Abstract
A large brain can offer several cognitive advantages. However, brain tissue has an especially high metabolic rate. Thus, evolving an enlarged brain requires either a decrease in other energetic requirements, or an increase in overall energy consumption. Previous studies have found conflicting evidence for these hypotheses, leaving the metabolic costs and constraints in the evolution of increased encephalization unclear. Mormyrid electric fishes have extreme encephalization comparable to that of primates. Here, we show that brain size varies widely among mormyrid species, and that there is little evidence for a trade-off with organ size, but instead a correlation between brain size and resting oxygen consumption rate. Additionally, we show that increased brain size correlates with decreased hypoxia tolerance. Our data thus provide a non-mammalian example of extreme encephalization that is accommodated by an increase in overall energy consumption. Previous studies have found energetic trade-offs with variation in brain size in taxa that have not experienced extreme encephalization comparable with that of primates and mormyrids. Therefore, we suggest that energetic trade-offs can only explain the evolution of moderate increases in brain size, and that the energetic requirements of extreme encephalization may necessitate increased overall energy investment.
Keywords: brain size, brain evolution, mormyrids, energetic trade-off
1. Introduction
Larger brains are generally associated with an increase in cognitive abilities [1–3]. Brain tissue is metabolically expensive, raising questions about the energetic cost of increased encephalization [4]. Two prominent, non-exclusive hypotheses have addressed evolutionary mechanisms for accommodating the energetic cost of increasing brain size. The direct metabolic constraints hypothesis predicts an increase in total basal metabolic rate (BMR) to pay for the energetic cost of a larger brain [5], whereas the energetic trade-off hypothesis predicts that the energetic cost of a large brain is met by reducing energy allocation to other expensive organs or functions [6,7]. Some studies in mammals have found evidence in support of the direct metabolic constraints hypothesis [5,8–10], but other studies have found trade-offs between gut size and brain size in primates [6], anurans [11] and different lineages of fish [2,12], and between locomotor costs and brain mass in birds [13].
However, many of these studies did not focus on extreme encephalization, which may entail different costs and arise through different mechanisms compared with more moderate variation in brain size. Extreme encephalization, where brain size greatly deviates from a lineage's allometric relationship between brain and body mass, is rare [14]. In studies of highly encephalized primates, both hypotheses are hotly debated [10], with some studies favouring the direct metabolic constraints hypothesis [5,9], and others favouring the energetic trade-off hypothesis [6,15]. Further, due to a lack of comparative studies of extreme encephalization in non-primate lineages, the generality of these hypotheses remains unclear.
To study general patterns of energetic costs related to extreme encephalization, we studied mormyrid electric fishes from Africa, which present an excellent system for studying the costs of extreme encephalization [16]. One species, Gnathonemus petersii, has a brain that constitutes approximately 3% of its body mass, comparable with human brains at 2–2.5% [12,17]. Further, there are more than 200 mormyrid species [18,19]. Anecdotal evidence suggests that other mormyrid species have large brains [20,21], but it is unclear how brain size varies across the family. It is also unclear how variation in brain size relates to metabolic demand. Metabolic rate can be determined by measuring the rate of oxygen consumption over time. Metabolic demand can also be assessed by measuring sensitivity to changes in environmental energy availability [22–25]. In aquatic environments, oxygen concentration can vary greatly throughout time and space [26,27], and this can impose limits on metabolic activity [17,28]. In this study, we measured brain size variation among 30 mormyrid species and four outgroup species. We compared brain size variation with the sizes of other organs, resting oxygen consumption and sensitivity to decreases in ambient oxygen (hypoxia).
2. Results
(a). Relative brain size varies widely among mormyrids
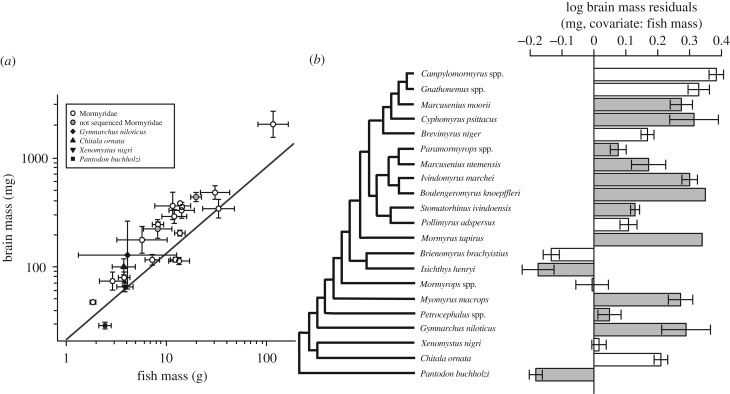
A linear model that incorporates Brownian evolution best fitted the variation in brain mass against body mass among lineages (AICBrownian = −16.27, AICOU = −14.41; electronic supplementary material, tables S1 and S2). We incorporated this model into a phylogenetic generalized least-squares (PGLS) analysis of the relationship between brain size and body size, which revealed a negative allometric pattern across lineages (y = axb, a = 21.53, b = 0.79, p < 10−7; figure 1a; electronic supplementary material, table S1). To obtain a measure of relative brain size corrected for this scaling with body size, we calculated brain mass residuals from this regression. Phylogenetic relatedness shifted the y-intercept of the regression, resulting in more positive brain size residuals than negative (SM2); however, these residuals were normally distributed (Shapiro–Wilk normality test: p = 0.14). Relative brain size varied widely among mormyrid lineages (figure 1b).
