Abstract
Urban ecosystems are an increasingly dominant feature of terrestrial landscapes. While evidence that species can adapt to urban environments is accumulating, the mechanisms through which urbanization imposes natural selection on populations are poorly understood. The identification of adaptive phenotypic changes (i.e. clines) along urbanization gradients would facilitate our understanding of the selective factors driving adaptation in cities. Here, we test for phenotypic clines in urban ecosystems by sampling the frequency of a Mendelian-inherited trait—cyanogenesis—in white clover (Trifolium repens L.) populations along urbanization gradients in four cities. Cyanogenesis protects plants from herbivores, but reduces tolerance to freezing temperatures. We found that the frequency of cyanogenic plants within populations decreased towards the urban centre in three of four cities. A field experiment indicated that spatial variation in herbivory is unlikely to explain these clines. Rather, colder minimum winter ground temperatures in urban areas compared with non-urban areas, caused by reduced snow cover in cities, may select against cyanogenesis. In the city with no cline, high snow cover might protect plants from freezing damage in the city centre. Our study suggests that populations are adapting to urbanization gradients, but regional climatic patterns may ultimately determine whether adaptation occurs.
Keywords: adaptation, cyanogenesis, herbivory, natural selection, urban evolution, white clover
1. Introduction
Urban environments are a novel and increasingly dominant feature of terrestrial landscapes in which more than half of the world's human population now reside [1]. Relative to non-urban areas, urban areas have dramatically different environmental conditions, such as warmer air temperatures [2] and altered ecological communities [3]. Although urbanization can have large ecological effects on plant and animal communities [4,5], the evolutionary consequences of urbanization are still poorly understood [6–8]. In particular, very little is known about whether species exhibit clinal evolution along urbanization gradients and about the ecological mechanisms that cause evolution in urban ecosystems. Here, we address these gaps in our knowledge using a model plant system (Trifolium repens) for studying adaptive clines.
Our understanding of the evolutionary ecology of urban ecosystems remains poor because of a paucity of data on the subject [7]. Although there have been relatively few studies, researchers have identified several cases of adaptive phenotypic differentiation between paired urban–rural populations. For example, a study of seed dispersal traits in the plant Crepis sancta found that urban populations in Montpellier, France, have evolved to produce a higher proportion of heavy, non-dispersing seeds than plants in non-urban areas [9]. Similarly, a study of Anolis lizards in Puerto Rico suggests that the morphology of urban lizard populations is adapted to more effectively climb on artificial smooth surfaces [10]. Unfortunately, little is known about the underlying genetic basis of such adaptations. By contrast, studies of genotypic evolution in urban areas typically do not identify the phenotype(s) under selection [11,12]. For example, a study of Peromyscus leucopus mice transcriptomes in New York City (NYC) identified several plausible adaptive changes associated with urbanization, but did not quantify phenotypic changes in the mice. Overall, these studies show that urbanization can drive phenotypic and genetic differentiation, but studies that integrate both the phenotypic and genetic basis of adaptation to urban environments are lacking.
It is also unclear if urbanization gradients—which are an important feature of urban ecosystems [13]—drive the evolution of clines, and whether parallel clines occur across distinct cities [7]. The study of adaptive clines along urbanization gradients has promise to advance our understanding of evolution in urban ecosystems, because clines are excellent systems in which the ecological drivers of adaptation can be investigated [14–16]. Thus, our goal was to test for parallel phenotypic clines along urbanization gradients in a trait with a known genetic basis, and to investigate the ecological mechanisms that drive the evolution of these clines.
To investigate parallel evolution along urbanization gradients, we used the globally distributed perennial plant, white clover (T. repens L.), as a model (electronic supplementary material, text S1). White clover exhibits a Mendelian-inherited polymorphism for cyanogenesis—the production of hydrogen cyanide (HCN) following tissue damage [17]. HCN is a chemical defence against herbivores that inhibits cellular respiration and is toxic to both plants and animals. Cyanogenesis is often polymorphic within populations; cyanogenic genotypes produce HCN following tissue damage, whereas HCN is absent from acyanogenic genotypes [17]. The cyanogenesis polymorphism is caused by two genes: CYP79D15 (hereafter Ac), which is required for the production of the cyanogenic glucoside substrate (i.e. linamarin and lotaustralin), and Li, which encodes the hydrolyzing enzyme, linamarase. Plants must have dominant alleles at both loci to produce HCN [18]. The two components (i.e. cyanogenic glycosides and linamarase) are stored separately in cells to avoid self-toxicity, and only form HCN when they are brought together following tissue damage. Previous research found that cyanogenesis is most frequent in populations at low latitudes and elevations [19,20], and it has been hypothesized that herbivores select for cyanogenesis in these warm environments [17,21]. In cold climates, cyanogenesis is selected against because freezing temperatures lyse cells and trigger HCN release, causing self-toxicity in cyanogenic plants [17,22,23]. Our understanding of temperature-driven clines in cyanogenesis makes the cyanogenesis polymorphism of T. repens an ideal system in which the evolution of clines in urban ecosystems can be quantified and studied.
Environmental change associated with urbanization may alter natural selection on cyanogenesis. Air temperatures are generally warmer in urban areas relative to non-urban areas [24], whereas herbivores are not consistently affected by urbanization [3]. This may cause urban populations to experience fewer freezing events than non-urban populations during winter [25], and consequently weaken selection against cyanogenesis in cities. Thus, we predicted that cyanogenesis would increase in frequency with increasing proximity to urban centres.
Here, we test the hypothesis that urbanization has driven the evolution of parallel adaptive clines in cyanogenesis. We first asked whether there is parallel evolution across multiple urbanization gradients in a single city by surveying populations of white clover along three transects in Toronto, Canada. Next, we evaluated whether parallel evolution has occurred across multiple cities by sampling transects in NYC, Boston and Montreal. Last, we used a field experiment, in situ temperature measurements, remote sensing and historical climate records to test competing mechanistic hypotheses that could explain the observed patterns: a change in the benefits of cyanogenesis owing to clines in herbivore pressure, and/or a change in the cost of cyanogenesis owing to clines in freezing temperature. Our results provide insight into how natural populations can evolve in response to previously undocumented features of urban environments.
