Abstract
Insects and fungi have a long history of association in shared habitats. Fungus-feeding, or mycophagy, is remarkably widespread in beetles (Coleoptera) and appears to be a primitive feeding habit that preceded feeding on plant tissues. Numerous Mesozoic beetles belonging to extant fungus-associated families are known, but direct fossil evidence elucidating mycophagy in insects has remained elusive. Here, we report a remarkable genus and species, Vetuproteinus cretaceus gen. et sp. nov., belonging to a new tribe (Vetuproteinini trib. nov.) of the extant rove beetle subfamily Proteininae (Staphylinidae) in Mid-Cretaceous Burmese amber. The mouthparts of this beetle have a markedly enlarged protruding galea bearing an apparent spore brush, a specialized structure we infer was used to scrape spores off surfaces and direct them into the mouth, as in multiple modern spore-feeding beetles. Considering the long evolutionary history of Fungi, the Mid-Cretaceous beetles likely fed on ancient Basidiomycota and/or Ascomycota fungi or spore-producing organisms such as slime moulds (Myxomycetes). The discovery of the first Mesozoic proteinine illustrates the antiquity of the subfamily, and suggests that ancestral Proteininae were already diverse and widespread in Pangaea before the supercontinent broke up.
Keywords: Coleoptera, Staphylinidae, Proteininae, Cretaceous, Burmese amber, mycophagy
1. Introduction
Insects and fungi have a long history of association in shared habitats [1]. Among various types of insect–fungal associations, mycophagy, or fungus-feeding, is widespread among insects [2] and well known in fungus-growing attine ants and termites [3,4]. Mycophagy in modern beetles (Coleoptera), the most diverse insect group, is likewise widespread and takes different forms, including spore-grazing, mastication of fungal hyphae, and liquid-feeding via preoral digestion [5]. The occurrence of this feeding habit in basal clades of many lineages [5–7] suggests that consumption of fungi is a primitive feeding habit that preceded feeding on plant tissues. Although representatives of multiple fungus-associated beetle groups are known from the Mesozoic [8–11], direct fossil evidence elucidating mycophagy in beetles has been lacking.
The rove beetle subfamily Proteininae Erichson, with about 230 described species in 11 genera, is a relatively small group among the hyperdiverse Staphylinidae (more than 62 550 extant species; [12–14], AF Newton 13 December 2016, unpublished data). Proteinines are small (usually less than 3 mm long) beetles that are usually found in fungi, under bark, in decaying vegetation, and in forest leaf litter [13,14] throughout most of the world. To date, no fossil proteinines have been described or named, although Larsson [15] reported a ‘Proteininae g. sp.’ from the Late Eocene Baltic amber (ca. 33–37 Ma). This specimen (ZMUC 900081, in the Zoological Museum, Statens Naturhistoriske Museum, Copenhagen) was later further identified by one of us (AFN) as a species of Proteinus Latreille. Here, we describe a remarkable new tribe belonging to the Proteininae in Mid-Cretaceous amber (ca. 99 Ma) from northern Myanmar. The discovery represents the oldest record for the subfamily, shedding light on the biogeographic history of the group. More significantly, the fossil beetle bears a markedly enlarged protruding galea bearing an apparent spore brush, a specialized structure probably used to scrape spores off surfaces and direct them into the mouth. It is likely that these early insects fed on ancient Basidiomycota and/or Ascomycota fungi or other spore-producing organisms, such as slime moulds (Myxomycetes), which provide evidence of early insect–fungal associations.
2. Results
(a). Systematic palaeontology
(i). Insecta
Order Coleoptera Linnaeus, 1758
Family Staphylinidae Latreille, 1802
Subfamily Proteininae Erichson, 1839
Vetuproteinini Cai, Newton & Thayer, trib. nov.
(Type genus: Vetuproteinus gen. nov.)
Vetuproteinus Cai, Newton & Thayer, gen. nov.
(Type species: V. cretaceus sp. nov.)
Vetuproteinus cretaceus Cai, Newton & Thayer, sp. nov. (figures 1 and 2).
Figure 1.