Figure 1.
Osteoglossomorph fishes display wide variation in relative brain size among lineages. (a) A Brownian PGLS regression of lineage-averaged brain mass against lineage-averaged fish mass shows a negative allometric relationship. Points show the mean ± s.e.m. of brain mass residuals. Grey circles are mormyrid lineages that do not have sequence data and are not included in the PGLS. (b) Residuals of log brain mass were determined from the PGLS regression of log brain mass versus log body mass (a) for each specimen. Bars show the mean ± s.e.m. of brain mass residuals. White bars indicate lineages used in respirometry and hypoxia experiments. Cladogram is based on consensus trees from Sullivan et al. [18] (12S, 16S, cytochrome b, and RAG2 sequences) and Lavoué et al. [29] (12S, 16S, cytochrome b, and RAG2).
(b). Relative brain size does not correlate linearly with the relative sizes of other organs
A linear Ornstein–Uhlenbeck (OU) evolution model best fitted the variation in gonad mass against body mass (electronic supplementary material, tables S1 and S2). For all other organs, a linear Brownian model was the best fit (electronic supplementary material, tables S1 and S2). For each organ, we incorporated the best-fit model into a PGLS analysis of the relationship between organ size and body size, which revealed the allometric scaling of each organ (y = axb, a = 3.28–19.82, b = 0.78–1.03, p < 10−2–10−18; electronic supplementary material, table S1).
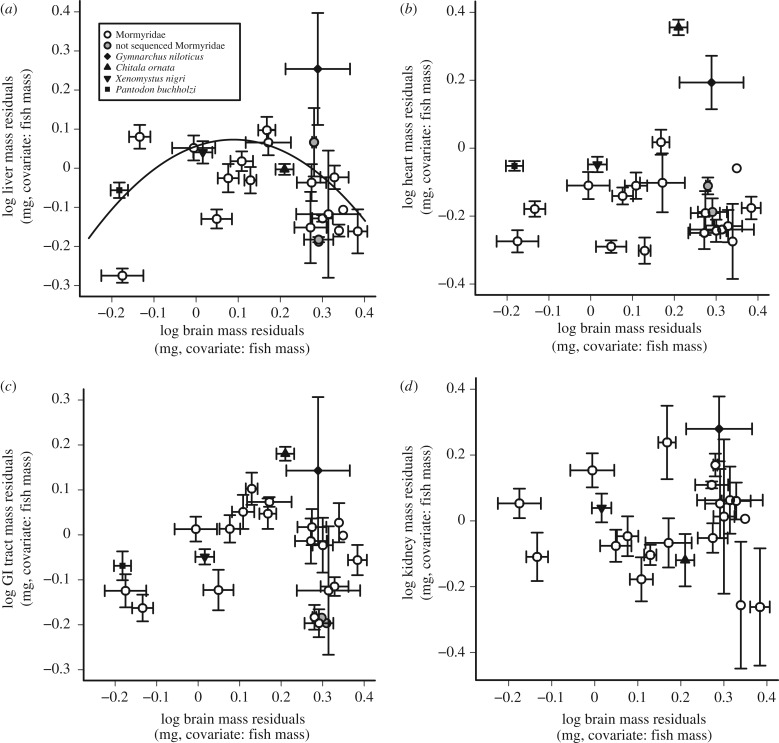
To obtain measures of relative organ size corrected for scaling with body size, we calculated organ mass residuals from the best-fit PGLS linear regression (Brownian or OU) for each organ. We then tested for correlations between relative brain size and the relative sizes of all other organs. There were no linear correlations between the relative sizes of the brain and other organs using either Brownian or OU models (PGLS: p = 0.10–0.84; figure 2; electronic supplementary material, table S3). There was, however, a weak, nonlinear relationship between relative liver size and relative brain size, and this was best fit by an OU model (y = ax2 + bx + c, a = −2.09, b = 0.38, c = 0.06, p < 0.05; figure 2a; electronic supplementary material, table S3).
Figure 2.
Relative brain size does not correlate linearly with the relative sizes of other organs. (a–d) Plots of the lineage-averaged residuals from each log organ mass versus log body mass against the lineage-averaged residuals from log brain mass versus log body mass. All residuals are taken from a Brownian PGLS of organ mass versus body mass (electronic supplementary material, table S1). Grey circles are mormyrid lineages that do not have sequence data and are not included in the PGLS regression. There is a significant OU PGLS quadratic relationship between lineage-averaged liver and brain residuals (a, black line).
