Abstract
The spatial scale of animal space use, e.g. measured as individual home range size, is a key trait with important implications for ecological and evolutionary processes as well as management and conservation of populations and ecosystems. Explaining variation in home range size has therefore received great attention in ecological research. However, few studies have examined multiple hypotheses simultaneously, which is important provided the complex interactions between life history, social system and behaviour. Here, we review previous studies on home range size in ungulates, supplementing with a meta-analysis, to assess how differences in habitat use and species characteristics affect the relationship between body mass and home range size. Habitat type was the main factor explaining interspecific differences in home range size after accounting for species body mass and group size. Species using open habitats had larger home ranges for a given body mass than species using closed habitats, whereas species in open habitats showed a much weaker allometric relationship compared with species living in closed habitats. We found no support for relationships between home range size and species diet or mating system, or any sexual differences. These patterns suggest that the spatial scale of animal movement mainly is a combined effect of body mass, group size and the landscape structure. Accordingly, landscape management must acknowledge the influence of spatial distribution of habitat types on animal behaviour to ensure natural processes affecting demography and viability of ungulate populations.
Keywords: allometry, diet, group size, landscape effects, mating system, space use
1. Introduction
Any mobile organism faces the question of where to move. Individuals' movement decisions affect what they can eat [1,2], who they get eaten by [3,4] and who they can mate with [5,6]. The pattern of movement may therefore strongly influence individual differences in fitness [7] and hence generate spatio-temporal variation in population dynamics [8–10]. Accordingly, numerous studies have analysed the causes and consequences of variation in animal movement patterns [11,12].
A fundamental characteristic of an individual's movement pattern is its home range [13]. In one of the first attempts to relate home range to spatial scale of animal movement, it was defined as ‘that area traversed by the individual in its normal activities of food gathering, mating, and caring for the young’ [14]. This definition considers annual to multiannual temporal scales of movement of adult individuals, whereas restricted dispersal phases, e.g. by subadults, and shorter time periods, such as seasonal and diurnal ranges, do not necessarily capture all aspects of a species ‘normal activities’ [11, p. 351]. Some home range estimators, such as 100% minimum convex polygons (MCP), were found to be sensitive to ‘non-normal activities’, such as exploratory behaviour [15–17]. By contrast, estimators rejecting a certain proportion of the outermost locations (e.g. 95% MCP) or defining thresholds of probability of occurrence (e.g. 95% kernel density estimator [18]) are more in accordance with the original definition [14], excluding movement patterns such as exploratory behaviour from an individual's home range. Although choice of home range estimator may affect estimates of home range size, several studies suggest that home range estimators can provide valuable information about variation in animal space use [18–22], and that variation in estimated home range size owing to methodological differences is weaker than ecological signals with no qualitative influence on conclusions regarding ecological patterns ([23,24], but see [25]). Accordingly, knowledge about factors generating variation in home range size among populations and species may provide valuable information about the underlying ecological processes affecting intra- and interspecific variation in space use [26,27].
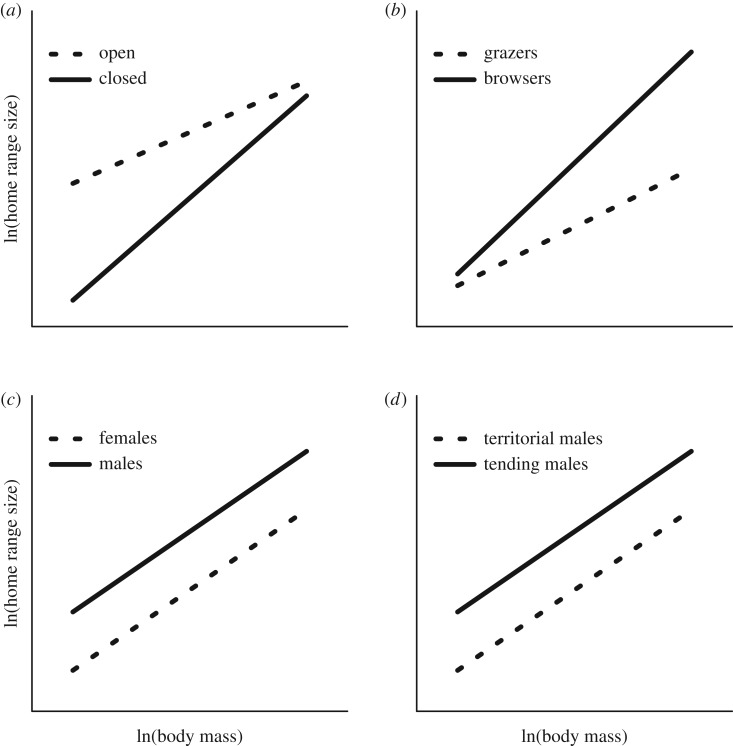
Ungulates, i.e. Perissodactyla and Artiodactyla, are a diverse group with respect to important ecological and behavioural traits. A large body of research on important factors such as relationships among metabolic requirements, diet, habitat preferences and social structures provides invaluable background knowledge about fundamental ecological processes [28–33]. Their potentially large impact on ecosystems [34] and high level of human–wildlife interactions [35] make them important parts of ecosystem management worldwide [36]. Ungulates are roughly classified as grazers, browsers or mixed foragers [37]. However, as with many other animal groups (e.g. [38,39]) diet and habitat use are often strongly associated, with grazers commonly found in open areas, whereas browsers more often use closed habitat [40–42]. Sexual size dimorphism is common among ungulates, with males being up to 2.6 times heavier than females [43]. Males do not take part in the care of offspring, and varying levels of polygamy by either harem-holding territorial males or tending males is the common mating system [44]. All the factors listed above are proposed to influence home range size in ungulates, as well as other animals (see figure 1 for predicted relationships). However, mechanisms may depend on each other, causing potentially complex relationships among species characteristics, environmental characteristics and spatial scale of movement as measured by the home range size (figure 1).
Figure 1.