2. Material and methods
(a). Patterns of clines in cyanogenesis
(i). Field sampling
To test whether clines in cyanogenesis occur along urbanization gradients, we sampled 2509 T. repens plants from 128 sites (hereafter ‘populations’) sequentially spaced approximately 1 km apart along three transects in the Greater Toronto Area (electronic supplementary material, figure S1). Transects extended west, north and east of Toronto. For both the east and west transects, we maintained a relatively constant distance from Lake Ontario. Trifolium repens is abundant on lawns found along roadsides, in parks and cemeteries, and similar habitats. All of our sampling was conducted near paved roads in human-occupied areas, which are all ploughed regularly during winter. Snow ploughed from roads may pile on roadsides and thus affect some of our sampled populations; however, this effect should not have been confounded with distance from the urban centre.
For each population, we recorded GPS coordinates and collected stolon cuttings from up to 20 plants; cuttings were approximately 4 cm in length and had at least two leaves. To reduce the likelihood of sampling a genotype twice, we ensured more than 2 m separated all collected stolons. We placed all stolons from a population into a plastic bag, and stored them in a cooler with ice. At the laboratory, we put one leaf from each plant into separate wells of 96-well plates, and put the remaining tissue in microcentrifuge tubes; we stored plates and tubes at −80°C. This freezing lyses cells, and thus makes the HCN reaction stronger when assays are conducted.
To determine if parallel clines in cyanogenesis occur in cities outside of Toronto, we sampled one 50 km transect in each of Montreal, QC, Canada (969 plants from 47 populations), Boston, MA, USA (876 plants from 43 populations) and NYC, NY, USA (946 plants from 48 populations), between 26 July and 1 August 2015 (electronic supplementary material, figures S4–S6). The sampling methods were identical to those for Toronto.
(ii). Cyanogenesis analysis
To estimate the relative frequency of cyanogenesis (i.e. proportion of cyanogenic versus acyanogenic plants) in natural populations of T. repens, we screened each plant for the presence of HCN using Feigl–Anger assays. Feigl–Anger assays determine the presence or absence of HCN in a sample using a colour change reaction [26]. We removed the filled 96-well plates from the freezer, allowed them to thaw, and then added 80 µl of water to each well. We used pipette tips to macerate the tissue samples, and secured Feigl–Anger test paper over the plates. We incubated the plates for 3 h at 37°C, and then scored each well for cyanide (i.e. cyanotype AcLi), which is indicated by a blue colour.
To confirm that clines were caused by selection on HCN and not independent functions of Ac or Li, we determined the frequency of Ac and Li in plants collected from the three Toronto transects. There was insufficient leaf tissue to screen each plant at both loci, so we screened all acyanogenic plants (with sufficient leaf tissue) in each population for either Ac or Li, alternating for sequential populations. Screening was conducted as above but instead of 80 µl of H2O, we added either 30 µl of 10 mM linamarin (Sigma-Aldrich 68264) and 50 µl H2O to each well to screen for linamarase (cyanotype acLi), or 80 µl of 0.2 EU ml−1 linamarase (LGC Standards CDX-00012238-100) with 20 µl of H2O to each well to screen for linamarin (cyanotype Acli). We previously confirmed that Feigl–Anger assays correspond to the presence/absence of the Ac and Li alleles [27], and thus these assays accurately reflect changes in genotype and allele frequency.
(iii). Statistical analysis
We used linear regression to determine if the relative frequency of cyanogenesis, or alleles at Ac and Li, is associated with urbanization. We first used the haversine formula—which calculates the distance between two sets of coordinates—to determine the distance from each sampled population to the urban centre [28] (see electronic supplementary material, figures S1, S4–S6). Next, we regressed the frequency of cyanogenesis in populations against the distance of those populations from the urban centre. All models had the proportion of cyanogenic plants in a population as the response variable, and distance to the urban centre as a predictor. We fitted a model for the Toronto data that included a transect × HCN interaction term; this interaction was non-significant, indicating that patterns do not differ across transects. Our conclusions are identical when generalized linear mixed-effects logistic regressions are used to analyse our data (analyses included in archived R script). Briefly, these models were fitted using the ‘glmer’ function in lme4, and were of the form HCN∼distance (km) + population, where distance is a continuous fixed effect and population is a discrete random effect. Analyses were conducted using R v. 3.0.3 [29]; R script is available online (see ‘Data accessibility’ section).
While distance to an urban centre might explain evolutionary patterns, it is important to distinguish large-scale factors associated with urbanization from finer-scale local factors [21]. To accomplish this, we retrieved 2015 human population density estimates from the NASA GPW4 dataset (resolution = 1 km) [30]. To determine if these variables explained variation in the frequency of cyanogenesis, we fitted linear models as before, but added a term for population density.
(b). Ecological causes of clines in cyanogenesis
Adaptive clines in cyanogenesis can arise because of spatial gradients in either the defensive benefits or the self-toxicity costs of cyanogenesis [21]. We tested two non-exclusive hypotheses that could explain the results of our natural population surveys: (i) cyanogenesis is more beneficial in rural areas because of increased herbivory, and (ii) cyanogenesis is more costly in urban areas because of colder winter ground temperatures. Urban areas typically have warmer air temperatures than non-urban areas (i.e. urban heat islands), and so it is non-intuitive that urban ground temperatures might be cold relative to non-urban areas. However, relative to non-urban areas, cities typically experience less snowfall and increased snowmelt [24]. Snow cover insulates the ground against freezing temperatures [31], and thus a reduction in urban snow cover may cause plants in urban areas to experience colder winter temperatures than those in rural areas.
(i). Field experiment
We conducted a field experiment in Toronto to test if herbivory is associated with urbanization, and if this could explain clines in cyanogenesis along urbanization gradients. We established 40 experimental populations of T. repens on private lawns that spanned from downtown Toronto to suburban and rural areas west of the city (electronic supplementary material, figure S7). The strongest individual cline in cyanogenesis was observed along this transect. From 4–6 June 2015, we placed 24 T. repens plants into solid plastic flats on a 1.5 m2 area of each lawn. Two populations were destroyed during the first month of the experiment and excluded from analyses (final n = 38 populations).
Each experimental population contained 12 cyanogenic (AcLi) and 12 acyanogenic (four Acli, four acLi, four acli) plants. Plants were initially propagated from stolons sourced from a group of 48 genotypes with cyanotypes determined using the methods outlined above. We propagated 5 cm stolons that had two to five leaves into 15 cm pots (AZE06000, ITML, Brantford, ON, Canada) filled with potting soil (Sunshine Mix #1; Sun Gro Horticulture, Agawam, MA, USA) and 0.5 g of slow-release fertilizer (Nutricote Total 13–13–13 With Minor Nutrients, Florikan, Sarasota, FL, USA). Plants were thoroughly watered and kept in a growth chamber set to 15.3 h of 500 µmol light, with a 25 : 20°C day:night temperature cycle. We randomly assigned plants to populations such that: (i) each genotype did not occur more than once in a population, (ii) each genotype occurred in 20 of 40 populations, and (iii) each population satisfied the cyanotype ratio described above.