(c). Relative brain size correlates with relative oxygen consumption
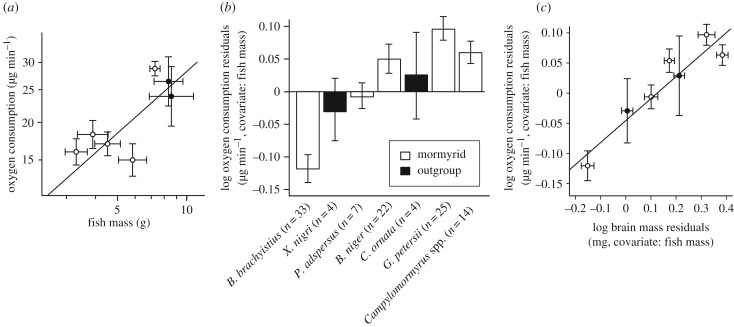
A linear model that incorporates Brownian evolution best fitted the variation in oxygen consumption rate against body mass among lineages (AICBrownian = −8.46, AICOU = −7.31; electronic supplementary material, table S1). In a PGLS analysis, oxygen consumption had a negative allometric relationship with body size (y = axb, a = 7.03, b = 0.60, p < 0.05; figure 3a; electronic supplementary material, figure S1). To obtain a measure of relative oxygen consumption rate corrected for scaling with body size, we calculated oxygen consumption residuals from this regression. An OU model best fitted the variation in oxygen consumption residuals versus brain size residuals (AICBrownian = −11.73, AICOU = −13.64; electronic supplementary material, table S3), and there was a significant linear correlation between relative oxygen consumption rates and relative brain size (PGLS: slope = 0.37, intercept = −0.04, p < 0.01; figure 3b,c; electronic supplementary material, table S3).
Figure 3.
Relative brain size correlates positively with relative oxygen consumption. (a) Lineage-averaged oxygen consumption against lineage-averaged fish mass shows a negative allometric relationship using Brownian PGLS. Mormyrid genera are shown in white, outgroup genera in black. A plot of log oxygen consumption versus log body mass for all individual specimens across lineages reveals a more continuous distribution than the means and standard errors between lineages suggest (electronic supplementary material, figure S1). (b) Residuals from the Brownian PGLS log oxygen consumption versus log body mass regression show the mean ± s.e.m. of relative oxygen consumption within genera. Lineages are arranged left to right from small to large relative brain mass. (c) Lineage-averaged residuals from log oxygen consumption versus log body mass against lineage-averaged residuals from log brain mass versus log body mass (error bars = s.e.m.) show a positive correlation using OU PGLS. Oxygen consumption and brain residuals are from Brownian PGLS analysis (electronic supplementary material, table S1).
(d). Large-brain mormyrids have relatively low hypoxia tolerance
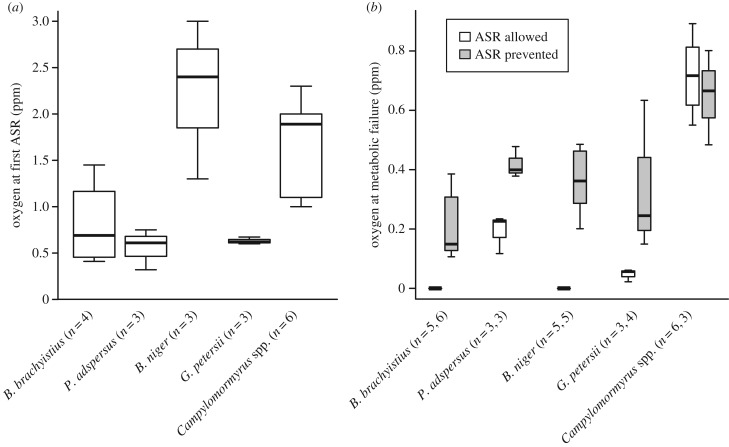
We performed two progressive hypoxia experiments [26]: one where aquatic surface respiration (ASR) was allowed and one where it was prevented. All fish performed ASR. Brevimyrus niger surfaced at a higher oxygen concentration than other species and surfaced repeatedly, whereas other species stayed at the surface. Oxygen concentration at first ASR was not related to brain size (ANOVA: F1,17 = 3.37, p = 0.08; figure 4a).
Figure 4.
Small-brained lineages are more hypoxia tolerant than large-brained lineages. Lineages are arranged left to right from small to large relative brain mass. (a) Box plot of the oxygen concentration at which fish first came to the surface for ASR. (b) Box plot of oxygen concentration at which fish experienced metabolic failure. White bars indicate the hypoxia experiment in which ASR was allowed, and grey bars indicate the experiment in which ASR was prevented. Sample sizes are different between panels (a) and (b) due to the camera malfunctioning during one video for B. brachyistius, and the high activity level for two B. niger made it unclear when ASR started.
Different genera experienced metabolic failure, defined here as losing the ability to remain upright, generate electric organ discharges (EODs) and swim, at different oxygen concentrations (two-way ANOVA: ASR allowed versus prevented: F1,1 = 2.32, p = 0.14; genus: F1,1 = 33.43, p < 1 × 10−5; interaction: F1,36 = 3.74, p = 0.06; figure 4b). When ASR was allowed, two species, B. brachyistius and B. niger, did not experience metabolic failure, even when oxygen concentrations were held at 0 ppm for 10 min. When ASR was prevented, however, all fish experienced metabolic failure. The lineage with the largest relative brain size, Campylomormyrus, experienced metabolic failure at the highest oxygen concentration, while the lineage with the smallest relative brain size, B. brachyistius, experienced it at the lowest oxygen concentration (figure 4b).