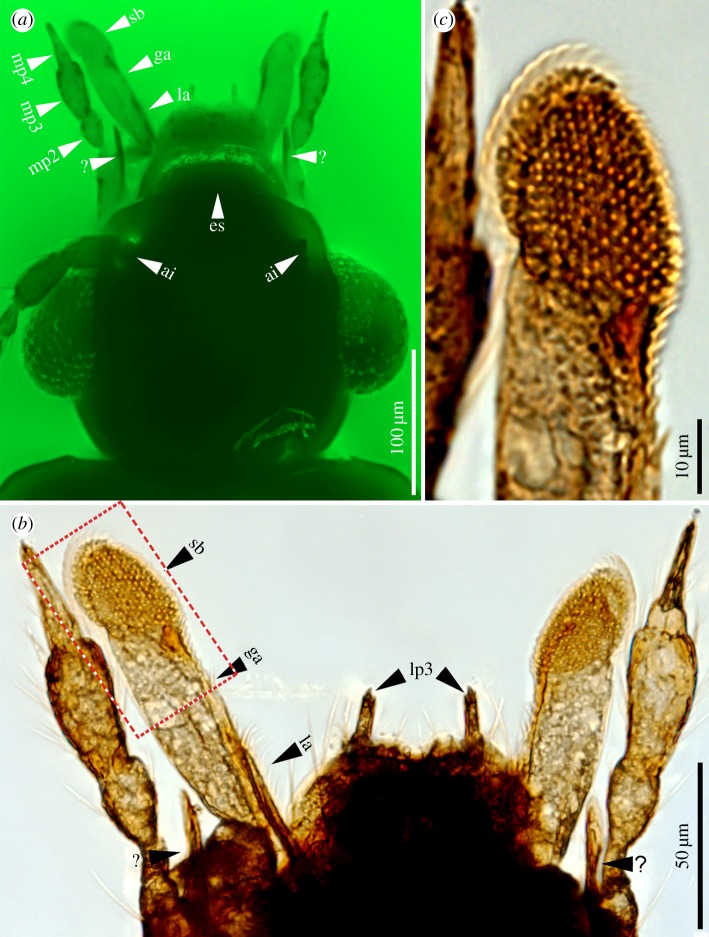
Vetuproteinus cretaceus gen. et sp. nov., holotype, NIGP164466. (a,b,g) Under normal transmitted light; others under green epifluorescence. (a) Dorsal view, (b) ventral view, (c) dorsal view. (d) Prothorax, ventral, showing the characteristic post-coxal process. (e) Left antenna. (f) Enlargement of left middle and hind legs, showing 4-segmented meso- and metatarsi. (g) Abdominal apex, dorsal, with internal sac of aedeagus everted. a8, antennomere 8; is, internal sac; lts, lateral tergal sclerite; mst, mesotarsomere; mtt, metatarsomere; pcp, post-coxal process; tIII–VIII, tergite III–VIII. (Online version in colour.)
Figure 2.
Details of specialized mouthparts of Vetuproteinus cretaceus gen. et sp. nov., holotype, NIGP164466. (a) Under green epifluorescence; others under normal transmitted light. (a) Enlargement of head, dorsal, showing details of mouthparts. (b) Enlargement of mouthparts, dorsal. (c) Enlargement of boxed area in (b), showing spore brush with apical rasp-like structure. ai, antennal insertion; es, epistomal suture; ga, galea; la, lacinia; lp, labial palpomere; mp, maxillary palpomere; sb, spore brush; ?, ‘spine-like process’. (Online version in colour.)
(ii). Diagnosis
Diagnosis of the tribe: Proteininae without distinct notch between eye and antennal insertion; antennal insertions exposed dorsally (autapomorphy); antennomeres 9–11 forming a slight loose club; maxillary palpomere 4 shorter than palpomere 3; apex of galea expanded and densely covered with short spinose projections, forming a spore brush (autapomorphy); spine-like processes present arising (possibly) from the stipes; pronotal hypomeron with large post-coxal process; mesospiracular peritreme not large and well sclerotized; tarsal formula ?-4-4.
Diagnosis of the genus and species: small, ovoid, slightly flattened Staphylinidae with: eyes distinctly protruding, coarsely faceted; epistomal suture present, straight, without median ‘stem’; notch between eye and antennal insertion evident only as a shallow impression; antennal insertions exposed dorsally; antennae long and setose, with 11 antennomeres, the last three forming a slight loose club; maxillary palpi with dilated palpomere 3 and acuminate palpomere 4; galea very long, apex with field of stiff projections forming an apparent spore brush; pronotum widest nearly at base, without sublateral carinae; elytra each with epipleural keel, relatively long, covering part of abdominal tergite III, exposing rest of abdomen; hind wings with staphylinid-type folding (hinge present in radial bar proximal to radial cell); protrochantin broadly exposed; pronotal hypomeron with large solid post-coxal process; mesospiracular peritreme not large and well sclerotized; pro- and metacoxae each contiguous, mesocoxae separated by about half of coxal width; femora weakly dilated, meso- and metatibiae very slender, protibiae slightly widened apicad; meso- and metatarsi 4-segmented (protarsi missing), with tarsomere 1 long, tarsomeres 1–3 successively shorter, tarsomere 4 elongate; metacoxal plate present, narrow, making metacoxae slightly excavate; abdominal segments III–VIII well-developed, at least III–VII with one pair of paratergites, tergites without basolateral ridges; abdominal spiracles not visible; abdominal intersegmental membranes apparently without microsclerites (synapomorphy with other Proteininae), moderately long (about one-third of tergal length between V and VI, intermediate between shorter membrane in other Proteininae and longer in related subfamilies); sternite VIII anteriorly with evidence of omaliine-type defensive gland structure [12].
(iii). Etymology
The genus-group name is a combination of Latin vetus, meaning ‘old’, and the genus Proteinus; it is masculine in gender. The species epithet is an adjective derived from Cretaceous, the age of the fossil.
(iv). Holotype, locality, and age
NIGP164466. Male. The type specimen is housed in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China. Preserved in Burmese amber (Burmite) from Hukawng Valley, Kachin State, northern Myanmar; earliest Cenomanian, absolute age 98.79 ± 0.62 Ma, established by uranium–lead (U–Pb) dating of zircons from associated matrix of the unprocessed amber [16].