We quantified herbivory, reproductive fitness and biomass for each plant during our experiment. To measure herbivory, we selected five of the oldest non-senesced leaves from each plant and visually quantified the proportion of leaf area consumed. Previous field experiments with T. repens determined that this method is sufficient to detect differences in herbivory between cyanogenic and acyanogenic plants [27]. We completed two herbivory surveys: early (July 9–13) and late (August 19–23) in the growing season. The patterns in herbivory were identical for both surveys, and so we used the mean damage observed between the two surveys as an estimate of herbivory for each plant. Our conclusions are unchanged if we analyse herbivory for each period separately. We harvested the aboveground biomass of each plant halfway through the experiment, leaving all tissue within 3 cm of the soil intact to facilitate regrowth. This harvesting mimicked intermittent mowing that plants experience on lawns, and prevented plants from rooting into adjacent pots. We dried harvested biomass for 72 h at 70°C and weighed it to the nearest 0.1 g. We collected all ripe inflorescences throughout the experiment. Final harvest was in late August, at which point we recorded the total number of inflorescences that each plant produced, and weighed the floral and vegetative tissue separately. After weighing and counting the inflorescences, we ground them through 1 mm and 0.5 mm sieves in series to isolate the seeds, which were weighed to the nearest 0.001 g as an estimate of maternal fitness.
Using the data from our field experiment we assessed whether: (i) cyanogenesis reduced herbivory in our experiment; (ii) herbivory changed along the urbanization gradient; and (iii) if patterns of herbivory along the urbanization gradients differed between cyanogenic and acyanogenic plants. We conducted the same analyses using vegetative biomass and reproductive fitness as the response variable. For these analyses, we fitted a linear mixed-effects model of the form: [Variable] = HCN + distance (km) + HCN × distance + population + genotype. In this model, [Variable] is the response variable of interest (i.e. herbivory, biomass or fitness), HCN is a fixed effect factor with two levels, distance is a continuous fixed effect, HCN × distance is an interaction term evaluating whether cyanogenesis influences the relationship between distance and the response variable, and population and genotype are factorial random effects. We analysed the model using ANOVA with type-III sums of squares and the Kenward–Roger method for denominator degrees of freedom approximation to test the significance of fixed effects.
To ensure that differences in herbivory were owing to HCN, and not caused by the effects of alleles at Ac, Li or linked genes, we fit separate two models for a dataset that consisted of only acyanogenic plants. These models were similar to those described above but instead of HCN, the predictor variable was the presence or absence of functional (i) Ac or (ii) Li alleles. There were no significant effects of the Ac and Li alleles and the results of these models are not reported below (but see online R script).
(ii). Ground temperature measurements
During the winter 2015, we recorded ground temperatures across our previously sampled east–west transect in Toronto (electronic supplementary material, figure S8) to determine if winter ground temperatures were affected by urbanization. On 27 January 2015, we placed iButton DS1921G thermochron devices (Maxim Integrated™, San Jose, USA) at 20 locations sequentially separated by 5 km. We placed the iButtons underneath trees and bushes so they were not directly exposed to sunlight. We retrieved all iButtons on 28 March 2015, and extracted hourly temperature measurements.
We used the temperature data to calculate a daily index of ‘relative urban coldness’. This index was calculated as the slope of minimum daily temperature on distance from the urban centre of Toronto (see electronic supplementary material, figure S9; inset figure 3a). Positive index values indicate that minimum temperatures were colder in urban areas relative to non-urban areas, whereas negative values indicate the opposite. We treated each index value (n = 59) as an independent observation and fit a 95% confidence interval (CI) loess curve to visually evaluate if temperatures were significantly colder or warmer in the city during winter.
Figure 3.
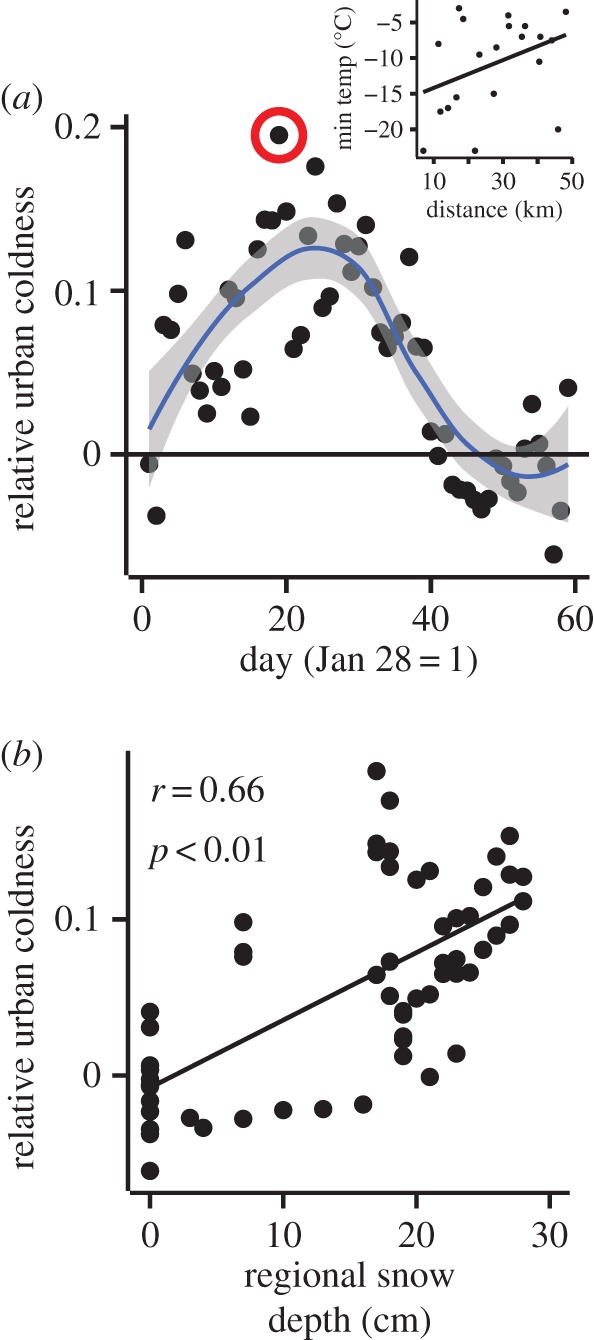
Urban ground temperatures were often colder than non-urban ground temperatures. (a) Analysis of daily relative urban coldness values (n = 59) reveals that it was often colder in Toronto during winter 2015 than in non-urban areas (curve is a 95% CI loess-smoothed surface). The inset shows the regression for the day circled in red. We hypothesized that relatively cold urban ground temperatures are caused by urban–rural snow depth gradients, and consistent with this expectation we found (b) a significant positive correlation between the daily relative urban coldness index value and regional snow depth on those days. (Online version in colour.)