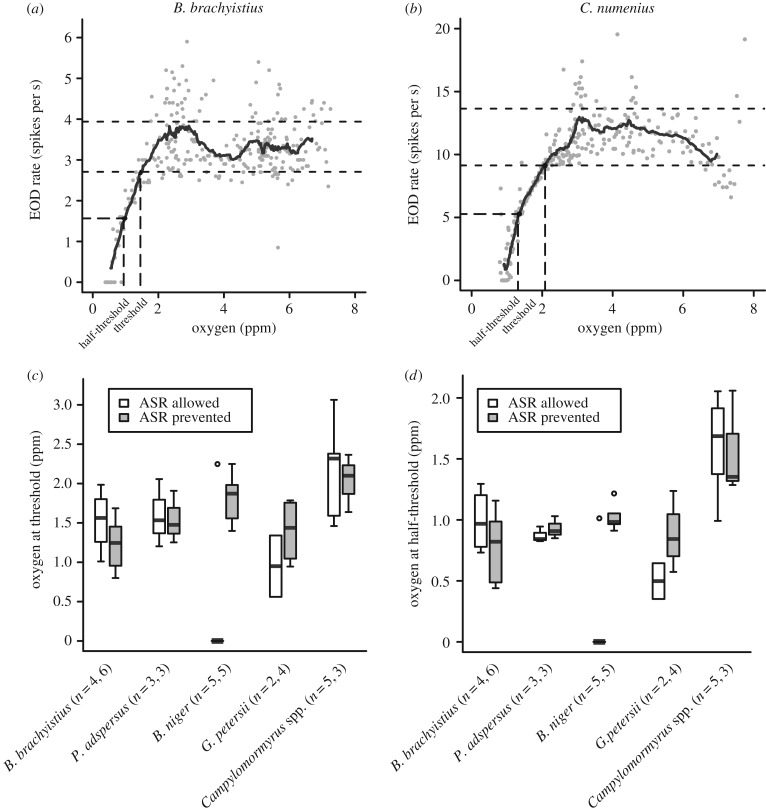
EOD rate can be used as a measure of behavioural activity in weakly electric fish [30]. As EOD rates can be highly variable [30,31], we calculated a running average of 10 adjacent time points before and after each point to obtain a smoothed curve of EOD activity. The threshold oxygen concentration was defined as the oxygen level at which the running average fell below one standard deviation of baseline EOD rate (figure 5a,b). We also calculated the half-threshold as the oxygen concentration at which the EOD rate was halfway between the threshold and the lowest EOD rate observed.
Figure 5.
The EOD rate of large-brained lineages is more sensitive to hypoxia than for small-brained lineages. Examples from individual fish in which EOD rate is plotted against oxygen concentration for (a) B. brachyistius and (b) C. numenius. The solid black line is the running average of EOD rates over 10 adjacent time points before and after. EOD rates measured between 4 and 8 ppm are considered baseline activity, and the mean ± s.d. of these rates (dotted lines) is used to determine oxygen threshold and half-threshold concentrations. (c) Box plot of oxygen threshold for each genus, and (d) box plot of oxygen half-threshold for each genus. Lineages in boxplots are arranged left to right from small to large relative brain size. White bars indicate the hypoxia experiment in which ASR was allowed, and grey bars indicate the experiment in which ASR was prevented (c,d). Sample sizes are different between figures 4 and 5 due to the signal-to-noise ratio being too low to reliably detect EODs in early experiments.
EOD rates decreased at low oxygen (approx. 0–3 ppm) in all species. There was significant variation in the threshold concentrations between lineages (two-way ANOVA: ASR allowed versus prevented: F1,5 = 1.34, p = 0.26; genus: F5,5 = 3.95, p < 0.01; interaction: F5,28 = 3.03, p < 0.05) and half-threshold oxygen concentrations between lineages (two-way ANOVA: ASR allowed versus prevented: F1,5 = 1.64, p = 0.21; genus: F5,5 = 13.25, p < 1 × 10−5; interaction: F5,28 = 3.69, p < 0.05; figure 5c,d). When ASR was prevented, EOD rate thresholds were highest in the lineage with the largest brain, Campylomormyrus, and lowest in the lineage with the smallest brain, B. brachyistius.
3. Discussion
We found that mormyrid lineages vary widely in relative brain size. Relative brain size did not correlate linearly with the relative sizes of other organs, but there was a significant nonlinear relationship with the size of the liver. This nonlinear relationship could indicate that evolution may favour an increase in liver size as the brain gets larger, but the extent of this increase may be subject to space or energetic constraints, leading to an energetic trade-off with liver as brain size increases further. However, this relationship was relatively weak compared with the strong correlation between relative brain size and relative oxygen consumption. Relative brain size also correlated negatively with hypoxia tolerance. These three lines of evidence suggest that the metabolic constraints hypothesis best explains evolutionary change in the brain sizes of mormyrids, consistent with previous findings in mammals [5,9,10]. However, we cannot rule out the possibility that energetic trade-offs could also play a role.
