Abstract
Deep-sea hydrothermal vents and methane seeps are inhabited by members of the same higher taxa but share few species, thus scientists have long sought habitats or regions of intermediate character that would facilitate connectivity among these habitats. Here, a network analysis of 79 vent, seep, and whale-fall communities with 121 genus-level taxa identified sedimented vents as a main intermediate link between the two types of ecosystems. Sedimented vents share hot, metal-rich fluids with mid-ocean ridge-type vents and soft sediment with seeps. Such sites are common along the active continental margins of the Pacific Ocean, facilitating connectivity among vent/seep faunas in this region. By contrast, sedimented vents are rare in the Atlantic Ocean, offering an explanation for the greater distinction between its vent and seep faunas compared with those of the Pacific Ocean. The distribution of subduction zones and associated back-arc basins, where sedimented vents are common, likely plays a major role in the evolutionary and biogeographic connectivity of vent and seep faunas. The hypothesis that decaying whale carcasses are dispersal stepping stones linking these environments is not supported.
Keywords: network analysis, marine biogeography, molluscs, stepping stones, evolutionary adaptation
1. Background
The adaptation to, and colonization of, new habitats is often achieved via geographically or ecologically intermediate ‘stepping stones’ [1–3]. Understanding the nature of these stepping stones is thus crucial for interpreting the history of colonizations and present-day distributions, for the design of protected areas and for predicting vectors of dispersal of invasive species and of infectious diseases [2–5]. Of particular interest for biogeographers and evolutionary biologists in this context are isolated and extreme habitats with faunas and floras showing endemism and unusual adaptations, such as island archipelagos [6,7], caves [8,9], seamounts [10,11], and deep-sea hydrothermal vents and methane seeps [12–14]. Among these, vents and seeps are characterized by harsh environmental conditions and host unique ecosystems in which the dominant species gain their nutrients from symbiotic chemoautotrophic bacteria and are independent of photosynthetic food chains [15,16]. Vents and seeps are inhabited by members of the same higher taxa, notably siboglinid tubeworms, bathymodiolin mussels, and vesicomyid clams, but overlap on the species level is limited. This is thought to result from the different tectonic settings and physical properties of vents and seeps: whereas many vents are located in open ocean settings and are characterized by volcanic rocks and hot, metal-rich fluids, methane seeps occur on continental margins and are characterized by cold fluids, soft sediment, and carbonate rocks [15]. Vents and seeps are thus perceived as two distinct types of ecosystems that have evolutionary connections, but the nature of these connections remains controversial. Because the number of shared species increases in areas where both habitat types occur in close proximity, for example, around Japan and in the Gulf of California, such areas are seen as evolutionary hotspots for the chemosymbiotic biota [17,18]. The discovery of vent-type taxa around whale carcasses on the deep-sea floor led to the idea that such ‘whale falls’ act as evolutionary and dispersal stepping stones for vent and seep taxa [19–21]. Findings of further habitat types fuelled by chemosynthetic primary production, such as sedimented vents, hydrothermal seeps, and serpentinization vents, recently led to the view of a continuum of reducing ecosystems in which habitats of intermediate character are seen as conducive to interactive evolution of vent and seep biota [22,23]. However, the importance of the various chemical, physical, and geographical factors for facilitating connectivity remains unclear [14], because biogeography and connectivity across all reducing ecosystems has never been analysed on a global scale. This shortcoming is addressed here by a network analysis of the largest biogeographic dataset of vent, seep, and whale-fall faunas assembled to date.
Network analysis is a powerful framework for deciphering the structure of biological systems composed of interacting units, with the units being anything from molecules to species to communities, and the links between them representing their interactions. This approach has, for example, been used to understand processes in shaping metabolic networks [24], mutualistic interactions between plants and animals [25], food web stability [26], and island biogeography [27]. It is free of a priori assumptions about the boundaries of biogeographic provinces [28] and instead allows quantification of both the importance of individual sites in connecting other sites (their centrality) and the strength of these connections. Overall, this approach uses a better representation of the dynamic and interactive nature of species distribution patterns than hierarchical clustering or pairwise interaction analysis [29].
This study has three main aims: (i) to resolve the biogeography and connectivity of vent, seep, and whale-fall faunas in combination and on a global scale, (ii) to identify sites or habitat types that play a major role in connecting the various types of reducing ecosystems and their biogeographic provinces, and (iii) to assess the role of water depth in the biogeography and connectivity of these faunas.
2. Data and methods
(a). Faunal data
The analysis is based on bivalves and gastropods, which are among the dominant and most diverse clades at vents and seeps [30] and includes all known taxa regardless of their physical size, relative abundance, and mode of life (i.e. epifaunal versus infaunal). It uses the genus level due to the high degree of endemism seen on the species level, as shown in previous studies of vent and seep biogeography [31–33]. The dataset was compiled from published faunal descriptions and site descriptions (electronic supplementary material, tables S1 and S2). One hundred and twenty-one genus-level taxa are recognized and the generic assignments of the respective species were updated using published taxonomic work [34–42] and taxonomic inferences made by myself from published phylogenies [43–47]. These are: species of the ‘“Bathymodiolus” childressi clade’ (cf. [43,48,49]) were treated as a separate genus, not as belonging to Bathymodiolus sensu strictu; ‘Bathymodiolus’ aduloides and ‘B.’ manusensis form a separate clade among bathymodiolins [43,44,49] and are also treated as a separate genus. Among the vesicomyids, I largely follow the recent classification by Krylova & Sahling [35], with the following exceptions. Because Phraeagena was not found to be monophyletic in recent molecular studies [46] its species are included in Archivesica; Archivesica further contains all species called ‘gigas/kilmeri complex’ or ‘gigas group’ in molecular studies, including ‘Ectenagena’ extenta (cf. [45–47,50,51]). ‘Calyptogena’ magnifica is considered as a separate genus [35]. The species assigned to Pliocardia are treated as three different genera: the two clades called ‘Pliocardia I’ and ‘Pliocardia II’ in the molecular phylogeny of Valdés et al. [45], and the remaining species assigned to this genus by Krylova & Sahling [35] as a third genus. This treatment may seem arbitrary but it is likely to reveal more biogeographic structure than if all species assigned to Pliocardia by Krylova & Sahling [35] were regarded as a single genus.