We hypothesized that clines in relative urban coldness are caused by differences in snow cover between the downtown core and outside non-urban areas. To test this hypothesis, we retrieved snow depth data for the central Toronto weather station (WMOID: 71508), which is located approximately 2 km from our urban centre reference point. We regressed the relative urban coldness index value against snow cover on the same day to evaluate whether regional snow cover influences relative urban coldness.
(iii). Remote sensing analysis
To assess whether our study populations occur along a snow cover gradient, and to make comparisons across cities, we used remote sensing analysis. We selected all Landsat images (earthexplorer.usgs.gov) that met the following criteria: (i) image captured in January or February; (ii) no cloud cover above the study area; (iii) image taken between 1980 and 2015; (iv) snow present along the transect (i.e. maximum normalized-difference snow index (NDSI) ≥ 0.4; see below); (v) less than 10 cm snowfall in the previous 5 days to allow sufficient time for the effects of urbanization on snow melt to occur. We focused our analyses on January and February because January isotherms have previously been implicated as major factors driving the evolution of clines in T. repens cyanogenesis [19,21], and there were insufficient images from January alone for robust analysis.
For images that met these criteria (list of images archived on Dryad), we extracted the NDSI [32] for each of our study populations using the point sampling tool in QGIS 2.12 [33]. NDSI is a quantitative measure of snow cover derived using the unique reflective and absorptive properties of snow in the green and short wave infrared wavelengths. Although Landsat data are at a resolution of 30 m × 30 m, many populations were within 30 m of roads or other artificial surfaces—especially in urban areas. Thus, using the raw population coordinates would often cause us to include snow-free surfaces in our analysis, which could bias our data towards low NDSI values in cities. We therefore manually moved all NDSI measurement points to the nearest large (more than 30 × 30 m) park or field before extracting NDSI.
We used linear mixed-effects models to test whether a change in snow cover was related to the distance of populations to the urban centre. We fit the following model: NDSI = distance + city + distance × city + month/year + population + image; distance is a continuous independent variable, city is a fixed effect, month and year are nested random effects, and population (sampling location) and image are both random effects. For each city, we extracted the slope of NDSI versus distance, and the intercept (figure 4a). To determine if mean NDSI was significantly different across cities, we used a Tukey HSD test.
Figure 4.
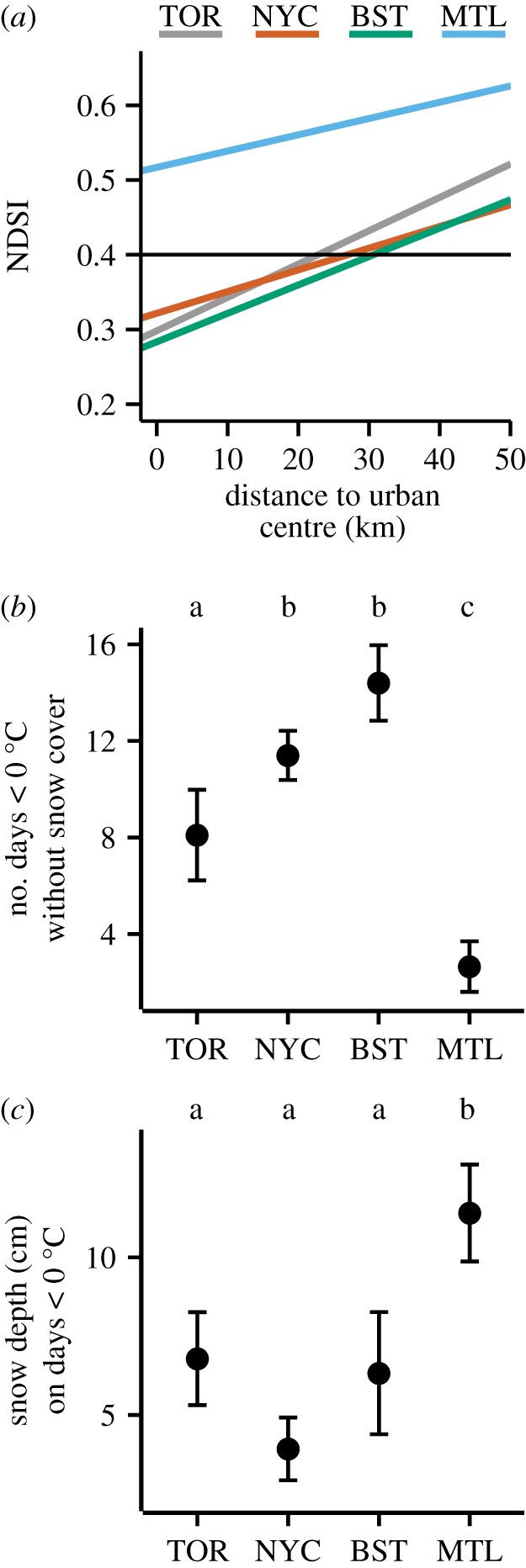
Historical patterns in January–February snow cover and temperature across the four cities sampled in this study. (a) The NDSI decreased toward the urban centre of each city. The horizontal line at NDSI = 0.4 represents the threshold value for the presence of snow [35]. Montreal (top line) has high snow cover along the entire transect. (b) Montreal (MTL) has fewer days when temperatures are below 0°C and there is no snow cover (±1 s.e.), and (c) deeper snow cover (±1 s.e.) on days where temperatures are less than 0°C, than Toronto (TOR), New York City (NYC) and Boston (BST). Different letters represent significant differences at p < 0.05 (Tukey HSD). (Online version in colour.)
(iv). Historical weather data
We used weather station data to compare snow cover and temperature across cities. All data were retrieved from the National Climatic Data Center (www.ncdc.noaa.gov/cdo-web/datatools/normals). We retrieved data for daily snow depth and minimum temperature for the period of 1980–2010. We retrieved data from one airport weather station per city (Toronto—YYZ, Montreal—YUL, NYC—LGA, Boston—BOS). We excluded dates when these data were not available for all four cities. While cities differ in where their airports are situated, our assumption is that inter-city climate variation overrides the effect of local variation in airport location. After filtering, we had data for 3545 days in each city, which represented 20 years (1980–1992, 1994, 1996, 1999–2002, 2004).
