Abstract
Cell fusion is a fundamental phenomenon observed in all eukaryotes. Cells can exchange resources such as molecules or organelles during fusion. In this paper, we ask whether a cell can also transfer an adaptive response to a fusion partner. We addressed this question in the unicellular slime mould Physarum polycephalum, in which cell–cell fusion is extremely common. Slime moulds are capable of habituation, a simple form of learning, when repeatedly exposed to an innocuous repellent, despite lacking neurons and comprising only a single cell. In this paper, we present a set of experiments demonstrating that slime moulds habituated to a repellent can transfer this adaptive response by cell fusion to individuals that have never encountered the repellent. In addition, we show that a slime mould resulting from the fusion of a minority of habituated slime moulds and a majority of unhabituated ones still shows an adaptive response to the repellent. Finally, we further reveal that fusion must last a certain time to ensure an effective transfer of the behavioural adaptation between slime moulds. Our results provide strong experimental evidence that slime moulds exhibit transfer of learned behaviour during cell fusion and raise the possibility that similar phenomena may occur in other cell–cell fusion systems.
Keywords: cell fusion, habituation, learned behaviour, Physarum polycephalum, slime moulds
1. Introduction
Cell fusion is a crucial process to the development and physiology of most living organisms, whether plants, fungi, protists or animals. It is involved in a large range of biological process such as mating, fertilization, muscle, bone and placenta organogenesis, immune response, tumorigenesis, and cell-mediated tissue regeneration (review in [1–5]). While cell fusion remains a relatively rare event restricted to particular cell types in animals, it constitutes a defining feature of the lifestyle of most filamentous fungi and slime moulds. In these organisms, cell fusion enhances foraging success [6,7] and provides the potential for cell assistance through resource sharing [8–11]. Here, using the unicellular slime mould Physarum polycephalum as a model system, we report a new feature of cell fusion never described before: the transmission of learned behaviour from one cell to another.
Physarum polycephalum belongs to the Amoebozoa, the sister group to fungi and animals (the opisthokonts), and presents the highest molecular complexity when compared with other sequenced Amoebozoa [12]. It is a giant multinucleated cell, which can extend up to hundreds of square centimetres. The cell forms pseudopods and creeps along various surfaces at a maximum speed of 4 cm per hour. P. polycephalum demonstrates amazing abilities, such as finding its way through a maze [13], building a smart network [14], solving complex nutritional challenges [15], avoiding being trapped [16] and anticipating periodic events [17]. In addition, P. polycephalum exhibits a form of self-signalling and shows chemoattractive movements towards other individuals [18]. When genetically or phenotypically identical slime moulds meet, they fuse to form a single individual [18]. Recently, we revealed that slime moulds showed an adaptive behavioural response when repeatedly exposed to a repellent, thus meeting the criteria for habituation, a simple form of learning [19]. Here, we describe a series of experiments that investigate whether this learned behaviour could be transferred from one individual to another during cell fusion.
2. Material and methods
(a). Species
Physarum polycephalum is an acellular slime mould that inhabits shady, temperate and damp areas, such as the forest litter. Its haplodiplophasic life cycle is composed of several stages, including spores, plasmodia, sclerotia and fruiting bodies [20]. In our experiments, we used the plasmodium, which is the vegetative, active, growing and feeding stage. The plasmodium is a vast multinucleate amoeboid cell that crawls over damp wood, leaves or soil, ingesting bacteria, moulds and fungi. When exploring its environment, the plasmodium extends tubular structures called pseudopods. Under adverse conditions, such as food shortage, desiccation or low temperatures, the plasmodium converts into a dormant stage called the sclerotium. This dehydrated and hardened structure may revert to a plasmodium when favourable environmental conditions return. Eight sclerotia of P. polycephalum were used to obtain cultures of plasmodia (Southern Biological, Victoria, Australia). The plasmodia were cultured in large Petri dishes (diameter = 135 mm) on a 1% agar gel containing 10% of blended oat flakes (Quaker Oats Company), at 25°C in the dark. All the experiments described below took place in temperature-controlled chambers set to 24°C. In each chamber, a high-definition camera (EOS 70D, Canon) took a picture every 5 min.