(v). Description
Body (figure 1a–c) very small, length 1.26 mm; ovoid, moderately flattened, pubescent; brown throughout, elytra slightly darker.
Head: 0.26 mm long and 0.27 mm wide (including eyes), without lateral or dorsal neck constriction; glabrous; antennal insertions (figure 2a) exposed dorsally, located at or slightly anterior to anterior margins of eyes, notch between eye and antennal insertion merely a shallow impression. Epistomal suture present, straight. Eye (figure 2a) large, strongly protruding, positioned laterally, coarsely faceted. Antenna (figure 1e) 11-segmented, setose, extending slightly beyond posterior margin of pronotum; antennomeres 1 and 2 elongate, slightly dilated, wider and longer than antennomere 3, antennomeres 4 and 5 almost the same shape and size, antennomeres 6–8 narrower, antennomere 8 smaller than 7 and 9, antennomeres 9–11 slightly dilated, forming a slight loose club, antennomere 11 fusiform, 1.7 times as long as antennomere 10. Labrum (figure 2a) transverse, nearly twice as wide as long, anterior margin slightly emarginate. Mandibles not visible. Maxillary palpus (figure 2a) relatively long, 4-segmented, all palpomeres elongate, palpomere 1 small, slender; palpomere 2 conical, wider than 1, clavate; palpomere 3 wider and about 1.5 times as long as palpomere 2; palpomere 4 acuminate, apparently glabrous, slightly shorter than 3. Galea (figure 2a,b) very long and dilated, apex with an oblique and oval uniform array of stiff spine-like projections which form a rasp-like structure (figure 2c) resembling the spore brush of extant fungus-feeding beetles, bordered externally by a thin translucent curved plate; lacinia (figure 2a,b) much shorter than galea, reduced to a slender lobe at inner side of galea. Labial palpus very small, 3-segmented, all segments elongate, palpomere 2 slightly longer than 1, palpomere 3 elongate, acuminate. Mentum sub-trapezoidal; gular sutures widely separated (figure 1b).
Thorax: Pronotum transverse, 0.37 mm wide and 0.22 mm long. Pronotum in dorsal view widest nearly at base, anterior margin nearly straight, posterior margin slightly sinuate. Pronotum without sublateral carina. Prosternum (figure 1d) transverse, procoxae contiguous, with a small prosternal process. Pronotal hypomeron developed, subtriangularly produced inwards (figure 1d, pcp). Mesocoxae slightly separated, metacoxae contiguous. Mesoscutellum sub-triangular, wider than long. Elytra slightly elongate, 0.39 mm long and each 0.21 mm wide, partly covering abdominal tergite III; surface with dense and fine microsetae, without striae; hind margin curved. Hind wings present, folded beneath elytra but visible by translucence, folding pattern of staphylinid type with first major fold of each wing a 90° hinge proximal to the radial cell. Epipleural keel present. Elytral lateral margins almost parallel to each other. Legs long, slender, densely setose; protrochantins broadly exposed; procoxae conical, protrochanters very small, profemora clavate, protibiae elongate, protarsi not preserved; mesotibiae very slender, narrower than protibiae, mesotarsi (figure 1f) 4-segmented, not lobed, tarsomere 1 very slender, about 2.6 times as long as tarsomere 2, tarsomeres 1–3 gradually decreasing in length, tarsomere 4 elongate; metacoxae large, sub-triangular, with narrow oblique metacoxal plates, metafemora slightly larger than mesofemora, metatibiae very slender, metatarsi (figure 1f) 4-segmented, tarsomere 1 about 2.6 times as long as tarsomere 2, tarsomere 3 shortest, tarsomere 4 longer than tarsomere 1. Pretarsal claws simple, slender; empodial setae absent.
Abdomen: broad, gradually tapering to apex, densely setose. Tergite III covered by elytra (abdomen artefactually slightly separated from metathorax). Basolateral ridges absent. Spiracles not detected on tergites IV–VI but possibly present on tergite VII. Abdominal terga without wing-folding patches. Segments IV–VII each with one pair of paratergites. Sternite II carinate at midline. Sternite VIII with apparent omaliine-type defensive gland [12] visible by translucence, similar in size and elongate shape of median projection to that of Neophonus Fauvel [17]. Lateral tergal sclerites IX (figure 1g) sub-triangular, apparently fused to one another at base in front of tergite X, each with a slender digitiform lateral projection bearing a long seta, and apices with dense long setae; tergite X with rounded base, elongate. Entire intestinal tract not clearly visible, but hindgut partly visible and containing dark solid matter (figure 1a).
Male: Aedeagus with partly everted internal sac exposed (figure 1g), elongate, spines visible inside, median lobe and parameres not everted and not visible.