To test the hypothesis that plants in the four cities differ in their exposure to freezing temperatures, we calculated the cumulative number of days in January and February of each year where temperatures were less than 0°C and there was no snow cover. Because snow depth influences insulation properties [31], we also compared snow depth on days when temperatures were less than 0°C. All models were simple mixed-effects models that had the following form: snow variable = city + year, where city was a fixed effect and year was a random effect.
3. Results
(a). Patterns of clines in cyanogenesis
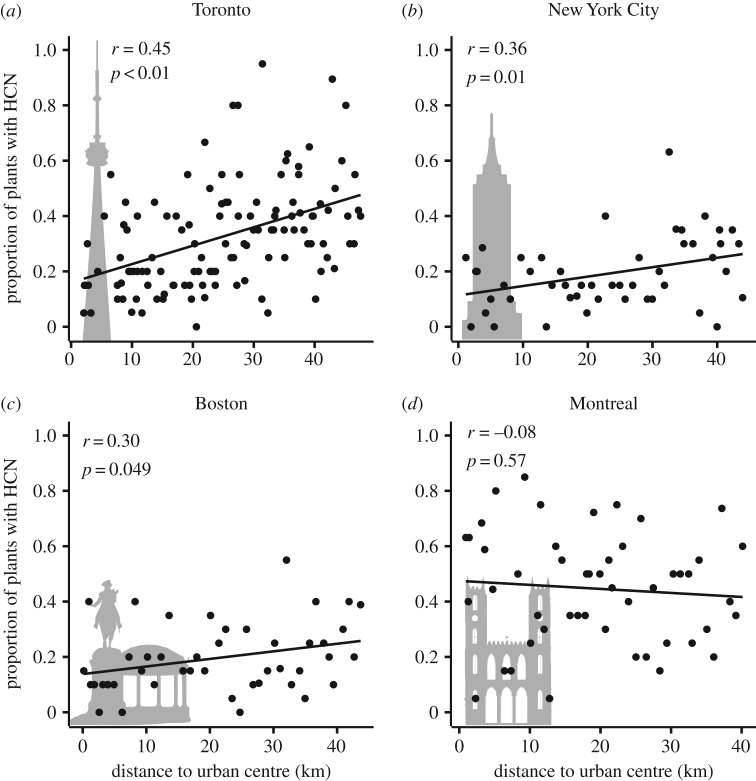
We found consistent changes in the frequency of cyanogenesis along each transect in Toronto, but clines were in the opposite direction to our prediction. The frequency of cyanogenesis within populations increased with greater distance from downtown Toronto at a rate of 0.65 ± 0.12% [s.e.] km−1 (F1,119 = 30.57, p < 0.001) (2379 plants from 121 populations) (figure 1a; electronic supplementary material, figures S1–S2). The frequency of dominant alleles at both Ac and Li also increased towards rural areas (Ac: 0.66 ± 0.17%, F1,63 = 15.38, p < 0.001; Li: 0.57 ± 0.19%, F1,62 = 9.26, p = 0.003) [34] (electronic supplementary material, figure S3). Human population density did not explain variation in cyanogenesis frequency in multiple regression models that also included distance from the urban centre (all p > 0.6). The frequency of cyanogenesis increased with distance from the urban centres in both NYC (0.34 ± 0.13% km−1; F1,46 = 6.88, p = 0.01) and Boston (0.27 ± 0.14% km−1; F1,42 = 4.09, p = 0.049), but there was no cline in Montreal (−0.14 ± 0.25% km−1; F1,47 = 0.33, p = 0.57) (figure 1b–d; electronic supplementary material, figures S4–S6).
Figure 1.
Urban populations of white clover, Trifolium repens, have evolved reduced cyanogenesis relative to non-urban populations in three cities. We observed parallel clines in cyanogenesis in: (a) Toronto (n populations = 121), (b) New York City (n = 48) and (c) Boston (n = 44), but not in (d) Montreal (n = 49). Each point represents the proportion of plants within a population that tested positive for cyanogenesis (mean n per population = 19.7 plants). Statistics were calculated using linear regression.
(b). Ecological causes of clines in cyanogenesis
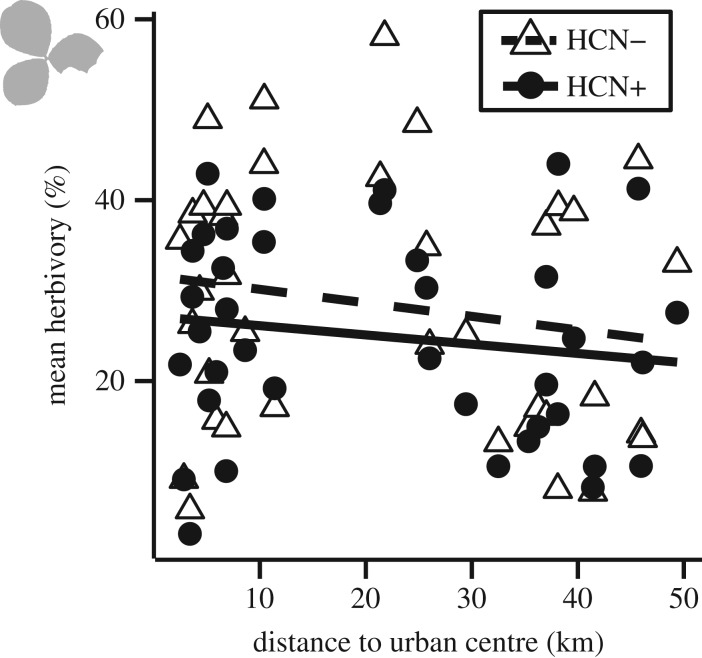
A field experiment conducted to test if the defensive benefits of cyanogenesis change along urbanization gradients did not provide support for our mechanistic hypothesis. We found that cyanogenesis was an effective defence against herbivores, such that cyanogenic plants received 15% less herbivore damage than acyanogenic plants (HCN main effect: F1,104 = 8.27, p = 0.004). However, there was no change in overall herbivory (distance main effect: β = −0.014 ± 0.011, F1,37 = 1.04, p = 0.30) or relative herbivory on cyanogenic plants compared to acyanogenic plants (HCN × distance interaction: β = 0.004 ± 0.004, F1,809 = 1.11; p = 0.29) along the urbanization gradient (figure 2).
Figure 2.