How interspecific factors are expected to affect home range size and the allometric relationship between body mass and home range size. (a) Because of higher predation risk in open habitats, species living in open habitats will have overall larger home ranges than species in closed habitats. However, the higher movement efficiency between foraging patches with increasing body size will result in a shallower allometric slope between body size and home range size in open compared to closed habitats. (b) Because of the more patchily distribution of browse compared with grass, browsers will have larger home ranges compared with grazers. However, larger bodied browsers have higher energetic demands and will need to include more and larger patches, which will be more dispersed and heterogeneous compared with the spatial distribution of food resources of larger grazers. This will give a steeper allometric slope between body mass and home range size for browsers than for grazers. (c) Females will have smaller home ranges than males owing to more selective foraging in heterogeneous landscapes, and because of movement constraints by calf at heal. However, increasing group size decreases sex difference in home range size (not shown in figure). (d) Owing to costs of defence and loss of mating benefits with increasing area, territorial males should have smaller home ranges than tending males.
Here, we review patterns and mechanisms of variation in home range size among ungulates, supplementing with a meta-analysis where we address several hypotheses simultaneously. Information about the meta-analysis can be found in the electronic supplementary material (S1: Data search for home range size in ungulates, S2: Statistical procedures and methodological considerations for analysing home range size variation in ungulates and S3: Detailed presentation of results from a meta-analysis of home range size variation in ungulates).
2. Metabolic requirements and home range size
The variation in home range size among [45] and within species [46] was originally considered to be a function of metabolic rate. Accordingly, the scaling between body mass and home range size should be comparable to the body mass scaling of forage intake rate of approximately 0.75 at the logarithmic scale [33,47,48]. On an arithmetic scale, this means that the positive relationship between home range size and body mass is steeper at smaller body masses, and decelerates with increasing body mass. Later studies found support for steeper allometric slopes of approximately 1.0 [20,24,47,49–52], which was explained by dietary differences [51], length of the biological time scale included in the study [47] and higher level of scramble competition from conspecifics for species with larger body mass [20]. Our meta-analysis revealed an overall allometric slope of 1.06 (95% CI = 0.67; 1.41, electronic supplementary material, table S4), which is in accordance with previous estimates among large herbivores of 1.06 [52], 1.02 [20] and 1.08 [53]. Hence, the predicted relationship between home range size and body mass of 0.75 [45] seems not to be valid in ungulates. The larger coefficient could be the result of environmental and/or species characteristics, other than body mass, affecting the cost–benefit ratio of movement. However, it can also be related to the fact that digestion efficiency increases with body mass [28]. The increased digestion efficiency of larger animals comes at the costs of a more time-restricted activity budget [29], which again may increase the home range [54]. This emphasizes how the complex interactions between body mass and ecological factors may amplify or cancel out each other's effects on home range size, and should therefore be considered jointly (see sections below).
For group-living animals, the functional unit at which space use scale is measured becomes the group rather than the individual [52]. Through increased scramble competition one therefore expects a similar relationship between home range and group size as for body mass [47]. Previous studies of home range size have often omitted or controlled for variation in group size prior to analyses [20,47,55,56], making it difficult to determine its effect. In our meta-analysis, we found a decelerating increase in home range size with increasing group size, but with a high uncertainty in the parameter estimate (electronic supplementary material, table S4). This could be because a group's size is an imprecise measure of the costs of scramble competition [52], and hence that group size is not a suitable measure for relating metabolic requirements to home range variation in animals.
3. Habitat
The habitat explains important factors of the foraging niche, such as the quantity and quality of forage [57,58]. It also encompasses a range of other biotic and abiotic variables and their spatial distribution so that different foraging strategies may lead to the same habitat [59]. However, within a habitat different diets may result in different energy trade-offs [59], or alternatively the habitat may determine the diet [60], making the distinction between the two important. Moreover, habitat affects the mortality risk associated with predation [39,61], and exposure to extreme weather conditions [62,63]. Habitat composition also affects movement among foraging patches [64,65], and thereby factors such as resource encounter rate and scale of perception [66]. Scale of perception, which is increased in low-dimensional patches such as open areas [64], may strongly reduce distance moved between patches [65,67]. As such, in combination with body mass (figure 1), habitat composition may strongly affect individual movement patterns and thereby shape the spatial scale of space use [64,68].
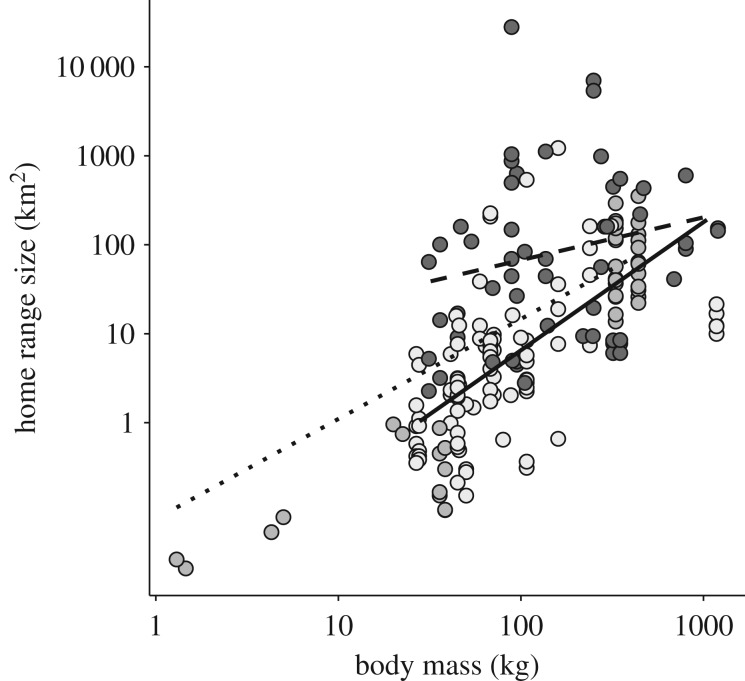
It seems to be a general pattern that species using open habitats have larger home ranges for a given body mass than species living predominantly in closed habitats ([69], figure 2; electronic supplementary material, table S4). Two mechanisms may explain this pattern (figure 1). Firstly, ungulate space use is not only a result of food, but may also be related to protection from predators and harsh weather [70]. Accordingly, ungulates foraging in open habitats may have to increase their home range to include habitats offering cover [71–75]. Secondly, plants growing under shady conditions, such as in closed habitats, develop fewer secondary compounds and have a lower fibre content, resulting in a higher quality herbivore forage [76–78]. This may lead to increased energy gain for a given intake rate [79] regardless of diet. As a consequence, closed habitats may provide sufficient food within smaller home ranges for a given body mass [49,80]. Our meta-analyses revealed that the size of home ranges of ungulates in mixed habitats was even smaller than that of ungulates in closed habitats (figure 2; electronic supplementary material, table S4). Possibly, this is because mixed habitats include more edges where animals can optimize the cost–benefit trade-off between food and cover at even smaller spatial scales than in open or closed habitats [81,82].