3. Discussion
(a). Systematic position of Vetuproteinus
With over 62 000 described species placed in one extinct and 32 extant subfamilies, the family Staphylinidae is the most diverse beetle family [18,19]. On the basis of morphology, the subfamilies of Staphylinidae have been organized into four informal groupings [20,21]: the Omaliine group, Tachyporine group, Oxyteline group, and Staphylinine group. In phylogenetic studies, however, some of these groups have been shown to be paraphyletic [22,23], and in the most recent morphological treatment, the subfamilies Apateticinae and Trigonurinae (previously treated as members of the Oxyteline group), were suggested to be the sister group of the remaining four major groups mentioned above [24]. We compared Vetuproteinus in detail with all major family-groups (electronic supplementary material, SI File 01), and by a process of elimination, attribute Vetuproteinus to the extant subfamily Proteininae by its general habitus, acuminate maxillary palpomere 4, the absence of paired ocelli, and relatively short abdominal intersegmental membranes without a pattern of minute sclerites (the last character being a synapomorphy for the subfamily [12]). A detailed comparison between Vetuproteinus and all extant proteinine genera (electronic supplementary material, SI File 01) indicates that the extinct genus has both probably plesiomorphic and highly derived features. It has a reduced post-antennal notch, larger post-coxal process and longer abdominal intersegmental membranes (but shorter than in related subfamilies), which are probably symplesiomorphies within Proteininae [12]. Vetuproteinus also has exposed antennal insertions and a specialized galeal spore brush, both features unique in Proteininae and unusual in the Omaliine group. The unique character combination of Vetuproteinus justifies the proposal of a new tribe, Vetuproteinini trib. nov., for Vetuproteinus.
(b). Specialized morphological trait and insect–fungal association
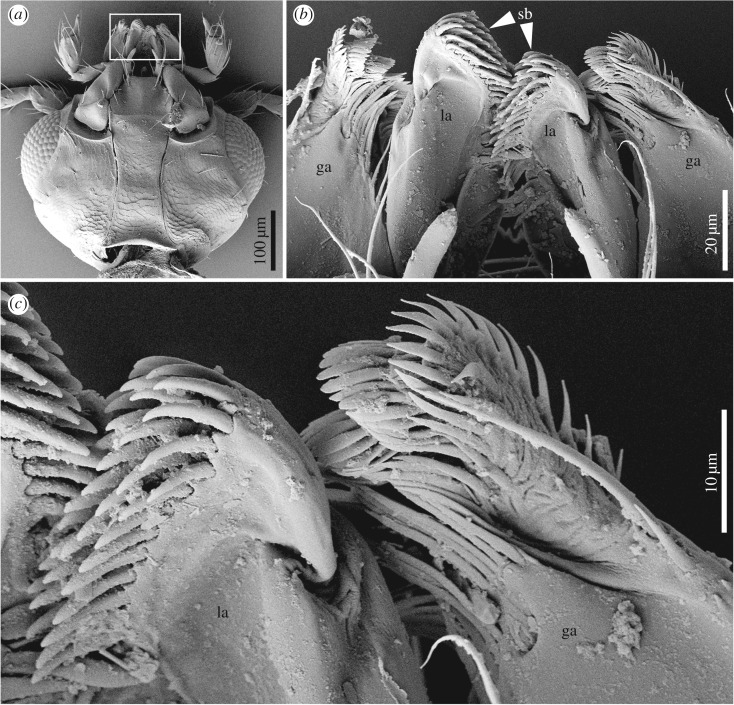
The most intriguing feature of Vetuproteinus is the rasp-like structure or spore brush on the galea. Closely similar structures occurring in some other staphylinids and other beetles are used to scrape spores or other microparticulate matter off surfaces and direct them into the mouth [6,25–27]. Among Staphylinoidea, such structures occur on the maxillae of Dasycerus Brongniart (Dasycerinae), many Gyrophaenina (Aleocharinae) and Agyrtodini (Leiodidae) adults where they are used to scrape spores off the spore-bearing surface (hymenium) of polypore fungi, although in those groups the brush is on the lacinia rather than galea (figure 3) [6,25,28]. In particular, all gyrophaenine rove beetles are obligatory inhabitants of fresh fruiting bodies of gilled and polypore mushrooms, and both adults and larvae feed exclusively by ‘grazing’ on the spore-producing layer [25]. The principal structural adaptations of gyrophaenines to mushrooms involve modifications of the mouthparts. The maxilla (figure 3a) appears to be the main feeding structure and is highly modified. The lacinial spore brush (figure 3b) is modified for scraping the hymenial surface of fresh mushrooms and the galeal setae (figure 3b) form a cap over the apex of the spore brush, possibly preventing loss of material removed from the hymenium [25]. The mandibles are inconspicuous or hidden in Gyrophaenina (figure 3a), as in Vetuproteinus. In Neophonus (Neophoninae) adults, a similar brush, also on the lacinia but with more specialized curved setae, appears to be used for sweeping leaf surfaces for microparticulate matter including fungal spores [17]. Similar spore-scraping structures are found in some fungus-associated larvae, e.g. on the mala (fused galea + lacinia) of many Gyrophaenina [25] and Sepedophilus Gistel (Tachyporinae) [29] or even on the mandibles of Scaphisoma Leach and Baeocera Erichson (Scaphidiinae) and Dasycerus [30]. The presence of a probably functionally analogous structure on the galea of Vetuproteinus strongly suggests that this genus had similar habits and ingested fungal spores or similar microparticulate matter; the presence of what looks like unconsolidated loose matter compacted within the hindgut (figure 1a) is similar to what is seen in typical gut dissections of spore-feeders [6,26] and is consistent with mycophagy. Newton [6] and Newton & Thayer [12] noted that adults and larvae of modern Proteininae (except the carnivorous Anepiini) are often associated with decaying fungi and are probably mycophagous or saprophagous, although they lack evident spore