical studies linking species richness and reduced species similarity with improved system-level performance [30,31]. The benefits of large behavioural hypervolumes may result from adaptive division of labour [32], reduced competition among community constituents [33] or other more subtle mechanisms of trait complementarity [34]. Positive effects of within-group behavioural diversity have been documented for a variety of animal societies of a single species (e.g. as a consequence of adaptive social niche specialization [35–37]). We predict that a similar positive relationship between behavioural-trait diversity and collective outcomes will scale up to the community level when examining mixed species assemblages [38–40].
The facultatively social spider Anelosimus studiosus (Araneae, Theridiidae) constructs three-dimensional webs that serve as habitat for more than 50 other species of spiders [41,42]. These foreign spiders typically contribute a modest amount to the architecture of a shared chimaeric web, but A. studiosus is the nuclear species of the association [43]. If the resident A. studiosus perish or disperse, then the community quickly disbands and vanishes [41]. Generally, more species-rich communities exhibit higher rates of extinction and disbandment in this system [41,42]. Yet, whether or how behavioural-trait variability per se contributes to the extinction and disbandment of a community is unknown. Here we evaluate the degree to which the behavioural hypervolumes occupied by communities of spiders influence individual- and community-level performance metrics. In particular, we test the hypotheses that: (i) communities that occupy a larger behavioural hypervolume will be less likely to disband; (ii) the size of the behavioural hypervolume occupied by a community will be positively associated with the average mass gained by all community constituents; and (iii) if communities that occupy a larger behavioural hypervolume are more successful, and thus are more profitable to join, then behaviourally diverse communities will attract a larger number of spider immigrants into the chimaeric web.
2. Material and methods
(a). Collection and behavioural assays
Adult females of five different species of web-building spiders were collected along a riparian habitat in eastern Tennessee in May 2015 (Melton Hill, 35°59′29.76″ N, 84°11′44.55″ W). The five focal species were Leucauge venusta, Barronopsis texana, Theridion murarium, Theridion flavonotatum and A. studiosus. These species were selected because they represent some of the most common species in these communities [42,44]. We ran 52 individuals of each species through three behavioural assays to assess individual differences in boldness, foraging aggressiveness and activity level. Boldness was assessed using individuals' latency to resume movement following an aversive stimulus (detailed in [45,46]). Foraging aggressiveness was determined using individuals' latency to attack prey (termite workers) placed into the spider's web (detailed in [47,48]). Activity level was the distance (no. of gridlines) traversed by spiders in a novel open field environment over 10 min (detailed in [13,49]). Some animal personality researchers have used this assay as a metric of exploratory behaviour. To measure behavioural repeatability, 12 individuals from each species were run through all three assays once daily for 10 consecutive days. The remaining 40 individuals per species were randomly assigned to 40 experimental communities created using one individual of each of the five species. This produced 40 replicate communities that were identical in species composition, but varied in community behavioural hypervolume.
(b). Community creation and establishment
Communities were initiated by placing one A. studiosus individual in a 1.5 l container with a tangled ball of poultry wiring to facilitate web construction. Each individual A. studiosus was first given one week to construct a web before adding any other spiders. We then added one spider into the community each day for 4 consecutive days. To limit predation, all spiders were fed to satiation 24 h prior to being added to the community. Spiders were added in the following sequence, with one spider added every 24 h: T. murarium, T. flavonotatum, L. venusta and B. texana. All individuals were uniquely marked using fast-drying modelling paint atop their cephalothorax and weighed using an analytical balance. After all five individuals were added we allowed the community a 48 h settling period before deploying it in the field. To deploy communities at naturally appropriate locations, we first identified pre-existing A. studiosus webs, removed them using pruning snips and fastened an experimental community to the same location using topiary guide wires. Fourteen of the 40 experimental communities were either destroyed by predatory ants or were somehow dislodged and fell into the river within 48 h of being deployed. The remaining 26 communities were used for our analyses here.
To determine community success we collected all the experimental communities after 20 days. Instances where the resident A. studiosus dispersed always resulted in community disbandment, where all of the other species of spiders dispersed into adjacent foliage and constructed independent webs. The precise moment at which these disbandment events occurred is unknown; however, we did not observe community disbandment events during the first 8 days of the experiment. When possible, we collected all the original tagged spiders whether or not the community had disbanded. We then measured the mass of each spider to determine its change in body mass, which was our estimate of individual performance. In the 15 communities that disbanded, the resident A. studiosus died in two instances (carcass recovered) and dispersed to adjacent foliage in the 13 other cases. When the community remained intact, we counted the number of individuals of non-focal spiders that had recruited to the community during the 20 days of our experiment. These new spiders were primarily small subadult theridiids of genus Theridion.