Many studies have shown that there is an energetic trade-off between brain size and other energetically expensive organs and processes [2,11,13]. However, many of these studies focused on animals with small to medium encephalization. In cases of extreme encephalization, support for energetic trade-offs is less clear. Early studies suggested that extreme encephalization in humans was not associated with an increase in metabolic rate [32], but instead a trade-off between gut and brain mass [6]. However, more recent studies have criticized these early studies for considering a limited diversity of mammals and not using appropriate phylogenetic methods, and instead suggest that increased encephalization in primates is partially paid for through an increase in net energy intake [9,10,15]. Our data provide an independent test case for understanding the evolution of extreme encephalization outside of mammals. As data from both mormyrids and primates support the metabolic constraints hypothesis, we suggest that energetic trade-offs are insufficient to accommodate energetic demands when brains become extremely large, and thus metabolic rate must vary. Energetic trade-offs may be more important in moderate encephalization, for which reducing energetic demands elsewhere can provide sufficient energy to support the brain.
A greater metabolic rate requires greater intake of energy and thus may be correlated with an increase in time spent foraging, as well as more intense competition for limited resources [6]. The active electric sense of mormyrids may improve their foraging efficiency [33,34]. In addition, three of the largest-brained genera, Gnathonemus, Campylomormyrus and Mormyrus, have morphological adaptations to help them forage for food. Gnathonemus petersii has an elongated, flexible chin appendage called a Schnauzenorgan, which may increase both motor and electrolocation efficiency while foraging [35]. Campylomormyrus and Mormyrus spp. both have a tube-snout, which acts as a specialized feeding appendage for extracting aquatic invertebrates from narrow crevices [36,37]. These adaptations may help provide the energy required for a higher metabolic rate.
Because lineages with large brains have low hypoxia tolerance, oxygen constraints may also limit the evolution of large brain size. Oxygen concentration can be highly variable, and is affected by environmental factors such as vegetation, light, temperature and pH [27]. Other mechanisms may help large-brained species avoid or deal with stress from low-oxygen environments, such as migration, phenotypic plasticity or ASR [38–40]. In some species, fish from well-oxygenated environments have larger brains than conspecifics from low-oxygen environments [41]. These differences could be due to divergent adaptation or phenotypic plasticity. Alternatively, large brain size may limit species distributions exclusively to environments where oxygen concentrations are consistently high such as large, fast-moving rivers [42], while small-brained species may be generalists capable of living in many different environments.
While it is probably difficult to lower the energetic requirements of brain tissue, there are other energetic expenses that are more easily reduced in environments with limited energy supplies. Producing an electric signal is energetically costly, as shown in several species of gymnotiform electric fish [43,44], so decreasing EOD rate would be a way to temporarily lower energetic expenses at the cost of decreased active sampling of the environment. Indeed, all mormyrid species we studied decreased their EOD rate at low oxygen concentrations, but large-brained lineages did so at higher oxygen concentrations than small-brained lineages.
Our results show that increased metabolic demand and decreased tolerance to environmental energy limitations could play a large role in constraining the evolution of extreme encephalization. These findings may help explain why extreme encephalization is rare, and suggest that high-energy environmental conditions must be present for extreme encephalization to evolve.
4. Experimental procedures
(a). Organ size measurements
We dissected 132 specimens, representing 30 mormyrid species and four non-mormyrid osteoglossomorph species. Seventy specimens were obtained from the Cornell University Museum of Vertebrates, which had been immersion-fixed in 10% phosphate-buffered formalin and stored in 70% ethanol. The rest were acquired live through the aquarium trade. Fish were euthanized in 300 mg l−1 MS-222 (tricaine methanesulfonate) until gilling ceased, transferred to 4% paraformaldehyde in 0.1 M phosphate buffer for immersion-fixation, and transferred to 70% ethanol after two weeks.
Before dissection, we rehydrated the specimens in 0.1 M phosphate buffer. We measured full wet body mass, and removed and measured the masses of the heart, gonads, kidney, liver, gastrointestinal (GI) tract and brain. We removed all stomach contents from the GI tract before measuring its mass. We were unable to obtain kidney masses for P. buchholzi or gonad masses for C. ornata due to their small size. We found that fixation did not affect our measurements of relative organ sizes (SM1).
(b). Phylogenetic comparisons and correlations
We used a bootstrapped maximum-likelihood tree from 73 cytb osteoglossomorph sequences built in MEGA v. 5.1 [45]. To include organ data from species that have not been sequenced, we grouped data from multiple species within monophyletic genera and chose the species sequence with the shortest phylogenetic distance from the genus node. Hippopotamyrus sp. and Marcusenius sanagaensis organ data were not used in evolutionary models, because these genera are polyphyletic, and these species have not been sequenced [18]. We pruned lineages for which we did not have organ data (electronic supplementary material, table S4).
To account for the effects of phylogeny, we fitted linear regressions of the log of each organ's mass and oxygen consumption against log body mass using two evolutionary models: Brownian and OU (electronic supplementary material, table S1). We also modelled nonlinear allometric relationships (SM4; electronic supplementary material, table S2). For models that were significant, we determined the model of best fit using the Akaike information criterion (AIC) (electronic supplementary material, table S1). Residuals were taken from the linear regression line of the best-fit model of each organ, or oxygen consumption, versus body size. We then tested for linear and quadratic correlations between residuals using Brownian and OU models (electronic supplementary material, table S3). All phylogenetic analyses were performed in R using the ape, caper and nlme packages [46–49].