(b). Site data
The ‘sites’ consist of 79 sites or composite sites with at least three taxa present, and include vents, seeps, whale-falls, a hydrothermal seep, and the Pleistocene Ghost City serpentinization vent site. In some cases, geographically close sites were grouped and treated as a single unit (composite site), including: seep sites in the Gulf of Mexico, the Gulf of Cadiz, off of the Caribbean coast of Colombia, and the Pacific coast of Costa Rica; vent sites on the northern and southern East Pacific Rise; and vent and seep sites in the Nankai and Okinawa troughs. A description of the sites and composite sites is provided in the electronic supplementary material, table S2. To assess the impact of water depth on the biogeographic distributions, the sites were binned into three depth ranges: less than 1 500 m, 1 500–3 000 m, and more than 3 000 m. When composite sites had depth ranges spanning two of these depth bins, they were assigned to the bin encompassing the larger proportion of the composite site's depth range.
(c). Network analysis
Weighted networks were constructed from the presence–absence data, in which each site or composite site represents a node, and the links between the nodes are constructed and weighted based on the Bray–Curtis dissimilarity index. A thresholding approach was used to detect biogeographic provinces. By decreasing the threshold, links weaker than the threshold are successively removed from the fully connected network, so that only the more important links are retained. The result is that the network is broken up into ‘compartments’ that have strong links within the compartments, but only weak links between them; these compartments were then used to delineate biogeographic provinces [32,52]. The ‘betweenness centrality’ is calculated for each node, describing the importance of the node in connecting other nodes through the shortest path. The average clustering coefficent 〈CC〉 was calculated for the entire network; for an individual node, the clustering coefficient indicates the proportion of neighbouring nodes that are neighbours of each other, and these values are then averaged over the entire network. This coefficient is compared to a null model: the average 〈CC〉 of networks with the same number of nodes and links but the links randomly rewired. The network analysis was carried out using the software package EDENetworks v. 2.18 [52]. Randomizations were performed using NetworkRandomizer [53] running on the network analysis software platform Cytoscape [54]. Further statistics were calculated with the software package PAST v. 2.17 [55].
3. Results
(a). Biogeography
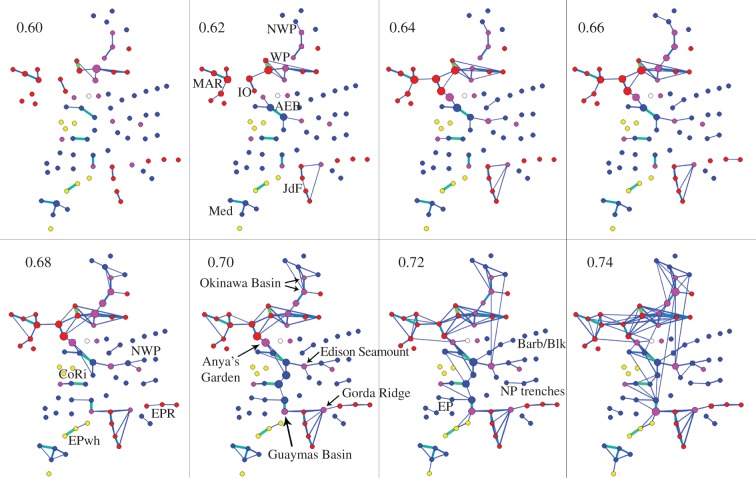
At a threshold of 0.7, the average clustering coefficient of the network (〈CC〉 = 0.31) is one order of magnitude higher than for the null model (〈CC0〉 = 0.02), indicating the presence of substructures within the network, with greater internal connectivity than would be expected to occur purely by chance. Visual inspection of the topologies of networks of increasing thresholds (i.e. with the continuous addition of weaker links; figure 1) allows the delineation of biogeographic provinces and the connectivity within and between them. Eight biogeographic provinces are apparent: (i) the Indian Ocean Ridges, (ii) the tropical western Pacific Ocean (WP), (iii) the Juan de Fuca Ridge, (iv) the East Pacific Rise plus the Galapagos Ridge, (v) the Mid-Atlantic Ridge (MAR) plus the East Scotia Ridge, (vi) NE Pacific back-arc basin vents plus seeps from the same region, (vii) seeps in the Gulf of Mexico and on the African west coast, also called the Atlantic Equatorial Belt; tightly linked are two sedimented vent sites: Anya's Garden on the MAR and Edison Seamount in the WP, and (viii) seeps in the Mediterranean Sea (figures 1 and 2). Overall, vent sites are much more tightly linked to each other than seep sites. For example, at a threshold of 0.7 all but one bare-rock vent site and one sedimented vent site (out of 33 vent sites) are connected to at least one other site, whereas among the seeps, 13 out of 38 sites remain unconnected; among the whale-fall sites, more than half remain unconnected at this threshold.
Figure 1.
Networks of the deep-sea vent and seep fauna at increasing thresholds. Colour coding by habitat type: red, bare-rock vents (mostly mid-ocean ridges); purple, sedimented vents in back-arc settings and the hydrothermal seep; blue, methane seeps; yellow, whale falls; white, serpentinization vent; node size by betweenness centrality; thickness of links indicate their weight. AEB, Atlantic Equatorial Belt; Barb/Blk, Barbados and Blake Ridge; CoRi, Costa Rica; EP, East Pacific; EPR, East Pacific Rise; EPwh, East Pacific whale falls; IO, Indian Ocean; JdF, Juan de Fuca Ridge; MAR, Mid-Atlantic Ridge; Med, Mediterranean Sea; NWP, Northwest Pacific; WP, West Pacific.
Figure 2.
Biogeographic network of the deep-sea vent and seep fauna at a threshold of 0.7. Colour coding by habitat type as in figure 1; unconnected nodes removed; EPR, East Pacific Rise; JdF, Juan de Fuca Ridge.
