Abstract
Anthropogenic activities are causing species extinctions, raising concerns about the consequences of changing biological communities for ecosystem functioning. To address this, we investigated how dung beetle communities influence seed burial and seedling recruitment in the Brazilian Amazon. First, we conducted a burial and retrieval experiment using seed mimics. We found that dung beetle biomass had a stronger positive effect on the burial of large than small beads, suggesting that anthropogenic reductions in large-bodied beetles will have the greatest effect on the secondary dispersal of large-seeded plant species. Second, we established mesocosm experiments in which dung beetle communities buried Myrciaria dubia seeds to examine plant emergence and survival. Contrary to expectations, we found that beetle diversity and biomass negatively influenced seedling emergence, but positively affected the survival of seedlings that emerged. Finally, we conducted germination trials to establish the optimum burial depth of experimental seeds, revealing a negative relationship between burial depth and seedling emergence success. Our results provide novel evidence that seed burial by dung beetles may be detrimental for the emergence of some seed species. However, we also detected positive impacts of beetle activity on seedling recruitment, which are probably because of their influence on soil properties. Overall, this study provides new evidence that anthropogenic impacts on dung beetle communities could influence the structure of tropical forests; in particular, their capacity to regenerate and continue to provide valuable functions and services.
Keywords: plant recruitment, biodiversity–ecosystem functioning, soil, ecosystem processes, defaunation
1. Introduction
Human activities over the past 500 years have driven a dramatic decline in biodiversity [1,2]. The loss of species is of concern for the maintenance of functioning ecosystems [3]. So too is the ongoing decline in the abundances of individuals that remain. It is increasingly recognized that this erosion of biodiversity will lead to the breakdown of species interactions and a loss of associated ecosystem functions and services [3,4].
The geographical pattern of species loss is non-random [5], with tropical forests displaying the highest rates of declines in biodiversity [1], caused by unsustainable hunting in conjunction with habitat loss and modification [6–8]. Decreases in vertebrate populations within tropical forests are of particular concern because top-down trophic cascades can affect plants through changes in the abundance of frugivores, granivores and folivores [9]. For example, in this edition, Bregman et al. [10] demonstrate that land use change negatively impacts primary seed dispersers, which could influence the long-term regeneration of tropical forests. However, most biodiversity–ecosystem function experiments focus on bottom-up processes governed by terrestrial plant communities, demonstrating that diversity is important for resource capture and ecosystem resilience [11–13]. We therefore have a poor understanding of direct effects of diversity within higher trophic levels or the indirect, cascading effects of biodiversity loss across tropic levels (but see [14]). There is mounting evidence that changes in forest vertebrate communities can lead to direct top-down consequences for plant demography, community composition and diversity [15–22], with knock-on effects for forest services and resilience [23,24]. However, because the indirect, multitrophic consequences of changing mammal communities are rarely experimentally tested, we have limited understanding of the ecosystem-wide consequences of anthropogenic impacts on tropical forests.
The secondary dispersal of seeds by dung beetles is an example of an indirect tropic interaction between vertebrates and plants, which probably impacts seedling recruitment [25]. Seeds within mammalian dung are frequently relocated to beneath the soil surface because dung beetles move and bury faeces for feeding and nesting purposes [26]. This can benefit seeds by placing them in a more suitable microsite for germination [27,28], avoidance of density-dependent competition [29] and through escape from predation [27,30]. However, if seeds are placed too deep, burial by beetles can result in seed mortality [27,30,31]; suggesting there exists a species-specific optimal seed burial depth.
According to the International Union for Conservation of Nature (IUCN) Redlist, approximately 20% of mammals globally are considered vulnerable, endangered or critically endangered, with the highest numbers of declining species occurring within tropical forests [1,32]. As dung beetles depend on mammalian faeces, this pervasive decline in mammal populations and biomass can cascade through ecosystems, reducing dung beetle body size and species richness [33]. At the same time, positive links have been established between dung beetle taxonomic and functional diversity and the burial and dispersion of seeds [34–36], and large-bodied beetles have a disproportionally important role in seed and dung burial [35,37]. Therefore, it is probable that top-down, cascading declines in dung beetle diversity and changes to community structure will impact the germination and establishment of secondarily dispersed seeds, with potential implications for forest regeneration and ecosystem resilience to environmental change. However, to our knowledge this has not yet been experimentally tested.
Therefore, in this study we investigate how dung beetle community composition (biomass, taxonomic and functional diversity) influences the burial, germination and survival of seeds in a tropical forest, and explore whether the presence of dung, and the burial depths of beetle dispersed seeds, influences seedling emergence. To do this, we carried out three sets of experiments, each testing a different hypothesis/prediction. First, because large-bodied dung beetles are instrumental in the dispersal of large seeds [35], we predicted that large-seeded species are more sensitive to reductions in dung beetle biomass and diversity than smaller seeds. To test this, we carried out an experiment in which beads (seed mimics) were buried by naturally assembled beetle communities. Second, because dung beetle diversity has been shown to positively influence the likelihood of bead burial and dispersion throughout the soil profile [36], we used real seeds to test the hypothesis that beetle functional diversity and species richness positively influences seedling emergence and survival. This is because: (i) burial decreases seed predation [27,30] and (ii) the greater the dispersal distance of seeds from a central point, the higher the likelihood that each individual seed will be placed in its optimal species-specific microsite for recruitment. Finally, experiments were complemented by germination trials to establish the optimal burial depth for experimental seeds and allow interpretation of any patterns observed between beetle activity and seedling emergence/survival. We predicted that highest germination would occur in microsites near the surface (from 1 cm to 4 cm), deep enough to reduce predation, yet shallow enough to avoid soil depth preventing emergence following germination (cf. [27,28]).
2. Material and methods
(a). Using seed mimics to examine burial
Experiments were conducted in the 17 000 km2 Jari Florestal landholding, located in the State of Pará, north-eastern Brazilian Amazon (0°53 S, 52°36 W). Unlike many regions of the Amazon, the predominant anthropogenic disturbance in this area is forest clearance for Eucalyptus plantations rather than clearance for pasture land and cattle ranching. As such, the region consists of a matrix of Eucalyptus plantations, regenerating secondary forests, and large areas of largely undisturbed primary terra firme rainforest that do not provide viable habitat for any domesticated ungulates. Within this landscape, experiments were established in three primary forest sites (see [36], for full site description).