Figure 2.
The relationship between home range size (in square kilometres) and body mass (in kilograms) for species belonging to Artiodactyla and Perissodactyla with respect to the dominating habitat the species inhabit (closed, grey/dotted; mixed, light grey/solid; open, dark grey/dashed). Predicted lines accounts for estimate uncertainty and use the mean group size (ln-transformed) across species.
Because larger bodied herbivores are better at using low-quality forage, such as fibre-rich grass found in open areas, the scaling between body mass and intake rate is expected to be weaker in such habitats compared to habitats with high-quality forage where the nutritional benefit of a given intake rate is weaker related to body mass [28,33]. Interactions between habitat composition and animals' movement patterns [66,67] can also lead to habitat-specific patterns in the allometric relationship between body mass and home range size. Specifically, a steeper allometric relationship is found for species in closed habitats compared to species in open habitats ([69]: open habitats β = 0.69, closed habitat β = 1.23), or animals found in high- versus low-dimensional habitats [64,67]. This is confirmed by our meta-analysis (βOpen = 0.48 [−0.08; 1.09], βClosed = 1.13 [0.39; 1.79], βMixed = 1.55 [0.88; 2.03], figure 2; electronic supplementary material, table S4). The allometric slope of species in mixed habitat was similar to that of species in closed habitat. The shallower allometric slope in open habitats may reflect that larger ungulates in open habitats can move more efficiently owing to larger perception range [52,83,84], or faster and more efficient because of longer legs [69,85,86]. However, it can also be related to dietary differences between species in open and closed/mixed habitats, although habitat use as an explanatory factor received stronger statistical support than diet for explaining home range size variation among ungulates (electronic supplementary material, table S3).
4. Diet
A broad classification used to explain differences in ungulates body-mass scaling properties, as well as other ecological factors, is to separate between browsers and grazers [33,37,87]. In general, grass is considered low-quality forage, but is normally abundant over large areas in open landscapes [37]. Browse is considered easier to digest, but is more patchily distributed, and more abundant in closed habitats [37,88]. According to optimal foraging theory [89], increased spatial heterogeneity is expected to increase the movement rate between patches [90–92], resulting in larger home range size among browsers. In line with this, several studies have reported larger home ranges for browsers compared with grazers [56,93]. However, a larger home range among browsers compared with grazers may also be explained by grazers having a slightly higher retention time [94], resulting in less time spend on movement and a weaker allometric relationship among grazers than among browsers [33]. The allometric slope of browsers may be further steepened owing to the need of relatively larger patches to sustain larger bodied browsers [56,93]. A decreasing patch encounter rate with increasing patch size [64] makes it necessary to increase movement to obtain the required intake rate given the body mass.
Diet and habitat use are inevitable linked; what you eat affects where you go and where you are affects what you can eat. For instance, grazers and browsers are often found in open and closed habitats, respectively [40], making it challenging to distinguish the relative contribution of these factors for explaining variation in the spatial scale of space use. The predicted relationship with home range size of habitat and diet differ; we expect species in closed habitat (i.e. typically browsers) to have smaller home ranges than species in open habitats, but also that a diet consisting mainly of browse should lead to a larger home range than a grass-dominated diet (figure 1). From theory, the predicted difference in the allometric relationship, however, is similar; browsers, which are mainly living in closed habitats, should have a steeper allometric relationship than open-living grazers (figure 1). Our meta-analysis, which is one of the first attempts of relating variation in home range size to habitat and diet simultaneously, suggests that habitat has a stronger explanatory power than diet (electronic supplementary material, S3 and table S3). Accordingly, it appears that when it comes to species variation in space use patterns, the browser–grazer dichotomy is not as suited as for other ecological phenomenon. Instead, observed relationship between scales of space use can be explained by habitat characteristics influencing the distribution of components of species' foraging niche [69].
5. Sex
If home range size was only a result of metabolic requirements, males and females would have similar home range sizes after accounting for differences in body mass, diet and habitat. However, several other factors are predicted to generate sex differences in ungulate space use patterns such as home range size, with mixed support from empirical studies [32]. First, females have the cost of lactation and fending for offspring. On the one hand, increased need for resources can increase home range size [71,95,96], whereas constrained movement and increased mortality risk have been found to decrease home range size [24,97,98]. Although the movement may be restricted only for a limited period after giving birth, the magnitude in differences between the males' and females' movement rate [24] may be sufficient to cause sex differences in annual home range size. Second, males are more willing to trade off forage quality for quantity if the costs of locating high-quality forage are high [49]. Accordingly, with decreasing forage quality males may compensate by increasing their home range, whereas females compensate by becoming more selective [4,32,99] and spend more time hiding or foraging to offset lactation costs [51]. Based on support from other studies on space use patterns in large herbivores [32], the general prediction is larger home ranges in males compared with females after accounting for sexual size dimorphism (figure 1c).
Our meta-analysis did not support sex-specific variation in home range size after accounting for size (electronic supplementary material S3 and tables S3), and supports previous findings that sex differences in the spatial scale of movement are caused by sexual size dimorphism [32,56]. Moreover, the meta-analysis did not support sex differences in allometric slopes between home range size and group size. Accordingly, it appears that any difference in the spatial scale of space use of male and female ungulates is explained by body size, and that it relates similarly to variation in group size, body mass, habitat or any other characteristics of the species or environment. The lack of distinct sex-specific differences may, however, be caused by different factors cancelling each other's effect on home range size. For instance, female movement may be constrained by calves at heal, but a calf may also increase the need for cover, forcing females living in open habitats to increase their space use to include more closed habitats to provide shelter for their offspring. However, such mechanisms have yet to be tested, but should be feasible with increasingly more available movement data at the individual level [100,101].