(b). Connectivity
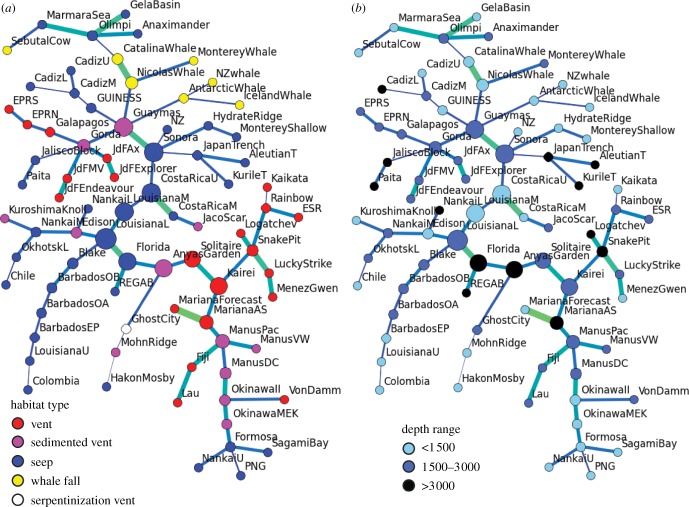
The average betweenness centrality of sedimented vents is much higher than that of seeps and of bare-rock vents (figure 3), at least in networks that include links between different habitat types and between the major biogeographic provinces (at thresholds more than 0.65; see also figure 1). The betweenness centrality of the other habitat types (hydrothermal seep, serpentinization vent, and whale falls) is negligibly low, and in the fully connected network (at thresholds more than 0.91), average betweenness centrality values are low and similar between all habitat types because virtually all the nodes are interconnected. The network topology shows no direct links between bare-rock vent, sites and seep sites, even at high thresholds (up to 0.75); links between the two habitat types are always via sedimented vent sites (figure 1). Also in the minimum spanning tree, links between bare-rock vents and seeps are only via sedimented vents (figure 4a). Whale-fall sites show mostly links among each other and to nearby seep and sedimented vent sites, and up to a threshold of 0.75 there are no links to bare-rock vents. The Jaco Scar ‘hydrothermal seep’ site is linked to the nearby Costa Rica seeps and up to a threshold of 0.75 it has no links to bare-rock vents and only a weak link to the sedimented Guaymas Basin vents. The Ghost City serpentinization vent site is mostly linked to vent sites on the Mid-Atlantic Ridge, including the sedimented Anya's Garden site, but only at high thresholds.
Figure 3.
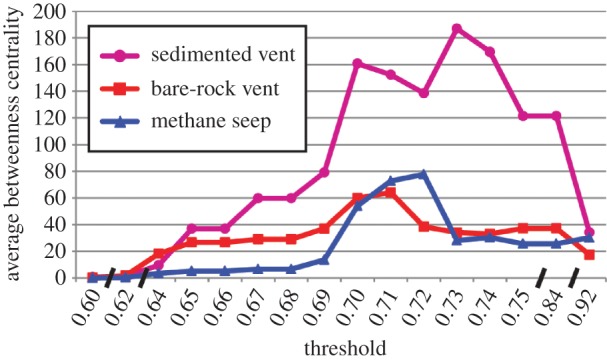
Betweenness centrality of the nodes averaged by habitat type, at increasing thresholds; note breaks in scale. (Online version in colour.)
Figure 4.
Minimum spanning trees of the network. (a) Colour coded by habitat type; all links between vents and seeps are via sedimented vents. (b) Colour coded by depth range. See the electronic supplementary material, table S1 for details on site names.
(c). Depth
Both in the minimum spanning tree and in the network at a threshold of 0.7 (when most major biogeographic provinces are connected) the numbers of links within and across depth ranges are statistically indistinguishable from the expected numbers (using the Spearman rank correlation test to compare the observed number of links to the total number of possible links; electronic supplementary material, table S3). Notably, though, in both test cases there are almost twice as many links between sites from the deepest depth range as expected, whereas for the upper range the numbers were lower than expected; in the network at a threshold of 0.7, there are about one-and-a-half times as many links between sites from the middle range as expected (electronic supplementary material, table S3). Network topology indicates that sorting by depth is particularly poor on the MAR, among seeps in the northeast Atlantic, and in the Mediterranean Sea, but better among most seep sites belonging to the Atlantic Equatorial Belt, and among many seeps and sedimented vents in the West Pacific back-arc basins (figure 4b).
4. Discussion
(a). Biogeographic provinces
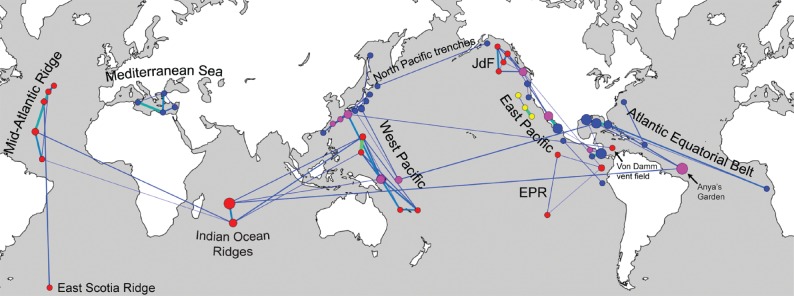
The biogeography of vent, seep, and whale-fall faunas is here analysed for the first time in combination and on a global scale. The analysis retrieved the same biogeographic provinces among the vent faunas as previous studies [12,14,31–33]. It also shows that, when analysed with the same methods and thresholds, the seep sites group into much less clearly defined faunal provinces than the vent fauna, and most groupings include sedimented vent sites as well (figure 1). Furthermore, the constituents of many previously recognized ‘seep provinces’ [56–59] are scattered across several network compartments, are frequently linked to vent and seep sites across the globe, and many sites remain unconnected even at high thresholds (figure 1). A typical example for this is the Atlantic Equatorial Belt [56,60], where seeps in the Gulf of Mexico show a close link to the Edison Seamount in the West Pacific even at low thresholds, but only weak links to nearby seep sites on the Blake Ridge and the Barbados Prism. Exceptions to the low provinciality of the seep fauna include the Mediterranean seep fauna, which shows an even higher level of distinctiveness than do the vent faunal provinces, and seeps from the deepest trenches.
This high distinctiveness of the Mediterranean seep fauna was considered to be due to the Messinian Salinity Crisis about 6 Ma, which caused severe extinction in the Mediterranean Basin [61,62], and Mediterranean seeps were thought to have subsequently been recolonized by taxa from the adjacent Atlantic Ocean [57]. Surprisingly, however, links between the Mediterranean seep fauna and that in the Gulf of Cadiz just outside the Strait of Gibraltar are not apparent in the present analysis. This indicates that the processes leading to the unique character of the Mediterranean seep fauna are not yet completely understood.