brushes and are usually associated with decaying soft fungi rather than sporulating polypore fungi or mushrooms. The varied morphological locations of the spore brushes mentioned above and the absence of such brushes in most close relatives of these taxa substantiate the multiple independent origins of such structures in these groups, and this is certainly the case in the uniquely placed galeal brush of Vetuproteinus. At least within Staphylinoidea, this is the first inference of such a galeal feeding mechanism based on mouthpart morphology and preservation of material in the hindgut. Further study of fossil Dasycerinae is needed to see whether they share it with their extant relatives, as Yamamoto [31] did not mention any corresponding mouthpart character for the Cretaceous Protodasycerus Yamamoto (and the very dark colour of that specimen precludes seeing whether it has solid gut contents, such as fungal spores or hyphae). The fungal fossil record, though patchy, is consistent with the possibility of Vetuproteinus feeding on ancient Basidiomycota (the group including polypores, mushrooms, and other taxa) or Ascomycota (including the hyperdiverse Ascomycetes), since both of those sister taxa have been found in Cretaceous deposits [32–34], including Burmese as well as New Jersey amber. Although the structure of the spore brush in Vetuproteinus most closely resembles those of extant staphylinoids known to feed on polypore fungi (Basidiomycota), such as Dasycerus and some Gyrophaenina and Agyrtodini as mentioned above, the possibility remains that Vetuproteinus fed on some other spore-producing organisms such as Myxomycetes, which are probably more ancient than true fungi but lack fossils [35], or it may have been a generalized saprotroph. Nevertheless, the specialized mouthparts of Vetuproteinus suggest an early association between insects and spore-producing organisms such as Ascomycota, Basidiomycota, or Myxomycetes, an association that was probably well established before the Mid-Cretaceous.
Figure 3.
Scanning electron micrographs (SEMs) of mouthparts of an extant representative of Gyrophaenina (Staphylinidae: Aleocharinae). (a) Ventral view of head. (b) Enlargement of boxed area of (a), showing specialized galeae and laciniae. (c) Details of spore brush at apex of lacinia. ga, galea; la, lacinia; sb, spore brush.
Another mouthpart feature deserving attention is the unknown maxillary structure that we call a spine-like process, which we think arises from the stipes. This could be either a true spine arising from the stipes and associated with the specialized spore-feeding mechanism, or it could be part of a sclerotized rim within the stipes itself and appearing as a spine as an artefact of preservation. It may also arise from another location, not the stipes. Additional specimens are required to determine the exact nature of this structure.
(c). Evolutionary and biogeographic implications
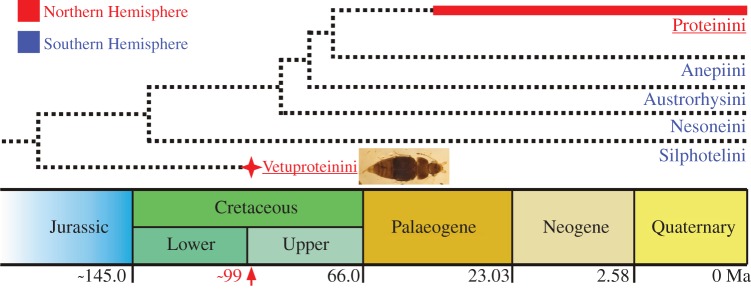
The discovery of Vetuproteinus cretaceus from Mid-Cretaceous Burmese amber pushes the fossil record of the subfamily Proteininae back in time significantly, and has several phylogenetic and evolutionary implications. The Burmite fossil provides direct evidence that the modern subfamily Proteininae and its sister group have minimum ages of about 99 Myr. This is consistent with recently described fossils of Glypholomatinae (sister group of all other Omaliine-group subfamilies together [14]) and Omaliinae (with Microsilphinae forming a sister group to most other Omaliine-group subfamilies, including Proteininae) from much older Middle Jurassic deposits of China (Daohugou Beds, 165 Ma, [10,36]) and the Late Jurassic of New South Wales, Australia (Talbragar Fish Beds, [37]). In addition, definite and diverse rove beetles belonging to later-branching Omaliine-group subfamilies other than Proteininae, such as Dasycerinae and Pselaphinae, have also been found recently in Mid-Cretaceous Burmese amber [31,38]. It is therefore possible that Proteininae had already originated well before 99 Ma (figure 4).
Figure 4.
Phylogeny of Proteininae, modified from [12]. Red star indicates the extinct tribe and red arrow its temporal occurrence; thick red bar shows the currently known time range of Proteinini. Red underlined font indicates groups occurring almost entirely in the Northern Hemisphere; blue font indicates groups occurring in the Southern Hemisphere. Geological scale after an updated version of the International Commission on Stratigraphy (ICS) International Chronostratigraphic Chart [39]. (Online version in colour.)