During July and August 2012, we established a grid of 30 mesocosms, separated by 100 m, at each experimental site (n = 90 in total). Mesocosms were created by burying nylon netting 10 cm vertically into the soil in a 50 × 50 cm square (electronic supplementary material, appendix S1) and were baited with a 100 g mixture of 50 : 50 human and pig dung containing 20 plastic seed mimics (beads) of four different sizes: two large (20 mm diameter, 4.12 g), six medium (10 mm diameter, 0.50 g), six small (5 mm diameter, 0.09 g) and six very small (2 mm diameter, 0.06 g). The dung and beads were placed on the floor within the plots, protected from rain by a plastic cover and left open for beetle colonization for between 12 and 24 h. After baiting, mesocosms were closed using pegs to hold the netting together, ensuring beetles could not leave and preventing further colonization by beetles that had not buried the dung. Each mesocosm also contained an internal, non-baited pitfall trap (13.5 cm width, 9 cm depth), buried flush with the ground surface and filled with a saltwater solution. Internal traps were opened when mesocosms were closed to capture the beetle community that had buried the dung and beads following emergence from the soil. After closure, mesocosms were left for 7–14 days before the soil beneath the dung was destructively sampled to a depth of 50 cm in search of the beads buried by beetles. This difference in time that mesocosms were left before sampling had no impact on the numbers of beads buried [36]. Internal pitfall traps were removed and beetles oven dried for laboratory processing (see [36], for detailed experimental design and rationale).
(b). Evaluating seedling emergence and survival
Following the procedure described above, in February 2014, we created a further 90 mesocosms in one of the sites (0°38′46.418″ S, 52°34′11.125″ W) with clay textured Oxisols (mean clay content ± s.e.: 67.3 ± 1.5%, silt: 14.4 ± 1%, sand: 14.1 ± 1.1%). This site was selected because previous work demonstrated that dung beetle diversity strongly influenced the dispersal of seed mimics in this site compared with other sites in the region [36]. We therefore designed this experiment to investigate if the observed patterns between dung beetle diversity and the burial of seed mimics influence the success of real seeds. Each mesocosm was baited with a 100 g mixture of 50 : 50 human and pig dung containing two seeds each of five animal-dispersed, Amazonian fruit species: Genipa americana, Malpighia emarginata, Myrciaria dubia, Psidium guajava and Rubus chamaemorus.
Dung and seeds were placed on the forest floor at the centre of the mesocosms between 07.00 and 09.00, protected from rain by a plastic cover. To enhance variation in the diversity of dung beetle communities, we randomly assigned mesocosms to one of three experimental treatments (n = 30 in each): control: baited and closed immediately, preventing any beetles from accessing dung and seeds; partial exclusion treatment: a 50 × 50 cm wire cage placed over the dung and seeds (mesh size 15 × 8 mm) within mesocosms; and open treatment: baited and left open for colonization by all beetles. The partial exculsion treatment prevented the largest beetles from entering plots and created a greater spread in diversity between mesocosms, while maintaining naturally assembled communities (electronic supplementary material, appendix S2 for treatment effects on dung beetle communities). During the establishment of mesocosms, nine were baited each day for 10 days (n = 3 per treatment, per day). The partial exclusion and open treatments were left for 24 h following baiting before closure.
Internal pitfall traps were opened when mesocosms were closed to capture the beetle community that had buried dung and seeds following emergence from the soil. Mesocosms were left closed for two weeks, during which time internal pitfall traps were emptied of beetles and refilled with saltwater once. After two weeks, we removed the pitfall traps and nylon netting covering mesocosms. The leaf litter and exposed soil was inspected to recover any beetles that remained within the mesocosms but had not fallen into the pitfall traps. All beetles recovered from within the mesocosms were dried and stored for laboratory processing. After baiting, mesocosms were monitored weekly for 18 weeks to assess emergence and survival of seedlings.
(c). Germination trials
To facilitate the interpretation of any patterns observed from the seed emergence and survival experiments in 2014, we created nine plots in the field to assess how burial depth and the presence of dung influenced emergence and survival of experimental seedlings. In each 120 × 200 cm plot, we planted seeds at 10 different depths (n = 40 per species; n = 200 seeds per plot): above the leaf litter, below the leaf litter, 1 cm, 2 cm, 3 cm, 5 cm, 7 cm, 10 cm, 15 cm and 20 cm. At each depth, seeds were either planted alone or in the centre of a 1 g ball of dung (n = 2 per treatment, per depth). Plots were divided into 10 cm2 sections, seeds were assigned a depth x treatment (dung or alone) and placed randomly within the plots (n = 200 seeds × 9 plots). Following planting, plots were monitored weekly for 18 weeks to assess the emergence and survival of seedlings.
Fifty-seven per cent of M. dubia seeds emerged from within mesocosms and 18% from within germination plots, compared with an emergence success of less than 10% and 5% from mesocosms and germination plots respectively for the other four species. Therefore, we focus results on only M. dubia (similar in dimensions to the medium bead used in burial trials: bead weight = 0.5 g, width = 10 mm, length = 10 mm; M. dubia mean weight = 0.45 g ± 0.03 g, mean width = 10.68 mm ± 0.26 mm, mean length = 13.76 g ± 0.26 g, calculated from 15 seeds) because emergence of the other species was too low to allow analyses (see the electronic supplementary material, appendix S3, for further explanation for exclusion of seed species). Myrciaria dubia (HBK) McVaugh, is a small, dicotyledonous tree, belonging to the Myrtaceae family that produces spherical fruits 2–5 cm in diameter, each containing two seeds [38]. It is widely distributed across the north-eastern Brazilian Amazon [39].