Faunal exchange through the Isthmus of Panama has often been suggested in vent and seep biogeography, especially in respect to the colonization of the Atlantic Ocean [12,14,33], but a recent network analysis of the vent fauna alone rejected such connections [32]. Here, however, Atlantic seep faunas and the Von Damm vent fauna in the Caribbean Sea show links to sedimented vents in the western Pacific Ocean, indicating that dispersal across the now-closed Isthmus of Panama indeed played an important role in shaping the composition and biogeography of the vent and seep fauna in the Atlantic Ocean. The fauna of the East Scotia Ridge near Antarctica shows a weak link to vent faunas of the Mid-Atlantic Ridge. A previous assessment based on decapods and barnacles indicated links to the Pacific Ocean [63], suggesting that molluscs and crustaceans may show different biogeographic patterns. Indeed, when individual phyla were investigated separately in a previous network analysis of the vent fauna [32], the molluscan biogeographic patterns differed somewhat from those of the entire fauna, and also differed from those of annelids and arthropods. However, molecular phylogenetic studies of several vent/seep annelid clades revealed individual species inhabiting both seeps and sedimented vents along the eastern margin of the Pacific Ocean (i.e. Archinome levinae [64] and Amphisamytha fauchaldi [65]) indicating that sedimented vents also play an important role at the species level for clades other than molluscs.
(b). Depth
Water depth was suggested to be more important in shaping seep biogeographic provinces than geographical distance [60,66–69]. Previous studies found that the degree of endemism of the vent and seep fauna increases with increasing water depth [68,70,71]. This pattern is most probably responsible for the observed (though not statistically significant) greater number of links between sites from the deepest and the middle depth ranges compared with the total number of possible links. Seeps in shallower water can potentially be colonized from a large pool of taxa pre-adapted to sulfide-rich sediments due to the higher diversity of such taxa (and taxa in general) in shallower water, whereas in the abyss such taxa are more rare. The low impact of depth on biogeographic patterns observed here might result from (i) the existence of a few provinces with very poor sorting by depth, which might mask a more general pattern, (ii) the existence of different depth zones in different ocean basins, or (iii) the existence of depth zones but along other boundaries than drawn in this analysis (or combinations of all of the above).
(c). Implications of network connectivity
Sedimented vent sites play a much larger role in overall network connectivity than sites of any other habitat type, and the network topology shows that they are the main connections between seep and bare-rock vent habitats. The network is constructed based on a distance measure between sites, thus the sedimented vents have a central position in the network, because they share a higher proportion of taxa with seeps and with bare-rock vents, than seeps and bare-rock vents share with each other. Vents, seeps, whale falls, and similar settings are seen as a ‘continuum of reducing habitats’ in which those of intermediate character play a central role in connectivity among the end members [22,23,72]. In this scenario, the intermediate habitat is the place where taxa inhabiting the intermediate and one end member could acquire adaptations that enable them to colonize also another end member.
This study indicates that mainly sedimented vents have the physico-chemical properties to play this central role, whereas other habitat types do not. For example, a ‘hydrothermal seep’ site with a diffuse flow of shimmering, warm fluids with high methane concentrations was initially regarded as being conducive for interactive evolution between vent and seep biota [22]. Here, however, this site plays no significant role in connecting global or local vent and seep faunas but shows links to nearby seeps only. Likewise, the Ghost City serpentinization vent site in the North Atlantic Ocean shows weak links to other vents in the North Atlantic but is not a major connector. Lastly, whale falls have long been considered as dispersal stepping stones for vent and seep taxa [19,21,73], but here they have only weak links within the biogeographic network. This sheds further doubt on their role as dispersal stepping stones; rather, the available data suggest that the whale-fall fauna might have its own biogeographic history, with some input from the vent and seep fauna.
The intermediate character of sedimented vents has previously been noted in a few regional and global analyses [17,74,75], and in phylogenetic studies of individual clades [64,65]. But what are the physico-chemical properties of sedimented vents that result in their crucial position in the network of reducing habitats? It may be the combination of sulfide-rich soft sediment that mimics the situation at methane seeps, and the hot metal-rich fluids that typically characterize mid-ocean ridge-type vents. In particular, this latter point sets sedimented vents apart from other perceived intermediates such as whale falls, sepentinization vents, and hydrothermal seeps.
The central role of sedimented vents challenges our current understanding of the biogeographic and evolutionary connectivity of reducing habitats. Several studies considered the close geographical proximity of vent, seep, and other reducing habitats in the West Pacific to facilitate connectivity among, and adaptations to, vents and seeps in this region [17,32,43,76]. However, two results of the present network analysis indicate that the abundance of sedimented vents in the West Pacific, rather than geographical proximity, facilitates connectivity: (i) even within small geographical areas, links between ‘bare-rock vents’ and seeps are via sedimented vents and not directly from vent to seep, as would be expected if geographical proximity alone would facilitate connectivity and (ii) the sedimented vents also connect vent and seep sites located in different ocean basins, such as the Gulf of Mexico and the Caribbean Sea, and the western Pacific and the Indian Ocean. Furthermore, the sedimented vents in the Guaymas Basin and on the Gorda Ridge play a prominent role in connecting vents and seeps along the East Pacific margin. This indicates that the tectonically active margins of the Pacific Ocean in general play an important role in the evolutionary connectivity of vent and seep faunas, especially considering that the Pacific margins have been tectonically active for tens to hundreds of millions of years [77].
By contrast, the Atlantic Ocean has been bound by passive continental margins ever since its opening about 200 Ma [77]. Remarkably, the Von Damm vent field in the Caribbean Sea shows only weak links to seep faunas in the Caribbean region and the Gulf of Mexico, despite their geographical proximity, and seep faunas on the eastern and western side of the Atlantic Equatorial Belt show virtually no links to vent faunas on the Mid-Atlantic Ridge that lie geographically between them [60]. An exception to this is the sedimented Anya's Garden site in the Logatchev vent field, which has links to seep faunas in the Gulf of Mexico. Interestingly though, the Anya's Garden site is also linked to a mid-ocean ridge vent site in the Indian Ocean rather than to nearby Mid-Atlantic Ridge vents. Given the importance of sedimented vents, especially in back-arc basins, in connecting both types of ecosystems, it appears that the scarcity of sedimented vents in most of the Atlantic Ocean is the main reason behind the much clearer separation of its vent and seep faunas compared with the Pacific Ocean. This adds a new dimension to the importance of tectonic plate boundaries in the evolution and biogeography of vent and seep faunas: while the changing distribution of mid-ocean ridges throughout the Earth's history has long been identified as the main factor shaping the biogeography of vent faunas [12,31,78], the distribution of subduction zones and associated back-arc basins with sedimented vents appears to play a major role in the interaction between vent and seep ecosystems.