6. Mating system
The mating system of a population is expected to greatly influence animal space use patterns and vice versa through several mechanisms [102]. These can have different strength on males and females. For instance, both harem-holding and territorial species are spatially polygynous owing to female clustering, and because of the benefit of occupying rutting sites in due time before the rutting season [103,104], males may have relatively small home ranges. By contrast, tending species are temporally polygynous as males roam about and sequentially guard and mate with females [102], resulting in the benefit of increasing home range size for males [6]. This suggests larger home ranges in tending males compared to territorial males (figure 1d), but with no predicted effects on females. This is supported by earlier studies covering the costs of defending a territorial home range across a range of taxa [105]. The increased costs of defending a larger home range outweigh the benefits, and resulted in a smaller home range for territorial species. Our meta-analysis did not provide support to the prediction that tending males have larger home ranges than territorial males. This may partly be due to interspecific correlation in characteristics such as habitat use and mating systems. For instance, it has been suggested that polygamy and diet coevolved during the development of open grasslands [41,44]. Thus, monogamous species tend to be browsers and reside in closed habitats while polygamous species tend to be grazers in open landscapes. Still, the fact that habitat was included in all the highest ranked models whereas other species traits only were included sporadically in the top models (electronic supplementary material, table S3), supports that habitat use, rather than other traits, is the key driver for variation in home range size given that variation in body mass and group size is accounted for.
7. Concluding remarks
Conservation of threatened species must account for important processes shaping their spatial distribution. Our review of the literature on home range size in ungulates, supplemented with a meta-analysis, suggests that only habitat association of a species provide a significant effect after accounting for metabolic requirements (i.e. body mass and group size). Our study also emphasizes the importance of methodological and phylogenetical considerations when assessing ecological patterns based on meta-analyses (electronic supplementary material, S3, table S3 and table S4). Most importantly, several methodological factors such as accounting for estimate uncertainty or phylogeny, have only weak signals on the ecological patterns and can be considered ignored at the gain of increased sample size (electronic supplementary material, S2). This may increase the statistical power to detect general ecological relationships across species or taxa.
The impact of habitat elaborates the importance of landscape characteristics on essential ecological and evolutionary processes related to spatial scale, such as the spatial scale of population fluctuations [106], population viability [107] and gene flow [108,109]. Currently, habitat loss and fragmentation are among the biggest threats to global biodiversity [11], and like in many other studies [110–112], our results emphasize the importance of conserving areas of sufficient size and complexity for maintaining viable populations. Optimizing the costs and benefits associated with the conservation of populations and species therefore hinges on joined landscape and wildlife management [29].
Supplementary Material
Acknowledgements
We thank all authors and researches that willingly shared data and provided additional information.
Data accessibility
The studies included in the meta-analysis presented in figure 2 are listed in the electronic supplementary material, table S1.
Authors' contributions
B.-E.S. initiated the study. E.G.O. performed the literature search, developed and ran all analyses. E.G.O., I.H., B.-E.S. and E.J.S. wrote the paper.
Competing interests
We have no competing interests.
Funding
This work was supported by the Norwegian Environment Agency, the European Research Council (ERC-2010-AdG 268562), the Research Council of Norway (SFF-III 223257/F50) and the Norwegian University of Science and Technology (NTNU).
References
- 1.Herfindal I, Tremblay J-P, Hansen BB, Solberg EJ, Heim M, Sæther B-E. 2009. Scale dependency and functional response in moose habitat selection. Ecography 32, 849–859. ( 10.1111/j.1600-0587.2009.05783.x) [DOI] [Google Scholar]
- 2.Patterson BR, Messier F. 2001. Social organization and space use of coyotes in eastern Canada relative to prey distribution and abundance. J. Mammal. 82, 463–477. ( 10.1644/1545-1542(2001)082%3C0463:SOASUO%3E2.0.CO;2) [DOI] [Google Scholar]
- 3.van Beest FM, Vander Wal E, Stronen AV, Paquet PC, Brook RK. 2013. Temporal variation in site fidelity: scale-dependent effects of forage abundance and predation risk in a non-migratory large herbivore. Oecologia 173, 409–420. ( 10.1007/s00442-013-2647-2) [DOI] [PubMed] [Google Scholar]
- 4.Beier P, McCullough DR. 1990. Factors influencing white-tailed deer activity patterns and habitat use. Wildl. Monogr. 109, 1–51. [Google Scholar]