(b). Habituation assay
In a first experiment, we verified that slime moulds have the ability to habituate to a repellent [19].
Two hundred and forty slime moulds were first accustomed to the experimental set-up. They were taken from the culture using a template (H = 2 mm, diameter = 18 mm) and introduced in an experimental arena (Petri dish diameter = 90 mm). The plasmodia were then connected to a food patch (1% agar gel containing 10% of blended oat flakes, H = 2 mm, diameter = 18 mm) using an agar gel bridge (1% agar gel, H = 2 mm, L = 13 mm, W = 15 mm). The slime moulds first explored the bridge by expanding pseudopods, then found the food and started to exploit it. After 24 h, the slime moulds had fully covered the food patch and were transferred to new experimental arenas to start the habituation experiment. We followed the habituation protocol we developed in a previous study using salt (NaCl) as a repellent [21] instead of quinine or caffeine [19]. The experiment lasted 9 days and was organized in four different phases: the habituation phase (days 1–5), test phase 1 (day 6), the recovery phase (days 7–8) and test phase 2 (day 9). During the habituation phase, 120 slime moulds referred to as habituated (H) were required to cross an agar gel bridge with a repellent (150 mM NaCl) to exploit a new food patch every day for 5 days, whereas 120 slime moulds referred to as unhabituated (U) had to cross a bridge without repellent. Typically, every day, each slime mould crossed the bridge, exploited the food and then was transferred to another arena the next day, and so on. Each daily transfer was done at the exact same time of the day. During test phase 1 (day 6), all slime moulds were required to cross a bridge with the repellent to reach the food and were then transferred to a new arena for the recovery phase. During the recovery phase, all slime moulds had to cross a plain agar bridge to find the food once a day for 2 days. During test phase 2, all slime moulds were again constrained to cross an agar gel bridge with the repellent to obtain the food.
(c). Transfer of learned behaviour
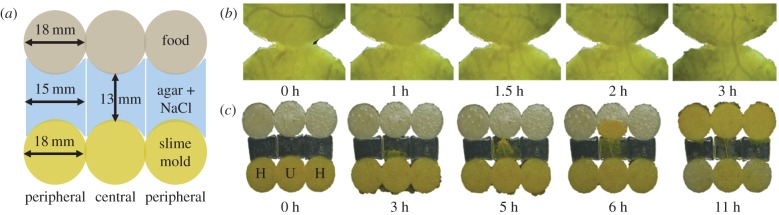
In a second experiment, we explored whether slime moulds can transfer learned behaviour to other individuals during fusion. First, slime moulds referred to as habituated slime moulds (H, n = 2190) were acclimated to a repellent (NaCl) following the 5-day habituation phase described above. At the exact same time, others slime moulds referred to as unhabituated slime moulds (U, n = 2190) followed the same protocol but without the repellent. Second, on day 6, habituated and unhabituated slime moulds were allowed to fuse and the merged entity was required to cross an agar gel bridge with the repellent to reach the food (figure 1a). Fusion was allowed by bringing the slime moulds into contact in an experimental arena (figure 1b). One hour after the first contact, we checked that fusion happened between the slime moulds and we introduced the bridge and the food. In total, we tested 19 configurations varying both the number of slime moulds used to form the merged entities (from 2 to 4) and the number of habituated slime moulds within the merged entities (from 0 to 4). The sizes of the bridge and the food source were both adjusted to the size of the merged entity.
Figure 1.
Transfer of learned behaviour. (a) Schematic of set-up for three slime moulds. (b) Photographs illustrate the fusion of two slime moulds. Close up of the point of contact between two slime moulds. Veins between the two slime moulds are apparent after 2 h. (c) Transfer of learned behaviour experiment where the merged entity resulted from the fusion of two habituated slime moulds (H) located at the periphery and one unhabituated slime mould (U) located at the centre of the merged entity. In this example, the pseudopod crossing the bridge comes from the unhabituated slime mould. (Online version in colour.)