The benefits of cyanogenesis as a defence against herbivores do not explain the observed clines. Our field experiment revealed that cyanogenic plants (HCN+) received less leaf herbivory than acyanogenic plants (HCN−) (p = 0.004, ANOVA), but mean herbivory on both cyanotypes changed little within experimental populations (each n = 38) along an urbanization gradient in Toronto (HCN × distance interaction p = 0.29).
Data from temperature probes provide support for the mechanistic hypothesis that clines in cyanogenesis are caused by spatial variation in freezing temperatures. Specifically, we found that minimum ground surface temperatures were often coldest in urban areas (figure 3a). By examining data for regional snow depth during our observation period, we found that ground temperatures in urban areas were colder than in non-urban areas when there was deep snow in the region, and relatively warmer when there was little or no snow in the region (F1,57 = 56.76; r = 0.66, p < 0.001) (figure 3b).
High regional snow cover probably provides the necessary conditions for clines in snow depth—and thus ground temperature—to arise along urbanization gradients. To test whether the white clover populations we sampled in the field occur along a snow cover gradient, we used remote sensing to quantify the NDSI [35] for our sampled populations. We found that NDSI decreased (i.e. less snow) toward the urban centre in each city (β = 3.70 × 10−3 ± 1.02 × 10−3, F1, 228 = 30.03; p < 0.01) (figure 4a).
(c). Comparison of winter climate features across cities
We sought to explain why clines in cyanogenesis occur in Toronto, NYC and Boston, but not Montreal. Because we hypothesized that insulation provided by snow cover could weaken selection against cyanogenesis, we predicted that urban plants are most insulated by snow cover in Montreal. We found that mean NDSI was significantly higher in Montreal (i.e. more snow) than all other cities (Tukey HSD, all p < 0.05), whereas the remaining three cities did not differ from each other (Tukey HSD, all p > 0.9) (figure 4a). Analysis of weather station data revealed that Toronto, Boston and NYC each have more days with freezing temperatures and no snow cover than Montreal (Tukey HSD, all p < 0.01) (figure 4b). On days when temperatures are less than 0°C, snow is more than 5 cm deeper in Montreal than the other three cities (Tukey HSD, all p < 0.01) (figure 4c). Moreover, cyanogenesis frequency is highest in Montreal (ANOVA, p < 0.001). These results support the hypothesis that greater snow cover in Montreal protects plants from freezing temperatures.
4. Discussion
Our results demonstrate that parallel clines in cyanogenesis have evolved in response to urbanization. We originally hypothesized that clover populations would exhibit adaptive clines along urbanization gradients with increasing cyanogenesis toward the urban centre. Instead, populations have evolved clines of decreasing cyanogenesis toward the urban centres of three cities, and no cline in a fourth. Data from a field experiment suggest that the defensive benefits of cyanogenesis are unlikely to explain the observed clines. Rather, we found evidence that snow cover was reduced and minimum winter temperatures were coldest in urban centres. Such ‘winter urban cold islands’ are expected to select against cyanogenic genotypes within cities. Our results are consistent with the interpretation that populations have rapidly adapted to urbanization gradients over fine spatial scales.
(a). Ecological causes of clines in cyanogenesis
Our conclusions are consistent with several studies of T. repens that experimentally demonstrate selection against cyanogenesis by freezing temperatures [23,36–38]. For example, field experiments conducted by Daday [36] and Dirzo & Harper [37] have demonstrated that cyanogenic T. repens plants experienced more frost damage than acyanogenic plants during the winter months; Olsen & Ungerer [23] partially replicated this pattern in the laboratory. In addition, decades of agricultural research has led to the conclusion that reduced cyanogenesis in T. repens is an adaptation to tolerate freezing temperatures [38]. Our observation of no cline in Montreal is also consistent with the hypothesis that high snow cover prevents the evolution of clines in cyanogenesis [39]. Further evidence that snow cover weakens selection against cyanogenesis comes from the observation that cyanogenesis frequencies are higher in Montreal (44.7%) than Toronto (32.3%), NYC (19.1%) and Boston (19.7%), despite Montreal having the coldest climate. Thus, we conclude that adaptation in response to winter urban cold islands is consistent with the established literature on selection against cyanogenesis.
Urbanization did not influence patterns of herbivory in our experiment. Although cyanogenic plants received less damage than acyanogenic plants, relative and absolute herbivory were unrelated to distance from the city. From this result, we conclude that spatial variation in herbivory is unlikely to explain the observed clines in cyanogenesis. Our results are consistent with other studies that find no consistent trend in herbivory along urbanization gradients (reviewed in [3]). Herbivory can vary both spatially and temporally [40], and so it is possible that our results could change if we had chosen different locations or conducted the experiment in another year. However, the large number of experimental populations (n = 38) and the lack of any clear association in herbivory with the urbanization gradient make this explanation unlikely. The ecological causes of clines in cyanogenesis can vary for different clines [16], and we acknowledge that gradients in herbivory could cause clines in other cities where we did not study herbivory.
(b). Alternative mechanisms causing clines in cyanogenesis
Our data allow us to rule-out several alternative mechanisms that could explain our results. First, because clines did not differ among multiple transects in Toronto, it is unlikely that the specific choice of transect in each city influenced whether or not we detected a cline. Second, clines in cyanogenesis can be driven by selection favouring alleles at either of the two underlying loci (Ac and Li), rather than cyanogenesis itself. Our observation that overall gene frequencies at both Ac and Li increase toward the urban centre suggests that cyanogenesis itself is under selection. Ac and Li gene frequencies in acyanogenic plants have a weaker association with urbanization than in cyanogenic plants (electronic supplementary material, figure S3c,d), suggesting that Ac and Li are under much stronger selection in cyanogenic plants. Third, because we conducted an experiment under natural field conditions during the summer, and observed no advantage of acyanogenic phenotypes in cities, it is unlikely that natural selection during the summer months drives the evolution of cyanogenesis clines (electronic supplementary material, text S2). Fourth, an experiment evaluating the effects of simulated mowing and nutrient supplementation on relative fitness of cyanogenic and acyanogenic plants found equal effects of both treatments on the growth of cyanogenic and acyanogenic plants (electronic supplementary material, text S3). Last, preliminary population genetic data demonstrate an absence of population structure at neutral markers along urbanization gradients (A. Nelson, K. Thompson and M. Johnson 2016, unpublished data), and thus genetic drift associated with population bottlenecks and stepping-stone invasions is unlikely to explain our results.