- 5.Komers PE, Brotherton PNM. 1997. Female space use is the best predictor of monogamy in mammals. Proc. R. Soc. Lond. B 264, 1261–1270. ( 10.1098/rspb.1997.0174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spritzer MD, Solomon NG, Meikle DB. 2005. Influence of scramble competition for mates upon the spatial ability of male meadow voles. Anim. Behav. 69, 375–386. ( 10.1016/j.anbehav.2004.03.015) [DOI] [Google Scholar]
- 7.Cattarino L, McAlpine CA, Rhodes JR. 2016. Spatial scale and movement behaviour traits control the impacts of habitat fragmentation on individual fitness. J. Anim. Ecol. 85, 168–177. ( 10.1111/1365-2656.12427) [DOI] [PubMed] [Google Scholar]
- 8.Ranta E, Lundberg P, Kaitala V. 2005. Ecology of populations. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Lande R, Engen S, Sæther B-E. 2003. Stochastic population dynamics in ecology and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Banks SC, Lindenmayer DB. 2014. Inbreeding avoidance, patch isolation and matrix permeability influence dispersal and settlement choices by male agile antechinus in a fragmented landscape. J. Anim. Ecol. 83, 515–524. ( 10.1111/1365-2656.12128) [DOI] [PubMed] [Google Scholar]
- 11.Clobert J, Baguette M, Benton TG, Bullock JM. 2012. Dispersal ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Milner-Gulland EJ, Fryxell JM, Sinclair ARE. 2011. Animal migration: a synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Börger L, Dalziel BD, Fryxell JM. 2008. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol. Lett. 11, 637–650. ( 10.1111/j.1461-0248.2008.01182.x) [DOI] [PubMed] [Google Scholar]
- 14.Burt WH. 1943. Territoriality and home range concepts as applied to mammals. J. Mammal. 24, 346–352. ( 10.2307/1374834) [DOI] [Google Scholar]
- 15.Hansteen TL, Andreassen HP, Ims RA. 1997. Effects of spatiotemporal scale on autocorrelation and home range estimators. J. Wildl. Manage. 61, 280–290. ( 10.2307/3802583) [DOI] [Google Scholar]
- 16.Dalke PD. 1942. Cottontail rabbits in Connecticut. In A report on the work of the Connecticut Wildlife Research Unit (ed. Hosley NW.), pp. 1935–1938. Connecticut, USA: State Geological Natural History Survey of Connecticut. [Google Scholar]
- 17.Mohr CO. 1947. Table of equivalent populations of North American small mammals. Am. Midl. Nat. 37, 223–249. ( 10.2307/2421652) [DOI] [Google Scholar]
- 18.Worton BJ. 1987. A review of models of home range for animal movement. Ecol. Modell. 38, 277–298. ( 10.1016/0304-3800(87)90101-3) [DOI] [Google Scholar]
- 19.Börger L, Franconi N, De Michele G, Gantz A, Meschi F, Manica A, Lovari S, Coulson T. 2006. Effects of sampling regime on the mean and variance of home range size estimates. J. Anim. Ecol. 75, 1393–1405. ( 10.1111/j.1365-2656.2006.01164.x) [DOI] [PubMed] [Google Scholar]
- 20.Jetz W, Carbone C, Fulford J, Brown JH. 2004. The scaling of animal space use. Science 306, 266–268. ( 10.1126/science.1102138) [DOI] [PubMed] [Google Scholar]
- 21.Signer J, Balkenhol N, Ditmer M, Fieberg J. 2015. Does estimator choice influence our ability to detect changes in home-range size? Anim. Biotelem. 3, 1–9. ( 10.1186/s40317-015-0051-x) [DOI] [Google Scholar]
- 22.Woodroffe R, Ginsberg JR. 1998. Edge effects and the extinction of populations inside protected areas. Science 280, 2126–2128. ( 10.1126/science.280.5372.2126) [DOI] [PubMed] [Google Scholar]
- 23.Nilsen EB, Pedersen S, Linnell JDC. 2008. Can minimum convex polygon home ranges be used to draw biologically meaningful conclusions? Ecol. Res. 23, 635–639. ( 10.1007/s11284-007-0421-9) [DOI] [Google Scholar]
- 24.van Beest FM, Rivrud IM, Loe LE, Milner JM, Mysterud A. 2011. What determines variation in home range size across spatiotemporal scales in a large browsing herbivore? J. Anim. Ecol. 80, 771–785. ( 10.1111/j.1365-2656.2011.01829.x) [DOI] [PubMed] [Google Scholar]
- 25.Laver PN, Kelly MJ. 2008. A critical review of home range studies. J. Wildl. Manage. 72, 290–298. ( 10.2193/2005-589) [DOI] [Google Scholar]
- 26.Linnell JDC, Andersen R, Kvam T, Andren H, Liberg O, Odden J, Moa PF. 2001. Home range size and choice of management strategy for lynx in Scandinavia. Environ. Manage. 27, 869–879. ( 10.1007/s002670010195) [DOI] [PubMed] [Google Scholar]
- 27.Kowalczyk R, Schmidt K, Jędrzejewski W. 2012. Do fences or humans inhibit the movements of large mammals in Białowieża Primeval Forest? In Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? (eds Somers MJ, Hayward M), pp. 235–243. New York, NY: Springer. [Google Scholar]
- 28.Demment MW, Van Soest PJ. 1985. A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am. Nat. 125, 641–672. ( 10.1086/284369) [DOI] [Google Scholar]
- 29.Hanley TA. 1982. The nutritional basis for food selection by ungulates. J. Range Manage. 35, 146–151. ( 10.2307/3898379) [DOI] [Google Scholar]
- 30.McNaughton SJ, Georgiadis NJ. 1986. Ecology of African grazing and browsing mammals. Annu. Rev. Ecol. Syst. 17, 39–66. ( 10.1146/annurev.es.17.110186.000351) [DOI] [Google Scholar]
- 31.McNaughton SJ, Oesterheld M, Frank DA, Williams KJ. 1989. Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature 341, 142–144. ( 10.1038/341142a0) [DOI] [PubMed] [Google Scholar]
- 32.Ruckstuhl KE, Neuhaus P. 2002. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol. Rev. 77, 77–96. ( 10.1017/S1464793101005814) [DOI] [PubMed] [Google Scholar]
- 33.Shipley LA, Gross JE, Spalinger DE, Hobbs NT, Wunder BA. 1994. The scaling of intake rate in mammalian herbivores. Am. Nat. 143, 1055–1082. ( 10.1086/285648) [DOI] [Google Scholar]