(d). Dung beetle traits and diversity metrics
We identified beetles to species using a reference collection at the Universidade Federal de Lavras, Brazil, and identification keys developed by T. A. Gardner and F. Z. Vaz-de-Mello. To calculate functional diversity, we used species median values of four continuous morphological traits: biomass (measured using a Shimatzu AY220 balance), biomass adjusted pronotum volume, biomass adjusted front leg area, back : front leg length (each measured using a Leica M250 microscope and Life Measurement software; electronic supplementary material, appendix S4); as well as three behavioural traits: nesting strategy (tunneller, roller, dweller [26]), diurnal activity (diurnal, nocturnal, crepuscular or generalist) and diet (coprophagus or generalist). Categorical trait information was gathered from [40] and [41]. These seven traits were selected because they have been linked to dung beetle-mediated seed dispersal [36] (see the electronic supplementary material, appendix S5, for details of the dung beetle communities and trait values).
We calculated species richness, total biomass, functional richness and the community-weighted means (CWM) of the continuous traits (biomass, biomass adjusted pronotum volume, biomass adjusted front leg area, back : front leg length) for all mesocoms that contained beetles. Functional richness, is a multidimensional measure of the range of traits in a biological community [42] and was calculated using median biomass, biomass adjusted pronotum volume, biomass adjusted front leg area, back : front leg length, nesting strategy, diurnal activity. CWMs describe the mean value of each trait in the communities, weighted by the relative abundances of the species carrying that trait [43]. Functional richness and CWM traits were calculated using the ‘FD’ package in R v. 3.0.2 [44,45].
(e). Statistical analyses
Analyses were carried out in R v. 3.0.2 [45]. Our first hypothesis was that large seeds are more sensitive to reductions in dung beetle biomass and diversity than smaller seeds. To test this, we used generalized linear mixed effects models (GLMMs) from the ‘lme4’ package [46] to investigate if bead size, beetle community metric and the interaction between the two factors affected probability of bead buried (2012 experiment). Each community metric was included in a separate model and mesocosm was nested within site as random factors. Our second hypothesis was that dung beetle diversity positively influences the emergence and survival of real seeds. We used linear models (LMs) to investigate if treatment (open or partial exclusion) succeeded in enhancing the variety in beetle community metrics across mesocosms (2014 experiment, electronic supplementary material, appendix S2). We then used GLMMs to assess how beetle community metrics within mesocosms influenced the probability of seed emergence and survival until the end of the 18-week experimental period. Mesocosm was included as a random factor. Our final goal was to assess the optimal burial depth of M. dubia seeds and to investigate if the presence of dung influences seedling emergence or survival. Here, we used GLMMs to ascertain if burial depth, the presence of dung and the interaction between the two factors influenced probability that seeds emergence from the soil and subsequently survived until the end of the 18-week monitoring period. We then used GLMMs to investigate if the week that seedlings emerged influenced the likelihood that they survived until the end of the experimental period to ensure that any observed correlations between burial depth and seedling survival were not an artefact of the seedlings having emerged at different times. Germination plot was a random factor in LMs and GLMMs.
Within GLMMs assessing the likelihood of bead burial, beads were assigned a 1 if they were buried and a 0 if they remained on the soil surface; in seed emergence models, seeds were assigned a 1 if they emerged from the soil surface and a 0 if they did not; in models assessing the likelihood of survival, seedlings that emerged were assigned a 1 if they survived until the end of the monitoring period and a 0 if they did not. As such, a binary error distribution with a logit link function was specified for all GLMMs. All community metrics were log10-transformed to ensure models satisfied assumptions of normality. Models were created using all fixed terms and interactions, we then used a top-down approach to arrive at the best descriptive model [47] in which only significant terms (p < 0.05) remained. χ2-likelihood ratio tests (LRT) were used within the ‘drop1’ function in R for GLMMs and ANOVAs for LMs to assess the loss of explanatory power following removal of an interaction or a single term predictor.
3. Results
(a). Using seed mimics to examine burial
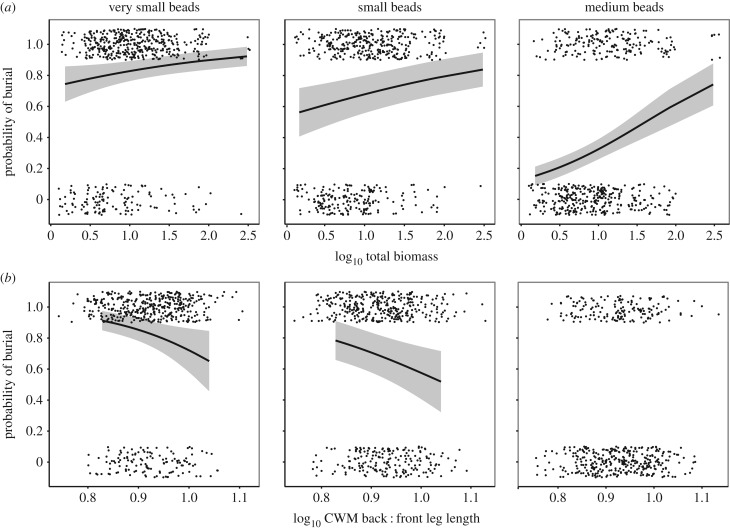
Bead size had a highly significant impact on the likelihood that dung beetles buried beads (LRT = 398.98, d.f. = 3, p < 0.0001) and significantly affected the depth at which they were placed within the soil (LRT = 325.91, d.f. = 3, p < 0.0001). Both the proportion of beads buried and burial depth decreased with increasing bead size (electronic supplementary material, appendix S6). Dung beetle total biomass and CWM back : front leg lengths were the only community metrics that significantly affected probability of bead burial. Biomass had a consistent positive effect on the likelihood that beads of all sizes were buried (LRT = 4.53, d.f. = 3, p = 0.033). However, the effect was stronger for the burial of medium sized beads: probability of burial increased from around 20% at the lowest biomass values to around 70% at the highest values for medium beads, compared with an increase from 70% to 90% for very small beads and a 60% to 80% increase for small beads (figure 1a). There was a significant interaction between CWM back : front leg length and bead size (LRT = 9.23, d.f. = 3, p = 0.026). An increase in CWM back : front leg length had a negative effect on the likelihood that small and very small beads were buried (a reduction of 80–55% and 90–65%, respectively), but did not affect the probability that medium beads were buried (figure 1b). The effect of beetle community metrics on the likelihood of burial of the large beads could not be assessed because too few were buried (less than 10%) to allow model testing.
Figure 1.