(c). Statistical approach
Repeatabilities and 95% CI for each behavioural trait were estimated using the rptR package in R [50]. For Gaussian error distributions, rptR uses GLMMs and restricted maximum likelihood to calculate repeatability values, bootstrapping to estimate the 95% confidence intervals (number of simulations = 1000), and permutation tests to assess significance (number of permutations = 1000). We designated individual ID as a random effect in these models. Behavioural data were log-transformed to achieve a normal distribution for most species. However, we found that the latency to attack for L. venusta was highly bimodal; thus we estimated the repeatability values using both the log-transformed latencies and a Gaussian error distribution as well as binomial transformed responses and a binomial error distribution. Using a binomial error distribution yielded a slightly lower repeatability (0.34 versus 0.40), but the estimate was still significant (p = 0.001, 95% CI 0.033–0.556). For ease of comparison, we present the results of the log-transformed data (electronic supplementary material, S1). We tested for behavioural syndromes via pair-wise Spearman correlations of subjects' average behavioural response across trials.
To quantify the behavioural diversity of the experimental communities, we computed behavioural hypervolumes. To allow for comparison across behaviours, we first standardized each behavioural trait by subtracting from it the mean value of all observations and dividing the difference by the standard deviation. In this analysis, each individual becomes a single point in a three-dimensional behavioural space, and the behavioural hypervolume of the community was estimated as the volume captured within the minimum convex polyhedron created by these five points. Volumes were computed using the convhulln function in MATLAB. To circumvent the potential sensitivity to sampling effort of the behavioural hypervolume, which relies on extreme values, we compared communities with an identical number of constituents (n = 5).
We assessed the predictive value of community hypervolume against a variety of rival measures using a series of univariate models. Each rival model contained only one predictor variable and one response variable. This procedure allowed us to pit the predictive value of behavioural hypervolumes against simpler extrema statistics, like the range of each behavioural measure, without issues of multicollinearity. Models were compared using the Akaike information criterion (AIC) and Akaike weights; lower AICs and higher Akaike weights convey superior (more informative) models [51,52]. Predictor variables included the highest and lowest values, and the ranges of activity level, foraging aggressiveness or boldness exhibited by any community constituent. We also examined the ranges of principal component axes 1 and 2 (details of our PCA analysis are provided in table 1). Finally, we tested volume-based measures including the product of the ranges of principal component axes 1 and 2, the community volume assuming a right rectangular prism shape (the product of the three ranges) and the actual community volume of the irregular convex polyhedron occupied by the community in behavioural-trait space (i.e. its behavioural hypervolume). Response variables included average mass gained by all community constituents recovered, colony disbandment (modelled with a binomial error distribution) and the recruitment of additional spiders into the community. Model comparisons were run using JMP 12.0.
Table 1.
Results of our principal component analysis. Factors included were spiders' activity level, foraging aggressiveness (latency to attack prey) and boldness.
| eigenvalues |
loadings |
||||
|---|---|---|---|---|---|
| eigenvalue | % var explained | activity | foraging | boldness | |
| PC1 | 1.32 | 44.08 | −0.70 | 0.75 | 0.52 |
| PC2 | 0.93 | 30.89 | 0.44 | −0.17 | 0.84 |
| PC3 | 0.75 | 25.03 | 0.56 | 0.64 | −0.17 |
To evaluate the degree to which individual variation in body size (mass) is associated or conflated with variation in behaviour, we tested for associations between mass and each of the three behavioural metrics for each of the five focal species using general linear models. An independent analysis was conducted for each combination of species and behavioural trait. To examine whether spiders' starting mass might impact their change in mass within each community, we used a general linear model with percentage change in body mass as our response variable and individuals' starting mass as our predictor variable.