- 34.Côté SD, Rooney TP, Tremblay J-P, Dussault C, Waller DM. 2004. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 35, 113–147. ( 10.1146/annurev.ecolsys.35.021103.105725) [DOI] [Google Scholar]
- 35.Putman R, Apollonio M. 2014. Behaviour and management of European ungulates. Dunbeath, UK: Whittles Publishing. [Google Scholar]
- 36.Putman R, Apollonio M, Andersen R. 2011. Ungulate management in Europe: problems and practices. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 37.Gordon IJ, Prins HH. 2008. The ecology of browsing and grazing. Stanford, CA: Springer. [Google Scholar]
- 38.Husseman JS, Murray DL, Power G, Mack C, Wenger CR, Quigley H. 2003. Assessing differential prey selection patterns between two sympatric large carnivores. Oikos 101, 591–601. ( 10.1034/j.1600-0706.2003.12230.x) [DOI] [Google Scholar]
- 39.Gorini L, Linnell JDC, May R, Panzacchi M, Boitani L, Odden M, Nilsen EB. 2012. Habitat heterogeneity and mammalian predator–prey interactions. Mamm. Rev. 42, 55–77. ( 10.1111/j.1365-2907.2011.00189.x) [DOI] [Google Scholar]
- 40.Janis CM. 2008. An evolutionary history of browsing and grazing ungulates. In The ecology of browsing and grazing (eds Gordon IJ, Prins HHT), pp. 21–45. Berlin, Germany: Springer. [Google Scholar]
- 41.Jarman PJ. 1974. The social organisation of antelope in relation to their ecology. Behaviour 48, 215–267. ( 10.1163/156853974X00345) [DOI] [Google Scholar]
- 42.Stewart KM, Bowyer RT, Kie JG, Cimon NJ, Johnson BK. 2002. Temporospatial distributions of elk, mule deer, and cattle: resource partitioning and competitive displacement. J. Mammal. 83, 229–244. ( 10.1644/1545-1542(2002)083%3C0229:Tdoemd%3E2.0.Co;2) [DOI] [Google Scholar]
- 43.Pérez-Barbería FJ, Gordon IJ. 2000. Differences in body mass and oral morphology between the sexes in the Artiodactyla: evolutionary relationships with sexual segregation. Evol. Ecol. Res. 2, 667–684. [Google Scholar]
- 44.Pérez-Barbería FJ, Gordon IJ, Pagel M. 2002. The origins of sexual dimorphism in body size in ungulates. Evolution 56, 1276–1285. ( 10.1111/j.0014-3820.2002.tb01438.x) [DOI] [PubMed] [Google Scholar]
- 45.McNab BK. 1963. Bioenergetics and the determination of home range size. Am. Nat. 97, 133–140. ( 10.1086/282264) [DOI] [Google Scholar]
- 46.Gompper ME, Gittleman JL. 1991. Home range scaling: intraspecific and comparative trends. Oecologia 87, 343–348. ( 10.1007/Bf00634589) [DOI] [PubMed] [Google Scholar]
- 47.Lindstedt SL, Miller BJ, Buskirk SW. 1986. Home range, time, and body size in mammals. Ecology 67, 413–418. ( 10.2307/1938584) [DOI] [Google Scholar]
- 48.Nagy KA, Girard IA, Brown TK. 1999. Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 19, 247–277. ( 10.1146/annurev.nutr.19.1.247) [DOI] [PubMed] [Google Scholar]
- 49.Harestad AS, Bunnel FL. 1979. Home range and body-weight: a reevaluation. Ecology 60, 389–402. ( 10.2307/1937667) [DOI] [Google Scholar]
- 50.Kelt DA, Van Vuren D. 1999. Energetic constraints and the relationship between body size and home range area in mammals. Ecology 80, 337–340. ( 10.1890/0012-9658(1999)080%5B0337:ECATRB%5D2.0.CO;2) [DOI] [Google Scholar]
- 51.Mace GM, Harvey PH. 1983. Energetic constraints on home-range size. Am. Nat. 121, 120–132. ( 10.1086/284043) [DOI] [Google Scholar]
- 52.Makarieva AM, Gorshkov VG, Li B-L. 2005. Why do population density and inverse home range scale differently with body size? Implications for ecosystem stability. Ecol. Complex. 2, 259–271. ( 10.1016/j.ecocom.2005.04.006) [DOI] [Google Scholar]
- 53.Kelt DA, Van Vuren DH. 2001. The ecology and macroecology of mammalian home range area. Am. Nat. 157, 637–645. ( 10.1086/320621) [DOI] [PubMed] [Google Scholar]
- 54.Van Moorter B, Rolandsen CM, Basille M, Gaillard J-M. 2016. Movement is the glue connecting home ranges and habitat selection. J. Anim. Ecol. 85, 21–31. ( 10.1111/1365-2656.12394) [DOI] [PubMed] [Google Scholar]
- 55.Gittleman JL, Harvey PH. 1982. Carnivore home-range size, metabolic needs and ecology. Behav. Ecol. Sociobiol. 10, 57–63. ( 10.1007/BF00296396) [DOI] [Google Scholar]
- 56.Mysterud A, Pérez-Barbería FJ, Gordon IJ. 2001. The effect of season, sex and feeding style on home range area versus body mass scaling in temperate ruminants. Oecologia 127, 30–39. ( 10.1007/s004420000562) [DOI] [PubMed] [Google Scholar]
- 57.Beyer HL, Gurarie E, Börger L, Panzacchi M, Basille M, Herfindal I, Van Moorter B, Lele SR, Matthiopoulos J. 2016. ‘You shall not pass!’: quantifying barrier permeability and proximity avoidance by animals. J. Anim. Ecol. 85, 43–53. ( 10.1111/1365-2656.12275) [DOI] [PubMed] [Google Scholar]
- 58.Lele SR, Merrill EH, Keim J, Boyce MS. 2013. Selection, use, choice and occupancy: clarifying concepts in resource selection studies. J. Anim. Ecol. 82, 1183–1191. ( 10.1111/1365-2656.12141) [DOI] [PubMed] [Google Scholar]
- 59.Shipley LA, Forbey JS, Moore BD. 2009. Revisiting the dietary niche: when is a mammalian herbivore a specialist? Integr. Comp. Biol. 49, 274–290. ( 10.1093/icb/icp051) [DOI] [PubMed] [Google Scholar]
- 60.Hanley TA, Hanley KA. 1982. Food resource partitioning by sympatric ungulates on Great Basin rangeland. J. Range Manage. 35, 152–158. ( 10.2307/3898380) [DOI] [Google Scholar]