Effects of dung beetle total biomass (a) and CWM back : front leg length (b) on the probability of seed mimic burial. Very small beads (left panels), small beads (middle panels) and medium beads (right panels). Significance determined by generalized linear mixed effects models. Predicted values (solid black lines) ± s.e. (ribbons) are displayed along with individual seeds (black points), which were either buried (1) or remained on the soil surface (0).
(b). Evaluating seedling emergence and survival
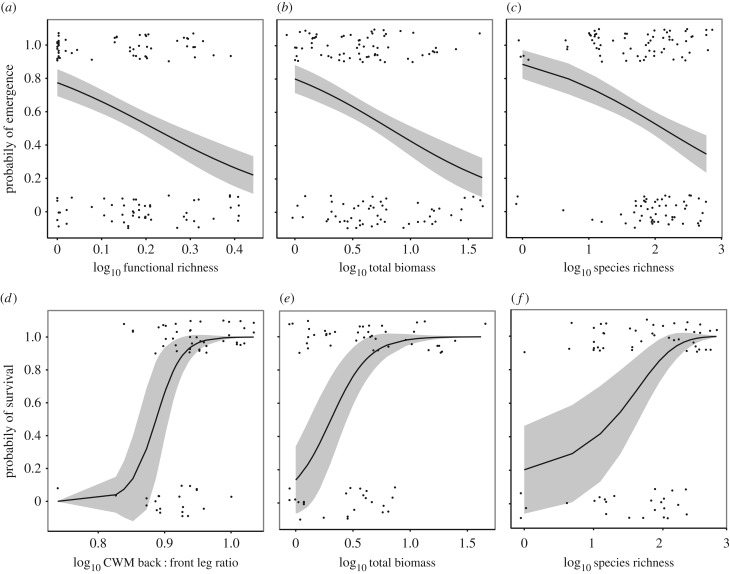
Functional richness, species richness and total biomass had a significant negative effect on the likelihood of M. dubia emergence. Eighty per cent of seeds emerged from mesocosms displaying the lowest values for functional richness, species richness and total biomass, compared with around 20% emergence from mesocosm displaying the highest values for functional richness, species richness and total biomass. Community-weighted mean biomass, pronotum volume, front leg area and back : front leg length had no significant effect on emergence success (table 1; figure 2a–c).
Table 1.
Generalized linear mixed effects model (GLMM) outputs to assess the influence of dung beetle community attributes on the probability of seed emergence (left section) and seedling survival until the end of the 18-week experimental period (right section). (Dung beetle community attributes that significantly affected emergence or survival (p < 0.005) are highlighted in italics.)
| GLMM(seed emergence ∼ beetle community) | LRT | d.f. | p-values | GLMM(seedling survival ∼ beetle community) | LRT | d.f. | p-values |
|---|---|---|---|---|---|---|---|
| functional richness | 6.3 | 1 | 0.0124 | CWM back : front leg length | 8.4 | 1 | 0.0038 |
| total biomass | 5.7 | 1 | 0.017 | total biomass | 6.5 | 1 | 0.0107 |
| species richness | 4.6 | 1 | 0.0326 | species richness | 3.9 | 1 | 0.0495 |
| CWM biomass | 0.3 | 1 | 0.6119 | CWM front leg area | 1.8 | 1 | 0.18 |
| CWM pronotum volume | 0.1 | 1 | 0.7924 | CWM biomass | 1.3 | 1 | 0.2598 |
| CWM front leg area | 0.1 | 1 | 0.7416 | CWM pronotum volume | 0.9 | 1 | 0.3373 |
| CWM back : leg length | 0 | 1 | 0.9733 | functional richness | 0.7 | 1 | 0.3994 |
Figure 2.
Significant negative effect of dung beetle functional richness (a), total biomass (b) and species richness (c) on the probability of seed emergence (a–c) and the significant positive effect of community-weighted mean (CWM) back : front leg length (d), total biomass (e) and species richness (e) on the likelihood that emerged seedlings survived until the end of the 18-week experimental period (d–f). Significance was determined by generalized linear mixed effects models. Predicted values (solid black lines) ± s.e. (ribbons) are displayed along with individual seeds (black points, jittered to avoid overlap), which either emerged (1) or did not emerge (0); and survived (1) or died after emergence (0).
By contrast, CWM back : front leg length, total biomass and species richness had a significant positive effect on the likelihood that emerged seedlings survived until the end of the 18-week monitoring period (figure 2d–f). The strongest predictor of seedling survival was CWM back : front leg length (table 1): 0% of seedlings buried by beetle communities displaying the lowest CWM back : front leg length values survived until the end of the monitoring period, whereas 100% of seedlings within mesocosms with the highest values were alive at the end of the experiment. Functional richness, CWM biomass, CWM front leg area and CWM pronotum volume had no effect on seedling survival (table 1), nor did the week that seedlings emerged from the soil surface (LRT = 1.19, d.f. = 1, p = 0.275).
(c). Germination trials
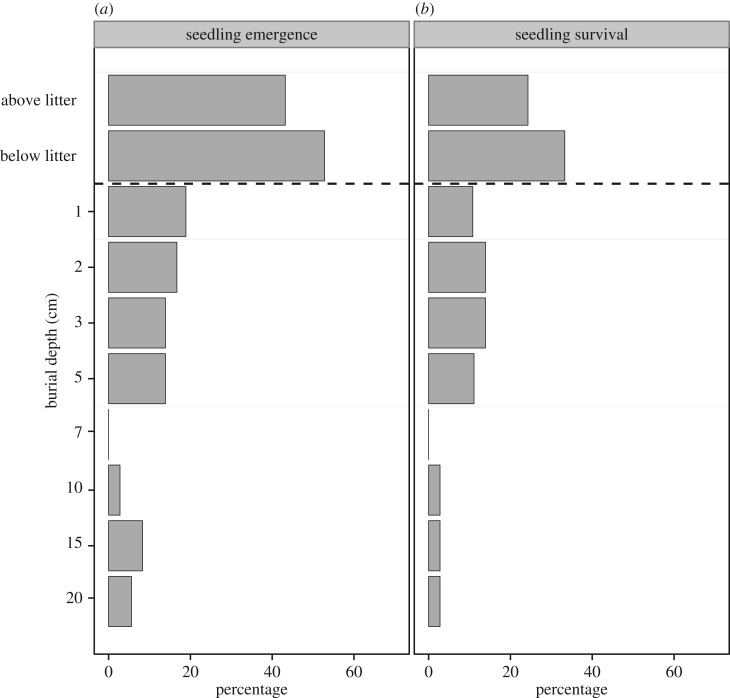
Burial depth was the only factor that significantly influenced the likelihood of emergence (LRT = 69.4, d.f. = 9, p < 0.0001); the presence of dung had no significant effect. Seeds that were buried below the soil surface were less likely to emerge as seedlings than those placed above or below the leaf litter: there was a 44.4% and 52.8% emergence rate for seeds above and below the litter respectively, compared to between 19.4% and 5.6% for seeds buried at 1 cm and 20 cm, respectively (figure 3). No factor or interaction had a significant effect on the probability of seedling survival. Emergence week had no effect on the probability that seedlings survived to the end of the monitoring period (LRT = 2.8, d.f. = 1, p = 0.0921). No seeds emerged from mesocosms after week 16 or from germination plots later than week 14 (electronic supplementary material, appendix S7). As such, we are confident that all emergence events were captured during the monitoring period.