3. Results
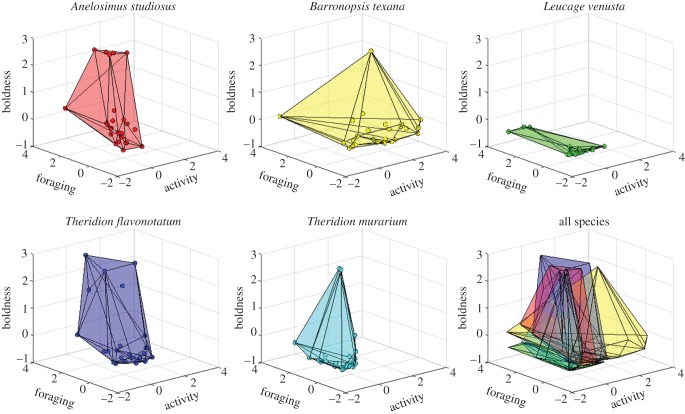
Our measures of activity level, foraging aggressiveness and boldness were repeatable for all traits and species (electronic supplementary material, S1 and table S1): A. studiosus (all r > 0.28), B. texana (all r > 0.36), T. flavonotatum (all r > 0.35), L. venusta (all r > 0.22) and T. murarium (all r > 0.55). Using all 52 animals per species, we detected some significant correlations in behaviour for three species: A. studiosus, B. texana and T. flavonotatum. We found positive correlations between activity level and foraging aggressiveness for all three of these species (electronic supplementary material, S1 and table S2) and a positive correlation between foraging aggressiveness and boldness in T. flavonotatum (electronic supplementary material, S1 and table S2). These syndromes yield a diamond-shaped behavioural hypervolume for all three species (figure 1).
Figure 1.
Behavioural hypervolumes of five species of web-building spiders. Points depict the activity level, foraging aggressiveness (latency to attack prey) and boldness of individual spiders. Polyhedrons are the behavioural hypervolume occupied by each species.
The species' behavioural hypervolumes varied across species (figure 1), with B. texana occupying the largest region in behavioural space and L. venusta the smallest: B. texana (13.05), T. flavonotatum (11.13), A. studiosus (4.89), T. murarium (3.83) and L. venusta (0.56). Post hoc inspection of our data indicated that the behavioural hypervolumes of some species (e.g. B. texana, T. murarium) might have been driven by one or a few individuals possessing extreme phenotypes. When removing the most extreme 5% of individuals (as done when creating two-dimensional 95% minimum convex polygons [53]), the resulting species behavioural hypervolumes had a similar rank-order as the full volumes: T. flavonotatum (8.38), B. texana (2.59), A. studiosus (2.21), T. murarium (2.05) and L. venusta (0.40).
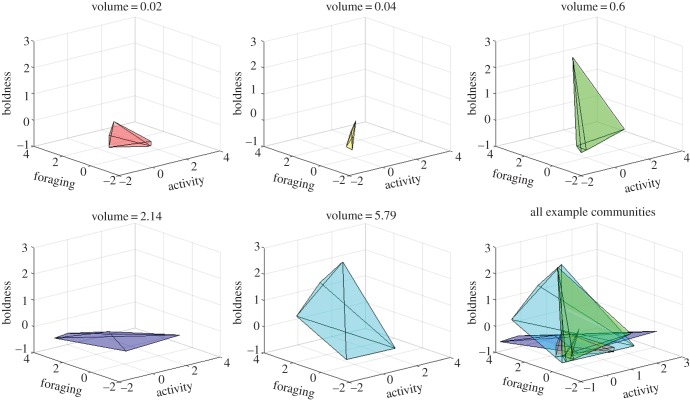
Example communities exhibiting a wide range of hypervolumes are depicted in figure 2. Communities varied a great deal in their behavioural hypervolumes. Some communities were composed of constituents of very similar multidimensional behavioural tendencies (BHVcommunity = 0.02) while other communities were composed of individuals that exhibited very different multidimensional behavioural tendencies (BHVcommunity = 5.79). The most behaviourally diverse communities exhibited behavioural hypervolumes that exceeded those exhibited by whole species (e.g. A. studiosus, T. murarium, L. venusta). This variability among communities raises the question of whether this variation has any functional consequences for community behaviour or performance.
Figure 2.
Behavioural hypervolumes of five example communities. The points of these polygons depict the activity level, foraging aggressiveness (latency to attack prey) and boldness of individual spiders. Communities were composed of five individual spiders, one from each of the following species: A. studiosus, B. texana, L. venusta, T. murarium and T. flavonotatum. Polyhedrons are the behavioural hypervolume occupied by each community.
The behavioural hypervolume occupied by a community was the best predictor of community disbandment (Akaike weight = 0.69; figure 3) and the average mass gain of all community constituents (Akaike weight = 0.29; figure 4). Both of these models had Akaike weights at least twice that of the second best model (electronic supplementary material, S2). Post hoc inspection of the regression of average mass gain on community hypervolume indicated a possible curvilinear relationship. However, post hoc comparisons of linear versus various nonlinear fits confirmed a better fit for the positive linear model (all ΔAICc > 2.2). To assess whether the relationship between mass gain and behavioural hypervolume was driven by communities that disbanded, we repeated the analysis with only the communities that remained together for the duration of the study (n = 12). Here again we detected a positive association between a community's behavioural hypervolume and the average mass gain of its constituents (L-R χ2 = 9.51, p = 0.002). Notably, many communities with relatively small behavioural hypervolumes (less than 0.70) lost weight during the course of our study (figure 4).