The discovery of Vetuproteinini revises inferences of the biogeographic history of Proteininae. Four of the five extant proteinine tribes—Anepiini Steel, Austrorhysini Newton and Thayer, Nesoneini Steel, and Silphotelini Newton and Thayer—are restricted to temperate areas of the Southern Hemisphere, while Proteinini occurs in the Northern Hemisphere, tropics, and some southern temperate areas [13] (figure 4). According to the phylogenetic analyses of Newton & Thayer [12] ([Silphotelini (Nesoneini (Austrorhysini (Anepiini + Proteinini)))], figure 4) and McKenna et al. [23] ([Anepiini ((Austrorhysini + Nesoneini) (Silphotelini, Proteinini)) and Silphotelini ((Nesoneini + Austrorhysini), (Proteinini, Anepiini)) in two different analyses]), Proteinini is always nested among the extant austral clades. Members of Proteinini occur virtually worldwide except where the other tribes occur, with only a few species of Megarthrus occurring in parts of the former Gondwana (South Africa and New Caledonia). Based only on those distributions, the subfamily would have had a Gondwanan origin, with the occurrence of Proteinini in the northern continents resulting either from isolation on Laurasia after the ancient Pangaean break-up (and after multiple splits within proto-Gondwana) or a much later dispersal from former Gondwana into former Laurasia, followed by diversification there. The Mid-Cretaceous occurrence of Vetuproteinini in present southeastern Asia (part of Laurasia) and its status as the putative sister of all extant Proteininae tribes—the more basal four of which are known only from austral areas—support the idea that ancestral Proteininae were already diverse and widespread in Pangaea before its fragmentation into northern and southern continents. The current distribution of Proteinini and its allopatry with its several successive sister tribes suggest that Proteinini also arose in the context of Pangaean break-up. On the basis of morphological disparity among the tribes, it seems that there has been significant extinction during the evolution of Proteininae, and the distributions of its subtaxa may also reflect this.
4. Material and methods
The Burmese amber specimen described here originated from the Hukawng Valley in Tanaing Township, Myitkyina District of Kachin State, Myanmar. It was prepared using a razor blade, polished with emery papers with different grit sizes and finally polished with diatomite mud. The specimen was then mounted between two microscopic coverslips with Canada balsam as a mounting medium. Observations and photographs were taken using a Zeiss Discovery V20 stereo microscope and a Zeiss Axio Imager 2 light microscope with a digital camera attached. Photomicrographs with green background (figures 1c–f, 2a) were taken using green epifluorescence as the light source attached to a Zeiss Axio Imager 2 light microscope. Focus stacking software (Helicon Focus 3.10) was used to increase depth of field. Photomicrographs of mouthparts of extant Gyrophaenina (figure 3) were taken with a LEO1530VP scanning electron microscope after preparations (dehydration and gold-coating). All images were processed using Adobe Photoshop™.
Supplementary Material
Acknowledgements
We thank Prof. Dany Azar (Lebanese University) for preparing the amber specimen here studied. We also thank C. Labandeira and two anonymous reviewers for their helpful comments.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material, SI File 01.
Authors' contributions
C.C., A.F.N, M.K.T., and D.H. conceived the study, acquired and analysed data. C.C. processed the photomicrograph data. C.C., A.F.N., M.K.T., and R.A.B.L. interpreted data and drafted the manuscript. All authors commented on the manuscript and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The work has been supported by National Basic Research Program of China (2012CB821903), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB18030501), the Ministry of Science and Technology (2016YFC0600406), the National Natural Science Foundation of China (41602009 and 91514302), and the National Natural Science Foundation of Jiangsu Province (BK20161091). R.A.B.L. was funded in part by core funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment's Science and Innovation Group.
References
- 1.Vega FE, Blackwell M. 2005. Insect–fungal associations: ecology and evolution, p. 333 New York, NY: Oxford University Press. [Google Scholar]
- 2.Hammond PM, Lawrence JF. 1989. Mycophagy in insects: a summary. In Insect–fungus interactions. 14th Symp. of the Royal Entomological Society of London (eds N Wilding, NM Collins, PM Hammond, JF Webber), pp. 275–324. London, UK: Academic Press.
- 3.Mueller UG, Rehner SA, Schultz TR. 1998. The evolution of agriculture in ants. Science 281, 2034–2038. ( 10.1126/science.281.5385.2034) [DOI] [PubMed] [Google Scholar]
- 4.Aanen DK, Eggleton P, Rouland-Lefevre C, Guldberg-Frøslev T, Rosendahl S, Boomsma JJ. 2002. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc. Natl Acad. Sci. USA 99, 14 887–14 892. ( 10.1073/pnas.222313099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence JF. 1989. Mycophagy in the Coleoptera: feeding strategies and morphological adaptations. In Insect–Fungus Interactions. 14th Symp. of the Royal Entomological Society of London in collaboration with the British Mycological Society (eds N Wilding, NM Collins, PM Hammond, JF Webber), pp. 1–23. London, UK: Academic Press.