Figure 3.
Percentage of M. dubia that emerged from the soil surface after being experimentally planted to 10 different depths, n = 36 at each depth; (a) and percentage of emerged M. dubia seedlings at each burial depth that survived until the end of the 18-week experimental period (b). The soil surface is shown with a horizontal dashed line.
4. Discussion
In this study, we investigated the consequences of changes in dung beetle community composition (biomass, taxonomic and functional diversity) for secondary seed dispersal and the emergence and survival of tropical seedlings. We found a stronger positive effect of beetle biomass on the likelihood of burial for medium-sized beads compared to smaller beads, suggesting that anthropogenic driven reductions of large-bodied dung beetles [48] will have the greatest relative effect on the secondary dispersal of large-seeded plant species. Furthermore, we found a negative relationship between dung beetle species richness, functional richness and biomass, and the likelihood that seedlings emerged from the soil surface. These results suggest that secondary seed dispersal by dung beetles could inhibit, rather than promote the emergence of some tropical species. Conversely, we found that seedling survival was positively influenced by beetle species richness, biomass and the CWM of back : front leg length. It is worth noting here the possibility that unmeasured microsite variation could be driving or interacting with some of the reported significant correlations. Nevertheless, these results provide new evidence that changes in the richness and composition of dung beetle communities could impact seedling recruitment in tropical forests (here defined as seed germination and the short-term survival of seedlings until the end of our experimental period), potentially affecting future vegetation composition. As dung beetle communities are inherently linked to mammalian dung, our results suggest that changes in mammal communities, such as the loss of large-bodied primates [49], caused by anthropogenic pressures could impact tropical forest regeneration through top-down trophic cascades involving below-ground fauna.
The relative effect of dung beetle biomass on the probability of seed mimic burial was strongest for medium beads. Previous work has demonstrated that large beetles are functionally more efficient in the removal of dung and seeds compared to smaller species and that they are instrumental in the movement of large seeds [35,37]. It is likely, therefore, that the stronger relationship we observed between biomass and medium bead burial, compared with small bead burial, is caused by the presence of large beetles in high biomass communities driving the burial of large seeds. This is important because large-bodied dung beetle species are known to be more prone to extinction and decline than smaller bodied species [33,48]. These results therefore support our first hypothesis that changes in dung beetle community structure are likely to differentially affect the secondary dispersal of seeds depending on their size. This adds weight to suggestions that large-seeded trees are most affected by the extinction of animal–plant interactions as a result of human pressures (cf. [16]).
Secondary dispersal by dung beetles has been demonstrated on a number of occasions to be beneficial to buried seeds [27,28,50]. However, contrary to our predictions, we show that functional richness, species richness and total biomass of beetle communities are negatively correlated to the emergence success of seedlings, suggesting that dung beetle activity may be detrimental for some species. Previous beetle-mediated seed dispersal experiments in tropical forests demonstrate that burial depths of between 1 cm and 4 cm result in increased germination success compared with seeds that remained on the soil surface or were buried to deeper depths [27,38]. We show that M. dubia emergence rates within germination plots were highest when seeds were placed either above or below the leaf litter, but immediately reduced by over 50% when seeds were buried within the soil profile. Therefore, it is probable that the negative relationship between beetle community attributes and emergence of M. dubia seeds is a consequence of higher biomass and diversity, resulting in higher rates of seed burial (cf. [36]) and net disadvantages to the fitness of this species. Furthermore, results from our bead burial and retrieval experiments demonstrate that small-seeded species are buried deeper than larger seeds; given that only large seeds have been shown to germinate from burial depths of 10 cm or more [27], we also expect negative consequences of beetle activity for many other smaller seeded species. It is therefore possible that seed burial by intact dung beetle communities may reduce the prevalence of small-seeded species, thus reducing competition experienced by larger seeds.
Seed predator escape is a key mechanism underpinning the increased germination success observed in seeds secondarily dispersed by dung beetles in tropical forests [27,28]. We found no evidence for this process in this investigation. However, our experiments were carried out in a primary forest with relatively low hunting pressure, and a full complement of large mammals [49]. More heavily disturbed forests differ in that they can harbour large populations of seed predators and hence higher seed predation pressure [20,51]. If seed predation was sufficiently high, burial by beetles could impart net benefits rather than disadvantages to M. dubia. It is possible, therefore, that seed predator escape may be relatively more important in more heavily disturbed forests, and that this result underestimates the importance of dung beetle-mediated seed burial in an increasing human-modified world. Furthermore, although M. dubia is a fleshy fruit dispersed by a wide range of forest vertebrates [52], it is also a riparian species and its seeds can be dispersed by water, which may explain its preference for being close to the soil surface. While these results highlight some interesting linkages across trophic levels, finding general patterns will require additional work using a broader range of plant species, and repeating the experiments in forests with differing levels of predation pressure.