- 61.Dussault C, Ouellet J-P, Courtois R, Huot J, Breton L, Jolicoeur H. 2005. Linking moose habitat selection to limiting factors. Ecography 28, 619–628. ( 10.1111/j.2005.0906-7590.04263.x) [DOI] [Google Scholar]
- 62.Conradt L, Clutton-Brock TH, Guinness FE. 2000. Sex differences in weather sensitivity can cause habitat segregation: red deer as an example. Anim. Behav. 59, 1049–1060. ( 10.1006/anbe.2000.1409) [DOI] [PubMed] [Google Scholar]
- 63.Melin M, Matala J, Mehtätalo L, Tiilikainen R, Tikkanen OP, Maltamo M, Pusenius J, Packalen P. 2014. Moose (Alces alces) reacts to high summer temperatures by utilizing thermal shelters in boreal forests: an analysis based on airborne laser scanning of the canopy structure at moose locations. Glob. Change. Biol. 20, 1115–1125. ( 10.1111/gcb.12405) [DOI] [PubMed] [Google Scholar]
- 64.Ritchie ME. 1998. Scale-dependent foraging and patch choice in fractal environments. Evol. Ecol. 12, 309–330. ( 10.1023/A:1006552200746) [DOI] [Google Scholar]
- 65.Fronhofer EA, Hovestadt T, Poethke H-J. 2013. From random walks to informed movement. Oikos 122, 857–866. ( 10.1111/j.1600-0706.2012.21021.x) [DOI] [Google Scholar]
- 66.Nilsen EB, Finstad AG, Næsje TF, Sverdrup-Thygeson A. 2013. Using mass scaling of movement cost and resource encounter rate to predict animal body size–population density relationships. Theor. Popul. Biol. 86, 23–28. ( 10.1016/j.tpb.2013.03.003) [DOI] [PubMed] [Google Scholar]
- 67.Whitehead H, Walde SJ. 1992. Habitat dimensionality and mean search distances of top predators: implications for ecosystem structure. Theor. Popul. Biol. 42, 1–9. ( 10.1016/0040-5809(92)90002-B) [DOI] [Google Scholar]
- 68.Aarts G, Fieberg J, Brasseur S, Matthiopoulos J. 2013. Quantifying the effect of habitat availability on species distributions. J. Anim. Ecol. 82, 1135–1145. ( 10.1111/1365-2656.12061) [DOI] [PubMed] [Google Scholar]
- 69.Janis CM, Wilhelm PB. 1993. Were there mammalian pursuit predators in the tertiary? Dances with wolf avatars. J. Mamm. Evol. 1, 103–125. ( 10.1007/BF01041590) [DOI] [Google Scholar]
- 70.Krebs JR, Kacelnik A. 1991. Decision-making. In Behavioural ecology—an evolutionary approach (eds. Krebs JR & Davies NB), pp. 105–137. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 71.Tufto J, Andersen R, Linnell J. 1996. Habitat use and ecological correlates of home range size in a small cervid: the roe deer. J. Anim. Ecol. 65, 715–724. ( 10.2307/5670) [DOI] [Google Scholar]
- 72.Cibien C, Sempere A. 1989. Food availability as a factor in habitat use by roe deer. Acta Theriol. 34, 111–123. ( 10.4098/AT.arch.89-7) [DOI] [Google Scholar]
- 73.Jeppesen JL. 1989. Activity patterns of free-ranging roe deer (Capreolus capreolus) at Kalø. Dan. Rev. Game Biol. 13, 32. [Google Scholar]
- 74.Blaxter KL, Hamilton WJ. 1980. Reproduction in farmed red deer. 2. Calf growth and mortality. J. Agric. Sci. 95, 275–284. ( 10.1017/S0021859600039290) [DOI] [PubMed] [Google Scholar]
- 75.White KS, Berger J. 2001. Antipredator strategies of Alaskan moose: are maternal trade-offs influenced by offspring activity? Can. J. Zool. 79, 2055–2062. ( 10.1139/cjz-79-11-2055) [DOI] [Google Scholar]
- 76.Jonasson S, Bryant JP, Chapin FS III, Andersson M. 1986. Plant phenols and nutrients in relation to variations in climate and rodent grazing. Am. Nat. 128, 394–408. ( 10.1086/284570) [DOI] [Google Scholar]
- 77.Laine KM, Henttonen H. 1987. Phenolics/nitrogen ratios in the blueberry Vaccinium myrtillus in relation to temperature and microtine density in Finnish Lapland. Oikos 50, 389–395. ( 10.2307/3565500) [DOI] [Google Scholar]
- 78.Reich PB, Peterson DW, Wedin DA, Wrage K. 2001. Fire and vegetation effects on productivity and nitrogen cycling across a forest-grassland continuum. Ecology 82, 1703–1719. ( 10.1890/0012-9658(2001)0821703:FAVEOP%5D2.0.CO;2) [DOI] [Google Scholar]
- 79.Bø S, Hjeljord O. 1991. Do continental moose ranges improve during cloudy summers? Can. J. Zool. 69, 1875–1879. ( 10.1139/z91-260) [DOI] [Google Scholar]
- 80.Morellet N, et al. 2013. Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within Europe. J. Anim. Ecol. 82, 1326–1339. ( 10.1111/1365-2656.12105) [DOI] [PubMed] [Google Scholar]
- 81.Saïd S, Servanty S. 2005. The influence of landscape structure on female roe deer home-range size. Landsc. Ecol. 20, 1003–1012. ( 10.1007/s10980-005-7518-8) [DOI] [Google Scholar]
- 82.Laundré J, Loxterman J. 2007. Impact of edge habitat on summer home range size in female pumas. Am. Midl. Nat. 157, 221–229. ( 10.1674/0003-0031(2007)157%5B221:Ioehos%5D2.0.Co;2) [DOI] [Google Scholar]
- 83.Forero-Medina G, Vieira MV. 2009. Perception of a fragmented landscape by neotropical marsupials: effects of body mass and environmental variables. J. Trop. Ecol. 25, 53–62. ( 10.1017/S0266467408005543) [DOI] [Google Scholar]
- 84.Mech SG, Zollner PA. 2002. Using body size to predict perceptual range. Oikos 98, 47–52. ( 10.1034/j.1600-0706.2002.980105.x) [DOI] [Google Scholar]
- 85.Tamburello N, Côté IM, Dulvy NK. 2015. Energy and the scaling of animal space use. Am. Nat. 186, 196–211. ( 10.1086/682070) [DOI] [PubMed] [Google Scholar]
- 86.Bejan A, Marden JH. 2006. Unifying constructal theory for scale effects in running, swimming and flying. J. Exp. Biol. 209, 238–248. ( 10.1242/jeb.01974) [DOI] [PubMed] [Google Scholar]
- 87.Spalinger DE, Hobbs NT. 1992. Mechanisms of foraging in mammalian herbivores: new models of functional response. Am. Nat. 140, 325–348. ( 10.1086/285415) [DOI] [PubMed] [Google Scholar]