(d). Time required for the transfer of learned behaviour
In a third experiment, we estimated how long the fusion had to last for an effective transfer of learned behaviour. As before, 250 slime moulds were habituated (H) with the repellent (NaCl, 150 mM) following a 5-day period of habituation, whereas 250 slime moulds were unhabituated (U). On day 6, pairs of slime moulds (UU, UH and HH) were formed and allowed to fuse. One hour or 3 h after the fusion, the slime moulds were then gently separated at the point of contact using a spatula. Three hours was chosen as a maximum because merged entities started to form a pseudopod 3 h after fusion in the previous experiment (electronic supplementary material, figure S1b). Then, all slime moulds were tested individually for habituation and were required to cross an agar gel bridge with NaCl to reach the food source.
(e). Variable recorded
The slime mould aversive behaviour was quantified by measuring the time needed to cross the bridge every day, for each slime mould and each experiment [19]. The measurements started when a pseudopod appeared on the bridge and stopped as soon as it contacted the food. To estimate the level of habituation, we developed an index (HI) inspired from the animal literature about habituation. The index was calculated as HI = (TŪ – TF)/TŪ, where TF is the time to cross the bridge for the focal merged entity and TŪ is the mean time to cross the bridge for the unhabituated merged entities. Values close to 0 indicate an aversion towards the repellent, whereas values clearly above zero indicate habituation to the repellent. TŪ was computed for each sclerotium and each experiment to take into account inherent speed variation. Regarding the merged entity, we recorded the provenance of the pseudopod that first contacted the food. In other words, for each replicate, we noted if the pseudopod came from a habituated or an unhabituated slime mould (figure 1c).
(f). Statistical analysis
The data were analysed using (i) generalized linear models to compare the time to cross the bridge and (ii) exact binomial tests to compare the provenance of the pseudopod that reached first the food source. Nonlinear fitting models were used to study the habituation dynamics and the habituation index response as a function of the proportion of habituated slime moulds (see the electronic supplementary material). All statistical analyses were conducted using R v. 3.2.3.
3. Results
(a). Habituation assay
On day 1, habituated slime moulds, facing the repellent for the first time, showed a clear aversive behaviour, crossing the bridge slowly, whereas unhabituated slime moulds, encountering plain agar, crossed the bridge twice as fast (figure 2a; linear model F1,238 = 344.7 p < 0.001; electronic supplementary material, table S1). The following days of the habituation phase, habituated slime moulds crossed the bridge quicker and quicker (electronic supplementary material, table S1). The decrease in responsiveness follows an inverse power function of the number of repellent presentations, a hallmark of habituation [22] (nonlinear model, R2 = 0.93, p < 0.001; electronic supplementary material, table S1). On day 6, habituated and unhabituated slime moulds were tested for habituation and were required to cross an agar gel bridge containing the repellent. Unhabituated slime moulds, which encountered the repellent for the first time, showed a strong aversive behaviour and a habituation index equal to 0. In contrast, habituated slime moulds, encountering the repellent for the sixth time, showed no aversive behaviour and a much higher habituation index (linear model, F1,214 = 131.80 p < 0.001; figure 1b; electronic supplementary material, table S1). On days 7 and 8, habituated and unhabituated slime moulds were allowed to recover from habituation and were required to cross a plain agar bridge to reach the food once a day. During this phase, the behavioural responses of habituated and unhabituated slime moulds were not statistically different. All slime moulds crossed the plain agar bridge quickly (mean time ± CI95: 130.1 ± 3.6 min and 136.7 ± 4.6 min for habituated and unhabituated slime moulds, respectively). On day 9, habituated and unhabituated slime moulds were tested for recovery and had to cross an agar gel bridge containing the repellent. All slime moulds showed a similar aversive behaviour towards the repellent and their habituation indexes were not statistically different (linear model, F1,238 = 0.14, p = 0.714; figure 2b; electronic supplementary material, table S1). The behavioural response of the habituated slime moulds after recovery was similar to their behaviour when they encountered the repellent for the first time on day 1, indicating that habituated slime moulds had recovered from the habituation.