We found a positive relationship between seedling survival and dung beetle total biomass, species richness and CWM back : front leg length. Results from our seed germination trials demonstrated that the presence of dung did not influence the survival of M. dubia seedlings. This suggests that the mechanisms driving increased seedling survival extend beyond simply the presence of dung surrounding seeds. There are myriad processes acting both above-ground and below-ground that influence whether a seedling lives or dies following germination (e.g. [53]). A plausible way in which beetles could influence seedling survival is through simultaneous effects on both soil resource (nutrients and water) availability and the soil physical environment. Owing to their small root system, recently emerged seedlings are reliant on the nutrient and water availability in their immediate surroundings [54]. Bang et al. [55] demonstrated that dung beetle activity had a positive effect on soil permeability in surface layers, which is positively associated with air and water movement, and greater soil pore space [56]. These soil characteristics could facilitate greater root and shoot growth. Furthermore, nitrogen is a mineral element that can become insufficient in seed reserves [57]. Dung beetles have been shown to positively influence rates of nitrogen (N) mineralization and concentrations of inorganic N in soil, as well as the availability of other limiting nutrients such as phosphorus (P) and potassium (K) [58,59]. Therefore, dung burial by beetles could concurrently alter soil biogeochemistry and physical structure so as to increase the availability of limiting nutrients, while facilitating the ease with which roots can access these resources. It is important to note, however, that past studies investigating dung beetle impacts on soil nutrient availability and physical structure have been exclusively carried out in grassland and heathlands, which differ in their soil properties to tropical forests [60,61]; hence, making inferences about the role of dung beetles in modifying tropical soils based on evidence from temperate systems is problematic. Future investigations are therefore needed to elucidate the small-scale impact of dung beetles on tropical soils, where highly heterogeneous distributions in soil nutrients are important factors structuring plant communities [62].
The only dung beetle trait that was positively associated with seedling survival was the CWM of back : front leg length. The abundance of dwelling dung beetle species, which do not bury dung or seeds but feed and nest within the dung [26], within these communities was positively related to CWM back : front leg length (electronic supplementary material, appendix S8); as such, an increase in the ratio between back and front leg lengths indicates an increase in the number of dwellers present. The burial of beads similar in size to M. dubia was low compared with smaller beads and was always unaffected by leg length. Therefore, it is unlikely that the relationship we found between seedling survival and CWM back : front leg length is a consequence of dwellers decreasing the likelihood that seeds are buried. Instead, it is likely that processing of dung on the soil surface increases with an increase in the abundance of dwelling species. This could give rise to similar processes described above, altering soil nutrient availability and physical environment in a way that provides benefits to seedling growth and survival. We are not aware of any studies to date that have investigated how the morphological traits of dung beetles influence soil properties and plant growth.
5. Conclusion
This investigation aimed to better understand the role of dung beetle communities in maintaining ecosystem functioning in tropical forests, through studying their impact on secondary seed dispersal and seedling establishment. Conceptual frameworks predict that large-seeded species are mostly at risk from the negative impacts of defaunation owing to the extirpation of their large-bodied primary dispersers [23,24]. Here, we demonstrate that large seeds may also be differentially vulnerable to the loss of their secondary dispersers through anthropogenic driven reductions in large-bodied dung beetles [33,48]. However, our results also suggest that decreases in dung beetle biomass and diversity could result in net advantages to some small-seeded species because seed burial can negatively impact their emergence success. Furthermore, we present novel experimental evidence suggesting that dung beetle activity could modify conditions within the soil and/or dung in a way that promotes seedling survival. Combined, these results demonstrate the complexities of predicting how anthropogenic driven changes biological communities can cause top-down cascading effects on ecosystem functioning; point to new avenues for future experimental work into the mechanisms driving plant responses to shifts in the community composition of their secondary dispersers, through alteration of the soil environment; and demonstrate ways in which dung beetle activity could impact forest regeneration and future forest composition. We therefore provide further evidence of the value of biodiversity for the maintenance of ecosystem functions and self-sustaining natural systems.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank our wonderful research assistants Edivar Dias Correa, Jucelino Alves dos Santos, Filipe Franca, Daniel Tregidgo and Cristiane Souza for countless hours of hard work in the field. We are also grateful to Jari Forestal for permission to work on their landholding and logistical support throughout the duration of the project. Finally, thank you very much to Nathalie Seddon and the anonymous referees for extremely helpful comments on the manuscript.
Ethics
Sampling did not involve any endangered species and permission to collect zoological material was granted to J.L. by the Instituto Brasileiro do Meio Ambiente dos Recursos Naturais Renováveis (IBAMA).
Data accessibility
Data can be accessed through Dryad http://dx.doi.org/10.5061/dryad.d20g3 [63].
Authors' contributions
H.M.G. and J.B. conceived and designed the experiments, with contributions from R.D.B. and J.L. to the development and framing of research questions; H.M.G. carried out the field work (with the help of her wonderful field assistants); H.M.G. analysed the data and wrote the paper, with input from all authors.
Competing interests
We have no competing interests that might have influenced this work.
Funding
This work was funded by a studentship awarded to H.M.G. by the National Environment Research Council (U.K.) and CNPq-PELD site 23.
References
- 1.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 2.Seddon N, Mace GM, Pigot AL, Naeem S, Mouillot D, Tobias JA, Walpole M, Vause J. 2016. Biodiversity in the Anthropocene: prospects and policy. Proc. R. Soc. B 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 4.Valiente-Banuet A, et al. 2014. Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29, 1–8. ( 10.1111/1365-2435.12356) [DOI] [Google Scholar]
- 5.Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO. 2014. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 ( 10.1126/science.1246752) [DOI] [PubMed] [Google Scholar]
- 6.Peres CA, Palacios E. 2007. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: implications for animal-mediated seed dispersal. Biotropica 39, 304–315. ( 10.1111/j.1744-7429.2007.00272.x) [DOI] [Google Scholar]
- 7.Parry L, Barlow JOS, Peres CA. 2009. Hunting for sustainability in tropical secondary forests. Conserv. Biol. 23, 1270–1280. ( 10.1111/j.1523-1739.2009.01224.x) [DOI] [PubMed] [Google Scholar]
- 8.Barlow J, et al. 2016. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535, 144–147. ( 10.1038/nature18326) [DOI] [PubMed] [Google Scholar]
- 9.Estes JA, et al. 2011. Trophic downgrading of Planet Earth. Science 333, 301 ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 10.Bregman 2016. Proc. R. Soc. B283.