The less clear-cut biogeographic provinces among the seep fauna compared with the vent fauna might provide further insights into their biogeographic and evolutionary connectivity. Sulfide-rich soft sediments are widespread along the world's continental margins, presumably implying that there is a large pool of taxa with pre-adaptations to chemosynthetic habitats that can potentially colonize seeps and thus blur biogeographic boundaries. By contrast, sulfide-rich hard substrate habitats are rare outside the vent environment and hence there are probably only a few taxa with pre-adaptations to those habitats, resulting in more clear-cut biogeographic provinces. Examples of sulfidic hard substrates include whale and wood falls—bones and wood parcels that emit sulfide due to the anaerobic breakdown of organic matter [20,79]. This might be the reason why taxa requiring a hard substrate, such as the largely epifaunal mussels, took wooden steps to deep-sea vents [2]. Indeed, it is tempting to speculate that vent/seep taxa requiring hard substrates generally took such a pathway of adaptation, whereas taxa inhabiting soft sediment can adapt to living at seeps without the need for wooden stepping stones.
(d). Limitations
A potential limitation of this study is that no distinction was made between seep faunas inhabiting a soft substrate, on the one hand, and carbonate rocks, on the other. Thus, a ‘seep site’ as used here may encompass higher habitat heterogeneity than sites classified as bare-rock vents. Making this distinction, also in the case of whale and wood falls, could potentially provide further insights into the role of hard substrates in the biogeographic and evolutionary connectivity among these faunas. Another limitation, as in any biogeographic study, is sampling density. The world's oceans are far from being evenly or comprehensively sampled for vents and seeps [80]. Seeps from the Southern Hemisphere are particularly poorly known; for example, there is not a single comprehensive description of a seep fauna from the entire Indian Ocean. As more studies on these and other faunas become available, our understanding of their biogeography will improve. Finally, low numbers of taxa per site could potentially lead to spurious results in such an analysis (i.e. artificially low or high similarities), which is why sites with fewer than three taxa were excluded here. However, virtually nothing changed when the analysis was re-run with a minimum of five taxa per site instead of three (electronic supplementary material, figure S1), suggesting that the results are robust even with low numbers of taxa per site.
5. Conclusion
The present analysis indicates that sedimented vent sites are the most important evolutionary and biogeographic links between vents and seeps. This has major implications for understanding the origin and biogeographic evolution of vent and seep faunas. The western Pacific Ocean hosts the highest number of active sedimented back-arc vents known on our planet, resulting in closer biogeographic ties between vent and seep faunas in this region than in other ocean basins. In the eastern Pacific Ocean, active spreading ridges are located close to continental margins and hence they receive large amounts of sediment, which also facilitates links between vents and seeps in this region. By contrast, the Atlantic Ocean has been characterized by passive continental margins and a scarcity of sedimented back-arc basins since its opening about 200 Ma, which offers an explanation for the stronger separation between vent and seep faunas in this ocean basin. Thus, the distribution of subduction zones and associated back-arc basins likely plays a major role in the evolutionary and biogeographic connectivity of vent and seep faunas. Whale falls appear not to function as dispersal stepping stones between vent and seep sites, rather, the limited available data suggests that the whale-fall fauna likely has its own biogeographic history, with some input from the vent and seep fauna.
Supplementary Material
Acknowledgements
Many thanks to Luciana Génio (Aveiro) for help with assembling the dataset of gastropods from the Gulf of Cadiz, Mikko Kivelä (Helsinki) for help with EDENetworks, and the three anonymous reviewers whose input considerably improved the manuscript.
Data accessibility
All data associated with this manuscript are available as the electronic supplementary material.
Competing interests
I have no competing interests.
Funding
Financial support was provided by the FWF – Der Wissenschaftsfont (Austria) through Lise-Meitner-fellowship M 1779-N29.
References
- 1.Vermeij GJ, Dudley R. 2000. Why are there so few evolutionary transitions between aquatic and terrestrial ecosystems? Biol. J. Linn. Soc. 70, 541–554. ( 10.1111/j.1095-8312.2000.tb00216.x) [DOI] [Google Scholar]
- 2.Distel DL, Baco AR, Chuang E, Morrill W, Cavanaugh CM, Smith CR. 2000. Do mussels take wooden steps to deep-sea vents? Nature 403, 725–726. ( 10.1038/35001667) [DOI] [PubMed] [Google Scholar]
- 3.Saura S, Bodin Ö, Fortin M-J. 2014. Stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J. Appl. Ecol. 51, 171–182. ( 10.1111/1365-2664.12179) [DOI] [Google Scholar]
- 4.Guimerà R, Mossa S, Turtschi A, Amaral LAN. 2004. The worldwide air transportation network: Anomalous centrality, community structure, and cities’ global roles. Proc. Natl Acad. Sci. USA 102, 7794–7799. ( 10.1073/pnas.0407994102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floerl O, Inglis GJ, Dey K, Smith A. 2009. The importance of transport hubs in stepping-stone invasions. J. Appl. Ecol. 46, 37–45. ( 10.1111/j.1365-2664.2008.01540.x) [DOI] [Google Scholar]