(c). Oxygen consumption rate measurements
We measured oxygen consumption in six mormyrid and two non-mormyrid osteoglossomorph species using closed-chamber respirometry [17,26]. Fish were placed in a 1 or 2 l Erlenmeyer flask inside a 45 l tank. To minimize microbial respiration artefacts, we used fresh deionized water and added aquarium salts to yield conductivity of 175–225 µS cm−1 and pH of 6–7. The flask was closed using a rubber stopper with a dissolved oxygen probe (DOX; Analytical Sensors) inserted through it to measure oxygen concentration. To ensure even oxygen concentration in the flask, a stir bar was spun in the bottom of the flask and plastic mesh was used to separate the fish from the stir bar. The temperature of the water in the tank and flask was kept at 26–28°C using tubing that circulated the tank and had heated water pumped through it from a separate bucket. Oxygen concentrations were saved using a dO2 isoPod, e-corder 210 and the program Chart (eDAQ). The oxygen probe was calibrated to 100% of ambient O2 using an airstone bubbled in a beaker of tank water for 15 min and to 0% oxygen using a solution of 2% sodium sulfite in deionized water. Fish were starved for at least 24 h prior to the experiment, and acclimated to the flask for 200 min before closing the flask and measuring oxygen consumption over the course of 2–5 h.
In some recordings, we calculated oxygen consumption by comparing two time points, one immediately after the chamber was closed and one after 3 h of closure. In others, we took oxygen concentration measurements every second throughout the course of the recording, and calculated oxygen consumption using the linear slope of oxygen concentration over time. Oxygen consumption rates were determined using the total volume of the flask minus the volume of the fish. To ensure that there was not a change in oxygen consumption rate throughout the course of the experiment, we compared the slope of oxygen consumption at half-hour increments for each fish. We found no significant difference in oxygen consumption among these samples (two-way ANOVA: time: F6,88 = 1.019, p = 0.4186; genus: F4,88 = 2.083, p = 0.0898; interaction: F21,88 = 0.772, p = 0.7443). We measured fish mass by gently dabbing fish with a paper towel to remove excess moisture, and then adding the fish to a beaker partially filled with water to measure the resulting change in mass.
(d). Determining hypoxia tolerance
Experiments were performed in an 11 l tank filled with water having the same chemistry as described above. Tubing with heated water pumped through it was placed at the bottom of the tank beneath a plastic mesh barrier to keep the tank at constant temperature. A small water pump in the corner of the tank surrounded by a mesh barrier was used to ensure thorough mixing of tank water. A clear plastic tube provided shelter during the experiment. To prevent ASR, clear netting was placed on both ends of the tube. We measured oxygen concentrations using the dissolved oxygen probe set in one corner of the tank. We recorded EODs using two electrodes placed on opposite ends of the tank, connected to an A-M Systems Model 3000 AC/DC differential amplifier with 1000× gain, band-pass filtering (0.1–20 kHz) and notch filtering for 60 Hz noise. EODs were digitized by the eDAQ e-corder 210 once every minute for 20 s at a sampling rate of 20 kHz. A Logitech HD Webcam c270 placed directly in front of the tank recorded behaviour.
The fish were starved for at least 24 h and acclimated to the tank for one hour before sodium sulfite was added. We recorded behaviour, EODs and oxygen concentrations for 20 min during the acclimation. We added 50 ml of a 500 mM solution of sodium sulfite to decrease oxygen concentration at a rate of approximately 2 ppm h−1. Experiments were stopped once the fish experienced metabolic failure, or oxygen concentration remained at 0 ppm for 10 min, whichever happened first. In native environments, oxygen concentration can vary from fully oxygenated to less than 1 ppm depending on season, time of day, water flow and vegetative growth [26,27], so this experiment encompasses the full range of possible variation a mormyrid species might encounter.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank John P. Sullivan and John P. Friel at the Cornell University Museum of Vertebrates for loaning us specimens used in this study. Carlos A. Botero provided helpful guidance on phylogenetic comparative methods.
Ethics
All procedures were in accordance with guidelines established by the National Institutes of Health and were approved by the Animal Care and Use Committee at Washington University in St. Louis.
Data accessibility
GenBank accession numbers are in the electronic supplementary, table S4. The datasets supporting this article have been uploaded to the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.s3vq7) [50].
Authors' contributions
K.V.S. and B.A.C. contributed to conceptualization and design of experiments. K.V.S., M.K.F. and R.W. performed experiments and analyses. The manuscript was drafted by K.V.S. and edited and revised by B.A.C. K.V.S., M.K.F., R.W. and B.A.C. approved final version of manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by NSF IOS 1255396.