- 11.Cardinale BJ, Matulich KL, Hooper DU, Byrnes JE, Duffy E, Gamfeldt L, Balvanera P, O'Connor MI, Gonzalez A. 2011. The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 572–592. ( 10.3732/ajb.1000364) [DOI] [PubMed] [Google Scholar]
- 12.Balvanera P, Pfisterer AB, Buchmann N, He J-S, Nakashizuka T, Raffaelli D, Schmid B. 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156. ( 10.1111/j.1461-0248.2006.00963.x) [DOI] [PubMed] [Google Scholar]
- 13.Turnbull 2016. Proc. R. Soc. B283.
- 14.Soliveres S, et al. 2016. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536, 456–459. ( 10.1038/nature19092) [DOI] [PubMed] [Google Scholar]
- 15.Kurten EL. 2013. Cascading effects of contemporaneous defaunation on tropical forest communities. Biol. Conserv. 163, 22–32. ( 10.1016/j.biocon.2013.04.025) [DOI] [Google Scholar]
- 16.Harrison R, Tan S, Plotkin J, Slik F. 2013. Consequences of defaunation for a tropical tree community. Ecol. Lett. 16, 687–694. ( 10.1111/ele.12102) [DOI] [PubMed] [Google Scholar]
- 17.Wright SJ, Hernandéz A, Condit R. 2007. The bushmeat harvest alters seedling banks by favoring lianas, large seeds, and seeds dispersed by bats, birds, and wind. Biotropica 39, 363–371. ( 10.1111/j.1744-7429.2007.00289.x) [DOI] [Google Scholar]
- 18.Wright SJ, Zeballos H, Dominguez I, Gallardo MM, Moreno M, Roberto I. 2000. Poachers alter mammal abundance, seed dispersal and seed predation in a neotropical forest. Conserv. Biol. 14, 227–239. ( 10.1046/j.1523-1739.2000.98333.x) [DOI] [Google Scholar]
- 19.Wright SJ, Duber HC. 2001. Poachers and forest fragmentation alter seed dispersal, seed survival, and seedling recruitment in the palm Attalea butyraceae, with implications for tropical tree diversity. Biotropica 33, 583–595. ( 10.1111/j.1744-7429.2001.tb00217.x) [DOI] [Google Scholar]
- 20.Terborgh J, et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926. ( 10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 21.Nunez-Iturri G, Howe HF. 2007. Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in Western Amazonia. Biotropica 39, 348–354. ( 10.1111/j.1744-7429.2007.00276.x) [DOI] [Google Scholar]
- 22.Beck H, Snodgrass JW, Thebpanya P. 2013. Long-term exclosure of large terrestrial vertebrates: implications of defaunation for seedling demographics in the Amazon rainforest. Biol. Conserv. 163, 115–121. ( 10.1016/j.biocon.2013.03.012) [DOI] [Google Scholar]
- 23.Peres CA, Emilio T, Schietti J, Desmoulière SJM, Levi T. 2016. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc. Natl Acad. Sci. USA 113, 892–897. ( 10.1073/pnas.1516525113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bello C, Galetti M, Pizo MA, Magnago LFS, Rocha MF, Lima RAF, Peres CA, Ovaskainen O, Jordano P. 2015. Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 ( 10.1126/sciadv.1501105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Culot L, Huynen M, Heymann EW. 2014. Partitioning the relative contribution of one-phase and two- phase seed dispersal when evaluating seed dispersal effectiveness. Methods Ecol. Evol. 6, 178–186. ( 10.1111/2041-210X.12317) [DOI] [Google Scholar]
- 26.Hanski I, Cambefort Y. 1991. Dung Beetle Ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 27.Shepherd VE, Chapman CA. 1998. Dung beetles as secondary seed dispersers: impact on seed predation and germination. J. Trop. Ecol. 14, 199–215. ( 10.1017/S0266467498000169) [DOI] [Google Scholar]
- 28.Andresen E, Levey DJ. 2004. Effects of dung and seed size on secondary dispersal, seed predation, and seedling establishment of rain forest trees. Oecologia 139, 45–54. ( 10.1007/s00442-003-1480-4) [DOI] [PubMed] [Google Scholar]
- 29.Lawson CR, Mann DJ, Lewis OT. 2012. Dung beetles reduce clustering of tropical tree seedlings. Biotropica 44, 271–275. ( 10.1111/j.1744-7429.2012.00871.x) [DOI] [Google Scholar]
- 30.Estrada A, Coates-Estrada R. 1991. Howler monkeys (Alouatta palliata), dung beetles (Scarabaeidae) and seed dispersal: ecological interactions in the tropical rain forest of Los Tuxtlas, Mexico. J. Trop. Ecol. 7, 459–474. ( 10.1017/S026646740000585X) [DOI] [Google Scholar]
- 31.Koike S, Morimoto H, Kozakai C, Arimoto I, Soga M, Yamazaki K, Koganezawa M. 2012. The role of dung beetles as a secondary seed disperser after dispersal by frugivore mammals in a temperate deciduous forest. Acta Oecologica 41, 74–81. ( 10.1016/j.actao.2012.04.009) [DOI] [Google Scholar]
- 32.Hoffmann M, et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330, 1503–1509. ( 10.1126/science.1194442) [DOI] [PubMed] [Google Scholar]
- 33.Culot L, Bovy E, Zagury Vaz-de-Mello F, Guevara R, Galetti M. 2013. Selective defaunation affects dung beetle communities in continuous Atlantic rainforest. Biol. Conserv. 163, 79–89. ( 10.1016/j.biocon.2013.04.004) [DOI] [Google Scholar]
- 34.Braga RF, Korasaki V, Andresen E, Louzada J. 2013. Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: a rapid assessment of ecological functions associated to biodiversity. PLoS ONE 8, e57786 ( 10.1371/journal.pone.0057786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slade EM, Mann DJ, Villanueva JF, Lewis OT. 2007. Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. J. Anim. Ecol. 76, 1094–1104. ( 10.1111/j.1365-2656.2007.01296.x) [DOI] [PubMed] [Google Scholar]
- 36.Griffiths HM, Louzada JNC, Bardgett RD, Beiroz W, França F, Tregidgo D, Barlow J. 2015. Biodiversity and environmental context predict dung beetle-mediated seed dispersal in a tropical forest field experiment. Ecology 96, 1607–1619. ( 10.1890/14-1211.1) [DOI] [Google Scholar]
- 37.Gregory N, Gómez A, Maria T, Oliveira FDS, Nichols E. 2014. Big dung beetles dig deeper: trait-based consequences for faecal parasite transmission. Int. J. Parasitol. 45, 101–105. ( 10.1016/j.ijpara.2014.10.006) [DOI] [PubMed] [Google Scholar]
- 38.Cavalcante P. 1991. Frutas Comestíveis da Amazônia. CEJUP Editions. [Google Scholar]
- 39.Peters CM, Balick MJ, Kahn F, Anderson AB. 1989. Oligarchic forests of economic plants in Amazonia: utilization and conservation of an important tropical resource. Conserv. Biol. 3, 341–349. ( 10.1111/j.1523-1739.1989.tb00240.x) [DOI] [PubMed] [Google Scholar]
- 40.Nichols E, et al. 2013. Trait-dependent response of dung beetle populations to tropical forest conversion at local and regional scales. Ecology 94, 180–189. ( 10.1890/12-0251.1) [DOI] [PubMed] [Google Scholar]
- 41.Beiroz W. 2013. Resposta da diversidade funcional de scarabaeinae (Coleoptera scarabaeidae) aos diferentes usos de solo na Amazôia. MSc thesis, Universidade Federal de Lavras, Minas Gerais, Brazil.