- 6.Grant PR. 1986. Ecology and evolution of Darwin's finches. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Losos JB, Ricklefs RE. 2009. Adaptation and diversification on islands. Nature 457, 830–836. ( 10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 8.Culver DC, Master LL, Christman MC, Hobbs HH. 2000. Obligate cave fauna of the 48 contiguous United States. Conserv. Biol. 14, 386–401. ( 10.1046/j.1523-1739.2000.99026.x) [DOI] [Google Scholar]
- 9.Iliffe TM, Wilkens H, Parzefall J, Williams D. 1984. Marine lava cave fauna: composition, biogeography, and origins. Science 225, 309–311. ( 10.1126/science.225.4659.309) [DOI] [PubMed] [Google Scholar]
- 10.McClain CR, Lundsten L, Ream M, Barry JP, DeVogelaere A. 2009. Endemicity, biogeography, composition, and community structure on a Northeast Pacific seamount. PLoS ONE 4, e4141 ( 10.1371/journal.pone.0004141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shank TM. 2010. Seamounts: deep-ocean laboratories of faunal connectivity, evolution, and endemism. Oceanography 23, 108–122. ( 10.5670/oceanog.2010.65) [DOI] [Google Scholar]
- 12.Tunnicliffe V, Fowler MR, McArthur AG. 1996. Plate tectonic history and hot vent biogeography. In Tectonic, magmatic, hydrothermal and biological segmentation of Mid-Oceanic ridges (eds MacLeod JC, Tyler PA, Walker CL), pp. 225–238. Geological Society of London, Special Publication 118. [Google Scholar]
- 13.Tunnicliffe V. 1992. The nature and origin of the modern hydrothermal vent fauna. Palaios 7, 338–350. ( 10.2307/3514820) [DOI] [Google Scholar]
- 14.Van Dover CL, German CR, Speer KG, Parson LM, Vrijenhoek RC. 2002. Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295, 1253–1257. ( 10.1126/science.1067361) [DOI] [PubMed] [Google Scholar]
- 15.Van Dover CL. 2000. The ecology of deep-sea hydrothermal vents, 424 p. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Kiel S. 2015. Did shifting seawater sulfate concentrations drive the evolution of deep-sea vent and seep ecosystems? Proc. R. Soc. B 282, 20142908 ( 10.1098/rspb.2014.2908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe H, Fujikura K, Kojima S, Miyazaki J-I, Fujiwara Y. 2010. Japan: vents and seeps in close proximity. In The vent and seep biota (ed. Kiel S.), pp. 379–402. Heidelberg, Germany: Springer. [Google Scholar]
- 18.Van Dover CL, Grassle JF, Boudrias M. 1990. Hydrothermal vent fauna of Escanaba Trough (Gorda Ridge). In Gorda ridge (ed. McMurray GR.), pp. 285–287. New York, NY: Springer. [Google Scholar]
- 19.Smith CR, Glover AG, Treude T, Higgs ND, Amon DJ. 2015. Whale-fall ecosystems: recent insights into ecology, paleoecology, and evolution. Annu. Rev. Mar. Sci. 7, 571–596. ( 10.1146/annurev-marine-010213-135144) [DOI] [PubMed] [Google Scholar]
- 20.Smith CR, Baco AR. 2003. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. Annu. Rev. 41, 311–354. [Google Scholar]
- 21.Smith CR, Kukert H, Wheatcroft RA, Jumars PA, Deming JW. 1989. Vent fauna on whale remains. Nature 341, 27–28. ( 10.1038/341027a0) [DOI] [Google Scholar]
- 22.Levin LA, et al. 2012. A hydrothermal seep on the Costa Rica margin: middle ground in a continuum of reducing ecosystems. Proc. R. Soc. B 279, 20120205 ( 10.1098/rspb.2012.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portail M, et al. 2015. Comparative study of vent and seep macrofaunal communities in the Guaymas Basin. Biogeosci. Disc. 12, 8497–8571. ( 10.5194/bgd-12-8497-2015) [DOI] [Google Scholar]
- 24.Guimerà R, Amaral LAN. 2005. Functional cartography of complex metabolic networks. Nature 433, 895–900. ( 10.1038/nature03288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bascompte J, Jordano P. 2007. Plant-animal mutualistic networks: the architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 38, 567–593. ( 10.1146/annurev.ecolsys.38.091206.095818) [DOI] [Google Scholar]
- 26.Krause AE, Frank KA, Mason DM, Ulanowicz RE, Taylor WW. 2003. Compartments revealed in food-web structure. Nature 426, 282–285. ( 10.1038/nature02115) [DOI] [PubMed] [Google Scholar]
- 27.Kougioumoutzis K, Simaiakis SM, Tiniakou A. 2014. Network biogeographical analysis of the central Aegean archipelago. J. Biogeogr. 41, 1848–1858. ( 10.1111/jbi.12342) [DOI] [Google Scholar]
- 28.Hausdorf B. 2002. Units in biogeography. Syst. Biol. 51, 648–652. ( 10.1080/10635150290102320) [DOI] [PubMed] [Google Scholar]
- 29.Proulx SR, Promislow DEL, Phillips PC. 2005. Network thinking in ecology and evolution. Trends Ecol. Evol. 20, 345–353. ( 10.1016/j.tree.2005.04.004) [DOI] [PubMed] [Google Scholar]
- 30.Desbruyères D, Segonzac M, Bright M. 2006. Handbook of deep-sea hydrothermal vent fauna. Second completely revised version. Denisia 18, 1–544. [Google Scholar]
- 31.Tunnicliffe V, Fowler MR. 1996. Influence of sea-floor spreading on the global hydrothermal vent fauna. Nature 379, 531–533. ( 10.1038/379531a0) [DOI] [Google Scholar]
- 32.Moalic Y, Desbruyères D, Duarte CM, Rozenfeld AF, Bachraty C, Arnaud-Haond S. 2012. Biogeography revisited with network theory: Retracing the history of hydrothermal vent communities. Syst. Biol. 61, 127–137. ( 10.1093/sysbio/syr088) [DOI] [PubMed] [Google Scholar]
- 33.Bachraty C, Legendre P, Desbruyères D. 2009. Biogeographic relationships among deep-sea hydrothermal vent faunas at global scale. Deep-Sea Res. I 56, 1371–1378. ( 10.1016/j.dsr.2009.01.009) [DOI] [Google Scholar]
- 34.Warén A, Bouchet P. 2001. Gastropoda and Monoplacophora from hydrothermal vents and seeps; new taxa and records. Veliger 44, 116–231. [Google Scholar]