Figure 3.
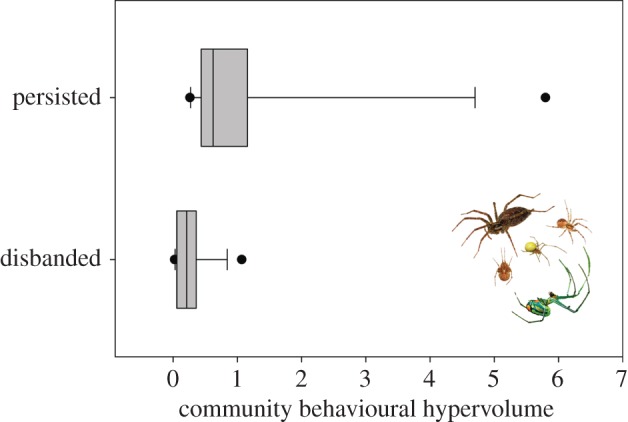
The behavioural hypervolume occupied by communities that persisted or disbanded over the course of our field study. The centre line designates the median, grey boxes give the interquartile range and vertical lines are the 90th and 10th percentiles. Note that community disbandment (x-axis) was the dependent variable for this analysis. Species positions and physical descriptions are as follows: B. texana (top left, large brown spider), A. studiosus (top right, small rust-coloured spider), T. flavonotatum (centre, yellow spider), T. murarium (bottom left, small brown spider), L. venusta (bottom right, large green spider).
Figure 4.
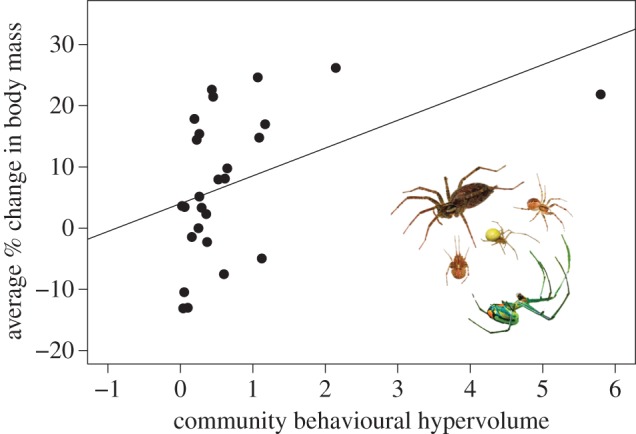
Average mass gain of community constituents in relations to community behavioural hypervolumes. Species positions and physical descriptions are as in figure 3.
Given that community-wide behavioural diversity had such large effects on the average mass gain of the community constituents, we conducted a post hoc analysis to determine whether individuals with behavioural tendencies unlike those of the rest of their community enjoyed superior performance, as one would predict if greater behavioural overlap caused greater competition. To conduct this analysis, we found the centre of gravity of the behavioural polyhedral of each community using the centroid function in MATLAB and calculated the Euclidean distance of each individual in the community from this centroid. This measure provided an estimate of each individual's behavioural dissimilarity from the rest of its community. We failed to detect an association between individuals' mass gain and their behavioural dissimilarity from their community (electronic supplementary material, S3).
Community hypervolume was also the best predictor for the mass gained by T. flavonotatum (Akaike weight = 0.20) and A. studiosus (Akaike weight = 0.23) (figure 5; electronic supplementary material, S2). Situations where these two species lost weight nearly always involved communities with small behavioural hypervolumes (less than 1.1) (figure 5). Community behavioural hypervolume was poorer than other measures at predicting the mass gain of the other species (depicted in the electronic supplementary material, S4) or the recruitment of additional spiders into the community.
Figure 5.
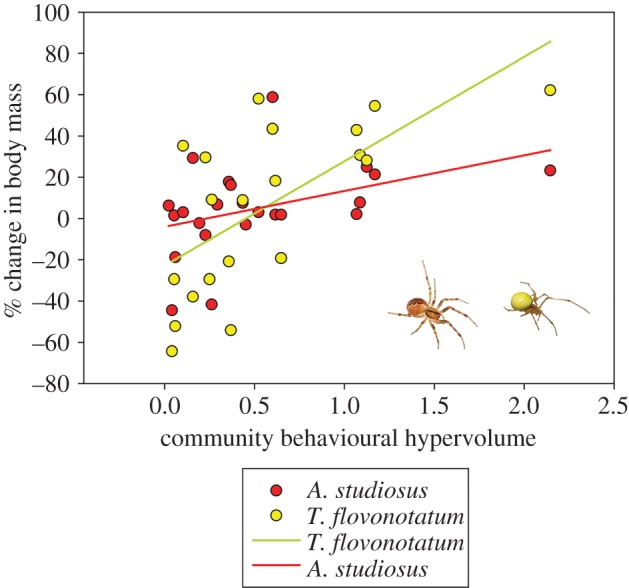
Percentage mass gain of A. studiosus (red dots, rust-coloured abdomen) and T. flavonotatum (yellow dots, yellow abdomen) in relation to community behavioural hypervolume.