References
- 1.Reader S, Laland K. 2002. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA 99, 4436–4441. ( 10.1073/pnas.062041299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brannstrom I, Immler S, Maklakov A, Kolm N. 2013. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. ( 10.1016/j.cub.2012.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sol D, Duncan R, Blackburn T, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca-Azevedo K, Herculano–Houzel S. 2012. Metabolic constraing imposes tradeoff between body size and number of brain neurons in human evolution. Proc. Natl Acad. Sci. USA 109, 18 571–18 576. ( 10.1073/pnas.1206390109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong E. 1983. Relative brain size and metabolism in mammals. Science 220, 1302–1304. ( 10.1126/science.6407108) [DOI] [PubMed] [Google Scholar]
- 6.Aiello L, Wheeler P. 1995. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36, 199–221. ( 10.1086/204350) [DOI] [Google Scholar]
- 7.Isler K, van Schaik C. 2009. The expensive brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol. 57, 392–400. ( 10.1016/j.jhevol.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 8.Isler K. 2011. Energetic trade-offs between brain size and offspring production: marsupials confirm a general mammalian pattern. Bioessays 33, 173–179. ( 10.1002/bies.201000123) [DOI] [PubMed] [Google Scholar]
- 9.Isler K, van Schaik C. 2006. Metabolic costs of brain size evolution. Biol. Lett. 2, 558–560. ( 10.1098/rsbl.2006.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pontzer H, et al. 2016. Metabolic acceleration and the evolution of human brain size and life history. Nature 533, 390–392. ( 10.1038/nature17654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao W, Lou S, Zeng Y, Kotrshal A. 2016. Large brains, small guts: the expensive tissue hypothesis supported within anurans. Am. Nat. 188, 693–700. ( 10.1086/688894) [DOI] [PubMed] [Google Scholar]
- 12.Kaufman J. 2003. On the expensive-tissue hypothesis: independent support from highly encephalized fish. Curr. Anthropol. 44, 705–707. ( 10.1086/379258) [DOI] [Google Scholar]
- 13.Isler K, van Schaik C. 2006. Costs of encephalization: the energy trade-off hypothesis tested on birds. J. Hum. Evol. 51, 228–243. ( 10.1016/j.jhevol.2006.03.006) [DOI] [PubMed] [Google Scholar]
- 14.Boddy A, McGowen M, Sherwood C, Grossman L, Goodman M, Wildman D. 2012. Comparative analysis of encephalization in mammals reveals relaxed constraints on anthropoid primate and cetacean brain scaling. J. Evol. Biol. 25, 981–994. ( 10.1111/j.1420-9101.2012.02491.x) [DOI] [PubMed] [Google Scholar]
- 15.Navarrete A, van Schaik C, Isler K. 2011. Energetics and the evolution of human brain size. Nature 480, 90–94. ( 10.1038/nature10629) [DOI] [PubMed] [Google Scholar]
- 16.Carlson B, Arnegard E. 2011. Neural innovations and the diversification of African weakly electric fishes. Commun. Integr. Biol. 4, 720–725. ( 10.4161/cib.17483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson G. 1996. Brain and body oxygen requirements of Gnathonemus petersii, a fish with an exceptionally large brain. J. Exp. Biol. 199, 603–607. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan J, Lavoue S, Hopkins C. 2000. Molecular systematics of the African electric fishes (Mormyroidea: Teleostei) and a model for the evolution of electric organs. J. Exp. Biol. 203, 665–683. [DOI] [PubMed] [Google Scholar]
- 19.Robosky D, Santini F, Eastman J, Smith S, Sidlauskas B, Chang J, Alfaro M. 1958. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat. Commun. 4, 1958 ( 10.1038/ncomms2958) [DOI] [PubMed] [Google Scholar]
- 20.Nieuwenhuys R, Nicholson C. 1969. A survey of the general morphology, the fiber connections, and the possible functional significance of the gigantocerebellum of mormyrid fishes. In Neurobiology of cerebellar evolution and development (ed. RR Llinas), pp. 107–134. Chicago, IL: American Medical Association. [Google Scholar]
- 21.Erdl M. 1846. Über das Gehirn der Fischgattung Mormyrus Gelehrte Anzeigen. d. K. Bayer Akad. d. Wiss. 22, 403–407. [Google Scholar]
- 22.Isler K, van Schaik C. 2014. How humans evolved large brains: comparatie evidence. Evol. Anthropol. 23, 65–75. ( 10.1002/evan.21403) [DOI] [PubMed] [Google Scholar]
- 23.Sol D, Garcia N, Iwaniuk A, Davis K, Meade A, Boyle WA, Szekely T. 2010. Evolutionary divergence in brain size between migratory and resident birds. PLoS ONE 5, e9617–e9625. ( 10.1371/journal.pone.0009617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Woerden JT, Willems EP, van Schaik CP, Isler K. 2011. Larger brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution 66, 191–199. ( 10.1111/j.1558-5646.2011.01434.x) [DOI] [PubMed] [Google Scholar]
- 25.Pontzer H, Kamilar JM. 2009. Great ranging associated with greater investment in mammal. Proc. Natl Acad. Sci. USA 106, 192–196. ( 10.1073/pnas.0806105106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman L, Chapman C. 1998. Hypoxia tolerance of the mormyrid Petrocephalus catastoma: implications for persistence in swamp refugia. Copia 3, 762–768. ( 10.2307/1447812) [DOI] [Google Scholar]