- 42.Villéger S, Mason NWH, Mouillot D. 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. ( 10.1890/07-1206.1) [DOI] [PubMed] [Google Scholar]
- 43.Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882–892. ( 10.1111/j.0030-1299.2007.15559.x) [DOI] [Google Scholar]
- 44.Laliberté AE, Shipley B, Laliberté ME. 2012. Measuring functional diversity (FD) from multiple traits, and other tools for functional ecology. See http://cran.r-project.org/web/packages/FD/.
- 45.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 46.Bates D, Maechler M, Bolker B. 2012. lme4: Linear mixed-effecs models using S4 classes. See https://cran.r-project.org/web/packages/lme4/index.html.
- 47.Zuur A, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 48.Gardner TA, Hernandez MIM, Barlow J, Peres CA. 2008. Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. J. Appl. Ecol. 45, 883–893. ( 10.1111/j.1365-2664.2008.01454.x) [DOI] [Google Scholar]
- 49.Parry L, Barlow J, Peres CA. 2007. Large-vertebrate assemblages of primary and secondary forests in the Brazilian Amazon. J. Trop. Ecol. 23, 653–662. ( 10.1017/S0266467407004506) [DOI] [Google Scholar]
- 50.Santos-Heredia C, Andresen E, Zarate DA. 2010. Secondary seed dispersal by dung beetles in a Colombian rain forest: effects of dung type and defecation pattern on seed fate. J. Trop. Ecol. 26, 355–364. ( 10.1017/s0266467410000192) [DOI] [Google Scholar]
- 51.Asquith NM, Wright SJ, Clauss MJ. 1997. Does mammal community composition control recruitment in neotropical forests? Evidence from Panama. Ecology 78, 941–946. ( 10.1890/0012-9658(1997)078%5B0941:DMCCCR%5D2.0.CO;2) [DOI] [Google Scholar]
- 52.Gressler E, Pizo MA, Morellato LPC. 2006. Polinização e dispersão de sementes em Myrtaceae do Brasil. Rev. Bras. Botânica 29, 509–530. ( 10.1590/S0100-84042006000400002) [DOI] [Google Scholar]
- 53.Khurana E, Singh JS. 2001. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest : a review. Environ. Conserv. 28, 39–52. ( 10.1017/S0376892901000042) [DOI] [Google Scholar]
- 54.Poorter L, Hayashida-Oliver Y. 2000. Effects of seasonal drought on gap and understorey seedlings in a Bolivian moist forest. J. Trop. Ecol. 16, 481–498. ( 10.1017/S026646740000153X) [DOI] [Google Scholar]
- 55.Bang HS, Lee JH, Kwon OS, Na YE, Jang YS, Kim WH. 2005. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil Ecol. 29, 165–171. ( 10.1016/j.apsoil.2004.11.001) [DOI] [Google Scholar]
- 56.Marshall TJ, Holmes JW, Rose CW. 1996. Soil physics, 3rd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 57.Fenner M. 1986. A bioassay to determine the limiting minerals for seeds from nutrient-deprived Senecio vulgaris plants. J. Ecol. 74, 497–505. ( 10.2307/2260270) [DOI] [Google Scholar]
- 58.Yokoyama K, Kai H, Koga T, Aibe T. 1991. Nitrogen mineralization and microbial populations in cow dung, dung balls and underlying soil affected by paracoprid dung beetles. Soil Biol. Biochem. 23, 649–653. ( 10.1016/0038-0717(91)90078-X) [DOI] [Google Scholar]
- 59.Yamada D, Imura O, Shi K, Shibuya T. 2007. Effect of tunneler dung beetles on cattle dung decomposition, soil nutrients and herbage growth. Grassl. Sci. 53, 121–129. ( 10.1111/j.1744-697X.2007.00082.x) [DOI] [Google Scholar]
- 60.Townsend AR, Cleveland CC, Asner GP, Bustamante MMC. 2007. Controls over foliar N:P ratios in tropical rain forests. Ecology 88, 107–118. ( 10.1890/0012-9658(2007)88%5B107:cofnri%5D2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 61.Vitousek PM, Porder S, Houlton BZ, Chadwick OA. 2010. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 20, 5–15. ( 10.1890/08-0127.1) [DOI] [PubMed] [Google Scholar]
- 62.John R, et al. 2007. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl Acad. Sci. USA 104, 864–869. ( 10.1073/pnas.0604666104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griffiths HM, Bardgett RD, Lauzada J, Barlow J. 2016. Data from: The value of trophic interactions for ecosystem function: dung beetle communities influence seed burial and seedling recruitment in tropical forests. Dryad Digital Repository. ( 10.5061/dryad.d20g3) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be accessed through Dryad http://dx.doi.org/10.5061/dryad.d20g3 [63].