Individual variation in body size was not significantly associated with individual variation in behaviour regardless of the species or behavioural trait considered (all p > 0.10; electronic supplementary material, S5). Thus, while individual variation in body size may interact with variation in behaviour in interesting ways, the behavioural results noted above cannot merely be attributed to variation in individual body size alone. With the exception of L. venusta (F1,19 = 7.23, R2 = 0.27, p = 0.014), individual variation in starting mass was unrelated to spiders' percentage change in body mass over the course of our experiment (all p > 0.43). For L. venusta, individual spiders that were larger at the start of the experiment gained proportionally more mass by the end of our study.
4. Discussion
The multidimensional behavioural diversity of communities of web-building spiders was strongly associated with community-wide outcomes such as likelihood of disbandment and collective mass gain. Interestingly, disbandment was also linked with community performance: communities tended to remain intact and gain more mass when they were more behaviourally diverse. Whether dispersing individuals were able to detect the behavioural hypervolume of their community directly or indirectly (e.g. via low mass gains, poor prey capture efficiency, etc.) is uncertain. However, we observed that greater behavioural diversity was positively associated with community-wide mass gain even when we restricted our analysis to only those communities that remained together for the duration of the study. This result conveys that more behaviourally diverse groups of heterospecific spiders outperform groups composed of behaviourally similar individuals independent of community disbandment. We discuss possible mechanisms for these findings below. Although positive effects of within-group behavioural variability have been noted for a variety of single-species social groups [35,36], our work represents a case where community-wide behavioural diversity has a positive impact on the stability and success of entire communities. Thus, we propose that positive effects of behavioural diversity could be a general phenomenon that occurs across multiple scales of biological organization.
The performance of A. studiosus and T. flavonotatum, both small-bodied theridiids, appears to be particularly susceptible to community-wide behavioural diversity. Both species gained more mass when occupying more behaviourally diverse communities. A positive relationship between behavioural diversity and enhanced performance has been detected previously in multi-female colonies of A. studiosus. Conspecific groups of behaviourally diverse A. studiosus outperform homogeneous groups [54], and dyadic interactions between A. studiosus and other species of spider (Larinioides and Theridion) often switch from parasitic to mutualistic when interactors exhibit unlike personality types [34]. Whether this stems from trait-mediated reductions in competition, enhanced division of labour or some other element of trait complementarity remains elusive. Single-species studies on A. studiosus groups suggest that the first two factors play at least some role in the enhanced performance of behaviourally diverse societies [55]. Given that A. studiosus is also the foundation species for this community, it is plausible that the positive effect of community-wide behavioural diversity on community performance stems from the fact that these are the same conditions where A. studiosus (the host species) does best. This hypothesis is consistent with prior studies which showed that community persistence is linked with the performance of A. studiosus [41]. We also cannot fully eliminate the hypothesis that species differences in body size could be responsible for the enhanced benefits of large behavioural hypervolumes for small-bodied species. We at present simply do not have enough species to critically evaluate this hypothesis. However, the hypothesis does not seem particularly viable merely because T. murarium, which is another small-bodied theridiid, failed to exhibit a similar pattern.
Finally, our results convey that a deep understanding of the relationship between behavioural variation and the ecology of A. studiosus's chimaeric webs calls for a multispecies and multidimensional perspective [56]. At present, it appears that the individual behavioural types of multiple interactors (conspecifics and heterospecifics) might work together in a multidimensional manner to shape the rise, disbandment and persistence of both colonies and whole communities in this system. Our inability to detect a direct relationship between the mass gain of each individual and its behavioural dissimilarity from its community suggests that synergistic, rather than additive, effects underlie the relationship between community mass gain and its behavioural hypervolume. We further note that more common multivariate statistical methods (PCAs) were consistently outperformed by behavioural hypervolume metrics when predicting community outcomes (electronic supplementary material, S2). This finding suggests that the most phenotypically extreme species or extreme individuals within communities may play a disproportionately large role in determining collective outcomes [57,58]. Therefore, it appears that behavioural hypervolumes could be a useful tool for deconstructing and assessing multi-tiered and species-rich systems, like most biological communities, of which the silken reefs of A. studiosus represent but one example.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are indebted to Niels Dingemanse, Alecia Carter, Emilie Snell-Rood and three anonymous reviewers for comments on previous versions of this manuscript.