Figure 2.
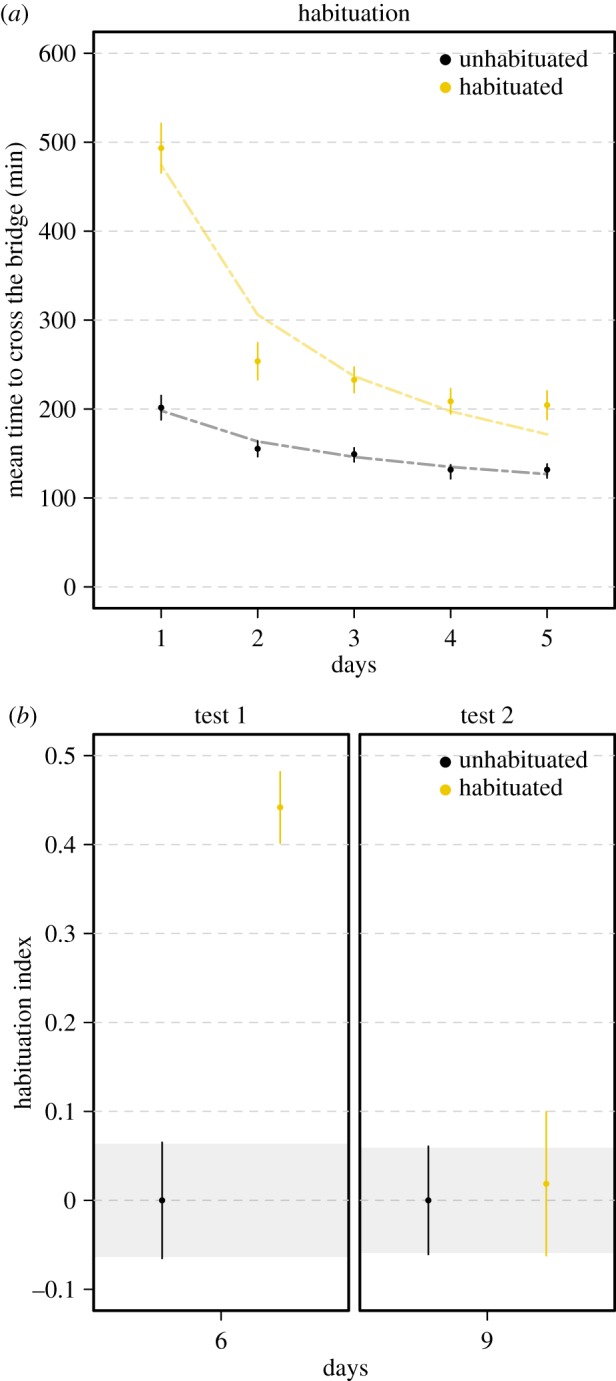
Habituation: slime moulds learn to ignore a repellent. (a) Time to cross the bridge during the habituation phase for each treatment. Slime moulds were required every day to cross an agar gel bridge with a repellent NaCl (150 mM; habituated treatment) or a plain agar gel bridge without the repellent (unhabituated treatment) to reach a food source. High values of time to cross the bridge indicate an aversive response. The dashed lines show the nonlinear fittings for the habituation dynamic. The habituated slime moulds stop responding to the repellent after 5 days. The decrease in responsiveness is shown as an inverse power function of the number of repellent presentations (R2 = 0.93, p < 0.00; electronic supplementary material, table S1). (b) Habituation index computed after the habituation phase (test 1) and after the recovery phase (test 2). During the test phases, all slime moulds had to cross a bridge with the repellent. Values close to 0 indicate an aversion to the repellent while values clearly above 0 indicate habituation. n = 240 slime moulds in total. Error bars indicate +95% CI. The CI of the unhabituated slime moulds are delineated by shaded areas. (Online version in colour.)
In this first experiment, using chemotaxis as the behavioural output and NaCl as a repellent stimuli, we demonstrated that slime moulds stopped responding to the aversive stimulus when it was repeated, but responded again when it was withheld, thus meeting the established criteria for habituation [22].