- 35.Krylova EM, Sahling H. 2010. Vesicomyidae (Bivalvia): current taxonomy and distribution. PLoS ONE 5, e9957 ( 10.1371/journal.pone.0009957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki T, Okutani T, Fujikura K. 2005. Molluscs from hydrothermal vents and cold seeps in Japan: a review of taxa recorded in twenty recent years. Venus 64, 87–133. [Google Scholar]
- 37.Oliver PG, Rodrigues CF, Cunha MR. 2011. Chemosymbiotic bivalves from the mud volcanoes of the Gulf of Cadiz, NE Atlantic, with descriptions of new species of Solemyidae, Lucinidae and Vesicomyidae. Zookeys 113, 1–38. ( 10.3897/zookeys.113.1402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krylova EM, Sellanes J, Valdés F, D'Elía G. 2014. Austrogena: a new genus of chemosymbiotic bivalves (Bivalvia; Vesicomyidae; Pliocardiinae) from the oxygen minimum zone off central Chile described through morphological and molecular analyses. Syst. Biodivers. 12, 225–246. ( 10.1080/14772000.2014.900133) [DOI] [Google Scholar]
- 39.Krylova EM, Cosel Rv. 2011. A new genus of large Vesicomyidae (Mollusca, Bivalvia, Vesicomyidae, Pliocardiinae) from the Congo margin with the first record of the subfamily Pliocardiinae in the Bay of Biscay (northeastern Atlantic). Zoosystema 33, 83–99. ( 10.5252/z2011n1a4) [DOI] [Google Scholar]
- 40.Krylova EM, Sahling H, Janssen R. 2010. Abyssogena: a new genus of the family Vesicomyidae (Bivalvia) from deep water vents and seeps. J. Moll. Stud. 76, 107–132. ( 10.1093/mollus/eyp052) [DOI] [Google Scholar]
- 41.Taylor JD, Glover EA. 2013. New lucinid bivalves from shallow and deeper water of the Indian and West Pacific Oceans (Mollusca, Bivalvia, Lucinidae). Zookeys 326, 69–90. ( 10.3897/zookeys.326.5786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor JD, Glover EA. 2009. New lucinid bivalves from hydrocarbon seeps of the Western Atlantic (Mollusca: Bivalvia: Lucinidae). Steenstrupia 30, 111–124. [Google Scholar]
- 43.Lorion J, Kiel S, Faure BM, Masaru K, Ho SYW, Marshall BA, Tsuchida S, Miyazaki J-I, Fujiwara Y. 2013. Adaptive radiation of chemosymbiotic deep-sea mussels. Proc. R. Soc. B 280, 20131243 ( 10.1098/rspb.2013.1243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thubaut J, Puillandre N, Faure BM, Cruaud C, Samadi S. 2013. The contrasted evolutionary fates of deep-sea chemosynthetic mussels (Bivalvia, Bathymodiolinae). Ecology and Evolution 3, 4748–4766. ( 10.1002/ece3.749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valdés F, Sellanes J, D'Elía G. 2013. Phylogenetic position of vesicomyid clams from a methane seep off central Chile (∼36°S) with a molecular timescale for the diversification of the Vesicomyidae. Zool. Stud. 51, 1154–1164. [Google Scholar]
- 46.Audzijonyte A, Krylova EM, Sahling H, Vrijenhoek RC. 2012. Molecular taxonomy reveals broad trans-oceanic distributions and high species diversity of deep-sea clams (Bivalvia: Vesicomyidae: Pliocardiinae) in chemosynthetic environments. Syst. Biodivers. 10, 403–415. ( 10.1080/14772000.2012.744112) [DOI] [Google Scholar]
- 47.Decker C, Olu K, Cunha RL, Arnaud-Haond S. 2012. Phylogeny and diversification patterns among vesicomyid bivalves. PLoS ONE 7, e33359 ( 10.1371/journal.pone.0033359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustafson RG, Turner RD, Lutz RA, Vrijenhoek RC. 1998. A new genus and five new species of mussels (Bivalvia: Mytilidae) from deep-sea sulfide/hydrocarbon seeps in the Gulf of Mexico. Malacologia 40, 63–112. [Google Scholar]
- 49.Samadi S, Quéméré E, Lorion J, Tillier A, Cosel Rv, Lopez P, Cruaud C, Couloux A, Boisselier-Dubayle M-C. 2007. Molecular phylogeny in mytilids supports the wooden steps to deep-sea vents hypothesis. C. R. Biol. 330, 446–456. ( 10.1016/j.crvi.2007.04.001) [DOI] [PubMed] [Google Scholar]
- 50.Peek AS, Gustafson RG, Lutz RA, Vrijenhoek RC. 1997. Evolutionary relationships of deep-sea hydrothermal vent and cold-water seep clams (Bivalvia: Vesicomyidae): results from mitochondrial cytochrome oxidase subunit I. Mar. Biol. 130, 151–161. ( 10.1007/s002270050234) [DOI] [Google Scholar]
- 51.Kojima S, Fujikura K, Okutani T. 2004. Multiple trans-Pacific migrations of deep-sea vent/seep-endemic bivalves in the family Vesicomyidae. Mol. Phylo. Evo. 32, 396–406. ( 10.1016/j.ympev.2004.02.016) [DOI] [PubMed] [Google Scholar]
- 52.Kivelä M, Arnaud-Haond S, Saramäki J. 2015. EDENetworks: a user-friendly software to build and analyse networks in biogeography, ecology and population genetics. Mol. Ecol. Resour. 15, 117–122. ( 10.1111/1755-0998.12290) [DOI] [PubMed] [Google Scholar]
- 53.Tosadori G, Bestvina I.. 2016. Network Randomizer - A tool for creating random networks and comparing them to the real ones. see http://apps.cytoscape.org/apps/networkrandomizer. [DOI] [PMC free article] [PubMed]
- 54.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. ( 10.1101/gr.1239303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Palaeontological Statistics software package for education and data analysis. Palaeont. Electronica 4, 9. [Google Scholar]
- 56.Tyler PA, German CR, Ramirez-Llodra E, Van Dover CL. 2003. Understanding the biogeography of chemosynthetic ecosystems. Oceanolog. Acta 25, 227–241. ( 10.1016/S0399-1784(02)01202-1) [DOI] [Google Scholar]
- 57.Olu K, Sibuet M, Fiala-Médoni A, Gofas S, Salas C, Mariotti A, Foucher J-P, Woodside J. 2004. Cold seep communities in the deep eastern Mediterranean Sea: composition, symbiosis and spatial distribution on mud volcanoes. Deep-Sea Res. I 51, 1915–1936. ( 10.1016/j.dsr.2004.07.004) [DOI] [Google Scholar]