Data accessibility
Data described in this manuscript are available on Dryad (http://dx.doi.org/10.5061/dryad.ps383 [59]).
Authors' contributions
J.N.P. and N.P.-W. designed and implemented the experiments and composed the original manuscript. All authors assisted in conducting statistical analyses and revising the manuscript.
Competing interests
We declare no competing interests.
Funding
Funding for this research was generously provided by NSF IOS grant no. 1352705 to J.N.P., NSF IOS grant nos. 1455895 and 1456010 to J.N.P. and N.P.-W., NIH GM115509 to N.P.-W. and J.N.P., and the University of California—Santa Barbara and the University of California—Los Angeles to J.N.P. and N.P.-W., respectively.
References
- 1.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG. 2003. Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424, 303–306. ( 10.1038/nature01767) [DOI] [PubMed] [Google Scholar]
- 2.Ellner SP, Becks L. 2011. Rapid prey evolution and the dynamics of two-predator food webs. Theor. Ecol. 4, 133–152. ( 10.1007/s12080-010-0096-7) [DOI] [Google Scholar]
- 3.Fogarty S, Cote J, Sih A. 2011. Social personality polymorphism and the spread of invasive species: a model. Am. Nat. 177, 273–287. ( 10.1086/658174) [DOI] [PubMed] [Google Scholar]
- 4.Phillips BL, Brown GP, Webb JK, Shine R. 2006. Invasion and the evolution of speed in toads. Nature 439, 803 ( 10.1038/439803a) [DOI] [PubMed] [Google Scholar]
- 5.Griffen BD, Toscano BJ, Gatto J. 2012. The role of individual behavior type in mediating indirect interactions. Ecology 93, 1935–1943. ( 10.1890/11-2153.1) [DOI] [PubMed] [Google Scholar]
- 6.Toscano BJ, Griffen BD. 2014. Trait-mediated functional responses: predator behavioural type mediates prey consumption. J. Anim. Ecol. 83, 1469–1477. ( 10.1111/1365-2656.12236) [DOI] [PubMed] [Google Scholar]
- 7.Hughes AR, Inouye BD, Johnson MTJ, Underwood N, Vellend M. 2008. Ecological consequences of genetic diversity. Ecol. Lett. 11, 609–623. ( 10.1111/j.1461-0248.2008.01179.x) [DOI] [PubMed] [Google Scholar]
- 8.Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313, 966–968. ( 10.1126/science.1128326) [DOI] [PubMed] [Google Scholar]
- 9.Mattila HR, Seeley TD. 2007. Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317, 362–364. ( 10.1126/science.1143046) [DOI] [PubMed] [Google Scholar]
- 10.Hart SP, Schreiber SJ, Levine JM. 2016. How variation between individuals affects species coexistence. Ecol. Lett. 19, 825–838. ( 10.1111/ele.12618) [DOI] [PubMed] [Google Scholar]
- 11.Pruitt JN, Ferrari MCO. 2011. Intraspecific trait variants determine the nature of interspecific interactions in habitat forming species. Ecology 92, 1902–1908. ( 10.1890/11-0701.1) [DOI] [PubMed] [Google Scholar]
- 12.McGhee KE, Pintor LM, Bell AM. 2013. Reciprocal behavioral plasticity and behavioral types during predator-prey interactions. Am. Nat. 182, 704–717. ( 10.1086/673526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeney K, Cusack B, Armagost F, O'Brien T, Keiser CN, Pruitt JN. 2013. Predator and prey activity levels jointly influence the outcome of long-term foraging bouts. Behav. Ecol. 24, 1205–1210. ( 10.1093/beheco/art052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiRienzo N, Pruitt JN, Hedrick AV. 2013. The combined behavioural tendencies of predator and prey mediate the outcome of their interaction. Anim. Behav. 86, 317–322. ( 10.1016/j.anbehav.2013.05.020) [DOI] [Google Scholar]
- 15.Biro PA, Post JR. 2008. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl Acad. Sci. USA 105, 2919–2922. ( 10.1073/pnas.0708159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riechert SE, Hedrick AV. 1993. A test for correlations among fitness-linked behavioral traits in the spider Agelenopsis-aperta (Araneae, Agelenidae). Anim. Behav. 46, 669–675. ( 10.1006/anbe.1993.1243) [DOI] [Google Scholar]
- 17.Riechert SE. 1991. Prey abundance vs diet breadth in a spider test system. Evol. Ecol. 5, 327–338. ( 10.1007/BF02214236) [DOI] [Google Scholar]
- 18.Carter AJ, Goldizen AW, Tromp SA. 2010. Agamas exhibit behavioral syndromes: bolder males bask and feed more but may suffer higher predation. Behav. Ecol. 21, 655–661. ( 10.1093/beheco/arq036) [DOI] [Google Scholar]