(b). Transfer of learned behaviour
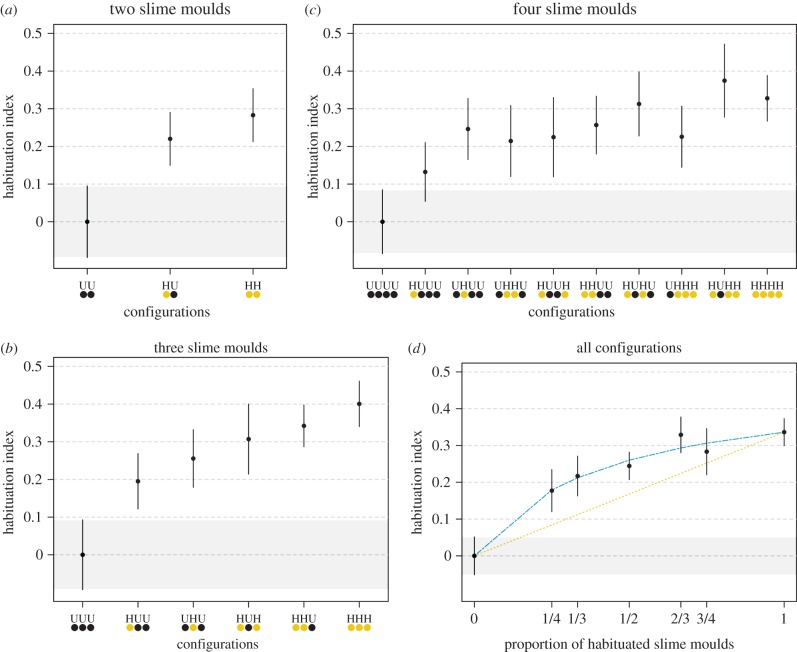
Having rigorously established that slime moulds can learn to ignore a repellent, we investigated whether these slime moulds can transfer this learned behaviour to other individuals. Habituated slime moulds were paired with unhabituated ones and allowed to interact. When the slime moulds came into contact, they fused to form a single plasmodium. Veins connected the two slime moulds and extensive protoplasmic mixing took place (figure 1b; electronic supplementary material, movie S1). Following the fusion, the merged entity was required to cross an agar gel bridge with NaCl as the repellent. Merged entities that included at least one habituated slime mould had a lower aversive response to NaCl than merged entities comprising only unhabituated slime moulds (generalized linear model, p < 0.001 for all configurations; electronic supplementary material, table S2). Increasing the proportion of habituated slime moulds within the merged entity just slightly increased its level of habituation (figure 3a–c). The relationship between the proportion of habituated slime moulds PH within the merged entity and the habituation index is nonlinear, ruling out the hypothesis that the behavioural response is passively quenched by cytoplasm dilution within the merged entity (nonlinear model, R2 = 0.97, p < 0.001; figure 3d; electronic supplementary material, table S3). When PH < 0.4, the habituation index increases linearly with PH. In contrast, when PH > 0.4, the habituation index becomes independent of PH and asymptotically approaches its maximum value (HImax = 0.47). Unexpectedly, the pseudopod that first reached the food source was often formed by an unhabituated slime mould rather than by a habituated one (probabilities of contacting the food first for an unhabituated slime moulds: 0.72 ± 0.03; electronic supplementary material, figure S1b and table S4).
Figure 3.
Transfer of learned behaviour. Habituation index for merged entities required to cross an agar gel bridge with a repellent (NaCl 150 mM) to reach a food source. Values close to 0 indicate an aversion to NaCl, whereas values largely above 0 indicate habituation to the repellent. The merged entities resulted from the fusion of (a) two, (b) three or (c) four slime moulds. The number of habituated (H) and unhabituated slime moulds (U) within the merged entity varied from 0 to 4. The habituated and the unhabituated slime moulds were either at the periphery or at the centre of the merged entity. Habituated slime moulds were habituated to the repellent (NaCl) for 5 days before the fusion. Unhabituated slime moulds followed the same habituation protocol but without the repellent. (d), Habituation index as a function of the proportion of habituated slime moulds (all configuration considered). The dashed line shows the predicted values of the nonlinear fitting (R2 = 0.97, p < 0.001). The dotted line represents the predicted value for the linear model (simple dilution of the response). The CI of the unhabituated merged entities (UU, UUU and UUUU) are delineated by shaded areas. n= 4380 slime moulds tested in total. Error bars indicate +95% CI. (Online version in colour.)