(c). Future research
Future research will aim to test the predictions generated by this study. First, a snow-removal experiment will provide empirical data to test the hypothesis that snow cover weakens selection against cyanogenesis—a hypothesis that we tested indirectly. In addition, sampling clines in cyanogenesis in cities along a latitudinal gradient will evaluate whether clines occur in a ‘goldilocks zone’ where snowfall is neither too prevalent nor too rare. Studies of clines in cities of various sizes but similar climate will determine whether adaptive clines are specific only to large cities, where gene flow between urban and non-urban areas may be reduced [8]. Quantification of population genetic structure will evaluate whether clines can evolve by neutral—as opposed to adaptive—processes. Together, these studies will advance our understanding of the conditions that are necessary and sufficient for clines in cyanogenesis to arise along urbanization gradients.
5. Conclusion and perspectives
Our results contribute to the growing body of knowledge on evolution in urban ecosystems. We build upon previous studies demonstrating adaptive evolution in cities by demonstrating that adaptive clines occur along urbanization gradients, and by identifying a pattern of phenotypic evolution with a known genetic basis. We also provide evidence that parallel evolution—one of the hallmarks of evolution by natural selection [41]—occurs along urbanization gradients within and among cities. Importantly, however, we demonstrate that parallel evolution does not necessarily occur in all urban areas. Rather, our data suggest that unique features of individual cities, such as climate, may influence the strength of selection imposed by urbanization.
Our study suggests that wild plant populations are rapidly adapting to the unique environmental conditions associated with urbanization, and highlights white clover as a model system for the study of adaptation in urban ecosystems. Clines in cyanogenesis arise from spatial sorting of standing genetic variation at cyanogenesis loci [18], which probably explains the rapid evolution of these clines [42]. White clover is the most abundant source of nectar for pollinators in many temperate ecosystems [43]. Thus, the ability of this species to rapidly adapt to urban environments may ultimately facilitate diverse and abundant pollinator communities in urban ecosystems. Despite recent progress in documenting evolution in urban environments, it is currently unclear whether the capacity to adapt to urban environments is a general phenomenon. More taxa and more traits must be studied to understand the long-term effects of urbanization on biological diversity.
Supplementary Material
Acknowledgements
We thank C. Cameron, M. Escobar, M. Ganguli, R. Mittal, C. Prashad, J. Santangelo and M. Urquhart-Cronish for help in the laboratory and field, and many people who donated their lawns (electronic supplementary material, text S3). We thank A. Agrawal, S. Barrett, F. Gould, Y. He, P. Kotanen, J. Losos, J. Merilä, D. Saini, D. Schluter, anonymous reviewers and the EvoEco Lab, for providing comments on the paper.
Data accessibility
The data and analysis script used to produce the results of this study are archived on Dryad (http://dx.doi.org/10.5061/dryad.15d2p) [44].
Authors' contributions
K.A.T. and M.T.J.J. conceived and designed the study. All authors participated in field collections. K.A.T. conducted the field experiment with assistance from M.T.J.J. and M.R. M.R conducted the growth chamber experiment with input from K.A.T. K.A.T. and M.T.J.J. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
K.A.T. was supported by an NSERC CGS-M and a QEII Scholarship. The project was supported by an NSERC Discovery Grant to M.T.J.J.
References
- 1.United Nations Department of Economics and Social Affairs. 2014. World urbanization prospects: the 2014 revision, highlights (ST/ESA/SER.A/352).
- 2.Oke TR. 1973. City size and the urban heat island. Atmos. Environ. 7, 769–779. ( 10.1016/0004-6981(73)90140-6) [DOI] [Google Scholar]
- 3.Raupp MJ, Shrewsbury PM, Herms DA. 2010. Ecology of herbivorous arthropods in urban landscapes. Annu. Rev. Entomol. 55, 19–38. ( 10.1146/annurev-ento-112408-085351) [DOI] [PubMed] [Google Scholar]
- 4.Hahs AK, et al. 2009. A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 12, 1165–1173. ( 10.1111/j.1461-0248.2009.01372.x) [DOI] [PubMed] [Google Scholar]
- 5.Lambert MR, Giller GSJ, Barber LB, Fitzgerald KC, Skelly DK. 2015. Suburbanization, estrogen contamination, and sex ratio in wild amphibian populations. Proc. Natl Acad. Sci. USA 112, 201501065 ( 10.1073/pnas.1501065112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberti M. 2015. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126. ( 10.1016/j.tree.2014.11.007) [DOI] [PubMed] [Google Scholar]
- 7.Donihue CM, Lambert MR. 2014. Adaptive evolution in urban ecosystems. Ambio 44, 194–203. ( 10.1007/s13280-014-0547-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson MTJ, Thompson KA, Saini HS. 2015. Plant evolution in the urban jungle. Am. J. Bot. 102, 1951–1953. ( 10.3732/ajb.1500386) [DOI] [PubMed] [Google Scholar]
- 9.Cheptou P-O, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proc. Natl Acad. Sci. USA 105, 3796–3799. ( 10.1073/pnas.0708446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winchell KM, Reynolds RG, Prado-Irwin SR, Puente-Rolón AR, Revell LJ. 2016. Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evolution 70, 1009–1022. ( 10.1111/evo.12925) [DOI] [PubMed] [Google Scholar]
- 11.Mueller JC, Partecke J, Hatchwell BJ, Gaston KJ, Evans KL. 2013. Candidate gene polymorphisms for behavioural adaptations during urbanization in blackbirds. Mol. Ecol. 22, 3629–3637. ( 10.1111/mec.12288) [DOI] [PubMed] [Google Scholar]
- 12.Harris SE, O'Neill RJ, Munshi-South J. 2015. Transcriptome resources for the white-footed mouse (Peromyscus leucopus): new genomic tools for investigating ecologically divergent urban and rural populations. Mol. Ecol. Resour. 15, 382–394. ( 10.1111/1755-0998.12301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonnell MJ, Pickett STA. 1990. Ecosystem structure and function along urban–rural gradients: an unexploited opportunity for ecology. Ecology 71, 1232–1237. ( 10.2307/1938259) [DOI] [Google Scholar]