- 19.Smith BR, Blumstein DT. 2010. Behavioral types as predictors of survival in Trinidadian guppies (Poecilia reticulata). Behav. Ecol. 21, 919–926. ( 10.1093/beheco/arq084) [DOI] [Google Scholar]
- 20.Barber I, Dingemanse NJ. 2010. Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077–4088. ( 10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pamminger T, Scharf I, Pennings PS, Foitzik S. 2011. Increased host aggression as an induced defense against slave-making ants. Behav. Ecol. 22, 255–260. ( 10.1093/beheco/arq191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sih A, Cote J, Evans M, Fogarty S, Pruitt JN. 2012. Ecological implications of behavioral syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 23.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. ( 10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 24.Roughgarden J. 1974. Niche width: biogeographic patterns among Anolis lizard populations. Am. Nat. 108, 429–442. ( 10.1086/282924) [DOI] [Google Scholar]
- 25.Whittaker RH, Levine SA, Root RB. 1973. Niche, habitat, and ecotope. Am. Nat. 107, 321–338. ( 10.1086/282837) [DOI] [Google Scholar]
- 26.Funk JL, et al. In press. Revisiting the holy grail: using plant functional traits to understand ecological processes. Biol. Rev. ( 10.1111/brv.12275) [DOI] [PubMed] [Google Scholar]
- 27.Wilson DS, Coleman K, Clark AB, Biederman L. 1993. Shy bold continuum in pumpkinseed sunfish (Lepomis gibbosus)—an ecological study of a psychological trait. J. Comp. Psychol. 107, 250–260. ( 10.1037/0735-7036.107.3.250) [DOI] [Google Scholar]
- 28.Michelena P, Jeanson R, Deneubourg J-L, Sibbald AM. 2010. Personality and collective decision-making in foraging herbivores. Proc. R. Soc. B 277, 1093–1099. ( 10.1098/rspb.2009.1926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegel O, Leu ST, Sih A, Godfrey SS, Bull CM. 2015. When the going gets tough: behavioural type-dependent space use in the sleepy lizard changes as the season dries. Proc. R. Soc. B 282, 20151768 ( 10.1098/rspb.2015.1768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilman D. 1999. The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80, 1455–1474. ( 10.1890/0012-9658(1999)080%5B1455:tecoci%5D2.0.co;2) [DOI] [Google Scholar]
- 31.Stachowicz JJ, Whitlatch RB, Osman RW. 1999. Species diversity and invasion resistance in a marine ecosystem. Science 286, 1577–1579. ( 10.1126/science.286.5444.1577) [DOI] [PubMed] [Google Scholar]
- 32.Beshers SN, Fewell JH. 2001. Models of division of labor in social insects. Annu. Rev. Entomol. 46, 413–440. ( 10.1146/annurev.ento.46.1.413) [DOI] [PubMed] [Google Scholar]
- 33.Bergmuller R, Taborsky M. 2010. Animal personality due to social niche specialisation. Trends Ecol. Evol. 25, 504–511. ( 10.1016/j.tree.2010.06.012) [DOI] [PubMed] [Google Scholar]
- 34.Keiser CN, Pruitt JN. 2014. Spider aggressiveness determines the bidirectional consequences of host–inquiline interactions. Behav. Ecol. [Google Scholar]
- 35.Modlmeier AP, Liebmann JE, Foitzik S. 2012. Diverse societies are more productive: a lesson from ants. Proc. R. Soc. B 279, 2142–2150. ( 10.1098/rspb.2011.2376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burns JG, Dyer AG. 2008. Diversity of speed-accuracy strategies benefits social insects. Curr. Biol. 18, R953–R954. ( 10.1016/j.cub.2008.08.028) [DOI] [PubMed] [Google Scholar]
- 37.Seeley TD, Tarpy DR. 2007. Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. B 274, 67–72. ( 10.1098/rspb.2006.3702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farine DR, Aplin LM, Garroway CJ, Mann RP, Sheldon BC. 2014. Collective decision making and social interaction rules in mixed-species flocks of songbirds. Anim. Behav. 95, 173–182. ( 10.1016/j.anbehav.2014.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farine DR, Downing CP, Downing PA. 2014. Mixed-species associations can arise without heterospecific attraction. Behav. Ecol. 25, 574–581. ( 10.1093/beheco/aru023) [DOI] [Google Scholar]