(c). Time required for the transfer of learned behaviour
Having demonstrated that slime moulds can transfer learned behaviour, we estimated how long the fusion had to last for the transfer to be successful. When fusion lasted 1 h, unhabituated slime moulds, which had fused with habituated ones, showed no sign of habituation and behaved like unhabituated slime moulds (linear model, p = 0.999; figure 4; electronic supplementary material, table S5). In contrast, when fusion was lengthened to 3 h, unhabituated slime moulds allowed to fuse with habituated ones demonstrated similar level of habituation as habituated slime moulds (linear model, p = 0.991; figure 4; electronic supplementary material, table S5). This last experiment definitively proves that transfer of learned behaviour happened between the two slime moulds during fusion.
Figure 4.
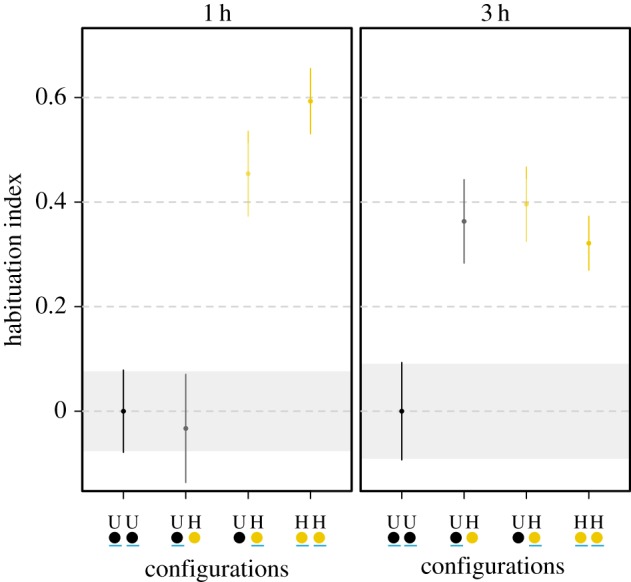
Time required for the transfer of learned behaviour. Habituation index according to fusion length. Slime moulds were allowed to fuse for either 1 or 3 h before being separated again and tested individually with the repellent. Habituated slime moulds (H) were habituated to the repellent (NaCl) for 5 days before the fusion. Unhabituated slime moulds (U) followed the same habituation protocol but without the repellent. Values close to 0 indicate an aversion to NaCl, whereas values largely above 0 indicate a habituation to the repellent. The underlined dot represents the slime mould under scrutiny. CI of the unhabituated slime moulds are delineated by shaded areas. n = 500 slime moulds in total. Error bars indicate +95% CI. (Online version in colour.)
4. Discussion
Our results show that non-neural organisms can learn to avoid a repellent and directly transfer this behavioural response to other individuals during cell fusion. The nonlinear relationship between the proportion of habituated slime moulds within the merged entity and the level of habituation precludes a simple dilution of the response via cytoplasm mixing [23], and reveals that a minimum of habituated individuals is required for the merged entity to show an adaptive behavioural response. The transfer of learned behaviour is emphasized by two facts. First, the pseudopod that crossed the bridge often originated from the unhabituated slime mould. Second, an unhabituated slime mould, which was first allowed to fused with a habituated one and then separated, still showed an adaptive behavioural response.