- 14.Cook LM. 2003. The rise and fall of the Carbonaria form of the peppered moth. Q. Rev. Biol. 78, 399–417. ( 10.1086/378925) [DOI] [PubMed] [Google Scholar]
- 15.Endler JA. 1977. Geographic variation, speciation, and clines. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 16.Kooyers NJ, Olsen KM. 2013. Searching for the bull's eye: agents and targets of selection vary among geographically disparate cyanogenesis clines in white clover (Trifolium repens L.). Heredity 111, 495–504. ( 10.1038/hdy.2013.71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes MA. 1991. The cyanogenic polymorphism in Trifolium repens L. (white clover). Heredity 66, 105–115. ( 10.1038/hdy.1991.13) [DOI] [Google Scholar]
- 18.Olsen KM, Kooyers NJ, Small LL. 2014. Adaptive gains through repeated gene loss: parallel evolution of cyanogenesis polymorphisms in the genus Trifolium (Fabaceae). Phil. Trans. R. Soc. B 369, 20130347 ( 10.1098/rstb.2013.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daday H. 1954. Gene frequencies in wild populations of Trifolium repens I. Distribution by latitude. Heredity 8, 61–78. ( 10.1111/evo.12900) [DOI] [Google Scholar]
- 20.Daday H. 1954. Gene frequencies in wild populations of Trifolium repens II. Distribution by altitude. Heredity 8, 377–384. ( 10.1038/hdy.1954.40) [DOI] [Google Scholar]
- 21.Kooyers NJ, Olsen KM. 2012. Rapid evolution of an adaptive cyanogenesis cline in introduced North American white clover (Trifolium repens L.). Mol. Ecol. 21, 2455–2468. ( 10.1111/j.1365-294X.2012.05486.x) [DOI] [PubMed] [Google Scholar]
- 22.Daday H. 1958. Gene frequencies in wild populations of Trifolium repens L. III. World distribution. Heredity 12, 169–184. ( 10.1038/hdy.1958.22) [DOI] [Google Scholar]
- 23.Olsen KM, Ungerer MC. 2008. Freezing tolerance and cyanogenesis in white clover (Trifolium repens L. Fabaceae). Int. J. Plant Sci. 169, 1141–1147. ( 10.1086/591984) [DOI] [Google Scholar]
- 24.Landsberg HE. 1981. The urban climate. New York, NY: Academic Press. [Google Scholar]
- 25.Parris KM, Hazell DL. 2005. Biotic effects of climate change in urban environments: the case of the grey-headed flying-fox (Pteropus poliocephalus) in Melbourne, Australia. Biol. Conserv. 124, 267–276. ( 10.1016/j.biocon.2005.01.035) [DOI] [Google Scholar]
- 26.Gleadow R, Bjarnholt N, Jørgensen K, Fox J, Miller R. 2011. Cyanogenic glycosides. In Research methods in plant sciences volume 1: soil allelochemicals (eds Narwal S, Szajdak L, Sampietro D), pp. 283–310. Houston, TX: Studium Press. [Google Scholar]
- 27.Thompson KA, Johnson MTJ. 2016. Antiherbivore defenses alter natural selection on plant reproductive traits. Evolution 70, 796–810. ( 10.1111/evo.12900) [DOI] [PubMed] [Google Scholar]
- 28.Sinnott RW. 1984. Virtues of the haversine. Sky Telescope 68, 159. [Google Scholar]
- 29.R Core Team. 2014. R: A language and environment for statistical computing. Vienna, Austria. See http://www.R-project.org/.
- 30.CIESIN. 2015. Gridded Population of the World, Version 4 (GPWv4): Population Density Adjusted to Match 2015 Revision of UN WPP Country Totals.
- 31.Goodrich LE. 1982. The influence of snow cover on the ground thermal regime. Can. Geotech. J. 19, 421–432. ( 10.1139/t82-047) [DOI] [Google Scholar]
- 32.Salomonson VV, Appel I. 2004. Estimating fractional snow cover from MODIS using the normalized difference snow index. Remote Sens. Environ. 89, 351–360. ( 10.1016/j.rse.2003.10.016) [DOI] [Google Scholar]
- 33.QGIS Development Team. 2014. QGIS Geographic Information System. Open Source Geospatial Foundation Project. See http://qgis.osgeo.org. QGIS Geogr. Inf. Syst. Open Source Geospatial Found. Proj. http//qgis.osgeo.org .
- 34.Kakes P. 1987. On the polymorphism for cyanogenesis in natural populations of Trifolium repens L. in the Netherlands I. Distribution of the genes Ac and Li. Acta Bot. Neerl. 36, 59–70. ( 10.1111/j.1438-8677.1987.tb01967.x) [DOI] [Google Scholar]
- 35.Shimamura Y, Izumi T, Matsuyama H. 2006. Evaluation of a useful method to identify snow-covered areas under vegetation—comparisons among a newly proposed snow index, normalized difference snow index, and visible reflectance. Int. J. Remote Sens. 27, 4867–4884. ( 10.1080/01431160600639693) [DOI] [Google Scholar]
- 36.Daday H. 1965. Gene frequencies in wild populations of Trifolium repens L. IV. Mechanism of natural selection. Heredity 20, 355–365. ( 10.1038/hdy.1965.49) [DOI] [Google Scholar]
- 37.Dirzo R, Harper JLJ. 1982. Experimental studies on slug–plant interactions: IV. The performance of cyanogenic and acyanogenic morphs of Trifolium repens in the field. J. Ecol. 70, 119–138. ( 10.2307/2259868) [DOI] [Google Scholar]
- 38.Caradus JR, Eerens JPJ. 1989. Genetic adaptation to frost tolerance in white clover. Proc. Annu. Conf. Agron. Soc. N. Z. 22, 103–109. [Google Scholar]
- 39.Till-Bottraud I, Gouyon P-H. 1992. Intra- versus interplant Batesian mimicry? A model on cyanogenesis and herbivory in clonal plants. Am. Nat. 139, 509–520. ( 10.2307/2462495) [DOI] [Google Scholar]
- 40.Karban R, Baldwin IT. 1997. Induced responses to herbivory. Chicago, IL: University of Chicago Press. [Google Scholar]
- 41.Schluter D, Clifford EA, Nemethy M, McKinnon JS. 2004. Parallel evolution and inheritance of quantitative traits. Am. Nat. 163, 809–822. ( 10.1086/383621) [DOI] [PubMed] [Google Scholar]
- 42.Barrett RDH, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44. ( 10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 43.Baude M, Kunin WE, Boatman ND, Conyers S, Davies N, Gillespie MAK, Morton RD, Smart SM, Memmott J. 2016. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 530, 85–88. ( 10.1038/nature16532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson KA, Renaudin M, Johnson MTJ. 2016. Data from: Urbanization drives the evolution of parallel clines in plant populations. Dryad Digital Repository. ( 10.5061/dryad.15d2p) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and analysis script used to produce the results of this study are archived on Dryad (http://dx.doi.org/10.5061/dryad.15d2p) [44].