- 6.Newton AF., Jr 1984. Mycophagy in Staphylinoidea (Coleoptera). In Fungus–insect relationships: perspectives in ecology and evolution (eds Wheeler Q, Blackwell M), pp. 302–353. New York, NY: Columbia University Press. [Google Scholar]
- 7.Leschen RAB, Buckley TR. 2007. Multistate characters and diet shifts: evolution of Erotylidae (Coleoptera). Syst. Biol. 56, 97–112. ( 10.1080/10635150701211844) [DOI] [PubMed] [Google Scholar]
- 8.Cai C, Hsiao Y, Huang D. 2016. A new genus and species of polypore fungus beetle in Upper Cretaceous Burmese amber (Coleoptera, Tetratomidae, Eustrophinae). Cretaceous Res. 60, 275–280. ( 10.1016/j.cretres.2015.12.010) [DOI] [Google Scholar]
- 9.Cai C, Huang D. 2014. Diverse oxyporine rove beetles from the Early Cretaceous of China (Coleoptera: Staphylinidae). Syst. Entomol. 39, 500–505. ( 10.1111/syen.12069) [DOI] [Google Scholar]
- 10.Cai C, Huang D, Thayer MK, Newton AF. 2012. Glypholomatine rove beetles (Coleoptera: Staphylinidae): a Southern Hemisphere recent group recorded from the Middle Jurassic of China. J. Kansas Entomol. Soc. 85, 239–244. ( 10.2317/JKES120531.1) [DOI] [Google Scholar]
- 11.Yue Y, Ren D, Solodovnikov A. 2011. The oldest fossil species of the rove beetle subfamily Oxyporinae (Coleoptera: Staphylinidae) from the Early Cretaceous (Yixian Formation, China) and its phylogenetic significance. J. Syst. Palaeontol. 9, 467–471. ( 10.1080/14772019.2010.493049) [DOI] [Google Scholar]
- 12.Newton AF Jr, Thayer MK. 1995. Protopselaphinae new subfamily for Protopselaphus new genus from Malaysia, with a phylogenetic analysis and review of the Omaliine Group of Staphylinidae including Pselaphidae. In Biology, phylogeny, and classification of Coleoptera: Papers celebrating the 80th birthday of Roy A. Crowson, vol. 2 (eds Pakaluk J, Ślipiński SA), pp. 219–320. Warszawa, Poland: Muzeum i Instytut Zoologii PAN. [Google Scholar]
- 13.Newton AF, Thayer MK, Ashe JS, Chandler DS. 2000. Staphylinidae Latreille, 1802. In American beetles, vol. 1 (eds Arnett RH, Thomas MC), pp. 272–418. Boca Raton, FL: CRC Press. [Google Scholar]
- 14.Thayer MK. 2016. Staphylinidae Latreille, 1802. In Coleoptera, Beetles. Vol. 1: Morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). 2nd edition. Handbook of Zoology; Arthropoda: Insecta (eds Beutel RG, Leschen RAB), pp. 394–442. Berlin, Germany: De Gruyter. [Google Scholar]
- 15.Larsson SG. 1978. Baltic amber: a palaeobiological study. Entomonograph 1, p. 192. Klampenborg, Denmark: Scandinavian Science Press Ltd. [Google Scholar]
- 16.Shi G, Grimaldi DA, Harlow GE, Wang J, Wang J, Yang M, Lei W, Li Q, Li X. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Res. 37, 155–163. ( 10.1016/j.cretres.2012.03.014) [DOI] [Google Scholar]
- 17.Thayer MK. 1987. Biology and phylogenetic relationships of Neophonus bruchi, an anomalous south Andean staphylinid (Coleoptera). Syst. Entomol. 12, 389–404. ( 10.1111/j.1365-3113.1987.tb00209.x) [DOI] [Google Scholar]
- 18.Bouchard P, et al. 2011. Family-group names in Coleoptera (Insecta). ZooKeys 88, 1–972. ( 10.3897/zookeys.88.807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman LH. 2001. Catalog of the Staphylinidae (Insecta: Coleoptera). 1758 to the end of the second millennium, Parts I–VII. Bull. Am. Mus. Nat. Hist. 265, 1–4218 (in 7 vols.) ( 10.1206/0003-0090(2001)264%3C0003:NCITSI%3E2.0.CO;2) [DOI] [Google Scholar]
- 20.Lawrence JF, Newton AF. 1982. Evolution and classification of beetles. Annu. Rev. Ecol. Syst. 13, 261–290. ( 10.1146/annurev.es.13.110182.001401) [DOI] [Google Scholar]
- 21.Lawrence JF, Newton AF Jr. 1995. Families and subfamilies of Coleoptera (with selected genera, notes, references and data on family-group names). In Biology, phylogeny and classification of Coleoptera: papers celebrating the 80th birthday of Roy A Crowson, vol 2 (eds Pakaluk J, Ślipiński SA), pp. 779–1006. Warszawa, Poland: Muzeum i Instytut Zoologii PAN. [Google Scholar]
- 22.Hansen M. 1997. Phylogeny and classification of the staphyliniform beetle families (Coleoptera). Biologiske Skrifter, Det Kongelige Danske Videnskabernes Selskab 48, 1–339. [Google Scholar]