- 58.Taviani M, Angeletti L, Ceregato A, Foglini F, Froglia C, Trincardi F. 2013. The Gela Basin pockmark field in the strait of Sicily (Mediterranean Sea): chemosymbiotic faunal and carbonate signatures of postglacial to modern cold seepage. Biogeosciences 10, 4653–4671. ( 10.5194/bg-10-4653-2013) [DOI] [Google Scholar]
- 59.Baco AR, Rowden AA, Levin LA, Smith CR, Bowden DA. 2010. Initial characterization of cold seep faunal communities on the New Zealand Hikurangi margin. Mar. Geol. 272, 251–259. ( 10.1016/j.margeo.2009.06.015) [DOI] [Google Scholar]
- 60.Olu K, Cordes EE, Fisher CR, Brooks JM, Sibuet M, Desbruyères D. 2010. Biogeography and potential exchanges among the Atlantic equatorial belt cold-seep faunas. PLoS ONE 5, e11967 ( 10.1371/journal.pone.0011967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taviani M. 2002. The Mediterranean benthos from late Miocene up to present: ten million years of dramatic climatic and geologic vicissitudes. Biol. Mar. Mediterr. 9, 445–463. [Google Scholar]
- 62.Monegatti P, Raffi S. 2010. The Messinian marine molluscs record and the dawn of the eastern Atlantic biogeography. Palaeogeogr. Palaeoclimat. Palaeoecol. 297, 1–11. ( 10.1016/j.palaeo.2010.06.023) [DOI] [Google Scholar]
- 63.Rogers, et al. 2012. The discovery of new deep-sea hydrothermal vent communities in the Southern Ocean and implications for biogeography. PLoS Biol. 10, e1001234 ( 10.1371/journal.pbio.1001234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borda E, et al. 2013. Cryptic species of Archinome (Annelida: Amphinomida) from vents and seeps. Proc. R. Soc. B 280, 20131876 ( 10.1098/rspb.2013.1876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stiller J, Rousset V, Pleijel F, Chevaldonne P, Vrijenhoek RC, Rouse GW. 2013. Phylogeny, biogeography and systematics of hydrothermal vent and methane seep Amphisamytha (Ampharetidae, Annelida), with descriptions of three new species. Syst. Biodivers. 11, 35–65. ( 10.1080/14772000.2013.772925) [DOI] [Google Scholar]
- 66.Krylova EM, Sahling H. 2006. Recent bivalve molluscs of the genus Calyptogena (Vesicomyidae). J. Moll. Stud. 72, 359–395. ( 10.1093/mollus/eyl022) [DOI] [Google Scholar]
- 67.Goffredi SK, Hurtado LA, Hallam SJ, Vrijenhoek RC. 2003. Evolutionary relationships of deep-sea vent and cold seep clams (Mollusca: Vesicomyidae) of the ‘pacifica/lepta’ species complex. Mar. Biol. 142, 311–320. ( 10.1007/s00227-002-0941-3) [DOI] [Google Scholar]
- 68.Sahling H, Galkin SV, Salyuk A, Greinert J, Foerstel H, Piepenburg D, Suess E. 2003. Depth-related structure and ecological significance of cold-seep communities—a case study from the Sea of Okhotsk. Deep-Sea Res. I 50, 1391–1409. ( 10.1016/j.dsr.2003.08.004) [DOI] [Google Scholar]
- 69.Tarasov VG, Gebruk AV, Mironov AN, Moskalev LI. 2005. Deep-sea and shallow-water hydrothermal vent communities: two different phenomena? Chem. Geol. 224, 5–39. ( 10.1016/j.chemgeo.2005.07.021) [DOI] [Google Scholar]
- 70.Levin LA, Dayton PK. 2009. Ecological theory and continental margins: where shallow meets deep. Trends Ecol. Evol. 24, 606–617. ( 10.1016/j.tree.2009.04.012) [DOI] [PubMed] [Google Scholar]
- 71.Carney RS. 1994. Consideration of the oasis analogy for chemosynthetic communities at Gulf of Mexico hydrocarbon vents. Geo-Mar. Let. 14, 149–159. ( 10.1007/BF01203726) [DOI] [Google Scholar]
- 72.Levin LA, et al. 2016. Hydrothermal vents and methane seeps: rethinking the sphere of influence. Front. Mar. Sci. 3, 72 ( 10.3389/fmars.2016.00072) [DOI] [Google Scholar]
- 73.Kiel S, Goedert JL. 2006. Deep-sea food bonanzas: early Cenozoic whale-fall communities resemble wood-fall rather than seep communities. Proc. R. Soc. B 273, 2625–2631. ( 10.1098/rspb.2006.3620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levin LA, Mendoza GF, Konotchick T, Lee R. 2009. Macrobenthos community structure and trophic relationships within active and inactive Pacific hydrothermal sediments. Deep-Sea Res. II 56, 1632–1648. ( 10.1016/j.dsr2.2009.05.010) [DOI] [Google Scholar]
- 75.Tunnicliffe V, McArthur AG, McHugh D. 1998. A biogeographical perspective of the deep-sea hydrothermal vent fauna. Adv. Mar. Biol. 34, 353–442. ( 10.1016/S0065-2881(08)60213-8) [DOI] [Google Scholar]
- 76.Lorion J, Duperron S, Gros O, Cruaud C, Samadi S. 2008. Several deep-sea mussels and their associated symbionts are able to live both on wood and on whale falls. Proc. R. Soc. B 276, 177–185. ( 10.1098/rspb.2008.1101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scotese CR, Gahagan LM, Larson RL. 1988. Plate tectonic reconstruction of the Cretaceous and Cenozoic ocean basins. Tectonophysics 155, 27–48. ( 10.1016/0040-1951(88)90259-4) [DOI] [Google Scholar]
- 78.Herrera S, Watanabe H, Shank TM. 2015. Evolutionary and biogeographical patterns of barnacles from deep-sea hydrothermal vents. Mol. Ecol. 24, 673–689. ( 10.1111/mec.13054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiel S. 2008. Fossil evidence for micro- and macrofaunal utilization of large nekton-falls: examples from early Cenozoic deep-water sediments in Washington State, USA. Palaeogeogr. Palaeoclimat. Palaeoecol. 267, 161–174. ( 10.1016/j.palaeo.2008.06.016) [DOI] [Google Scholar]
- 80.German CR, Ramirez-Llodra E, Baker MC, Tyler PA, ChEss Scientific Steering Committee. 2011. Deep-water chemosynthetic ecosystem research during the Census of Marine Life decade and beyond: a proposed deep-ocean road map. PLoS ONE 6, e23259 ( 10.1371/journal.pone.0023259) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this manuscript are available as the electronic supplementary material.