- 27.Talling J. 1965. The chemical composition of African lake waters. Int. Rev. Hydrobiol. 50, 421–463. ( 10.1002/iroh.19650500307) [DOI] [Google Scholar]
- 28.Chapman LJ, Chapman CA, Nordlie FG, Rosenberger AE. 2002. Physiological refugia: swamps, hypoxia tolerance and maintenance of fish diversity in the Lake Vicoria region. Comp. Biochem. Physiol. 133, 421–437. ( 10.1016/S1095-6433(02)00195-2) [DOI] [PubMed] [Google Scholar]
- 29.Lavoué S, Sullivan J, Hopkins C. 2003. Phylogenetic utility of the first two introns of the S7 ribosomal protein gene in African electric fishes (Mormyroidea: Teleostei) and congruence with other molecular markers. Biol. J. Linn. Soc. 78, 273–292. ( 10.1046/j.1095-8312.2003.00170.x) [DOI] [Google Scholar]
- 30.Carlson B. 2002. Electric signaling behavior and the mechanisms of electric organ discharge production in mormyrid fish. J. Physiol. Paris 96, 405–419. ( 10.1016/S0928-4257(03)00019-6) [DOI] [PubMed] [Google Scholar]
- 31.Teyssèdre C, Boudinot M, Minisclou C. 1987. Categorisation of interpulse intervals and stochastic analysis of discharge patterns in resting weak-electric mormyrid fish (Gnathonemus petersii). Behaviour 102, 264–282. ( 10.1163/156853986X00162) [DOI] [Google Scholar]
- 32.McNab BK, Eisenberg JF. 1989. Brain size and its relation to the rate of metabolism in mammals. Am. Nat. 133, 157–167. ( 10.1086/284907) [DOI] [Google Scholar]
- 33.Arnegard ME, Carlson BA. 2005. Electric organ discharge patterns during group hunting by a mormyrid fish. Proc. R. Soc. B 272, 1305–1314. ( 10.1098/rspb.2005.3101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von der Emde G. 1999. Active electrolocation of objects in weakly electric fish. J. Exp. Biol. 202, 1205–1215. [DOI] [PubMed] [Google Scholar]
- 35.Engelmann J, Nöbel S, Röver T, von der Emde G. 2009. The Schnauzenorgan-response of Gnathonemus petersii. Front. Zool. 6, 21 ( 10.1186/1742-9994-6-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrero C, Winemiller K. 1993. Tube-snouted gymnotiform and mormyriform fishes: convergence of a specialized foraging mode in teleosts. Environ. Biol. Fish. 38, 299–309. ( 10.1007/BF00007523) [DOI] [Google Scholar]
- 37.Macdonald W. 1956. Observations on the biology of chaoborids and chironomids in Lake Victoria and on the feeding habits of the ‘elephant-snout fish’ (Mormyrus kannume Forsk.). J. Anim. Ecol. 25, 36–53. ( 10.2307/1849) [DOI] [Google Scholar]
- 38.Crispo E, Chapman L. 2010. Geographic variation in phenotypic plasticity in response to dissolved oxygen in an African cichlid fish. J. Evol. Biol. 23, 2091–2103. ( 10.1111/j.1420-9101.2010.02069.x) [DOI] [PubMed] [Google Scholar]
- 39.Kramer D, McClure M. 1982. Aquatic surface respiration, a widespread adapatation to hypoxia in tropical freshwater fishes. Environ. Biol. Fish. 7, 47–55. ( 10.1007/BF00011822) [DOI] [Google Scholar]
- 40.Blake BF. 1977. Aspects of the reproductive biology of Hippopotamyrus pictus from Lake Kainji, with notes on four other mormyrid species. J. Fish. Biol. 11, 437–445. ( 10.1111/j.1095-8649.1977.tb04138.x) [DOI] [Google Scholar]
- 41.Chapman LJ, Hulen KG. 2001. Implications of hypoxia for the brain size and gill morphometry of mormyrid fishes. J. Zool. Lond. 254, 461–472. ( 10.1017/S0952836901000966) [DOI] [Google Scholar]
- 42.Feulner P, Kirschbaum F, Mamonekene V, Ketmaier V, Tiedemann R. 2007. Adaptive radiation in African weakly electric fish (teleostei: Mormyridae: Camplyomormyrus): a combined molecular and morphological approach. J. Evol. Biol. 20, 403–414. ( 10.1111/j.1420-9101.2006.01181.x) [DOI] [PubMed] [Google Scholar]
- 43.Stoddard P, Salazar V. 2011. Energetic cost of communication. J. Exp. Biol. 214, 200–205. ( 10.1242/jeb.047910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salazar VL, Krahe R, Lewis JE. 2013. The energetics of electric organ discharge generation in gymnoiform weakly electric fish. J. Exp. Biol. 216, 2459–2468. ( 10.1242/jeb.082735) [DOI] [PubMed] [Google Scholar]
- 45.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. ( 10.1093/molbev/msr121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Core Team. 2012. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 47.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2012. Caper: comparative analyses of phylogenetics and evolution in R. R package version 0.5. See https://cran.r-project.org/web/packages/caper/index.html. [Google Scholar]
- 48.Pinheiro J, Bates D, DebRoy S, Sarkar D. 2015. nlme: linear and nonlinear mixed effects models. R package version 3.1–120. See https://cran.r-project.org/web/packages/nlme/index.html. [Google Scholar]
- 49.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 50.Sukhum KV, Freiler MK, Wang R, Carlson BA. 2016. Data from: The costs of a big brain: extreme encephalization results in higher energetic demand and reduced hypoxia tolerance in weakly electric African fishes. Dryad Data Repository. ( 10.5061/dryad.s3vq7) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
GenBank accession numbers are in the electronic supplementary, table S4. The datasets supporting this article have been uploaded to the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.s3vq7) [50].