- 23.McKenna DD, et al. 2015. Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: forest litter as a stepping stone for diversification of nonphytophagous beetles. Syst. Entomol. 40, 35–60. ( 10.1111/syen.12093) [DOI] [Google Scholar]
- 24.Grebennikov VV, Newton AF. 2012. Detecting the basal dichotomies in the monophylum of carrion and rove beetles (Insecta: Coleoptera: Silphidae and Staphylinidae) with emphasis on the Oxyteline group of subfamilies. Arthropod Syst. Phylo. 70, 133–165. [Google Scholar]
- 25.Ashe JS. 1984. Major features of the evolution of relationships between gyrophaenine staphylinid beetles (Coleoptera: Staphylinidae: Aleocharinae) and fresh mushrooms. In Fungus–insect relationships: perspectives in ecology and evolution (eds Wheeler Q, Blackwell M), pp. 227–255. New York, NY: Columbia University Press. [Google Scholar]
- 26.Leschen RAB. 1993. Evolutionary patterns of feeding in selected Staphylinoidea (Coleoptera): shifts among food textures. In Functional Morphology of Insect Feeding. (eds Schaefer CW, Leschen RAB), pp. 59–104. Lanham, USA: Thomas Say Publications, ESA. [Google Scholar]
- 27.Betz O, Thayer MK, Newton AF. 2003. Comparative morphology and evolutionary pathways of the mouthparts in spore-feeding Staphylinoidea (Coleoptera). Acta Zool. 84, 179–238. ( 10.1046/j.1463-6395.2003.00147.x) [DOI] [Google Scholar]
- 28.Seago AE. 2009. Revision of Agyrtodes Portevin (Coleoptera: Leiodidae). Coleopt. Soc. Monogr. 7, 1–73. ( 10.1649/0010-065X-63.sp7.1) [DOI] [Google Scholar]
- 29.Leschen RAB, Beutel RG. 2001. Pseudotracheal tubes, larval head, and mycophagy in Sepedophilus (Coleoptera: Staphylinidae: Tachyporinae). J. Zool. Syst. Evol. Res. 39, 25–36. ( 10.1046/j.1439-0469.2001.00149.x) [DOI] [Google Scholar]
- 30.Newton AF., Jr 1991. Dasyceridae; Scaphidiidae (Staphylinoidea). In Immature Insects, vol. 2 (ed Stehr FW.), pp. 335–339. Dubuque, IA: Kendall/Hunt Publishing Co. [Google Scholar]
- 31.Yamamoto S. 2016. The first fossil of dasycerine rove beetle (Coleoptera: Staphylinidae) from Upper Cretaceous Burmese amber: phylogenetic implications for the omaliine group subfamilies. Cretaceous Res. 58, 63–68. ( 10.1016/j.cretres.2015.09.022) [DOI] [Google Scholar]
- 32.Berbee ML, Taylor JW. 2010. Dating the molecular clock in fungi – how close are we? Fungal Biol. Rev. 24, 1–16. ( 10.1016/j.fbr.2010.03.001) [DOI] [Google Scholar]
- 33.Kiecksee AP, Seyfullah LJ, Dorfelt H, Heinrichs J, Suss H, Schmidt AR. 2012. Pre-Cretaceous Agaricomycetes yet to be discovered: reinvestigation of a putative Triassic bracket fungus from southern Germany. Fossil Rec. 15, 85–89. ( 10.1002/mmng.201200006) [DOI] [Google Scholar]
- 34.Schmidt AR, Dorfelt H, Struwe S, Perrichot V. 2010. Evidence for fungivory in Cretaceous amber forests from Gondwana and Laurasia. Palaeontographica Abteilung B 283, 157–173. [Google Scholar]
- 35.Stephenson SL, Schnittler M, Novozhilov YK. 2008. Myxomycete diversity and distribution from the fossil record to the present. Biodivers. Conserv. 17, 285–301. ( 10.1007/s10531-007-9252-9) [DOI] [Google Scholar]
- 36.Cai C-Y, Huang D-Y. 2013. Sinanthobium daohugouense, a tiny new omaliine rove beetle (Coleoptera: Staphylinidae) from the Middle Jurassic of China. Can. Entomol. 145, 496–500. ( 10.4039/tce.2013.33) [DOI] [Google Scholar]
- 37.Cai C, Yan EV, Beattie R, Wang B, Huang D. 2013. First rove beetles from the Jurassic Talbragar Fish Bed of Australia (Coleoptera, Staphylinidae). J. Paleontol. 87, 650–656. ( 10.1666/12-136) [DOI] [Google Scholar]
- 38.Parker J. 2016. Emergence of a superradiation: Pselaphine rove beetles in mid-Cretaceous amber from Myanmar and their evolutionary implications. Syst. Entomol. 41, 541–566. ( 10.1111/syen.12173) [DOI] [Google Scholar]
- 39.Cohen KM, Finney SC, Gibbard PL, Fan J-X. 2013. (updated) The ICS International Chronostratigraphic Chart. Episodes 36, 199–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material, SI File 01.