Recently, we discovered that non-neural organisms are able to learn to ignore an innocuous stimulus when this stimulus is repeated. This result raised the exciting possibility that mechanisms for learning might pre-date the evolution of nervous systems and could be emulated through physiological adaptation in non-neuronal organisms. In this paper, we went a step further and we showed that learned behaviour could be transferred from one individual to another during cell fusion. The ability of slime moulds to share an adaptive behavioural response directly via cell fusion provides a rapid and efficient means for slime moulds to adapt to their environment. Slime moulds that have been habituated to an environmental repellent can relay by fusion any potential changes in gene expression or physiology that occur during habituation. Recipient slime moulds then become pre-habituated to environmental repellents before the repellents are even encountered. Thus, fusion can confer resistance to naive slime moulds that otherwise would be susceptible to this repellent.
Content exchange between cells is often observed as a strategy for eukaryotic organisms to cope with environmental stress [24]. For example, transfer of mitochondria between cells provides a way to rescue recipient cells under respiratory stress [25]. Here, we show that to combat environmental stress, survival strategies of cells might extend to transfer of adaptive behavioural response, a notion that will stimulate further studies on other eukaryotic cells. Physarum polycephalum not only has the natural ability to form multinucleate giant cells via fusion, but it also possesses a complex network of signalling molecules common to all eukaryotic cells [12]. This combination of attributes made it an interesting model system to investigate cell fusion in more complex, multicellular species.
Supplementary Material
Acknowledgements
We are grateful to E. Haenel for pilot studies. We thank Mathieu Lihoreau for fruitful discussions and R. Jeanson for his comments on earlier version of the manuscript. D.V. is supported by the Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture and A.D. by the CNRS.
Data accessibility
Data have been deposited in the Dryad Digital Repository [26].
Authors' contributions
A.D. conceived the study. D.V. and A.D designed the experiments. D.V. conducted the experiments and the analysis. D.V. and A.D wrote the paper.
Competing interests
No competing interests.
Funding
We received no funding for this study.
References
- 1.Chen EH, Olson EN. 2005. Unveiling the mechanisms of cell–cell fusion. Science 308, 369–373. ( 10.1126/science.1104799) [DOI] [PubMed] [Google Scholar]
- 2.Ogle BM, Cascalho M, Platt JL. 2005. Biological implications of cell fusion. Nat. Rev. Mol. Cell Biol. 6, 567–575. ( 10.1038/nrm1678) [DOI] [PubMed] [Google Scholar]
- 3.Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B, Wang H, Wong M. 2013. Genetic basis of cell–cell fusion mechanisms. Trends Genet. 29, 427–437. ( 10.1016/j.tig.2013.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez-Vargas J, Krey T, Valansi C, Avinoam O, Haouz A, Jamin M, Raveh-Barak H, Podbilewicz B, Rey FA. 2014. Structural basis of eukaryotic cell–cell fusion. Cell 157, 407–419. ( 10.1016/j.cell.2014.02.020) [DOI] [PubMed] [Google Scholar]
- 5.Herzog S, Schumann MR, Fleißner A. 2015. Cell fusion in Neurospora crassa. Curr. Opin. Microbiol. 28, 53–59. ( 10.1016/j.mib.2015.08.002) [DOI] [PubMed] [Google Scholar]
- 6.Richard F, Glass NL, Pringle A. 2012. Cooperation among germinating spores facilitates the growth of the fungus, Neurospora crassa. Biol. Lett. 8, 419–422. ( 10.1098/rsbl.2011.1141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastiaans E, Debets AJM, Aanen DK. 2015. Experimental demonstration of the benefits of somatic fusion and the consequences for allorecognition. Evolution (N.Y.) 69, 1091–1099. ( 10.1111/evo.12626) [DOI] [PubMed] [Google Scholar]