- 40.Farine DR, Aplin LM, Sheldon BC, Hoppitt W. 2015. Interspecific social networks promote information transmission in wild songbirds. Proc. R. Soc. B 282, 20142804 ( 10.1098/rspb.2014.2804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruitt JN, Modlmeier AP. 2015. Animal personality in a foundation species drives community divergence and collapse in the wild. J. Anim. Ecol. 84, 1461–1468. ( 10.1111/1365-2656.12406) [DOI] [PubMed] [Google Scholar]
- 42.Pruitt JN, Riechert SE. 2011. Within-group behavioral variation promoted biased task performance and the emergence of a defensive caste in a social spider. Behav. Ecol. Sociobiol. 65, 1055–1060. ( 10.1007/s00265-010-1112-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perkins TA, Riechert SE, Jones TC. 2007. Interactions between the social spider Anelosimus studiosus (Araneae, Theridiidae) and foreign spiders that frequent its nests. J. Arachnol. 35, 143–152. ( 10.1636/T06-43.1) [DOI] [Google Scholar]
- 44.Pruitt JN, Cote J, Ferrari MCO. 2012. Behavioural trait variants in a habitat-forming species dictate the nature of its interactions with and among heterospecifics. Funct. Ecol. 26, 29–36. ( 10.1111/j.1365-2435.2011.01922.x) [DOI] [Google Scholar]
- 45.Keiser CN, Jones DK, Modlmeier AP, Pruitt JN. 2014. Exploring the effects of individual traits and within-colony variation on task specialization and collective behavior in a desert social spider. Behav. Ecol. Sociobiol. 68, 839–850. ( 10.1007/s00265-014-1696-9) [DOI] [Google Scholar]
- 46.Keiser CN, Modlmeier AP, Singh N, Jones DK, Pruitt JN. 2014. Exploring how a shift in the physical environment shapes individual and group behavior in two social contexts. Ethology 120, 825–833. ( 10.1111/eth.12256) [DOI] [Google Scholar]
- 47.Hedrick AV, Riechert SE. 1989. Genetically-based variation between 2 spider populations in foraging behavior. Oecologia 80, 533–539. ( 10.1007/BF00380078) [DOI] [PubMed] [Google Scholar]
- 48.Pruitt JN, Riechert SE, Jones TC. 2008. Behavioural syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim. Behav. 76, 871–879. ( 10.1016/j.anbehav.2008.05.009) [DOI] [Google Scholar]
- 49.Keiser CN, Slyder JB, Carson WP, Pruitt JN. 2015. Individual differences in predators but not producers mediate the magnitude of a trophic cascade. Arthropod-Plant Interact. 9, 225–232. ( 10.1007/s11829-015-9377-9) [DOI] [Google Scholar]
- 50.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 51.Akaike H. 1987. Factor-analysis and AIC. Psychometrika 52, 317–332. ( 10.1007/BF02294359) [DOI] [Google Scholar]
- 52.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 53.Kenward RE. 1987. Wildlife radio tagging: equipment, field techniques and data analysis. Orlando, FL: Academic Press. [Google Scholar]
- 54.Pruitt JN, Riechert SE. 2011. How within-group behavioral variation and task efficiency enhance fitness in a social group. Proc. R. Soc. B 278, 1209–1215. ( 10.1098/rspb.2010.1700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright CM, Holbrook CT, Pruitt JN. 2014. Animal personality aligns task specialization and task proficiency in a spider society. Proc. Natl Acad. Sci. USA 111, 9533–9537. ( 10.1073/pnas.1400850111) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Villeger S, Mason NWH, Mouillot D. 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. ( 10.1890/07-1206.1) [DOI] [PubMed] [Google Scholar]
- 57.Modlmeier AP, Keiser CN, Watters JV, Sih A, Pruitt JN. 2014. The keystone individual concept: an ecological and evolutionary overview. Anim. Behav. 89, 53–62. ( 10.1016/j.anbehav.2013.12.020) [DOI] [Google Scholar]
- 58.Chang AT, Sih A. 2013. Multilevel selection and effects of keystone hyperaggressive males on mating success and behavior in stream water striders. Behav. Ecol. 24, 1166–1176. ( 10.1093/beheco/art044) [DOI] [Google Scholar]
- 59.Pruitt J, Bolnick DI, Sih A, DiRienzo N, Pinter-Wollman N. 2016 doi: 10.5061/dryad.ps383. Data from: Behavioural hypervolumes of spider communities predict community performance and disbandment. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in this manuscript are available on Dryad (http://dx.doi.org/10.5061/dryad.ps383 [59]).