- 88.Van Soest PJ. 1994. Nutritional ecology of the ruminant. New York, NY: Cornell University Press. [Google Scholar]
- 89.Charnov EL. 1976. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136. ( 10.1016/0040-5809(76)90040-X) [DOI] [PubMed] [Google Scholar]
- 90.Calcagno V, Mailleret L, Wajnberg É, Grognard F. 2014. How optimal foragers should respond to habitat changes: a reanalysis of the marginal value theorem. J. Math. Biol. 69, 1237–1265. ( 10.1007/s00285-013-0734-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calcagno V, Grognard F, Hamelin FM, Wajnberg É, Mailleret L. 2014. The functional response predicts the effect of resource distribution on the optimal movement rate of consumers. Ecol. Lett. 17, 1570–1579. ( 10.1111/ele.12379) [DOI] [PubMed] [Google Scholar]
- 92.Barraquand F, Benhamou S. 2008. Animal movements in heterogeneous landscapes: identifying profitable places and homogeneous movement bouts. Ecology 89, 3336–3348. ( 10.1890/08-0162.1) [DOI] [PubMed] [Google Scholar]
- 93.Owen-Smith N. 1992. Megaherbivores: the influence of very large body size on ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 94.Clauss M, Schwarm A, Ortmann S, Streich WJ, Hummel J. 2007. A case of non-scaling in mammalian physiology? Body size, digestive capacity, food intake, and ingesta passage in mammalian herbivores. Comp. Biochem. Phys. A 148, 249–265. ( 10.1016/j.cbpa.2007.05.024) [DOI] [PubMed] [Google Scholar]
- 95.Saïd S, et al. 2005. Ecological correlates of home-range size in spring–summer for female roe deer (Capreolus capreolus) in a deciduous woodland. J. Zool. 267, 301–308. ( 10.1017/s0952836905007454) [DOI] [Google Scholar]
- 96.Bjørneraas K, Herfindal I, Solberg EJ, Sæther B-E, van Moorter B, Rolandsen CM. 2012. Habitat quality influences population distribution, individual space use and functional responses in habitat selection by a large herbivore. Oecologia 168, 231–243. ( 10.1007/s00442-011-2072-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ford RG. 1983. Home range in a patchy environment: optimal foraging predictions. Am. Zool. 23, 315–326. ( 10.1093/icb/23.2.315.) [DOI] [Google Scholar]
- 98.Cederlund G, Sand H. 1994. Home-range size in relation to age and sex in moose. J. Mammal. 75, 1005–1012. ( 10.2307/1382483) [DOI] [Google Scholar]
- 99.Relyea RA, Lawrence RK, Demarais S. 2000. Home range of desert mule deer: testing the body-size and habitat-productivity hypotheses. J. Wildl. Manage. 64, 146–153. ( 10.2307/3802984) [DOI] [Google Scholar]
- 100.van Beest FM, McLoughlin PD, Mysterud A, Brook RK. 2015. Functional responses in habitat selection are density dependent in a large herbivore. Ecography 39, 515–523. ( 10.1111/ecog.01339) [DOI] [Google Scholar]
- 101.Bjørneraas K, et al. 2011. Moose Alces alces habitat use at multiple temporal scales in a human-altered landscape. Wildl. Biol. 17, 44–54. ( 10.2981/10-073) [DOI] [Google Scholar]
- 102.Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 103.Hardenberg A, Bassano B, Peracino A, Lovari S. 2000. Male alpine chamois occupy territories at hotspots before the mating season. Ethology 106, 617–630. ( 10.1046/j.1439-0310.2000.00579.x) [DOI] [Google Scholar]
- 104.Vanpé C, Morellet N, Kjellander P, Goulard M, Liberg O, Hewison AJM. 2009. Access to mates in a territorial ungulate is determined by the size of a male's territory, but not by its habitat quality. J. Anim. Ecol. 78, 42–51. ( 10.1111/j.1365-2656.2008.01467.x) [DOI] [PubMed] [Google Scholar]
- 105.Grant JWA, Chapman CA, Richardson KS. 1992. Defended versus undefended home range size of carnivores, ungulates and primates. Behav. Ecol. Sociobiol. 31, 149–161. ( 10.1007/BF00168642) [DOI] [Google Scholar]
- 106.Grøtan V, Sæther BE, Engen S, Solberg EJ, Linnell JDC, Andersen R, Broseth H, Lund E. 2005. Climate causes large-scale spatial synchrony in population fluctuations of a temperate herbivore. Ecology 86, 1472–1482. ( 10.1890/04-1502) [DOI] [Google Scholar]
- 107.Rybicki J, Hanski I. 2013. Species–area relationships and extinctions caused by habitat loss and fragmentation. Ecol. Lett. 16, 27–38. ( 10.1111/ele.12065) [DOI] [PubMed] [Google Scholar]
- 108.Shafer AB, Northrup JM, White KS, Boyce MS, Côté SD, Coltman DW. 2012. Habitat selection predicts genetic relatedness in an alpine ungulate. Ecology 93, 1317–1329. ( 10.1890/11-0815.1) [DOI] [PubMed] [Google Scholar]
- 109.Weckworth BV, Musiani M, DeCesare NJ, McDevitt AD, Hebblewhite M, Mariani S. 2013. Preferred habitat and effective population size drive landscape genetic patterns in an endangered species. Proc. R. Soc. B 280, 20131756 ( 10.1098/rspb.2013.1756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andrén H. 1994. Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71, 355–366. ( 10.2307/3545823) [DOI] [Google Scholar]
- 111.Debinski DM, Holt RD. 2000. A survey and overview of habitat fragmentation experiments. Conserv. Biol. 14, 342–355. ( 10.1046/j.1523-1739.2000.98081.x) [DOI] [Google Scholar]
- 112.Lindenmayer DB, Fischer J. 2013. Habitat fragmentation and landscape change: an ecological and conservation synthesis. Washington, DC: Island Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The studies included in the meta-analysis presented in figure 2 are listed in the electronic supplementary material, table S1.