- 8.Marwan W. 2003. Detecting functional interactions in a gene and signaling network by time-resolved somatic complementation analysis. Bioessays 25, 950–960. ( 10.1002/bies.10342) [DOI] [PubMed] [Google Scholar]
- 9.Walter P, Hoffmann X-K, Ebeling B, Haas M, Marwan W. 2013. Switch-like reprogramming of gene expression after fusion of multinucleate plasmodial cells of two Physarum polycephalum sporulation mutants. Biochem. Biophys. Res. Commun. 435, 88–93. ( 10.1016/j.bbrc.2013.04.043) [DOI] [PubMed] [Google Scholar]
- 10.Glass NL, Rasmussen C, Roca MG, Read ND. 2004. Hyphal homing, fusion and mycelial interconnectedness. Trends Microbiol. 12, 135–141. ( 10.1016/j.tim.2004.01.007) [DOI] [PubMed] [Google Scholar]
- 11.Read ND, Fleißner A, Roca MG, Glass NL. 2010. Hyphal fusion. In Molecular biology of filamentous fungi (eds KA Borkovich, D Ebbole), pp. 260–273. St Paul, MN: APS Press. ( 10.1128/9781555816636.ch19) [DOI] [Google Scholar]
- 12.Schaap P, et al. 2016. The Physarum polycephalum genome reveals extensive use of prokaryotic two-component and metazoan-type tyrosine kinase signaling. Genome Biol. Evol. 8, 109–125. ( 10.1093/gbe/evv237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagaki T, Yamada H, Tóth A. 2000. Maze-solving by an amoeboid organism. Nature 407, 470 ( 10.1038/35035159) [DOI] [PubMed] [Google Scholar]
- 14.Tero A, Takagi S, Saigusa T, Ito K, Bebber DP, Fricker MD, Yumiki K, Kobayashi R, Nakagaki T. 2010. Rules for biologically inspired adaptive network design. Science 327, 439–442. ( 10.1126/science.1177894) [DOI] [PubMed] [Google Scholar]
- 15.Dussutour A, Latty T, Beekman M, Simpson SJ. 2010. Amoeboid organism solves complex nutritional challenges. Proc. Natl Acad. Sci. USA 107, 4607–4611. ( 10.1073/pnas.0912198107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid CR, Latty T, Dussutour A, Beekman M. 2012. Slime mold uses an externalized spatial ‘memory’ to navigate in complex environments. Proc. Natl Acad. Sci. USA 109, 17 490–17 494. ( 10.1073/pnas.1215037109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saigusa T, Tero A, Nakagaki T, Kuramoto Y. 2008. Amoebae anticipate periodic events. Phys. Rev. Lett. 100, 1–4. ( 10.1103/PhysRevLett.100.018101) [DOI] [PubMed] [Google Scholar]
- 18.Vogel D, Nicolis SC., Perez-Escudero A, Nanjundiah V, Sumpter DJT, Dussutour A. 2015. Phenotypic variability in unicellular organisms: from calcium signalling to social behaviour. Proc. R. Soc. B 282, 20152322 ( 10.1098/rspb.2015.2322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boisseau RP, Vogel D, Dussutour A. 2016. Habituation in non-neural organisms: evidence from slime moulds. Proc. R. Soc. B 283, 20160446 ( 10.1098/rspb.2016.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller HW, et al. 2008. Myxomycete plasmodia and fruiting bodies: unusual occurrences and user-friendly study techniques. Fungi 1, 24. [Google Scholar]
- 21.Adamatzky A. 2010. Routing Physarum with repellents. Eur. Phys. J. E 31, 403–410. ( 10.1140/epje/i2010-10589-y) [DOI] [PubMed] [Google Scholar]
- 22.Thompson RF. 2009. Habituation: a history. Neurobiol. Learn. Mem. 92, 127–134. ( 10.1016/j.nlm.2008.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamiya N, Allen RD, Yoshimoto Y. 1988. Dynamic organization of Physarum plasmodium. Cell Motil. Cytoskeleton 10, 107–116. ( 10.1002/cm.970100115) [DOI] [PubMed] [Google Scholar]
- 24.Vassallo CN, Wall D. 2016. Tissue repair in myxobacteria: A cooperative strategy to heal cellular damage. Bioessays 38, 306–315. ( 10.1002/bies.201500132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spees JL, Olson SD, Whitney MJ, Prockop DJ. 2006. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283–1288. ( 10.1073/pnas.0510511103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel D, Dussutour A.. 2016. Data from: Direct tranfer of learned behaviour via cell fusion in non-neural organisms. ( 10.5061/dryad.p3720) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been deposited in the Dryad Digital Repository [26].